Abstract
Heart disease is a major cause of death worldwide. Chronic Chagas cardiomyopathy (CCC) caused by infection with Trypanosoma cruzi leading to high mortality in adults, and rheumatic heart disease (RHD), resulting from infection by Streptococcus pyogenes affecting mainly children and young adults, are amongst the deadliest heart diseases in low-middle income countries. Despite distinct etiology, the pathology associated with both diseases is a consequence of inflammation. Here we compare systemic immune profile in patients with these cardiopathies, to identify particular and common characteristics in these infectious heart diseases. We evaluated the expression of 27 soluble factors, employing single and multivariate analysis combined with machine-learning approaches. We observed that, while RHD and CCC display higher levels of circulating mediators than healthy individuals, CCC is associated with stronger immune activation as compared to RHD. Despite distinct etiologies, univariate analysis showed that expression of TNF, IL-17, IFN-gamma, IL-4, CCL4, CCL3, CXCL8, CCL11, CCL2, PDGF-BB were similar between CCC and RHD, consistent with their inflammatory nature. Network analysis revealed common inflammatory pathways between CCC and RHD, while highlighting the broader reach of the inflammatory response in CCC. The final multivariate model showed a 100% discrimination power for the combination of the cytokines IL-12p70, IL-1Ra, IL-4, and IL-7 between CCC and RHD groups. Thus, while clear immunological distinctions were identified between CCC and RHD, similarities indicate shared inflammatory pathways in these infectious heart diseases. These results contribute to understanding the pathogenesis of CCC and RHD and may impact the design of immune-based therapies for these and other inflammatory cardiopathies that may also share immunological characteristics.
Keywords: Chagas disease, Rheumatic heart disease, Inflammation, Systemic immune profile, Pathology, Immunoregulation
1. Introduction
Heart disease is the leading cause of death worldwide with a disproportional burden in underserved populations. The cardiomyopathy resulting from Chagas disease (ChD), caused by infection with the intracellular parasite Trypanosoma cruzi, is amongst the most debilitating and deadly heart diseases in adults, leading to over 10,000 deaths/year [1]. Rheumatic heart disease (RHD) is caused by an immune response triggered by infection with type A Streptococcus, is responsible for the highest number of disability-adjusted life-years among 10–14-year-olds, and is the second highest number among children 5–9 years old [2]. The cardiac involvement in both diseases is progressive, and clinical management relies on palliative therapies to ameliorate symptoms. There are no vaccines to prevent these diseases.
Chronic Chagas disease cardiopathy (CCC) is characterized by ventricular dilation and dysfunction, thromboembolic events, and conductive alterations. The severity of left ventricular systolic dysfunction is the main factor influencing prognosis [3,4]. In contrast, RHD is characterized by progressive damage to the heart valves, leading to valvular regurgitation and/or stenosis [5]. Despite the differences, these diseases share several aspects. The pathogenesis of both diseases involves immune reactions caused by antigenic mimicry to heart structures, especially cardiac myosin, tropomyosin, laminin, and vimentin [6–9]. Additionally, the presence of bacterial DNA in the valve tissue of patients with RHD and of T. cruzi antigens in the myocardium of Chagas patients suggest the additional role of the continuous antigenic stimulus in inflammatory injury and cardiovascular sequelae in both diseases [10,11]. Anti-host and anti-pathogen reactivity leads to persistent T cell activation and inflammation, causing tissue destruction [6,8,12,13]. The inflammatory infiltrate observed in the heart tissue of CCC patients is composed mainly of activated CD8+ T cells that produce inflammatory cytokines such as TNF [14]. The affected valves in RHD also contains pro-inflammatory cytokines such as TNF, IFN-gamma, IL-1beta, and IL-17, but is mainly composed of CD4+ T cells and macrophages that orchestrate the production of mediators related to tissue fibrosis [15]. The mechanisms underlying the recruitment of distinct effector cells to the myocardial and valvular tissues are not completely understood. However, it is known that chemokines orchestrate migration of cells associated with the inflammatory response and severity in CCC and RHD [16–18]. Chronic inflammation is key for the development of pathology, by regulating the hypertrophy and apoptosis of cardiomyocytes, as well as fibrosis [17,19,20].
Given that both heart diseases are characterized by a continuous inflammatory process that can lead to heart failure, we evaluated the profile of the systemic soluble immune-mediators in patients with CCC and RHD by determining the plasma levels of cytokines, chemokines, and growth factors. The identification of immunological characteristics specific to and shared by CCC and RHD provides essential information to understanding their pathogenesis, as well as to potentially guide specific or general intervention strategies for infectious heart disease.
2. Study population, materials, and methods
2.1. Study population
This was an observational study, with cross-sectional analysis of circulating molecules including a total of 67 individuals. Plasma samples from 38 patients with CCC, from areas endemic for Chagas disease in Brazil and with a positive serological test for Trypanosoma cruzi (age range between 28 and 70 years, 58% of men and 42% women) were used to measure circulating soluble factors. Detailed evaluations, including physical examinations, electrocardiogram, chest X-rays and echocardiogram were performed in order to characterize the clinical status of the CCC patients as previously defined by us [21]. CCC patients presented right and/or left ventricular dilation, global left ventricular dysfunction, alterations in the cardiac electric impulse generation and conduction upon eletrocardiogram, chest x-rays and echocardiography. Plasma samples from 17 patients with RHD with symptomatic severe mitral stenosis (age range between 28 and 64 years; 11.8% men and 88.2% women) were also included in this evaluation. RHD patients presented with late RHD characterized by valve dysfunction resulting from rheumatic involvement confirmed by echocardiography. All patients were undergoing cardiological clinical follow-up at the Hospital das Clínicas of Universidade Federal de Minas Gerais (UFMG). Samples from 12 healthy individuals not infected with T. cruzi and with no previous events related to rheumatic fever, nor evidence of cardiac involvement were included in the control group (CONTROL; age range between 17 and 18 years, 58 % men and 42% women).
All patients who accepted to participate voluntarily in the research were informed about the objectives of our study and signed the Informed Consent Form. This study was approved by the Research Ethics Committee of Universidade Federal de Minas Gerais (COEP-UFMG – ETIC006 / 05) and National Research Ethics Commission (CONEP n° 2.809.859) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Luminex Bioassay
To evaluate the soluble factors present in the plasma of the different study groups, the Bio-Plex ProTM Human Cytokine Standard 27-plex Kit (Bio-Rad – Hercules, CA, USA) was used to measure the following molecules: cytokines (IL-1beta, IL1- Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, IFN-gamma, TNF) chemokines (CCL2, CCL3, CCL4, CCL5, CXCL8, CXCL10, CCL11) and growth factors (G-CSF, GM-CSF, PDGF-BB, VEGF, basic FGF). Experiments were performed according to manufacturer’s instructions. The data were acquired by the Bio-Plex 200 instrument equipped with the Manager software and the results were expressed as the mean fluorescence intensity (MFI), after background subtraction.
2.3. Protein-protein interaction network and enriched pathways analysis
Network was constructed using NetworkAnalyst.ca. through direct relationships between proteins and altered soluble factors in CCC and RHD. Pairwise correlation prediction was determined based on IMEx database. Resulting high-scoring genes were used to identify hub genes. Enriched pathway analysis emerging as a result of interconnections in the network were generated using Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.4. Statistical analysis
To compare the plasma levels of the molecules between the study groups, the results were obtained using the Graphpad Prism 7 software (GraphPad Software, La Jolla - CA, USA). The parametric data were subjected to the one-way ANOVA multiple comparison test followed by the Tukey post-test, and represented by the mean and standard deviation. Nonparametric data were analyzed using the Kruskal-Wallis test, followed by Dunn’s post-test and represented by the median and interquartile range (25 and 75 percentile range).
