Abstract
Nanoparticles (NPs) have shown potential in cancer therapy, while a single administration conferring a satisfactory outcome is still unavailable. To address this issue, the dissolving microneedles (DMNs) were developed to locally deliver functionalized NPs with combined chemotherapy and photothermal therapy (PTT). α-Tocopheryl polyethylene glycol succinate (TPGS)/hyaluronic acid (HA) dual-functionalized PLGA NPs (HD10 NPs) were fabricated to co-load paclitaxel and indocyanine green. HD10 NPs significantly enhanced the cytotoxicity of low-dose paclitaxel because of active and mitochondrial targeting by HA and TPGS, respectively. PTT could further sensitize tumor cells toward chemotherapy by promoting apoptosis into the advanced period, highly activating caspase 3 enzyme, and significantly reducing the expression of survivin and MMP-9 proteins. Further, the anti-tumor effects of HD10 NPs delivered through different administration routes were conducted on the 4T1 tumor-bearing mice. After a single administration, HD10 NPs delivered with DMNs showed the best anti-tumor effect when giving chemotherapy alone. As expected, the anti-tumor effect was profoundly enhanced after combined therapy, and complete tumor ablation was achieved in the mice treated with DMNs and intra-tumor injection. Moreover, DMNs showed better safety due to moderate hyperthermia. Therefore, the DMNs along with combined chemo-photothermal therapy provide a viable treatment option for superficial tumors.
KEY WORDS: Functionalized PLGA nanoparticles, TPGS, Hyaluronic acid, Chemotherapy, Photothermal therapy, Dissolving microneedles, Superficial tumor
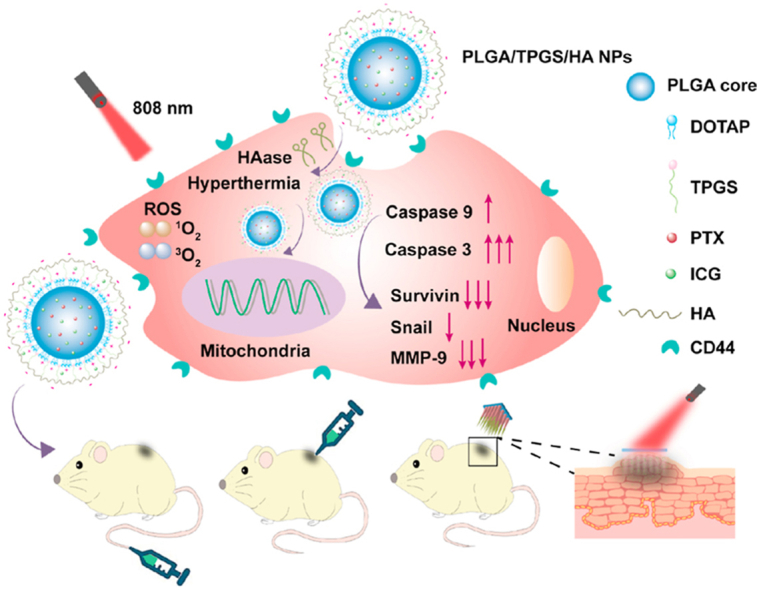
Graphical abstract
Dissolving microneedles encapsulating TPGS/HA functionalized PLGA nanoparticles provide a highly efficient local treatment for superficial tumor by integrating chemotherapy and photothermal therapy (PTT). The functionalized nanoparticles significantly enhanced the cytotoxicity, and sensitized tumor cells toward chemotherapy through multiple pathways.
1. Introduction
Tumors have become the leading cause of the threat to health and death in humans. Chemotherapy is the most widely used and classical clinical regimen for cancer therapy. However, some malignant tumors, such as triple-negative breast cancer (TNBC) and melanoma, are insensitive to conventional chemotherapy, and thus, some combination therapies are urgently needed to improve the efficacy of chemotherapy. Recently, the combination of chemotherapy and photothermal therapy (PTT) mediated by nanoparticles (NPs) has emerged as a new modality for tumor treatment, since NPs are uniquely advantageous in controlling drug release, improving drug efficacy, and enhancing photothermal effect.
Among diverse nanocarriers, poly (lactic-co-glycolic acid) (PLGA) NPs have gained great attention due to their desirable properties of good biocompatibility, versatile modification, and simple production1,2. However, bare PLGA NPs with strong hydrophobicity usually involves several drawbacks, including (1) easy recognition and uptake by the reticuloendothelial system, leading to a short circulation time in vivo3, (2) poor adhesion to cells and low permeability to the cell membrane4, and (3) low encapsulation efficiency of hydrophilic drugs5.
To address the intrinsic dilemma, surface modification of PLGA NPs with poly (ethylene glycol) (PEG)6,7, lipids (lecithin, DSPE-PEG)8, or surfactants α-tocopheryl polyethylene glycol succinate (TPGS)9,10, poloxamer11 and enclosure of PLGA NPs with cell membranes (erythrocyte12, platelet13, and cancer-derived cells14) are commonly used strategies. Obviously, it was more feasible to modify PLGA NPs using the commercially available materials, rather than the naturally derived lipids. Until now, there has been no report on the use of TPGS-functionalized PLGA NPs to co-deliver chemotherapeutic drug and photosensitizer for combined chemo-photothermal therapy. TPGS also offers unique advantages over other PEGylated material or lipids by targeting mitochondria to trigger cell apoptosis and amplifying the apoptosis-inducing effect of PTT15,16. Therefore, we conjecture that the TPGS-modified PLGA NPs with combined chemo-photothermal therapy can maximize the anti-tumor efficacy to the fullest.
Herein, indocyanine green (ICG) and paclitaxel (PTX) were used as the photosensitizer and chemotherapeutic drug, respectively. Unfortunately, the conventional PLGA NPs showed a relatively low encapsulation efficiency of ICG at about 20%–40%5,8. Therefore, a cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) was introduced to provide a positively charged surface to the hybrid NPs and further increase the encapsulation efficiency (EE%) of negatively charged ICG. Hyaluronic acid (HA) with the negative charge was further used to coat the hybrid NPs, and hence neutralize the positive charge of NPs to avoid plasma adsorption and rapid clearance from blood circulation17,18. Meanwhile, the HA-coated hybrid NPs are expected to achieve active tumor targeting by specifically interacting with CD 44 receptors, which are overexpressed on many types of tumor cells18, 19, 20. However, multiple and frequent injections of these NPs at high dosage are commonly required to achieve a satisfying anti-tumor outcome, which may cause severe adverse effects and patient discomfort.
The recent development of dissolving microneedles (DMNs) has revolutionized the treatment modality of superficial tumors because it provides a platform for local treatment with reduced dosing, enhanced safety, and promising anti-tumor outcome21, 22, 23, 24. Compared to intravenous and intratumor injections, DMNs, which contain multiple needles as drug depots, are more susceptible to releasing cargoes and realizing uniform heat distribution from PTT over tumors, thus diminishing tumor recurrence and inferior outcome.
Based on this, our study aimed to develop composite DMNs loading NPs, with the advantages of both polymeric NPs and DMNs, for treating superficial tumors with a combination of chemotherapy and PTT. We firstly constructed the TPGS/HA dual-functionalized PLGA NPs and evaluated the underlying cell apoptotic mechanism. Next, mixtures of polyvinylpyrrolidone (PVP) and polyvinyl alcohol (PVA) were used as needle material to prepare NPs-loaded DMNs because of their hydrophilic nature and appropriate mechanical strength25,26. Finally, we established the animal model of 4T1 tumor-bearing mice to compare the anti-tumor efficacy of NPs delivered through tail vein injection, intratumor injection, and DMNs.
