ABSTRACT
This study examined the humoral and cellular response of cattle vaccinated with two commercial leptospiral vaccines, Leptavoid and Spirovac, and a novel bacterin vaccine using Seppic Montanide oil emulsion adjuvant. Vaccination was followed by experimental challenge. All vaccinated cattle were protected from colonization of the kidney and shedding of Leptospira in urine, as detected by culture and immunofluorescence assay. Agglutinating antibody titers were detected in vaccinated cattle at 4 weeks following vaccination, with small anamnestic response detected following experimental challenge. Only animals vaccinated with the oil emulsion-adjuvanted bacterin produced significant IgG2 titers following vaccination, and nonvaccinated animals produced serum IgA titers after experimental challenge. CD4+ and γδ T cells from vaccinated cattle proliferated when cultured with antigen ex vivo. Cellular responses included a marked proliferation of γδ T cells immediately following experimental challenge in vaccinated cattle and release of gamma interferon (IFN-γ), interleukin 17a (IL-17a), and IL-12p40 from stimulated cells. Proliferative and cytokine responses were found not just in peripheral mononuclear cells but also in lymphocytes isolated from renal lymph nodes at 10 weeks following experimental challenge. Overall, effects of leptospirosis vaccination and infection were subtle, resulting in only modest activation of CD4+ and γδ T cells. The use of Seppic Montanide oil emulsion adjuvants may shorten the initiation of response to vaccination, which could be useful during outbreaks or in areas where leptospirosis is endemic.
IMPORTANCE Leptospirosis is an underdiagnosed, underreported zoonotic disease of which domestic livestock can be carriers. As a reservoir host for Leptospira borgpetersenii serovar Hardjo, cattle may present with reproductive issues, including abortion, birth of weak or infected calves, or failure to breed. Despite years of study and the availability of commercial vaccines, detailed analysis of the bovine immune response to vaccination and Leptospira challenge is lacking. This study evaluated immunologic responses to two efficacious commercial vaccines and a novel bacterin vaccine using an adjuvant chosen for enhanced cellular immune responses. Antigen-specific responsive CD4 and γδ T cells were detected following vaccination and were associated with release of inflammatory cytokines IFN-γ and IL-17a after stimulation. CD4 and γδ cells increased in the first week after infection and, combined with serum antibody, may play a role in clearance of bacteria from the blood and resident tissues. Additionally, these antigen-reactive T cells were found in the regional lymph nodes following infection, indicating that memory responses may not be circulating but are still present in regional lymph nodes. The information gained in this study expands knowledge of bovine immune response to leptospirosis vaccines and infection. The use of oil emulsion adjuvants may enhance early immune responses to leptospiral bacterins, which could be useful in outbreaks or situations where leptospirosis is endemic.
KEYWORDS: Leptospira, vaccine, cattle, immune response, adjuvant, adjuvants, vaccines, veterinary vaccine development
INTRODUCTION
The true prevalence of leptospirosis in humans and domestic livestock is unknown. While a reportable disease in human health in many countries (including the United States) (https://wwwn.cdc.gov/nndss/), lack of appropriate diagnostics, lack of distinguishing characteristics from other endemic tropical diseases, lack of knowledge, and lack of infrastructure all contribute to underreporting of human infection (1). In livestock, leptospirosis outbreaks are not required to be reported to the World Organization for Animal Health (OIE), and thus the prevalence is unknown in most areas. There are serovars that are commonly associated with specific mammals, and asymptomatic chronic carriage of various serovars is common in wild or domestic animals. During disruptions in public services or during natural disasters (high rainfall or flooding) are commonly when marked increase in human cases occur; mainly from spillover from animal reservoirs. Leptospirosis should be addressed using the One Health concept, as it is a global zoonotic disease, impacted by climate change, and has many of the other hallmarks of neglected tropical diseases.
While often asymptomatic in reservoir hosts, leptospirosis is a leading cause of reproductive failure in cattle worldwide. Leptospirosis can significantly impact cattle production by decreasing milk production and causing reproductive failures (infertility, abortion, stillbirths, and the birth of weak calves). With few other clinical signs, cattle are a chronic host of serologically identical but genetically distinct members of serovar Hardjo, namely Leptospira borgpetersenii serovar Hardjo (type Hardjobovis), and Leptospira interrogans serovar Hardjo (type Hardjoprajitno) (2). Cattle are the major reservoir of these agents, which potentially infect humans and other animals, in which this serovar can cause acute disease (3). In North America, L. borgpetersenii serovar Hardjo type Hardjobovis is most commonly isolated from cattle (4), whereas in other regions, serovar Hardjoprajitno is more common (5). In comparison, a serologic study in India indicated that serovars Australis, Ballum, Hardjo, Hebdomadis, and Pomona were all found in cattle and buffaloes (Bubalus bubalis) (6). The L. interrogans serovar Pomona, usually associated with pigs (domestic and wild [Sus scrofa]) can also be found in cattle, resulting in acute kidney disease and abortion (6). Serovar Pomona can also be transmitted from cattle to dogs and humans, which occurred in an outbreak in Israel (7). Overall data does not suggest that long-term implementation of vaccination has shifted serovars, as can occur in human pneumococcal disease (8). For example, the dominant serovars in an outbreak in Australia in 1996 were the same as those observed in the 1960s, namely Hardjo, Tarassovi, Pomona, and Szwajizak, despite the widespread use of Hardjo and Pomona vaccines (9). Vaccination is a proven method for interrupting livestock-human transmission, and they can also reduce the impact of leptospirosis for improving animal production. In Brazil, vaccinating cattle for Leptospira spp. prior to breeding significantly increased conception rates and pregnancy retention (10).
Since the 1950s, various bacterins have been used in numerous studies to vaccinate cattle against leptospirosis. Most studies used killed bacterins of one or more serovars combined with an adjuvant, usually aluminum hydroxide, and measures immune responses by only monitoring antibody using a microscopic agglutination test (MAT) or enzyme-linked immunosorbent assay (ELISA). Several studies evaluated cellular proliferative responses and measured cytokine responses after stimulation, indicating cellular response, and most studies did not characterize cell populations responding after vaccination. One study found that γδ T cells with WC1 receptors were the dominant cell type after vaccination with a monovalent leptospirosis bacterin and produced high levels of gamma interferon (IFN-γ) (11). However, this work evaluated leptospirosis vaccination, but not responses after an experimental challenge, and it did not specifically evaluate the characteristics of a protective immunologic response. Use of recently developed bovine-specific immunologic reagents will afford understanding of the reservoir host response and allow development of improved vaccine formulations and performance.
The objective of the current study was to characterize immune responses of cattle to vaccination with commercial Leptospira vaccines or with a novel bacterin combined with a novel adjuvant or after experimental challenge. An overall schematic of the experimental timeline is given in Fig. 1. Twenty-four cattle were allotted into four groups of six cattle each, with each group receiving one of the vaccines (Spirovac, Leptavoid, or Seppic-adjuvanted bacterin) or adjuvants only (alum and Seppic). After 32 weeks, cattle were challenged with Leptospira borgpetersenii serovar Hardjo strain 203. Immune response during the vaccine phase and the challenge phase was analyzed.
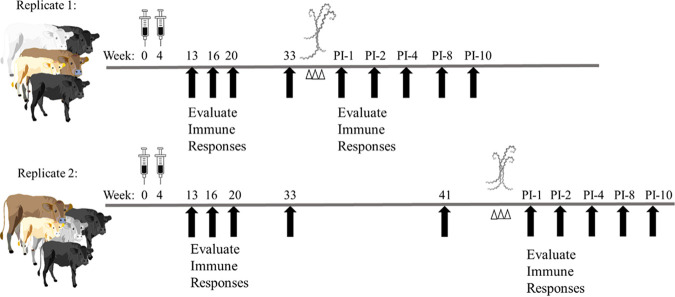
FIG 1.
Depiction of experimental timeline with vaccination, challenge (Δ), and blood collection for immune response evaluation (↑, black arrows) marked at approximate weeks after vaccination or experimental/infectious challenge (postinfection [PI]). A total of 24 crossbred heifers were assigned to 4 groups, vaccine or nonvaccinated control group, with 6 cattle per group. Due to space constraints in the agricultural biosafety level 2 (Ag-BSL-2) containment facility, half of the cattle were challenged in replicate 1 and half of the cattle were challenged in replicate 2.
RESULTS
Leptospiral shedding and histologic lesions after experimental challenge.
All cattle in the control treatment shed Leptospira in their urine based on culture and immunofluorescence assay (IFA) testing (Table 1). In 4 of 6 controls, Leptospira were recovered by culture from urine or kidney homogenates, indicating shedding of live bacteria. For control animals from which live bacteria were not recovered, one animal was IFA positive for 2 weeks early in the infection, and the second animal was IFA positive in urine for multiple weeks and was kidney IFA positive at necropsy. No animal in any of the vaccine groups was culture positive or IFA positive in any urine or kidney samples.
