Abstract
Endometrial cancer is the most common gynecological cancer in developed countries, and its incidence has increased. The majority of patients with endometrial cancer have an early disease and favorable prognosis; however, a significant proportion of endometrial cancer, which mainly comprises high-grade or type II endometrial cancer such as serous, clear cell, and carcinosarcoma, shows advanced/recurrent disease and dismal prognosis. Novel therapeutic development is required for patients with aggressive endometrial cancers. Recent genomic and immunohistochemical analyses revealed human epidermal growth factor receptor 2 (HER2) overexpression/gene amplification in 20%-40% of patients with type II endometrial cancer. Historically, HER2 targeted therapy has been developed for various major cancers, including breast and gastric cancer. Notably, recent advances in HER2 targeted therapy for patients with type II endometrial cancer are also expected to change. Simultaneously, an optimized HER2 test for endometrial cancer as companion diagnostics should be established. In this review, we summarize the recent findings on endometrial cancer, current treatment, optimized HER2 testing, key clinical trials on HER2 targeted therapy, and future directions in aggressive endometrial cancer, including serous carcinoma and carcinosarcoma.
Keywords: Endometrial cancer, Serous carcinoma, Carcinosarcoma, Human epidermal growth factor receptor 2, Chemotherapy, Antibody-drug conjugates
Core Tip: Endometrial cancer is the most common gynecological cancer in developed countries, and its incidence has increased. A significant proportion of endometrial cancer, which mainly comprises high-grade or type II endometrial cancer, including serous carcinoma and carcinosarcoma, shows a dismal prognosis. Recent molecular analyses revealed human epidermal growth factor receptor 2 (HER2) overexpression/ gene amplification in 20%-40% of patients with type II endometrial cancer. Notably, HER2 targeted therapy for type II endometrial cancer has been dramatically developed. We review the recent findings on endometrial cancer, current treatment, optimized HER2 testing, key clinical trials on HER2 targeted therapy, and future directions in these aggressive endometrial cancers.
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy, with 65000 cases diagnosed and 12000 deaths in the United States in 2020[1]. In Japan, approximately 16000 cases are diagnosed, 2500 deaths occur annually, and the number of patients is increasing[2,3]. The prognosis is relatively good, with a 5-year survival rate of 80%, and many cases are diagnosed at the localized stage[4]. However, a significant proportion of patients with endometrial cancer still have an advanced or recurrent disease and have a dismal prognosis.
Endometrial cancers can be classified into two types. Type I shows endometrioid morphology, which accounts for 80% of endometrial cancers, and type II shows non-endometrioid morphology, such as serous carcinoma, clear cell carcinoma, and carcinosarcoma, which account for the remaining 20%. Each type of carcinoma is known to have a different background and clinical course. Type I is reportedly caused by unopposed estrogen, and obesity is a trigger for carcinogenesis; it is diagnosed at a young age, has low grade and early stage, and is usually curable by surgery. In contrast to type I, type II is more common in elderly patients, occurs on a background atrophic endometrium, is diagnosed at an advanced stage, and is resistant to chemotherapy, resulting in a poor prognosis[5].
An analysis of The Cancer Genome Atlas revealed different molecular genetic pathways that are present in these two types. The results show that endometrial cancers can be divided into four clusters: POLE ultramutated (cluster 1), microsatellite instability hypermutated (cluster 2), copy-number low, endometrioid (cluster 3), and copy-number high; serous-like (cluster 4). Uterine serous carcinoma (USC), classified as Type II, accounts for the majority of cluster 4 cases[6]. Differences in common genomic alterations by cluster were characterized by PTEN, PIK3R1, FBXW7, and KRAS alterations in cluster 1, KRAS alterations in cluster 2, CTNNB1 alterations in cluster 3, TP53, FBXW7, PPP2R1A alterations, ERBB2, and CCND1 amplification in cluster 4[6].
Surgical treatment is appropriate for early-stage endometrial cancer, and chemotherapy and radiation therapy should be considered according to the risk of recurrence after resection. Women with low-risk endometrial cancer undergoing surgical treatment alone and no adjuvant treatment are indicated. For women with high-risk endometrial cancers undergoing surgery, adjuvant chemotherapy with or without radiation should be offered[7,8]. Although there is no consensus on adjuvant treatment among intermediate-risk cancers, some clinicians may offer adjuvant chemotherapy and/or radiation[5]. For advanced or recurrent uterine cancer, carboplatin, paclitaxel, adriamycin, and cisplatin are often chosen as chemotherapy regimens based on the GOG 122L[9], GOG 209L[10], and JGOG 2043 trials[11]. Furthermore, low-grade endometrial cancer with positive estrogen receptor and progesterone receptor can be expected to benefit from hormone therapy[12].
Molecular targeted agents for patients with endometrial cancer have been developed, similar to other solid tumors. In recent years, the efficacy of pembrolizumab has been demonstrated in solid tumors of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR)[13,14]. MSI-H/dMMR is found in 20%-30% of endometrial cancers[6,15]. The combination of pembrolizumab and lenvatinib has been shown to be effective in patients with microsatellite stable/mismatch repair proficient, regardless of histologic subtype, with 40% of participants having type II, serous/clear cell carcinoma, and type I[16]. Notably, human epidermal growth factor receptor 2 (HER2) targeted therapy for patients with endometrial cancer has demonstrated practice-changing efficacy in the recent decade. In this review, we aim to overview recent advances in HER2 targeted therapeutics and HER2 testing for endometrial cancer.
HER2 AMPLIFICATION AND OVEREXPRESSION IN ENDOMETRIAL CANCER
HER2 is a cell surface receptor named after human epidermal growth factor receptor (EGFR)-related 2 in the 1980s. It is encoded by the ERBB2 gene on chromosome 17. It is known to belong to the EGFR family that includes other receptors: EGFR, HER3, and HER4. When HER2 is amplified, HER2 is overexpressed and forms active dimers that induce the downstream RAS/RAF/MAPK and PI3K/AKT pathways without ligand stimulation. This, in turn, activates the downstream pathway, contributing to oncogenesis[17,18].