2. Materials and methods
PTX (purity>99%, MW = 853.91), coumarin-6, TPGS and PVA-103 were purchased from Sigma–Aldrich (St. Louis, MO, USA). Free-acid terminated PLGA (MW = 5000–15,000 Da) with a 50:50 monomer ratio was gained from Daigang Biological engineering Co., Ltd. (Jinan, China). ICG (laser grade) was procured from Acros Organics (Belgium, USA). 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) was obtained from Avanti Polar Lipid, Inc. (Alabaster, AL, USA). Small molecular HA (purity>99%, MW = 10,000) was obtained from Ronlabs Co., Ltd. (Shanghai, China). PVP K30 and K90 was kindly donated by BASF AG (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM, Gibco™) and fetal bovine serum (FBS, Gibco™) were obtained from Thermo Fisher Scientific (MA, USA). Cell Counting Kit-8 (CCK-8) was gained from Dojindo Molecular Technologies, Inc. (Kyushu, Japan). Caspase 9 and 3 activity assay kits were obtained from Biyotime Biotechnology Co., Ltd. (Shanghai, China). Annexin V-FITC/propidium iodide (PI) apoptosis detection kit was purchased from Lianke Biotechnology Corp. Ltd. (Hangzhou, China). All other reagents were used as received.
2.1. Preparation and characterization of TPGS and TPGS/HA functionalized PLGA NPs
TPGS-modified PLGA NPs [termed as D10 NPs, with 10% (w/w) DOTAP of polymer weight] were prepared by the nanoprecipitation method. Briefly, PLGA (3.6 mg/mL), DOTAP (0.4 mg/mL), and PTX (0.2 mg/mL) dissolved in acetone were used as the organic phase. The mixtures of TPGS (2.0 mg/mL, 2.5 mL) in 4% (w/w) ethanol aqueous solution, and ICG (1.0 mg/mL, 100 μL) in 20% (v/v) methanol aqueous solution were used as the aqueous phase and heated to 65 °C. D10 NPs were generated by dripping 0.5 mL of acetone solution dropwise into the aqueous solution under magnetic stirring (Magnetic Stirrer, WH-610D) for 20 min at 35 °C. Then, HD10 NPs were prepared by slowly adding HA solution (1 mg/mL) into the fresh D10 NPs at a 2:3 volume ratio, depending on the electrostatic force between HA and D10 NPs. Finally, the residual organic solvent and free PTX or ICG were removed by washing the D10 NPs or HD10 NPs thrice using an Amicon Ultra-4 centrifugal filter (MWCO 10 kDa, Millipore, Billerica, MA, USA). The resultant NPs were stored in the dark at 4 °C until further use.
Characterization of NPs in terms of particle size, zeta potential, EE%, morphology, spectroscopic properties, fluorescent intensity, photothermal effect, and in vitro drug release study, is provided in the Supporting Information.
2.2. Cellular study
The cytotoxicity evaluation of different formulations with or without laser irradiation (LS) was carried out on the MDA-MB-231 cells. The mechanism of cell death induced by chemotherapy or chemo-photothermal combined therapy was investigated by determining apoptosis, intracellular localization, caspase 3 and 9 activity, and the expression of proteins related to tumor metastasis or recurrence. Detailed evaluations are provided in the Supporting Information.
2.3. Preparation and characterization of DMNs
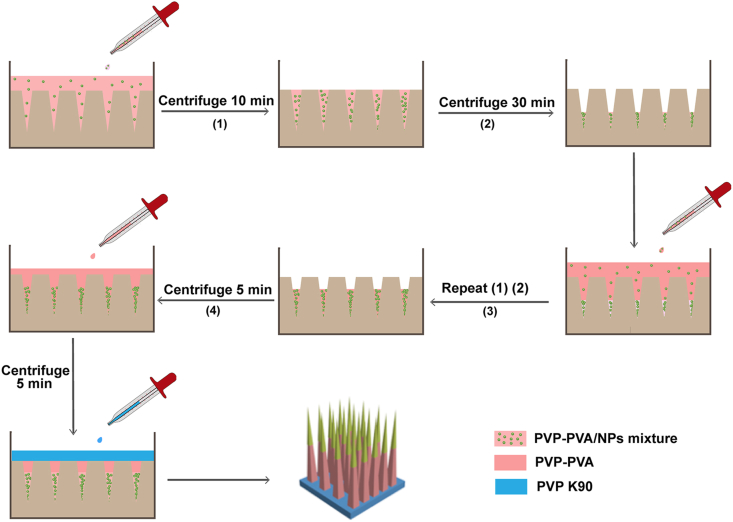
DMNs were prepared by a stepwise centrifugation method to concentrate the NPs in the tip of needles and increase the dug loading of DMNs (Fig. 1)27. The detailed procedure and characterization of DMNs in terms of morphology, skin insertion ability, and photothermal conversion efficiency are provided in the Supporting Information.
Figure 1.
Schematic illustration of preparing HD10 NP-loaded DMNs.
2.4. In vivo studies
The 4T1 tumor-bearing mice with tumors of 100–150 mm3 volume were used to evaluate and compare the in vivo photothermal effect and anti-tumor efficacy of different administration routes (intravenous injection, intra-tumor injection, and DMNs) of HD10 NPs. Major organs were histologically evaluated to determine the safety of various treatments. All animal experiments were conducted under the protocols approved by the Institution Animal Care and Use Committee of Sun Yat-sen University, Guangzhou, China (Approval No. SYSU-IACUC-2019-000114). Detailed information is summarized in the Supporting Information.
2.5. Statistical analysis
Data are reported as mean±standard deviations (SD). For multiple comparisons, data were analyzed by one-way analysis of variance (ANOVA) with Turkey's test (for pairwise comparison with each other) or Dunnett's test (with the untreated or defined group as a control) using GraphPad Prism 7.0 software (GraphPad Software Inc., San Diego, CA, USA). Differences with P < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Preparation and characterization of NPs
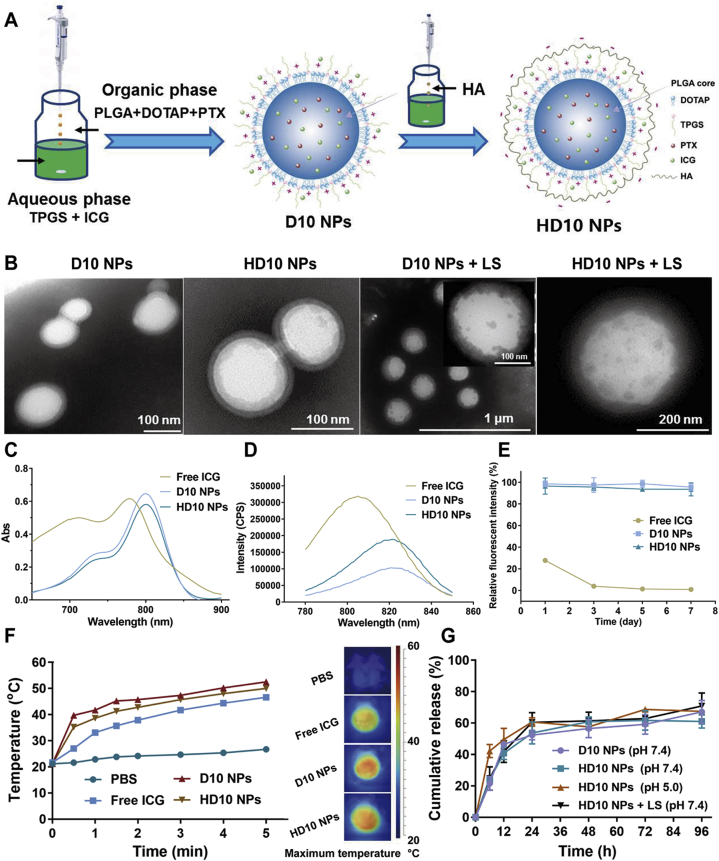
The schematic illustration of D10 and HD10 NPs preparation is displayed in Fig. 2A. D10 NPs were prepared by nanoprecipitation, where the organic phase loading PLGA/DOTAP/PTX was slowly added to the aqueous solution containing TPGS/ICG. PLGA was precipitated with the diffusion of the organic solvent into the aqueous solution, to form the polymer matrix, while DOTAP and TPGS were simultaneously aligned and deposited around the PLGA core due to their amphiphilic nature. As a consequence, D10 NPs with a core–shell structure was formed. Then, HD10 NPs with a core–shell–shell structure were prepared by HA coating of D10 NPs by electrostatic crosslinking.