To represent the general profile of the soluble factors evaluated, data from MFI medians were used for making radar charts using Microsoft Excel Software. Each axis of the graph represents the percentage contribution for each molecule within each functional group (cytokines, chemokines, and growth factors). Additionally, for qualitative representation of data, analysis of the t-distributed stochastic neighbor-embedding (t-SNE) algorithm was performed, a tool that allows the visualization of multidimensional data in smaller dimensions (t-SNE1 and t-SNE2) [22]. Initially, the MFI values of the molecules evaluated by Luminex Multiplex Assay were tabulated in Excel spreadsheets in xls format, converted into a csv file, imported and converted into an Fcs file in the Flowjo software. Then, the t-SNE analysis was generated by selecting the 27 molecules evaluated in our study, using the Barnes-Hut algorithm with 1000 interactions and perplexity parameter of 30. Subsequently, the analysis was performed by the FlowSOM algorithm for the detection of clusters on the islands generated by tSNE [23]. Next, an analysis was performed by the cluster explorer algorithm to represent the relative intensity expression profile of the molecules evaluated in the clusters found by the FlowSom algorithm.
To assess a possible pattern of differentiation between groups using the soluble factors, a representative heatmap analysis was performed using the Clustvis software using the heatmap package R-version 0.7.7, where it is possible to analyze the grouping amongst samples and to define the homogeneity or heterogeneity in the distribution of data amongst groups. Rows and columns are grouped using the correlation distance and the mean link.
To identify possible predictors of distinct immune responses between the evaluated heart diseases, a logistic regression model adjusted by the LASSO method was used. This method of analysis is suitable for situations in which there is a large number of potential predictors as well as variables that can be correlated. Due to their right-skewed distributions, cytokine values were log-transformed and standardized to ensure the proper identification of predictors in the regression models. The analysis was conducted using the statistical software R version 3.6.3, with the aid of the tidyverse (data manipulation), foreign (data reading), ggplot2, corrplot, gridExtra and GGally packages (graphs), glmnet (adjustment of the models via LASSO), and pROC (calculation of the area under the ROC curve). Initially, the original data were randomly divided into a training set (80% of the data) to adjust the model and test (remaining 20%), to calculate the predicted probabilities and evaluate the predictive performance of the model. The results obtained were shown as a set of variables that, in association, can explain the event, in our study, the power to distinguish between the two heart diseases. The impact of the variable on the desired response (differentiation between heart diseases) was evidenced as an s0 coefficient that translates a directly proportional association when positive and an inversely proportional association when negative. The results were represented using violin plot graph exploring the relationship of each molecule with the groups evaluated, by the ROC curve to determine the power of discrimination between the groups (CCC and RHD) and by a correlation graph between the covariates selected in the final model of the method LASSO.
3. Results
3.1. Plasma levels of cytokines, chemokines, and growth factors indicate greater immunological activation in patients with Chagas cardiomyopathy as compared to rheumatic heart disease.
Analysis of plasma levels of circulating mediators comparing CCC patients with individuals in the CONTROL group demonstrated an increase in a wide variety of soluble factors. Amongst the cytokines, only IL-6 and TNF were not significantly higher in CCC plasma as compared to CONTROL (Fig 1 and supplementary table 1). All chemokines and growth factors were higher in CCC plasma as compared to CONTROL (Figs 2A and 2B and supplementary table 1). On the other hand, plasma levels of the different immune mediators in the samples from patients with RHD compared to the CONTROL group showed an increase only in IL-12, IFN-gamma, IL-17, IL-4, IL-1Ra, CCL4 and PDGF-BB (Fig 1, Figs 2A and 2B and supplementary table 1).
Fig. 1. Comparative analysis of plasma cytokines levels between the study groups.
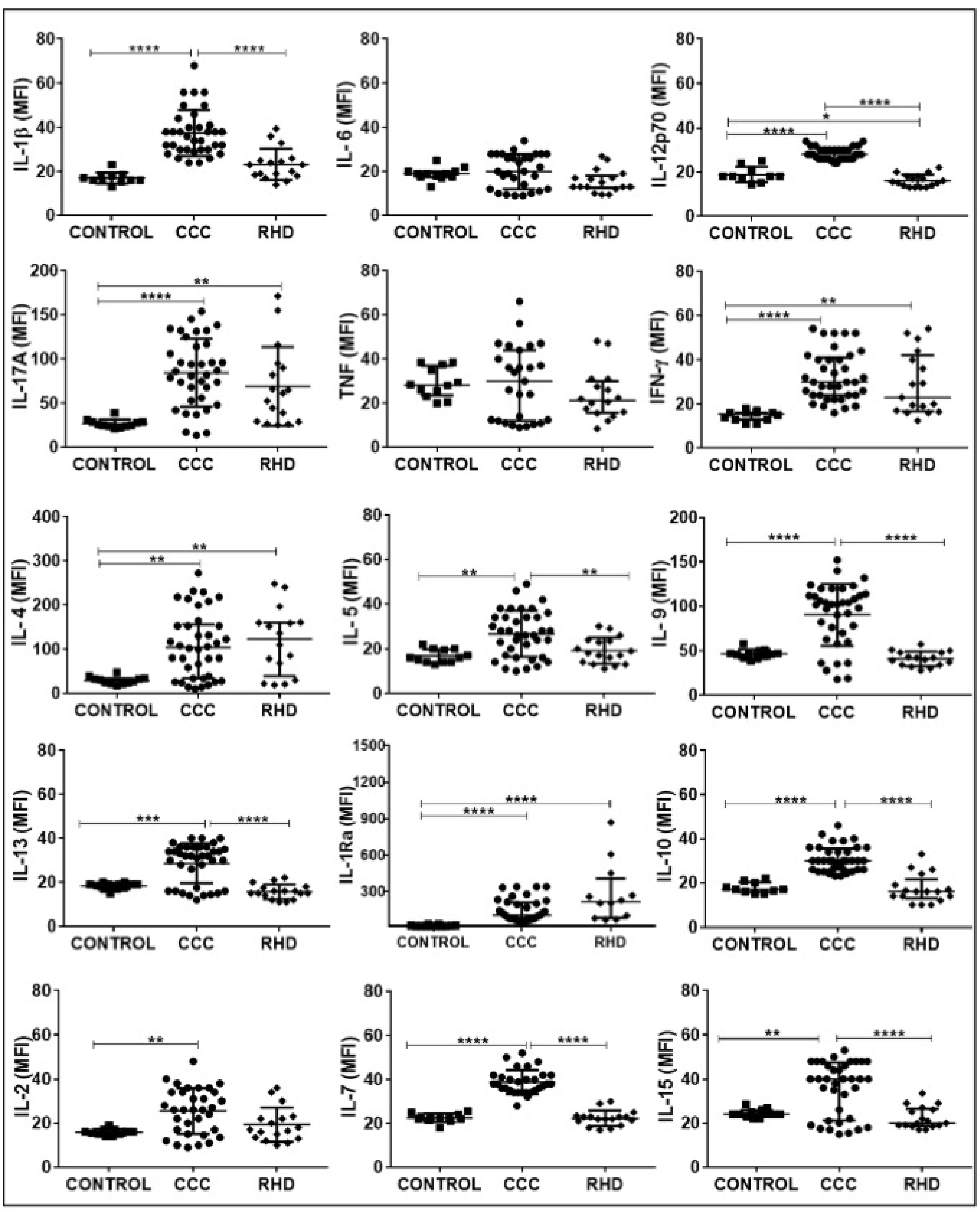
The groups evaluated were CONTROL (n = 12), CCC (Chagas cardiomyopathy, n = 38) and RHD (rheumatic heart disease, n = 17). p values <0.05 were considered statically significant. * p <0.05, ** p <0.005 *** and **** p <0.0001. Plasma soluble cytokine levels were measured using the Bio-Plex ProTM Human Cytokine Standard 27-plex Kit and results are expressed in MFI, as described in Materials and Methods. IL: Interleukin. TNF: Tumor necrosis factor. IFN: Interferon Gamma. IL-1Ra: Interleukin-1 receptor antagonist.
Fig. 2. Comparative analysis of levels of plasma chemokines (A) and growth factors (B) between the study groups.
