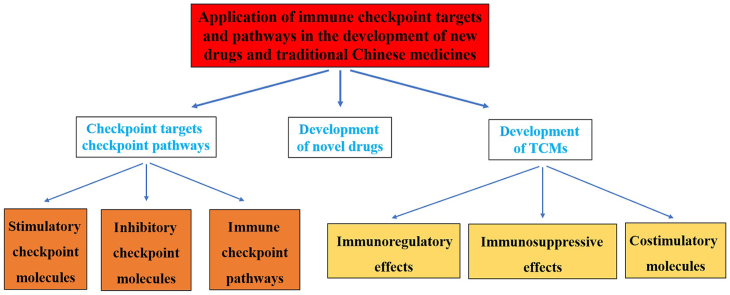
Abstract
Immune checkpoints are the crucial regulators of immune system and play essential roles in maintaining self-tolerance, preventing autoimmune responses, and minimizing tissue damage by regulating the duration and intensity of the immune response. Furthermore, immune checkpoints are usually overexpressed in cancer cells or noninvasive cells in tumor tissues and are capable of suppressing the antitumor response. Based on substantial physiological analyses as well as preclinical and clinical studies, checkpoint molecules have been evaluated as potential therapeutic targets for the treatment of multiple types of cancers. In the last few years, extensive evidence has supported the immunoregulatory effects of traditional Chinese medicines (TCMs). The main advantage of TCMs and natural medicine is that they usually contain multiple active components, which can act on multiple targets at the same time, resulting in additive or synergistic effects. The strong immune regulation function of traditional Chinese medicine on immune checkpoints has also been of great interest. For example, Astragalus membranaceus polysaccharides can induce anti-PD-1 antibody responses in animals, and these antibodies can overcome the exhaustion of immune cells under tumor immune evasion. Furthermore, many other TCM molecules could also be novel and effective drug candidates for the treatment of cancers. Therefore, it is essential to assess the application of immune checkpoints in the development of new drugs and TCMs. In this review, we focus on research progress in the field of immune checkpoints based on three topics: (1) immune checkpoint targets and pathways, (2) development of novel immune checkpoint-based drugs, and (3) application of immune checkpoints in the development of TCMs.
KEY WORDS: Immune checkpoint, Therapeutic target, Signaling pathway, Drug development, Traditional Chinese medicine, Immunoregulatory, Cancer immunity
Graphical abstract
In this review, we provide a discussion of the applications of immune checkpoints in the development of novel drugs and TCMs.
1. Introduction
In cancer and chronic viral infections, T cells are exposed to persistent antigen stimulation, leading to the expression of multiple inhibitory receptors, also called “immune checkpoints”. Immune checkpoints are the regulators of immune system and are involved in self-tolerance, which prevents the immune system from attacking cells indiscriminately. The concept of immune checkpoint molecules was proposed many years ago. The immune function of the human body is activated after stimulation; however, overactivation is prevented through immune checkpoint molecule applying a “brake” in order to maintain normal activation of the immune system1.
CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T-helper (Th) 1, Th2, and Th17 cells are all subtypes of effector T cells that induce protective immunity in response to causative agents, parasites, and tumors. These T cells can also promote inflammation and participate in other immunopathology and/or autoimmunity processes. Moreover, various regulatory cells [regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2-type macrophages] and secreted cytokines [interleukin (IL)-2, IL-10, transforming growth factor (TGF)-β, interferon (IFN)-γ] in the innate and adaptive immune systems mediate immune regulation and facilitate immune integration to affect cancer and acute and chronic infections; therefore, abnormal integration is an essential therapeutic target in clinical diagnosis and treatment2. Abnormal expression and function of immune checkpoint molecules are important causes of many diseases. For example, overexpression or hyperactivation of immune checkpoint molecules can block immune function, increasing the risk of cancer. Conversely, if immune suppression of checkpoint molecules is poor or the immune checkpoint are regulated by targeted checkpoint molecule inhibitors, the body's immune function is enhanced. Currently approved checkpoint inhibitors block cytotoxic T lymphocyte antigen-4 (CTLA4), programmed cell death 1 protein (PD-1), and PD-1 ligand (PD-L1)1. James P. Allison and TasukuHonjo won the Tang Prize in Biopharmaceutical Science and the Nobel Prize in Physiology or Medicine in 2018 for research related to these discoveries3,4.
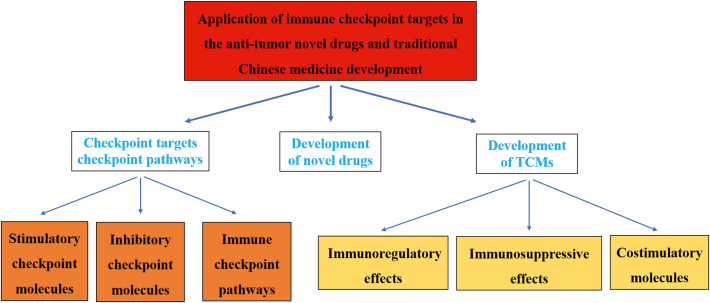
In this review, we provide a discussion of the applications of immune checkpoints in the development of novel drugs and TCMs based on the following three topics: (1) immune checkpoint targets and pathways; (2) development of novel immune checkpoint-based drugs; and (3) development of immune checkpoint-related TCMs.
2. Checkpoint targets and pathways with different effects
2.1. Stimulatory checkpoint molecules
The activation of stimulatory immune checkpoints can augment the impact of the immune response and immune subclasses of the tumor microenvironment. For example, enhancement of the activation, proliferation, and activities of CD8+ T cells, CD4+ T cells, natural killer (NK) cells, and macrophages can lead to increased production of inflammatory agents. Moreover, these activities inhibit the proliferation and function of MDSCs and Tregs. Five stimulatory checkpoint molecules, i.e., CD27, CD40, OX40, glucocorticoid-induced tumor necrosis factor (TNF) receptor (TNFR)-related protein (GITR), and CD137, are members of the TNFR superfamily, whereas two other stimulatory checkpoint molecules, i.e., CD28 and inducible T-cell costimulatory (ICOS), belong to the B7-CD28 superfamily (Table 15, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49).
Table 1.
Checkpoint targets with different effects and its pathways.
| Checkpoint molecule | Molecule expression | Application | |
|---|---|---|---|
| Stimulatory checkpoint molecules | CD27 | (1) The molecule supports antigen-specific expansion of naïve T cells and is vital for the generation of T cell memory5; (2) A memory marker of B cells6, the activity is governed by the transient availability of its ligand, CD70, on lymphocytes and dendritic cells7; (3) CD27 co-stimulation is known to suppresses Th17 effector cell function8. |
Celldex Therapeutics is working on CDX- 1127, an agonistic anti-CD27 monoclonal antibody which in animal models has been shown to be effective in the context of T cell receptor stimulation9,10 |
| CD28 | (1) The molecule is constitutively expressed on almost all human CD4+ T cells and on around half of all CD8 T cell11; (2) Binding with its two ligands are CD80 and CD86, expressed on dendritic cells, prompts T cell expansion11. |
CD28 was the target of the TGN1412 (superagonist) which caused severe inflammatory reactions in the first-in-man study11 | |
| CD40 | (1) The molecule, found on a variety of immune system cells including antigen presenting cells has CD40L, as CD154 and transiently expressed on the surface of activated CD4+ T cells, as its ligand12; (2) CD40 signaling is known to “license” dendritic cells to mature and thereby trigger T-cell activation and differentiation12. |
VLST in-licensed an anti-CD40 agonist monoclonal antibody in 201213 | |
| CD122 | The molecule, which is the interleukin-2 receptor β subunit, is known to increase proliferation of CD8+ effector T cells14. | Therapeutics is working on NKTR-214, a CD122-biased immunestimulitory cytokine phase I results announced in 201615,16 | |
| CD137 | The molecule (4-1BB) is bound by CD137 ligand, the result is T-cell proliferation. CD137-mediated signaling is known to protect T cells, and in particular, CD8+ T cells from activation-induced cell death17. | It has developed an engineered lipocalin that is bi-specific for CD137 and HER218 | |
| OX40 | CD134 has OX40L, or CD252, as its ligand. OX40 promotes the expansion of effector and memory T cells, it is noted for its ability to suppress the differentiation and activity of T-regulatory cells, and for its regulation of cytokine production19. | OX40 as a drug target primarily lies in the fact that, being transiently expressed after T-cell receptor engagement, it is to upregulate on the most antigen-activated T cells within inflammatory lesions20 Anti-OX40 antibodies have been used in advanced cancer21 Three drugs have in development for targeting therapy22 |
|
| GITR | The molecule is glucocorticoid-induced TNFR family related gene, prompts T cell expansion, including Treg-expansion. The ligand for GITR is mainly expressed on antigen presenting cells. Antibodies to GITR shown to promote an anti-tumor response through loss of Treg lineage stability23, 24, 25. | Transgenic (TG) therapeutics is working on anti-GITR antibodies26 | |
| ICOS | CD278 is expressed on activated T cells. Its ligand is ICOSL, expressed mainly on B cells and dendritic cells. The molecule seems to be important in T cell effector function27. | Therapeutics is developing an ICOS agonist27 | |
| Inhibitory checkpoint molecules | A2AR | Adenosine A2A receptor (A2AR) is regarded as an important checkpoint in cancer therapy because adenosine in the immune microenvironment, leading to the activation of the A2a receptor. | A2AR is negative immune feedback loop and the tumor microenvironment has relatively high concentrations of adenosine28 |
| B7-H3 | B7-H3 (CD276) is originally understood to be a co-stimulatory molecule29 but is now regarded as co-inhibitory30. | MGA271 of Macro-Genics is an Fc-optimized monoclonal antibody that targets B7-H3. B7-H3 receptors have not yet been identified31,32 | |
| B7-H4 | B7-H4 (VTCN1) is expressed by tumor cells and tumor-associated macrophages33. | B7-H4 plays a role in tumor escape33 | |
| BTLA | CD272, short for B and T lymphocyte attenuator has HVEM (herpesvirus entry mediator) as its ligand. Surface expression of BTLA is downregulated during differentiation of human CD8+ T cells from the naive to effector cell phenotype34. | BTLA is downregulated during differentiation of human CD8+ T cells from the naive to effector cell phenotype. The tumor-specific human CD8+ T cells express high levels of BTLA34 | |