HER2 amplification/overexpression is known to occur in a variety of solid tumors, including about 20% in breast cancer[17], 10%-20% in gastric cancer, 2%-10% in colorectal cancer, 5%-20% in biliary tract cancer, 10% in bladder cancer, and 2%-5% in lung cancer[19,20]. In all endometrial cancers, HER2 overexpression and amplification have been reported 18%-80% and 4%-69%[19]. HER2 positivity differs according to histopathological subtype. Although HER2 overexpression and amplification are rarely seen in low-grade endometrioid adenocarcinoma, USC has the highest prevalence of HER2 positivity. The rates of HER2 overexpression and amplification by histologic subtype are shown in Table 1.
Table 1.
Human epidermal growth factor receptor 2 overexpression and gene amplification in endometrial cancer
|
Histological type
|
HER2 overexpression
|
HER2
gene amplification
|
Ref.
|
| Overall | 4%-69% | 18%-80% | [19,45,55] |
| Endometrioid (low grade) | rare | rare | [29,45] |
| Endometrioid (high grade) | 8%-31% | 15%-29% | [29,45] |
| Serous carcinoma | 20%-60% | 13%-29% | [29,42,43,45] |
| Clear cell carcinoma | 33%-70% | 22%-50% | [29,55] |
| Carcinosarcoma | 0-25% | 13%-20% | [38,48-51] |
HER2-targeted drugs are being used and developed for various malignancies. Currently, trastuzumab, pertuzumab, lapatinib, neratinib, tucatinib[21], trastuzumab emtansine, and trastuzumab deruxtecan[22] have shown clinical activity in HER2-positive breast cancer[19]. For HER2-positive gastric cancer, trastuzumab[23] and trastuzumab deruxtecan[24] have also shown clinical activity. In other carcinomas, HER2-targeted drug therapies are being developed for tumors with HER2 overexpression, amplification, or mutations[25-28]. In endometrial cancer, treatment options are very limited, especially for type II uterine carcinoma, which is resistant to cytotoxic chemotherapy. Therefore, new therapeutic targets and effective new drugs are required.
HER2 overexpression/amplification is currently assessed using immunostaining and fluorescent in situ hybridization (FISH)/dual-color in situ hybridization. However, there is variability in positive/negative results depending on the type of cancer. In this article, we summarize the methods used to assess HER2 in type II endometrial cancer and the related development of HER2-targeted drugs.
HER2 TESTING IN ENDOMETRIAL CANCER; PATHOLOGICAL PERSPECTIVE
A significant proportion of endometrial cancers show HER2 overexpression or gene amplification[19,29-31]. The percentage of HER2 positive cases varies by histological type, with serous carcinomas having the highest prevalence of HER2 positivity[29-31]. Standardization of HER2 testing accompanied with evidence-based treatment has not been performed for all endometrial cancers; thus, HER2 testing methods are currently optimized for each histological type, specifically in serous carcinoma and carcinosarcoma.
HER2 testing as companion diagnostics has been best established for breast and gastric cancers, and ASCO/CAP guidelines have been provided[32,33]. The HER2 assessment algorithm is based on the detection of protein overexpression by immunohistochemistry (IHC) and gene amplification by ISH to determine HER2-positive/-negative cases[32,33]. Although HER2 overexpression has been reported in other types of carcinomas, in most cases, these two HER2 testing methods are used[19,34] or modified (e.g., in colorectal cancer[35,36]).
In endometrial cancer, HER2 testing has become more clinically relevant in serous carcinoma[37] and carcinosarcoma[38,39], with the accumulating knowledge of HER2 testing accompanied by ongoing therapeutic developments.
HER2 TESTING IN SEROUS CARCINOMA
Serous carcinoma accounts for up to 10% of endometrial cancers; however, it is aggressive and responsible for almost 40% of endometrial cancer deaths[40]. Histopathologically, serous carcinoma is usually composed of tumor cells with marked nuclear pleomorphism and presents a complex papillary pattern of typically small, bud-like papillae, irregular slit-like glands, an endometrioid-like glandular pattern, solid nests and sheets, and microcysts[41]. HER2 is overexpressed in 20%-60% of serous carcinomas[29,42-45]; however, less frequent HER2 gene amplification has been reported[43,44]. Notably, the staining pattern of HER2 in serous carcinoma cells has been well studied; approximately 75% of HER2 expressing carcinoma cells reportedly show a basolateral/Lateral membranous staining pattern, similar to that of gastric adenocarcinoma[37]. HER2 assessment of serous carcinoma has been performed using various methods, mainly based on the breast cancer criteria[42-44]. However, an optimal HER2 testing method specific for endometrial serous carcinoma has not been established in the clinical trial setting.
