Abstract
Genomic instability remains an enabling feature of cancer and promotes malignant transformation. Alterations of DNA damage response (DDR) pathways allow genomic instability, generate neoantigens, upregulate the expression of programmed death ligand 1 (PD-L1) and interact with signaling such as cyclic GMP–AMP synthase-stimulator of interferon genes (cGAS–STING) signaling. Here, we review the basic knowledge of DDR pathways, mechanisms of genomic instability induced by DDR alterations, impacts of DDR alterations on immune system, and the potential applications of DDR alterations as biomarkers and therapeutic targets in cancer immunotherapy.
KEY WORDS: DNA damage response, DNA repair, Immunotherapy, Genomic instability, Tumor microenvironment, PD-1, PD-L1, cGAS–STING
Abbreviations: ATM, ataxia-telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; BAP1, BRCA1-associated protein 1; BER, base excision repair; BRAF, v-RAF murine sarcoma viral oncogene homologue B; BRCA, breast cancer susceptibility gene; cGAS, cyclic GMP–AMP synthase; CHEK, cell-cycle checkpoint kinase; CHK1, checkpoint kinase 1; DAMP, damage-associated molecular patterns; DDR, DNA damage response; DR, direct repair; DSBs, double-strand breaks; GSK3β, glycogen synthase kinase 3β; HMGB1, high mobility group box-1; HRR, homologous recombination repair; ICI, immune checkpoint inhibitor; IFNγ, interferon gamma; IHC, immunohistochemistry; IRF1, interferon regulatory factor 1; JAK, Janus kinase; MAD1, mitotic arrest deficient-like 1; MGMT, O6-methylguanine methyltransferase; MLH1, MutL homolog 1; MMR, mismatch repair; MNT, MAX network transcriptional repressor; MSH2/6, MutS protein homologue-2/6; MSI, microsatellite instability; MUTYH, MutY homolog; MyD88, myeloid differentiation factor 88; NEK1, NIMA-related kinase 1; NER, nucleotide excision repair; NGS, next generation sequencing; NHEJ, nonhomologous end-joining; NIMA, never-in-mitosis A; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PALB2, partner and localizer of BRCA2; PARP, poly-ADP ribose polymerase; PCR, polymerase chain reaction; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PFS, progression-free survival; RAD51C, RAD51 homolog C; RB1, retinoblastoma 1; RPA, replication protein A; RSR, replication stress response; SCNAs, somatic copy number alterations; ssDNA, single-stranded DNA; STAT, signal transducer and activator of transcription; STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1; TILs, tumor-infiltrating lymphocytes; TLR4, Toll-like receptor 4; TMB, tumor mutational burden; TME, tumor microenvironment; TP53, tumor protein P53; TRIF, Toll-interleukin 1 receptor domain-containing adaptor inducing INF-β; FDA, United State Food and Drug Administration; XRCC4, X-ray repair cross complementing protein 4
Graphical abstract
This review summarizes basic knowledge of DNA damage response (DDR) pathways, mechanisms of genomic instability induced by DDR alterations, impacts of DDR alterations on immune system, and its potential applications as therapeutic targets in cancer immunotherapy.
1. Introduction
Cancer is a main public health problem in the world with a high incidence of estimated 18.1 million new-diagnosed cases, and a mortality of 9.6 million deaths reported in 2018 global cancer statistics1. With the continuous improvement of early diagnosis and treatments, the rate of cancer related death decreased continuously since 1991, leading to a 29% overall decline through 20172. Great success has been achieved in immune checkpoint inhibitor (ICI)-based immunotherapies3,4. Two programmed death 1 (PD-1) blockades, nivolumab and pembrolizumab, were approved in 2014 by the United State Food and Drug Administration (U.S. FDA) for treating patients with metastatic melanoma who progressed after receiving ipilimumab, and patients with v-RAF murine sarcoma viral oncogene homologue B (BRAF)-mutated melanoma. In the combination of these PD-1 blockades, objective response was observed in 30%–40% of patients, and major responses were durable5. Besides, in patients with advanced solid tumors, an elegant study of Marabelle and colleagues6 found that objective response rate was 29% in the tissue tumor mutational burden (TMB)-high group when treated with pembrolizumab monotherapy. However, there were also some non-responders reported, and potential adverse events as well as increased cost were seen during immunotherapy5. These findings suggest a great need for exploring biomarkers and therapeutic strategies to treat cancers.
Genomic instability remains an enabling feature of cancer and promotes malignant transformation7. Hereditary cancer predisposition syndromes (such as Lynch syndrome) associated with genetic mutations of the core DNA repair genes are the initial evidence suggesting the importance of genome stability in tumorigenesis8. DNA damage response (DDR) pathways aim to protect cells against some acquired genome changes and monitor exogenous or endogenous DNA damage9. Cytotoxic agents targeting DDR pathways have been used as anti-cancer therapies. Many mechanisms of tumor cells’ resistance and sensitivity to cytotoxic radiotherapy and chemotherapy are controlled by DDR pathways. Herein, we review the basic knowledge of DDR pathways, mechanisms of genomic instability induced by DDR alterations, impacts of DDR alterations on immune system, and the potential applications of DDR alterations as biomarkers and therapeutic targets in cancer immunotherapy.
2. Basis of DDR pathway
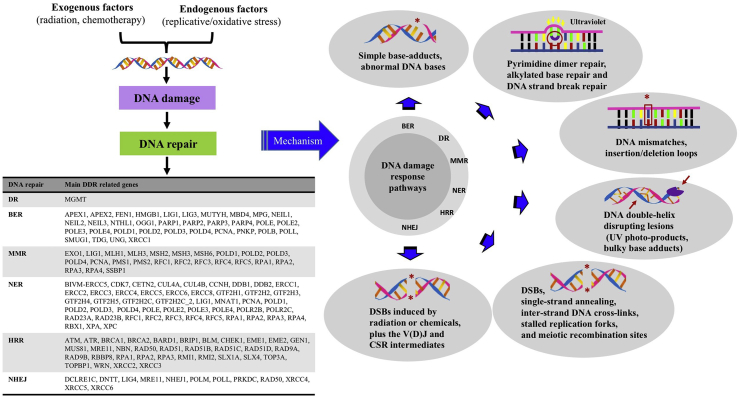
DNA integrity affects the transmission of genetic information. DNA damage may occur from either endogenous or exogenous sources, and DNA repair are required for maintaining genomic integrity10,11. Beyond direct repair (DR), multiple pathways compose the DDR system: base excision repair (BER), mismatch repair (MMR), nucleotide excision repair (NER), homologous recombination repair (HRR) and nonhomologous end-joining (NHEJ, Fig. 1)12.
Figure 1.
The components and mechanisms of DDR pathway. DNA damage may occur from either endogenous or exogenous sources, which can impair genomic instability. Therefore, DNA repair is crucial for maintaining genome stability. Multiple mechanisms involve DNA damage response (DDR) system: direct repair (DR), base excision repair (BER), mismatch repair (MMR), nucleotide excision repair (NER), homologous recombination repair (HRR) and nonhomologous end-joining (NHEJ).