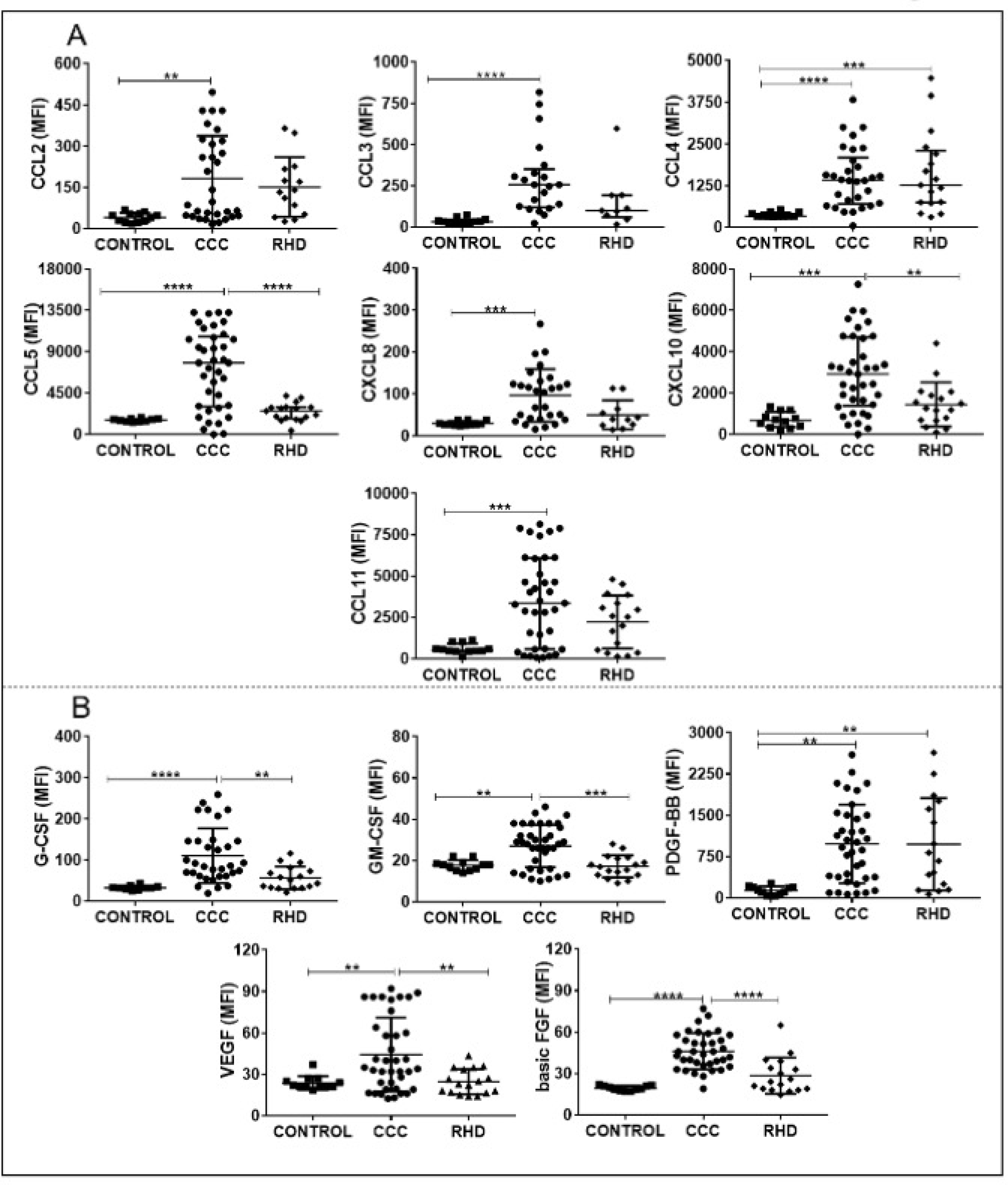
The groups evaluated were CONTROL (n = 12), CCC (Chagas cardiomyopathy, n = 38) and RHD (rheumatic heart disease, n = 17). p values <0.05 were considered statically significant. * p <0.05, ** p <0.005 *** and **** p <0.0001. Plasma soluble cytokine levels were measured using the Bio-Plex ProTM Human Cytokine Standard 27-plex Kit and results are expressed in MFI, as described in Materials and Methods. CCL: chemokine (C-C motif) ligand. CXCL: chemokine (C-X-C motif) ligand. G-CSF: Granulocyte colony-stimulation factor. GM-CSF: Granulocyte macrophage colony-stimulating factor. PDGF: Platelet- derived growth factor. VEGF: vascular endothelial growth factor. FGF basic: Fibroblast growth factor.
Analysis of the levels of soluble factors amongst patients with different heart diseases (CCC x RHD) showed increased levels of cytokines (IL1-beta, IL-12, IL-5, IL-9, IL-13, IL-10, IL-7, IL-15), chemokines (CCL5, CXCL10), and growth factors (G-CSF, GM-CSF, VEGF, basic FGF) in CCC (Fig 1, Figs 2A, 2B and supplementary table 1).
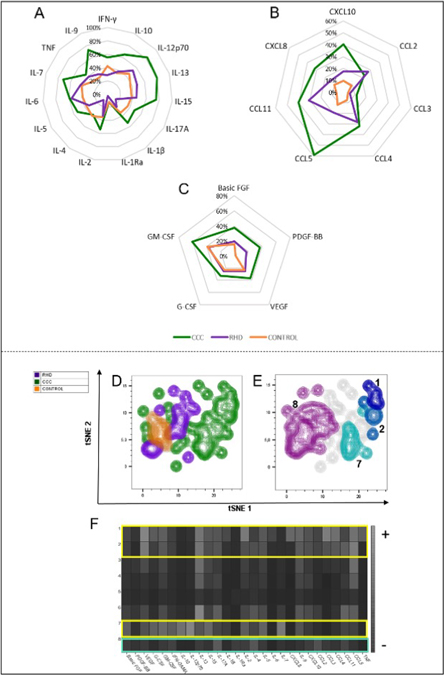
This highly activated immunological profile observed in CCC patients is depicted in a radar graph analysis, showing increased relative levels of cytokines, chemokines, and growth factors (Figs 3A, 3B, and 3C respectively), as compared to the CONTROL group and RHD. These data were reinforced by the tSNE analysis in Fig 3D, demonstrating the stratification between the different groups as seen by separation of distinct islands formed on the map generated by the algorithm. Clusters 1, 2 and 7 in Fig 3E were identified by the FlowSOM analysis and projected onto the tSNE map showing their location in the region occupied by CCC group (observed in Fig 3D, green islands to the right). Group 8 (Fig 3E) is distributed amongst the regions occupied by primarily RHD and CONTROL groups, with some contribution from the CCC group (observed in Fig 3D, purple, orange, and a few green islands to the left). The profile of relative intensity values identified by the cluster explorer showed a profile of greater expression of cytokines, chemokines, and growth factors by clusters 1, 2, and 7 located in the CCC group (Fig 3F, yellow brackets). In contrast, lesser expression of the evaluated molecules was represented by cluster 8, identified predominantly in the RHD and CONTROL groups (Fig 3F, turquoise bracket).
Fig. 3. General profile of the evaluated soluble factors represented by radar graphs and tSNE analysis.
Representative analysis by radar chart with the median plasma levels of (A) cytokines, (B) chemokines and (C) growth factors in samples from CONTROL group (orange line), patients with CCC (green line) or RHD (purple line). (D) Generated tSNE map, showing the stratification between the different groups evaluated after overlapping on the islands formed by the algoritm. (E) Clusters detected by the FlowSOM analysis and projected on the tSNE map to identify the distribution within the groups evaluated. (F) Heat map representation of the relative intensity expression of the molecules performed by the cluster explorer algorithm.
3.2. Protein-protein interaction network analysis reveals enriched pathways associated with cardiac Chagas and rheumatic heart disease.
