Abstract
Background: The preoperative systemic inflammation response index (SIRI), based on peripheral neutrophil (N), monocyte (M), and lymphocyte (L) counts, has shown mounting evidence as an effective prognostic indicator in some malignant tumors. The aim of the present study was to evaluate the prognostic significance of pre-treatment SIRI in gastric cancer patients who received neoadjuvant chemotherapy (NACT).
Methods: This retrospective study comprised 107 patients with advanced gastric cancer treated with NACT between July 2007 and September 2015 in our hospital. SIRI was calculated from peripheral venous blood samples obtained prior to treatment. The best cutoff value for SIRI by receiver operating characteristic (ROC) curve was 1.2 (low SIRI <1.21, high SIRI ≥1.21). The clinical outcomes of disease-free survival (DFS) and overall survival (OS) were analyzed by Kaplan-Meier survival analysis and compared using the log-rank test. Univariate and multivariate analyses were performed by the Cox proportional hazards regression model.
Results: The results demonstrated that the low SIRI group was statistically associated with gender, primary tumor site, white blood cell, neutrophil, and monocyte counts, NLR (neutrophil to lymphocyte ratio), MLR (monocyte to lymphocyte ratio), and PLR (platelet to lymphocyte ratio). The SIRI was predictive for DFS and OS by univariate and multivariate analysis; the low SIRI group had better median DFS and OS than the high SIRI group (median DFS 27.03 vs. 22.33 months, median OS 29.73 vs. 24.43 months). The DFS and OS in the low SIRI group were longer than the high SIRI group.
Conclusions: SIRI may qualify as a useful, reliable, and convenient prognostic indicator in patients with advanced gastric cancer to help physicians to provide personalized prognostication for gastric cancer patients treated with NACT.
Keywords: prognosis, neoadjuvant chemotherapy, advanced gastric cancer, systemic inflammation response index (SIRI), tumor indicator
Introduction
Globally, gastric cancer (GC) is the sixth most common cancer and the third leading cause of cancer-related death; clearly, it remains a critical public health problem (1). According to worldwide statistics from 2020, a total of 1,089,000 new cases were diagnosed, and about 769,000 cases resulted in death in which many cases had advanced-stage disease at the time of diagnosis (2). Although the incidence and mortality rates of gastric cancer have declined over the last few decades in most parts of the world, the occurrence of the diffuse type of gastric cancer has increased, and a trend to a younger age of onset has been reported (3). Moreover, the prognosis of gastric cancer remains poor, with an average five-year survival rate of less than 30% and a median overall survival of less than 1 year (4). Complete surgical resection continues to form the basis of treatment, and the rate of surgical resection can be improved with the addition of radiotherapy and chemotherapy (5). Neoadjuvant chemotherapy (NACT), like in other malignancies, now plays an important role in the treatment of gastric cancer (6). NACT has increased overall survival in gastric cancer and improved the pathological complete response rate (7).
Inflammation has been identified as a critically important factor in carcinogenesis, and systemic inflammatory cells likely play important roles in the development, progression, and metastasis of malignant tumors (8). These cells, including neutrophils, monocytes, platelets, and lymphocytes, may present potential immuno-therapeutic targets in gastric cancer, as well as yielding prognostic information. Our understanding of the full role of these cells as well as their relative interaction, such as NLR (neutrophil to lymphocyte ratio), MLR (monocyte to lymphocyte ratio), and PLR (platelet to lymphocyte ratio), remains incomplete; these systemic inflammatory response markers have been widely used to determine the prognosis of tumors (9-11).
In recent years, the preoperative systemic inflammation response index (SIRI), based on peripheral neutrophil, monocyte, and lymphocyte counts, has gained credibility as an effective prognostic indicator in some malignancies (12, 13). However, the SIRI has rarely been studied in patients with advanced gastric cancer who received NACT. Moreover, the efficacy of systemic inflammatory response markers to help predict which patients would benefit from specific neoadjuvant chemotherapeutic agents must be considered. Therefore, the present study intended to investigate the prognostic significance of the preoperative SIRI in patients with advanced gastric cancer undergoing NACT.
Materials and Methods
Patient Selection
This retrospective study comprised 107 gastric cancer patients, enrolled at our hospital from July 2007 to September 2015, who were treated with neoadjuvant chemotherapy. All cancers were histologically confirmed, and clinical data were extracted from the medical records. Our study was approved by the ethics committee of Harbin Medical University Cancer Hospital, and informed consent was obtained from all enrolled patients.
The inclusion criteria included the following (1): patients with advanced histologically-confirmed gastric cancer (excluding distant metastasis) (2); surgical treatment (3); overall survival time ≥3 months (4); no cancer treatment, such as chemotherapy or radiotherapy, prior to evaluation at our hospital; and (5) complete clinical data and postoperative follow-up. The exclusion criteria included the following (1): co-existing malignancies (2); surgical complications or infection (3); co-existing inflammatory or autoimmune disease; and (4) patients who had received blood product transfusion or peripheral blood tests were not available.
Treatment Methods for NACT
All enrolled patients received preoperative NACT, and the standard regimens for advanced gastric cancer included SOX and XELOX regimens. For the SOX regimen, Oxaliplatin was 130 mg/ m2 on the first day and S-1 60 mg twice daily for 2 weeks. For the XELOX regimen, Oxaliplatin 130 mg/ m2 on the first day and Capecitabine 1,500 mg twice daily for 2 weeks. Cycles were repeated every 3 weeks.