TABLE 1.
Presence of Leptospira in urine and kidneys as determined by culture and specific immunofluorescence antibody labeling
| Exptl group | Shedding in urine (no. positive/total)a |
Kidneyb (no. positive/total)a |
||
|---|---|---|---|---|
| Positive culture (2 or more wks) | IFA positive (2 or more wks) | Positive culture | IFA positive | |
| Adjuvant only/infected | 4/6 | 6/6 | 4/6 | 5/6 |
| Spirovac | 0/6 | 0/6 | 0/6 | 0/6 |
| Leptavoid | 0/6 | 0/6 | 0/6 | 0/6 |
| Seppic bacterin | 0/6 | 0/6 | 0/6 | 0/6 |
Number of cattle positive/total number of cattle in group.
Kidney was assayed at necropsy, 10 weeks postchallenge.
Serum antibody responses after vaccination and experimental challenge.
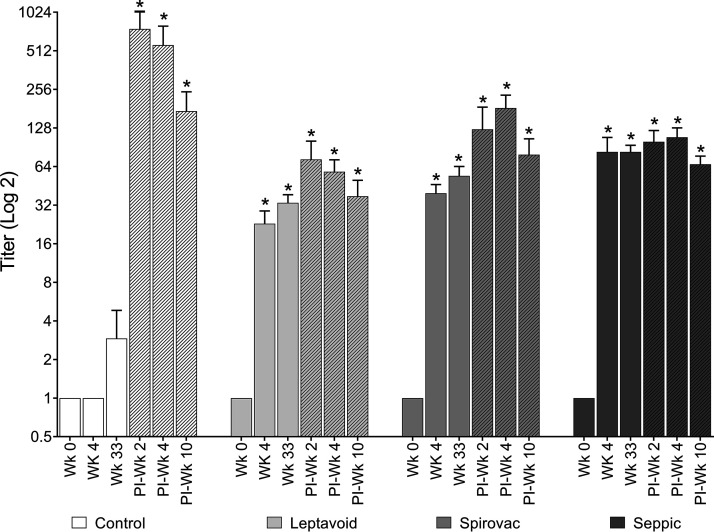
Microscopic agglutination test (MAT) titers against Leptospira in the three vaccination groups were greater (P < 0.05) at 4 and 33 weeks after vaccination compared to responses prior to vaccination (week 0) (Fig. 2). After experimental challenge, cattle in the control treatment had increased (P < 0.05) MAT titers, but titers did not differ between samples obtained at 2, 4, or 10 weeks. In comparison, MAT titers in cattle in all 3 vaccination groups did increase post-experimental challenge compared to responses of samples obtained prior to challenge at 33 weeks postvaccination.
FIG 2.
Microscopic agglutinating test. Serum antibody titer to strain 203 was measured at the given time points postvaccination and postinfectious challenge (PI, shaded bars). Group means (n = 6) + standard error of the mean (SEM). An asterisk (*) indicates statistical difference from week 0 within vaccine group and statistical difference between vaccine groups and control group within a time point (week); P ≤ 0.05.
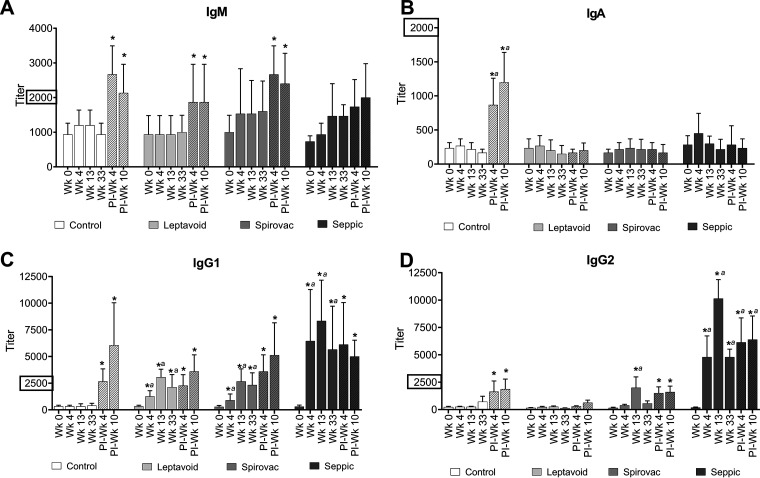
The control, Leptavoid, and Spirovac groups demonstrated increased (P < 0.05) IgM ELISA responses at 4 and/or 10 weeks after experimental challenge compared to serum obtained prior to vaccination (Fig. 3). The control treatment was the only group that demonstrated an increase (P < 0.05) in IgA at 4 and 10 weeks postchallenge. All three vaccine groups (Leptavoid, Spirovac, and Seppic) showed increases in IgG1 titers (P < 0.05) following vaccination in comparison to week 0 and to the control group for weeks 4, 13, and 33 (Fig. 3). All groups, including the control group, showed increases in IgG1 titer following experimental challenge (postinfection [PI] weeks 4 and 10). The Spirovac group demonstrated an IgG2 response postvaccination at week 13. The Seppic treatment group demonstrated more robust IgG2 responses (P < 0.05) at 4, 13, and 33 weeks postvaccination. Control, Spirovac, and Seppic groups all had increases in IgG2 titers following experimental challenge (P < 0.05).
FIG 3.
Serum antibody enzyme-linked immunosorbent assay (ELISA) for IgM, IgA, IgG1, and IgG2 isotypes binding to plate-bound serovar Hardjo 203 whole-cell sonicate antigen at weeks 0, 4, 13, and 33 postvaccination and postinfection (PI) weeks 4 and 10 (shaded bars). Mean (n = 6) + SEM. An asterisk (*) indicates statistical difference from week 0 within vaccine group; a indicates statistical difference between control and vaccine groups within time point (week); P ≤ 0.05. (A, B, C, and D) y axis scales are not all equivalent.
Expression of surface markers on PBMCs after vaccination and experimental challenge.
No differences were detected in total percentage of lymphocytes or T-cell surface markers in unstimulated peripheral blood mononuclear cells (PBMCs) isolated from vaccinated or control cattle (see Fig. S2 in the supplemental material). There was an increase in B cells (CD21+ cells) (P < 0.05) at week 1 following experimental challenge for the vaccinated groups and at weeks 4 and 10 for the control groups. Furthermore, PBMCs from vaccinated cattle were found to have increased expression of CD45RO+ on γδ T-cell receptor (TCR) lymphocytes 1 week after experimental challenge (see Fig. S3 in the supplemental material), whereas in control cattle, an increase in CD45RO+ on γδ TCR lymphocytes was not observed until weeks 4 and 8 postchallenge.
Peripheral blood mononuclear cell (PBMC) phenotype. Peripheral blood mononuclear cells isolated from vaccinated and infected cattle at 16 and 33 weeks postvaccination and 1, 4, and 8 weeks (shaded bars) post-experimental challenge. Minimum of 2,000 uncultured, unstimulated cells were analyzed for phenotype (CD4+, CD8+, γδ TCR+, and CD21+) per sample. Mean (n = 6) + SEM; *, statistical difference from other time points within the vaccine group; P ≤ 0.05. Download FIG S2, TIF file, 1.6 MB (1.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Activation state of uncultured PBMCs. Peripheral blood mononuclear cells isolated from vaccinated and infected cattle at 16 and 33 weeks postvaccination and 1, 4, and 8 weeks (shaded bars) post-experimental challenge. Minimum of 2,000 uncultured, unstimulated cells were analyzed for phenotype (CD4, CD8, and γδ TCR) and expression of activation marker (CD25, and CD45RO) by flow cytometry. Mean (n = 6) + SEM; #, statistical difference between vaccine groups and the control group within a time point (week); *, statistical difference from prechallenge time points (weeks 16 and 33) within the vaccine group (P ≤ 0.05); a indicates statistical difference of vaccinated group from control group at that time point (week 1 postinfection [PI]) (P ≤ 0.05). Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
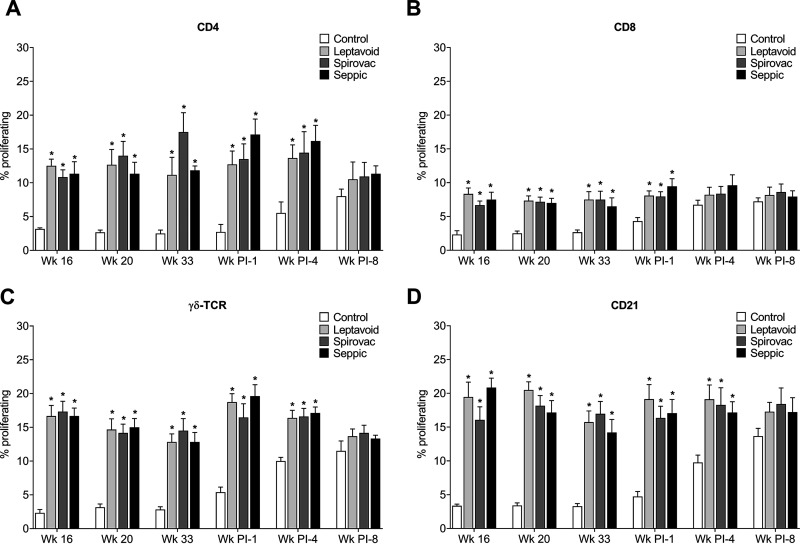
PBMCs were isolated from vaccinated and control cattle, stimulated in vitro with L. borgpetersenii strain 203 antigens, and analyzed for phenotype and proliferation. Cells from cattle in the vaccine groups had increased proportions of proliferating CD4+, CD8+, γδ TCR+, and CD21+ cells when stimulated with antigen at 16, 20, and 33 weeks following vaccination and following 1 and 4 weeks following experimental challenge (Fig. 4). At 8 weeks following challenge, the control group had an increased proportion of proliferating cells, statistically equivalent to the vaccine groups. Values for nonstimulated wells were between 0.5 and 3%, with no difference between vaccine group, time points, and cell types (data not shown). Values for concanavalin A (ConA; mitogen)-stimulated wells were between 30 and 80% depending on cell type, with no difference between vaccine groups or time points (data not shown).