Although some damages can be repaired by direct reversal mediated via proteins such as O6-methylguanine methyltransferase (MGMT), most are subject to DNA repairs mediated by a series of protein-mediated catalytic events. In the BER pathway, DNA glycosylase can recognize a damage base, which mediates base removal, while polymerase, nuclease, and ligase proteins repair the lesion13. The MMR system mainly detects mismatches as well as insertion or deletion loops, which induces a single-strand incision, followed by DNA repair through polymerase, nuclease, and ligase enzymes14. In the NER pathway, recognition of the helix-distorting lesions is mediated by two different mechanisms: one is transcription-coupled NER (specifically targeting transcription-blocked lesions), the other is global-genome NER. The NER system often excises a oligonucleotide with 22–30 bases to remove damage, which triggers a single-stranded DNA (ssDNA) production15. DNA polymerases as well as related factors would act upon it before ligase completes the repair15.
HRR and NHEJ are mechanisms used in double-strand breaks (DSBs). In HRR, sister-chromatid sequences are used as the template for DNA repair, which restricts HRR to S and G2. Several sub-pathways are involved in HRR16. Nevertheless, its initiation is always mediated by ssDNA generation, promoted by proteins such as MRE11A–RAD50–NBN (MRN) complex. Then the ssDNA executes strand invasion of an undamaged partner chromosome, before DNA synthesis ensues16. As for NHEJ, DSBs are detected by KU70/80 proteins. This complex then binds to DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to activate it, which results in recruitment as well as activation of the end-processing enzymes, polymerases and ligase17,18.
3. Mechanisms of DDR pathway defects
Methods including genetic and epigenetic inactivation mediate deficiencies of DDR pathways. Germline or somatic mutations are important mechanisms in genetic inactivation at the level of DNA sequence. Germline alterations in DDR pathways associate with incidence of some hereditary syndromes prone to form cancer, such as hereditary nonpolyposis colorectal cancer (Lynch syndrome) caused by MMR-deficiency, as well as hereditary breast and ovarian cancer caused by defective HRR. Several less common syndromes including ataxia-telangiectasia, fanconi anemia, xeroderma pigmentosum and MutY homolog (MUTYH)-associated polyposis need biallelic germline mutations to form full predisposition phenotype of cancer19, 20, 21. Beyond these hereditary syndromes, in some sporadic cancers, DDR-related germline mutations were also detected in population-based studies. DDR-associated genes can also undergo somatic mutations at high frequency. A pan-cancer analysis in 17 tumor types identified somatic mutations among 72 key DDR genes, which were mutated in at least 1% samples in a given tumor and were presented in more than one type of cancer22. Another study on biliary tract cancer demonstrated 63.5% patients with germline or somatic inactivation in DDR genes such as ataxia-telangiectasia mutated (ATM), ataxia telangiectasia and Rad3 related (ATR), breast cancer susceptibility gene 1/2 (BRCA 1/2), mismatch repair genes MutL homolog 1 (MLH1) and MutS protein homologue-2/6 (MSH2/6), cell-cycle checkpoint kinase (CHEK), and BRCA1-associated protein 1 (BAP1)23.
The most-characteristic epigenetic mechanism involved in the regulation of DDR pathways is DNA methylation. An analysis of DNA methylation identified 5%–10% of the promoter CpG islands (normally un-methylated) were methylated abnormally in human cancers24, which led to the silencing of related genes at the transcriptional level. Promoter methylation causes loss of somatic function in DNA repair genes such as MGMT of the DR pathway, MLH1 of the MMR pathway, BRCA1 and RAD51 homolog C (RAD51C) of the HRR pathway. Promoter methylations of MGMT and MLH1 have been shown as the early events in the multistep tumorigenesis, as they were observed in premalignant polyps and the normal epithelium of colon that was adjacent to the tumor. Studies on breast, as well as ovarian cancers, demonstrated hypermethylation of BRCA1 and RAD51C was associated with HRR deficiency, resulting in tumor predisposition25,26. However, impact of epigenetic inactivation of genes BRCA1 and RAD51C remains debated, since not all gene methylation appears marked effects at the expression level. In addition, epigenetic mechanisms involved in the regulation of DNA repair include histone modification, RNA-mediated targeting, and nucleosome remodeling. For example, depletion of TRRAP can impair the hyperacetylation of DNA-damage related histone H4 as well as the accumulation of repair molecules at DSB sites, which leads to defective HRR27.
4. Defective/downregulated DDR pathway allows genomic instability
Absence of specific DDR pathway may lead to consequences such as mutations or chromosomal rearrangements, promoting genomic instability and tumor progression. Multiple cellular processes may impair genetic stability. For example, the stalled, reversed and folded replication forks can cause deregulation of DNA replication, which may result in replication stress and trigger chromosomal rearrangements and the formation of DNA DSB28. Telomere maintenance contributes to the prevention of genome instability, and chromosomal instability may be produced due to upcapping or erosion of telomere29,30. Beyond DNA replication stress and telomere maintenance, processes including chromosome segregation, RNA processing and epigenetic mechanisms also relate to genome instability. The defects of RNA processing destabilize genomes mediated by forming mutagenic R-loop structure and altering gene expressions that are crucial in maintaining genome stability31.
5. Tumor microenvironment (TME) regulates genomic stability via DDR pathways
TME is characterized by vascular abnormalities, hypoxia, as well as acidic pH, and the major components of it include tumor cells, many non-malignant cells (such as T cells, dendritic cells and tumor associated fibroblasts), extracellular matrix, and blood vessels32. Through TME, tumor can affect pathways of DNA damage and repair correspondingly33. Tumor progression is accompanied with multiple complex interactions between cancer cells and TME, and TME functions in malignant transformation via inhibiting DDR pathways34,35. For example, hypoxia was observed to downregulate c-MYC, a proliferation-promoting transcription factor that bound to promoters of MMR genes Mlh1 and Mlh2, leading to decrease of Mlh1 and Mlh2 at the transcriptional level36. Besides, hypoxia also increased the binding of inhibitory transcription factors (MAX network transcriptional repressor (MNT) and mitotic arrest deficient-like 1 (MAD1)) to promoters of Mlh1 and Mlh2 genes. Therefore, the expression of MMR genes was downregulated. In addition, MMR proteins also relate to apoptosis, indicating that TME can promote the survival of tumor cells indirectly mediated by inhibiting some DDR pathways. Moreover, BRCA1 and RAD51 genes in the HRR pathway are also associated with hypoxia37,38, which may link to the increase of genomic instability. Thus, TME can negatively regulate genomic stability by repressing DDR pathways.