Figure 2.
Preparation and characterization of D10 NPs and HD 10 NPs: (A) the schematic illustration of preparing D10 NPs by nanoprecipitation and HD 10 NPs by titration, (B) TEM morphology of NPs before and after LS, (C) UV–Vis absorbance spectra, (D) the fluorescence emission spectra, (E) the fluorescent stability of free ICG, D10 NPs and HD10 NPs aqueous solution incubated at 37 °C over 7 days (Data are presented as mean ± SD, n = 3), (F) in vitro photothermal effect, and (G) in vitro drug release profiles (Data are presented as mean ± SD, n = 3).
3.1.1. Particle size, zeta potential, and encapsulation efficiency of NPs
The particle size, zeta potential, and encapsulation efficiency (EE, %) of NPs are presented in Table 1. D10 NPs and H10 NPs had a particle size of about 140 and 160 nm, respectively. The zeta potential of D10 NPs was 9.20 mV, while that of HD10 NPs shifted to −19.40 mV. The increased particle size and negative charge of HD10 NPs confirmed the adsorption of HA onto the D10 NPs. Both D10 and H10 NPs showed an EE% of PTX (>85%) and ICG (>47%). PTX could be effectively encapsulated into the PLGA core, since TPGS has a good solubilizing ability to PTX, and PLGA interacted with PTX through a hydrophobic force. When the mass ratio of TPGS/PLGA was ≥3, the EE% of PTX was up to 85%10. The potential causes for high EE% of ICG were (1) ICG being an amphiphilic compound, can not only be encapsulated into PLGA core through hydrophobic interaction with PLGA but also form a self-assembled complex with TPGS to deposit as the outer shell28; (2) the positively charged DOTAP in the shell can adsorb ICG via electrostatic force (the amount of DOTAP in the physicochemical properties of NPs is described in Supporting Information Fig. S2); (3) ICG and PTX can interact with each other through the electrostatic force and π‒π stacking, which further improved the EE% of ICG29.
Table 1.
The physical characteristics of D10 NPs and HD 10 NPs.
| NP | Z-average (nm) | PDI | Zeta potential |
EE (%) |
|
|---|---|---|---|---|---|
| (mV) | PTX | ICG | |||
| D10 NPs | 142.40 ± 4.30 | 0.14 ± 0.01 | 9.20 ± 0.17 | 87.01 ± 1.11 | 50.23 ± 4.14 |
| HD10 NPs | 162.30 ± 1.80 | 0.15 ± 0.01 | ‒19.40 ± 0.79 | 85.93 ± 4.29 | 47.43 ± 1.89 |
Data are presented as mean ± SD (n = 3).
3.1.2. Morphologies of NPs before and after LS
The morphologies of NPs before and after LS are shown in Fig. 2B. D10 NPs show a well-defined spherical shape with a core–shell structure, indicating the successful coating of PLGA core by TPGS shell and a good agreement with the outcomes of a previous report10. The core–shell–shell structure of HD10 NPs further confirmed the successful coating of D10 NPs with HA by electrostatic interaction. However, after LS, the outer TPGS shell showed noticeable ablation, while the PLGA core remained intact or showed slight ablation. This was mainly because the melting point of TPGS (41.6 °C) is relatively low; however, the increased temperature during LS was insufficient to corrode the PLGA core to split it into smaller pieces.
3.1.3. Spectroscopic properties
Compared to the free ICG solution, the absorption spectra of ICG-loaded NPs exhibited a red-shift from 778 to 800 nm (Fig. 2C), which agreed well with previous studies30,31. Likewise, the fluorescence emission spectrum of ICG-loaded NPs (Fig. 2D) red-shifted from 805 to 822 nm at the excitation wavelength of 763 nm.
3.1.4. Fluorescent intensity
The fluorescence intensity decreased to 27.8% and 4.0% of its initial value on the 1st and 3rd day, respectively (Fig. 2E). It was reported that ICG had poor stability and could degrade to its free form because ICG concentration exceeding 2 μg/mL could cause its self-quenching by forming aggregated particles through the van der Waals force and hydrophobic interaction force between the ICG molecules32. The relative fluorescence intensity of ICG loaded NPs was more than 94% during storage, indicating that encapsulation of ICG into the NPs successfully avoided rapid aggregation, degradation, and self-quenching in aqueous solution, thereby remarkably improving the fluorescence stability of the ICG aqueous solution33,34.
3.1.5. In vitro photothermal effect
The temperature changes and typical thermal images of different solutions during LS are presented in Fig. 2F. The temperature of all solutions except PBS increased with an increase in irradiation time. The highest temperature of PBS, free ICG, D10 NPs, and HD10 NPs was 26.7, 46.6, 52.5 and 50 °C, respectively, showing that the encapsulation of ICG in NPs increased the photothermal conversion efficiency of ICG. The major reasons for enhanced heating efficacy of NPs were: (1) increased photothermal stability of ICG35, (2) higher local ICG concentration from NPs31, (3) closer absorption peak of ICG-loaded NPs to 808 nm resulting in more efficient heat production36.
3.1.6. In vitro drug release profiles
The in vitro release profiles of various NPs are shown in Fig. 2G. The release rates of D10 and HD10 NPs were comparable, indicating that HA coating of D10 NPs did not affect the diffusion of PTX from the NPs into the dissolution buffer because HA could dissolve rapidly upon contact with dissolution buffer. However, the release rate of HD10 NPs after LS increased slightly, possibly due to the minor ablation in the PLGA core. While on the one hand, HD10 NPs did not show sensitive light-triggered release behavior, on the other hand, PEGylation of PLGA NPs with TPGS did not hinder the contact of PLGA with the release medium and delayed drug release, while drug release retardation with PEGylated PLGA NPs has been reported in previous studies37,38. Additionally, at pH 5.0, HD10 NPs exhibited remarkably faster drug release within 12 h than that at pH 7.4, probably due to increased PLGA degradation in acidic medium39.