| CTLA-4 | CTLA-4 is known as CD152, is the target of melanoma drug, expression of CTLA-4 on Treg cells serves to control T cell proliferation35,36. | Yervoy is gained FDA approval35,36 | |
| IDO | IDO is a tryptophan catabolic enzyme with immune–inhibitory properties. Another important molecule is TDO, tryptophan 2,3-dioxygenase37,38. | IDO is known to suppress T and NK cells, generate and activate Treg and myeloid-derived suppressor cells, and promote tumor angiogenesis37,38 | |
| KIR | KIR is a receptor for MHC class I molecules on natural Killer cells. | Lirilumab, a monoclonal antibody to KIR, is developing | |
| LAG3 | LAG3 works to suppress an immune response by action to Treg and direct effects on CD8+ T cells36,38,39. | The phase I clinical study of anti-LAG3 (BMS-986016) is carried out40 | |
| Nicotinamide adenine dinucleotide phosphate NADPH oxidase isoform 2 (NOX2) | NOX2 is an enzyme of myeloid cells that generates immunosuppressive reactive oxygen species. Genetic and pharmacological inhibition of NOX2 in myeloid cells improves anti-tumor functions of adjacent NK cells and T cells and also triggers autoimmunity in humans and experimental animals36,41. | Ceplene has gained approval for use in acute myeloid leukemia within the EU36,41 | |
| PD-1 | PD-1 receptor, has two ligands, PD-L1 and PD-L2. This checkpoint is the target of melanoma drug Keytruda36,42. | An advantage of targeting PD-1 can restore immune function in the tumor microenvironment36,42 | |
| TIM-3 | TIM-3, expresses on activated human CD4+ T cells and regulates Th1 and Th17 cytokines43. | TIM-3 acts as a negative regulator of Th1/Tc1 function by triggering cell death upon interaction with ligand (galectin-9)36,44 | |
| VISTA | VISTA is primarily expressed on hematopoietic cells45. | The consistent expression of VISTA on leukocytes within tumors may allow VISTA blockade to be effective across a broad range of solid tumors36,46 | |
| Sialic acid-binding immunoglobulin-type lectin (SIGLEC) | SIGLEC7 is designated as CD328 and SIGLEC9 is designated as CD329, and are proteins found on the surface of various immune cells, including natural killer cells and macrophages or neutrophils, macrophages, dendritic cells and activated T-cells47. | SIGLECs 7 and 9 suppress the immune function of these cells by binding to terminal sialic acid on glycans that cover the surface of cells48,49 | |
| Immune checkpoint target | Immune checkpoint pathway | |
|---|---|---|
| Co-Stimulatory immune checkpoint targets and pathways | CD155/PVR, CD226/DNAM-1 | CD266 & CD155 |
| CD40/TNFRSF5, CD40L/CD154/TNFSF5 | CD40 & CD40L | |
| OX40/CD134, OX40L/TNFSF4/CD252 | OX40 & OX40L | |
| HVEM/TNFRSF14, TNFSF14/LIGHT/CD258 | HVEM & LIGHT | |
| CD28/TP44, CD80/B7-1, CD86/B7-2 | CD28 & CD80 (CD86) | |
| GITR/TNFRSF18, GITR Ligand/TNFSF18 | GITR & GITR ligand | |
| CD27, CD70/CD27L/TNFSF7 | CD27 & CD70 | |
| CD137/4-1BB, 4-1BBL/CD137L | 4-1BB & 4-1BBL | |
| ICOS/AILIM/CD278, ICOS ligand/B7-H2 | ICOS & ICOS ligand | |
| Co-inhibitory immune checkpoint targets and pathways | PD1/PDCD1/CD279, PD-L1/B7-H1/CD274 | PD1, PD-L1 |
| CTLA-4/CD152, CD80/B7-1, CD86/B7-2 | CTLA-4, CD80 (CD86) | |
| B7-H3/CD276 | B7-H3/CD276 | |
| B7-H4/B7S1/B7x | B7-H4/B7S1/B7x | |
| HVEM/TNFRSF14, BTLA | HVEM/BTLA | |
| HVEM/TNFRSF14, CD160 | HVEM/CD160 | |
| LAG3/CD223 | LAG3/CD223 | |
| Galectin-9/LGALS9, TIM-3/HAVCR2 | Galectin-9, TIM-3 | |
| Indoleamine2,3-dioxygenase/IDO | Indoleamine2,3-dioxygenase/IDO | |
| VISTA/B7-H5/GI24 | VISTA/B7-H5/GI24 | |
| CEACAM1/CD66a | CEACAM1/CD66a | |
| SIRPalpha/CD172a, CD47 | SIRPalpha, CD47 | |
| 2B4/CD244, CD48/SLAMF2 | 2B4, CD48 | |
| TIGIT/VSTM3, CD155/PVR | TIGIT, CD155 |
2.2. Inhibitory checkpoint molecules
In the last decade, inhibitory immune checkpoints have emerged as pivotal molecules preventing tumor attack by the immune system. Cancer cells exploit immune-inhibitory molecules to contrast immune interventions and induce tumor tolerance. Additionally, inhibitory checkpoint molecules are targets for cancer immunotherapy because of their potential for use in multiple types of cancers. Molecular agents that target these checkpoints represent a new frontier for cancer treatment. Immune checkpoint-targeted therapy has been shown to be helpful for the treatment of selected and even histologically different types of cancer. Unlike most currently approved antibodies that are used in cancer therapy, antibodies for blocking immune checkpoints do not target tumor cells directly; instead, they target lymphocyte receptors or their ligands to enhance endogenous antitumor activity. ThemoAb-based immunotherapy, which targets PD-1, PD-L1, or CTLA4, is the most frequently used antibody therapy. PD-1, a transmembrane receptor found on T cells from the immunoglobulin B7-CD28 family, consists of an extracellular segment, a hydrophobic transmembrane region, and an intracellular segment50. After PD-1 binds to its ligand, the immunoreceptor tyrosine motif of the PD-1 intracellular domain is phosphorylated, and tyrosine phosphatase is recruited to the intracellular region. These phosphatases dephosphorylate key proteins in the T-cell antigen receptor (TCR) signaling pathway; inhibit downstream phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), RAS/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), and other signaling pathways; and block the proliferation and differentiation of T cells and the production of cytokines51. To date, many new immune checkpoints have been discovered and developed as potential therapeutic targets (Table 1).
2.3. Immune checkpoint pathways
Under normal conditions, immune checkpoint molecules maintain self-tolerance and prevent immunopathology; however, their sustained expression deteriorates T-cell function. Recent advances in cancer immunotherapy involve blockade of CTLA4 (also known as CD279) and PD-1 in order to reverse T-cell exhaustion and reinvigorate immunity in metastatic melanoma and lung cancer. First, T-cell responses rely on TCR-dependent recognition of MHC/peptide complexes on antigen-presenting cells (APCs). Further engagement of costimulatory and co-inhibitory molecules guarantees the onset and limitation of T-cell activities, supporting the functions of immune checkpoints. Initial studies focused on relieving the immunosuppressive brake applied by the co-inhibitory CTLA-4 and PD-1 receptors52. CTLA-4 is expressed by activated Tregs and exhibits competitive binding with stimulatory CD28 ligands (CD80/CD86). PD-1 is expressed by activated and exhausted T cells, and binding to its ligands PD-L1 and PDL-2 directly inhibits TCR signaling through SHP2-mediated dephosphorylation of proximal signaling elements. Notably, recent findings have identified CD28 as a convergent regulatory target for both CTLA-4 and PD-1 and have demonstrated the regulation of intratumoral T-cell trafficking by PD-1. A schematic of these characteristics of the immune checkpoint pathway is presented in Fig. 1.
Figure 1.
The mechanism of immunotherapy regulation based on immune checkpoint pathways (PD-1 and PD-L1).
For therapeutic vaccines against chronic infections, e.g., human immunodeficiency virus (HIV), human papilloma virus (HPV), hepatitis B virus, and hepatitis C virus, adjunct checkpoint blockade strategies are required, including blockade of other inhibitory receptors (e.g., T-cell immunoreceptor) with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibitory motif-domains, e.g., T-cell Ig and mucin domain 3 (TIM-3), lymphocyte activation gene 3 (LAG3), and V-domain Ig-containing suppressor of T-cell activation (VISTA). Different chronic viral infections and cancers are likely to influence the level, composition, and pattern of inhibitory receptors expressed by responding T cells, thereby affecting checkpoint antibody blockade strategies. Recent advances have identified co-inhibitory receptors and new antibody-blockade therapeutic targets for T-cell exhaustion in chronic viral infections and cancer. Therefore, understanding the mechanisms of T-cell exhaustion in response to infections and cancer and the characteristics of T-cell responses will contribute to further improvement of immune checkpoint blockade strategies.
Suppression of co-inhibitory receptors shows unprecedented efficacy in the treatment of some tumors, and characterization of immunotherapy targets may increase the number of patients who can be assisted by these drugs. Immunotherapy research programs are now exploring a wide range of both co-inhibitory (e.g., LAG3/TIGIT/TIM-3) and costimulatory (e.g., GITR/4-1BB/OX40) receptors as individual or combination therapies. The complex immune system is regulated by a broad network of co-inhibitory and costimulatory receptors that control the type, scale, and duration of immune responses. Thus, these receptors are now recognized as promising immunotherapeutic targets for the treatment of cancers and autoimmune diseases. Table 1 lists the costimulatory and co-inhibitory immune checkpoint targets and pathways.
3. Immune checkpoints in the development of novel drugs
Inflammatory immune responses are often difficult to treat specifically because of their highly complex multitarget networks. There are two well-known immune checkpoint receptors that have been actively studied. One of the most widely studied anticancer targets is the important immune checkpoint molecule PD-1 and its ligand PD-L1. Another important checkpoint molecule is CTLA-4. Antibodies targeting these molecules are currently the most effective treatments available. Corresponding antibodies can inhibit the functions of the receptors and enhance the immunity of antitumor cells. Moreover, multiple additional immune checkpoints are being studied as promising targets for anticancer therapy.
Novel immune checkpoint molecules and drugs that regulate the expression or function of these molecules may have promising clinical applications. Drugs or drug candidates that inhibit or block inhibitory checkpoint molecules are sometimes known as checkpoint inhibitors or the immune checkpoint blockade1.