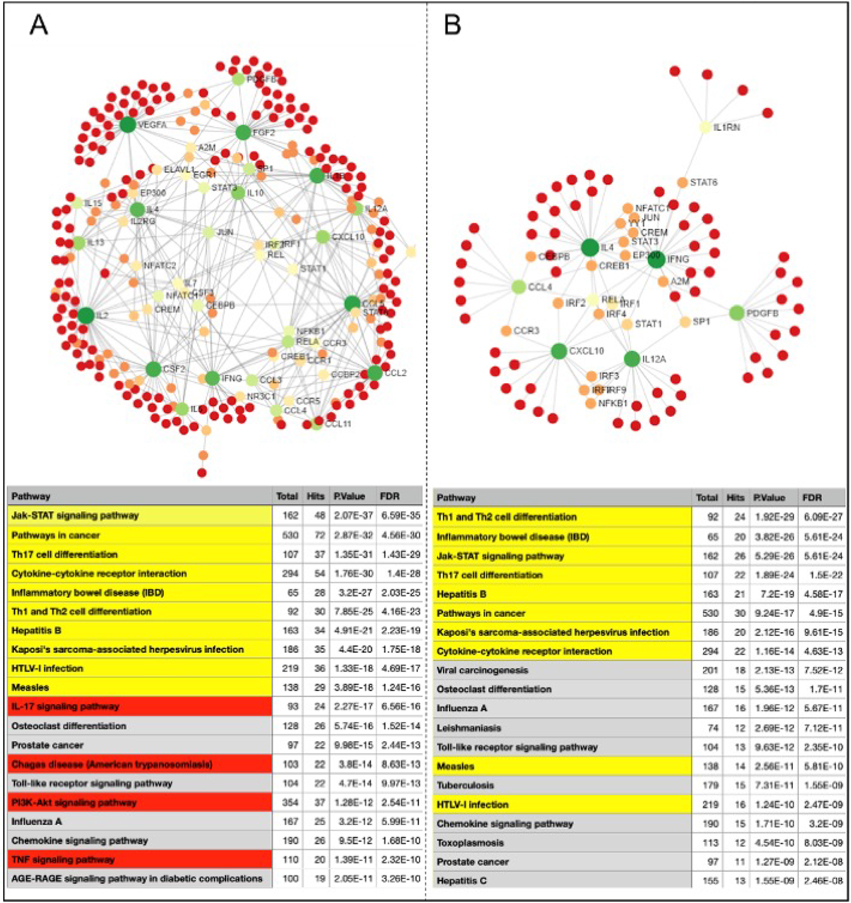
Network analysis using the molecules that were altered in CCC (Fig. 4A) and RHD (Fig. 4B) as compared to CONTROL group shows a greater number of interactions in the CCC network as compared to RHD, given the presence of 9 versus 2 nodes with high centrality degrees (dark green), respectively. In addition, CCC displays connections with 267 molecules potentially influenced by the main node molecules, while RHD displays 90 connections. Interestingly, enriched pathway analysis emerging as a result of interconnections in the network generated by KEGG, showed a predominance of inflammatory networks in both diseases (Fig.4, bottom tables). In addition, while the first 10 networks with lowest false discovery rate (FDR, adjusted p value) in CCC were also found amongst the first 20 of RHD (yellow rows), IL-17, PI3K-Akt and TNF signaling inflammatory pathways were specific for CCC (red rows).
Fig. 4. Network and pathway interactions in Chagas and rheumatic herat diseases.
Protein-protein network interactions were performed considering the molecules altered in CCC and RHD as compared to CONTROL group. Top panels of (A) and (B) show the networks resulting of the analysis for CCC and RHD, respectively. Dark and light green represent rub nodes. Different colors distinguish nodes with different numbers of interactions. Bottom tables show the pathways found using the generated by the Kyoto Encyclopedia of Genes and Genomes (KEGG) algorithm, showing general common pathways between CCC and RHD (grey rows), the correspondence between the top ten hits for CCC in the top 20 for RHD (yellow rows) and the pathways exclusive for CCC (red rows).
3.3. Soluble immune mediators segregate individuals with the different cardiomyopathies.
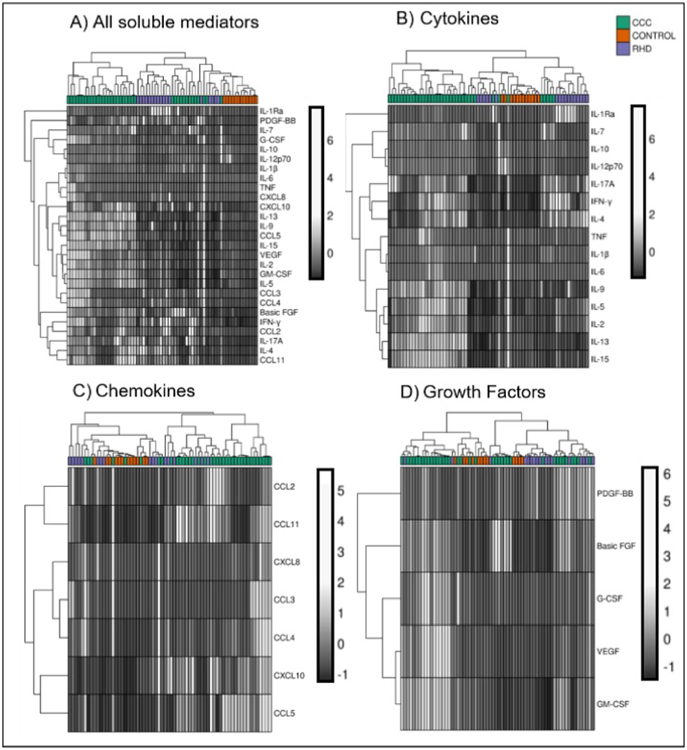
Cluster analysis including all soluble immune mediators segregates patients from RHD, CCC, and the CONTROL group (Fig 5A). Also, strong associations between IL-10 and IL-12, IL-1beta and IL-6, TNF and CXCL8, IL-9 and CCL5, GM-CSF and IL-5, CCL3 and CCL4, IFN-gamma and CCL2, IL-4 and CCL11 were observed (Fig 5A). Cytokine analysis led to an improved clustering of CCC patients and revealed correlations between IL-10 and IL-12, IFN-gamma and IL-4, IL-1beta and IL-6, IL-2 and IL-5, IL - 13 and IL-15 (Fig 5B). The chemokine cluster analysis was less capable of segregating the groups. However, associations were observed between CCL2 and CCL11, CCL3 and CCL4, CCL5 and CXCL10 (Fig 5C). Likewise, the analysis of growth factors showed less capability for segregating the groups. The correlation analysis revealed association only between VEGF and GM-CSF (Fig 5D).
Fig. 5. Cluster analysis of the soluble immune mediators.
(A) Heatmap representation of the plasma levels of all soluble mediators evaluated; and individual analysis by group of molecules: (B) cytokines, (C) chemokines, and (D) growth factors in samples from CONTROL group (n = 12), patients with CCC (n = 38) or RHD (n = 17). Both rows and columns are clustered using correlation distance and average linkage. Light gray indicates higher plasma levels, while dark grey indicates lower plasma level.
3.4. The cytokines IL-12p70, IL1Ra, IL-4, and IL-7 are the main predictors of segregation of the systemic immune response between Chagas and rheumatic heart disease.
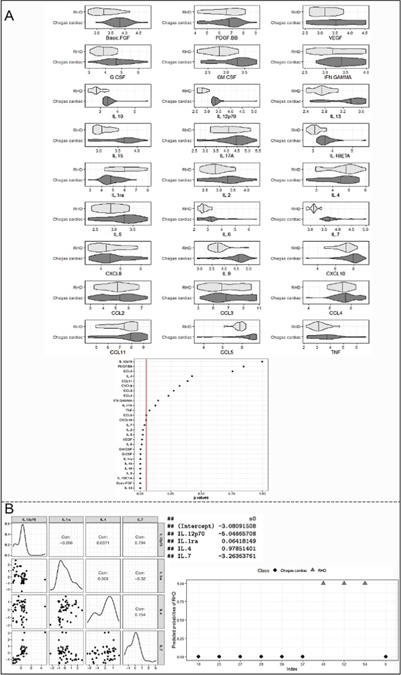
Expression of all immune mediators were compared between CCC and RHD and confirmed factors that are distinct between the diseases, but also showed parameters that did not show statistical significance between groups, suggesting similarities between CCC and RHD. Together, these data were used for a predictive analysis performed by the logistic regression model adjusted by the LASSO method, which demonstrated a complete separation for the cytokine IL-12p70 (Fig 6A). On the other hand, TNF, IL-17, IFN-gamma, CCL4, CCL3, CXCL8, CCL11, IL-4, CCL2, and PDGF-BB molecules did not show statistically significant differences between CCC and RHD, which may indicate immunological characteristics shared between these heart diseases (Fig 6A, p values in bottom panel).
Fig. 6. Identification of predictors of immunological segregation between heart diseases.