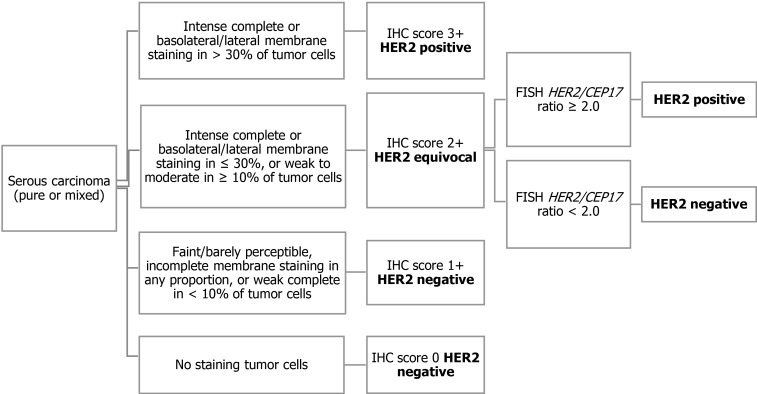
Recently, a randomized phase 2 clinical trial of carboplatin/paclitaxel vs carboplatin/ paclitaxel/trastuzumab in advanced or recurrent HER2 positive serous carcinoma demonstrated the survival benefit of adding trastuzumab[46]. Both prolonged progression-free survival (PFS) and overall survival (OS) were observed in the trastuzumab arm. For patient enrollment in this clinical trial, HER2 status was determined based on the modified 2007 ASCO/CAP breast criteria (Figure 1)[37]. Serous carcinoma showing intense complete or lateral/basolateral membranous HER2 staining in more than 30% of tumor cells were classified as score 3+, and score 2+ was assigned when intense complete or lateral/basolateral membrane staining was seen in ≤ 30%, or weak to moderate staining in ≥ 10% of tumor cells. FISH was performed only in tumors with an IHC score of 2+, and a HER2/CEP17 ratio of ≥ 2.0 was considered as amplified. Although this HER2 assessment criterion was justified in the context of the treatment effect of trastuzumab-containing regimens, it is still uncertain whether this HER2 testing algorithm should be used in patients receiving HER2 targeted drugs with a different mode of action from that of trastuzumab. For example, HER2-antibody drug conjugate (ADC) has been shown to be clinically effective in patients with low-level HER2 expressing breast cancer who have been assessed as HER2-negative according to the ASCO/CAP breast cancer criteria established for the prediction of response to trastuzumab[47]. The HER2 testing algorithm was optimized to maximize patient benefit and safety for each HER2 targeted therapy.
Figure 1.
Human epidermal growth factor receptor 2 testing for serous carcinoma used in the Phase 2 clinical trial[37,46]. IHC: Immunohistochemistry; FISH: Fluorescent in situ hybridization.
HER2 TESTING IN UTERINE CARCINOSARCOMA
Uterine carcinosarcoma (UCS) is a rare and high-grade subtype of endometrial cancer (WHO2020) and is characterized by the presence of both carcinomatous and sarcomatous components that are usually intimately admixed. The carcinomatous component is usually a high-grade endometrioid, serous, clear, or nonspecific carcinoma. HER2 is a promising therapeutic target for UCS. HER2 gene amplification has been reported in 13%-20% of UCS cases[48,49]. Additionally, HER2 overexpression (IHC score 3+) reportedly ranges from 0 to 25%[38,49-51].
An evidence-based HER2 testing protocol for UCS has not been fully established; thus, several researchers have adopted HER2 testing protocols and assessment methods based on ASCO/CAP recommendations for HER2 testing for breast cancer and gastroesophageal cancer.
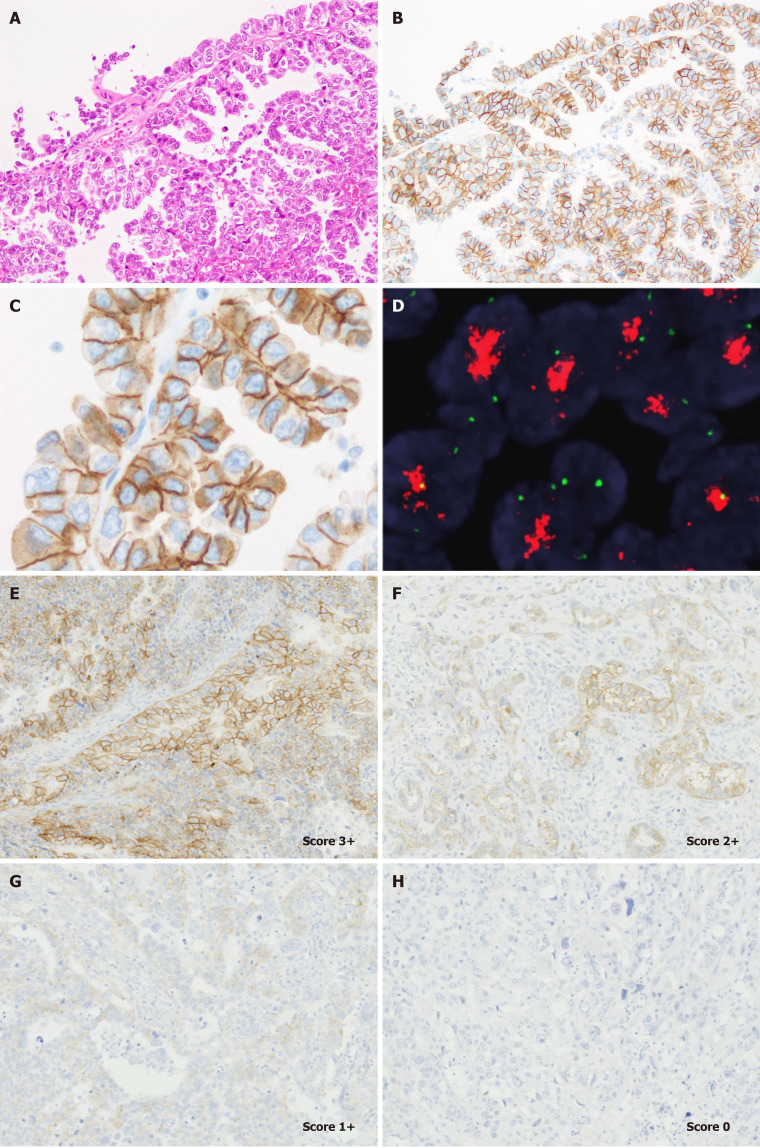
Recently, we proposed several requirements for HER2 testing in UCSs[39]. Our evaluation method has been used in the ongoing phase 2 clinical trial of HER2 ADC in patients with recurrent/metastatic UCS (STATICE trial)[52]. In this study, we identified that most UCS showed lateral/basolateral staining patterns (Figure 2), similar to endometrial serous carcinoma and gastric adenocarcinoma[39]. Based on our previous observations, we concluded that a HER2 testing protocol for UCS should contain the following requirements[39]: (1) Established pre-analytical factors of HER2 IHC[32,33] should be carefully controlled; (2) One representative section containing carcinoma components should be submitted from the hysterectomy specimen; (3) HER2 IHC should be performed using an IVD kit or a laboratory-developed test with appropriate quality control[32,33]; (4) Positive lateral/basolateral membranous staining patterns should be considered as positive, according to the 2016 ASCO/CAP gastric cancer criteria[32]; and (5) The proportion of HER2 expressing tumor cells should be determined as an approximate number of HER2-positive tumor cells divided by that of total tumor cells (both carcinoma and sarcomatous elements). Unfortunately, we did not provide supporting data for the best scoring system based on the patient clinical outcomes. However, we would report the correlation between treatment efficacy and HER2 score in patients with UCS in 1-2 years.