3.2. Cellular study of NPs
3.2.1. Cell viability
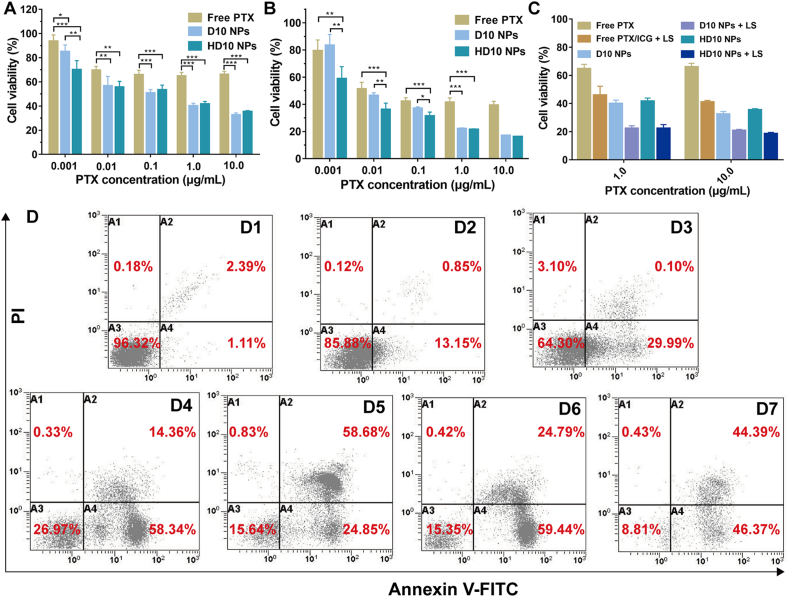
The cell viability of MDA-MB-231 cells with different treatments is shown in Fig. 3A–C, and the determination of half-maximal inhibitory concentration (IC50) of PTX is displayed in Table 2. The cytotoxicity of PTX was significantly enhanced after D10/HD10 NP encapsulation. This was attributed to the increase in the cellular uptake of PTX, and TPGS-mediated synergistic inhibition of cell proliferation with PTX by reducing drug efflux as an inhibitor of P-gp40,41, and activation of mitochondrial apoptotic pathways in cells with enhanced intracellular ROS generation, mitochondrial depolarization, and functional degradation42,43. There was no significant difference in the 24 h cytotoxicity of D10 and HD10 NPs, while the 48 h cytotoxicity was greater in HD10 NPs than in D10 NPs at low-dose PTX (0.001–0.1 μg/mL). This may be because HA recognized the highly expressed CD44 receptor on MDA-MB-231 cells, and increased the uptake of NPs by receptor-mediated endocytosis44.
Figure 3.
Cytotoxicity evaluation of MDA-MB-231 cells incubated with different formulations. Cell viability at 24 h (A) and 48 h (B) without LS, and 24 h with LS (C). Data are presented as mean ± SD (n = 5), ∗P < 0.05, ∗∗P < 0.05 and ∗∗∗P < 0.001. (D) Cell apoptosis detected by the flow cytometry: (D1) untreated group, (D2) free PTX + ICG, (D3) free PTX + ICG + LS, (D4) D10 NPs, (D5) D10 NPs + LS, (D6) HD10 NPs, and (D7) HD10 NPs + LS.
Table 2.
Inhibitory concentration (IC50) of PTX in MDA-MB-231 cells.
| Formulation | IC50 (μg/mL) |
|
|---|---|---|
| 24 h | 48 h | |
| Free PTX | >10 | 0.02211 |
| D10 NPs | 0.1008 | 0.01221 |
| HD10 NPs | 0.1248 | 0.00312 |
Irrespective of formulation and dosage, the chemo-photothermal combined therapy displayed higher cytotoxicity than the chemo-monotherapy (Fig. 3C). The mechanism of PTT enhancing the cytotoxicity of PTX occurred primarily in two ways: (1) the generated heat could rupture lysosomes and further induce programmed cell apoptosis45, and (2) the produced ROS, such as hydroxyl and singlet oxygen, damaged the cells by inducing necrosis46,47. This enhancing effect was more noticeable in the D10/HD10 NPs than in free PTX/ICG, implying that incorporation of ICG in NPs could augment the anti-tumor efficacy of PTT due to increased photothermal conversion efficiency and cellular uptake.
3.2.2. Cell apoptosis
Cell apoptosis was quantified by flow cytometry. As shown in Fig. 3D, the ability of different treatments to induce apoptosis followed the order of HD10 NPs + LS > HD10 NPs > D10 NPs + LS > D10 NPs > Free PTX + ICG + LS > Free PTX + ICG. The result indicates that the encapsulation of PTX in NPs, especially in HD10 NPs, profoundly enhanced the apoptotic effect. Also, the combination of chemotherapy and PTT showed remarkably enhanced apoptosis-inducing capacity in comparison to chemotherapy alone, which is consistent with the result of cytotoxicity evaluation. Interestingly, PTT induced more cells to enter the late apoptosis for the D10 and HD10 NPs, but more cells to the early apoptosis for the free PTX/ICG.
3.2.3. Subcellular localization of the NPs
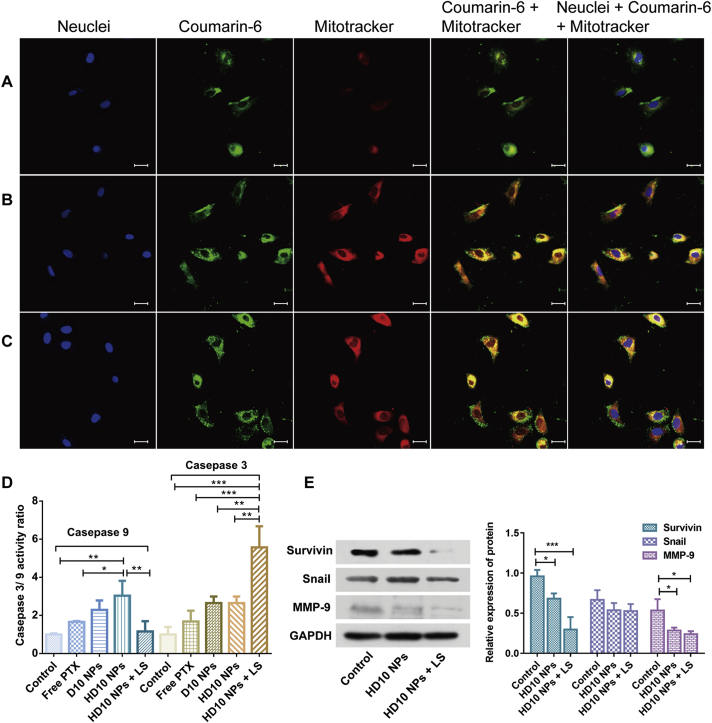
The mitochondrial specificity of D10/HD10 NPs was monitored using confocal laser scanning microscopy (CLSM). Free coumarin-6 and coumarin-6 loaded D10/HD10 NPs displayed an intrinsic green fluorescence, and Mitotracker Deep Red was used to label mitochondria with red fluorescence. According to Fig. 4A–C, free coumarin-6 displayed a distinguished separation from the mitochondria but presented a high co-localization with lysosomes (Supporting information Fig. S5), indicating that free coumarin-6 could internalize into the cells and get entrapped by the lysosome. An opposite phenomenon was observed in the D10/HD10 NPs, where the green fluorescence signal overlapped well with the red fluorescence signal, resulting in a strong yellow fluorescence signal. This result demonstrates the primary accumulation of NPs in the mitochondria after cellular uptake. The mitochondrial targeting capacity of the NPs could be benefited from the TPGS coating, as an outer shell of NPs42, and the positively charged DOTAP, recruiting the NPs to the negatively charged mitochondrial site by electrostatic interaction48.
Figure 4.
The distribution of coumarin-6 in the nucleus and mitochondria of MDA-MB-231 cells after treatment with free coumarin-6 (A), D10 NPs (B), and HD10 NPs (C). Scale bar = 30 μm. (D) and (E) Mechanistic investigation of apoptosis in the MDA-MB-231 cells after receiving different treatments. (D) Caspases 9 and 3 activations and (E) the relative protein expression of surviving, snail and MMP-9 protein. Data are presented as mean ± SD (n = 3), ∗P < 0.05, ∗∗P < 0.05 and ∗∗∗P < 0.001.