Checkpoint inhibitors have been evaluated by various pharmaceutical companies as potential anticancer agents53. The U.S. Food and Drug Administration (FDA) has approved two anti-CTLA-4 antibodies, i.e., ipilimumab (Yervoy) developed by Bristol-Myers-Squibb54 and tremelimumab developed by Pfizer55, for the treatment of melanoma and mesothelioma, respectively. Immunotherapies targeting PD-1/PD-L1 stimulates the body's antitumor immune function by blocking the PD-1/PD-L1 signal pathway (Table 2). FDA has approved four PD-1/PD-L1 antibodies, i.e., nivolumab (Opdivo) developed by Bristol-Myers-Squibb56, pembrolizumab (Keytruda) by MSD57, atezolizumab (Tecentriq) by Roche58, and avelumab (Bavencio) by Merck and Pfizer59. Clinical trials for advanced melanoma, non-small cell lung cancer, kidney cancer, Hodgkin's lymphoma, head and neck squamous cell carcinoma, bladder cancer, and metastatic Meckel's cell cancer are ongoing for a variety of other tumors, including liver cancer and bowel cancer. In the European Union, nivolumab60 has also been approved for the treatment of locally advanced or metastatic squamous non-small cell lung cancer with prior chemotherapy, and pembrolizumab has been approved for the treatment of previously and untreated unresectable or metastatic melanoma and advanced non-small cell lung cancer. In addition to the above two main immune checkpoints, immunotherapies targeting other molecules, such as TIM-3, LAG3, killer-cell IG-like receptor (KIR), GITR, VISTA, indoleamine-pyrrole 2,3-dioxygenase (IDO), 4-1BB, and tryptophan 2,3-dioxygenase, are also being explored. For example, LAG-3 is an important immune checkpoint in vivo and plays a balanced regulatory role in the human immune system. LAG-3 negatively regulates T lymphocytes by binding to the extracellular domain of the ligand, thus avoiding autoimmunity caused by excessive activation of T cells. Currently, there are no drugs to target LAG-3 in the global market, but 12 drugs are in clinical research. Among them, IMP321 developed by Prima BioMed/Immutep has the fastest clinical research progress and is in stage IV treatment of breast cancer61. VISTA is a member of the B7 family. Unlike other negative checkpoint molecules, it is constitutively expressed on naïve T cells. The lack of VISTA leads to a breakdown of self-tolerance and the development of inflammatory T cell self-reactive responses. CA-170, an orally delivered dual inhibitor of VISTA and PD-L1, has shown to have clinical efficacy in phase I and II clinical trials from different advanced solid tumor types. However, further data are needed to determine whether this drug can become a new therapeutic option of cancer patients expressing VISTA62. TIM-3 antibodies include TSR-022 from Tesaro for the treatment of advanced or metastatic solid tumors alone or in combination with PD-1 antibody and TSR-022 from Novartis for treatment alone or in combination with PD-1 antibody PDR001 MBG-453 to target advanced malignant tumors currently in clinical trials63.
Table 2.
Research progress of new drugs to target PD-1.
| Medicine | Research institute | Application | Clinical phase |
|---|---|---|---|
| Nivolum (Opdivo) | Ono Pharmaceutical and Bristol-Myers-Squibb | Non-small cell lung cancer, malignant melanoma, renal cell carcinoma, head and neck squamous cell carcinoma, urothelium carcinoma, colorectal cancer, liver cancer, classical hodgkin lymphomas | 2014-06 (Marketing) |
| Pembrolizumab (Keytruda) | Merck | Non-small cell lung cancer, malignant melanoma, renal cell carcinoma, head and neck squamous cell carcinoma, urothelium carcinoma, colorectal cancer, liver cancer, classical hodgkin lymphomas | 2014-09 (Marketing) |
| Cemiplimab (Libtayo) | Sanofi and Regeneron | Metastatic squamous cell carcinoma of the skin | 2018-09 (Marketing) |
| Toripalimab | Shanghai Junshi Biosciences (China), Suzhou Zhonghe Biomedical Technology (China) | Hepatocellular carcinoma, melanoma | 2018-12 (Marketing) |
| Sintilimab | Innovent (China), Eli Lilly and Company | Hodgkin lymphomas | 2018-12 (Marketing) |
| Camrelizumab | Jiangsu Hengrui Medicine (China) | Esophageal squamous cell carcinoma, advanced solid-tumor, hepatocellular carcinoma, hodgkin lymphomas | 2019-05 (Marketing) |
| Tislelizumab | Beigene (China), Celgene, Boehringer-Ingelheim | Urothelium carcinoma, hodgkin lymphomas | 2019-12 (Marketing) |
| CX-188 | CytomX Therapeutics | Solid tumors | Phase I clinical trial |
| Sym-021 | Symphogen | Solid tumors, lymphomas | Phase I clinical trial |
| STW204 | Stainwei Biotech (China) | Solid tumors | Phase I clinical trial |
| Millamolecule | Bristol-Myers-Squibb | Immune diseases | Phase I clinical trial |
| XmAb20717 | Xencor | Solid tumors | Phase I clinical trial |
| CC-90006 | AnaptysBio, Celgene | Autoimmune diseases, psoriasis | Phase I clinical trial |
| YBL-006 | Y Biologics | Advanced solid-tumor | Phase I clinical trial |
| ONO-4685 | Merus, Ono Pharmaceutical | Autoimmune diseases | Phase I clinical trial |
| MGD-019 (MacroGenics) | MacroGenics | Solid tumors | Phase I clinical trial |
| SL-279252 | Shattuck Labs | Gastric adenocarcinoma, non-small cell lung cancer, diffuse large b cell lymphoma, renal cell carcinoma, urothelium carcinoma, solid tumors, head and neck squamous cell carcinoma, hodgkin lymphomas, adeno-carcinoma of esophagogastric junction, melanoma | Phase I clinical trial |
| RG-6084 | Roche | Hepatitis B | Phase I clinical trial |
| AMP-224 | Amplimmune, MedImmune, National Cancer Institute, GlaxoSmithKline | Solid tumors, colorectal cancer | Phase I clinical trial |
| PD-1 knockout engineered T cells | Cell Biotech | Renal cell carcinoma, prostatic cancer bladder cancer | Phase I clinical trial |
| IMU-201 | Imugene | Non-small cell lung cancer | Phase I clinical trial |
| MEDI-5752 | MedImmune | Solid tumors | Phase I clinical trial |
| JTX-4014 | Jounce Therapeutics | Cancer | Phase I clinical trial |
| RO-7247669 | Roche | Solid tumors | Phase I clinical trial |
| Budigalimab | Abbvie | Non-small cell lung cancer, solid tumors, colorectal cancer, ovarian cancer | Phase I clinical trial |
| LY-3434172 | Eli Lilly and Company | Solid tumors | Phase I clinical trial |
| INCB-086550 | Incyte | Solid tumors | Phase I clinical trial |
| MEDI-0680 | MedImmune | B cell lymphoma | Phase II clinical trial |
| EDP-1503 | The University of Chicago, Evelo Biosciences, Merck Sharp & Dohme | Melanoma | Phase II clinical trial |
| BCD-217 | Biocad | Melanoma | Phase II clinical trial |
| Balstilimab | Agenus, Ludwig Institute for Cancer Research | Solid tumors, cervical cancer | Phase II clinical trial |
| BI-754091 | Boehringer-Ingel heim | Solid tumors | Phase II clinical trial |
| TY-101 | Tayu Biotech | Solid tumors, lymphomas | Phase II clinical trial |
| GS-4224 | Gilead Sciences | Solid tumors | Phase II clinical trial |
| CA-170 | Aurigene, Curis | Cancer | Phase II clinical trial |
| MGD-013 | MacroGenics, ZaiLab (China) | Gastric cancer, esophageal cancer | Phase III clinical trial |
| Sasanlimab | Pfizer | Bladder cancer | Phase III clinical trial |
| Retifanlimab | MacroGenics, Incyte, ZaiLab (China) | Gastric cancer, esophageal cancer | Phase III clinical trial |
| Spartalizumab | Novartis | Melanoma | Phase III clinical trial |
| Cetrelimab | Johnson & Johnson | Multiple myeloma | Phase III clinical trial |
| Nivolumab/Relatlimab | Bristol-Myers-Squibb | Melanoma | Phase III clinical trial |
| Dostarlimab | AnaptysBio, Tesaro, GlaxoSmithKline | Fallopian tube cancer, endometrial cancer, peritoneal cancer, ovarian cancer | BLA |
| Zimberelimab | Gloria Pharmaceuticals (China), WuXi AppTec (China), Arcus Biosciences | Triple negative breast cancer, gastric cancer, solid tumors, liver cancer, hodgkin lymphomas | BLA |
In the clinical setting, some anti-PD-1 therapies are not effective in certain patients. This has led to the development of related detection technology. In addition to cancer treatment, anti-PD-1 immune checkpoint therapy is also effective in the treatment of other diseases. For example, chronic inflammation represents a central component in the pathogenesis of Alzheimer's disease; however, animal model data do not support further evaluation of PD-1 checkpoint inhibition as a therapeutic modality for Alzheimer's disease64. Therefore, further studies are needed to assess the application of anti-PD-1 therapies in the treatment of other diseases.
Understanding the key steps in the regulation of T-cell responses has led to the groundbreaking development of immune checkpoint blocking monoclonal antibodies (mAbs) to fight cancer. The first FDA-approved mAbs have resulted in unprecedented remission in melanoma and non-small cell lung cancer, although response rates vary dramatically (10%–90%), and significant toxicity has been noted65,66. This revolution in cancer therapy is now the basis for new immune checkpoint-based curative strategies.
Therapeutic immune checkpoint-blocking mAbs have dual activities inherent to their structure; the variable regions bind to immune checkpoint-epitopes, whereas the “fragment crystallizable” (Fc) region mediates targeted cell death through selective interaction with the complement molecule C1q (called complement-dependent cytotoxicity) and the Fc receptors on innate effector cells (called antibody-dependent cellular cytotoxicity or antibody-dependent cellular phagocytosis)67. To date, two types of approved immune checkpoint-mAbs, i.e., IgG1s and IgG4s, have been developed to protect or kill target cells, depending on the specific need68. The anti-CTLA-4 antibody ipilimumab and the anti-PDL1 antibody tezolizumab are IgG1s that are expected to cause preferential Treg and tumor cell depletion, respectively52,65. In contrast, the anti-PD1 antibodies nivolumab and pembrolizumab are modified IgG4s with low effector functions and mainly function by blocking PD-1 interaction with its ligands65,68. Taken together, these immune checkpoint-mAbs allow better activation of effector T cells, and the combination of anti-CTLA4 and anti-PD-1/PD-L1 antibodies improves survival52,65.
Immune checkpoint immunotherapy is used to improve mAb response rates in many cancers through various approaches. The first approach is to modulate the functions of mAbs through Fc engineering69. For example, tezolizumab is a nonglycosylated IgG1 that retains its blocking activity but lacks cytotoxic functions52. New anti-CLTA-4 mAbs with increased or dampened effector functions have also been developed and are currently being evaluated in clinical trials64. Other strategies targeting more co-inhibitory molecules on T cells (e.g., LAG3, TIM-3, TIGIT, and VISTA) have entered clinical trials, although their biological roles are not fully understood52,70. Additionally, next-generation therapeutic mAbs also include agonist agents targeting costimulatory molecules (e.g., OX40, ICOS, GITR, 4-1BB, and CD40) on T cells to potentiate effector responses52,70. Importantly, a combination of the above-mentioned approaches may be quite effective70.
