Abstract
Background and Objectives
CSF in antibody-defined autoimmune encephalitis (AE) subtypes shows subtype-dependent degrees of inflammation ranging from rare and often mild to frequent and often robust. AEs with NMDA receptor antibodies (NMDAR-E) and leucine-rich glioma-inactivated protein 1 antibodies (LGI1-E) represent opposite ends of this spectrum: NMDAR-E with typically frequent/robust and LGI1-E with rare/mild CSF inflammation. For a more in-depth analysis, we characterized CSF findings in acute, therapy-naive NMDAR-E and LGI1-E in a multicentric, retrospective, cross-sectional setting.
Methods
Eighty-two patients with NMDAR-E and 36 patients with LGI1-E from the GErman NEtwork for Research of AuToimmune Encephalitis (GENERATE) with lumbar puncture within 90 days of onset and before immunotherapy were included. CSF parameters comprised leukocytes, oligoclonal bands (OCBs), and CSF/serum ratios for albumin, immunoglobulin G (IgG), A (IgA), and M (IgM), the latter 3 converted to Z scores according to Reiber formulas. The MRZ reaction was tested in 14 patients with NMDAR-E and 6 patients with LGI1-E, respectively.
Results
CSF was abnormal in 94% of NMDAR-E but only in 36% of LGI1-E patients. Robust quantitative intrathecal immunoglobulin synthesis (IIS, IgG > IgM >> IgA) was characteristic for NMDAR-E, but absent in LGI-E. In NMDAR-E, CSF leukocytes were higher when IIS was present or more pronounced. In addition, in NMDAR-E, CSF leukocytes were lower and IIS occurred less often and if so to a lesser degree at older age. Patients with NMDAR-E with severe functional impairment more often had positive OCBs. In CSF obtained later than 3 weeks of onset, leukocytes were lower. In parallel, the correlation of leukocytes with IIS disappeared as IIS was partially independent of disease duration. The MRZ reaction was positive in 5 (36%) patients with NMDAR-E. All these associations were completely absent in LGI1-E. Here, younger patients showed more blood-CSF barrier dysfunction. In LGI1-E, but not in NMDAR-E, the blood-CSF barrier was more dysfunctional when CSF leukocytes were higher.
Discussion
NMDAR-E and LGI-E differ in their typical extent of CSF inflammation. In addition, the patterns formed by the different inflammatory CSF parameters and their relationship with disease severity, age, and disease duration are subtype-characteristic. Moreover, signs for multiple sclerosis-like chronic inflammation are present in a subgroup of patients with NMDAR-E. These CSF patterns might be markers for the different immunopathogeneses of LGI1-E and NMDAR-E.
The 2 most common subtypes of autoimmune encephalitis (AE) are AE with antibodies against NMDA receptors (NMDAR-E) and AE with leucine-rich glioma inactivated protein-1 (LGI1-E).1,2 NMDAR-E and LGI1-E are quite different: NMDAR-E mostly affects young women,3 whereas LGI1-E tends to occur more frequently at older age and in men.2 NMDAR-E typically progresses to a global encephalitic syndrome with decreased consciousness, stereotypic movements, and vegetative dysfunction,3 whereas LGI1-E is a typical limbic AE.2 On cranial MRI, many patients with LGI1-E show mesiotemporal T2-hyperintensities,2 whereas in NMDAR-E, the MRI is frequently normal, although heterogeneous lesions also involving white matter are found in about half of the patients.3 A recent systematic analysis of diverse AE subtypes with regard to published basic CSF parameters comprising leukocytes, total protein, and oligoclonal bands (OCBs) revealed 2 different clusters: together with AEs with contactin-associated protein-like 2 (CASPR2), γ-aminobutyric acid (GABAA), and glycine receptor antibodies, LGI1-E typically showed scarce and infrequent CSF inflammation, whereas robust and frequent inflammation was characteristic for NMDAR-E and AEs with dipeptidyl-peptidase-like protein-6 (DPPX, GABAB, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antibodies.4 To complement this systematic analysis,4 we performed a multicentric retrospective analysis of the detailed inflammatory CSF findings in therapy-naive patients with LGI1- and NMDAR-E enrolled in the registry of the German Network of Research on Autoimmune Encephalitis (GENERATE) with CSF obtained within 90 days from clinical onset. The parameters included not only CSF leukocytes, blood-CSF barrier function, and OCBs but also quantitative intrathecal immunoglobulin synthesis for immunoglobulin G (IgG), A (IgA), and M (IgM). For a subset of patients with CSF/serum samples still available, an analysis of the MRZ reaction (M = measles, R = rubella, Z = varicella zoster [VZV]), a marker for polyspecific intrathecal synthesis of pathogen-specific IgG typical of MS was analyzed. The mutual interactions of different CSF parameters and their associations with disease duration, severity, and age were analyzed.