3.2.4. Caspase activation
Caspase activation is closely linked to the apoptosis in mitochondria49. The activation of caspases 9 and 3 act as the initiator and ultimate effector in the cascade reaction, respectively50. In general, D10 and HD10 NPs enhanced the activity of caspases 9 and 3 much better than free PTX (Fig. 4D). The activity ratio of caspase 9 in the cells treated with HD10 NPs showed a 1.82-fold and 1.33-fold of free PTX and D10 NPs, respectively. A similar trend was also found in the activity of caspase 3, and HD10 NPs showed the highest activity ratio when the cells were subject to chemotherapy alone. It was demonstrated that TPGS/HA dual-functionalized NPs were more capable in inducing apoptosis than TPGS single functionalized NPs. Although the activity ratio of caspase 9 in the cells treated with HD10 NPs and LS was significantly lower than that of HD10 NPs, the caspase 3 activity ratio of the HD10 NPs with LS group was 2.1-fold of HD10 NPs. This result further confirms that the combination of chemotherapy and PTT greatly elevated the cellular caspase 3 activity, and promoted the cells to advance into the late apoptosis.
3.2.5. Western blotting
The expression of proteins related to the invasion, metastasis, and prognosis of TNBC was evaluated by Western blotting (Fig. 4E). Compared with normal cells, the expression of MMP-9 was significantly reduced in cells treated with HD10 NPs or HD10 NPs plus LS. MMP-9 is one of the extremely crucial proteolytic enzymes involved in cell invasion and migration for the degradation of the extracellular matrix and basement membrane51. Besides, snail overexpresses in the MDA-MB-231 cells52, as a member of the upstream transcription factors during epithelial–mesenchymal transition (EMT), and participates in the invasion and migration of tumor53. However, the expression of snail did not show a noticeable reduction after treatment with either chemotherapy or chemo-photothermal combined therapy. However, the expression of survivin was significantly reduced after giving LS, consistent with the outcomes of the previous report54. Survivin is known to mediate the metastasis and prognosis of TNBC, and the decrease in its expression was advantageous in terms of suppressed tumor metastasis55 and sensitized cancer cells toward chemotherapeutic drugs56. These results, therefore, suggest that the combination of chemotherapy and PTT could not only lower tumor invasion and metastasis but also potentially improve TNBC prognosis.
3.3. Characterization of DMNs loading NPs
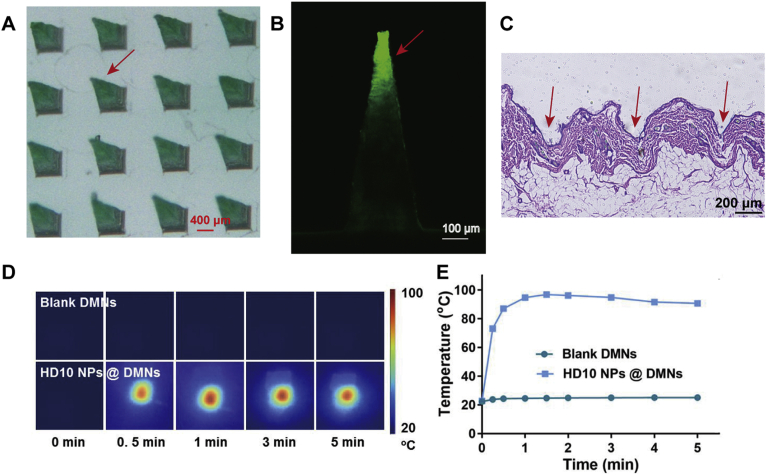
The fabricated array of DMNs had a regular pyramid shape with a needle height approaching 800 μm (Fig. 5A). Besides, only the needle tip was in green color and showed green fluorescence (Fig. 5B). Since the ICG-loaded NPs are green and emit green fluorescence at the excitation wavelength of 675 nm, it was deduced that NPs were successfully enriched in the needle tip. Such DMNs are conducive to delivering drugs to the dermis and avoiding waste of drug residues on the skin surface.
Figure 5.
Characterization of HD10 NPs loaded DMNs: (A) bright-field photograph under a stereo fluorescence microscope, (B) CLSM image, (C) H&E-stained skin histologic section to evaluate skin insertion ability, (D) near-infrared thermal imaging and (E) the maximum temperature change curves of blank DMNs and HD10 NPs loaded DMNs under LS to assess the in vitro photothermal conversion efficiency.
The Hematoxylin & Eosin (H&E) staining of the punctured skin (Fig. 5C), revealed that obvious microchannels were created in the skin with DMNs application, and the insertion depth was 250–350 μm, available to reach the dermis for efficient drug delivery. Therefore, the produced DMNs exhibited good skin penetration ability. Besides, the far shorter insertion depth than the height of DMNs could be ascribed to two aspects57: (1) the inserted skin could shrink due to its elasticity, and (2) the needle tips adsorbed physiological fluid and dissolved quickly (Supporting Information Fig. S6).
The temperature increase of DMNs during LS (Fig. 5D and E) was used to evaluate their photothermal conversion capacity. The blank DMNs showed a slightly increased temperature during LS, with a maximum temperature increase of 2.8 °C, indicating that the preparation material of DMNs basically did not exert heating capacity. However, the temperature of HD10 NPs containing DMNs increased rapidly to 94.7 °C within 1 min after LS, and the temperature could be maintained above 90 °C for 4 min. These results indicate that DMNs loaded with NPs have excellent photothermal conversion efficiency, which further benefits to ensure the heating efficiency in vivo. Furthermore, the superiority of DMNs to HD10 NPs solution in heating efficacy implies that the enrichment of in the needle offered a feasible way to improve the photothermal conversion efficiency of ICG. The reason for this could be the soild-state of the NPs loaded in the DMNs, which benefits by increasing the photostability and local concentration of ICG than that in the solution state.