(A) Violin Plot depict the relationship of each molecule in the study groups (Chagas cardiac and RHD); p-values are shown in bottom graph (B) marginal distribution of the selected covariates and their correlations. In the main diagonal of the figure, graphs of the marginal distribution (smoothed) of each covariate are presented, while below the diagonal, scatter plots are represented for each pair. Above the diagonal, the estimated correlations are shown. Coefficient values S0 are shown on the side. Graph on the right-hand side shows the discrimination between CCC (diamonds) and RHD (triangles) patients, based on the expression of IL-12p70, IL-1Ra, IL-4 and IL-7. Y-axis shows the predicted probability of RHD and X-axis displays the individual patient ID.
In the final model using all variables, data demonstrated a 100% discrimination power for the combination of the cytokines IL-12p70, IL-1Ra, IL-4, and IL-7 among CCC and RHD groups (Figure 6B, right graph). The left panel in Fig 6B shows the distribution of the selected discriminating variables and their correlations. The data showed a strong positive correlation only between IL-12 and IL-7. In contrast, a weak correlation was demonstrated between IL-12 and IL-4; 1L-1Ra and IL-4; IL-4, and IL-7. A negative correlation was observed between IL-12 and IL-1Ra; IL-1Ra and IL-7. The values of the coefficients s0 were shown next to the figure.
4. Discussion
Heart disorders are the number one killer in the world, encompassing diseases with several distinct etiologies. Cardiopathies of infectious nature disproportionally affect underserved populations, causing millions of deaths and severe morbidity with a tremendous social and economic impact in already impoverished populations. CCC is the deadliest consequence of T. cruzi infection due to progressive conductive and contractile heart alterations[21]. RHD, while rare in developed countries, is still highly prevalent in poorer areas globally and causes thousands of deaths mainly due to heart valve compromise [24]. Despite distinct etiologies and pathologies, the inflammatory immune response plays a crucial role in both diseases. However, whether the pattern of immune response is similar or different in these infectious heart diseases is not clear. This is an important point to address. It will provide information about the pathogenesis of these diseases, provide disease markers, and indicate therapeutic targets for each or both diseases. The findings may also illuminate the understanding of other infectious and inflammatory heart diseases.
In our study, we evaluated the systemic release of soluble mediators in the plasma of individuals with CCC and RHD. Our data point to a strong immunological activation in patients with CCC as shown by elevated plasma levels of cytokines, chemokines, and growth factors in comparison to RHD. This hyperactivation was confirmed by comparative network analysis that showed the broader extent of the immune response in CCC as compared to RHD. Using machine learning analysis, we found that the combination of positive association between IL-12 and IL-7 and a negative association between IL-1Ra and IL-4 completely segregated CCC and RHD patients. These distinctions indicate the involvement of particular immunological pathways in these infectious cardiopathies, providing insight regarding their pathogenesis. Importantly, in addition to identifying immunological distinctions between CCC and RHD, our data also pointed to similarities in the expression of the mediators comparing the two groups, which may suggest common pathways in infectious heart diseases.
Previous studies by our group and others have reported increased levels of inflammatory cytokines in plasma, in peripheral blood mononuclear cells and hearts of patients with CCC [25–30], as well as RHD [31,32]. These molecules can be released in response to lesions in the myocardium, being present locally or systemically and, since they might be responsible in the long term for cardiac dysfunction, they can predict a worse prognosis in cardiovascular diseases [33–35]. Thus, evaluating their levels systemically can be informative of the disease development.
Our data demonstrated an increase in plasma levels of the inflammatory cytokines IL-1beta, IL-12, IL-17A, IFN-gamma in CCC patients as compared to CONTROL. While levels of IL-12, IL-17A and IFN-gamma were also increased in RHD as compared to CONTROL, IL-12, which is mainly derived from innate cells, was even higher in CCC patients as compared to RHD. Analysis of cytokine levels by association dendrogram revealed a strong hierarchical link between the inflammatory cytokines IL-6 and IL-1beta and subsequently with TNF, all mainly derived from innate cells, in CCC but not RHD. This suggests a strong activation of innate cells resulting in the production of inflammatory cytokines in CCC, which corroborates our previous data using distinct study groups [36].
While levels of IL-17, a T-cell derived cytokine, were higher in CCC and RHD in comparison with CONTROL, they did not differ between the cardiopathies. The functional response of IL-17 in RHD is still controversial, being associated with protective response [37] or significant rheumatic mitral stenosis in RHD [38]. In CCC, IL-17 by T lymphocytes is consistently correlated with better ventricular function [39,40]. Thus, clarifying the role of IL-17 in RHD and CCC will help to elucidate whether this cytokine plays similar or antagonistic roles in CCC and RHD. If associated with inflammation in these cardiomyopathies, the inhibition of function of these cytokines may emerge as therapeutic strategies, as described in other non-infectious inflammatory diseases such as psoriasis and rheumatoid arthritis [41–43].
IL-4, IL-5, IL-9, and IL-13 are associated with Th2 response, which are key for modulating Th1-mediated responses [44]. Our results showed increased levels of these molecules in the plasma of CCC patients compared to control and RHD. We also observed increased levels of IL-10 in CCC as compared to RHD and CONTROL. It is interesting that the increase in regulatory cytokines is observed in the CCC patients, which displays a more intense inflammatory profile as compared to RHD. It is possible that these cytokines are induced as an attempt to control inflammation. To some extent they do so, given the long-lasting nature of CCC. However, given the progressive nature of CCC, it is also possible that these cytokines while present, are not fully active due to an altered expression of their receptors. This mechanism was previously shown by us in human leishmaniasis, where the severe mucosal clinical form displays high expression of IL-10, despite presenting a hyperactivated inflammatory response associated with low expression of IL-10 receptor [45]. Further studies regarding cytokine receptor expression and signaling will address this issue. In addition to potential Th1 control, IL-4, IL-9 and IL-13 have also been associated with the formation of tissue fibrosis, thus potentially involved in disease pathology. There was an association of IL-4 with fibrosis and cardiac remodeling in patients with heart fialure [46]. Furthermore, increased levels of IL-9 in patients with ischemic heart disease were associated with left ventricular dysfunction and disease progression [47], and high IL-13 was observed in the plasma of individuals with severe CCC corroborating this hypothesis [48]. These data demonstrate the need for studies regarding the use of anti-IL-4 and anti-IL-13 antibodies in an attempt to neutralize the pro-fibrotic effect of these molecules as a useful therapeutic strategy to limit cardiac fibrosis. Clinical studies targeting the blockade of the IL-4/IL-13 axis have already been reported in other diseases, such as atopic dermatitis and allergic asthma, but they have not yet been studied in the cardiomyopathies evaluated in our study [49,50]. On the other hand, it is important to highlight that the therapeutic blockade of these cytokines can induce an immune imbalance that can exacerbate the Th1 response, emphasizing the importance of further studies.
Our data demonstrated that the plasma levels of proliferative T-cell-derived cytokines IL-2, IL-7, and IL-15 are predominant in CCC as compared to CONTROL. Increased serum IL-2 levels have been described and associated with impaired cardiac function in CCC [51]. IL-7 and IL-15 have been described as important factors for the expansion of CD8 T cells in vitro and maintenance of infiltrating T cells in the heart of CCC patients [52]. Our data also showed that the levels of IL-7 and IL-15 are increased in the plasma of individuals with CCC as compared to RHD, emphasizing a role for these molecules in CCC.
Growth factors are potential modulators of the inflammatory and fibrotic immune response in chronic diseases [20,53,54]. In our study, circulating levels of VEGF, G-CSF, and GM-CSF, involved with the induction of inflammatory mediators, as well as basic FGF, associated with the fibrotic and hypertrophic response, are increased in CCC as compared to CONTROL and RHD, possibly reflecting the dilated nature of CCC. Elevated levels of PDGF-BB were observed in both heart diseases. The accumulation of this molecule in the systemic environment may reflect the fibrotic effect observed in the valvular and heart tissue of patients RHD and CCC, respectively [20,55,56].