Methods
Patient Identification
GENERATE is a multicentric, combined retrospective and prospective registry for patients with AE in Germany (generate-net.de/) recruiting since 2013. For this project, patients were selected according to the following criteria: (1) enrollment before January 1, 2017, (2) NMDAR antibodies in CSF or LGI1 antibodies in serum and/or CSF positive, (3) no recent infectious encephalitis, and (4) complete first CSF examination, including leukocyte count, OCB, and CSF/serum ratios for albumin (QAlb), IgG (QIgG), IgA (QIgA), and IgM (QIgM) obtained within 90 days after onset without prior immunomodulatory therapy (Figure 1). Basic demographic variables, clinical presentation, MRI and EEG findings, as needed to test the fulfillment of recently suggested diagnostic criteria for AE,5 and severity of functional impairment at the time of lumbar puncture (LP) as the modified Rankin Scale (mRS) score6 were extracted from the GENERATE database.
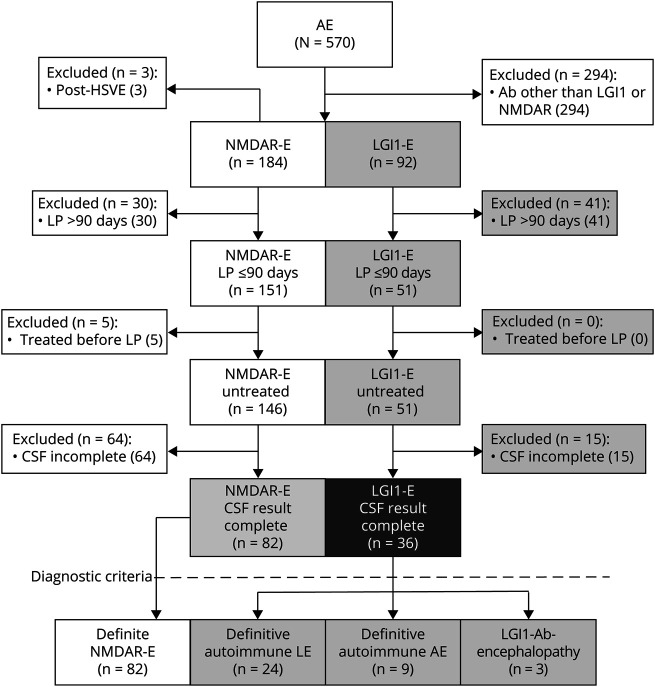
Figure 1. Study Profile.
Flowchart depicting the enrollment process. AE = autoimmune encephalitis; HSVE = herpes simplex virus encephalitis; AB = antibody.
Normal Values and Reiber Formulas
All laboratory analyses except pathogen-specific CSF/serum antibody indices (AIs) were performed locally at the time of LP. Pleocytosis was defined as >4 leukocytes/µL. Age-normalized QAlb (QAlb/Qlim) was calculated by dividing QAlb by the age-dependent upper limit (Qlim; 4+age/15 × 10−3).7 When CSF IgM was below the lower limit of quantification, QIgM was calculated with the CSF IgM set to the lowest level of detection of the respective laboratory. For 2 data points, the lower limits of quantification were not available. Thus, these data points were omitted from the univariate analysis and imputed as median QIgM of the respective AE subtype for multivariate analyses. The definitive quantitative IIS of IgG, IgA, and IgM for Table was diagnosed when QIgG, QIgA, and QIgM were higher than the upper limit, Qlim(high), Qmean + 3 SD according to Reiber8 formulas. Reiber diagrams were generated using the CSF Research Tool/Reibergrams (Albaum IT-Solutions, Frankfurt am Main, Germany). For IIS Z scores, the difference of each QIgG/A/M from Qmean was converted into the number of SDs above or below Qmean. Z scores were categorized as different IIS probabilities: Z > 3 definitive, 2 < Z ≤ 3 probable, 1 < Z ≤ 2 possible, and ≤1 unlikely (eFigure 1A, links.lww.com/NXI/A600). Z sores were graphically presented and adjusted as described in the Supplementary Methods Section (eFigures 1–4, links.lww.com/NXI/A600).