In cancer, immune checkpoints are prematurely activated at a stage when cancer cells are not completely eradicated, resulting in the escape of tumor cells from immune rejection. The first checkpoint inhibitor to be tested in the clinic was directed against CTLA-4 and showed impressive clinical results, including long-term survival in approximately 20% of patients with metastatic melanoma71. Second-generation checkpoint inhibitors (anti-PD-1 antibodies) show increased efficacy, not only in patients with melanoma but also in patients with advanced urothelial carcinoma, head and neck squamous cell carcinoma, renal cell carcinoma, and non-small cell lung cancer72, 73, 74, 75. Moreover, a study by Zhu et al.76 highlighted another mechanism that could account for reduced T-cell infiltration in tumors. Indeed, sufficient T-cell infiltration in tumor tissues is often a prerequisite for the response to immunotherapy. Interfering with the Fas/Fas ligand (Fas–FasL) pathway in the tumor microenvironment could increase the efficacy of cancer immunotherapy.
The clinical success of checkpoint inhibitors has supported the potential efficacy of cancer immunotherapies63,77, 78, 79. Future research challenges include the following: (1) increasing the efficacy of checkpoint blockers and the combination of checkpoint inhibitors to achieve good and lasting results in patients who cannot be treated with blockers alone; (2) studying the responses and resistance mechanisms of immune checkpoint co-inhibitors in order to understand the similarities and differences in co-inhibitory pathways and the synergistic mechanisms of combined co-inhibitory pathways with scientific evidence and to optimize the design of immune checkpoint combined therapy; (3) studying the mechanisms of durability of the immune checkpoint blockade and the time required for treatment; (4) evaluating biomarkers to predict the response to immune checkpoint blockade, help patients with stratified treatment, and predict the responses of patients to immune blockade alone therapy and assessing the necessity for combination therapy or other treatments; and (5) developing effective combination therapies of immune checkpoint blockades in order to enhance efficacy and reduce side effects, performing research on reducing tumor evasive immunity, and understanding the necessity for immunosuppression in the tumor microenvironment.
The future of antitumor immunotherapy relies on the induction of responses at multiple levels, including harnessing of other effector cells (e.g., NK cells and neutrophils) in addition to T cells. As an example, the anti-NKG2A antibody monalizumab blocks inhibitory signaling in NK cells and subsets of cytotoxic T cells and further potentiates the effects of other therapeutic mAbs80. The identification of reliable predictive biomarkers and the combination of immune checkpoint therapies with other immunotherapies (such as adoptive T-cell therapy, oncolytic viruses, agonists for pattern recognition receptors), and radiotherapies/chemotherapies may lead to maximization of response rates81.
4. Immune checkpoints in the development of TCMs
4.1. Immunoregulatory effects of TCMs
Recent research progress on the immunomodulatory effects of TCMs on immune organs, immune cells, and immune molecules and the inhibitory effects of TCMs on inflammatory reactions, hypersensitivity reactions, autoimmune diseases, and rejection reactions has highlighted the potential use of TCMs as immune checkpoint modulators in cancer. With increasing need for scientific rigor, these traditional medicine therapies have been widely studied in recent years. Given the recent successes of immunotherapies and checkpoint blockades, there is a renewed interest in identifying novel drugs, including TCMs, with immunomodulatory effects.
TCMs can affect various immune molecules, including those that regulate human immune function and participate in various inflammatory reactions82. For example, Lemmon et al.83 studied the effects of high-molecular-weight polysaccharides from American ginseng on human immune cells and found that American ginseng could upregulate various cytokines, including interferon-γ (IFN-γ), IL-6, and IL-23a, and downregulate other cytokines, including TNF-β and IL-13. Nakada et al.84 treated two mouse strains (C57BL/6 and BALB/c) with the same amount of ginseng nourishing decoction to observe changes in cytokine levels in the spleen; they showed that the amount of IL-4 in spleen cells in C57BL/6 mice increased significantly, whereas the concentration of IFN-γ decreased slightly. In contrast, IFN-γ secretion increased significantly in spleen cells from BALB/c mice. These findings demonstrated that ginseng nourishing decoction could maintain the stability of the immune system by regulating the secretion of cytokines in mouse spleen cells. TCM immune-enhancers have different effects on NK cells, mononuclear macrophages, red blood cells, hematopoietic stem cells, and other immune cells. Many TCMs, such as Astragalus membranaceus, Codonopsis, Ginseng, Atractylodes macrocephala, Poriacocos, Angelica sinensis, and Acanthopanax, have the effect of promoting antibody generation85,86. Flavonoid ingredients in Astragalus seeds have been shown to have immunoregulatory effects on NK cells in vitro87. Moreover, by enhancing IFN-γ levels and increasing the expression of CD25 and CD69, flavonoids can significantly promote the proliferation and cytotoxicity of NK-92 cells.
Fujiwara et al.88 demonstrated that MDSCs isolated from the spleens of tumor-bearing mice prevent the proliferation of CD4-and CD8-positive T cells. Additional studies have indicated that immature MDSCs are important drug development targets in the treatment for tumors and chronic inflammation. Chemical phytochemicals of different herbal categories, including flavonoids, terpenoids, retinoids, curcumins, and β-glucans, possess MDSC-dependent antitumor and anti-inflammatory properties in both in vitro and in vivo experiments. A randomized phase II study investigated the immunological efficacy of the herbal medicines Hochu-ekki-to (a spray-dried powder extract composed of equal volumes of Cinnamomum Cortex, Hoelen, Moutan Cortex, Paeoniae Radix, and Persicae Semen) and Keishi-bukuryo-gan (a spray-dried powder extract made from 10 medical herbs) in combination with a personalized peptide vaccination (PPV) for curing castration-resistant prostate cancer; in this study, Tregs were defined as CD4+ CD25+ FOXP3+ cells in lymphocytes, and MDSCs were identified as CD33+ 11b+ cells from lineage markers (CD3, CD19, CD56, and CD16) and HLA-DR cells. The monocytic subset was identified as CD14+ by Noriko et al.89; they found that the frequencies of Mo-MDSCs and levels of IL-6 in the PPV-alone group were significantly increased. The study results also suggested that the combined use of herbal medicines had the potential to prevent immunosuppression induced by Mo-MDSCs or IL-6 during immunotherapy.
4.2. Immunosuppressive effects of TCMs
TCMs inhibit hypersensitivity, which is an abnormal and excessive immune response that interacts with antigenic substances under certain conditions to produce sensitized lymphocytes; these responses, if combined with re-entering antigens, can lead to disruption of the physiological functions of the body and damage to tissues90. Li et al.91 studied the regulatory and immunosuppressive mechanisms of artemisinin by establishing a model of delayed hypersensitivity in mice; they found that artemisinin could reduce the degree of ear swelling in model mice, indicating that artemisinin could inhibit the effects of the delayed hypersensitivity reaction.
Autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and autoimmune liver, are diseases caused by the immune response to the body's own components. Tripterine has been widely used in the treatment of autoimmune diseases and inflammatory neurodegenerative diseases92.
Most patients with cancer exhibit unfavorable responses to PD-1/PDL-1 inhibitors; however, PD-1/PDL-1 inhibitors can show good antitumor effects and are approved by the FDA for use in various tumor cases. Thus, increasing evidence suggests that TCMs and combination therapies may be applied to overcome this limitation (Table 393, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105). Investigators have shown that prim-oglucosylcimifugin (POG) extracted from the Saposhnikovia root is an effective polymorphonuclear (PMN)-MDSC inhibitor. Indeed, POG can prevent the proliferation, metabolism, and immunosuppressive ability of PMN-MDSCs; enhance the tumor immunosuppressive microenvironment; and exert synergistic effects with PD-1 inhibitors in B16F10 and 4T1 mouse tumor models93. Additionally, Chen et al.94 showed that total glucosides of paeony (TGP) increase the expression of PD-1/PD-L1 in peripheral blood mononuclear cells by modulating regulatory T cells/Th cells17. Other natural plants, such as Ganoderma lucidum95, Astragalus polysaccharides (APS96, and icaritin97, also mediate the immunomodulatory effects of PD-1, resulting in regulation of immune cytokine and chemokine expression. Classical TCM formulas98, 99, 100, e.g., Gegen Qinlian decoction (GQD), combined with anti-mouse PD-1 antibodies potently inhibited the growth of CT26 tumors and significantly enriched gut microbiota. Two metabolic signaling pathways, i.e., glycerophospholipid metabolism and sphingolipid metabolism, were also altered by this treatment. Furthermore, association strategies significantly increased the proportion of CD8+ T cells in peripheral blood and tumor tissues, enhanced IFN-γ expression, downregulated PD-1, and increased IL-2 levels. These data suggested that application of the formula GQD based on PD-1 blockade may be a novel therapeutic strategy for patients with cancer98.
Table 3.