Evaluation of Response
The TNM stage system was used as per the eighth edition of the Union for International Cancer Control and the American Joint Committee on Cancer TNM stage classification (14). Response Evaluation Criteria in Solid Tumors (RECIST) guidelines was performed to evaluate the response (15), and included the following categories: 1) complete response (CR): all target lesions disappeared; 2) partial response (PR): the sum of the longest diameters of target lesions was decreased at least 30%; 3) stable disease (SD): between PR and PD; and 4) progression of disease (PD): the sum of the longest diameters of target lesions was increased by at least 20%, or one or more new lesions appeared. The toxicity of NACT was determined by the National Cancer Institute Common Toxicity Criteria (NCI-CTC) (16).
Peripheral Venous Blood Parameters
Peripheral venous blood was collected at defined points of time prior to NACT. All samples were collected into EDTA anticoagulant tubes and obtained whilst fasting. The SIRI was defined as follows: SIRI = N × M/L (the units were N (109/L), M (109/L), and L (109/L)) where N, M, and L are pretreatment peripheral neutrophil (N), monocyte (M), and lymphocyte (L) counts, respectively.
Follow up
All enrolled patients were followed regularly by telephone or as inpatients and outpatients. The postoperative schedule was every 3 months for the first and second years, every 6 months for the third through the fifth years, and then at 12-months intervals thereafter. Disease-free survival (DFS) was defined as the interval from the surgical date to relapse (local recurrence or distant metastases). Overall survival (OS) was defined as the interval from the surgical date to death from any cause or last follow-up.
Statistical Analysis
All statistical analyses were performed using SPSS software 17.0 (Chicago, IL, United States) and GraphPad prism software 8.0 (La Jolla, CA, United States). The best cutoff value for SIRI was determined by ROC analysis. The Chi-square test and Fisher’s exact test were used to analyze the relationship between SIRI and clinicopathological features. The clinical outcomes of DFS and OS were analyzed by Kaplan-Meier survival analysis and compared using the log-rank test. Univariate and multivariate analyses were performed by the Cox proportional hazards regression model. A two-tailed p < 0.05 was considered statistically significant.
Results
Demographic and Clinicopathological Characteristics
The demographic and clinicopathological characteristics of the 107 patients are shown in Table 1. There were 82 males and 25 females, with an age range from 32 to 73 years (median, 56 years). The best cutoff value by ROC analysis for pre-treatment SIRI was 1.21; 62 patients (57.9%) in the low SIRI group and 45 patients (42.1%) in the high SIRI group. Compared to the high SIRI group, the low SIRI group was significantly associated with gender (χ2 = 12.090, p < 0.001) and primary tumor site (χ2 = 6.237, p = 0.047). (Table 1).
TABLE 1.
Baseline clinicopathological characteristics of all enrolled patients.
| Parameters | Low SIRI <1.21 | High SIRI ≥1.21 | χ2 | P Value | |
|---|---|---|---|---|---|
| Cases (n) | Number (%) | 62 | 45 | ||
| Age (years) | 0.116 | 0.733 | |||
| <56 | 52 (48.6%) | 31 (50.0%) | 21 (46.7%) | ||
| ≥56 | 55 (51.4%) | 31 (50.0%) | 24 (53.3%) | ||
| Gender | <0.001 a | ||||
| Male | 82 (76.6%) | 40 (64.5%) | 42 (93.3%) | ||
| Female | 25 (23.4%) | 22 (35.5%) | 3 (6.7%) | ||
| BMI | 2.823 | 0.093 | |||
| <22.10 | 53 (49.5%) | 35 (56.5%) | 18 (40.0%) | ||
| ≥22.10 | 54 (50.5%) | 27 (43.5%) | 27 (60.0%) | ||
| ABO blood type | 2.337 | 0.523 a | |||
| A | 30 (28.0%) | 17 (27.4%) | 13 (28.9%) | ||
| B | 36 (33.6%) | 19 (30.6%) | 17 (37.8%) | ||
| O | 31 (29.0%) | 18 (29.1%) | 13 (28.9%) | ||
| AB | 10 (9.4%) | 8 (12.9%) | 2 (4.4%) | ||
| Radical resection | 5.331 | 0.070 | |||
| R0 | 60 (56.1%) | 38 (61.3%) | 22 (48.9%) | ||
| R1 | 24 (22.4%) | 9 (14.5%) | 15 (33.3%) | ||