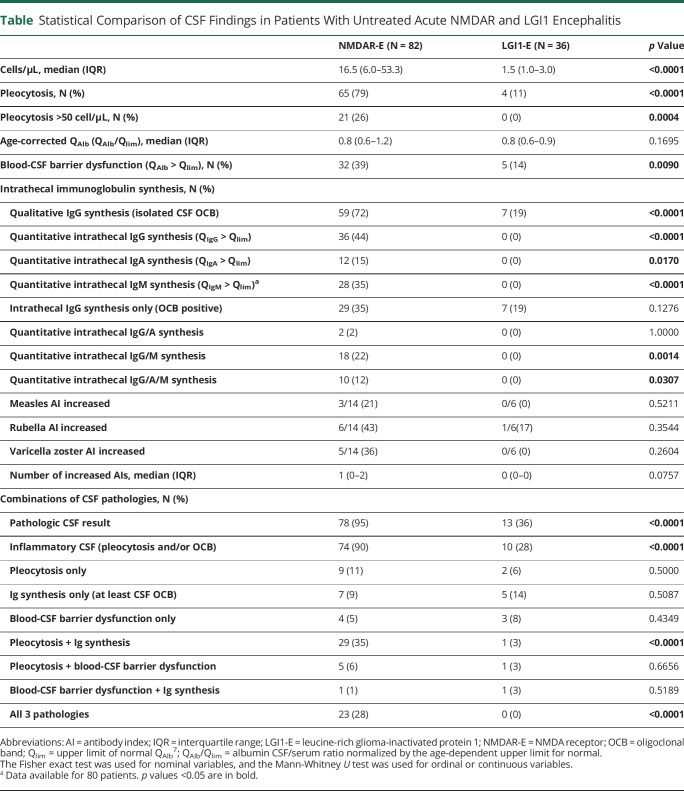
Table.
Statistical Comparison of CSF Findings in Patients With Untreated Acute NMDAR and LGI1 Encephalitis
Quantification of Pathogen-Specific CSF/Serum Antibody Indices
Pathogen-specific AIs for measles, rubella, and VZV from the initial CSF/serum samples were determined centrally and retrospectively using ELISAs (Virion\Serion, Würzburg, Germany) according to the manufacturer with the following modifications: sera were diluted 1:5,000 and 1:20,000, CSF 1:25 and 1:100, incubated for 3 hours at 37°C under constant agitation (250 rpm), and detection antibody was replaced by a horse-radish peroxidase-coupled rabbit anti-human IgG (Dako Agilent, Waldbronn Germany, #P0214). AIs were calculated as described with an upper normal limit of 1.4.9 The data for the 14 patients with NMDAR-E and 6 patients with LGI1-E reported here are analyzed in more detail with additional patients and pathogen-specific AIs in the accompanying publication.10
Statistics
Statistical analysis was performed using GraphPad Prism (GraphPad Inc., La Jolla, US) and SAS, version 9.4 (SAS Institute Cary, NC). Categorical variables were analyzed using the Fisher exact test or χ2 test as appropriate. For ordinal and continuous variables, the median and interquartile range (IQR) were calculated. The Mann-Whitney U test was used for 2 and the Kruskal-Wallis test followed by the Dunn multiple comparisons test for more than 2 groups. The Spearman rank correlation and the Pearson correlation coefficient were used to investigate associations between 2 continuous variables as appropriate. The mRS score was dichotomized into 2 groups, 0–2 (mild impairment) and 3–5 (severe impairment). Because of the differences in group size and range in ages, the NMDAR-E cohort was trichotomized according to age (≤20 years, 21–40 years, and >40 years), whereas the LGI-E cohort was dichotomized (≤60 years vs >60 years), a strategy that led to subcohorts not disproportionately different in size for univariate analysis. Multiple logistic regression and multiple linear regression analyses were performed to investigate the effect of different parameters on categorical and continuous variables, respectively. For these analyses, patients with NMDAR-E were dichotomized by age applying a cutoff of 20 years as indicated by the univariate analyses. Complex associations are graphically presented as results from locally weighted scatterplot smoothing. A 2-sided p value of <0.05 was regarded as statistically significant. In addition, p values ≥0.05 but <0.1 were categorized as just failing to reach statistical significance. Because of the explorative nature of this study, all results from statistical tests have to be interpreted as hypothesis generating.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available but can be obtained by qualified researchers from the corresponding author on reasonable request.
Ethics
All patients or their legal representatives gave their informed consent. The study was approved by the Institutional Review Board of the University of Schleswig-Holstein (#13-162).
Results
Study Cohorts
At database lock, 570 patients with AE were enrolled in the GENERATE registry, of whom 184 and 92 were documented as NMDAR-E and LGI1-E, respectively (Figure 1). Of those, 82 documented as NMDAR-E and 36 documented as LGI1-E fulfilled all inclusion criteria. According to recent criteria,5 all patients with NMDAR-E had definitive NMDAR-E, whereas 24 of 36 patients documented as LGI1-E had definitive autoimmune limbic AE (67%) and only 9 patients with LGI1-E fulfilled the criteria for definitive AE (25%). Three patients with LGI1-E (8%) were diagnosed with anti–LGI1-associated cognitive impairment11 (Figure 1). The selected subcohorts were largely representative of all NMDAR and LGI1 antibody-positive patients (eTable 1, links.lww.com/NXI/A600).