The recruitment of activated leukocytes to the sites of inflammatory injury is mediated by the interaction between chemokines and their receptors [57]. Our data showed an increase in the levels of all chemokines in plasma samples from CCC patients compared to CONTROL, reflective of intense inflammation. Cluster analysis demonstrated a strong association between CCL3 and CCL4 in CCC, which bind CCR5 receptor. CCR5 has been associated with CCC [58,59] and is involved in the recruitment of CD8+ T cells, a predominant cell subpopulation in the myocardium of CCC patients [14]. The levels of CCL5 and CXCL10 were higher in CCC as compared to RHD. Additionally, the cluster heatmap analysis revealed a strong association between these molecules. CXCL10 is a protein produced in response to IFN-gamma, which recruits Th1 cells, contributing to the expansion of inflammation [60]. IFN-gamma has been associated with cardiopathy progression in CCC [61]. Interestingly, in RHD, there was an increase as compared to CONTROL only in CCL4, a chemokine associated with the preferential recruitment of CD4+ T lymphocytes, the main subpopulation of T cells found in the inflammatory valve infiltrate of patients with this heart disease [31]. These results are consistent with the differential cellular recruitment to lesion sites in CCC and RHD and indicate potential points of intervention in these diseases.
The LASSO analysis demonstrated that the concomitant positive association between IL-12 and IL-7 and negative association between IL-1Ra and IL-4 were the main predictors of segregation of the immune response between CCC and RHD with a power of 100% discrimination. Of note is the negative association observed with the regulatory cytokines, which may indicate that the pathways for IL-1Ra and IL-4 may be compromised in CCC.
The wealth of information contained within the systemic immune profile is well exploited by implementation of pathway and network analyses, thereby permitting us to investigate potential disease pathways that promote CCC and RHD. These pathway networks provide a global view of the multiple processes involved in disease pathogenesis where clusters of similar pathways emerging as a result of strong interconnections between them allow us to identify predominant biological processes. Our network analysis showed that CCC displays a greater number of node molecules with high centrality, with 267 nodes overall, while RHD displayed 90. This suggests a broader interference in associated immune molecules in CCC and, therefore, involvement of many more pathways. KEGG enrichment analyses identified inflammatory pathways common in both diseases, while IL-17, TNF, and PI3K-Akt signaling pathways are exclusive of CCC. Interestingly, PI3K signaling pathway has been associated with control of parasitism in hearts of mice infected with T. cruzi, as well as in CCC patients [62]. Exploiting the pathways involving these molecules may provide potential targets to treat CCC.
While many differences were observed between the profile in CCC and RHD, our data also shows similarities amongst these diseases. This was not necessarily expected, since the diseases have distinct etiologies and pathology, and suggests that despite such differences, there are pathways commonly activated. Thus, this may have implications for other infectious cardiopathies as well. Univariate analysis of the expression of TNF, IL-17, IFN-gamma, CCL4, CCL3, CXCL8, CCL11, IL-4, CCL2, PDGF did not show statistically significant differences between CCC and RHD, suggesting that these molecules may represent a common pathway in inflammatory cardiopathies. Extending these studies to other heart diseases will be of great value to unveil new pathways of intervention to prevent disease progression.
One limitation of our study was that a correlation analysis between the increased levels of the molecules and the cardiac function of the patients included in the study was not performed. This was mainly due to the fact that the criteria for disease severity are distinct in CCC and RHD. While in CCC ejection fraction is a key measure, this function is not necessarily associated with severity in RHD, in which the clinical parameters of abnormalities involve the echocardiographic evaluation of valve morphology [63,64].
As a conclusion, our findings suggest that heart disease due to Chagas disease is associated with stronger immunological activation as compared to RHD. Despite the infectious and inflammatory nature of both diseases, our study identified factors that can clearly discriminate the immune response between them, unveiling singular pathways in these diseases. In addition, we observed similarities between CCC and RHD that may indicate shared immunological pathways between these heart diseases, and potentially others. Taken together, our data shows the first indication of potential immunological targets for specific and broad intervention in CCC and RHD.
Supplementary Material
8. Acknowledgments
We thank Ana Carolina C. Azevedo and Lorena J.S. Santos for their assistance in conducting the Luminex Bioassay. Lucas Rizzo for assistance with t-SNE analyzes.
6. Funding Statement
This work was supported by grants from NIH, NIAID, FAPEMIG, CAPES, INCT-DT. KJG, WOD, MCPN, are CNPq fellows. NIH and NIAID provided funding for the acquisition of the Luminex bioassay and materials for conducting the experiment. EGAN is a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior) and FAPEMIG (Fundação de Amparo à pesquisa de Minas Gerais) fellow.
Abbreviations:
- CCC
Chronic Chagas disease cardiomyopathy
- RHD
rheumatic heart disease
- ChD
Chagas disease
- KEGG
Kyoto encyclopedia of genes and genomes
- t-SNE
t-distributed stochastic neighbor-embedding
Footnotes
7. Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- [1].World Health Organization. Chagas disease (American trypanosomiasis). https://www.who.int/chagas/epidemiology/en/. (acessed 03 September 2020).
- [2].Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. , Disability-adjusted life years ( DALYs ) for 291 diseases and injuries in 21 regions, 1990 – 2010 : a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380 (2014) 1990–2010. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- [3].Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P, The reality of heart failure in Latin America, J. Am. Coll. Cardiol 62 (2013) 949–958. 10.1016/j.jacc.2013.06.013. [DOI] [PubMed] [Google Scholar]
- [4].Pereira Nunes MDC, De Melo Barbosa M, Andrade Brum VA, Da Costa Rocha MO, Morphofunctional characteristics of the right ventricle in Chagas’ dilated cardiomyopathy, Int. J. Cardiol 94 (2004) 79–85. 10.1016/j.ijcard.2003.05.003. [DOI] [PubMed] [Google Scholar]
- [5].Guilherme L, Oshiro SE, Faé KC, Cunha-Neto E, Renesto G, Goldberg AC, Tanaka AC, Pomerantzeff PMA, Kiss MH, Silva C, Guzman F, Patarroyo ME, Southwood S, Sette A, Kalil J, T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients, Infect. Immun 69 (2001) 5345–5351. 10.1128/IAI.69.9.5345-5351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J, Autoimmunity in Chagas’ disease: Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient, J. Clin. Invest 98 (1996) 1709–1712. 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bach-Elias M, Bahia D, Teixeira DC, Cicarelli RMB, Presence of autoantibodies against small nuclear ribonucleoprotein epitopes in Chagas’ patients’ sera, Parasitol. Res 84 (1998) 796–799. 10.1007/s004360050490. [DOI] [PubMed] [Google Scholar]
- [8].Cunha-Neto E, Duranti M, Gruber A, Zingales B, De Messias I, Stolf N, Bellotti G, Patarroyo ME, Pilleggi F, Kalil J, Autoimmunity in Chagas disease cardiopathy: Biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen, Proc. Natl. Acad. Sci. U. S. A 92 (1995) 3541–3545. 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cunha-Neto E, Bilate AM, Hyland KV, Fonseca SG, Kalil J, Engman DM, Induction of cardiac autoimmunity in Chagas heart disease: A case for molecular mimicry, Autoimmunity. 39 (2006) 41–54. 10.1080/08916930500485002. [DOI] [PubMed] [Google Scholar]