3.4. In vivo study of NPs and DMNs
3.4.1. In vivo photothermal effect
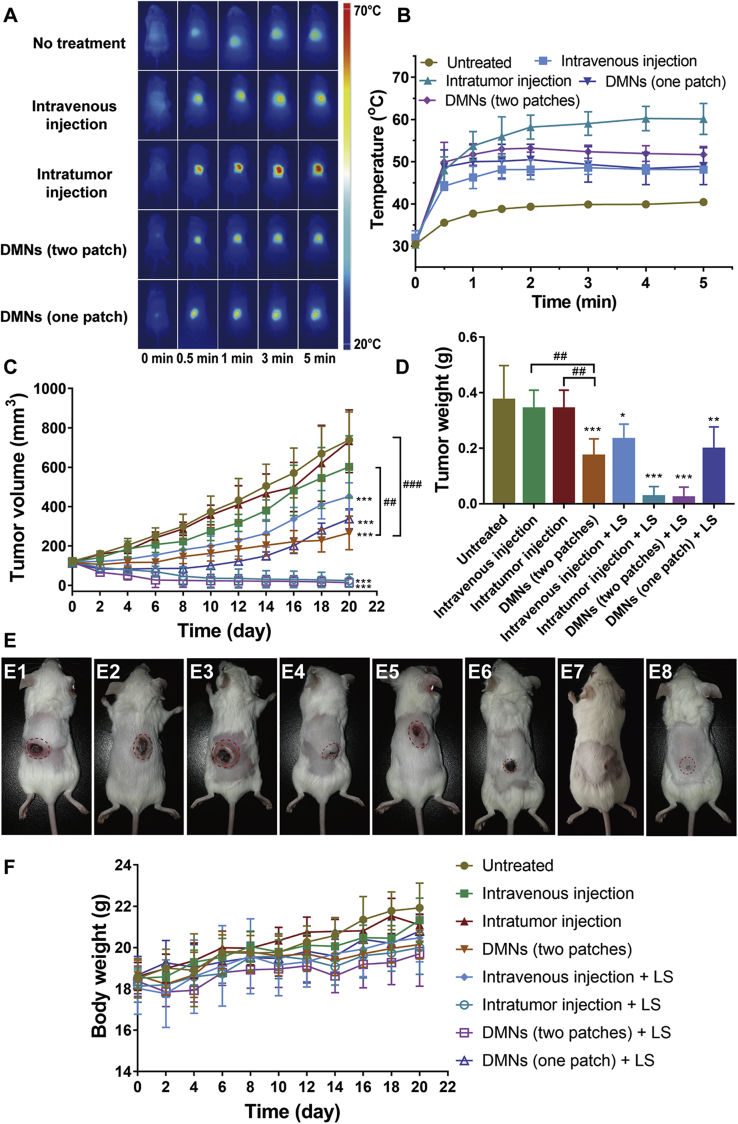
For a guarantee in vivo anti-tumor effect, an effective increase in temperature is essential. As shown in Fig. 6A and B, all groups showed the increased temperature in the local tumor, with the order of maximum temperature as intratumoral injection group > DMNs group (two patches) > DMNs (one patch) > caudal vein injection > untreated group, corresponding to an average temperature of 60.6, 53.7, 50.5, 48.9, and 40.4 °C. Concurrently, the temperature of untreated mice during LS did not exceed 41 °C, which was insufficient to promote tumor cell apoptosis for effective PTT. The temperature of both intratumoral injection and DMNs group exceeded the threshold (above 50 °C) required to cause irreversible damage to the tumor58, while the higher temperature achieved in the intratumoral injection group could result from a higher dose of ICG (5.4-fold to the single DMNS patch). Nevertheless, DMNs exhibited an excellent heating efficiency, as one DMN patch contained only 4.1 μg of ICG. In the caudal vein injection group, the temperature was elevated up to 45–50 °C, which could produce certain damage to tumor cells and risk of tumor recurrence35, as well as a successful accumulation of ICG in the tumor (Supporting Information Fig. S7). The inferior heating efficiency of caudal vein injection to DMNs maybe because the NPs through caudal vein injection could distribute throughout the whole body and dramatically diminish the ICG concentration before reaching the tumor tissue, while DMN is an in situ administration route that allows the NPs with condensed ICG to be released locally for highly efficient heat generation.
Figure 6.
Anti-tumor effect of HD10 NPs delivered with different administration routes. (A) In vivo near-infrared thermal imaging of mice receiving LS, (B) the temperature change curves of local tumors, (C) the tumor volume growth curve of 4T1 tumor-bearing mice within 20 days, (D) the tumor weight of mice on Day 20 post-administration, (E) the photographic images of the mice on Day 20 post-administration, which were successively designated as untreated group (E1), intravenous injection (E2), intratumor injection (E3), DMNs (two patches) (E4), intravenous injection + LS (E5), intratumor injection + LS (E6), DMNs (two patches)+LS (E7), and DMNs (one patch)+LS (E8), and (F) the change in body weight of 4T1 tumor-bearing mice within 20 days. Data are presented as mean ± SD for A (n = 3), B, C and E (n = 5). ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. the untreated group. ##P < 0.001 and ###P < 0.001 indicates the pairwise comparision of the intravenous injection, the intratumor injection and the DMNs, when chemotherapy alone was conducted.
3.4.2. In vivo anti-tumor effect
The therapeutic efficacy of HD10 NPs via different administration routes was compared and assessed on the 4T1-tumor-bearing mice. The tumor volume growth profile and tumor weight at the endpoint of the experiment are displayed in Fig. 6C and D, respectively. Compared with the untreated group, intratumorally injected HD10 NPs showed no anti-cancer effect because only one dosing was given, and most of the drug could leak into the surrounding subcutaneous tissue. Injection of HD10 NPs via caudal vein had a minimal inhibitory effect on tumor growth, which may be because the NPs enriched in the tumor tissue exerted certain tumor-suppressing effects in the early stage. This was consistent with earlier reports that the chemo-monotherapy by PTX is insensitive toward TNBC, and frequent injections are required to achieve a considerable anti-tumor effect59,60. Additionally, HD10 NPs delivered through DMNs significantly lowered the tumor growth rate, resulting in tumors of the average volume of 266 mm3 on Day 20. Overall, the capacity of different administration routes to suppress tumor growth followed the order of DMNs > tail vein injection > intratumoral injection. The greatest anti-cancer efficacy of DMNs could be the uniform and broad dispersion of PTX in the tumor tissue to maximize its anti-tumor effect without wastage due to drug leakage.
When chemotherapy and PTT were simultaneously administered, the tumor growth inhibition rate was further strengthened irrespective of the administration route. PTT enhanced the effect of chemotherapy by producing hyperthermia and destroying cancer cells. More importantly, most of the mice in the DMNs (two patches) group and the intratumoral injection group were completely ablated, mainly due to effectively localized PTT. However, we should bear in mind that the efficacy of PTT was also affected by dosage, as the single DMN patch group could effectively prohibit tumor growth in the early stage, but tumor regrew continuously after the 10th day, probably due to insufficient damage to cancer cells. Furthermore, in the mice receiving an intratumoral injection, skin tissue around tumor did not recover to normal and exhibited black scar (Fig. 6E), while, the skin (Fig. 6E) of mice treated by DMNs almost completely healed, suggesting that PTT through DMNs was a safe modality with good control over elevated temperature at a low-dose ICG (Supporting Information Fig. S8).
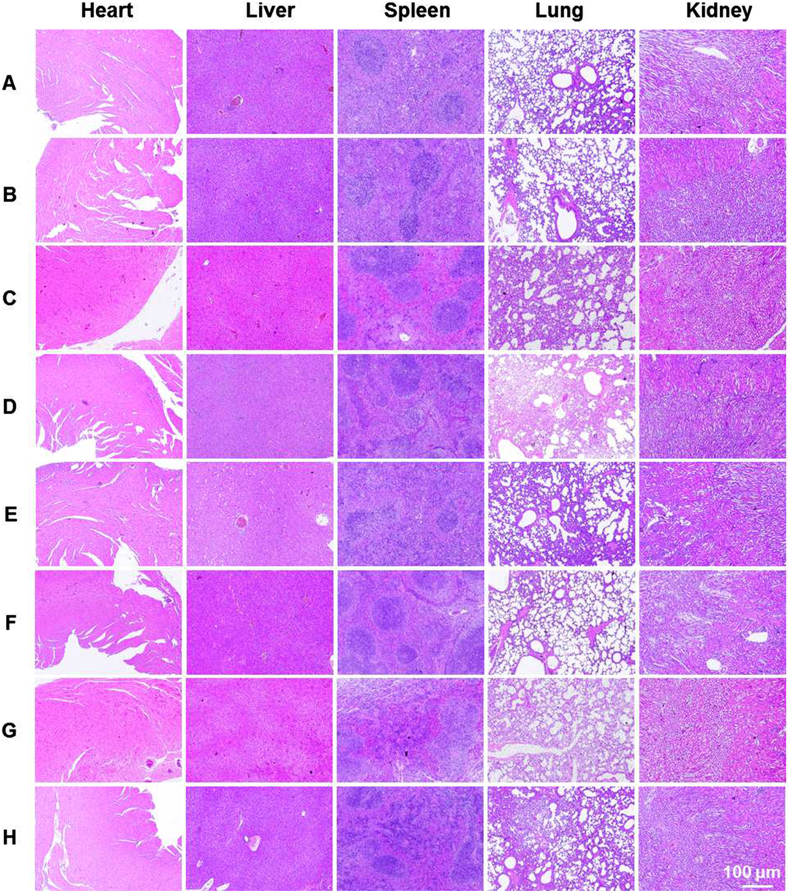
The change in mice body weight is a usual indicator to evaluate the safety of formulations. In general, no group showed any noticeable weight loss, albeit, an increasing trend in weight (Fig. 6F), implying that these treatments caused no damage to the mice. Additionally, the H&E staining of various groups (Fig. 7) showed similar results with no lesion and inflammation, which further demonstrated the safety of different treatments.