| R2 | 23 (21.5%) | 15 (24.2%) | 8 (17.8%) | ||
| Type of surgery | 2.708 | 0.243 a | |||
| distal gastrectomy | 60 (56.1%) | 35 (56.5%) | 25 (55.6%) | ||
| proximal gastrectomy | 7 (6.5%) | 2 (3.2%) | 5 (11.1%) | ||
| total gastrectomy | 40 (37.4%) | 25 (40.3%) | 15 (33.3%) | ||
| Differentiation | 3.587 | 0.168 a | |||
| poorly differentiated | 65 (60.8%) | 42 (67.8%) | 23 (51.1%) | ||
| moderately differentiated | 36 (33.6%) | 18 (29.0%) | 18 (40.0%) | ||
| well differentiated | 6 (5.6%) | 2 (3.2%) | 4 (8.9%) | ||
| Primary tumor site | 6.237 | 0.047 a | |||
| upper 1/3 | 9 (8.4%) | 2 (3.2%) | 7 (15.6%) | ||
| middle 1/3 | 39 (36.5%) | 23 (37.1%) | 16 (35.6%) | ||
| low 1/3 | 59 (55.1%) | 37 (59.7%) | 22 (48.8%) | ||
| Pathology | 2.311 | 0.467 a | |||
| normal (Tis) | 9 (8.4%) | 7 (11.3%) | 2 (4.4%) | ||
| Adenocarcinoma | 61 (57.0%) | 33 (53.2%) | 28 (62.2%) | ||
| mucinous carcinoma | 8 (7.5%) | 5 (8.1%) | 3 (6.7%) | ||
| signet ring cell carcinoma | 12 (11.2%) | 9 (14.5%) | 3 (6.7%) | ||
| mixed carcinoma | 17 (15.9%) | 8 (12.9%) | 9 (20.0%) | ||
| Clinical TNM classification | |||||
| T stage | 2.538 | 0.206 a | |||
| T3 | 9 (8.4%) | 5 (8.1%) | 4 (8.9%) | ||
| T4a | 68 (63.6%) | 36 (58.1%) | 32 (7.1%) | ||
| T4b | 30 (28.0%) | 21 (33.9%) | 9 (20.0%) | ||
| N stage | 1.145 | 0.551 a | |||
| N0 | 30 (28.0%) | 16 (25.8%) | 14 (31.1%) | ||
| N1 | 72 (67.3%) | 42 (67.7%) | 30 (66.7%) | ||
| N2 | 5 (4.7%) | 4 (6.5%) | 1 (2.2%) | ||
| TNM stage | 1.231 | 0.267 | |||
| II | 23 (21.5%) | 11 (17.7%) | 12 (26.7%) | ||
| III | 84 (78.5%) | 51 (82.3%) | 33 (73.3%) | ||
| Pathological TNM classification | |||||
| T stage | 4.201 | 0.299 a | |||
| Tis + T1 | 17 (15.9%) | 11 (17.7%) | 6 (13.3%) | ||
| T2 | 3 (2.8%) | 3 (4.8%) | 0 (0.0%) | ||
| T3 | 42 (39.3%) | 20 (32.3%) | 22 (48.9%) | ||
| T4a | 21 (19.6%) | 15 (24.2%) | 6 (13.3%) | ||
| T4b | 24 (22.4%) | 13 (21.0%) | 11 (24.4%) | ||
| N stage | 2.938 | 0.421 a | |||
| N0 | 32 (29.9%) | 21 (33.9%) | 11 (24.4%) | ||
| N1 | 23 (21.5%) | 15 (24.2%) | 8 (17.8%) | ||
| N2 | 18 (16.8%) | 8 (12.9%) | 10 (22.2%) | ||
| N3a | 25 (23.4%) | 13 (21.0%) | 12 (26.7%) | ||
| N3b | 9 (8.4%) | 5 (8.0%) | 4 (8.9%) | ||
| Metastasis | 0.096 | 0.756 a | |||
| M0 | 104 (97.2%) | 60 (96.8%) | 44 (97.8%) | ||
| M1 | 3 (2.8%) | 2 (3.2%) | 1 (2.2%) | ||
| TNM stage | 2.137 | 0.551 a | |||
| Tis + I | 15 (14.0%) | 11 (17.8%) | 4 (8.9%) | ||
| II | 31 (29.0%) | 16 (25.8%) | 15 (33.3%) | ||
| III | 58 (54.2%) | 33 (53.2%) | 25 (55.6%) | ||
| IV | 3 (2.8%) | 2 (3.2%) | 1 (2.2%) | ||
| Total lymph nodes | 1.127 | 0.288 | |||
| <27 | 53 (49.5%) | 28 (45.2%) | 25 (55.6%) | ||
| ≥27 | 54 (50.5%) | 34 (54.8%) | 20 (44.4%) | ||
| Positive lymph nodes | 1.670 | 0.434 | |||
| 0 | 33 (30.8%) | 21 (33.9%) | 12 (26.7%) | ||
| <3 | 20 (18.7%) | 13 (21.0%) | 7 (15.6%) | ||
| ≥3 | 54 (50.5%) | 28 (45.1%) | 26 (57.7%) | ||
| Lauren classification | 0.363 | 0.834 | |||
| Intestinal | 57 (53.3%) | 33 (53.2%) | 24 (53.3%) | ||
| Diffuse | 31 (29.0%) | 19 (30.7%) | 12 (26.7%) | ||
| Mixed | 19 (17.8%) | 10 (16.1%) | 9 (20.0%) | ||
| Borrmann classification | 1.122 | 0.925 a | |||
| Borrmann I | 1 (0.9%) | 1 (1.6%) | 0 (0.0%) | ||
| Borrmann II | 24 (22.4%) | 15 (24.2%) | 9 (20.0%) | ||
| Borrmann III | 67 (62.6%) | 37 (59.7%) | 30 (66.7%) | ||
| Borrmann IV | 15 (14.0%) | 9 (14.5%) | 6 (13.3%) | ||
| Tumor size (mm) | 0.047 | 0.829 | |||
| <50 | 56 (52.3%) | 33 (53.2%) | 23 (51.1%) | ||
| ≥50 | 51 (47.7%) | 29 (46.8%) | 22 (48.9%) | ||
Performed using the Fisher’s exact test.
Blood Parameters
We analyzed blood parameters by median value (Table 2). Compared with the high SIRI group, the low SIRI group was significantly associated with white blood cell (χ2 = 27.100, p < 0.001)、N (χ2 = 35.866, p < 0.001)、M (χ2 = 21.628, p < 0.001)、NLR (χ2 = 51.321, p < 0.001)、MLR (χ2 = 45.862, p < 0.001) and PLR (χ2 = 4.293, p < 0.05), however, the low SIRI group was not associated with hemoglobin (χ2 = 1.001, p > 0.05)、p (χ2 = 0.116, p > 0.05)、or L (χ2 = 0.449, p > 0.05). (Table 2).