6. Impacts of DDR alterations on immune system in cancers
6.1. DDR deficiencies activate innate immune system through cyclic GMP–AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway
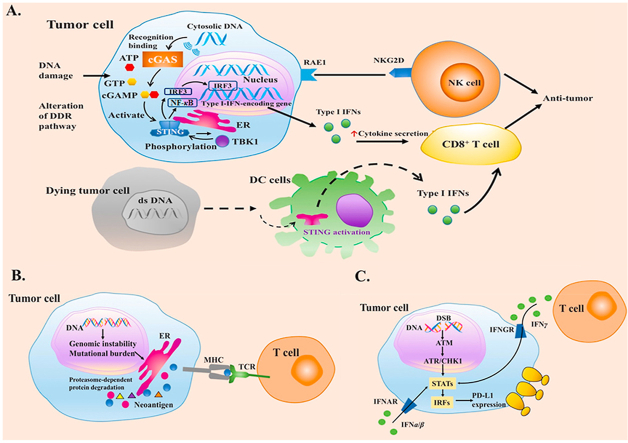
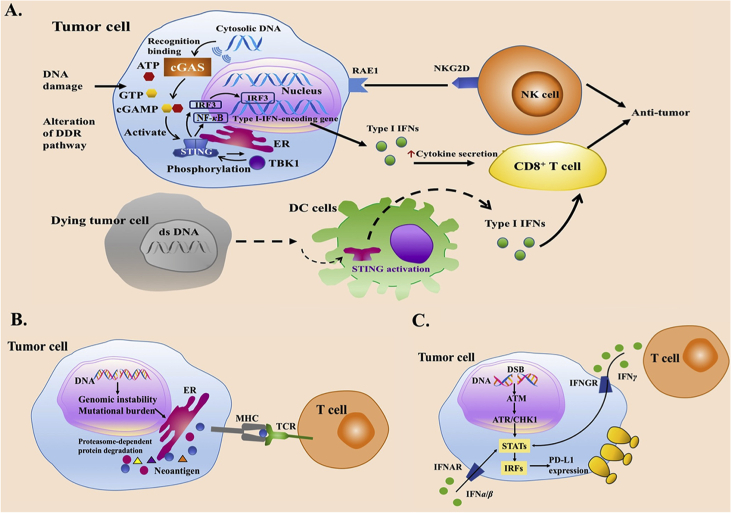
Before the recognition of adaptive immunity, innate immune system plays an important part in recruiting immune cells to tumors. Recent studies indicate the STING pathway as an important player in host innate immune system against cancer, which drives interferon (IFN) production and arouses T-cell responses39 (Fig. 2A40,41). When exposed to DNA-damaging agents such as clinical chemotherapy (etoposide and camptothecin), STING pathway can be activated42,43. On the other hand, DDR deficiency can also upregulate the activation of STING pathway. Emerging evidence suggests micronuclei produced by BRCA2-inactivation can initiate a cGAS/STING-mediated interferon response44. Enhanced IFN-related gene expression and higher abundance of tumor-infiltrating lymphocytes (TILs) were observed in DDR-deficient breast cancers compared with DDR-proficient tumors. Upregulated TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) phosphorylation was also observed in BRCA1/2-deficient cancers45.
Figure 2.
Impacts of DDR alterations on immune system in cancers. (A) Correlations of DNA damage and repair with STING pathway in tumor microenvironment. The STING pathway can be activated by DNA damaging agents or DDR alterations. Cytosolic DNA sensor cGAS can activate innate immune responses by catalyzing cGAMP synthesis, which roles as a second messenger in the activation of STING pathway40. The activation of STING pathway makes a conformation change of STING, which leads to an endoplasmic reticulum to perinuclear endosome shuttling. TBK1 can phosphorylate STING as well as IRF3, therefore, promoting the production of type I IFNs. Besides, expression of RAE1 can activate NK cells. A hypothesis regarding the mechanisms of STING in spontaneous anti-tumor immunity has been put forward: dying tumor cells are engulfed by DCs, while free tumor DNA is recognized by cGAS, leading to the secretion of IFN α/β to improve DCs' cross-presentation to enhance T cell activation41. DNA damage in tumor cells can also cause the activation of natural-killer cells mediated by the expression of retinoic acid early transcript 1 (RAE1) through STING pathway. (B) DDR deficiencies improve tumor recognition through generating neoantigens. The neoantigen hypothesis is that a non-synonymous mutation leads to the change of an amino acid, which produces a new peptide. Therefore, cancer cells with DDR-deficiency can be recognized as the foreign by immune system. (C) DNA damage signaling and DDR deficiencies role as important regulators in upregulating PD-L1 expression. Abbreviations: ATP, adenosine-triphosphate; cGAMP, cyclic GMP–AMP; cGAS, cyclic GMP–AMP synthase; DDR, DNA damage response; DC, dendritic cell; ds DNA, double-stranded DNA; ER, endoplasmic reticulum; GTP, guanosine triohosphte; IRF3, interferon regulatory factor 3, IFN, interferon; NF-κB, nuclear factor kappa-B; NKG2D, natural killer group 2 member D; NK, natural killer; RAE1, retinoic acid early transcript 1; STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1.
6.2. DDR deficiencies improve tumor recognition of adaptive immune system
In adaptive immune responses, activation of specific T cells is essential in the interaction among MHC molecules displayed on the surface of antigen presenting cells, T cell receptor and costimulatory molecules expressed on the surface of naïve T cells11. Accumulation of intratumoral T cells necessitates recognition of antigens (such as cancer testis-antigens, antigens from overexpressed oncogenes, and neoantigens). The quantity of tumor neoantigen is positively correlated with the number of non-synonymous mutations in a tumor, which varies in different development of cancer or across diverse cancers46. Neoantigen hypothesis is that a non-synonymous mutation leads to the change of an amino acid, which produces a new peptide. Therefore, cancer cells with DDR-deficiency can be recognized as foreign cells by immune system (Fig. 2B)47. DDR deficiencies may increase the number of non-synonymous mutations. MMR-defected tumors have been reported capable of inducing strong inflammatory responses, in which a markedly increased number of non-synonymous mutations and large numbers of small insertions or deletions play important roles, with high production of novel proteins48. BRCA1/2 are genes of HRR pathway. Given that high somatic mutation rate and chromosome copy number alterations have been reported in BRCA1/2 mutative tumors, it is possible that mutations in BRCA1/2 may also promote neoepitope formation12.
6.3. Regulation of programmed death ligand 1 (PD-L1) responds to DNA damaging and repair signaling in tumor cells
DNA damage has been studied capable of inducing the expression of PD-L1 mRNA, thereby increasing the expression of PD-L1 in cell surface (Fig. 2C)49, 50, 51. This regulation depends on the activity of the transduction of ATM-ATR/checkpoint kinase 1 (CHK1) signaling, and defective DDR may link to greater PD-L1 upregulation induced by DSB. Defective BRCA or KU70/80 could significantly upregulate PD-L1 expression after radiation50. HRR pathway requires BRCA2 to function in promoting the switch of RAD51 from replication protein A (RPA) on the ssDNA regions. Thus, BRCA2 deficiency would impair this switch ability, resulting in RPA accumulation at the DSB ends and continuous activation of ATR/CHK1 signal. Thus, upregulation of PD-L1 is increased in cells with BRCA2 deficiency, which can be inhibited when suppressing ATR/CHK1 signaling50. Deficiency of KU70/80 complex are also correlated with increased activation of ATR/CHK1 and upregulation of PD-L1 expression compared with control group. Under oxidative stress, deficiency of NTH1 (a key BER component) can also enhance PD-L1 upregulation, which indicates that oxidative stress may induce DNA damage signaling to upregulate PD-L1 expression49.
The downstream component of ATR/CHK1 signaling, signal transducer and activator of transcription 1/3 (STAT1/3)-interferon regulatory factor (IRF), is crucial for producing signal that can activate the generation of PD-L1 mRNA at the transcriptional level (Fig. 2C)50. In case of immune response, interferon gamma (IFNγ) binds to its receptor, followed by the phosphorylation of Janus kinase 1/2 (JAK1/2) and STAT1/3, which can increase the expression of interferon gamma inducible genes such as IRF1. Phosphorylation of STAT1/3 as well as the expression of IRF1 control the expression of PD-L152. IRF1 can bind to PD-L1 promoter to upregulate PD-L1 transcription52. DNA damage was reported to contribute to STAT1/3 phosphorylation and IRF1 expression50. Therefore, DNA damage signaling and DDR deficiencies play important roles in upregulating the expression of PD-L1.