FIG 4.
Proliferation of in vitro-stimulated peripheral blood mononuclear cells (PBMCs). Percentage of cellular phenotype proliferating in response to 203 antigen for (A) CD4-expressing population, (B) CD8-expressing population, (C) γδ T-cell receptor (γδ TCR)-expressing population, and (D) B cell- or CD21-expressing population. Mean (n = 6) + SEM; an asterisk (*) indicates a significant difference (P < 0.05) between control and vaccine groups within a time point (week).
Cytokine expression in PBMCs after in vitro stimulation with Leptospira antigens.
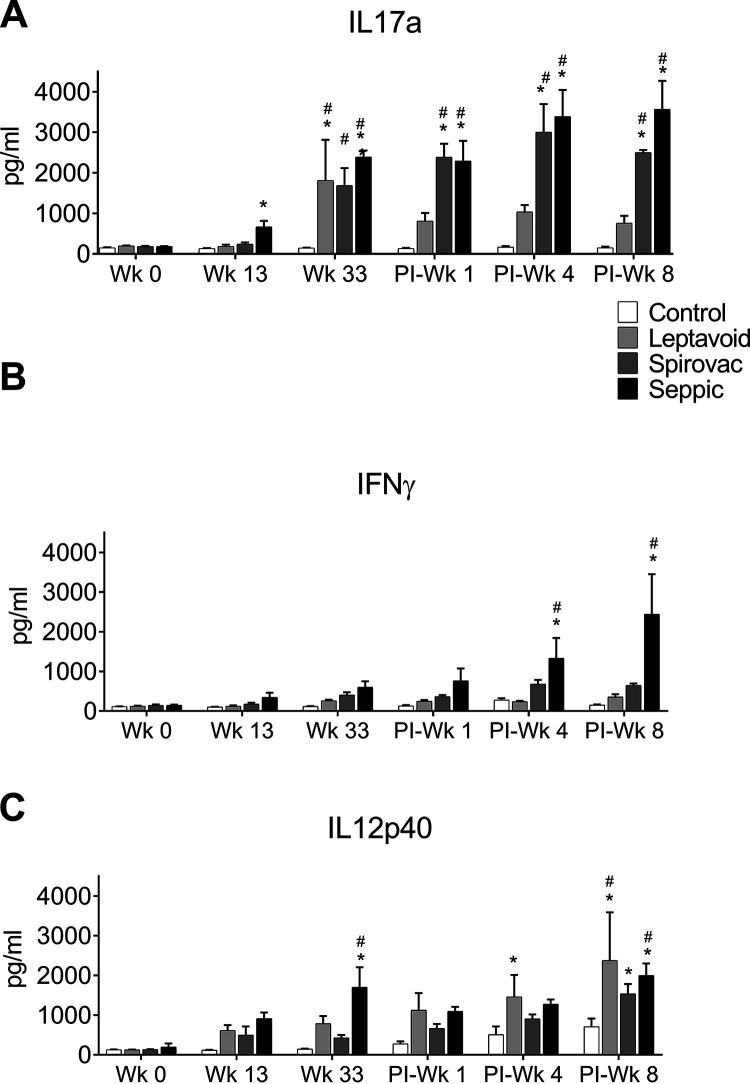
PBMCs from Spirovac and Seppic treatments demonstrated greater (P < 0.05) mean concentrations of IL-17 at 33 weeks after vaccination and at 1, 4, and 8 weeks after experimental challenge compared to responses by PBMCs from the control group and responses from PBMCs in Leptavoid vaccine treatments (Fig. 5). PBMCs from the Leptavoid treatment had increased (P < 0.05) IL-17 expression only at 33 weeks after vaccination. Increased in vitro expression of IFN-γ in supernatants after Leptospira antigen stimulation was only detected in PBMCs from the Seppic treatment obtained at 4 and 8 weeks after experimental challenge. Antigen-induced increases in IL-12 concentrations in supernatants, compared to responses in the control treatment, were detected at various times in Leptavoid (1 and 8 weeks after challenge), Spirovac (8 weeks after experimental challenge), and Seppic (33 weeks after vaccination and 8 weeks after experimental challenge) treatments. Expression by PBMCs of IL-6, IL-13, and IL-10 after in vitro stimulation with Leptospira antigens remained below assay detection limits at all time points for all treatments (data not shown).
FIG 5.
Cytokine release by antigen-stimulated PBMCs. Supernatants from PBMCs stimulated with 203 antigen were collected 48 h after stimulation and assayed for cytokines. Mean + SEM depicted. An asterisk (*) indicates a significant difference between control and vaccine groups within a time point (week) (P < 0.05). # indicates a significant difference between time points within treatment group (P < 0.05).
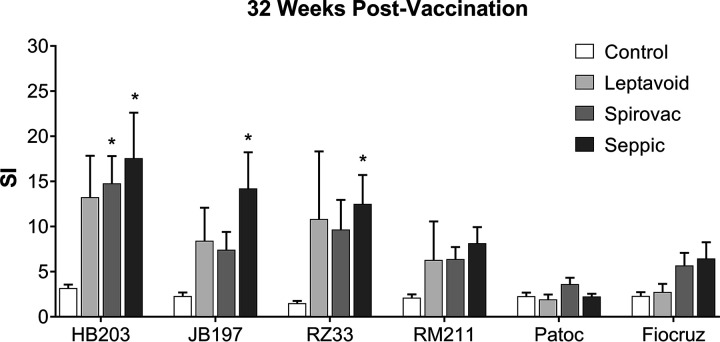
Cross-reactivity to antigens from homologous and heterologous Leptospira serovars.
Responses to antigens from homologous and heterologous Leptospira serovars were determined by in vitro stimulation of PBMCs collected at 33 weeks postvaccination and at 4 weeks postchallenge. PBMCs from vaccine and control treatments were incubated with whole-cell sonicates of strain 203, strain JB197, strain RZ33, L. interrogans serovar Pomona strain RM211, the nonpathogenic saprophyte Leptospira biflexa strain Patoc, and the human pathogen L. interrogans serovar Copenhageni strain Fiocruz LI-130 (Fig. 6). Proliferative responses were detected in PBMCs obtained from cattle in the Seppic vaccine group as measured by [3H]-thymidine incorporation, presented as stimulation index (SI), showed significant increase over the control group for homologous Hardjo strains 203, JB197, and RZ33, for the Seppic and Spirovac vaccination groups following vaccination (Fig. 6), but no significant differences for the heterologous Leptospira strains tested (L. interrogans serovar Pomona type kennewicki strain RM211, serovar Copehangeni strain Fiocruz LI-130, and the nonpathogenic L. biflexa strain Patoc), indicating little to no cross-species cross-reactivity.
FIG 6.
Cross-reactive response to other Leptospira strains. [3H]-Thymidine incorporation measurement of proliferation when stimulated with different Leptospira strains, serovars, and species. PBMCs collected from vaccinated and control cattle 32 weeks postvaccination. Stimulation index (SI) of pokeweed mitogen (PWM; positive-control mitogen) values between 50 and 300 (not shown). Mean (n = 6) + SEM; an asterisk (*) indicates that a group is significantly different (P < 0.05) from other groups.
Immune response in renal lymph nodes.