Figure 2.
Representative staining patterns of human epidermal growth factor receptor 2 and fluorescent in situ hybridization results in uterine carcinosarcoma. Serous-like carcinoma component (A) shows diffuse strong membranous staining of Human epidermal growth factor receptor 2 (HER2) (B). Most tumor cells show lateral or basolateral membranous staining patterns (C). HER2 amplification observed in these tumor cells using fluorescent in situ hybridization (D). Representative images of HER2 score according to ASCO/CAP gastric cancer criteria. Score 3+ (E), score 2+ (F), score 1+ (G), and score 0 (H) (× 200). A (H&E, × 100); B, E-G, and H (HER2, × 100); C (HER2, × 400). FISH: Fluorescent in situ hybridization; H&E: Hematoxylin and eosin.
FUTURE DIRECTION OF HER2 TESTING IN ENDOMETRIAL CANCER
Most quality assurance of HER2 testing should be performed in accordance with well-established methods for breast and gastric cancer. In addition, we should consider specific issues for HER2 testing in endometrial cancers, including correlation with specific treatment response, intratumoral heterogeneity of HER2 status, discordant HER2 status between the primary site and metastasis, improvement of interobserver reproducibility of HER2 assessment, and use of liquid biopsy in the future.
CURRENT THERAPIES AND FUTURE DIRECTIONS FOR HER2 TARGETED THERAPIES FOR UTERINE SEROUS CARCINOMA AND UTERINE CARCINOSARCOMA
Trastuzumab is the most popular HER2-targeted drug. Several case reports have shown that trastuzumab had clinical activity in patients with endometrial cancer[53,54]. Fleming et al[55] investigated the efficacy of trastuzumab as a single agent in HER2-positive endometrial carcinoma in a GOG study (Table 2). However, the overall response rate was 0%, and no significant clinical activity was observed. A possible issue was that many endometrioid carcinomas with low HER2 positivity were screened, and the study had early termination with poor accrual. Lapatinib is a dual inhibitor of EGFR and HER2 used in HER2-positive breast cancer. The GOG 229D[56] trial examined the efficacy of lapatinib in uterine cancer (n = 2 for HER2-positive) in a phase II trial. The response rate was 3%, the median PFS was 1.82 mo, and clinical activity could not be demonstrated. This lack of clinical activity could be explained by the unselected patient population. Therefore, further studies are warranted to highlight type II endometrial carcinoma patients with high HER2-positivity.
Table 2.
Human epidermal growth factor receptor 2-targeted therapies for uterine serous carcinoma and carcinosarcoma
|
Trial
|
Phase
|
Participants
|
Treatment
|
Efficacy/indentifer
|
| GOG181B[55] | II | HER2-positive EC (n = 33, endometrioid: 13, serous: 11, clear: 3, others: 6) | Trastuzumab | ORR 0%, mPFS 1.84 mo, mOS 7.8 mo |
| GOG229D[56] | II | Persistent or recurrent EC (n = 31, endometrioid: 16, serous: 7, clear: 3, others: 5, HER2-positive: n = 2) | Lapatinib | mPFS 1.82 mo, mOS 7.33 mo |
| Fader et al[46,57] | II | HER2-positive USC (n = 61) | carboplatin+paclitaxel+trastuzumab vs carboplatin+paclitaxel | mPFS 12.9 mo vs 8.0 mo, mOS 29.6 vs 24.4 mo |
| MyPathway[25] | Phase IIa Multiple basket study | Solid tumor(HER2-positive EC, n = 7) | Trastuzumab+pertuzumab | ORR 0%(EC) |
| Li et al[59] | II | HER2 amplified cancer (endometrial cancer: n = 18) | Trastuzumab emtansine | Endometrial cancers: CR 2, PR 2 |
| DESTINY-PanTumor02[77] | II | HER2 expressing tumor (urothelial, biliary tract, cervical, endometrial, ovarian, pancreatic, rare tumors) | Trastumab deruxtecan | NCT04482309 |
| Veneris[78] | I | HER2-positive USC | Trastuzumab deruxtecan+olaparib | NCT04585958 |
| Banerji et al[61] | I | Dose-expansion cohort: HER2-positive breast, gastric, urothelial, endometrial cancer | SYD-985 (Trastuzumab duocarmazine) | Endometrial cancer: ORR 39%, mPFS 4.3 mo |
| Koper[79] | I | HER2-positive solid tumor | SYD-985 (Trastuzumab duocarmazine)+Niraparib | NCT04235101 |
| Hendriks[80] | II | HER2-positive endometrial cancer | SYD-985 (Trastuzumab duocarmazine) | NCT04205630 |
| STATICE[52] | II | HER2-positive UCS | Trastuzumab deruxtecan | UMIN00002956 (NCCH1615) |
| Makker[81] | II | HER2-positive endometrial cancer, UCS | ZW25 | NCT04513665 |
| Ramos[82] | II | HER2-positive solid tumors | Tucatinib and trastuzumab | NCT04579380 |
EC: Endometrial cancer; USC: Uterine serous carcinoma; UCS: Uterine carcinosarcoma; CR: Complete response; mOS: Median overall survival; mPFS: Median progression-free survival; ORR: Objective response rate; PR: Partial response.