6.4. Excessive DNA damage arouses immune responses by cell death signals
Apoptosis or mitotic catastrophe-induced cell death may occur mediated by excessive DNA damage in cancer cells. High mobility group box-1 (HMGB1) is released from dying cells, to activate Toll-like receptor 4 (TLR4) pathway and increase myeloid differentiation factor 88 (MyD88)/Toll-interleukin 1 receptor domain-containing adaptor inducing INF-β (TRIF) signaling53, which can stimulate immune activity. Multiple types of DNA damage can be caused by chemotherapy or radiotherapy, and DSBs are associated closely with cell death. Tumors with deficiency of NHEJ or HRR, may show higher sensitivity to chemotherapy or radiotherapy54,55, and promote greater release of damage-associated molecular patterns (DAMPs) into TME after these treatments. For example, tumors with low expression of X-ray repair cross complementing protein 4 (XRCC4, a main component of the NHEJ pathway), might release more DAMPs after radiotherapy, with better therapeutic benefits55. Although great immune activity is stimulated by DAMPs, the TLR4/MyD88/TRIF signaling mediated by HMGB1 could also upregulate PD-L1 expression in the neighboring surviving tumor cells56. This process may inhibit immune activity to some extent after tumor receiving chemotherapy or radiotherapy.
6.5. Tumor aneuploidy and immune evasion
Genomic instability manifests as increased point mutation and chromosome instability. DDR pathway can also take a role in maintaining chromosome instability. For example, mitotic protein kinases, such as never-in-mitosis A (NIMA) in fungi, have been regarded to maintain genomic integrity. A component of DDR pathway named NIMA-related kinase 1 (NEK1) can maintain genomic integrity in mammalian ortholog. Research in NEK1-deficient cells showed faulty mitotic chromosome segregation, leading to aneuploidy. Inactivation of NEK1 could result in tumor formation without anchorage dependence57. Besides, somatic copy number alterations (SCNAs) are also correlated with immune evasion, including reduced adaptive immune signaling, cytotoxic T cell activity and cytokine signaling58. For example, based on TCGA dataset, a study identified more than 5000 tumors across 12 tumor types, and revealed high levels of SCNAs were correlated with decreased expression of cytotoxic immune cell markers59. In a trial on metastatic melanoma patients, high burden of SCNAs in non-responders to PD-1 and CTLA-4 blockade was identified associated with reduced expression of immune-related genes60.
Point mutations of DDR pathways may also correlate with tumor immune-evasion. BAP1 protein, a tumor suppressor mentioned before, is closely associated with DDR through interactions with BRCA1. Figueiredo and colleagues61 suggested BAP1 loss in uveal melanomas could cause upregulation of regulatory T cells, which could inhibit the cytotoxicity of effector T cells. They further found regulatory immune markers CD74 and CD38 were upregulated in tumors with BAP1 loss. Thus, compounds inhibiting CD38 and/or CD74 may potentially become adjuvant therapies for immunotherapy. Thus, how to elicit immune-evasion of DDR alterations in cancer immunotherapy should be considered carefully.
7. Application of DDR alterations in cancer immunotherapy
7.1. Potential of DDR pathway alterations in predicting ICI response
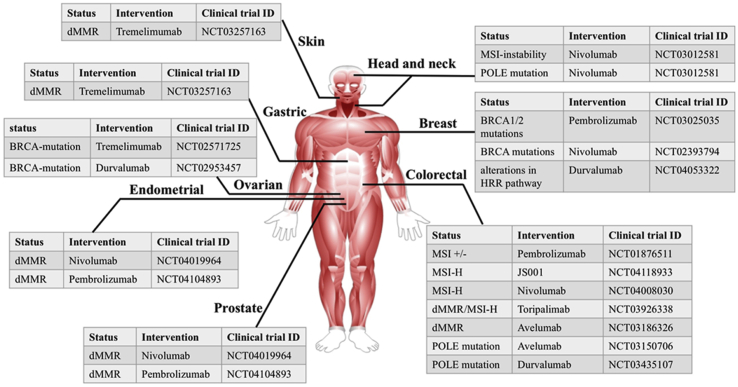
Much attention has been drawn to the association between DDR alterations and response to ICI immunotherapy in various cancer types (Table 162, 63, 64, 65, 66 and Fig. 3).
Table 1.
Association of the main DDR alterations with patients’ response to ICI immunotherapy.
| DDR pathway | Genomic biomarker | Tumor type | Immune characteristics | Clinical ICI response |
|---|---|---|---|---|
| DR | MGMT promoter methylation | Glioblastoma multiforme | – | Temozolomide and ICIs (Nivolumab)62 |
| BER | MUTYH mutations | Colorectal cancer | Immune cell infiltration (NK cells, CD3+, CD8+ T cells) | Not reported |
| NER | ERCC mutations (ERCC1 SNPs), mutations of xeroderma pigmentosum (XP)-related genes | ERCC mutations in NSCLC, XP-associated skin cancers | – | Nivolumab63 (NSCLC with ERCC mutations), Pembrolizumab, nivolumab64-66 (XP-associated skin cancers) |
| MMR | Mutations of MMR genes (MSH2, MSH6, MLH1, PMS2) | Colon, gastric, endometrial, ovarian, prostate, glioma, breast cancers, etc. | Immune cell infiltration, increased staining of PD-1, PD-L1 | Several clinical trials regarding response of patients with MMR mutations to ICIs are underway or planned (Fig. 3) |
| HRR | Mutations of HRR genes (BRCA1, BRCA2, PALB2, etc.) | Breast, ovarian, prostate, etc. | Immune cell infiltration (CD3+, CD8+ T cells), increased staining of PD-1, PD-L1 | Several clinical trials regarding response of patients with HRR mutations to ICIs are underway or planned (Fig. 3) |
| POLD1/POLE proofreading | POLD1/POLE mutations | Endometrial, colon, etc. | Immune cell infiltration (CD3+, CD8+ T cells), increased staining of PD-1, PD-L1 | Several clinical trials are ongoing or planed (Fig. 3) |
Figure 3.
Clinical trials of the efficacy of ICIs in tumors with DDR pathway alterations.
Defects of MMR genes characterized by microsatellite instability-high (MSI-H) status result in the accumulation of mutations as well as the production of neoantigens, which can enhance anti-cancer immune response. Bardelli's group67 presented an elegant research to knock out Mlh1 in the mouse model by transient Cas9 editing. When the Mlh1-deficient cells developed tumors in immunocompromised mice, these tumors were transplanted into immunocompetent mice. Under the treatment of ICIs, inactivation of Mlh1 in the transplanted tumors was observed to inhibit tumor growth, with higher levels of CD8+ T cells, higher clonal neoantigens and mutational load67. Besides, a phase 2 study from Le et al.68 evaluated the clinical efficacy of pembrolizumab in 41 patients with progressive metastatic carcinoma. In this study, MMR-deficient colorectal cancers showed 40% immune-associated objective response rate (ORR) and 78% 20-week immune-associated progression-free survival (PFS) rate, which were higher than MMR-proficient colorectal tumors (0% ORR and 11% PFS rate respectively).
Beyond MMR genes, analyses of TCGA dataset in ovarian tumors showed patients harboring higher neoantigens in BRCA2-mutated types, with elevated PD-1/PD-L1 expression and TILs12. Increased neoantigens in tumors enhanced patients' overall survival (OS). In addition, a preliminary phase II trial of PD-1 inhibitor pembrolizumab in 258 melanoma patients demonstrated patients with enriched BRCA1/2 mutations showed higher response to ICI therapy. The ORR was 5% in patients regardless of PD-L1 expression, while 12% ORR was shown in patients with BRCA1/2 mutations69. Patients with DNA polymerase epsilon (POLE)-mutated cancers also exhibit stronger response to ICIs. A study identified the association between patients’ response to PD-1 inhibitor and POLE-mutation in endometrioid endometrial cancers. The result of a heat map demonstrated there were more immune-related genes in POLE-mutated group than POLE-wild type (WT) tumors. Interestingly, POLE-mutated tumors also showed higher expression of immune checkpoint proteins (such as PD-L1) and T cell markers (such as PD-1) compared with POLE-WT tumors. This study suggested POLE mutations might become good biomarkers for ICI immunotherapy70. Additionally, ICI immunotherapy was suggested useful in MUTYH-associated colorectal cancers as well, as increased lymphocyte infiltration was observed71.