TABLE 2.
Correlation between SIRI and hematological parameters.
| Parameters | Low SIRI<1.21 | High SIRI≥1.21 | χ2 | P Value |
|---|---|---|---|---|
| Cases (n) | 62 | 45 | ||
| White blood cell (×109/L) | 27.100 | <0.001 | ||
| <6.42 | 44 (71.0%) | 9 (20.0%) | ||
| ≥6.42 | 18 (29.0%) | 36 (80.0%) | ||
| Hemoglobin (×109 /L) | 1.001 | 0.317 | ||
| <121 | 27 (43.5%) | 24 (53.3%) | ||
| ≥121 | 35 (56.5%) | 21 (46.7%) | ||
| Neutrophils (×109 /L) | 35.866 | <0.001 | ||
| <3.82 | 46 (74.2%) | 7 (15.6%) | ||
| ≥3.82 | 16 (25.8%) | 38 (84.4%) | ||
| Monocyte (×109 /L) | 21.628 | <0.001 | ||
| <0.45 | 42 (67.7%) | 10 (22.2%) | ||
| ≥0.45 | 20 (32.3%) | 35 (77.8%) | ||
| Platelet (×109 /L) | 0.116 | 0.733 | ||
| <285 | 31 (50.0%) | 21 (46.7%) | ||
| ≥285 | 31 (50.0%) | 24 (53.3%) | ||
| Lymphocyte (×109 /L) | 0.449 | 0.503 | ||
| <1.72 | 29 (46.8%) | 24 (53.3%) | ||
| ≥1.72 | 33 (53.2%) | 21 (46.7%) | ||
| NLR | 51.321 | <0.001 a | ||
| <2.18 | 49 (79.0%) | 4 (8.9%) | ||
| ≥2.18 | 13 (21.0%) | 41 (91.1%) | ||
| MLR | 45.862 | <0.001 | ||
| <0.28 | 48 (77.4%) | 5 (11.1%) | ||
| ≥0.28 | 14 (22.6%) | 40 (88.9%) | ||
| PLR | 4.293 | 0.038 | ||
| <164 | 36 (58.1%) | 17 (37.8%) | ||
| ≥164 | 26 (41.9%) | 28 (62.2%) | ||
Performed using the Fisher’s exact test.
Correlation Between SIRI and Chemotherapy
All patients were received neoadjuvant chemotherapy, and the results indicated that the SIRI was associated with NACT regimens (χ2 = 7.151, p = 0.028). After the operation, 93 patients were received postoperative chemotherapy, and the results have shown that SIRI was not associated with postoperative chemotherapy regimens (χ2 = 2.103, p = 0.717). (Table 3).
TABLE 3.
Correlation between SIRI and chemotherapy.
| Parameters | Low SIRI <1.21 | High SIRI ≥1.21 | χ2 | P Value | |
|---|---|---|---|---|---|
| Cases (n) | Number (%) | 62 | 45 | ||
| NACT regimens | |||||
| SOX | 35 (32.7%) | 17 (27.4%) | 18 (40.0%) | 7.151 | 0.028 |
| XELOX | 56 (52.3%) | 39 (62.9%) | 17 (37.8%) | ||
| Others a | 16 (15.0%) | 6 (9.7%) | 10 (22.2%) | ||
| Preoperative chemotherapy times | |||||
| <3 | 57 (53.3%) | 38 (61.3%) | 19 (42.2%) | 3.809 | 0.051 |
| ≥3 | 50 (46.7%) | 24 (38.7%) | 26 (57.8%) | ||
| Postoperative chemotherapy regimens | |||||
| SOX | 32 (29.9%) | 16 (25.8%) | 16 (35.5%) | 2.103 | 0.717 |
| XELOX | 41 (38.3%) | 26 (41.9%) | 15 (33.3%) | ||
| Others a | 20 (18.7%) | 13 (21.0%) | 7 (15.6%) | ||
| No | 14 (13.1%) | 7 (11.3%) | 7 (15.6%) | ||
| Postoperative chemotherapy times | |||||
| 0 | 14 (13.1%) | 7 (11.3%) | 7 (15.6%) | 2.198 | 0.333 |
| <4 | 51 (47.7%) | 27 (43.5%) | 24 (53.3%) | ||
| ≥4 | 42 (39.2%) | 28 (45.2%) | 14 (31.1%) | ||
| Response | |||||
| CR | 9 (8.4%) | 7 (11.3%) | 2 (4.4%) | 7.488 | 0.112 b |
| PR | 72 (67.3%) | 45 (72.6%) | 27 (60.0%) | ||
| SD | 7 (6.5%) | 4 (6.4%) | 3 (6.7%) | ||
| PD | 19 (17.8%) | 6 (9.7%) | 13 (28.9%) | ||
DCF, docetaxel, cisplatin, and fluorouracil and other fluoropyrimidine-based adjuvant chemotherapy; ECF, epirubicin, cisplatin, and fluorouracil; FOLFOX, folinic acid, oxaliplatin, and fluorouracil; TCF, paclitaxel, cisplatin, and fluorouracil; TF, docetaxel and fluorouracil; TS, paclitaxel and S-1.
Performed using the Fisher’s exact test.