- [10].Guilherme L, Dulphy N, Douay C, Coelho V, Cunha-Neto E, Oshiro SE, Assis RV, Tanaka AC, Pomerantzeff PMA, Charron D, Toubert A, Kalil J, Molecular evidence for antigen-driven immune responses in cardiac lesions of rheumatic heart disease patients, Int. Immunol 12 (2000) 1063–1074. 10.1093/intimm/12.7.1063. [DOI] [PubMed] [Google Scholar]
- [11].Benvenuti LA, Roggério A, Nishiya AS, Campos SV, Fiorelli AI, Levi JE, Trypanosoma cruzi persistence in the native heart is associated with high-grade myocarditis, but not with Chagas’ disease reactivation after heart transplantation, J. Hear. Lung Transplant 33 (2014) 698–703. 10.1016/j.healun.2014.01.920. [DOI] [PubMed] [Google Scholar]
- [12].Fae KC, da Silva DD, Oshiro SE, Tanaka AC, Pomerantzeff PMA, Douay C, Charron D, Toubert A, Cunningham MW, Kalil J, Guilherme L, Mimicry in Recognition of Cardiac Myosin Peptides by Heart-Intralesional T Cell Clones from Rheumatic Heart Disease, J. Immunol 176 (2014) 5662–5670. 10.4049/jimmunol.176.9.5662. [DOI] [PubMed] [Google Scholar]
- [13].Guilherme L, Oshiro SE, Faé KC, Cunha-Neto E, Renesto G, Goldberg AC, Tanaka AC, Pomerantzeff PMA, Kiss MH, Silva C, Guzman F, Patarroyo ME, Southwood S, Sette A, Kalil J, T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients, Infect. Immun 69 (2001) 5345–5351. 10.1128/IAI.69.9.5345-5351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reis DD, Jones EM, Tostes S, Lopes ER, Gazzinelli G, Colley DG, McCurley TL, Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: Presence of tumor necrosis factor-α+ cells and dominance of granzyme A+, CD8+ lymphocytes, Am. J. Trop. Med. Hyg 48 (1993) 637–644. 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- [15].El-Demellawy M, El-Ridi R, Guirguis NI, Alim MA, Kotby A, Kotb M, Preferential recognition of human myocardial antigens by T lymphocytes from rheumatic heart disease patients, Infect. Immun 65 (1997) 2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomes JAS, Bahia-Oliveira LMG, Rocha MOC, Busek SCU, Tekeira MM, Silva JS, Correa-Oliveira R, Type 1 chemokine receptor expression in Chagas’ disease correlates with morbidity in cardiac patients, Infect. Immun 73 (2005) 7960–7966. 10.1128/IAI.73.12.7960-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cunha-Neto E, Nogueira LG, Teixeira PC, Ramasawmy R, Drigo SA, Goldberg AC, Fonseca SG, Bilate AM, Kalil J, Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy, Mem. Inst. Oswaldo Cruz 104 (2009) 252–258. 10.1590/S0074-02762009000900032. [DOI] [PubMed] [Google Scholar]
- [18].Guilherme L, Kalil J, Rheumatic Heart Disease: Molecules Involved in Valve Tissue Inflammation Leading to the Autoimmune Process and Anti-S. pyogenes Vaccine, Front. Immunol 4 (2013) 1–6. 10.3389/fimmu.2013.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Klinkhammer BM, Floege J, Boor P, PDGF in organ fibrosis, Mol. Aspects Med 62 (2018) 44–62. 10.1016/j.mam.2017.11.008. [DOI] [PubMed] [Google Scholar]
- [20].Gallini R, Lindblom P, Bondjers C, Betsholtz C, Andrae J, PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice, Exp. Cell Res 349 (2016) 282–290. 10.1016/j.yexcr.2016.10.022. [DOI] [PubMed] [Google Scholar]
- [21].Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, Dutra WO, Gascon J, Morillo CA, Oliveira-Filho J, Ribeiro ALP, Marin-Neto JA, Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association, 2018. 10.1161/cir.0000000000000599. [DOI] [PubMed] [Google Scholar]
- [22].Belkina AC, Ciccolella CO, Anno R, Halpert R, Snyder-cappione JE, stochastic neighbor embedding improve visualization and analysis of large datasets, Nat. Commun 10 (2019) 1–12. 10.1038/s41467-019-13055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, Saeys Y, FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data, Cytom. Part A 87 (2015) 636–645. 10.1002/cyto.a.22625. [DOI] [PubMed] [Google Scholar]
- [24].Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA, Global, regional, & national burden of rheumatic heart disease, 1990–2015, N. Engl. J. Med 377 (2017) 713–722. 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- [25].Menezes CAS, Rocha MOC, Souza PEA, Chaves ACL, Gollob KJ, Dutra WO, Phenotypic and functional characteristics of CD28+ and CD28- cells from chagasic patients: Distinct repertoire and cytokine expression, Clin. Exp. Immunol 137 (2004) 129–138. 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Passos LSA, Magalhães LMD, Soares RP, Marques AF, do C M.Nunes P, Gollob KJ, Dutra WO, Specific activation of CD4–CD8– double-negative T cells by Trypanosoma cruzi-derived glycolipids induces a proinflammatory profile associated with cardiomyopathy in Chagas patients, Clin. Exp. Immunol 190 (2017) 122–132. 10.1111/cei.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abel LCJ, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D, Bocchi EA, Teixeira HC, Mady C, Kalil J, Cunha-Neto E, Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-γ response to Trypanosoma cruzi infection, J. Autoimmun 17 (2001) 99–107. 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- [28].Reis MM, Higuchi MDL, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, Pileggi F, An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: Correlation with the presence of myocardial Trypanosoma cruzi antigens, Clin. Immunol. Immunopathol 83 (1997) 165–172. 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- [29].Sousa GR, Gomes JAS, Fares RCG, Damásio MPDS, Chaves AT, Ferreira KS, Nunes MCP, Medeiros NI, Valente VAA, Corrêa-Oliveira R, Rocha MODC, Plasma cytokine expression is associated with cardiac morbidity in chagas disease, PLoS One. 9 (2014) 1–9. 10.1371/journal.pone.0087082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dutra WO, Gollob KJ, Pinto-Dias JC, Gazzinelli G, Correa-Oliveira R, Coffman RL, Carvalho-Parra JF, Cytokine mRNA Profile of Peripheral Blood Mononuclear Cells Isolated from Individuals with Trypanosoma cruzi Chronic Infection, Scand. J. Immunol 45 (1997) 74–80. [DOI] [PubMed] [Google Scholar]
- [31].Toor D, Vohra H, Immune responsiveness during disease progression from acute rheumatic fever to chronic rheumatic heart disease, Microbes Infect. 14 (2012) 1111–1117. 10.1016/j.micinf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- [32].Ellis NMJ, Li Y, Hildebrand W, Fischetti VA, Cunningham MW, T Cell Mimicry and Epitope Specificity of Cross-Reactive T Cell Clones from Rheumatic Heart Disease, J. Immunol 175 (2005) 5448–5456. 10.4049/jimmunol.175.8.5448. [DOI] [PubMed] [Google Scholar]
- [33].Zarrouk-Mahjoub S, Zaghdoudi M, Amira Z, Chebi H, Khabouchi N, Finsterer J, Mechmeche R, Ghazouani E, Pro- and anti-inflammatory cytokines in post-infarction left ventricular remodeling, Int. J. Cardiol 221 (2016) 632–636. 10.1016/j.ijcard.2016.07.073. [DOI] [PubMed] [Google Scholar]
- [34].Deten A, Volz HC, Driest W, Zimmer HG, Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats, Cardiovasc. Res 55 (2002) 329–340. 10.1016/S0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- [35].Kumar S, Maulik S, Role of Cytokines in Heart Failure Journal of Pharmacological Reports, J. Pharmacol. Reports 2 (2017) 1–6. [Google Scholar]
- [36].Souza PEA, Rocha MOC, Rocha-Vieira E, Menezes CAS, Chaves ACL, Gollob KJ, Dutra WO, Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity, Infect. Immun 72 (2004) 5283–5291. 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Diamantino Soares AC, Araújo Passos LS, Sable C, Beaton A, Ribeiro VT, Gollob KJ, Dutra WO, Nunes MCP, Circulating cytokines predict severity of rheumatic heart disease, Int. J. Cardiol 289 (2019) 107–109. 10.1016/j.ijcard.2019.04.063. [DOI] [PubMed] [Google Scholar]