Immune mechanism of TCMs as PD-1/PDL-1 inhibitors.
| TCM | Immune mechanism |
|---|---|
| Saposhnikovia root extract Prim-O-glucosylcimifugin (POG)93 | Inhibited the proliferation, metabolism and immunosuppressive ability of PMN-MDSCs, improved the tumor immunosuppressive microenvironment Created a synergistic effect with PD-1 inhibitors |
| Total glucosides of paeony (TGP)94 | Increased the expression of PD-1/PD-L1 in the peripheral blood mononuclear cells Downregulated Treg cells/T helper 17 cells |
| Ganoderma lucidum95 | Mediated the immunomodulation effect of PD-1 increased the expression of CCL5 chemokine in the cultured B-lymphocytes |
| Astragalus polysaccharides (APS) extracted from astragalus membranaceus96 | Inhibited MOG35-55-specific T cell proliferation and reduced the immune cytokines expression of IFN-γ, TNF-α, IL-2, IL-17, IL-4 and IL-10 Up-regulated the expression of PD-1 and PD-L1 |
| Icaritin isolated from Epimedium97 | Effectively decrease tumor burden in a T-cell dependent manner and increased CD8 T-cell infiltration and increased effector memory T-cell frequency Reduced frequency of CD11b+ Gr1+ MDSCs infiltration and downregulation of PD-L1 expression and the expression of PD-L1 on neutrophils |
| Gegen Qinlian decoction98 | The combination of GQD and anti-mouse PD-1 could potently inhibit the growth of CT26 tumors in a xenograft model Enriched gut microbiota and altered metabolic signaling pathways Increased the proportion of CD8+ T cells in peripheral blood and tumor tissues, increased the expression of IFN-γ, downregulated PD-1 and increased IL-2 levels |
| Ginseng and Astragalus granules99 | Increased the level of insulin and reduced the level of blood glucose Increased both CD4+FoxP3+ and CD8+CD122+PD-1+ Treg numbers in both spleens and lymph nodes of NOD mice Reversed a decline in CD4+FoxP3+ Tregs The percentage of effector/memory CD8+ T cells (CD44highCD62Llow) was significantly reduced and attenuated cellular infiltration and lowered CD3+ T cell numbers around and in islets |
| SORICM02 (Curcumae Rhizoma, Radix Paeoniae Rubra, Rhizoma Smilacis Glabrae, Mume Fructus, and Sarcandrae Herba)100 | Significantly inhibited murine skin allograft rejection and reduced graft-infiltration of CD3+ T cells increased CD8+CD122+PD-1+ Treg frequency with CD4+FoxP3+ Tregs remaining unchanged Hindered CD11c+ DC maturation post transplantation Induced CD8+CD122+PD-1+ Tregs Suppressed T cell proliferation in vitro and inhibited both mTOR and NF-κB signaling pathways increased IL-10 production, reduced IFN-γ level |
| PG2 isolated from Astragalus membranaceus101 | Inhibited the expression of PD-L1 on the cell surface by the protein kinase B (AKT)/mammalian target of rapamycin (mTOR)/ribosomal protein S6 kinase beta-1 (p70S6K) pathway |
| Indirubin, an active ingredient of Indigofera tinctoria L.102 | Repaired the expression of PD1 and phosphatase and tensin homolog (PTEN) on the CD4+ T cells of ITP patients |
| PSORI-CM02 consisting of five herbs (Curcumae Rhizoma, Radix Paeoniae Rubra, Rhizoma Smilacis Glabrae, Mume Fructus, and Sarcandrae Herba)103 | Increased CD8+CD122+PD-1+ Treg frequency in vivo Inhibited mTOR and NF-κB signaling pathways in vitro |
| Damingyin, an herb formulation104 | Improved visual acuity of patients with proliferative diabetic retinopathy by inhibiting the expression of PD1/PD-L1, then repaired the function of monocytes |
| Yin Zi Huang, an herbal medicine105 | Inhibited the expression of CD25, CD69, PD-1, and ICOS by stimulated CD4+ T cells in mouse |
4.3. Stimulatory effects of TCMs on costimulatory molecules
TCMs are complex systems with multiple components and targets, resulting in multitarget effects in the body; these multitarget effects could provide new directions for the treatment of immune diseases. Compared with mAbs, TCMs are less expensive, simpler to obtain, induce fewer adverse reactions, and are abundantly available. However, most studies of TCMs for the treatment of immune diseases are still in the basic research phase, and few human trials have been conducted. Accordingly, additional, higher-quality clinical trials are needed to evaluate the immunoregulatory mechanisms of TCMs in the human body. Identifying the active components and drug targets in TCMs could provide important clues for the control and treatment of immune diseases.
Activation of immune cells is an important part of the immune response. Previous studies have shown that the activation of T cells requires multiple signals; that is, the TCR recognizes the MHC-antigen peptide complex on the surface of the APC and then generates the first signal. Other molecular interactions between the two provide a second signal. If only the first signal is detected, T-cell response incompetence or even programmed death could occur. Thus, costimulatory molecules are essential for inducing effective immune responses106,107.
Costimulatory molecules can be divided into positive and negative types. The former produces costimulatory signals that initiate or sustain the immune response, whereas the latter is mainly involved in termination of the immune response in a timely and effective manner to restrict the immune response. Negative type costimulatory molecules include CTLA-4-b7-1/b7-2 and FasL. In the later stages of the initial immune response, CTLA-4 is induced and binds with the corresponding ligand B7 to downregulate the immune response. In addition, these costimulatory signals regulate body functions in a bidirectional and holistic manner. These findings are consistent with the immunomodulatory mechanisms of TCMs and costimulatory molecules. Indeed, elucidation of the regulatory effects of TCMs on costimulatory molecules is required to understand the immunomodulatory roles of TCMs.
Many TCMs or active components have regulatory or even bidirectional regulatory effects on adhesion molecules, such as Ig108, 109, 110, 111, 112, 113, 114 and intercellular adhesion molecule 1 (ICAM-1)115, 116, 117. Peony-glucosides, ginsenosides, Astragalus membranaceus, and Chailing decoction118 all have bidirectional regulatory effects on TNF. Some costimulatory molecules are members of the Ig or TNF/TNFR superfamily, whereas some are adhesion molecules, such as ICAM-1; thus, TCMs can regulate the expression of costimulatory molecules, particularly induced costimulatory molecules. Indeed, TCMs have regulatory effects on many cytokines, such as IL-1, IL-4, IL-5, IL-10, IL-12, IFN-γ, and TGF-β, and can also indirectly regulate the expression of costimulatory molecules by mediating cytokine expression. For example, taxifolin, a natural catechol-type flavonol element (also known as dihydroquercetin) has strong antioxidant and antiglycation activities and has been reported to clear cerebrovascular amyloid-β deposits. In vivo, taxifolin suppresses inflammation (via IL-1β, IL-6, IL-10, TNF-α/β, TGF-β, and vascular endothelial growth factor) and alleviates the accumulation of triggering receptor expressed on myeloid cell 2-expressing cells in the brain. Moreover, intracerebral production of amyloid-β can be inhibited by suppressing the ApoE/ERK1/2/amyloid-β precursor protein axis119. Vascular amyloid-β and 40-residue amyloid-β protein (amyloid-β1–40) deposits were decreased in taxifolin-treated Tg-SwDI mice120. In vitro studies have demonstrated that taxifolin has inhibitory effects on the aggregation of the 42-residue amyloid-β protein (amyloid-β1–42), which is involved in the pathogenesis of Alzheimer's disease121. Therefore, the biological component taxifolin may be a potential target for clinical applications to prevent or treat cerebral amyloid angiopathy.
Increasing researches have shown that cancers and tumor cells are associated with abnormal expression of costimulatory molecules. Low expression or even absence of co-stimulatory molecules is an important reason for immune escape of malignant tumors, such as breast cancer122, ovarian cancer123 and hematological malignancies124. TCMs has also been reported to inhibit angiogenesis and tumor growth of Lewis lung or prostate cancer125. For example, Xu et al.126 found that astragaloside IV extracted from Astragalus significantly restrained the reductive expression of CD206 and M2-related genes in the M2-type macrophage that polarized by IL-13 and IL-4, exhibited the inhibition of the invasion, migration and angiogenesis of A549 and H1299 cancer cells induced by M2-type macrophages, and suppressed AMPKα in M2-type macrophages activation. In addition, the in vivo experiments also showed that astragaloside IV could inhibit the tumor growth rate and cut down the metastasis of Lewis lung cancer. As shown in Table 4126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, there are some compounds or herbs from TCMs which can regulate the cancer immunity, especially in the tumor microenvironment. What’ s more, TCMs can exert the regulation of cancer immunity in clinic. Schwartzberg et al.143 discovered that a neutral oil extracted and isolated from Coix seed had a statistically significant improvement in progression-free survival (PFS) in pancreatic cancer patients, and showed anti-neoplastic activity. Rhodiola can improve the immunity in patients receiving postoperative chemotherapy for breast cancer and reduce the occurrence of chemotherapy-related oral ulcer. Rhodiola therefore has the potential to be used in conjunction with chemotherapy to reduce the incidence of oral ulcers127. TCMs has played, and still plays, an integral role in immunity of human health care all over the world. A “hot topic” has also been aroused in anti-cancer research by natural products derived from TCMs. Despite the unique immunity regulatory features of many compounds originated from TCMs, their clinical applications are disproportionally limited, and some TCMs are indeed highly toxic, particularly, their toxicity increases dramatically as herbs absorb a growing number of toxic substances in contaminated areas. Further, some herbal formulations contain too many herbs, which may cause additional toxicity. We believe that as the demonstration for immune regulation and toxicology research continue to be explored, which improve the comprehension about the mechanistic actions and clinical potential use of these compounds in the near future. TCMs will also serve as a huge community from which many promising compounds will be more extensively studied their applications in antitumor therapy for clinical use.
Table 4.
Regulation the cancer immunity of some compounds/herbs from TCMs.