Figure 7.
The H&E staining images of major organs extracted from the mice receiving different treatments: (A) untreated, (B) intravenous injection, (C) intratumor injection, (D) DMNs (two patches), (E) intravenous injection + LS, (F) intratumor injection + LS, (G) DMNs (two patches)+LS, and (H) DMNs (one patch)+LS.
4. Conclusions
To conclude, HD10 NPs significantly enhanced the cytotoxicity of low-dose PTX by increasing the cellular uptake of NPs and inducing apoptosis via the mitochondria. In addition, the combination of chemotherapy and PTT augmented ROS production to facilitate necrosis and the activity of apoptosis-related proteins to induce apoptosis. The HD10 NPs delivered with DMNs in a single administration provided a high efficacy and safe local treatment to the 4T1-bearing tumors. Taken together, the composite DMNs with chemo-photothermal integrated therapy showed favorable anti-cancer effect and great potential in cancer treatment by taking advantage of both NPs and DMNs.
Acknowledgments
The work was supported by the Fundamental Research Funds for the Central Universities (21620356, China), the Research and Development Plan for Key Areas in Guangdong Province (2019B020204002, China), and the National Natural Science Foundation (81803466, China).
Author contributions
Tingting Peng designed the research, performed the experiments, and wrote the manuscript. Yao Huang and Xiaoqian Feng participated part of the experiments. Chune Zhu, Yin Shi, Xinyi Wang, and Xuequn Bai provided data analysis. Xin Pan and Chuanbin Wu revised the manuscript. All authors have read and approved the final version of the article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.11.013.
Contributor Information
Xin Pan, Email: panxin2@mail.sysu.edu.cn.
Chuanbin Wu, Email: wuchuanb@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pandita D., Kumar S., Lather V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: design and delivery prospectives. Drug Discov Today. 2015;20:95–104. doi: 10.1016/j.drudis.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Bose R.J., Lee S.H., Park H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater Res. 2016;20:34. doi: 10.1186/s40824-016-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Bose R.J., Ahn J.C., Yoshie A., Park S., Park H., Lee S.H. Preparation of cationic lipid layered PLGA hybrid nanoparticles for gene delivery. J Control Release. 2015;213:92–93. doi: 10.1016/j.jconrel.2015.05.154. [DOI] [PubMed] [Google Scholar]
- 5.Zheng M., Yue C., Ma Y., Gong P., Zhao P., Zheng C. Single-step assembly of DOX/ICG loaded lipid–polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7:2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y., Sadoqi M., Shao J. Biodistribution of indocyanine green-loaded nanoparticles with surface modifications of PEG and folic acid. Int J Pharm. 2012;436:25–31. doi: 10.1016/j.ijpharm.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Rafiei P., Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomed. 2017;12:935–947. doi: 10.2147/IJN.S121881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao P., Zheng M., Yue C., Luo Z., Gong P., Gao G. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014;35:6037–6046. doi: 10.1016/j.biomaterials.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Wang G., Yu B., Wu Y., Huang B., Yuan Y., Liu C.S. Controlled preparation and antitumor efficacy of vitamin E TPGS-functionalized PLGA nanoparticles for delivery of paclitaxel. Int J Pharm. 2013;446:24–33. doi: 10.1016/j.ijpharm.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y., Yu B., Wang G., Wu Y., Zhang X., Chen Y. Enhanced antitumor efficacy of vitamin E TPGS-emulsified PLGA nanoparticles for delivery of paclitaxel. Colloids Surf B. 2014;123:716–723. doi: 10.1016/j.colsurfb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y.Y., Xiong X.Y., Cheng F., Gong Y.C., Li Z.L., Li Y.P. The targeting properties of folate-conjugated Pluronic F127/poly (lactic-co-glycolic) nanoparticles. Int J Biol Macromol. 2017;105:711–719. doi: 10.1016/j.ijbiomac.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 12.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Nat Acad Sc. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang R.H., Hu C.M., Luk B.T., Gao W., Copp J.A., Tai Y. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo R., Peng H., Tian Y., Shen S., Yang W. Mitochondria-targeting magnetic composite nanoparticles for enhanced phototherapy of cancer. Small. 2016;12:4541–4552. doi: 10.1002/smll.201601094. [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Lei Q., Qiu W.X., Liu L.H., Zheng D.W., Fan J.X. Mitochondria-targeting "nanoheater" for enhanced photothermal/chemo-therapy. Biomaterials. 2017;117:92–104. doi: 10.1016/j.biomaterials.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Souris J.S., Lee C.H., Cheng S.H., Chen C.T., Yang C.S., Ho J.A. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31:5564–5574. doi: 10.1016/j.biomaterials.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang T., Mo R., Bellotti A., Zhou J., Gu Z. Gel–liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv Funct Mater. 2014;24:2295–2304. [Google Scholar]
- 19.Luo Z., Dai Y., Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;9:1099–1112. doi: 10.1016/j.apsb.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R., Hu C., Yang Y., Zhang J., Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B. 2019;9:410–420. doi: 10.1016/j.apsb.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M.C., Lin Z.W., Ling M.H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10:93–101. doi: 10.1021/acsnano.5b05043. [DOI] [PubMed] [Google Scholar]
- 22.Dong L., Li Y., Li Z., Xu N., Liu P., Du H. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl Mater Interfaces. 2018;10:9247–9256. doi: 10.1021/acsami.7b18293. [DOI] [PubMed] [Google Scholar]
- 23.Lan X., She J., Lin D., Xu Y., Li X., Yang W. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl Mater Interfaces. 2018;10:33060–33069. doi: 10.1021/acsami.8b12926. [DOI] [PubMed] [Google Scholar]
- 24.Pei P., Yang F., Liu J., Hu H., Du X., Hanagata N. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater Sci. 2018;6:1414–1423. doi: 10.1039/c8bm00005k. [DOI] [PubMed] [Google Scholar]
- 25.Yang P., Lu C., Qin W., Chen M., Quan G., Liu H. Construction of a core–shell microneedle system to achieve targeted co-delivery of checkpoint inhibitors for melanoma immunotherapy. Acta Biomater. 2020;104:147–157. doi: 10.1016/j.actbio.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Lee I.C., He J.S., Tsai M.T., Lin K.C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J Math Chem B. 2015;3:276–285. doi: 10.1039/c4tb01555j. [DOI] [PubMed] [Google Scholar]
- 27.Peng T., Huang Y., Feng X., Zhu C., Ma X., Wang X. Dissolving microneedles loading TPGS biphasic functionalized PLGA nanoparticles for efficient chemo-photothermal combined therapy of melanoma. Adv Ther. 2020;3:1900190. [Google Scholar]