Univariate and Multivariate Cox Regression Survival Analyses
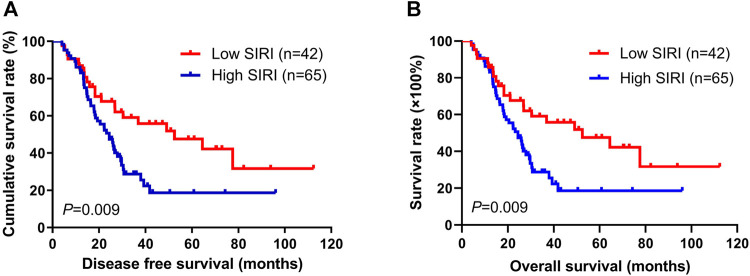
Using the best cutoff value of 1.21 for the SIRI, it significantly correlated with DFS and OS. In univariate analysis, low SIRI was correlated with prolonged DFS and OS (hazard ratio (HR): 3.437, 95% confidence interval (CI): 1.059-11.149, p = 0.009; HR: 3.331, 95% CI: 1.001-11.082, p = 0.021). In multivariate analysis, low SIRI was also correlated with prolonged DFS and OS (HR: 1.782, 95% CI: 1.241-3.942, p = 0.024; HR: 1.665, 95% CI: 1.302-3.613, p = 0.028; Supplementary Table S1). Kaplan-Meier survival curves for DFS and OS for the SIRI of all patients are shown in Figure 1.
FIGURE 1.
Kaplan-Meier analysis of DFS and OS for the SIRI of all patients with advanced gastric cancer.
Survival and Evaluation of SIRI
The 1-year, 3-years, and 5-years survival rates of DFS and OS in the low SIRI group were 71.0% (44/62), 30.6% (19/62), and 14.5% (9/62); and 85.5% (53/62), 32.3% (20/62), and 19.4% (12/62), respectively. The 1-year, 3-years, and 5-years survival rates of DFS and OS in the high SIRI group were 75.6% (34/45), 15.6% (7/45), and 2.2% (1/45); and 84.4% (38/45), 17.8% (8/45), and 2.2% (1/45), respectively (Table 4). Notably, patients in the high SIRI group had worse 3-years DFS and OS than the low SIRI group (χ2 = 3.228, p = 0.072; χ2 = 2.830, p = 0.093) and worse 5-years DFS and OS than the low SIRI group (χ2 = 4.651, p = 0.031; χ2 = 7.171, p = 0.007).
TABLE 4.
1-year, 3-years, and 5-years DFS and OS rates of patients with advanced gastric cancer.
| Parameters | Case (n) | 1-year (%) | DFS | OS | |||
|---|---|---|---|---|---|---|---|
| 3-years (%) | 5-years (%) | 1-year (%) | 3-years (%) | 5-years (%) | |||
| Low SIRI | 62 (59.8%) | 44 (71.0%) | 19 (30.6%) | 9 (14.5%) | 53 (85.5%) | 20 (32.3%) | 12 (19.4%) |
| High SIRI | 45 (40.2%) | 34 (75.6%) | 7 (15.6%) | 1 (2.2%) | 38 (84.4%) | 8 (17.8%) | 1 (2.2%) |
| χ2 | 0.278 | 3.228 | 4.651 | 0.022 | 2.830 | 7.171 | |
| P value | 0.598 | 0.072 | 0.031# | 0.882 | 0.093 | 0.007# | |
Association of SIRI and Borrmann Classification
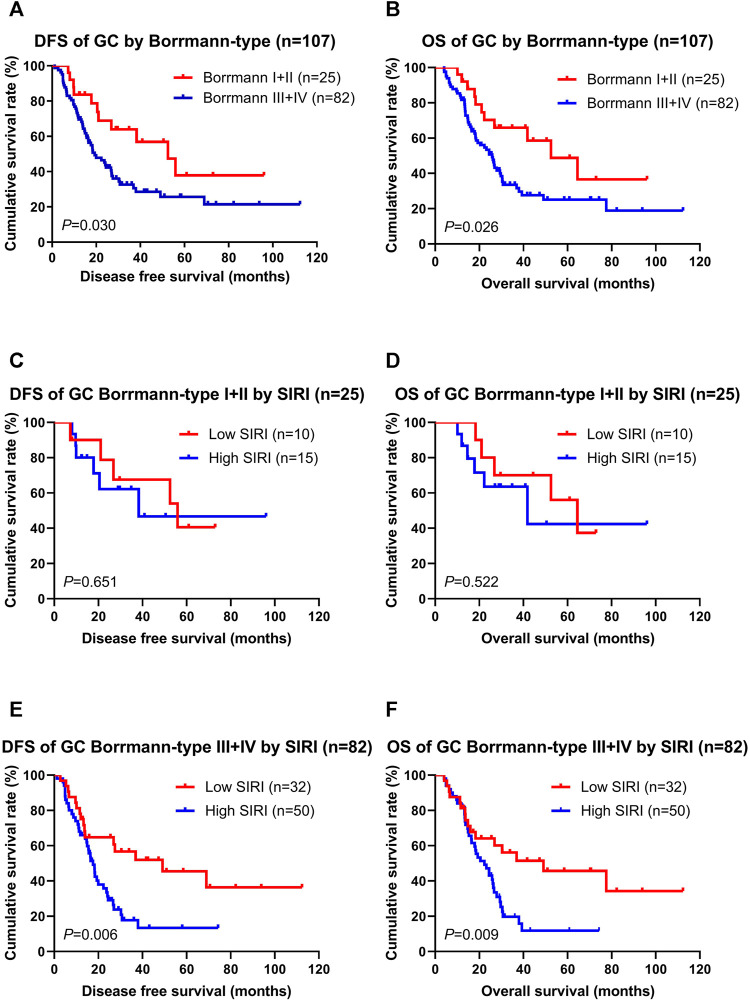
According to multivariate Cox regression model analyses, the Borrmann classification was a significant prognostic factor (Supplementary Table S1). We stratified into two groups, Borrmann I + II and Borrmann III + IV. The results showed that patients with Borrmann I + II had longer DFS and OS than Borrmann III + IV (χ2 = 4.690, p = 0.030; χ2 = 4.986, p = 0.026; Figures 2A,B). Moreover, the low SIRI group had longer DFS and OS than the high SIRI group in Borrmann I + II (χ2 = 0.204, p = 0.651; χ2 = 0.410, p = 0.522; Figures 2C,D), and longer DFS and OS than the high SIRI group in Borrmann III + IV (χ2 = 7.434, p = 0.006 and χ2 = 6.884, p = 0.009, respectively; Figures 2E,F).