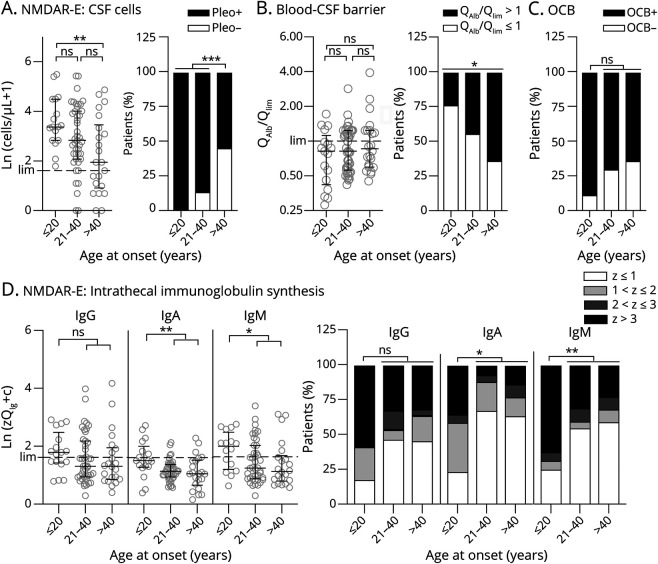
Higher CSF Leukocytes and More Frequent Blood-CSF Barrier Dysfunction and OCBs in NMDAR-E Compared With LGI1-E
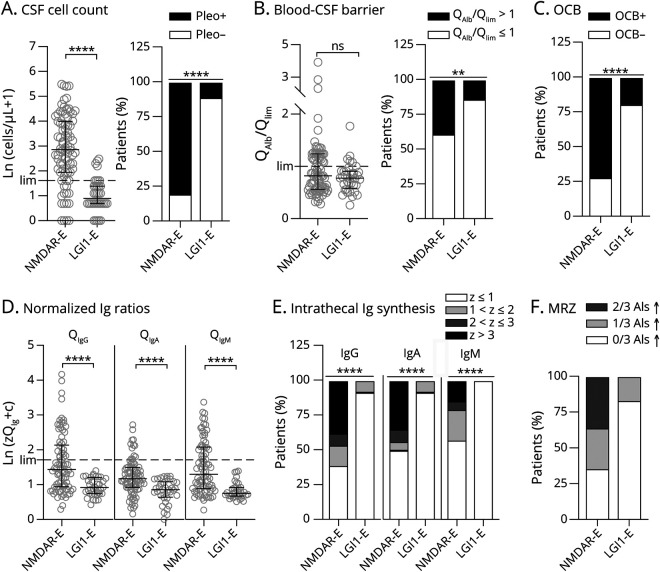
CSF leukocytes in NMDAR-E (median 16.5 cells/µL) were >10-fold higher than in LGI1-E (1.5 cells/µL, Table 1, Figure 2A). CSF leukocytes >50/µL were found exclusively in NMDAR-E (Table 1). Although the median age-adjusted QAlb in LGI1-E and NMDAR-E did not differ, increased QAlbs occurred twice as often in NMDAR-E (Table 1, Figure 2B). Finally, CSF-restricted OCBs were positive in two-thirds of NMDAR-E, but in less than one-fifth of the patients with LGI1-E (Table 1, Figure 2C). In LGI-E, but not in NMDAR-E, the age-adjusted QAlb was higher when CSF leukocytes were also higher (eFigure 5A, links.lww.com/NXI/A600). In contrast, CSF leukocytes in NMDAR-E, but not in LGI-E (eFigure 5B, links.lww.com/NXI/A600), were higher in OCB-positive patients (median, IQR; OCB−: 7/µL, 2–14/µL; OCB+: 27/µL, 9–73/µL). Overall, CSF was pathologic in 94% and 36% of patients with NMDAR-E and LGI1-E, respectively (Table 1).
Figure 2. CSF Findings Are Very Different in NMDAR-E Compared With LG1-E.