Some other DDR-related genes were also studied. Biallelic mutations of genes of DDR pathways such as ATM, partner and localizer of BRCA2 (PALB2), retinoblastoma 1 (RB1), and tumor protein P53 (TP53) were also correlated significantly with increased tumor immunogenicity72. Teo and colleagues73 analyzed 34 DDR genes in several pathways. In patients with metastatic urothelial carcinoma receiving atezolizumab (anti-PD-L1 antibody) or nivolumab (anti-PD-1 antibody), deleterious DDR alterations were related to longer survival. Recent studies suggested BAP1 might help to identify tumors that responded to cancer immunotherapy. In malignant mesothelioma, tumors with BAP1 mutations were distinctly correlated with an inflammatory TME, increased TILs and immune checkpoint activation74,75. To date, ICI trials for mesothelioma do not consider status of genomic biomarkers. Thus, BAP1 mutations should be considered as potential biomarkers to predict patients’ response to ICIs in mesothelioma. Although many DDR genes were studies, few studies about genes in the NHEJ pathway were reported. Additional studies are required to explore the role of these genes in cancer immunotherapy.
Multiple defects in different DDR pathways may lead to higher genomic instability76. Recently, Wang and colleagues77 prompted that co-mutations in DDR pathways might become a helpful biomarker in ICI immunotherapy. They mainly identified co-mutations in the HRR and MMR pathways (HRR-MMR) as well as HRR and BER pathways (HRR-BER). They collected data of whole-exome sequencing and mRNA expression across 29 tumors, with the observation of higher TMB and neoantigens in DDR-comutated groups. However, this study suggested that a single DDR pathway alteration could not predict higher TMB. They further investigated the response of cancer patients with co-mutations of DDR pathways to clinical ICIs, confirming the predictive role of co-mutations of DDR pathways in ICI immunotherapy77.
However, not all diagnosed DDR-deficient patients respond to ICI immunotherapies78. Interestingly, Touat and colleagues79 recently analyzed the mutational signatures in 10,294 gliomas. Although their results discovered MMR deficiency could be triggered by temozolomide, MMR-defected gliomas showed poor survival and low patients’ response to PD-1 inhibitors, with a lack of TIL abundance. Therefore, it remains a challenge to choose useful diagnostic assays of DDR deficiency. Take MMR testing as an example. Emerging study demonstrated that tumors with MMR deficiency determined by immunohistochemistry (IHC)- or polymerase chain reaction (PCR)-based MSI may not necessarily responded to ICIs80. In advanced prostate cancer, a study compared the association of MMR status determined by different assays. The results of MMR-mutated patients tested by IHC or PCR assay showed decreased OS, which was discordant compared with results based on next generation sequencing (NGS) testing. This study regarded NGS-based testing as an improved assay of MSI detection, but the challenge of cut-off definition was still maintained78.
Another challenge occurs because somatic mutations have time heterogeneity, highlighting the limitation to define DDR status in cancers. It would help a lot if a novel strategy can be designed to detect DNA repair functions dynamically.
7.2. Combinations of DNA-damaging agents with ICIs
The strategy to combine DNA-damaging agents with ICIs may enhance genomic instability and immunotherapy activity. Tumor immunogenicity may be increased by the standard care of DNA-damaging chemotherapy as well as radiotherapy, mediated via mechanisms such as DNA damage with cytosolic dsDNA production and immunogenic induction of cell death with increased antigen presentation. Therefore, several clinical trials were developed to investigate the efficacy of combining chemotherapy and ICIs, and a significantly longer survival has been observed in non-small cell lung cancer (NSCLC) patients treated with pembrolizumab co-administrated with chemotherapy in the first line81. In addition, a phase I clinical trial in NSCLC patients showed a marked clinical benefit when pembrolizumab combined with radiation therapy82.
A main limitation of DDR-targeted inhibitors is the development of acquired resistance. The significant correlation between DDR alterations and immune system in cancers suggests that combining DDR-targeted agents with ICI-based immunotherapies is very promising. Success of PD-1 inhibitor in tumors with MMR deficiency proves that neoantigen generation mediated by defective DDR can induce an immune response80. But unlike MMR-defective malignancies, some tumors may not produce sufficient neoantigen burden to arouse immune response, and inhibiting DDR proteins may also generate insufficient neoantigens. In this case, interactions between DDR proteins and some immunomodulators are potential to stimulate immune activity against cancer83.
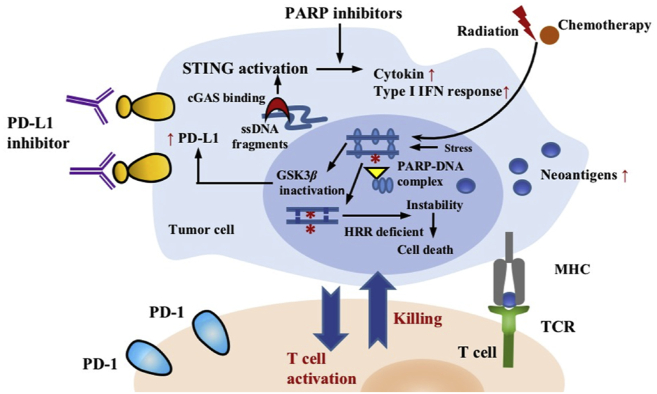
Proteins of the poly-ADP ribose polymerase (PARP) family play crucial roles in various cellular processes including DNA repair, aspects of replication stress response (RSR), and chromatin modulation84,85. As for DNA repair, PARP1 and PARP2 are important in the repair of DNA strand break through diverse DDR pathways, and cells with HRR defects are dependent on PARP activity for survival85,86. Application of PARP inhibitors may help the formation of inflammatory TME (Fig. 4). For example, in murine ovarian tumors with BRCA1 deficiency, treatment of a PARP1 inhibitor talazoparib (BMN 673) can increase CD8+ lymphocyte infiltration as well as promote the production of IFNγ and tumor necrosis factor (TNF)-α. Its combination with ICIs could further promote the establishment of immune system with higher lymphocyte infiltration and IFNγ production87,88. In addition, PARP inhibitors can also inactivate glycogen synthase kinase 3β (GSK3β) and enhance PD-L1 upregulation in a dose-dependent manner, therefore inhibiting T-cell activity (Fig. 4). PD-L1 blockade was found capable of re-sensitizing these tumor cells to T-cell killing89. In tumor models treated with alazaparib, olparib, and rucaparib, combining PARP inhibitor with anti-PD-L1 antibody resulted in greater antitumor activity than either agent alone89. The presence of cyto-plasmic DNA can also activate the cGAS–STING pathway to enhance innate immunity and drive T-cell priming90,91. Emerging clinical trials have studied the safety and clinical efficacy of the combination therapy of DDR-targeted inhibitors and ICIs, and published clinical trials suggested that these combinations could be used safely (Table 2).
Figure 4.