FIGURE 2.
DFS and OS for the SIRI of patients with gastric cancer in different Borrmann classification. Borrmann I + II group means patients with Borrmann I or Borrmann II (25 patients); Borrmann III + IV group means patients with Borrmann III or Borrmann IV (82 patients).
Association of Pathologic Stage and SIRI
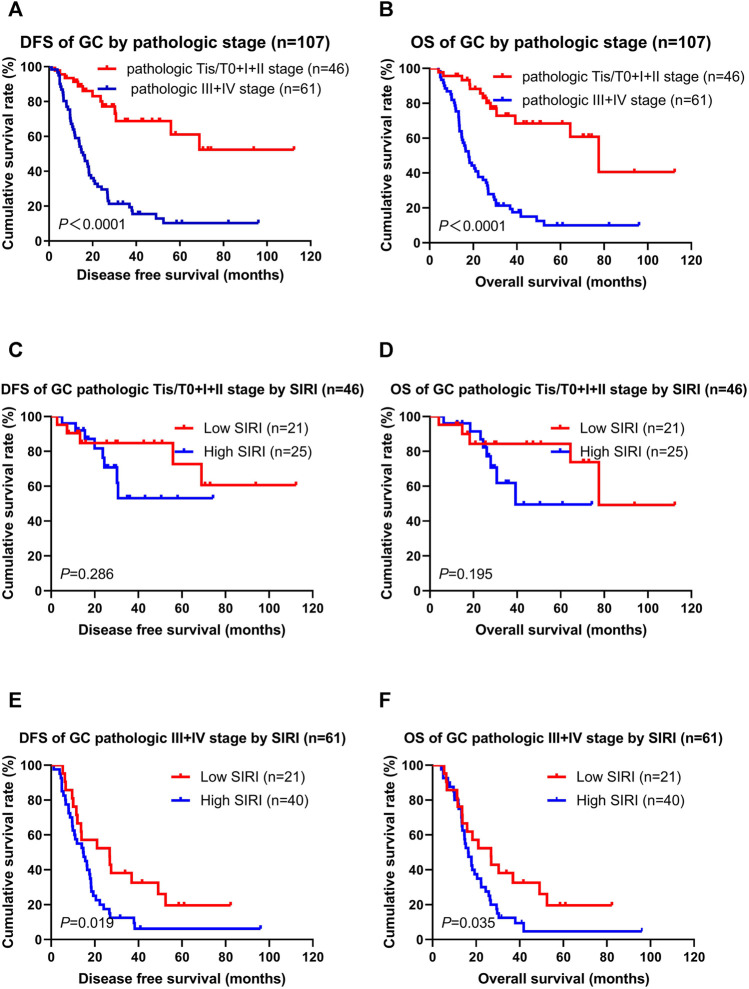
Not surprisingly, patients with the pathologic Tis/T0 + I + II stages had longer DFS and OS than the pathologic III + IV stages (χ2 = 28.850, p < 0.0001; χ2 = 31.030, p < 0.0001; Figures 3A,B). The low SIRI group had longer DFS and OS than the high SIRI group in the pathologic Tis/T0 + I + II stages (χ2 = 1.137, p = 0.286; χ2 = 1.683, p = 0.195; Figures 3C,D), and longer DFS and OS than the high SIRI group in the pathologic III + IV stages (χ2 = 5.503, p = 0.019; χ2 = 4.431, p = 0.035; Figures 3E,F).
FIGURE 3.
DFS and OS for the SIRI of patients with gastric cancer in different pathologic stages. Pathologic Tis/T0+I + II stage means patients with pathologic Tis/T0 or I or II stages (46 patients); pathologic III + IV stage means patients with pathologic III or IV stages (61 patients).
Correlation Between SIRI and Toxicity Assessment
NACT toxicity was evaluated following two treatment cycles. The most common toxicities were hematologic. There was no correlation with SIRI relative to anemia, leucopenia, neutropenia, myelosuppression, or gastrointestinal reaction (p > 0.05), but it did correlate with thrombocytopenia (p < 0.05).
Discussion
Despite advances in surgical techniques and adjuvant therapy in recent decades, gastric cancer overall continues to be associated with a poor outcome, rapid recurrence, and metastasis (17). NACT has evolved into a critical part of gastric carcinoma treatment, importantly without increasing postoperative complications (18). Therefore, in order to optimize chemotherapy selection, accurate prognostic indicators that have been assessed in patients treated with NACT are most valuable.