In 82 and 36 patients with therapy-naive NMDAR- and LGI1-E undergoing lumbar puncture within 90 days after onset of symptoms, basic CSF findings, cell count (A), age-adjusted blood-CSF barrier function (QAlb/Qlim, B), and the presence of isolated oligoclonal bands (OCB) in CSF as most sensitive proof for intrathecal IgG synthesis (C) were compared. (A) CSF leukocytes counts were logarithmized after adding 1, thus Ln(cell count +1) in patients with 0 cells/µL is 0. (B) The individual CSF/serum albumin ratio (QAlb) was normalized by division with the age-dependent upper limit [Qlim, 4 + age (yrs)/15]. (D) CSF/serum IgG, IgA, and IgM ratios (QIgG, QIgA, and QIgM) were compared with the expected mean CSF/serum ratio with regard to the individual QAlb calculated by the Reiber formulas (traditional Reiber diagrams can be found in eFigure 4, links.lww.com/NXI/A600). The distance from the mean QIg is expressed as the number of standard deviations (Z score), with a Z score >3 judged as a proof of intrathecal synthesis (black), a Z score ≤3 but >2 classified a probable intrathecal synthesis (dark gray), and a Z score ≤2 but >1 classified a possible intrathecal synthesis (light gray). The resulting Z scores were logarithmized after adding the correction factor c (2.51), resulting in Ln(zQIg + c) of 0 in the patient with LGI1-E with the lowest Z score for QIgA (additional information in eFigures 1 and 2, links.lww.com/NXI/A600). (D) Comparison of the quantitative intrathecal IgG, IgA, and IgM synthesis in patients with NMDAR-E with patients with LGI1-E. (E) Frequency of intrathecal IgG, IgA, and IgM synthesis classified as present, probable, possible, or absent in both groups of patients. (F) Detection of a polyspecific immune activation using 3 pathogen-specific AIs for measles, rubella, and varicella zoster virus in a subset of patients with NMDAR-E (N = 14) and LGI-E (N = 6). Two elevated AIs (>1.4): dark gray, 1 elevated AI: light gray, no elevated AI: clear. The upper normal limits (lim, A: 4 leukocytes/µL, B: QAlb/Qlim = 1.0, D: Qmean + 3 SD) are indicated as dashed lines. Statistical analysis was performed using the Mann-Whitney U test (A/B, left panels, D) and Fisher exact test of the unnormalized number of patients (A/B, right panel, C/E). ns = not significant, **p < 0.01, and ****p < 0.0001 (additional analyses in eFigures 5 and 7–11, links.lww.com/NXI/A600). AI = antibody index; LGI1-E = leucine-rich glioma-inactivated protein 1; NMDAR-E = NMDA receptor.
Relevant Quantitative Intrathecal IgG, IgA, and IgM Synthesis Is Characteristic for NMDAR-E but Not LGI-E
For many patients with NMDAR-E, Reiber diagrams showed definitive quantitative IIS (QIg > Qlim) not only for IgG and IgM but also for IgA (eFigure 6, links.lww.com/NXI/A600, Table 1). The corresponding Z scores (eFigure 1A, see eMethods, links.lww.com/NXI/A600) were significantly higher in NMDAR-E compared with LGI1-E for all Ig classes (Figure 2D). All IIS Z scores were strongly associated with each other in NMDAR-E (eFigure 7A, links.lww.com/NXI/A600), but not in LGI1-E (eFigure 8A, links.lww.com/NXI/A600). In NMDAR-E, Z scores >3 indicating definitive quantitative IgG, IgM, and IgA IIS occurred in 38%, 35%, and 15%, respectively (Figure 2E). None of the patients with LGI1-E showed either probable or definitive quantitative IIS. Overall, IIS, especially for IgM, was more pronounced in NMDAR-E patients with higher CSF leukocytes (eFigure 7B, links.lww.com/NXI/A600). No such association was found in LGI1-E (eFigure 8B, links.lww.com/NXI/A600). The observation that higher leukocytes coincided with more pronounced IIS in NMDAR-E across the whole data set resulted from values obtained within 1 week of onset (eFigure 14, links.lww.com/NXI/A600). This association disappeared when LP was performed at later time points. Of interest, IIS of NMDAR-specific IgG correlated neither with quantitative IIS for total IgG nor with the presence of OCB (eFigure 7D, links.lww.com/NXI/A600). The MRZ reaction measured in a subset of patients with the initial CSF/serum samples still available was positive for 5/14 patients with NMDARE (36%, Figure 2F), but for none of 6 patients with LGI-E. No major selection bias was identified when comparing the patients with NMDAR-E and LGI1-E tested for the MRZ reaction with patients without biosamples available (eFigures 9 and 10, links.lww.com/NXI/A600).