| Compound/herb | Immune cell | Cancer type | Regulation mechanism |
|---|---|---|---|
| Astragaloside IV extracted from Astragalus126 | M2-type macrophage | A549 and H1299 cells, Lewis lung cancer | Reduced the growth, invasion, migration, and angiogenesis of lung cancer by blocking the macrophages polarization partially through the AMPK signaling pathway |
| Rhodiola127 | Granulocyte-macrophage, lymphocytes | Breast cancer | Enhanced the levels of IL-2, IL-4 and granulocyte-macrophage colony-stimulating factor; increased the proliferation of lymphocytes and white blood cell (WBC) count |
| Crassocephalum crepidioides128 | RAW264.7 macrophages | S-180 cells | Decreased tumor growth, induced NF-κB signaling pathway for releasing nitric oxide (NO) |
| Soyasapogenols extracted from soybean129 | M2-type and M1-type macrophages | U373, SaOS2 and LM8 tumor cells | Inhibited the M2 polarization and increased the secretion of IL-12, suppressed the activation of STAT3 and the proliferation of these tumor cells |
| Soyasapogenols B129 | Macrophage | LM8 tumor cells | Delayed subcutaneous tumor development and lung metastasis, induced anti-tumor immune response |
| A standardized herbal extract130 | Macrophage | Low-risk prostate cancer | Stimulated phagocytosis and the expression of inflammatory mediators (C4b, CXCL3, lymphotoxin, NOS2, TLRI, TNE, and TNFSF14), suppressed the tumor size, increased splenocyte cytotoxicity and numbers of CD8 T cells, macrophages, and dendritic cells in the spleens |
| Astragalus polysaccharides and Codonopsis polysaccharides131 | Dendritic cells (DCs), CD4+ and CD8+ T cell | 4T1 breast cancer | Enhanced mouse CD4+ and CD8+ T-cell proliferation and anti-4T1 metastasis activity, increased expression of CD40, CD80 and CD86, activated immune cells by mediating the expression of cytokines/chemokines |
| Lupanol132 | NK cells | BGC823, N87 and HGC27 of human gastric cancer cells | Promoted the proliferation of NK cells, inhibit the proliferation of BGC823, N87 and HGC27, and increased the killing effect of NK cells on gastric cancer cells, via the activated of PI3K/AKT and WNT/B-catenin signaling pathways for increasing PFP, IFN-γ, and CD107a expression |
| Icariin or 3,5,7-trihydroxy-4′-methoxy-8- (3-hydroxy-3-methylbutyl)-flavone133 | MDSCs | 4T1-Neu tumors | Reduced MRP8/MRP14 and toll-like receptor 4 (TLR4) expression, inhibited tumor growth in 4T1-Neu tumor-bearing mice and decreased MDSC numbers in the spleen, restarted IFN-γ production, decreased the production of NO and reactive oxygen species (ROS) in MDSC, decreased the expression of S100A8/9 and inhibition of activation of STAT3 and AKT |
| Glycoprotein ZPDC extracted from Chinese prickly ash134 | NK cells | Liver cancer cells | Induced secretion of perforin and granzyme B, NK cells activity and apoptosis-related factors (BID, cytochrome c, and caspase-3) in liver tissues |
| Shugan Jianpi formula135 | CD8+ T cells and MDSCs | Breast cancer | Reduced CD8+ T lymphocyte apoptosis and tumor cell activity, increased immune surveillance capability, and inhibited MDSC proliferation |
| Shikonin136 | DCs | B16 tumor cells | Increased the population of skin DCs migrating into the draining lymph nodes and cytotoxic T lymphocyte activities in B16 tumor model |
| Ginsenoside or ginsenoside Rg3137,138 | DCs and naive T cells | H22 tumor | Enhanced CD1a, CD80, CD83, CD86 and HLA-DR expression and T cell stimulatory capacity, decreased endocytic activity, Naïve T cells turned into typical Th1 cells Inhibited the growth of H22 tumors, enhanced the cellular immunity of H22-bearing mice |
| Tetramethylpyrazine phosphate139 | Macrophages and Th2 cells | Lung cancer | Enhance the expression levels of IFN-γ, IL-2 and T-bet, but reduced type 2 cytokines (IL-4, IL-6, IL-10) and GATA3 |
| Polysaccharide extracted from Glycyrrhizia Radix140 | Treg cells | H22 tumor | Decreased the population of Treg cells and lymph node FOXP3 and IL-10 expression, decreased IL-10 and TGF-β level and increased IL-2 and IL-12p70 level |
| Prunella vulgare141 | B cells | TPC-1 and FTC-133 cell lines | Induced apoptosis in TPC-1 and FTC-133 cell lines, increased BCL-2-associated X protein and caspase-3 expression, and downregulated B-cell 1ymphoma-2 expression in TPC-1 and FTC-133 |
| Ganoderma lucidum polysaccharides142 | Teff/Treg/Th2 cells | Hepatocellular carcinoma | Suppressed tumor growth in hepatoma-bearing mice associated with an increase of the ratio of Teffs/Tregs, increased IL-2 secretion for eliminating Treg suppression of Teff proliferation, inhibited NOTCH1 and FOXP3 expression |
5. Conclusions
Immune checkpoints are critical regulators of T-cell responses in tissues. Accordingly, decreasing the function of immune checkpoints by antibody blockade in cancer or chronic disorders can increase the availability of ligands to permit the activation of potentially self-reactive T cells, thereby altering cellular homeostasis. Moreover, overexpression of numerous immune checkpoints on cells can stimulate or inhibit T-cell responses. The use of immune checkpoint inhibitors may help to reverse immunosuppression, which is commonly observed in chronic infections and can promote immune responses. Importantly, combining immune checkpoint blockade with therapeutic vaccination can enhance vaccine-induced immune responses by depleting Tregs or blocking the production or function of immunosuppressive cytokines. Although blocking Tregs or immune checkpoints is unlikely to enhance the efficacy of routine prophylactic vaccination, it may have the potential to increase the efficacy of therapeutic vaccines against many chronic infections, such as malaria, tuberculosis, and HIV. Thus, further studies of immune checkpoints could facilitate our understanding of the mechanisms through which TCMs and novel drugs regulate immune function.
Acknowledgments
This work was partly supported by National Natural Science Foundation of China for Key Projects (No. 81430096, China), National New Drug Innovation (No. 2017ZX09301062, China) and Tianjin Science and Technology Plan Project (No. 19YFSLQY00110, China).
Author contributions
He Huang and Changxiao Liu: proposition proposal, designed and final revision; Yuli Wang: organizational framework and construction, paper drafting; Xingyan Zhang, Wenjing Zhao and Huling Li: revision, analysis, final revision; Yuyan Wang, Lixing Zhang, Li Xinping: collected data and provided materials; Tiejun Zhang and Hongbing Zhang: revision.
Conflicts of interest
The authors report no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
He Huang, Email: huang@tju.edu.cn.
Changxiao Liu, Email: liuchangxiao@163.com.
References
- 1.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.S., Shen C.R. Immune checkpoint blockade therapy: the 2014 Tang Prize in biopharmaceutical science. Biomed J. 2015;38:5–8. doi: 10.4103/2319-4170.151150. [DOI] [PubMed] [Google Scholar]
- 4.Teillaud J.L. Cancer immunotherapy crowned with Nobel prize in physiology or medicine awarded to James Allison and Tasuku Honjo. Med Sci. 2019;35:365–366. doi: 10.1051/medsci/2019073. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks J., Gravestein L.A., Tesselaar K., Lier R.A.W.V., Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 6.Agematsu K. Memory B cells and CD27. Histol Histopathol. 2000;15:573–576. doi: 10.14670/HH-15.573. [DOI] [PubMed] [Google Scholar]
- 7.Borst J., Hendriks J., Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Coquet J.M., Middendorp S., Horst G.V.D., van der Horst G., Kind J., Veraar E.A.M., Xiao Y. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity. 2012;38:53–65. doi: 10.1016/j.immuni.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Thomas L.J., He L.Z., Marsh H., Keler T. Targeting human CD27 with an agonist antibody stimulates T-cell activation and antitumor immunity. OncoImmunology. 2014;3 doi: 10.4161/onci.27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L.Z., Prostak N., Thomas L.J., Vitale L., Weidlick J., Crocker A. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood D., Findlay L., Poole S., Bird C., Wadhwa M., Moore M. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effecter memory T-cells. Br J Pharmacol. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Sullivan B., Thomas R. CD40 and dendritic cell function. Crit Rev Immunol. 2003;23:83–107. doi: 10.1615/critrevimmunol.v23.i12.50. [DOI] [PubMed] [Google Scholar]
- 13.Austin P.J., Kim C.F., Perera C.J., Moalem-Taylor G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain. 2012;153:1916–1931. doi: 10.1016/j.pain.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 15.Merck Merck and MD Anderson Cancer Center announce strategic immuno-oncology research collaboration in solid tumors. Darmstadt: A Subsidiary of Merck & Co., Inc. [updated 2015 Aug 13; cited 2020 Nov 25]. Available from: https://www.merck.com/news/merck-and-md-anderson-cancer-center-announce-strategic-immuno-oncology-research-collaboration-in-solid-tumors/.
- 16.Langowski J.L., Pena R., Kirksey Y.M., Addepalli M.K., Hoch U., Zalevsky J. Abstract 558: durable antitumor activity of the CD122-biased immunostimulatory cytokine NKTR-214 combined with immune checkpoint blockade. Cancer Res. 2016;76:558. [Google Scholar]
- 17.Mittler R.S., Foell J., Mccausland M., Strahotin S., Niu L., Bapat A. Anti-CD137 antibodies in the treatment of autoimmune disease and cancer. Immunol Res. 2004;29:197–208. doi: 10.1385/IR:29:1-3:197. [DOI] [PubMed] [Google Scholar]
- 18.Hinner M.J., Aiba R.S.B., Wiedenmann A., Schlosser C., Allersdorfer A., Matschiner G. Costimulatory T cell engagement via a novel bispecific anti-CD137/anti-HER2 protein. J Immunother Cancer. 2015;3:P187. [Google Scholar]
- 19.Croft M., So T., Duan W., Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg A.D., Morris N.P., Kovacsovics-Bankowski M., Urba W.J., Curti B.D. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244:218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curti B.D., Kovacsovics-Bankowski M., Morris N., Walker E., Chisholm L., Floyd K. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspeslagh S., Postel-Vinay S., Rusakiewicz S., Soria J.C., Zitvogel L., Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Ronchetti S., Zollo O., Bruscoli S., Agostini M., Bianchini R., Nocentini G. Frontline: GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 24.Nocentini G., Ronchetti S., Cuzzocrea S., Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 25.Schaer D.A., Budhu S., Liu C., Bryson C., Malandro N., Cohen A. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res. 2013;1:320–331. doi: 10.1158/2326-6066.CIR-13-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scirka B., Szurek E., Pietrzak M., Rempala G., Kisielow P., Ignatowicz L. Anti-GITR antibody treatment increases TCR repertoire diversity of regulatory but not effector T cells engaged in the immune response against B16 melanoma. Arch Immunol Ther Exp. 2017;65:553–564. doi: 10.1007/s00005-017-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burmeister Y., Lischke T., Dahler A.C., Mages H.W., Coyle A.J., Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 28.Leone R.D., Lo Y.C., Powell J.D. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapoval A.I., Ni J., Lau J.S., Wilcox R.A., Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 30.Leitner J., Klauser C., Pickl W.F., Stöckl J., Majdic O., Bardet A. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baughman J., Loo D., Chen F., Moore P., Bonvini E., Vasselli J. A phase I, open-label, dose escalation study of MGA271 in combination with pembrolizumab in patients with B7-H3-expressing melanoma, squamous cell cancer of the head and neck, or squamous cell non-small cell lung cancer. J Immunother Cancer. 2015;3:P177. [Google Scholar]