Inflammation, as an important component of the tumor microenvironment (TME), is related to tumor development and progression (19). Systemic inflammation is an ancestral physiological response, and tumor cells influence proinflammatory mediators (20, 21). Increasingly, studies have provided convincing evidence that systemic inflammatory cells are associated with prognosis in many malignancies (22-24). Cellular components of the systemic inflammatory response may reflect and predict survival for gastric cancer. Moreover, inflammation and immune-based biomarkers, such as NLR, MLR, and PLR, have been statistically validated as prognostic markers (25-27). The combined cellular elements of SIRI provide a comprehensive balance of host immune and inflammatory status, reported to be associated with survival times and clinical outcomes. To date, however, there is no evidence whether SIRI can serve as a useful indicator to predict outcomes of gastric cancer patients that have received NACT.
In this study, the SIRI was a significant prognostic factor that could predict prognosis and treatment response. Low SIRI correlated well with DFS and OS. Therefore, SIRI appears to be a cost-effective, convenient, noninvasive, and reproducible biomarker for treatment response in patients with advanced gastric cancer treated with NACT and surgical resection.
Cancer-related inflammation is considered the seventh hallmark of cancer and several potential mechanisms might explain why a low SIRI was associated with better survival than the high SIRI group. Neutrophils can inhibit the immune system by releasing cytokines and chemokines, promote circulating tumor cells (CTCs), and stimulate tumor angiogenesis and progression (28, 29). Monocytes are released from the bone marrow, part of the tumor-derived secretome that can increase myelopoiesis and influence tumor proliferation, angiogenesis, and progression (30, 31). Lymphocytes are known to play an important role in tumor immune surveillance, induce cytotoxic cell death to defend against tumor cells, and inhibit tumor progression (IFN-γ) (32, 33). Simplistically synthesizing these, either increasing neutrophils and monocytes or decreasing lymphocytes, may disorder the immune balance, resulting in an elevation in the SIRI, which is correlated with a worse prognosis for gastric cancer patients.
The critical findings of this study, with a best cutoff value derived for SIRI, showed a statistical correlation of SIRI with DFS and OS: low SIRI, better survival; high SIRI, worse survival. That SIRI also correlated with Borrmann and pathologic stages is logical and further validates these findings. Finally, a comprehensive investigation of hematologic parameters in peripheral venous blood may help to discover new immunologic targets for individualized treatment.
Conclusion
SIRI may qualify as a useful, reliable, and convenient prognostic indicator in patients with advanced gastric cancer to help physicians provide personalized prognostication for gastric cancer patients treated with NACT and surgical resection. Certainly, further studies are needed to verify the preliminary results of SIRI in larger groups of gastric cancer patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Harbin Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HS, YX and SZ contributed to the study conception and design. LC and YC performed the collection of data and conducted the data interpretation. LC, XL and LZ prepared the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Funding of Department of Education of Heilongjiang Province (No: 12541458) and Clinical Research Foundation of Wu Jieping Medical Foundation (grant no. 320.6750.13105 and no. 320.6750.18278).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2021.1609811/full#supplementary-material
Abbreviations
DFS, disease-free survival; GC, gastric cancer; L, lymphocyte; M, monocyte; MLR, monocyte to lymphocyte ratio; .N, neutrophil; NACT, neoadjuvant chemotherapy; NCI-CTC, National Cancer Institute Common Toxicity Criteria; NLR, neutrophil to lymphocyte ratio; OS, overall survival; P, platelet; PLR, platelet to lymphocyte ratio; RECIST, Response Evaluation Criteria In Solid Tumors; ROC, receiver operating characteristic curve; SIRI, systemic inflammation response index.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA A Cancer J Clin (2020) 70(1):7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric Cancer. The Lancet (2020) 396(10251):635–48. 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 4. Lordick F, Shitara K, Janjigian YY. New Agents on the Horizon in Gastric Cancer. Ann Oncol (2017) 28(8):1767–75. 10.1093/annonc/mdx051 [DOI] [PubMed] [Google Scholar]
- 5. Neves Filho EHC, de Sant'Ana RO, Nunes LVSC, Pires APB, da Cunha Md. PSS. Histopathological Regression of Gastric Adenocarcinoma after Neoadjuvant Therapy: a Critical Review. APMIS (2017) 125(2):79–84. 10.1111/apm.12642 [DOI] [PubMed] [Google Scholar]