Effect of Disease Duration on Inflammatory CSF Changes in NMDAR-E and LGI1-E
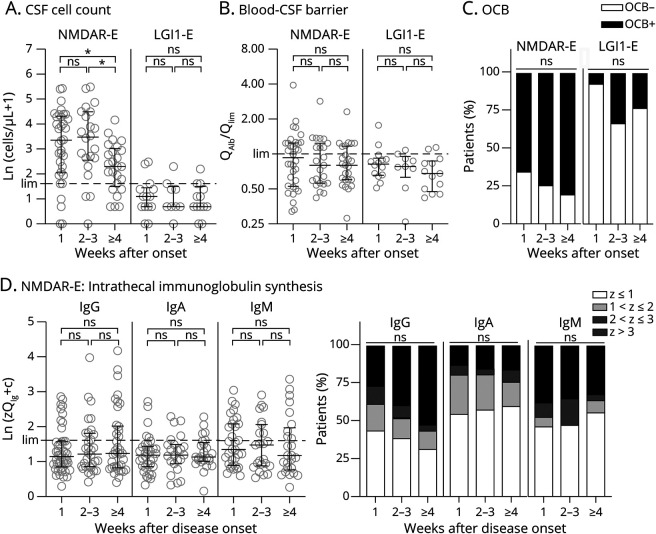
Patients were categorized according to disease duration (1 week, 2/3 weeks, ≥4 weeks) at the time of LP. The resulting subgroups were quite similar (χ2 test, p = 0.83, eFigure 12, A–C, links.lww.com/NXI/A600). CSF leukocytes in NMDAR-E, but not in LGI-E, were significantly lower when the LP was delayed (weeks 1–3: median 28.0 cells/µL, IQR 8.0–76.0; ≥4 weeks: median 9.0 cells/µL, 3.5–19.5; Mann-Whitney U test, p < 0.01; Figure 3A; eFigure 12D, links.lww.com/NXI/A600). In both NMDAR- and LGI-E, OCBs became slightly more frequent over time. However, these trends were not significant (Figure 3C). In NMDAR-E with early LP, quantitative IIS was virtually identical to those with later LP (Figure 3D), however, with a trend toward higher values for IgG (Z > 3 weeks 1–3, 18/57, 32%, vs ≥4 weeks, 13/25, 52%, Fisher exact test, p = 0.09, Figure 3D). Of interest, IIS was present even in a substantial proportion of patients with LP within the first 3 days after clinical onset (eFigure 13A, links.lww.com/NXI/A600). For IgG and IgA, the disappearance of the association between IIS and CSF leukocytes at later time points coincided with a relative increase in the ratio of IIS to CSF leukocytes (eFigure 14, links.lww.com/NXI/A600).
Figure 3. In NMDAR-E, Quantitative Intrathecal Immunoglobulin Synthesis IIS Is Largely Independent of Disease Duration, Whereas CSF Leukocytes Are Higher Early After Clinical Onset.
The 82 and 36 patients with therapy-naive NMDAR-E and LGI-E, respectively, were categorized with respect to the delay of lumbar puncture after onset of symptoms (week 1 = day 0–6; weeks 1/3 = day 7–20; ≥4 = day 21 and later). CSF leukocyte count, blood-CSF barrier function, and the presence of OCB are depicted as in Figure 2, A–C and quantitative intrathecal IgG, IgA, and IgM synthesis as in Figure 3, A and B. The upper normal limits (lim, cell count: 4 cells/µL, QAlb/Qlim: 1.0, QIg: Qmean + 3 SD/Z score = 3, lim) are indicated as dashed lines. Statistical analysis was performed using the Kruskal-Wallis test followed by the Dunn multiple comparisons test (A/B/D), the χ2 test of the unnormalized number of patients with and without OCB (C), or with or without intrathecal immunoglobulin synthesis of a Z score >3. ns = not significant and *p < 0.05 (additional analyses in eFigures 12–14, links.lww.com/NXI/A600). IIS = immunoglobulin synthesis; LGI1-E = leucine-rich glioma-inactivated protein 1; NMDAR-E = NMDA receptor.
At Younger Age, Pleocytosis and Intrathecal Ig Synthesis Are More Pronounced in NMDAR-E, Whereas the Blood-CSF Barrier Is More Dysfunctional in Younger Patients With LGI1-E
Patients with NMDAR-E were subgrouped into young (≤20, N = 17), intermediate (21–40 years; N = 43), and older (>40 years, N = 22) patients. To obtain roughly equal subgroup sizes, the LGI1-E cohort was dichotomized only (<60 years, N = 16; ≥60 years, N = 20). Although older patients were more likely to be male, the resulting subgroups for each AE subtype were quite similar (eFigure 15, A–C, links.lww.com/NXI/A600).