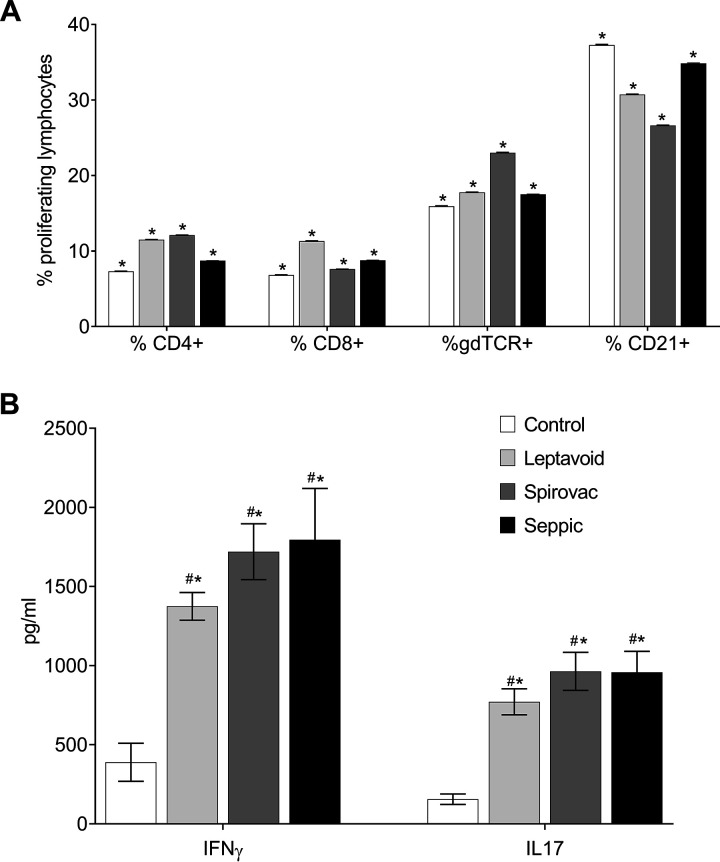
Recent observations in the rat chronic leptospirosis infection model indicated that immune memory responses may not reside in the periphery but in regional lymph nodes (12). For this reason, we evaluated proliferative responses and antigen-specific cytokine responses in lymphocytes from renal lymph nodes. At 10 weeks following challenge, there was no difference between groups in regard to percentage of proliferating lymphocytes, regardless of phenotype (CD4+, CD8+, γδ TCR, or CD21+) when stimulated with strain 203 antigen (Fig. 7A). To further characterize this response, supernatants of stimulated cells were collected and analyzed for cytokine release. Cells from all vaccine groups had greater (P < 0.05) concentrations of IFN-γ and IL-17 in supernatants at 48 h after incubation with Leptospira antigens compared to responses from cattle in the control treatment (Fig. 7B).
FIG 7.
(A) Proliferation of lymphocytes from renal lymph node. Percentage of proliferating lymphocytes, by phenotype, isolated from renal lymph node of strain 203-infected cattle, 10 weeks postinfection, stimulated ex vivo with 203 antigens. (B) Cytokine release from cultured renal lymphocytes collected for analysis 48 h poststimulation. Mean ± SEM. #, statistical difference (P < 0.05) between vaccine group and nonvaccinated control group; *, statistical difference from nonstimulated well within group (nonstimulated not shown).
DISCUSSION
The primary objective of this study was to use two previously known efficacious leptospiral vaccines and a novel Leptospira bacterin in an adjuvant chosen for directing strong cellular immune responses (Seppic Montanide ISA 201 VG) to conduct a detailed examination of the immune response to leptospirosis vaccination and subsequent infection in both vaccinated and unvaccinated cattle. The purpose was to try to identify potential correlates of immunity or a few key hallmarks of effective leptospiral vaccines. This information could enable future trials employing new vaccine constructs, adjuvants, and other vaccine technologies to be conducted without the expense or complexity of using live challenge, along with limiting the risk of leptospirosis infection in human personnel. The results of this study also further build upon previous bovine leptospirosis vaccination literature, conducted before some bovine reagents (e.g., IL-17 assays) were available.
Antibody responses were as expected and were similar to those observed in previous studies (13–15). It is of note that while there is a generally accepted belief that MAT vaccine titers of 1:100 are protective (16), in this experiment most titers were 1:50 or less (Fig. 2), yet upon challenge, all vaccinated animals were protected (Table 1). The MAT is subject to individual technician variability and differences in individual laboratories, as live Leptospira are used as the agglutinating antigen. Even so, it remains a popular assay, because the MAT shows, albeit indirectly, that a functional pathogen-binding antibody is produced. Cattle in all treatments, vaccination and control, had detectable IgM ELISA responses to Leptospira antigens prior to vaccination (Fig. 3). It should be noted that background humoral responses (e.g., IgM) to Leptospira antigens is expected in adult cattle housed in outdoor grassy paddocks, even in the absence of vaccination. The use of whole-cell sonicates (WCS) as the ELISA antigen could also have contributed to the high IgM titers, as WCS contain many bacterial epitopes that react with natural or bacterial cross-reacting antibodies (i.e., to flagella). There was an increase in antigen-specific IgM following infectious challenge, which also corresponded with increase in CD21+ B-cell populations in periphery of vaccinated cattle. The IgG2 titers trend closely with the cytokine release profiles, especially IFN-γ and IL-12 following challenge, supporting the link between Th1-type cytokines and IgG2 in bovine immune responses (2, 17–19). A somewhat surprising result was the detection of IgA titers in serum from the control cattle following infection. IgA has also been reported in other natural infections, including the vaginal secretions of heifers naturally infected with serovar Hardjo (20), in the serum of recently symptomatic human infections (21), and in the urine of infected canines (22). A mouse IgA monoclonal antibody to L. interrogans serovar Copenhageni strain H45 lipopolysaccharide was agglutinating, promoted opsonization, and provided passive protection in a guinea pig model when antibody was maintained at a serum titer of 16 to 32 (23). In cattle, the progression from IgM to IgA in naive B cells can occur in T cell-independent processes through switch intermediates if there is presence of sufficient antigen to cross-link B cell receptors and sufficient soluble factors (IFNs and tumor necrosis factor [TNF] family factors) from dendritic cells for activation (24). This would mean that a threshold of bacterial numbers or tissue damage needs to occur for the transition to happen, and could it occur locally in tissues or distally in lymphoid organs with or without T-cell help. The presence of IgA in the serum of infected but not vaccinated cattle should be further studied, as this presents an interesting possibility of using serum IgA as an infection biomarker in cattle.
Leptospirosis is a nearly silent infection, rarely inducing outward clinical signs in cattle, as was reflected in PBMC profiles. There was no observed skewing in PBMC profiles except for a slight expansion of CD21+ population in week 1 postinfection (see Fig. S2 in the supplemental material). Nor were these cells expressing differences in activation (increases or decreased in CD25+ or CD45RO), with the exception of increases in CD45RO on γδ T cells in vaccinated cattle 1 week-postchallenge (see Fig. S3 in the supplemental material). When PBMCs were antigen stimulated, similar profiles were observed, namely, increases in proliferating CD4, γδ T cells, and CD21+ B cells in vaccinated cattle 1 week following infectious challenge. This complements findings by Baldwin and others, who indicated that CD4 T cells responded first to leptospiral vaccine antigens and likely served as the long-lived memory cells (11, 25–27). γδ T cells, which have an exceptionally high affinity for leptospiral antigen, respond with CD4 T-cell help (11, 25, 27). It is theorized then that these antigen-responsive γδ T cells are part of an effector memory response and provide key protection shortly after infection. While much of the IFN-γ produced from antigen-stimulated PBMCs comes from CD4+ T cells (reference 15 and data not shown), it is theorized that these primed antigen responsive γδ T cells are a source for IL-17a in addition to CD4+ T cells (28). Thus, IgG1 antibody titers, antigen-responsive CD4+ T cells, γδ T cells, and release of IFN-γ and IL17-a, should all be considered hallmarks of effective vaccine formulations.
It is a limitation of this study that we did not include U.S. Standard or nonprotective vaccines for comparison of the immunological profile of a nonprotective vaccine. Previously published studies showed that cattle immunized with a less-effective pentavalent vaccine produced less IgG2 and had a lower proliferative response and a lower IFN-γ response than cattle immunized with either Spirovac or Leptavoid (27). Zuerner et al. showed that cattle immunized with a noneffective alum-based bacterin failed to generate antigen-specific proliferative CD4+ responses following vaccination and lacked the γδ TCR proliferative response shortly (3 weeks) after challenge (15). Thus, while we did not include the noneffective U.S. Standard or nonprotective vaccine, the publication record has enough evidence to confirm our observations. Effector γδ T cells producing IFN-γ and IL-17 and memory CD4 T cells are primed by vaccination. Together these two cell types are necessary for Leptospira clearance shortly following infection. Longevity of the vaccine response was also not addressed in this study. Longevity of leptospiral cattle vaccines has been studied and previously published (15, 17, 29). Initial studies, and subsequent studies examining Th1-type immune responses of Spirovac and Leptavoid, indicated that immunity was protective for 12 months (15, 17). In this study, immunity did not wane significantly between 33 and 41 weeks as the second group of cattle was waiting for challenge (data not shown). For both Spirovac and Leptavoid, the manufacturers recommend annual revaccination for optimal protection.