- [38].Bilik MZ, Kaplan I, Polat N, Akil MA, Akyüz A, Acet H, Yüksel M, Inci U, Kayan F, Toprak N, Serum levels of IL-17 and IL-23 in patients with rheumatic mitral stenosis, Med. (United States) 95 (2016) e3562. 10.1097/MD.0000000000003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Magalhães LMD, Villani FNA, do C M.Nunes P, Gollob KJ, Rocha MOC, Dutra WO, High interleukin 17 expression is correlated with better cardiac function in human Chagas disease., J. Infect. Dis 207 (2013) 661–665. 10.1093/infdis/jis724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guedes PMM, Gutierrez FRS, Silva GK, Dellalibera-Joviliano R, Rodrigues GJ, Bendhack LM, Rassi A, Rassi A, Schmidt A, Maciel BC, Marin Neto JA, Silva JS, Deficient regulatory T cell activity and low frequency of IL-17-producing T cells correlate with the extent of cardiomyopathy in human Chagas’ disease, PLoS Negl. Trop. Dis 6 (2012). 10.1371/journal.pntd.0001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, Armstrong AW, Stingl G, Kimball AB, Bachelez H, Wu JJ, Crowley J, Langley RG, Blicharski T, Paul C, Lacour J-P, Tyring S, Kircik L, Chimenti S, Callis Duffin K, Bagel J, Koo J, Aras G, Li J, Song W, Milmont CE, Shi Y, Erondu N, Klekotka P, Kotzin B, Nirula A, Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis, N. Engl. J. Med 373 (2015) 1318–1328. 10.1056/nejmoa1503824. [DOI] [PubMed] [Google Scholar]
- [42].Griffiths CEM, Reich K, Lebwohl M, Van De Kerkhof P, Paul C, Menter A, Cameron GS, Erickson J, Zhang L, Secrest RJ, Ball S, Braun DK, Osuntokun OO, Heffernan MP, Nickoloff BJ, Papp K, Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials, Lancet. 386 (2015) 541–551. 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- [43].Blanco H.R. Francisco J., Moricke Rudiger, Dokoupilova Eva, Christine Codding, Neal 4 Jeffrey, Andersson Mats, Rohrer Susanne, Secukinumab in Active Rheumatoid Arthritis, 69 (2017) 1144–1153. 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- [44].Spellberg B, Edwards JE, Type 1 / Type 2 Immunity in Infectious Diseases, Clin. Infect. Dis 90509 (2001) 76–102. [DOI] [PubMed] [Google Scholar]
- [45].Faria DR, Gollob KJ, Barbosa J, Schriefer A, Machado PRL, Lessa H, Carvalho LP, Romano-Silva MA, De Jesus AR, Carvalho EM, Dutra WO, Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis, Infect. Immun 73 (2005) 7853–7859. 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Roselló-Lletí E, Rivera M, Bertomeu V, Cortés R, Jordán A, González-Molina A, Interleukin-4 and Cardiac Fibrosis in Patients With Heart Failure, Rev. Española Cardiol. (English Ed. 60 (2007) 777–780. 10.1016/s1885-5857(08)60014-6. [DOI] [PubMed] [Google Scholar]
- [47].Cappuzzello C;, Di Vito L, Melchionna R, Melillo G, Silvestri L, Cesareo E, Crea F, Liuzzo G, Facchiano A, Capogross MC, Napolitano M, Increase of plasma IL-9 and decrease of plasma IL-5, IL-7, and IFN- in patients with chronic heart failure, J. Transl. Med 9 (2011) 1–7. http://www.translational-medicine.com/content/9/1/28%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=2011178315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].De Alba-Alvarado M, Salazar-Schettino PM, Jiménez-Álvarez L, Cabrera-Bravo M, García-Sancho C, Zenteno E, Vazquez-Antona C, Cruz-Lagunas A, Zúñiga J, Bucio-Torres MI, Th-17 cytokines are associated with severity of Trypanosoma cruzi chronic infection in pediatric patients from endemic areas of Mexico, Acta Trop. 178 (2018) 134–141. 10.1016/j.actatropica.2017.11.009. [DOI] [PubMed] [Google Scholar]
- [49].Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW, A critical evaluation of Anti-IL-13 and Anti-IL-4 strategies in severe asthma, Int. Arch. Allergy Immunol 170 (2016) 122–131. 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- [50].Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, Ardeleanu M, Weinstein SP, Pirozzi G, Guttman-Yassky E, Suárez-Fariñas M, Hager MD, Stahl N, Yancopoulos GD, Radin AR, Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis, N. Engl. J. Med 371 (2014) 130–139. 10.1056/nejmoa1314768. [DOI] [PubMed] [Google Scholar]
- [51].Ward LS, Guariento ME, Fernandes GA, Serum cytokines in chronic Chagas ‘ disease Citocinas séricas na forma crônica da doenca de Chagas, Rev. Soc. Bras. Med. Trop 32 (1999) 285–289. [DOI] [PubMed] [Google Scholar]
- [52].Fonseca SG, Reis MM, Coelho V, Nogueira LG, Monteiro SM, Mairena EC, Bacal F, Bocchi E, Guilherme L, Zheng XX, Liew FY, Higuchi ML, Kalil J, Cunha-Neto E, Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic chagas disease cardiomyopathy, Scand. J. Immunol 66 (2007) 362–371. 10.1111/j.1365-3083.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- [53].Shiomi A, Usui T, Pivotal Roles of GM-CSF in Autoimmunity and Inflammation, Mediators Inflamm. 2015 (2015) 1–13. 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Christensen AD, Haase C, Cook AD, Hamilton JA, Granulocyte colony-stimulating factor ( G-CSF ) plays an important role in immune complex-mediated arthritis, (2016) 1235–1245. 10.1002/eji.201546185. [DOI] [PubMed] [Google Scholar]
- [55].Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW, Kanzler S, Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice q, 45 (2006) 419–428. 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [56].Mo S, Fu M, Hellman U, Malm L, Ma L, Growth Factor PDGF-BB Stimulates Cultured Cardiomyocytes to Synthesize the Extracellular Matrix Component Hyaluronan, 5 (2010). 10.1371/journal.pone.0014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dusi V, Ghidoni A, Ravera A, De Ferrari GM, Calvillo L, Chemokines and Heart Disease : A Network Connecting Cardiovascular Biology to Immune and Autonomic Nervous Systems, 2016 (2016). 10.1155/2016/5902947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nogueira LG, Santos RHB, Ianni BM, Fiorelli AI, Mairena EC, Benvenuti LA, Frade A, Donadi E, Dias F, Saba B, Wang HTL, Fragata A, Sampaio M, Hirata MH, Buck P, Mady C, Bocchi EA, Stolf NA, Kalil J, Cunha-Neto E, Myocardial Chemokine Expression and Intensity of Myocarditis in Chagas Cardiomyopathy Are Controlled by Polymorphisms in CXCL9 and CXCL10, PLoS Negl. Trop. Dis 6 (2012). 10.1371/journal.pntd.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Batista AM, Alvarado-Arnez LE, Alves SM, Melo G, Pereira IR, de LA Ruivo S, da Silva AA, Gibaldi D, do T da Silva ESP, de Lorena VMB, de Melo AS, de AK Soares A, da M Barros S, Costa VMA, Cardoso CC, Pacheco AG, Carrazzone C, Oliveira W, Moraes MO, Lannes-Vieira J, Genetic polymorphism at CCL5 is associated with protection in Chagas’ heart disease: Antagonistic participation of CCR1+ and CCR5+ cells in chronic chagasic cardiomyopathy, Front. Immunol 9 (2018) 1–16. 10.3389/fimmu.2018.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Van Den Borne P, Quax PHA, Hoefer IE, Pasterkamp G, The multifaceted functions of CXCL10 in cardiovascular disease, Biomed Res. Int 2014 (2014). 10.1155/2014/893106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gomes JAS, Rocha MOC, Gazzinelli G, Fiocruz R, Evidence that Development of Severe Cardiomyopathy in Human Chagas ’ Disease Is Due to a Th1-Specific Immune Response, Infect. Immun 71 (2003) 1185–1193. 10.1128/IAI.71.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Silva MC, Davoli-ferreira M, Medina TS, Sesti-costa R, Silva GK, Lopes CD, Cardozo LE, Gava FN, Lyroni K, Dias FC, Frade AF, Baron M, Nakaya HI, Figueiredo F, Alves-filho JC, Cunha FQ, Tsatsanis C, Chevillard C, Cunha-neto E, Hirsch E, Silva JS, Cunha TM, Canonical PI3KÎ3 signaling in myeloid cells restricts Trypanosoma cruzi infection and dampens chagasic myocarditis, Nat. Commun 234 (2018). 10.1038/s41467-018-03986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].do C M.Nunes P, Barbosa MM, Ribeiro ALP, Fenelon LMA, Rocha MOC, Predictors of Mortality in Patients With Dilated Cardiomyopathy: Relevance of Chagas Disease as an Etiological Factor, Rev. Española Cardiol. (English Ed 63 (2010) 788–797. 10.1016/s1885-5857(10)70163-8. [DOI] [PubMed] [Google Scholar]
- [64].Tozatto M, Coelho B, Silva L, Passos A, Guarçoni FV, Marcelo J, Aguiar DS, Benjamim R, Mendonça T, De Paula N, Figueiredo R, Cecília M, Nassif L, Gomes NFA, Tan TC, Carmo M, Nunes P, Mini Review Rheumatic heart disease in the modern era : recent developments and current challenges, (2019) 1–9. 10.1590/0037-8682-0041-2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.