In NMDAR-E, at younger age CSF leukocytes were considerably higher (≤20 years: median 28.0 cells/µL, IQR 16.0–88.5, >40 years: median 6.0 cells/µL, IQR 1.5–31.5; Figure 4A, eFigure 16A, links.lww.com/NXI/A600). An increased age-adjusted QAlb was more frequent in older patients with NMDAR-E (Figure 4B). For pleocytosis and CSF-blood barrier dysfunction, rather gradual changes were observed across the 3 age groups (Figures 4, A and B). For OCBs and quantitative IIS, only the youngest age group differed from the 2 groups of older patients. The latter 2 were thus combined for statistical analysis (Figures 4, C and D). OCBs were >2.5-fold more frequently absent in older patients (21/65, 32%) compared with younger patients (2/17, 12%). Correspondingly, definitive IgG IIS (z > 3) was >1.5-fold more frequent in young patients with NMDAR-E (10/17, 59% vs 21/65, 32%). However, these differences were not statistically significant (p = 0.13 and p = 0.05, respectively). IgA and IgM Z scores and the frequencies of definitive IgA and IgM IIS were significantly lower in older patients with NMDAR-E (Figure 4D). In LGI1-E, the only age-related difference detected was a considerably higher age-adjusted QAlb in younger patients (eFigure 15, D–G, and eFigure 16, A and B, links.lww.com/NXI/A600).
Figure 4. With Increasing Age, Patients With NMDAR-E Become Less Likely to Show CSF Pleocytosis and Quantitative Intrathecal Immunoglobulin Synthesis, Whereas Blood-CSF Barrier Dysfunction Occurs More Frequently.
Patients with NMDAR-E were divided into 3 groups, those with the age of 20 years or younger (≤20 years), those older than 40 years (>40 years), and those in between (21–40 years). CSF leukocyte count and frequency of pleocytosis (A), blood-CSF barrier function (B), and the presence of OCB restricted to the CSF (C) are presented as in Figure 2, A–C, but only for NMDAR-E, intrathecal IgG, IgA, and IgM synthesis (D) IIS presented as in Figure 3D. Statistical analysis was performed using the Kruskal-Wallis test followed by the Dunn multiple comparisons test (A/B/D, left panels). For intrathecal immunoglobulin synthesis, patients with a Z score >3 were compared with those ≤3. When comparing all 3 groups (B, right panel, C, D right panel IgG and IgM), the χ2 test of the unnormalized number of patients was performed. If the criteria for a valid χ2 test were not met, 2 groups were combined, and a Fisher exact test was performed (A, right panel, D right panel—IgA). ns = not significant, *p < 0.05, **p < 0.01, and ***p<0.001 (additional analyses including age dependency of CSF findings in LGI-E can be found in eFigures 15–18, links.lww.com/NXI/A600). NMDAR-E = NMDA receptor.
Definitively inflammatory CSF can be most clearly defined as CSF with either pleocytosis or OCBs or both. In NMDAR-E, inflammatory CSF became less frequent with age (eFigure 16C, links.lww.com/NXI/A600). Only 58% (7/12) of patients with NMDAR-E aged >60 years showed inflammatory CSF compared with 94% (66/70) younger than 60 years (Fisher exact test, p < 0.01). In LGI1-E, only a trend toward less frequent inflammatory CSF with age was found (≤70 years; 9/26, 35%, vs >70 years: 1/10, 10%, Fisher exact test, p = 0.23; eFigure 16C, links.lww.com/NXI/A600). To exclude a major bias in CSF findings between NMDAR-E and LGI1-E because of the different ages of the cohorts, a subgroup analysis with patients with NMDAR- and LGI1-E aged >40 years was performed. The 2 groups were identical in age and sex distribution and the duration from disease onset to LP (eFigure 17, A–D, links.lww.com/NXI/A600). In patients aged >40 years, NMDAR-E still showed more pronounced inflammatory CSF changes (eFigure 17, E–I, links.lww.com/NXI/A600). In contrast, sex did not significantly affect CSF findings in either AE subtype (eFigure 18, links.lww.com/NXI/A600).