- 28.Zheng C., Zheng M., Gong P., Jia D., Zhang P., Shi B. Indocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imaging. Biomaterials. 2012;33:5603–5609. doi: 10.1016/j.biomaterials.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y., Jiang K., Shen Z., Zheng G., Fan L., Zhao R. A small molecule nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-infrared cancer theranostics. ACS Appl Mater Interfaces. 2017;9:43508–43519. doi: 10.1021/acsami.7b14755. [DOI] [PubMed] [Google Scholar]
- 30.Hu H., Xiao C. Nanocolloidosomes with selective drug release for active tumor-targeted imaging-guided photothermal/chemo combination therapy. ACS Appl Mater Interfaces. 2017;9:42225–42238. doi: 10.1021/acsami.7b14796. [DOI] [PubMed] [Google Scholar]
- 31.Shen X., Li T., Chen Z., Xie X., Zhang H., Feng Y. NIR-light-triggered anticancer strategy for dual-modality imaging-guided combination therapy via a bioinspired hybrid PLGA nanoplatform. Mol Pharm. 2019;16:1367–1384. doi: 10.1021/acs.molpharmaceut.8b01321. [DOI] [PubMed] [Google Scholar]
- 32.Saxena V., Sadoqi M., Shao J. Degradation kinetics of indocyanine green in aqueous solution. J Pharmacol Sci. 2003;92:2090–2097. doi: 10.1002/jps.10470. [DOI] [PubMed] [Google Scholar]
- 33.Wan Z., Mao H., Guo M., Li Y., Zhu A., Yang H. Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen-synergized chemotherapy for cancer eradication. Theranostics. 2014;4:399–411. doi: 10.7150/thno.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M., Zhao P., Luo Z., Gong P., Zheng C., Zhang P. Robust ICG theranostic nanoparticles for folate targeted cancer imaging and highly effective photothermal therapy. ACS Appl Mater Interfaces. 2014;6:6709–6716. doi: 10.1021/am5004393. [DOI] [PubMed] [Google Scholar]
- 35.Yan F., Wu H., Liu H., Deng Z., Liu H., Duan W. Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles. J Control Release. 2016;224:217–228. doi: 10.1016/j.jconrel.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 36.Li W., Wang X., Wang J., Guo Y., Lu S.Y., Li C.M. Enhanced photoacoustic and photothermal effect of functionalized polypyrrole nanoparticles for near-infrared theranostic treatment of tumor. Biomacromolecules. 2019;20:401–411. doi: 10.1021/acs.biomac.8b01453. [DOI] [PubMed] [Google Scholar]
- 37.Bershteyn A., Chaparro J., Yau R., Kim M., Reinherz E., Ferreira-Moita L. Polymer-supported lipid shells, onions, and flowers. Soft Matter. 2008;4:1787–1791. doi: 10.1039/b804933e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan J.M., Zhang L., Yuet K.P., Liao G., Rhee J.W., Langer R. PLGA-lecithin-PEG core–shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly(lactic acid)-co-(glycolic acid) Polym Int. 2005;54:36–46. [Google Scholar]
- 40.Assanhou A.G., Li W., Zhang L., Xue L., Kong L., Sun H. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015;73:284–295. doi: 10.1016/j.biomaterials.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Bao Y., Yin M., Hu X., Zhuang X., Sun Y., Guo Y. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J Control Release. 2016;235:182–194. doi: 10.1016/j.jconrel.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Neuzil J., Dong L.F., Ramanathapuram L., Hahn T., Chladova M., Wang X.F. Vitamin E analogues as a novel group of mitocans: anti-cancer agents that act by targeting mitochondria. Mol Aspect Med. 2007;28:607–645. doi: 10.1016/j.mam.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Neophytou C.M., Constantinou C., Papageorgis P., Constantinou A.I. D-Alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in survivin-overexpressing breast cancer cells. Biochem Pharmacol. 2014;89:31–42. doi: 10.1016/j.bcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Cerqueira B.B.S., Lasham A., Shelling A.N., Al-Kassas R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mat Sci Eng C-Mater. 2017;76:593–600. doi: 10.1016/j.msec.2017.03.121. [DOI] [PubMed] [Google Scholar]
- 45.Wang R., Han Y., Sun B., Zhao Z., Opoku-Damoah Y., Cheng H. Deep tumor penetrating bioparticulates inspired burst intracellular drug release for precision chemo-phototherapy. Small. 2018;14 doi: 10.1002/smll.201703110. [DOI] [PubMed] [Google Scholar]
- 46.Mundra V., Peng Y., Rana S., Natarajan A., Mahato R.I. Micellar formulation of indocyanine green for phototherapy of melanoma. J Control Release. 2015;220:130–140. doi: 10.1016/j.jconrel.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Wang G., Zhang F., Tian R., Zhang L., Fu G., Yang L. Nanotubes-embedded indocyanine green-hyaluronic acid nanoparticles for photoacoustic-imaging-guided phototherapy. ACS Appl Mater Interfaces. 2016;8:5608–5617. doi: 10.1021/acsami.5b12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Modica-Napolitano J.S., Weissig V. Treatment strategies that enhance the efficacy and selectivity of mitochondria-targeted anticancer agents. Int J Mol Sci. 2015;16:17394–17421. doi: 10.3390/ijms160817394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J., Zhao W.Y., Ma X., Ju R.J., Li X.Y., Li N. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34:3626–3638. doi: 10.1016/j.biomaterials.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 50.Liang D., Wang A.T., Yang Z.Z., Liu Y.J., Qi X.R. Enhance cancer cell recognition and overcome drug resistance using hyaluronic acid and alpha-tocopheryl succinate based multifunctional nanoparticles. Mol Pharm. 2015;12:2189–2202. doi: 10.1021/acs.molpharmaceut.5b00129. [DOI] [PubMed] [Google Scholar]
- 51.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 52.Xu P., Yin Q., Shen J., Chen L., Yu H., Zhang Z. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int J Pharm. 2013;454:21–30. doi: 10.1016/j.ijpharm.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 53.Smit M.A., Geiger T.R., Song J.Y., Gitelman I., Peeper D.S. A twist-snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun X.J., Jin Y., Wang H., Feng N., Li Z.H., Liu D.D. A NIR-light activated nanoplatform for sensitizing triple negative breast cancer against therapeutic resistance to enhance the treatment effect. J Math Chem B. 2018;6:6950–6956. doi: 10.1039/c8tb01723a. [DOI] [PubMed] [Google Scholar]
- 55.Xu G.C., Zhang P., Leng F., Pan L., Li Z.Y., Yu D.D. Inhibition of lymphatic metastases by a survivin dominant-negative mutant. Oncol Res. 2012;20:579–587. doi: 10.3727/096504013X13775486749416. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Wu Y., Zhao R., Nie G. Engineering the assemblies of biomaterial nanocarriers for delivery of multiple theranostic agents with enhanced antitumor efficacy. Adv Mater. 2013;25:1616–1622. doi: 10.1002/adma.201204750. [DOI] [PubMed] [Google Scholar]
- 57.Zhao X., Li X., Zhang P., Du J., Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J Control Release. 2018;286:201–209. doi: 10.1016/j.jconrel.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 58.Chu K.F., Dupuy D.E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 59.Yan F., Li L., Deng Z., Jin Q., Chen J., Yang W. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J Control Release. 2013;166:246–255. doi: 10.1016/j.jconrel.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Wang Y., Yang Y., Liu Y., Ruan S., Zhang Q. High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in creast cancer. ACS Appl Mater Interfaces. 2015;7:9691–9701. doi: 10.1021/acsami.5b01473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.