Fader et al[46] conducted a randomized phase II trial to evaluate the effect of trastuzumab on carboplatin and paclitaxel, the standard of care for HER2-positive USC. The median PFS [12.6 mo vs 8.0 mo; hazard ratio (HR) = 0.44, 90% confidence interval (CI): 0.26-0.76, P = 0.005] was significantly different between the two groups. Updated analysis (median PFS 12.9 mo vs 8.0 mo; HR = 0.46, 90%CI: 0.28-0.76; P = 0.005, median OS 29.6 mo vs 24.4 mo; HR = 0.58, 90%CI: 0.34-0.99; P = 0.46) showed clinically significant benefit[57]. The addition of trastuzumab to carboplatin and paclitaxel chemotherapy represents a new standard treatment for USC. In the NCCN guidelines, carboplatin, paclitaxel, and trastuzumab combination therapy are recommended in Category 2A for advanced or recurrent USC[58].
Several basket trials in HER2-positive solid tumors, including endometrial carcinoma, have been reported. MyPathway is a phase II, multiple basket study with patients with advanced refractory solid tumors harboring molecular alterations such as HER2, BRAF, EGFR, and the Hedgehog pathway[25]. Patients with HER2 alterations were treated with pertuzumab plus trastuzumab. Eight patients with HER2-positive endometrial carcinoma received trastuzumab plus pertuzumab. No responses were observed. The efficacy of trastuzumab emtansine has also been evaluated in a phase II basket trial, including HER2-positive endometrial carcinoma[59]. This trial demonstrated that 18 patients with uterine cancer (subtype unknown) were included, and two patients had a complete response and two had a partial response (PR), suggesting high efficacy.
Currently, several clinical trials of HER2-targeted drugs are ongoing. The DESTINY-PanTumor02 trial is a basket trial to evaluate the efficacy of trastuzumab deruxtecan in HER2-positive tumors, including endometrial carcinoma (NCT04482309). A phase I trial evaluating combination therapy with trastuzumab deruxtecan and olaparib in HER2-positive USC is also ongoing (NCT04585958). In HER2-positive UCS, the STATICE trial is a phase II trial to evaluate the efficacy of trastuzumab deruxtecan (UMIN00002956, NCCH1615)[52].
Novel therapeutic agents targeting HER2 have emerged. Trastuzumab duocarmazine (known as SYD985) is a novel HER2-targeted ADC that combines trastuzumab with duocarmazine, a DNA alkylating agent, as a payload. Trastuzumab duocarmazine has shown preclinical anti-tumor activity in USC[60]. Banerji et al[61] demonstrated phase 1 dose-escalation and dose-expansion study in breast, gastric, urothelial, and endometrial carcinomas that express HER2. Thirteen patients with endometrial cancer were included in the dose-expansion cohort. Five patients (39%) had PR, and the median PFS was 4.3 mo[61]. Treatment-related serious adverse events were reported in 11% of patients, and the frequency of cardiac toxicity did not increase compared with previous anti-HER2 drugs. Most patients had ocular adverse events, such as conjunctivitis, dry eye, and lacrimation. Trastuzumab duocarmazine has a manageable safety toxicity profile[61]. Further investigation of trastuzumab duocarmazine is ongoing in phase III trials for HER2-positive breast cancer (TULIP study, NCT03262935), phase II trials for HER2-positive endometrial cancer (NCT04205630), and phase I trial for HER2-positive solid tumor combination with niraparib (NCT04235101).
There are important considerations for future studies. Anti-HER2 therapy has been successful in HER2-positive breast cancer, but most of these drugs have not been successful in non-breast HER2-positive solid tumors. One explanation for these differences is the pattern of HER2 expression and heterogeneity within the tumor. Since the HER2 expression pattern of USC and UCS is similar to that of gastric cancer, but not breast cancer, it is possible that the same HER2-targeted therapy may have similar effects.
The first consideration may be to focus on combination therapies and ADC drugs rather than HER2 blockade alone in endometrial cancer. The second consideration is to overcome the resistance to HER2 targeted agents. Mechanisms of resistance to HER2 targeted therapy have been studied and can be classified into four groups: intratumoral heterogeneity, alterations in the binding site, activation of downstream signals, and overexpression of other HER2 family members[62]. The strategy against intratumoral heterogeneity could be a bystander effect of clinical activity against not only targeted cells but also surrounding cells. A novel HER2 ADC drug, trastuzumab deruxtecan, and trastuzumab duocarmazine showed a bystander effect in vitro and in vivo, and showed anti-tumor activity in clinical trials with low HER2 positivity[47,60,63-65]. Alteration in the binding site of HER2 and extracellular domain shedding could be overcome using irreversible inhibitors. In preclinical studies, afatinib and neratinib are irreversible pan-HER inhibitors that show anti-tumor activity in USC and gynecologic carcinosarcoma[66-68]. Tucatinib is an HER2 and HER3 kinase inhibitor, combination with trastuzumab are investigated in baskets study of Solid tumor with HER2 alterations, including uterine neoplasms. In downstream pathway alterations, the gain of function in PI3K is well known as resistance to HER2. Combined HER2 and PIK3CA dual inhibition using neratinib and taselisib were effective in cell lines and xenograft models of USC[69,70]. Of overexpression of other HER2 family members, HER3 overexpression plays an important role. The formation of HER2-HER3 heterodimers is most related to resistance to anti-HER2 therapy. Pertuzumab is an HER2 antibody that binds to a different epitope from that of trastuzumab and inhibits dimerization. In breast cancer, the combination of trastuzumab and pertuzumab improved clinical outcomes[71]. Pertuzumab and trastuzumab showed anti-tumor activity in USC cell lines[72]. Although these drug combinations showed no clinical benefit in the Mypathway trial with HER2 amplification in endometrial cancer[25], further investigation is warranted. Several HER2 bispecific antibodies have been developed that simultaneously bind to two distinct HER2 epitopes, the same domain as trastuzumab and pertuzumab[73]. Researchers demonstrated a phase 1 basket trial to evaluate ZW25, one of HER2 bispecific antibodies, in HER2 positive solid tumors, including endometrial cancer. Of 17 evaluable patients, seven patients (41%) had an objective response, and the median PFS was 6.2 mo[74]. ZW25 is investigated in phase 2 clinical trial of HER2 overexpressed advanced endometrial cancer and carcinosarcomas.