Therapeutic strategy of DDR alterations in cancer immunotherapy. Combination strategy: cancer cells treated with PARP inhibitors are sensitive to immune checkpoint inhibitors. PARP inhibitors increase genomic instability, activate immune pathway and upregulate the expression of PD-L1 on cancer cells, making the combination of PARP inhibitor with ICIs a promising strategy against cancer.
Table 2.
Combinations of DDR-targeted inhibitors with ICI immunotherapies.
| Clinical trial ID | Cancer type | DDR-targeted inhibitor | ICI | Phase |
|---|---|---|---|---|
| NCT04209686 | Advanced GAC | Olaparib | Pembrolizumab | Phase II |
| NCT04052204 | SCCHN, prostate cancer | Talazoparib | Avelumab | Phase II |
| NCT04034927 | Ovarian, fallopian tube and peritoneal cancer | Olaparib | Tremelimumab | Phase II |
| NCT03964532 | Breast cancer | Talazoparib | Avelumab | Phase I, phase II |
| NCT03958045 | SCLC | Rucaparib | Nivolumab | Phase II |
| NCT03951415 | Endometrial cancer | Olaparib | Durvalumab | Phase II |
| NCT03851614 | Colorectal cancer, pancreatic adenocarcinoma, leiomyosarcoma | Olaparib | Durvalumab | Phase II |
| NCT03834519 | Prostatic neoplasms | Olaparib | Pembrolizumab | Phase III |
| NCT03824704 | Solid tumors | Rucaparib | Nivolumab | Phase II |
| NCT03810105 | Prostate cancer | Olaparib | Durvalumab | Phase II |
| NCT03642132 | Ovarian cancer | Talazoparib | Avelumab | Phase III |
| NCT03639935 | Biliary tract cancer | Rucaparib | Nivolumab | Phase II |
| NCT03602859 | Ovarian cancer | Niraparib | Dostarlimab | Phase III |
| NCT03572478 | Prostate, endometrial cancer | Rucaparib | Nivolumab | Phase IIb |
| NCT03544125 | Metastatic triple negative breast cancer | Olaparib | Durvalumab | Phase I |
| NCT03522246 | Ovarian cancer | Rucaparib | Nivolumab | Phase III |
| NCT03404960 | Pancreatic adenocarcinoma | Niraparib | Nivolumab Ipilimumab |
Phase I, phase II |
| NCT03330405 | Solid tumors | Talazoparib | Avelumab | Phase II |
| NCT03308942 | Lung neoplasms | Niraparib | PD-1 Inhibitor | Phase II |
| NCT03167619 | Triple negative breast cancer | Olaparib | Durvalumab | Phase II |
| NCT03061188 | Ecurrent or refractory stage IV solid tumors | Veliparib | Nivolumab | Phase I |
| NCT02953457 | Ovarian, fallopian tube or primary peritoneal cancer | Olaparib | Durvalumab | Phase II |
| NCT02944396 | NSCLC | Veliparib | Nivolumab | Phase I |
| NCT02849496 | Breast cancer | Olaparib | Atezolizumab | Phase II |
| NCT02660034 | Solid tumors | Pamiparib | Tislelizumab | Phase I |
| NCT02657889 | Triple-negative breast cancer or ovarian cancer | Niraparib | Pembrolizumab | Phase I, phase II |
| NCT02571725 | BRCA-deficient ovarian cancer | Olaparib | Tremelimumab | Phase I, phase II |
DDR, DNA damage repair; GAS, gastric adenocarcinoma; ICIs, immune checkpoint inhibitors; NSCLC, non-small cell lung cancer; PD-1, programmed death 1; SCCHN, squamous cell carcinoma of the head and neck; SCLC, small cell lung cancer.
However, there are also some challenges in this combination including agent choice, dose of DNA damaging compounds, combination schedule and toxicity92. Different immunogenicity of DNA-damaging agents and dose-related myelosuppressive effects suggest the combination scheme should be considered carefully. Several successful anti-tumor studies have shown that treatment of receiving chemotherapy for a period of time before its combination with ICIs is an optional method93,94. Besides, many single arm trials suggest only a modest anti-cancer activity. Randomized clinical trials are required to determine whether these combination efficacies are superior to treatment of receiving ICI alone95,96.
Besides, combination of DDR inhibition and radiation can enhance inflammatory response. For example, inhibiting ATR in mice could potentiate type I IFN response induced by radiation, thereby increasing TILs97. Combining WEE1 inhibition and radiation could increase the killing of malignant cells by cytotoxic T lymphocytes in a granzyme B-dependent manner98. When adding anti-PD-L1 antibody to WEE1 inhibitor plus radiotherapy, increased survival was shown in mice with head and neck squamous cell carcinoma. Although preclinical studies about the combination of DNA-PK inhibition, ICIs and radiotherapy are lacking, clinical trials of this combination are already recruiting99. The use of DDR inhibition can increase the immunogenicity of radiation, and the use of ICIs can further negate immunosuppressive effects. Thus, this combination represents a promising therapeutic method. The combination of three drugs may make the interpretation of clinical findings more complicated; therefore, it is urgently to conduct more preclinical and clinical studies.
8. Conclusions and perspectives
DNA damage repair response of tumor cells has a very profound effect in creating inflammatory TME. Great progress has been made in understanding the potential correlations of DDR and immunity. Defective or downregulated DDR pathway allows genomic instability in the existence of exogenous or endogenous DNA damage, and tumor microenvironment also contributes to the regulation of genomic instability through DDR pathways. Alterations of DDR pathways can generate neoantigens promoted by degradation of abnormal proteins due to mutations in the open reading frames. MMR deficiency, defective DNA replication, as well as endogenous oxidative DNA damage may contribute to these mutations. When exposed to chemotherapy and/or radiotherapy, DDR signaling is activated by DSBs. Activation of ATM-ATR/CHK1 can result in the upregulation of PD-L1 in tumor cells. DNA fragments may also be generated from cells with DSBs, which can be recognized by cGAS and activate the STING pathway. Excessive DNA damage could make cancer cells die, inducing DAMP release and arousing immune response. Thus, DDR pathways and their alterations have strong application prospects in cancer immunotherapy.
Some proteins with DDR deficiency represent potential biomarkers for cancer immunotherapy in different types of cancers. Besides, interactions between DDR pathway and TME make it tempting to combine DDR-related therapies and immunotherapy to treat cancer. Given that explaining the efficacy of the combined strategy (DDR inhibitor/ICI immunotherapy) is complex, some biomarkers are being explored to predict patients’ response. For example, a NanoString-based method was developed as a predictive biomarker through detecting expression of interferon genes100. In addition, preclinical immunocompetent models are also needed to evaluate the efficacy of the combination between DDR inhibitors and immunotherapies. Moreover, novel therapies have also been developed. For example, the manganese-based nanoactivator [doxorubicin (DOX)-loaded and phospholipid (PL)-coated hybrid nanoparticles] can optimize cancer immunotherapy through inducing DNA damage and increasing innate immunity101. Thus, developing novel therapeutic strategies based on DNA damage and repair may be very potential for cancer immunotherapy. Meanwhile, it is also challenging to seek ways of potentiating the immune-promotive impacts of DDR pathways while reducing immune-suppressive effects. Inhibiting immune-suppressive effects through flexibly using adjuvant therapies for immunotherapy in DDR-altered tumors, or enhancing immunogenicity through using DDR inhibitors may represent promising therapeutic approaches against cancers.