The two commercial vaccines and our Seppic bacterin construct did induce some within-serogroup cross-reactivity to nonhomologous serovar Hardjo strains (Fig. 6) and even a trend toward some reactivity toward serovar Pomona. However, little cross-reactivity was seen to serovar Fiocruz or to saprophyte serovar Patoc. Using whole-cell sonicate has the disadvantage that conserved intracellular structures or potential immune-reactive epitopes of the bacteria are exposed, such as cytoskeletal components or flagella, and thus some cross-reactivity is expected; however, that cross-reactivity would be similar across all strains of the genus, including serovars Fiocruz and Patoc, which was not observed.
Furthermore, this is the first study to analyze local immune response, i.e., response from the renal lymph node. While all infected cattle 10 weeks after infection exhibited an antigen recall response in the renal lymph nodes, there was a difference in the mechanism behind the responses because only the stimulated lymphocytes from the renal lymph nodes of vaccinated cattle (Leptavoid, Spirovac, and Seppic) released significantly more proinflammatory cytokines IFN-γ and IL-17a than those from the control cattle. This indicates that these lymphocytes, while antigen responsive (Fig. 7A), were in different states of memory/activation from those in the control group. Additionally, the response in the renal lymph node was greater in magnitude (e.g., greater percentage of proliferating cells and increased concentration in supernatant IFN-γ; Fig. 7A and B), than that in PBMCs. This may be due to a greater percentage of antigen-responsive cells in the lymph node than in the circulating periphery. Following isolation processes as we did, expected cellular profile from PMBCs would be approximately 50% T cells (including γδ and natural killer T cells; Fig. S2), up to 30% B cells, 20% monocytes, and up to 20% granulocytes (30, 31). In contrast, the cellular makeup of lymphocytes isolated from a lymph node may exceed 65% T cells, less than 10% monocytes, and few if any granulocytes. This phenomenon was also observed in a rat model of chronic leptospirosis infection, with little to no antigen-specific response observed in the spleen, representing peripheral blood responses, but a robust response in the renal lymph nodes, local to the site of infection (12). Similar findings in both chronic leptospirosis infection models indicate that the local lymph nodes rather than the periphery are the sites for memory T cells. The local lymph node memory response will need to be further studied and the role of these cytokines elucidated in cattle or in other chronic infection host models such as the rat (32).
A secondary objective of this study was to evaluate an alternative adjuvant for leptospirosis vaccination in cattle. Seppic Montanide ISA 201 VG was chosen for its ability to induce rapid immunity and strong Th1-driven immune response (33). Our results show that the Seppic bacterin-vaccinated cattle did indeed exhibit an IgG1 and IgG2 antibody response at 4 weeks postvaccination by ELISA, before the booster was given. As IgG2 is strongly linked to IFN-γ, a cellular response cytokine, it may be inferred that a cellular response was also present at this early time point (19). This is similar to other reports using similar adjuvants. The use of the Montanide ISA 206 emulsion adjuvant greatly enhanced mean MAT titers to an experimental Leptospira bacterin containing locally relevant serovars in India (6). These titers were present as soon as 7 days postimmunization and persisted for the 6 months of that study. Measures of cellular immune responses (cellular proliferation, T-cell activation markers, and cytokine release) were equivalent between the Seppic bacterin- and the Spirovac-vaccinated groups in the later vaccination phase and postchallenge. Thus, the advantage of the Seppic adjuvant would be shortly following vaccination and in the early response postchallenge. Unfortunately, our study design did not capture the early cellular responses. Early effective immune responses, or even immune responses that are protective after a single dose, would be a great advantage to the cattle producer in an outbreak situation or in areas where bovine leptospirosis is endemic. In other vaccine studies from our group, Seppic bacterin prepared with lyophilized and sonicated Leptospira antigen performed equally well to or better than the Spirovac vaccine (14). In the current study, the Seppic bacterin protected cattle as well as the current commercial vaccines (Spirovac and Leptavoid) (Table 1). Taken together, this study, along with previous work including Seppic Montanide oil emulsion adjuvants, indicates that these adjuvants may provide a means to enhance the immune response and efficacy of Leptospira vaccines in cattle.
In conclusion, induction of a combined humoral and cellular response appears to correlate with protective leptospiral vaccines for cattle, marked by an increase of antigen-responsive γδ T cells in the PBMC response shortly after infectious challenge. IL-17a, as well as IFN-γ, is released from antigen-responsive cells expanding the previously held views of a Th1-dominated response to include proinflammatory antibacterial Th17-type response. In examining immune response from protective vaccines, proliferation of CD4 and γδ T cells with increases in CD45RO and cytokines IL-17 and IFN-γ appears to be consistent among the tested protective vaccines. Adjuvants such as Seppic Montanide oil emulsions can be used to enhance early vaccine responses without any differences in vaccine efficacy and should be considered in outbreak situations or where early immunity would be advantageous.
MATERIALS AND METHODS
Ethical statement.
All animal procedures were approved by National Animal Disease Center Institutional Animal Care and Use Committee in accordance with the standards established by Public Health Service Policy “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,” the Guide for the Care and Use of Laboratory Animals (National Research Council), the Animal Welfare Act (1966), and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (USDA, Federation of Animal Science Societies).
Animals.
Mixed-breed heifers (Holstein-Angus cross, n = 24), of approximately 18 months of age were used in this study. Heifers were prescreened for any preexisting antibodies to Leptospira using a microscopic agglutination test (MAT) as previously described (34). Cattle were housed in outdoor paddocks with ad lib access to pasture, grass hay, and water and supplemented with a grain/concentrate mix. The cattle were transferred into containment facilities for the experimental challenge. Inside the containment facility, animals were housed in rooms containing two individual pens with one animal in each pen and fed a concentrate diet and ad libitum cubed hay.
Bacterial culture.
Leptospira borgpetersenii serovar Hardjo strain 203, Leptospira borgpetersenii serovar Hardjo strain JB197, Leptospira borgpetersenii serovar Hardjo strain RZ33, Leptospira interrogans serovar Pomona type Kennewicki isolate RM211, Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130, and nonpathogenic Leptospira biflexa strain Patoc (ATCC 23582) were cultured in semisolid media as previously described (35, 36). Leptospiral strains were grown to the mid-log phase, the Dinger’s ring was collected and diluted in phosphate-buffered saline (PBS; pH 7.2), and bacterial cells were harvested by centrifugation. The bacterial pellet was washed 3 times in PBS (pH 7.2), and resuspended in sterile distilled water. Bacteria were frozen at −80°C overnight and lyophilized the next day. Lyophilized bacteria were stored at −20°. For use in assays, (ELISA and cellular stimulation), bacteria were resuspended at 10 mg dried weight per ml in sterile PBS, briefly sonicated on ice, and sterilized a using UV light (Stratalinker UV). Antigen used in assays was calculated based on dry weight of lyophilized bacteria.
For bacterin preparation, Leptospira borgpetersenii serovar Hardjo strain 203 less than 10 passages from cattle isolation was cultured in liquid T80/40/LH medium at 29°C to the mid-log phase, the Dinger’s ring was collected by pipette and diluted in phosphate-buffered saline (pH 7.2), and Leptospira cells harvested by centrifugation (36). The bacterial pellet was washed 3 times in PBS, number of cells was adjusted to 5 × 108 per ml, and bacteria were incubated overnight with sterile 10% merthiolate solution (final concentration, 1:10,000) while shaking at 37°C. Verification of inactivation was performed as previously described (37).
Bacteria used for experimental challenge were from a single-passage culture of Leptospira borgpetersenii serovar Hardjo strain 203 after incubation for 3 weeks at 29°C. Two mixed-breed heifers (approximately 24 months of age) were experimentally infected by administration of 107 Leptospira bacteria bilaterally on conjunctiva and intravaginally for three consecutive days as previously described (38). Urine was collected approximately 15 min after treatment with furosemide (10 mg), and shedding of Leptospira in urine was determined using fluorescent antibodies (36). Once urinary shedding was detected, cattle were euthanized, and bacterial cultures were obtained from kidney homogenates. The experimental challenge was conducted in two replications, with approximately half of animals from each vaccine group randomly assigned to each replicate. A schematic of the experimental timeline is given in Fig. 1. Primary cultures from kidney homogenates were used for experimental challenge in the first replicate, and isolates obtained from control animals were used to prepare the challenge inoculum for the second repetition.