In addition, there is rapidly growing HER2-directed immunotherapy in patients with HER2-positive solid tumors[19,75]. Several drugs with a different target, such as bispecific antibodies, immune-stimulating conjugates, vaccines, and adoptive T-cell therapies, are under investigation[76]. The bispecific HER2/CD3 antibodies BTRC4017A, GBR-1302 and M802 induce cytotoxic effect by interaction with HER2 on tumor cell and CD3 on cytotoxic T cell. NJH395 are immune-stimulating antibody conjugates which HER2 antibody links to payload as toll-like receptor 7 (TLR7) and TLR8. Stimulating TLR activated natural killer cells and antigen-presenting cells and facilitate invasion of CTLs to tumor tissues. PRS-343 increases tumor lymphocyte invasion via targeting HER2 and CD137 (4-1BB). CD137 is known as a co-stimulating factor of T cell activation[73]. We expect a further investigation of these drugs in patients with endometrial cancer.
CONCLUSION
In this review, we provided an overview of HER2-overexpression/amplification in endometrial cancer, pathological evaluation methods, and the current status of HER2-targeted therapies. With the advent of precision medicine, the development of therapies targeting biomarkers has become increasingly advanced. In the development of anti-HER2 inhibitors and ADC drugs targeting HER2, it may be important to develop not only a single drug but also combination therapies. Since there are limited therapeutic agents for endometrial cancer, especially for type II, the development of HER2-targeted therapy is urgently needed.
Footnotes
Conflict-of-interest statement: All the authors have no conflict of interest to declare.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Clinical Oncology; Japanese Society of Medical Oncology; Japanese Society of Pathologists; and The Japanese Cancer Society.
Peer-review started: February 26, 2021
First decision: June 7, 2021
Article in press: September 2, 2021
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu SY, Wu RC S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX
Contributor Information
Ayumi Saito, Department of Breast and Medical Oncology, National Cancer Center Hospital, Tokyo 1040045, Japan.
Hiroshi Yoshida, Department of Diagnostic Pathology, National Cancer Center Hospital, Tokyo 1040045, Japan. hiroyosh@ncc.go.jp.
Tadaaki Nishikawa, Department of Breast and Medical Oncology, National Cancer Center Hospital, Tokyo 1040045, Japan.
Kan Yonemori, Department of Breast and Medical Oncology, National Cancer Center Hospital, Tokyo 1040045, Japan.
References
- 1.National Cancer Institute. SEER Cancer Stat Facts: Uterine Cancer. [cited 23 January 2021]. Available from: https://seer.cancer.gov/statfacts/html/corp.html .
- 2.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Ministry of Health, Labour and Welfare, National Cancer Registry). [cited 23 January 2021]. Available from: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#a14 .
- 3.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan). [cited 23 January 2021]. Available from: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#a14 .
- 4.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, Katabuchi H. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32. doi: 10.3802/jgo.2017.28.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu KH, Broaddus RR. Endometrial Cancer. N Engl J Med. 2020;383:2053–2064. doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, Mannel RS, Kim JW, Salani R, DiSilvestro PA, Burke JJ, Rutherford T, Spirtos NM, Terada K, Anderson PR, Brewster WR, Small W, Aghajanian CA, Miller DS. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J Clin Oncol. 2019;37:1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron MH, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Carinelli S, Provencher D, Hanzen C, Lutgens LCHW, Smit VTHBM, Singh N, Do V, D'Amico R, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL PORTEC study group. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 10.Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, DiSilvestro P, Pearl ML, Argenta PA, Powell MA, Zweizig SL, Warshal DP, Hanjani P, Carney ME, Huang H, Cella D, Zaino R, Fleming GF. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209) J Clin Oncol. 2020;38:3841–3850. doi: 10.1200/JCO.20.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura H, Aoki D, Michimae H, Mizuno M, Nakai H, Arai M, Sasagawa M, Ushijima K, Sugiyama T, Saito M, Tokunaga H, Matoda M, Nakanishi T, Watanabe Y, Takahashi F, Saito T, Yaegashi N Japanese Gynecologic Oncology Group. Effect of Taxane Plus Platinum Regimens vs Doxorubicin Plus Cisplatin as Adjuvant Chemotherapy for Endometrial Cancer at a High Risk of Progression: A Randomized Clinical Trial. JAMA Oncol. 2019;5:833–840. doi: 10.1001/jamaoncol.2019.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17:964–978. doi: 10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 13.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, Romeo M, Bratos R, Brose MS, DiSimone C, Messing M, Stepan DE, Dutcus CE, Wu J, Schmidt EV, Orlowski R, Sachdev P, Shumaker R, Casado Herraez A. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020;38:2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med. 2019;381:1284–1286. doi: 10.1056/NEJMcibr1909386. [DOI] [PubMed] [Google Scholar]