Acknowledgments
This study was supported in part by a grant from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036, China) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131, China), National Key Research & Development Project (2016YFC0902300, China), major disease clinical skills enhancement program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A, China), “Dream Tutor” Outstanding Young Talents Program (fkyq1901, China), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012, China), grant of Shanghai Science and Technology Commission (16JC1405900, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yayi He, Email: 2250601@qq.com.
Caicun Zhou, Email: caicunzhoudr@126.com.
Author contributions
Minlin Jiang contributed significantly to analysis and manuscript preparation. Keyi Jia and Lei Wang provided suggestions for the improvement of manuscript. Wei Li, Bin Chen, Yu Liu, Hao Wang, Sha Zhao contributed to the correction of the manuscript. Minlin Jiang, Yayi He and Caicun Zhou contributed to the conception of the study. Yayi He and Caicun Zhou helped perform the analysis with constructive discussions.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Pan X., Zhang W., Guo H., Cheng S., He Q. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10:723–733. doi: 10.1016/j.apsb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Yu H., Rozeboom L., Rivard C.J., Ellison K., Dziadziuszko R. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J., Hodi F.S., Wolchok J.D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 6.Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Li S.K.H., Martin A. Mismatch repair and colon cancer: mechanisms and therapies explored. Trends Mol Med. 2016;22:274–289. doi: 10.1016/j.molmed.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Cleary J.M., Aguirre A.J., Shapiro G.I., D'Andrea A.D. Biomarker-guided development of DNA repair inhibitors. Mol Cell. 2020;78:1070–1085. doi: 10.1016/j.molcel.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domingo E., Freeman-Mills L., Rayner E., Glaire M., Briggs S., Vermeulen L. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol. 2016;1:207–216. doi: 10.1016/S2468-1253(16)30014-0. [DOI] [PubMed] [Google Scholar]
- 11.Suh W.K., Tafuri A., Berg-Brown N.N., Shahinian A., Plyte S., Duncan G.S. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J Immunol. 2004;172:5917–5923. doi: 10.4049/jimmunol.172.10.5917. [DOI] [PubMed] [Google Scholar]
- 12.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J.F. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David S.S., O'Shea V.L., Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 15.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 16.San Filippo J., Sung P., Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 17.Lieber M.R. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. doi: 10.1074/jbc.R700039200. [DOI] [PubMed] [Google Scholar]
- 18.Warren A.J. DNA-repair enzyme turns to translation. Nature. 2020;579:198–199. doi: 10.1038/d41586-020-00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Beebe-Dimmer J.L., Kapron A.L., Fraser A.M., Smith K.R., Cooney K.A. Risk of prostate cancer associated with familial and hereditary cancer syndromes. J Clin Oncol. 2020;38:1807–1813. doi: 10.1200/JCO.19.02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraemer K.H., Lee M.M., Andrews A.D., Lambert W.C. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 21.Renwick A., Thompson D., Seal S., Kelly P., Chagtai T., Ahmed M. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–875. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 22.Das S., Camphausen K., Shankavaram U. Pan-cancer analysis of potential synthetic lethal drug targets specific to alterations in DNA damage response. Front Oncol. 2019;9:1136. doi: 10.3389/fonc.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae H., Kim D., Yoo C., Kim K.P., Jeong J.H., Chang H.M. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur J Cancer. 2019;120:31–39. doi: 10.1016/j.ejca.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polak P., Kim J., Braunstein L.Z., Karlic R., Haradhavala N.J., Tiao G. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476–1486. doi: 10.1038/ng.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabano S., Azzollini J., Pesenti C., Lovati S., Costanza J., Fontana L. Analysis of BRCA1 and RAD51C promoter methylation in Italian families at high-risk of breast and ovarian cancer. Cancers. 2020;12:910. doi: 10.3390/cancers12040910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murr R., Loizou J.I., Yang Y.G., Cuenin C., Li H., Wang Z.Q. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 28.Burrell R.A., McClelland S.E., Endesfelder D., Groth P., Weller M.C., Shaikh N. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chene G., Tchirkov A., Penault-Llorca F. Early telomere shortening and genomic instability in tubo-ovarian preneoplastic lesions—response. Clin Cancer Res. 2013;19:5255. doi: 10.1158/1078-0432.CCR-13-1915. [DOI] [PubMed] [Google Scholar]
- 30.Jia P.P., Her C.T., Chai W.H. DNA excision repair at telomeres. DNA Repair. 2015;36:137–145. doi: 10.1016/j.dnarep.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan Y.A., Hieter P., Stirling P.C. Mechanisms of genome instability induced by RNA-processing defects. Trends Genet. 2014;30:245–253. doi: 10.1016/j.tig.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y., Xu C., Sun X., Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830–3852. doi: 10.1039/c6cs00592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds T.Y., Rockwell S., Glazer P.M. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- 34.Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J., Narayanan L., Rockwell S., Glazer P.M. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 36.Mihaylova V.T., Bindra R.S., Yuan J., Campisi D., Narayanan L., Jensen R. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bindra R.S., Gibson S.L., Meng A., Westermark U., Jasin M., Pierce A.J. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 38.Bindra R.S., Glazer P.M. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2007;26:2048–2057. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y., An X., Zhang X., Qiao Y., Zheng T., Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18:152. doi: 10.1186/s12943-019-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q., Sun L.J., Chen Z.J.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 41.Rivera Vargas T., Benoit-Lizon I., Apetoh L. Rationale for stimulator of interferon genes-targeted cancer immunotherapy. Eur J Cancer. 2017;75:86–97. doi: 10.1016/j.ejca.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brzostek-Racine S., Gordon C., Van Scoy S., Reich N.C. The DNA damage response induces IFN. J Immunol. 2011;187:5336–5345. doi: 10.4049/jimmunol.1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaston J., Cheradame L., Yvonnet V., Deas O., Poupon M.F., Judde J.G. Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget. 2016;7:77205–77224. doi: 10.18632/oncotarget.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heijink A.M., Talens F., Jae L.T., van Gijn S.E., Fehrmann R.S.N., Brummelkamp T.R. BRCA2 deficiency instigates cGAS-mediated inflammatory signaling and confers sensitivity to tumor necrosis factor-alpha-mediated cytotoxicity. Nat Commun. 2019;10:100. doi: 10.1038/s41467-018-07927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkes E.E., Walker S.M., Taggart L.E., McCabe N., Knight L.A., Wilkinson R. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw199. djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riaz N., Morris L., Havel J.J., Makarov V., Desrichard A., Chan T.A. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28:411–419. doi: 10.1093/intimm/dxw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiricny J. Postreplicative mismatch repair. Cold Spring Harb Perspect Biol. 2013;5:a012633. doi: 10.1101/cshperspect.a012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Permata T.B.M., Hagiwara Y., Sato H., Yasuhara T., Oike T., Gondhowiardjo S. Base excision repair regulates PD-L1 expression in cancer cells. Oncogene. 2019;38:4452–4466. doi: 10.1038/s41388-019-0733-6. [DOI] [PubMed] [Google Scholar]
- 50.Sato H., Niimi A., Yasuhara T., Permata T.B.M., Hagiwara Y., Isono M. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vendetti F.P., Karukonda P., Clump D.A., Teo T., Lalonde R., Nugent K. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest. 2018;128:3926–3940. doi: 10.1172/JCI96519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 54.Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. 2010;31:955–960. doi: 10.1093/carcin/bgq064. [DOI] [PubMed] [Google Scholar]