Vaccination.
Twenty-four heifers were randomly assigned to one of four vaccine groups (n = 6 cattle per group). The first group were subcutaneously inoculated in the cervical region with a commercial vaccine (Leptavoid-H; Intervet UK) in accordance with the label instructions and booster vaccinated at 4 weeks. Leptavoid contains 2.0 × 109 to 3.0 × 109 thimerosal-inactivated L. interrogans serovar Hardjo per ml in an alum adjuvant. The second group were subcutaneously vaccinated with a different commercial vaccine (Spirovac; Pfizer Animal Health, New York NY) in accordance with the label instructions and in a similar manner as group 1, and also booster vaccinated at 4 weeks. Spirovac contains chemically inactivated whole cultures of L. borgpetersenii serovar Hardjo (type Hardjobovis; undisclosed quantity) in an alum adjuvant. The third group (Seppic) were inoculated in a similar manner with approximately 5 × 109 formalin-fixed L. borgpetersenii serovar Hardjo strain 203 suspended in an oil-water-oil emulsion (Seppic Montanide ISA 201 VG; Seppic, Fairfield NJ) and booster vaccinated 4 weeks later. The Leptospira bacteria were fixed and evaluated for inactivation as previously described (39). The emulsion was created by passing the bacterial suspension and the Montanide adjuvant at least 30 times between two all-plastic syringes with a Luer lock bridge. The emulsion was verified by placing droplets in water and looking for dispersal patterns, and by looking for a ring with the oil droplet by dark-field microscopy as suggested by adjuvant manufacturer. The control group (adjuvant only or control) received 1 ml of alum-based adjuvant (Alhydrogel; InvivoGen, San Diego, CA) mixed with 1 ml of saline and 2 ml of a saline-Montanide ISA 201 VG emulsion prepared as described above for group 3 but without the Leptospira. The two inoculations were administered in separate subcutaneous sites and repeated 4 weeks later.
Urine culture postchallenge.
Urine samples were collected weekly from cattle after experimental challenge and evaluated for Leptospira shedding (36). Briefly, cattle were intravenously administered 10 mg furosemide, and the second void urine was collected by clean catch. For culture of urine, 1.5 ml was concentrated by centrifugation (10,000 × g, 7 min), the supernatant discarded, and the pellet suspended in 200 μl T80/40/LH liquid medium. The suspended pellet was used to inoculate T80/40/LH semisolid medium. In addition, 1 ml of urine was directly inoculated on T/80/40/LH semisolid medium. Urine cultures were conducted in triplicate for each animal at each sampling time. Inoculated media were incubated at 29°C and checked for growth weekly intervals for 1 month and then monthly for 4 months. Leptospira bacteria were identified by morphology under dark-field microscopy.
Recovery and histology of Leptospira at necropsy.
Cattle were euthanized 10 weeks after experimental challenge with virulent Leptospira. Kidneys were removed and transported to the laboratory on ice. An approximately 1-cm3 piece was aseptically removed and homogenized in 9 ml T80/40/LH liquid medium. The homogenate was directly inoculated on T80/40/LH semisolid medium (200 μl homogenate per 7-ml tube). Inoculated medium was incubated at 29°C and checked for growth by dark-field microscopy at weekly for 1 month and then monthly for 4 months.
Immunofluorescence antibody labeling assay postchallenge.
For immunofluorescence assay (IFA), 1.5 ml of urine was concentrated by centrifugation (10,000 × g, 7 min), and the assay was conducted as previously described (36). Kidney tissue homogenate was prepared as described above and used to directly inoculate slides, staining as previously described (36).
Microscopic agglutination test.
Blood was collected from the jugular vein of all animals at 8, 20, and 26 weeks following initial vaccination. After centrifugation, serum was collected and stored at −20°C. Microscopic agglutination text (MAT) assays were performed using strain 203 as antigen, at 2-fold dilutions from an initial dilution of 1:12.5 in accordance with OIE guidelines (34). Titers were reported as the reciprocal of the highest dilution in the well with a positive result. Sera testing negative at a 1:12.5 dilution were given a value of 1.
Leptospira-specific enzyme-linked immunosorbent assay.
Microtiter plates (96-well, Costar High Binding 9018; Corning Incorporated, Corning, NY) were coated overnight with 50 μl phosphate-buffered saline (PBS) containing 5 μg/ml L. borgpetersenii serovar Hardjo strain 203 whole-cell sonicate (WCS). After washing with PBS containing 0.05% Tween 20 (PBST), plates were blocked for 1 h at 37°C with PBST containing 5% casein (Sigma) and incubated at 4°C overnight. Serum samples were serially diluted and added to the wells (100 μl/well) in triplicate for each secondary antibody. PBS was added to one column as a negative control for each plate. After incubation for 1 h at 37°C followed by 4°C overnight, plates were washed three times with PBST. The wash step was followed by addition of 100 μl of a horseradish peroxidase-conjugated secondary antibody in PBST. Secondary antibodies (0.5 mg/ml diluted 1:1,000; KPL, Gaithersburg, MD) included goat anti-bovine IgM, goat anti-bovine IgA, goat anti-bovine IgG1, and goat anti-bovine IgG2. After a 1-h incubation at 37°C, plates were washed three times with PBST, and the colorimetric reaction was induced using TMB Sure Blue substrate (KPL) in accordance with manufacturer recommendations. Color development was stopped by addition of 50 μl of TMB Sure Blue stop reagent (KPL). Optical density (OD) was measured at 620 nm using a SpectraMax 190 plate reader (Molecular Devices, Sunnyvale, CA). Serum titers are reported as the reciprocal of the highest dilution giving an OD equal to or greater than average OD plus one standard deviation of PBS negative-control wells.
Blood collection, PBMC isolation, and in vitro culture.
A 90-ml aliquot of blood was collected by jugular vein into 10 ml of 2× acid-dextran-citrate anticoagulant. Blood was diluted 1:2 with sterile PBS and centrifuged to isolate peripheral blood mononuclear cells (PBMCs). PBMCs were purified on a density gradient (Histopaque, 1,077 g/ml density; Sigma) with any residual red blood cells lysed with ammonium-chloride-potassium (ACK) lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.01 mM Na2EDTA). Isolated PBMCs (5 × 106 cells) were labeled with 10 nM CellTrace violet (Invitrogen) and incubated in triplicate using wells of a 96-well flat-bottomed microtiter plate containing 25 μg/ml of leptospiral antigen, 10 μg/ml pokeweed mitogen (PWM), or 2 μg/ml concanavalin A (ConA) or medium alone in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 1% penicillin-streptomycin (Pen-Strep, 10,000 U/ml; Gibco, Gaithersburg MD), 25 mM HEPES buffer (Gibco), 1% nonessential amino acids (Gibco), 1% essential amino acids (Gibco), 1% sodium pyruvate (Gibco), 50 μM 2-β-mercaptoethanol, and 100 μg/ml gentamicin sulfate with sodium bicarbonate solution (Gibco) added to restore the pH to approximately 7. Plates were incubated at 39°C and 5% CO2 for 5 days. Replicate plates were inoculated with unlabeled cells from which 150 μl cell culture supernatants were collected at 24, 48, and 72 h poststimulation and frozen at −20°C until assayed.
Flow cytometry.
Isolated PBMCs from each collection time point both fresh and after in vitro stimulation for 5 days, were characterized by flow cytometric techniques. Antibodies, suppliers, and concentrations are given in the supplemental material (see Table S1). After 5 days of culture in vitro, cell trace labeled cells were harvested by centrifugation and labeled with live/dead discriminator dye (Zombie yellow; BioLegend), Cells were labeled with antibodies for surface markers given in Table S1 and analyzed on a FACSAria flow cytometer (BD). Data were analyzed using FlowJo software. An example of a gating scheme is given in Fig. S1 in the supplemental material. Cells were gated on viability dye exclusion followed by forward scatter/side scatter profile historically consistent with bovine lymphocytes. Lymphocyte populations were plotted against the fluorescent intensity of each antibody in the following pairs: CD4 versus B cell markers and CD8 versus γδ TCR. Positive marker gates for each subset were determined by a cell pool containing all antibodies except the antibody of interest and the isotype of the antibody of interest. An increase or decrease in the percentage of cells expressing a specific marker was determined by comparison to cells from wells without antigenic stimulation (background).