- 32.Mao Y., Li W., Chen K., Xie Y., Tao M. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6:3452–3461. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dangaj D., Lanitis E., Zhao A., Joshi S., Cheng Y., Sandaltzopoulos R. Novel recombinant human B7-H4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derré L., Rivals J.P., Jandus C., Pastor S., Speiser D.E. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolar P., Knieke K., Hegel J.K., Quandt D., Burmester G.R., Hoff H. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- 36.Syn N.L., Teng M.W.L., Mok T.S.K., Soo R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 37.Prendergast G.C., Smith C., Thomas S., Mandik-Nayak L., Laury-Kleintop L., Metz R. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C.T., Workman C.J., Flies D., Pan X., Marson A.L., Zhou G. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Grosso J.F., Kelleher C.C., Harris T.J., Maris C.H., Hipkiss E.L., Angelo D.M. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap T.A., Papadopoulos K.P., Lorusso P., Wong D.J., Hu-Lieskovan S., Holz J.B. A first-in-human phase I study of FS118, an anti-LAG-3/PD-L1 bispecific antibody in patients with solid tumors that have progressed on prior PD-1/PD-L1 therapy. J Clin Oncol. 2019;37:TPS2652. [Google Scholar]
- 41.Martner A., Aydin E., Hellstrand K. NOX2 in autoimmunity, tumor growth and metastasis. J Pathol. 2018;247:151–154. doi: 10.1002/path.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philips G.K., Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 43.Hastings W.D., Anderson D.E., Kassam N., Koguchi K., Greenfield E.A., Kent S.C. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu C., Anderson A.C., Kuchroo V.K. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2010;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Rubinstein R., Lines J.L., Wasiuk A., Ahonen C., Guo Y. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lines J.L., Pantazi E., Mak J., Sempere L.F., Wang L., O'Connell S. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanczak M.A., Siddiqui S.S., Trefny M.P., Thommen D.S., Boligan K.F., von Gunten S. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest. 2018;128:4912–4923. doi: 10.1172/JCI120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varki A., Paulson J.C., Crocker P.R. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 49.Paulson J.C., Crocker P.R., Macauley M.S. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hui E., Cheung J., Zhu J., Su X.L., Taylor M.J., Wallweber H.A. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei S.C., Colm R.D., James P.A. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 53.Clark D.P. Biomarkers for immune checkpoint inhibitors: the importance of tumor topography and the challenges to cytopathology. Cancer Cytopathol. 2018;126:11–19. doi: 10.1002/cncy.21951. [DOI] [PubMed] [Google Scholar]
- 54.Forde P.M., Scherpereel A., Tsao A.S. Use of immune checkpoint inhibitors in mesothelioma. Curr Treat Options Oncol. 2019;20:18–28. doi: 10.1007/s11864-019-0613-x. [DOI] [PubMed] [Google Scholar]
- 55.Kumar P., Saini S., Prabhakar B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. 2019:2–28. doi: 10.1016/j.semcancer.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Gupta V.G., Rangaraju R.R., Abbas W., Bajpai P., Khetrapal R. Immune checkpoint inhibitors: real-world experience from India in advanced solid cancers that have progressed on chemotherapy. South Afr J Cancer. 2019;8:65–68. doi: 10.4103/sajc.sajc_167_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blakeway E.A., Elshimy N., MuinonenMA, Marples M., Mathew B., Mitra A. Cutaneous lupus associated with pembrolizumab therapy for advanced melanoma: a report of three cases. Melanoma Res. 2019;29:338–341. doi: 10.1097/CMR.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 58.Pawel J., Bordoni R., Satouchi M., Fehrenbacher L., Cobo M., Han J.Y. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–132. doi: 10.1016/j.ejca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 59.Gupta A.K., Versteeg S.G., Abramovits W., Vincent K.D. Bavencio (avelumab)—a newly approved anti-PD-L1 IgG1 antibody. Skinmed. 2018;16:183–187. [PubMed] [Google Scholar]
- 60.Trefny M.P., Rothschild S.I., Uhlenbrock F., Rieder D., Kasenda B., Stanczak M.A. A variant of a killer cell immunoglobulin-like receptor is associated with resistance to PD-1 blockade in lung cancer. Clin Cancer Res. 2019;25:3026–3034. doi: 10.1158/1078-0432.CCR-18-3041. [DOI] [PubMed] [Google Scholar]
- 61.Shan C., Li X., Zhang J. Progress of immune checkpoint LAG-3 in immunotherapy. Oncol Lett. 2020;20:207. doi: 10.3892/ol.2020.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tagliamento M., Bironzo P., Novello S. New emerging targets in cancer immunotherapy: the role of VISTA. ESMO Open. 2020;4:683. doi: 10.1136/esmoopen-2020-000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson A., Joller N., Kuchroo V. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latta-Mahieu M., Elmer B., Bretteville A., Wang Y., Lopez-Grancha M., Goniot P. Systemic immune- checkpoint blockade with anti-PD1 antibodies does not alter cerebral amyloid-β burden in several amyloid transgenic mouse models. Glia. 2018;66:492–504. doi: 10.1002/glia.23260. [DOI] [PubMed] [Google Scholar]
- 65.Kavecansky J., Pavlick A.C. Beyond checkpoint inhibitors: the next generation of immunotherapy in oncology. AJHO. 2017;13:9–20. [Google Scholar]
- 66.Ribas A., Wolchock J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quast I., Peschke B., Lünemann J.D. Regulation of antibody effector functions through IgG Fc N-glycosylation. Cell Mol Life Sci. 2017;74:837–847. doi: 10.1007/s00018-016-2366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Almagro J.C., Daniels-Wells T.R., Perez-Tapia S.M., Penichet M.L. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front Immunol. 2018;8:1751–1770. doi: 10.3389/fimmu.2017.01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X.H., Mathieuet M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donini C., D'Ambrosio L., Grignani G., Aglietta M., Sangiolo D. Next generation immune-checkpoints for cancer therapy. J Thorac Dis. 2018;10:S1581–S1601. doi: 10.21037/jtd.2018.02.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.L., Fong L. KEYNOTE-024 investigators Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferris R.L., Blumenschein G.J., Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gettinger S.N., Horn L., Gandhi L., Spigel D.R., Antonia S.J., Rizvi N.A. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu J., Petit P.F., van den E.B.J. Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother. 2018;68:835–847. doi: 10.1007/s00262-018-2269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 78.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu W., Liu D., Jiao L.B., Zhang W., Hu X.Y., Gan S.T. Mechanism of Chinese herbal formula QHF against breast cancer MCF-7 cells invasion and migration. Chin Herb Med. 2018;10:405–410. [Google Scholar]
- 80.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743. doi: 10.1016/j.cell.2018.10.014. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marshall H.T., Djamgoz B.A. Immuno-oncology: emerging targets and combination therapies. Front Oncol. 2018;8:315–344. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marcais A., Viel S., Grau M. Regulation of mouse NK cell development and function by cytokines. Front iImmunol. 2013;4:450–464. doi: 10.3389/fimmu.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lemmon R., Sham J., Chau L.A. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. J Ethnopharmacol. 2012;142:1–13. doi: 10.1016/j.jep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Nakada T., Watanabe K., Jin G.B. Effect of Ninjin-Youei-To on Th1/Th2 type cytokine production in different mouse strains. Am J Chin Med. 2002;30:215–223. doi: 10.1142/S0192415X0200034X. [DOI] [PubMed] [Google Scholar]
- 85.Bao Y.L., Fan Y.L., Du J.L. Study on immune regulation of Huangqiyiqi granules. Chin J Veter Med. 2014:60–63. [Google Scholar]
- 86.Liu H., Lin J.L., Guo Y.Q. Study on immunomodulatory effect of Sijunzi decoction due to immunohistochemistry of traditional Chinese medicine. Chin J Exp Formulae. 2010;16:125–127. [Google Scholar]
- 87.Han R., Wu W.Q., Wu X.P., Liu C.Y. Effect of total flavonoids from the seeds of Astragali complanati on natural killer cell function. J Ethnopharmacol. 2015;173:157–165. doi: 10.1016/j.jep.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Fujiwara Y., Horlad H., Shiraishi D., Tsuboki J., Kudo R., Ikeda T. Onionin A, a sulfur-containing compound isolated fromonions, impairs tumor development and lung metastasis by inhibiting the protumoral and immunosuppressive functions of myeloid cells. Mol Nutr Food Res. 2016;60:2467–2480. doi: 10.1002/mnfr.201500995. [DOI] [PubMed] [Google Scholar]
- 89.Noriko K., Fukuko M., Kayoko W., Yamada A., Itoh K., Noguchi M. Immunological efficacy of herbal medicines in prostate cancer patients treated by personalized peptide vaccine. Cancer Sci. 2017;108:2326–2332. doi: 10.1111/cas.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy D.A., Osler A.G. Studies on the mechanism of hypersensitivity phenomena. XV. Enhancement of passive sensitization of human leukocytes by heparin. J Immunol. 1967;99:1062–1067. [PubMed] [Google Scholar]
- 91.Li Q., Chen H., Mei X., Na C.Q., Wei N., Cao B. The immunosuppressive and regulatory mechanism of artemisinin. Chin Pharmacol Bull. 2011;27:848–854. [Google Scholar]
- 92.Pan X.D., Chen X.C. Advances in the study of immunopharmacological effects and mechanisms of extracts of Tripterygium wilfordii Hook. f. in neuroimmunologic disorders. Acta Pharm Sin. 2008;43:1179–1185. [PubMed] [Google Scholar]
- 93.Gao W.F., Zhang X.Y., Yang W.D., Dou D.L., Zhang H., Tang Y.H. Prim-O-glucosylcimifugin enhances the antitumour effect of PD-1 inhibition by targeting myeloid-derived suppressor cells. J Immunother Cancer. 2019;7:231. doi: 10.1186/s40425-019-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y.Y., Wang Y., Xu L., Zhu W.N., Xu C.S., Xu M.M. Influence of total glucosides of paeony on PD-1/PD-L1 expression in primary Sjögren's syndrome. Int J Rheum Dis. 2019;22:200–206. doi: 10.1111/1756-185X.13391. [DOI] [PubMed] [Google Scholar]