- 55.Takada Y., Someya M., Matsumoto Y., Satoh M., Nakata K., Hori M. Influence of Ku86 and XRCC4 expression in uterine cervical cancer on the response to preoperative radiotherapy. Med Mol Morphol. 2016;49:210–216. doi: 10.1007/s00795-016-0136-5. [DOI] [PubMed] [Google Scholar]
- 56.Liu J., Hamrouni A., Wolowiec D., Coiteux V., Kuliczkowski K., Hetuin D. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y., Chen C.F., Chiang H.C., Pena M., Polci R., Wei R.L. Mutation of NIMA-related kinase 1 (NEK1) leads to chromosome instability. Mol Cancer. 2011;10:5. doi: 10.1186/1476-4598-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouw K.W., Goldberg M.S., Konstantinopoulos P.A., D'Andrea A.D. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017;7:675–693. doi: 10.1158/2159-8290.CD-17-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davoli T., Uno H., Wooten E.C., Elledge S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355 doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roh W., Chen P.L., Reuben A., Spencer C.N., Prieto P.A., Miller J.P. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9:eaah3560. doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Figueiredo C.R., Kalirai H., Sacco J.J., Azevedo R.A., Duckworth A., Slupsky J.R. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J Pathol. 2020;250:420–439. doi: 10.1002/path.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reardon D.A., Brandes A.A., Omuro A., Mulholland P., Lim M., Wick A. Effect of nivolumab vs. bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello M.M., Vigneri P.G., Bruzzi P., Verderame F., Paratore S., Restuccia N. Excision repair cross complementation group 1 (ERCC-1) gene polymorphisms and response to nivolumab in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2017;35 doi: 10.3389/fonc.2020.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambon F., Osdoit S., Bagny K., Moro A., Nguyen J., Reguerre Y. Dramatic response to nivolumab in xeroderma pigmentosum skin tumor. Pediatr Blood Cancer. 2018;65 doi: 10.1002/pbc.26837. [DOI] [PubMed] [Google Scholar]
- 65.Hauschild A., Eichstaedt J., Mobus L., Kahler K., Weichenthal M., Schwarz T. Regression of melanoma metastases and multiple non-melanoma skin cancers in xeroderma pigmentosum by the PD1-antibody pembrolizumab. Eur J Cancer. 2017;77:84–87. doi: 10.1016/j.ejca.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 66.Kraemer K.H., Tamura D., Khan S.G. Pembrolizumab treatment of a patient with xeroderma pigmentosum with disseminated melanoma and multiple nonmelanoma skin cancers. Br J Dermatol. 2018;178:1009. doi: 10.1111/bjd.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Germano G., Lamba S., Rospo G., Barault L., Magri A., Maione F. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–120. doi: 10.1038/nature24673. [DOI] [PubMed] [Google Scholar]
- 68.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Bono J.S., Goh J.C.H., Ojamaa K., Rodriguez J.M.P., Drake C.G., Hoimes C.J. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2018;36:abs5007. [Google Scholar]
- 70.Mehnert J.M., Panda A., Zhong H., Hirshfield K., Damare S., Lane K. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126:2334–2340. doi: 10.1172/JCI84940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colebatch A., Hitchins M., Williams R., Meagher A., Hawkins N.J., Ward R.L. The role of MYH and microsatellite instability in the development of sporadic colorectal cancer. Br J Cancer. 2006;95:1239–1243. doi: 10.1038/sj.bjc.6603421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidotto T., Nersesian S., Graham C., Siemens D.R., Koti M. DNA damage repair gene mutations and their association with tumor immune regulatory gene expression in muscle invasive bladder cancer subtypes. J Immunother Cancer. 2019;7:148. doi: 10.1186/s40425-019-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teo M.Y., Seier K., Ostrovnaya I., Regazzi A.M., Kania B.E., Moran M.M. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36:1685–1694. doi: 10.1200/JCO.2017.75.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ladanyi M., Vega F.S., Zauderer M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med. 2019;11:18. doi: 10.1186/s13073-019-0631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrestha R., Nabavi N., Lin Y.Y., Mo F., Anderson S., Volik S. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 2019;11:8. doi: 10.1186/s13073-019-0620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tubbs A., Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–656. doi: 10.1016/j.cell.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z.J., Zhao J., Wang G.Q., Zhang F., Zhang Z.M., Zhang F. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78:6486–6496. doi: 10.1158/0008-5472.CAN-18-1814. [DOI] [PubMed] [Google Scholar]
- 78.Rodrigues D.N., Rescigno P., Liu D., Yuan W., Carreira S., Lambros M.B. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J Clin Invest. 2018;128:5185. doi: 10.1172/JCI125184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Touat M., Li Y.Y., Boynton A.N., Spurr L.F., Iorgulescu J.B., Bohrson C.L. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580:517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 82.Shaverdian N., Lisberg A.E., Bornazyan K., Veruttipong D., Goldman J.W., Formenti S.C. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mouw K.W., D'Andrea A.D. DNA repair deficiency and immunotherapy response. J Clin Oncol. 2018;36:1710–1713. doi: 10.1200/JCO.2018.78.2425. [DOI] [PubMed] [Google Scholar]
- 84.Forment J.V., O'Connor M.J. Targeting the replication stress response in cancer. Pharmacol Ther. 2018;188:155–167. doi: 10.1016/j.pharmthera.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang B.Y., Lyu J.F., Yang E.J., Liu Y.F., Wu C.J., Pardeshi L. Class I histone deacetylase inhibition is synthetic lethal with BRCA1 deficiency in breast cancer cells. Acta Pharm Sin B. 2020;10:615–627. doi: 10.1016/j.apsb.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Higuchi T., Flies D.B., Marjon N.A., Mantia-Smaldone G., Ronner L., Gimotty P.A. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J., Wang L., Cong Z.Y., Amoozgar Z., Kiner E., Xing D.Y. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1–/– murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463:551–556. doi: 10.1016/j.bbrc.2015.05.083. [DOI] [PubMed] [Google Scholar]
- 89.Jiao S., Xia W., Yamaguchi H., Wei Y., Chen M.K., Hsu J.M. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang M., Chen P., Wang L., Li W., Chen B., Liu Y. cGAS–STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13:81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pantelidou C., Sonzogni O., De Oliveria Taveira M., Mehta A.K., Kothari A., Wang D. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722–737. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown J.S., Sundar R., Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118:312–324. doi: 10.1038/bjc.2017.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lesterhuis W.J., Salmons J., Nowak A.K., Rozali E.N., Khong A., Dick I.M. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynch T.J., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 95.Konstantinopoulos P.A., Waggoner S., Vidal G.A., Mita M., Moroney J.W., Holloway R. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vinayak S., Tolaney S.M., Schwartzberg L., Mita M., McCann G., Tan A.R. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dillon M.T., Bergerhoff K.F., Pedersen M., Whittock H., Crespo-Rodriguez E., Patin E.C. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin Cancer Res. 2019;25:3392–3403. doi: 10.1158/1078-0432.CCR-18-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel P., Sun L., Robbins Y., Clavijo P.E., Friedman J., Silvin C. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition. OncoImmunology. 2019;8 doi: 10.1080/2162402X.2019.1638207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLaughlin M., Patin E.C., Pedersen M., Wilkins A., Dillon M.T., Melcher A.A. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 100.Farkkila A., Gulhan D.C., Casado J., Jacobson C.A., Nguyen H., Kochupurakkal B. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun. 2020;11:1459. doi: 10.1038/s41467-020-15315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hou L., Tian C.Y., Yan Y.S., Zhang L.W., Zhang H.J., Zhang Z.Z. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano. 2020;14:3927–3940. doi: 10.1021/acsnano.9b06111. [DOI] [PubMed] [Google Scholar]