- 18.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 19.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 20.Fusco N, Bosari S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J Gastroenterol. 2016;22:7926–7937. doi: 10.3748/wjg.v22.i35.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 22.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I DESTINY-Breast01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 24.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 25.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein A, Beattie M, Kurzrock R. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol. 2018;36:536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 26.Kurzrock R, Bowles DW, Kang H, Meric-Bernstam F, Hainsworth J, Spigel DR, Bose R, Burris H, Sweeney CJ, Beattie MS, Blotner S, Schulze K, Cuchelkar V, Swanton C. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol. 2020;31:412–421. doi: 10.1016/j.annonc.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, Juric D, Quinn DI, Moreno V, Doger B, Mayer IA, Boni V, Calvo E, Loi S, Lockhart AC, Erinjeri JP, Scaltriti M, Ulaner GA, Patel J, Tang J, Beer H, Selcuklu SD, Hanrahan AJ, Bouvier N, Melcer M, Murali R, Schram AM, Smyth LM, Jhaveri K, Li BT, Drilon A, Harding JJ, Iyer G, Taylor BS, Berger MF, Cutler RE Jr, Xu F, Butturini A, Eli LD, Mann G, Farrell C, Lalani AS, Bryce RP, Arteaga CL, Meric-Bernstam F, Baselga J, Solit DB. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–194. doi: 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, Maxwell GL, Fowler JM. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24:2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 30.Peiró G, Mayr D, Hillemanns P, Löhrs U, Diebold J. Analysis of HER-2/neu amplification in endometrial carcinoma by chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, p53 and Ki-67 protein expression, and outcome. Mod Pathol. 2004;17:227–287. doi: 10.1038/modpathol.3800006. [DOI] [PubMed] [Google Scholar]
- 31.Vermij L, Horeweg N, Leon-Castillo A, Rutten TA, Mileshkin LR, Mackay HJ, Leary A, Powell ME, Singh N, Crosbie EJ, Smit VTHBM, Creutzberg CL, Bosse T. HER2 Status in High-Risk Endometrial Cancers (PORTEC-3): Relationship with Histotype, Molecular Classification, and Clinical Outcomes. Cancers (Basel) 2020;13 doi: 10.3390/cancers13010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, Carrato A, Gulley ML, Jain D, Kakar S, Mackay HJ, Streutker C, Tang L, Troxell M, Ajani JA. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:446–464. doi: 10.1200/JCO.2016.69.4836. [DOI] [PubMed] [Google Scholar]
- 33.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Shimada K, Kosuge T, Hiraoka N. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Arch. 2016;468:431–439. doi: 10.1007/s00428-015-1898-1. [DOI] [PubMed] [Google Scholar]
- 35.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 36.Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W, Bencardino K, Lonardi S, Zagonel V, Leone F, Noe J, Ciardiello F, Pinto C, Labianca R, Mosconi S, Graiff C, Aprile G, Frau B, Garufi C, Loupakis F, Racca P, Tonini G, Lauricella C, Veronese S, Truini M, Siena S, Marsoni S, Gambacorta M. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28:1481–1491. doi: 10.1038/modpathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 37.Buza N. HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand. Arch Pathol Lab Med. 2021;145:687–691. doi: 10.5858/arpa.2020-0207-RA. [DOI] [PubMed] [Google Scholar]
- 38.Rottmann D, Snir OL, Wu X, Wong S, Hui P, Santin AD, Buza N. HER2 testing of gynecologic carcinosarcomas: tumor stratification for potential targeted therapy. Mod Pathol. 2020;33:118–127. doi: 10.1038/s41379-019-0358-x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida H, Nishikawa T, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Hasegawa K, Yonemori K. Histopathological features of HER2 overexpression in uterine carcinosarcoma: proposal for requirements in HER2 testing for targeted therapy. Virchows Arch. 2021;478:1161–1171. doi: 10.1007/s00428-021-03017-5. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatius S, Matias-Guiu X. Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod Pathol. 2016;29 Suppl 1:S45–S58. doi: 10.1038/modpathol.2015.141. [DOI] [PubMed] [Google Scholar]
- 42.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013;26:1605–1612. doi: 10.1038/modpathol.2013.113. [DOI] [PubMed] [Google Scholar]
- 43.Mentrikoski MJ, Stoler MH. HER2 immunohistochemistry significantly overestimates HER2 amplification in uterine papillary serous carcinomas. Am J Surg Pathol. 2014;38:844–851. doi: 10.1097/PAS.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 44.Singh P, Smith CL, Cheetham G, Dodd TJ, Davy ML. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int J Gynecol Cancer. 2008;18:1344–1351. doi: 10.1111/j.1525-1438.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 45.Grushko TA, Filiaci VL, Mundt AJ, Ridderstråle K, Olopade OI, Fleming GF Gynecologic Oncology Group. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers SK, Secord AA, Havrilesky L, O'Malley DM, Backes F, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi KS, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi DA, Schwartz PE, Santin AD. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 47.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amant F, Vloeberghs V, Woestenborghs H, Debiec-Rychter M, Verbist L, Moerman P, Vergote I. ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol. 2004;95:583–587. doi: 10.1016/j.ygyno.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 49.Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol. 2006;100:101–106. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 50.Swisher EM, Gown AM, Skelly M, Ek M, Tamimi HK, Cain JM, Greer BE, Muntz HG, Goff BA. The expression of epidermal growth factor receptor, HER-2/Neu, p53, and Ki-67 antigen in uterine malignant mixed mesodermal tumors and adenosarcoma. Gynecol Oncol. 1996;60:81–88. doi: 10.1006/gyno.1996.0015. [DOI] [PubMed] [Google Scholar]
- 51.Saglam O, Husain S, Toruner G. AKT, EGFR, C-ErbB-2, and C-kit expression in uterine carcinosarcoma. Int J Gynecol Pathol. 2013;32:493–500. doi: 10.1097/PGP.0b013e31827fedef. [DOI] [PubMed] [Google Scholar]
- 52.Yonemori K. A clinical trial of DS-8201a in HER2 positive(HER2>=1) metastatic/recurrent uterine carcinosarcoma patients (NCCH1615,STATICE trial). [cited 27 September 2020]. Available from: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000033713 .