Antibodies used for cell phenotyping by flow cytometry. Download Table S1, DOCX file, 0.02 MB (16.8KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Flow cytometry gating strategy. (A) Isolated lymphocytes (cultured or uncultured) were gated on lymphocyte phenotype based on forward scatter (FSC) and side scatter (SSC) profile. (B) Cells were selected for CD4 or CD21 (B-cell) expression. (C) Cells were then selected for CD8 expression or γδ TCR expression. Cells expressing both CD8 and γδ TCR were considered to be γδ T cells. (D) Proliferating cells were selected on left-shift or dimming of CellTrace violet (Pacific Blue) as shown in this overlay example of cultured cells from a naïve animal stimulated with leptospiral antigen (green), ConA (blue), or media only (pink). Download FIG S1, TIF file, 1.0 MB (1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Cytokine measurement by AlphaLISA.
Supernatants were collected from bovine PBMCs after stimulation with Leptospira antigens, mitogens, or medium alone for 48 h. Supernatants were stored at −20°C and thawed at 4°C before assayed. Supernatants were analyzed for IL-6, IL-10, IL-12/p40, IL-13, IL-17a, IL-18, IFN-γ, and TNF-α per the manufacturer instructions using commercial kits (AlphaLISA, PerkinElmer).
Assay for homologous and heterologous reactivity.
PBMCs were purified as described above and incubated in triplicate using wells of a 96-well flat-bottomed microtiter plate containing 25 μg/ml of leptospiral antigen from Leptospira borgpetersenii serovar Hardjo strain 203, Leptospira borgpetersenii serovar Hardjo strain JB197, Leptospira borgpetersenii serovar Hardjo strain RZ33, Leptospira interrogans serovar Pomona type kennewicki isolate RM211, Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130, and the nonpathogenic Leptospira biflexa strain Patoc, or 2 μg/ml concanavalin A (ConA) or medium alone (no stimulation) in RPMI 1640 (Life Technologies, Carlsbad, CA), supplemented as above. Plates were incubated at 39°C and 5% CO2 for 5 days. Lymphocyte proliferation was determined by incorporation of [3H]-thymidine during an additional 18 h of incubation as previously described (40). Stimulation indices (SI) were calculated from raw counts by comparing the counts per minute incorporated by cells stimulated with antigen divided by the counts per minute incorporated in the absence of any stimulation (medium alone).
Renal lymph node lymphocyte analysis.
At 12 weeks following experimental challenge, all cattle were euthanized and renal lymph nodes (RLN) were sampled. RLN samples were homogenized using a Precision tissue grinder (Covidien, Mansfield, MA), passed through a 40-μm cell strainer (Falcon-Corning, Corning, NY). Lymphocytes isolated by density gradient (Histopaque, 1,077 g/ml density; Sigma) and cultured with mitogens or Leptospira antigen as described previously for PBMCs. Culture supernatants were assayed for IFN-γ and IL-17a after 48 h of incubation using commercial kits in accordance with manufacturer’s instructions (AlphaLISA, PerkinElmer). Cytokine values below the assay’s lower limit of detection (50 pg/ml for IFN-γ, 20 pg/ml for IL17a) were given the value of 10 for statistical analysis.
Statistics.
Results were analyzed using GraphPad Prism 7 statistical software. MAT and ELISA data were log transformed [Y = ln(y)] for analysis and were analyzed using a 2-way analysis of variance (ANOVA) with means separated by Tukey’s multiple comparisons for simple effects between vaccine groups at each time point and Tukey’s multiple-comparison test for simple effects within vaccine group by time point. Flow cytometric data were analyzed by 2 way-ANOVA with Sidak’s multiple-comparison posttest, comparing within vaccination groups the effect of well stimulation compared to background or no-stim wells and Dunnett’s multiple-comparison posttest for within-well stimulation between vaccine groups. Cytokine data were analyzed using a 2 way-ANOVA with Tukey’s multiple-comparison posttest, comparing within-group effects of well stimulation compared to background or control wells and Dunnett’s multiple-comparison posttest for within-well stimulation between groups, differences in treatment means were reported as significant when P ≤ 0.05. Preliminary analysis of serum titers and proliferation results of cattle in replicate 2, delayed infection due to space constraints, showed no statistical difference or decrease in immune response between weeks 42 and 33. Thus, data from replicates 1 and 2 were combined as a single experiment.
ACKNOWLEDGMENTS
Mention of trade names or commercial products in this study is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). The USDA is an equal opportunity employer.
We acknowledge Richard Hornsby, Ami Frank, and Jayne Wiarda for excellence in laboratory assistance in the generation of data used in this study. We also wish to acknowledge the NADC Animal Resource Unit for excellence in animal care, outdoors and in containment.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Work was completed by U.S. Department of Agriculture employees in the course of their assigned duties in relation to project number 5030-32000-223-00-D.
We have no competing interests to declare.
Contributor Information
Jennifer H. Wilson-Welder, Email: jennifer.wilson-welder@usda.gov.
David W. Pascual, University of Florida
REFERENCES
- 1.Haake DA, Levett PN. 2015. Leptospirosis in humans, p 65–97. In Adler B (ed), Leptospira and leptospirosis. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- 2.Ellis WA. 2015. Animal leptospirosis. Curr Top Microbiol Immunol 387:99–137. doi: 10.1007/978-3-662-45059-8_6. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfus A, Wilson P, Collins-Emerson J, Benschop J, Moore S, Heuer C. 2015. Risk factors for new infection with Leptospira in meat workers in New Zealand. Occup Environ Med 72:219–225. doi: 10.1136/oemed-2014-102457. [DOI] [PubMed] [Google Scholar]
- 4.Nally JE, Hornsby RL, Alt DP, Bayles D, Wilson-Welder JH, Palmquist DE, Bauer NE. 2018. Isolation and characterization of pathogenic leptospires associated with cattle. Vet Microbiol 218:25–30. doi: 10.1016/j.vetmic.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Martins G, Oliveira CS, Lilenbaum W. 2018. Dynamics of humoral response in naturally-infected cattle after vaccination against leptospirosis. Acta Trop 187:87–91. doi: 10.1016/j.actatropica.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan G, Roy P. 2014. Comparision of efficacy of two experimental bovine leptospira vaccines under laboratory and field. Vet Immunol Immunopathol 159:11–15. doi: 10.1016/j.vetimm.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Dadon Y, Haas EJ, Kaliner E, Anis E, Singer SR, Atiya-Nasagi Y, Cohen-Dar M, Avramovich E, King R, Sued O, Goshen T, Amit S, Miskin I, Gino E, Yishai R, Sheffer R, Grotto I, Moran-Gilad J. 2018. Outbreak of human leptospirosis linked to contaminated water bodies in Northern Israel, June to August 2018. Eurosurveillance 23:1800486. doi: 10.2807/1560-7917.ES.2018.23.38.1800486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tin Tin Htar M, Christopoulou D, Schmitt HJ. 2015. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis 15:419. doi: 10.1186/s12879-015-1147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black PF, Corney BG, Smythe LD, Dohnt MF, Norris MA, Symonds ML. 2001. Prevalence of antibodies of Leptospira serovars in beef cattle in central Queensland. Aust Vet J 79:344–348. doi: 10.1111/j.1751-0813.2001.tb12010.x. [DOI] [PubMed] [Google Scholar]
- 10.Aono FH, Cooke RF, Alfieri AA, Vasconcelos JL. 2013. Effects of vaccination against reproductive diseases on reproductive performance of beef cows submitted to fixed-timed AI in Brazilian cow-calf operations. Theriogenology 79:242–248. doi: 10.1016/j.theriogenology.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin CL, Hsu H, Chen C, Palmer M, McGill J, Waters WR, Telfer JC. 2014. The role of bovine γδ T cells and their WC1 co-receptor in response to bacterial pathogens and promoting vaccine efficacy: a model for cattle and humans. Vet Immunol Immunopathol 159:144–155. doi: 10.1016/j.vetimm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Nally JE, Wilson-Welder JH, Hornsby RL, Palmer MV, Alt DP. 2018. Inbred rats as a model to study persistent renal leptospirosis and associated cellular immune responsiveness. Front Cell Infect Microbiol 8:66. doi: 10.3389/fcimb.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naiman BM, Blumerman S, Alt D, Bolin CA, Brown R, Zuerner R, Baldwin CL. 2002. Evaluation of type 1 immune response in naive and vaccinated animals following challenge with Leptospira borgpetersenii serovar Hardjo: involvement of WC1+ γδ and CD4 T cells. Infect Immun 70:6147–6157. doi: 10.1128/iai.70.11.6147-6157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson-Welder JH, Boggiatto P, Nally JE, Wafa EI, Alt DP, Hornsby RL, Frank A, Jones DE, Olsen SC, Bowden NB, Salem AK. 2020. Bovine immune response to leptospira antigen in different novel adjuvants and vaccine delivery platforms. Vaccine 38:3464–3473. doi: 10.1016/j.vaccine.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 15.Zuerner RL, Alt DP, Palmer MV, Thacker TC, Olsen SC. 2011. A Leptospira borgpetersenii serovar Hardjo vaccine induces a Th1 response, activates NK cells, and reduces renal colonization. Clin Vaccine Immunol 18:684–691. doi: 10.1128/CVI.00288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler B. 2015. Vaccines against leptospirosis. Curr Top Microbiol Immunol 387:251–272. doi: 10.1007/978-3-662-45059-8_10. [DOI] [PubMed] [Google Scholar]