- 6. Rausei S, Bali CD, Lianos GD. Neoadjuvant Chemotherapy for Gastric Cancer. Has the Time to Decelerate the Enthusiasm Passed Us by. Semin Oncol (2020) 47(6):355–60. 10.1053/j.seminoncol.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 7. Lin JX, Yoon C, Desiderio J, Yi BC, Li P, Zheng CH, et al. Development and Validation of a Staging System for Gastric Adenocarcinoma after Neoadjuvant Chemotherapy and Gastrectomy with D2 Lymphadenectomy. Br J Surg (2019) 106(9):1187–96. 10.1002/bjs.11181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–e503. 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 9. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The Neutrophil-To-Lymphocyte Ratio (NLR) Predicts Short-Term and Long-Term Outcomes in Gastric Cancer Patients. Eur J Surg Oncol (2018) 44(5):607–12. 10.1016/j.ejso.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 10. Shoji F, Kozuma Y, Toyokawa G, Yamazaki K, Takeo S. Complete Blood Cell Count-Derived Inflammatory Biomarkers in Early-Stage Non-small-cell Lung Cancer. Atcs (2020) 26(5):248–55. 10.5761/atcs.oa.19-00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li B, Zhou P, Liu Y, Wei H, Yang X, Chen T, et al. Platelet-to-lymphocyte Ratio in Advanced Cancer: Review and Meta-Analysis. Clinica Chim Acta (2018) 483:48–56. 10.1016/j.cca.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Jin M, Shao Y, Xu G. (2019). Prognostic Value of the Systemic Inflammation Response Index in Patients with Adenocarcinoma of the Oesophagogastric Junction: A Propensity Score-Matched Analysis. Dis Markers, 2019 1, 9. 10.1155/2019/4659048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chao B, Ju X, Zhang L, Xu X, Zhao Y. A Novel Prognostic Marker Systemic Inflammation Response Index (SIRI) for Operable Cervical Cancer Patients. Front Oncol (2020) 10:766. 10.3389/fonc.2020.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More "personalized" Approach to Cancer Staging. CA: A Cancer J Clinicians (2017) 67(2):93–9. 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16. Caussanel J-P, Lévi F, Brienza S, Misset J-L, Itzhaki M, Adam R, et al. Phase I Trial of 5-Day Continuous Venous Infusion of Oxaliplatin at Circadian Rhythm-Modulated Rate Compared with Constant Rate. J Natl Cancer Inst (1990) 82(12):1046–50. 10.1093/jnci/82.12.1046 [DOI] [PubMed] [Google Scholar]
- 17. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic Inflammation Response Index (SIRI), Cancer Stem Cells and Survival of Localised Gastric Adenocarcinoma after Curative Resection. J Cancer Res Clin Oncol (2017) 143(12):2455–68. 10.1007/s00432-017-2506-3 [DOI] [PubMed] [Google Scholar]
- 18. Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, et al. Neoadjuvant Chemotherapy for Gastric Cancer. Is it a Must or a Fake. World. J. Gastroentrol. (2018) 24(2):274–89. 10.3748/wjg.v24.i2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140:883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related Inflammation. Nature (2008) 454(7203):436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 21. Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The Platelet-To-Lymphocyte Ratio versus Neutrophil-To-Lymphocyte Ratio: Which Is Better as a Prognostic Factor in Gastric Cancer. Ann Surg Oncol (2015) 22(13):4363–70. 10.1245/s10434-015-4518-z [DOI] [PubMed] [Google Scholar]
- 22. Li T-J, Jiang Y-M, Hu Y-F, Huang L, Yu J, Zhao L-Y, et al. Interleukin-17-Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin Cancer Res (2017) 23(6):1575–85. 10.1158/1078-0432.CCR-16-0617 [DOI] [PubMed] [Google Scholar]
- 23. Kılınçalp S, Ekiz F, Başar Ö, Ayte MR, Çoban Ş, Yılmaz B, et al. Mean Platelet Volume Could Be Possible Biomarker in Early Diagnosis and Monitoring of Gastric Cancer. Platelets (2014) 25(8):592–4. 10.3109/09537104.2013.783689 [DOI] [PubMed] [Google Scholar]
- 24. Eo WK, Jeong DW, Chang HJ, Won KY, Choi SI, Kim SH, et al. Absolute Monocyte and Lymphocyte Count Prognostic Score for Patients with Gastric Cancer. Wjg (2015) 21(9):2668–76. 10.3748/wjg.v21.i9.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ock C-Y, Nam A-R, Lee J, Bang J-H, Lee K-H, Han S-W, et al. Prognostic Implication of Antitumor Immunity Measured by the Neutrophil-Lymphocyte Ratio and Serum Cytokines and Angiogenic Factors in Gastric Cancer. Gastric Cancer (2017) 20(2):254–62. 10.1007/s10120-016-0613-5 [DOI] [PubMed] [Google Scholar]
- 26. Chen L, Hao Y, Zhu L, Li S, Zuo Y, Zhang Y, et al. Monocyte to Lymphocyte Ratio Predicts Survival in Patients with Advanced Gastric Cancer Undergoing Neoadjuvant Chemotherapy. Ott (2017) Vol. 10:4007–16. 10.2147/OTT.S140118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh C-H, Bhoo-Pathy N, Ng K-L, Jabir RS, Tan G-H, See M-H, et al. Utility of Pre-treatment Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio as Prognostic Factors in Breast Cancer. Br J Cancer (2015) 113(1):150–8. 10.1038/bjc.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolaczkowska E, Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat Rev Immunol (2013) 13(3):159–75. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 29. Tan KW, Chong SZ, Wong FHS, Evrard M, Tan SM-L, Keeble J, et al. Neutrophils Contribute to Inflammatory Lymphangiogenesis by Increasing VEGF-A Bioavailability and Secreting VEGF-D. Blood (2013) 122(22):3666–77. 10.1182/blood-2012-11-466532 [DOI] [PubMed] [Google Scholar]
- 30. Shi C, Pamer EG. Monocyte Recruitment during Infection and Inflammation. Nat Rev Immunol (2011) 11:762–74. 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated Regulation of Myeloid Cells by Tumours. Nat Rev Immunol (2012) 12:253–68. 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, et al. Comparison of Tumor-Infiltrating Lymphocytes between Primary and Metastatic Tumors in Breast Cancer Patients. Cancer Sci (2016) 107(12):1730–5. 10.1111/cas.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrone C, Dranoff G. Dual Roles for Immunity in Gastrointestinal Cancers. J. Clin. Oncol. (2010) 28(26):4045–51. 10.1200/JCO.2010.27.9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.