- 95.Wang G., Wang L., Zhou J., Xu X. The possible role of PD-1 protein in Ganoderma lucidum-mediated immunomodulation and cancer treatment. Integr Cancer Ther. 2019;18 doi: 10.1177/1534735419880275. 1534735419880275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y., Jing Y., Huang M., Ma J., Peng X., Wang J. The PD-1/PD-Ls pathway is up-regulated during the suppression of experimental autoimmune encephalomyelitis treated by Astragalus polysaccharides. J Neuroimmunol. 2019;332:78–90. doi: 10.1016/j.jneuroim.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 97.Hao H., Zhang Q., Zhu H., Wen Y., Qiu D., Xiong J. Icaritin promotes tumor T-cell infiltration and induces antitumor immunity in mice. Eur J Immunol. 2019;49:2235–2244. doi: 10.1002/eji.201948225. [DOI] [PubMed] [Google Scholar]
- 98.Lv J., Jia Y., Li J., Kuai W., Li Y., Guo F. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Xie Q., Liang C.L., Zeng Q., Dai Z. Chinese medicine Ginseng and Astragalus granules ameliorate autoimmune diabetes by upregulating both CD4+FoxP3+ and CD8+CD122+PD1+ regulatory T cells. Oncotarget. 2017;8:60201–60209. doi: 10.18632/oncotarget.18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu C., Liu H., Jin X., Chen Y., Liang C.L., Qiu F. Herbal components of a novel formula PSORI-CM02 interdependently suppress allograft rejection and induce CD8+CD122+PD-1+ regulatory T cells. Front Pharmacol. 2018;9:88. doi: 10.3389/fphar.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang H.L., Kuo Y.H., Wu L.H. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int J Med Sci. 2020;17:939–945. doi: 10.7150/ijms.42978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y., Han P., Liu L., Wang X.J., Xu P.C., Wang H.Y. Indirubin modulates CD4+ T-cell homeostasis via PD1/PTEN/AKT signalling pathway in immune thrombocytopenia. J Cell Mol Med. 2019;23:1885–1898. doi: 10.1111/jcmm.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qiu F., Liu H., Liang C.L., Nie G.D., Dai Z.A. New immunosuppressive molecule emodin induces both CD4+ FoxP3+ and CD8+ CD122+ regulatory T cells and suppresses murine allograft rejection. Front Immunol. 2017;8:1519. doi: 10.3389/fimmu.2017.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lv X.D., Cao J. Study on the mechanism of PD1/PDL1 signaling pathway in proliferative diabetic retinopathy treated by Chinese traditional medicine. Pharmacol Clin Chin Mater Med. 2017;33:136–140. [Google Scholar]
- 105.Chen X., Krakauer T., Oppenheim J.J., Howard O.M. Yin zi huang, an injectable multicomponent Chinese herbal medicine, is a potent inhibitor of T-cell activation. J Alternative Compl Med. 2004;10:519–526. doi: 10.1089/1075553041323687. [DOI] [PubMed] [Google Scholar]
- 106.Watts T.H., De Bendette M.A. T cell co-stimulatory molecules other than CD281J2. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X.G., Zhu J.K. In: New progress in immunology. Zhu X., editor. people's health publishing house; Beijing: 2002. Co-stimulatory molecules and their role in the immune response; p. 426. [Google Scholar]
- 108.Ho X.H., Xi X.S. People's Military medical Press; Beijing: 2002. Immunology of traditional Chinese medicine. [Google Scholar]
- 109.Wei P., Guo G.Q., Wang Y.Q. Research progress on immunopharmacology of polysaccharide traditional Chinese medicine. J Changchun Col Tradit Chin Med. 1997;13:66–67. [Google Scholar]
- 110.Yu S.C., Zhang Y.Z., Zhao H.J., Yu L.H. Experimental study on the immunomodulatory effect of Fructuslycii and Atractylodes macrocephala. Curr Immunol. 1994;14:12–14. [Google Scholar]
- 111.Zhou J.H. people's military medical press; Beijing: 1994. Immunopharmacology of traditional Chinese medicine. [Google Scholar]
- 112.Liu S.Y., Wang N.Q. Curative effect of liuweidihuang pill or jinguishenqi pill on small cell carcinoma. J Integr Tradit Chin West Med. 1990;10:720–722. [Google Scholar]
- 113.Zhang Y.S., Feng Y.W. Yupingfeng powder was used to treat 28 cases of herpes simplex viral keratitis. Shandong J Tradit Chin Med. 1995;14:409–410. [Google Scholar]
- 114.Wu W.W., Ye Z.J., Xu Y.M., Zhang J., Tang J.S. Ergosta-7,22-diene-2β,3α,9α-triol (EGDT) from Ganodermalucidum inhibits nasopharyngeal carcinoma cells by blocking EGFR signaling pathway. Chin Herb Med. 2018;10:34–39. [Google Scholar]
- 115.Wang S.S., Qi Q.H. Effect of dachengqi granule on serum NO, TNF and ICAM-1 in patients with abdominal malignancy. J Tradit Chin Med. 1999;40:293–294. [Google Scholar]
- 116.Bagnasco M., Pesce G., Caretto A., PaolieriI F., Pronzato C., Villaggio B. Follicular thyroid cells of autoimmune thyroiditis may coexpress ICAM-1 (CD54) and its natural ligand LFA-1 (CD11a/CD18) J Allergy Clin Immunol. 1995;95:1036–1043. doi: 10.1016/s0091-6749(95)70105-2. [DOI] [PubMed] [Google Scholar]
- 117.Nai Z., Geng X.Y., Wang S.Y. Effects of integrated traditional Chinese and Western medicine on immune function in patients with acute cerebral infarction. Chin J Integr Tradit Chin West Med. 1999;19:27–28. [PubMed] [Google Scholar]
- 118.Ho X.H., Xi X.S. People's Military Medical Press; Beijing: 2002. Immunology of traditional Chinese medicine; pp. 202–203. [Google Scholar]
- 119.Inoue T., Saito S., Tanaka M., Yamakage H., Kusakabe T., Shimatsu A. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2019;116:10031–10038. doi: 10.1073/pnas.1901659116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saito S., Yamamoto Y., Maki T., Hattori Y., Ito H., Mizuno K. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol Com. 2017;5:26. doi: 10.1186/s40478-017-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sato M., Murakami K., Uno M., Ikubo H., Nakagawa Y., Katayama S. Structure-activity relationship for (+)-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci Biotechnol Biochem. 2013;77:1100–1103. doi: 10.1271/bbb.120925. [DOI] [PubMed] [Google Scholar]
- 122.Fan P., Wang S., Liu X., Zhen L., Wu Z. Major histocompatibility complex class II antigen and costimulatory molecule expression on the surface of breast cancer cells. Chin J Oncol. 2002;24:327–330. [PubMed] [Google Scholar]
- 123.Cao S.J., Qian H.N., Feng J., Fu T.Y., Ye X. Expression of costimulatory molecules in ovarian cancer cells. J Peking Univ (Heal Sci) 2000;32:90–91. [Google Scholar]
- 124.Jiang D.F. Study on the role of co-stimulatory signal in immunotherapy of hematological malignancies. Clin Chem Lab Med. 2000;21:37–39. [Google Scholar]
- 125.Wang D.D., Wang S., Feng Y., Zhang L., Li Z., Ma J. Antitumor effects of Bulbus fritillariae cirrhosae on Lewis lung carcinoma cells in vitro and in vivo. Ind Crop Prod. 2014;54:92–101. [Google Scholar]
- 126.Xu F., Cui W.Q., Wei Y., Cui J., Qiu J., Hu L.L. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37:207. doi: 10.1186/s13046-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loo W.T., Jin L.J., Chow L.W., Cheung M.N., Wang M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expet Opin Invest Drugs. 2010;19:S91–S100. doi: 10.1517/13543781003727057. [DOI] [PubMed] [Google Scholar]
- 128.Tomimori K., Nakama S., Kimura R., Tamaki K., Ishikawa C., Mori N. Antitumor activity and macrophage nitric oxide producing action of medicinal herb, Crassocephalum crepidioides. BMC Compl Alternative Med. 2012;12:78. doi: 10.1186/1472-6882-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujiwara Y., Shiraishi D., Yoshitomi M., Ikeda T., Mizuta H., Takeya M. Soya sapogenols contained in soybeans suppress tumour progression by regulating macrophage differentiation into the protumoural phenotype. J Func Food. 2015;19:594–605. [Google Scholar]
- 130.Liang P., Guo J., Li S., Guan Q., Vanderheyden T., So A. Prevention of prostate tumor development by stimulation of antitumor immunity using a standardized herbal extract (Deep immune(R)) in TRAMP mice. Evid Complement Alternat Med. 2018;2018:9707543. doi: 10.1155/2018/9707543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang W.T., Lai T.H., Chyan Y.J., Yin S.Y., Chen Y.H., Wei W.C. Specific medicinal plant polysaccharides effectively enhance the potency of a DC based vaccine against mouse mammary tumor metastasis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu X.T., Liu J.Q., Lu X.T., Chen F.X., Zhou Z.H., Wang T. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharm. 2013;16:332–340. doi: 10.1016/j.intimp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Zhou J., Wu J., Chen X., Fortenbery N., Eksioglu E., Kodumudi K.N. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharm. 2011;11:890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee J., Lee S.J., Lim K.T. ZPDC glycoprotein (24 kDa) induces apoptosis and enhances activity of NK cells in N-nitrosodiethylamine-injected BALB/c. Cell Immunol. 2014;289:1–6. doi: 10.1016/j.cellimm.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 135.Lu Y.T., Li J., Qi X., Pei Y.X., Shi W.G., Lin H.S. Effects of Shugan Jianpi formulaon myeloid-derived suppression cells-mediated depression breast cancer mice. Chin J Integr Med. 2017;23:453–460. doi: 10.1007/s11655-016-2734-4. [DOI] [PubMed] [Google Scholar]
- 136.Chen H.M., Wang P.H., Aravindaram K., Chen Y.H., Yu H.H., Yang W.C. Shikonin enhances efficacy of a gene-based cancer vaccine via induction of RANTES. J Biomed Sci. 2012;19:42. doi: 10.1186/1423-0127-19-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takei M., Tachikawa E., Umeyama A. Dendritic cells promoted by ginseng saponins drive a potent Th1 polarization. Biomark Insights. 2008;3:269–286. doi: 10.4137/bmi.s585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu R., Ru Q., Chen L., Ma B., Li C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J Food Sci. 2014;79:H1430–H1435. doi: 10.1111/1750-3841.12518. [DOI] [PubMed] [Google Scholar]
- 139.Wei H., Sun R., Xiao W., Feng J., Zhen C., Xu X. Type two cytokines predominance of human lung cancer and its reverse by traditional Chinese medicine TTMP. Cell Mol Immunol. 2004;1:63–70. [PubMed] [Google Scholar]
- 140.He X., Li X., Liu B., Xu L., Zhao H., Lu A. Down-regulation of Treg cells and upregulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules. 2011;16:8343–8352. doi: 10.3390/molecules16108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin D.T., Lei M., Xu J., Li H., Wang Y., Liu Z. The Chinese herb Prunella vulgaris promotes apoptosis in human well-differentiated thyroid carcinoma cells via the B cell lymphoma-2/Bcl-2-associated X protein/caspase-3 signaling pathway. Oncol Lett. 2017;14:1309–1314. doi: 10.3892/ol.2017.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li A., Shuai X., Jia Z., Li H., Liang X., Su D. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J Transl Med. 2015;13:100. doi: 10.1186/s12967-015-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwartzberg L.S., Arena F.P., Bienvenu B.J., Kaplan E.H., Camacho L.H., Campos L.T. A randomized, open-label, safety and exploratory efficacy study of Kanglaite injection (KLTi) plus Gemcitabine versus Gemcitabine in patients with advanced pancreatic cancer. J Cancer. 2017;8:1872–1883. doi: 10.7150/jca.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]