- 17.Ellis W, McDowell S, Mackie D, Pollock J, Taylor M. 2000. Immunity to bovine leptospirosis. Proceedings of the 21st World Buiatrics Congress, Punte del Este, Uruguay. [Google Scholar]
- 18.Estes DM, Brown WC. 2002. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet Immunol Immunopathol 90:1–10. doi: 10.1016/s0165-2427(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 19.Estes DM, Closser NM, Allen GK. 1994. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol 154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 20.Dhaliwal GS, Murray RD, Dobson H, Montgomery J, Ellis WA. 1996. Presence of antigen and antibodies in serum and genital discharges of cows from dairy herds naturally infected with Leptospira interrogans serovar Hardjo. Res Vet Sci 60:163–167. doi: 10.1016/s0034-5288(96)90012-0. [DOI] [PubMed] [Google Scholar]
- 21.da Silva MV, Nakamura PM, Camargo ED, Batista L, Vaz AJ, Romero EC, Brandao AP. 1997. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG, and IgA antibodies. Am J Trop Med Hyg 56:650–655. doi: 10.4269/ajtmh.1997.56.650. [DOI] [PubMed] [Google Scholar]
- 22.Zaragoza C, Barrera R, Centeno F, Tapia JA, Mañé MC. 2003. Characterization of renal damage in canine leptospirosis by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting of the urinary proteins. J Comp Pathol 129:169–178. doi: 10.1016/s0021-9975(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 23.Jost BH, Adler B, Vinh T, Faine S. 1986. A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J Med Microbiol 22:269–275. doi: 10.1099/00222615-22-3-269. [DOI] [PubMed] [Google Scholar]
- 24.Estes DM. 2010. Regulation of IgA responses in cattle, humans and mice. Vet Immunol Immunopathol 138:312–317. doi: 10.1016/j.vetimm.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Blumerman SL, Herzig CT, Baldwin CL. 2007. WC1+ γδ T cell memory population is induced by killed bacterial vaccine. Eur J Immunol 37:1204–1216. doi: 10.1002/eji.200636216. [DOI] [PubMed] [Google Scholar]
- 26.Blumerman SL, Herzig CT, Wang F, Coussens PM, Baldwin CL. 2007. Comparison of gene expression by co-cultured WC1+ γδ and CD4+ αβ T cells exhibiting a recall response to bacterial antigen. Mol Immunol 44:2023–2035. doi: 10.1016/j.molimm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Brown RA, Blumerman S, Gay C, Bolin C, Duby R, Baldwin CL. 2003. Comparison of three different leptospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 21:4448–4458. doi: 10.1016/s0264-410x(03)00439-0. [DOI] [PubMed] [Google Scholar]
- 28.Peckham RK, Brill R, Foster DS, Bowen AL, Leigh JA, Coffey TJ, Flynn RJ. 2014. Two distinct populations of bovine IL-17+ T-cells can be induced and WC1+IL-17+γδ T-cells are effective killers of protozoan parasites. Sci Rep 4:5431. doi: 10.1038/srep05431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman AD, Springer EW, Barling KS, Buterbaugh RE, Pooley RD, Scholz DA, Rhoades JR, Chase CC. 2013. Immunity in heifers 12 months after vaccination with a multivalent vaccine containing a United States Leptospira borgpetersenii serovar Hardjo isolate. J Am Vet Med Assoc 242:1573–1577. doi: 10.2460/javma.242.11.1573. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton CA, Mahan S, Bell CR, Villarreal-Ramos B, Charleston B, Entrican G, Hope JC. 2017. Frequency and phenotype of natural killer cells and natural killer cell subsets in bovine lymphoid compartments and blood. Immunology 151:89–97. doi: 10.1111/imm.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hope JC, Howard CJ, Prentice H, Charleston B. 2006. Isolation and purification of afferent lymph dendritic cells that drain the skin of cattle. Nat Protoc 1:982–987. doi: 10.1038/nprot.2006.125. [DOI] [PubMed] [Google Scholar]
- 32.Putz EJ, Nally JE. 2020. Investigating the immunological and biological equilibrium of reservoir hosts and pathogenic leptospira: balancing the solution to an acute problem? Front Microbiol 11:2005. doi: 10.3389/fmicb.2020.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vordermeier HM, Dean GS, Rosenkrands I, Agger EM, Andersen P, Kaveh DA, Hewinson RG, Hogarth PJ. 2009. Adjuvants induce distinct immunological phenotypes in a bovine tuberculosis vaccine model. Clin Vaccine Immunol 16:1443–1448. doi: 10.1128/CVI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole JR, Jr, Sulzer CR, Pursell AR. 1973. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol 25:976–980. doi: 10.1128/AM.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson-Welder JH, Frank AT, Hornsby RL, Olsen SC, Alt DP. 2016. Interaction of bovine peripheral blood polymorphonuclear cells and Leptospira species; innate responses in the natural bovine reservoir host. Front Microbiol 7:1110. doi: 10.3389/fmicb.2016.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuerner RL. 2005. Laboratory maintenance of pathogenic Leptospira. Curr Protoc Microbiol Chapter 12:Unit 12E.1. [DOI] [PubMed] [Google Scholar]
- 37.Bolin CA, Cassells JA, Zuerner RL, Trueba G. 1991. Effect of vaccination with a monovalent Leptospira interrogans serovar Hardjo type Hardjo-bovis vaccine on type Hardjo-bovis infection of cattle. Am J Vet Res 52:1639–1643. [PubMed] [Google Scholar]
- 38.Bolin CA, Thiermann AB, Handsaker AL, Foley JW. 1989. Effect of vaccination with a pentavalent leptospiral vaccine on Leptospira interrogans serovar Hardjo type Hardjo-bovis infection of pregnant cattle. Am J Vet Res 50:161–165. [PubMed] [Google Scholar]
- 39.Bolin CA, Alt DP. 2001. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar Hardjo. Am J Vet Res 62:995–1000. doi: 10.2460/ajvr.2001.62.995. [DOI] [PubMed] [Google Scholar]
- 40.Olsen SC, Boyle SM, Schurig GG, Sriranganathan NN. 2009. Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. CVI 16:535–540. doi: 10.1128/CVI.00419-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral blood mononuclear cell (PBMC) phenotype. Peripheral blood mononuclear cells isolated from vaccinated and infected cattle at 16 and 33 weeks postvaccination and 1, 4, and 8 weeks (shaded bars) post-experimental challenge. Minimum of 2,000 uncultured, unstimulated cells were analyzed for phenotype (CD4+, CD8+, γδ TCR+, and CD21+) per sample. Mean (n = 6) + SEM; *, statistical difference from other time points within the vaccine group; P ≤ 0.05. Download FIG S2, TIF file, 1.6 MB (1.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Activation state of uncultured PBMCs. Peripheral blood mononuclear cells isolated from vaccinated and infected cattle at 16 and 33 weeks postvaccination and 1, 4, and 8 weeks (shaded bars) post-experimental challenge. Minimum of 2,000 uncultured, unstimulated cells were analyzed for phenotype (CD4, CD8, and γδ TCR) and expression of activation marker (CD25, and CD45RO) by flow cytometry. Mean (n = 6) + SEM; #, statistical difference between vaccine groups and the control group within a time point (week); *, statistical difference from prechallenge time points (weeks 16 and 33) within the vaccine group (P ≤ 0.05); a indicates statistical difference of vaccinated group from control group at that time point (week 1 postinfection [PI]) (P ≤ 0.05). Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Antibodies used for cell phenotyping by flow cytometry. Download Table S1, DOCX file, 0.02 MB (16.8KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Flow cytometry gating strategy. (A) Isolated lymphocytes (cultured or uncultured) were gated on lymphocyte phenotype based on forward scatter (FSC) and side scatter (SSC) profile. (B) Cells were selected for CD4 or CD21 (B-cell) expression. (C) Cells were then selected for CD8 expression or γδ TCR expression. Cells expressing both CD8 and γδ TCR were considered to be γδ T cells. (D) Proliferating cells were selected on left-shift or dimming of CellTrace violet (Pacific Blue) as shown in this overlay example of cultured cells from a naïve animal stimulated with leptospiral antigen (green), ConA (blue), or media only (pink). Download FIG S1, TIF file, 1.0 MB (1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.