- 53.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Jewell E, Secord AA, Brotherton T, Berchuck A. Use of trastuzumab in the treatment of metastatic endometrial cancer. Int J Gynecol Cancer. 2006;16:1370–1373. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 55.Fleming GF, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, Berek JS, Chapman JA, DiSilvestro PA, Horowitz IR, Fiorica JV. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, Schilder RJ, Walker JL, Tewari K, Hanjani P, Abulafia O, Rose PG. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers S, Secord AA, Havrilesky L, O'Malley DM, Backes FJ, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi K, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi DA, Schwartz PE, Santin AD. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu ( NCT01367002): Updated Overall Survival Analysis. Clin Cancer Res. 2020;26:3928–3935. doi: 10.1158/1078-0432.CCR-20-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Uterine Neoplasms. Version 1. 2021. [cited 24 January 2021] Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf .
- 59.Li BT, Makker V, Buonocore DJ, Offin MD, Olah ZT, Panora E, Shen R, Ho AL, Yaeger R, Iyer G, Ginsberg MS, Ulaner G, Solit DB, Hyman DM, Rudin CM, Berger MF, Baselga J, Scaltriti M, Arcila ME, Kris MG. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol. 2018;36:2502–2502. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, Predolini F, De Haydu C, Cocco E, Buza N, Hui P, Wong S, Lopez S, Ratner E, Silasi DA, Azodi M, Litkouhi B, Schwartz PE, Goedings P, Beusker PH, van der Lee MM, Timmers CM, Dokter WH, Santin AD. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Mol Cancer Ther. 2016;15:1900–1909. doi: 10.1158/1535-7163.MCT-16-0163. [DOI] [PubMed] [Google Scholar]
- 61.Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, Macpherson IR, Boni V, Rolfo C, de Vries EGE, Rottey S, Geenen J, Eskens F, Gil-Martin M, Mommers EC, Koper NP, Aftimos P. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 62.Erickson BK, Zeybek B, Santin AD, Fader AN. Targeting human epidermal growth factor receptor 2 (HER2) in gynecologic malignancies. Curr Opin Obstet Gynecol. 2020;32:57–64. doi: 10.1097/GCO.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, Damming D, Jacobs DC, Rouwette M, Egging DF, van den Dobbelsteen D, Beusker PH, Goedings P, Verheijden GF, Lemmens JM, Timmers M, Dokter WH. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther. 2015;14:692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- 64.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 65.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwab CL, Bellone S, English DP, Roque DM, Lopez S, Cocco E, Nicoletti R, Bortolomai I, Bonazzoli E, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Santin AD. Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo. Br J Cancer. 2014;111:1750–1756. doi: 10.1038/bjc.2014.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwab CL, English DP, Roque DM, Bellone S, Lopez S, Cocco E, Nicoletti R, Rutherford TJ, Schwartz PE, Santin AD. Neratinib shows efficacy in the treatment of HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014;135:142–148. doi: 10.1016/j.ygyno.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Schwab CL, English DP, Black J, Bellone S, Lopez S, Cocco E, Bonazzoli E, Bussi B, Predolini F, Ferrari F, Ratner E, Silasi DA, Azodi M, Rutherford T, Schwartz PE, Santin AD. Neratinib shows efficacy in the treatment of HER2 amplified carcinosarcoma in vitro and in vivo. Gynecol Oncol. 2015;139:112–117. doi: 10.1016/j.ygyno.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez S, Schwab CL, Cocco E, Bellone S, Bonazzoli E, English DP, Schwartz PE, Rutherford T, Angioli R, Santin AD. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014;135:312–317. doi: 10.1016/j.ygyno.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez S, Cocco E, Black J, Bellone S, Bonazzoli E, Predolini F, Ferrari F, Schwab CL, English DP, Ratner E, Silasi DA, Azodi M, Schwartz PE, Terranova C, Angioli R, Santin AD. Dual HER2/PIK3CA Targeting Overcomes Single-Agent Acquired Resistance in HER2-Amplified Uterine Serous Carcinoma Cell Lines In Vitro and In Vivo. Mol Cancer Ther. 2015;14:2519–2526. doi: 10.1158/1535-7163.MCT-15-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortés J CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102:134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Santis R. Anti-ErbB2 immunotherapeutics: struggling to make better antibodies for cancer therapy. MAbs. 2020;12:1725346. doi: 10.1080/19420862.2020.1725346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Effective in HER2-Positive Cancers. Cancer Discov. 2019;9:8. doi: 10.1158/2159-8290.CD-NB2018-162. [DOI] [PubMed] [Google Scholar]
- 75.Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J, Piha-Paul SA. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin Cancer Res. 2019;25:2033–2041. doi: 10.1158/1078-0432.CCR-18-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa RLB, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer. 2020;6:10. doi: 10.1038/s41523-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.AstraZeneca A Phase 2, Multicenter, Open-label Study to Evaluate the Efficacy and Safety of Trastuzumab Deruxtecan (T-DXd, DS-8201a) for the Treatment of Selected HER2 Expressing Tumors (DESTINY-PanTumor02). [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04482309. ClinicalTrials.gov Identifier: NCT04482309.
- 78.Veneris JL. Testing the Combination of DS-8201a and Olaparib in HER2-Expressing Cancers With Expansion in Patients With Endometrial Cancer. [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT04585958. ClinicalTrials.gov Identifier: NCT04585958.
- 79.Koper N. Phase I Study of SYD985 With Niraparib in Patients With Solid Tumors. [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT04235101. ClinicalTrials.gov Identifier: NCT04235101.
- 80.Hendriks M. SYD985 in Patients With HER2-expressing Recurrent, Advanced or Metastatic Endometrial Carcinoma. [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT04205630. ClinicalTrials.gov Identifier: NCT0425630.
- 81.Makker V. ZW25 in Women With Endometrial Cancers. [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT04513665. ClinicalTrials.gov Identifier: NCT04513665.
- 82.Ramos J. Basket Study of Tucatinib and Trastuzumab in Solid Tumors With HER2 Alterations. [Accessed January 25, 2021]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://ClinicalTrials.gov/show/NCT04579380. ClinicalTrials.gov Identifier: NCT04579380.