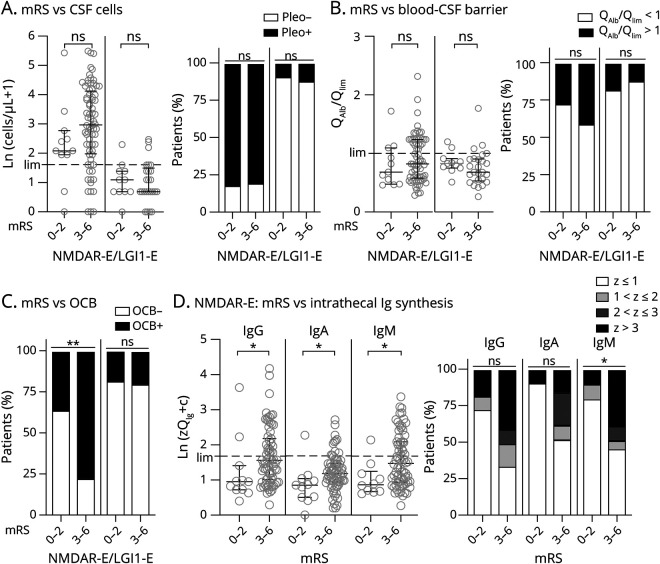
Intrathecal Ig Synthesis Is Associated With More Severe Functional Impairment in NMDAR-E But Not in LGI1-E
Patients with NMDAR-E with an mRS score of 0–2 showed less inflammatory CSF changes than those with mRS scores of 3 or more. However, inflammatory changes in those patients with mRS scores of 4 and 5 did not differ from those with scores of 3 (eFigure 19, links.lww.com/NXI/A600). Thus, patients were dichotomized accordingly with mRS scores < 3 considered mild and ≥3 severe functional impairment. Age, sex distribution, and duration from onset to LP were similar in the resulting subcohorts for both AE subtypes (eFigure 20, A–C, links.lww.com/NXI/A600). CSF leukocytes were nonsignificantly higher in severe compared with mild NMDAR-E (mRS score ≥ 3: median 19 cells/µL, IQR 7–63 cells/µL; mRS score < 3: median 7 cells/µL, IQR 6.0–15 cells/µL; Mann-Whitney U test p = 0.08; Figure 5A, left graphs). Negative OCBs were almost 3-fold more frequent in mild compared with severe disease (64%, 7/11, vs 23%, 16/71, Fisher exact test, p < 0.01, Figure 5C, left graphs). Z scores for quantitative IgG, IgA, and IgM IIS were moderately but significantly higher in NMDAR-E with mRS score ≥ 3 (Figure 5D, left graphs). Definitive quantitative IIS (Z > 3) was significantly more frequent at mRS score ≥ 3 for only IgM (Figure 5D, right graphs). In LGI1-E, no associations between CSF findings and functional impairment were found (Figure 5, A–C, right graphs, eFigure 20D, links.lww.com/NXI/A600).
Figure 5. In NMDAR-E, the Presence of CSF-Specific OCBs and Increased Quantitative IIS for IgG, IgA, and IgM Are the Only CSF Finding Associated With Disease Severity at the Time Point of Lumbar Puncture.
Patients with NMDAR-E and LGI1-E were dichotomized according to the degree of functional impairment using the mRS, with a score of 0–2 regarded as a low degree of impairment and 3–6 as a high degree of impairment (6 = death did not occur before lumbar puncture, eFigure 19, links.lww.com/NXI/A600). CSF leukocyte count and frequency of pleocytosis (A), age-adjusted blood-CSF barrier function (QAlb/Qlim) (B), frequency of OCBs restricted to the CSF (C), and the probability of quantitative intrathecal immunoglobulin synthesis (D) are presented as in Figure 2, A–C for A-C and in Figure 3, A and B for D. Statistical analysis was performed using the Mann-Whitney U test (A/B/D, left panels) and the Fisher exact test (A/B/D right panels). For intrathecal immunoglobulin synthesis, patients with a Z score >3 were compared with those ≤3. ns = not significant, *p < 0.05, and **p<0.01 (additional analyses can be found in eFigure 19, links.lww.com/NXI/A600). LGI1-E = leucine-rich glioma-inactivated protein 1; mRS = modified Rankin Scale; NMDAR-E = NMDA receptor.
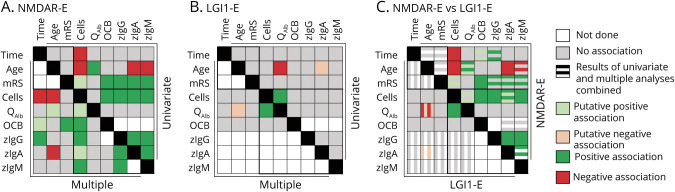
Comprehensively Analyzing the Interdependency of Inflammatory CSF Parameters Among Themselves and With Relevant Confounders Identifies Disease-Specific Patterns
For a more comprehensive description of the mutual interactions among CSF parameters, multiple models were calculated (eTable 1–5, links.lww.com/NXI/A600). These took into account the influences of disease duration and severity as well as age. Quantitative IIS was present only in NMDAR-E. Among CSF leukocytes, blood-CSF barrier function, and OCBs, only leukocytes distinguished between NMDAR-E and LGI1-E. Age was identified as a relevant confounder (eTable 2, links.lww.com/NXI/A600). Using CSF leukocytes, blood-CSF barrier function, and OCBs as target variables for LGI1-E and all 3 in addition to quantitative IgG, IgA, and IgM IIS for NMDAR-E, our multiple models largely confirmed the univariate analyses (eTables 2–5, links.lww.com/NXI/A600 Figure 6). A strong positive association of CSF leukocytes with IIS, either qualitative as OCB or quantitative, proved to be characteristic for NMDAR-E. For LGI1-E, more disturbed blood-CSF barrier function remained associated with higher CSF leukocytes. In NMDAR-E, disease duration, age, and functional impairment influenced CSF findings in a characteristic manner: shorter disease duration was associated with higher CSF leukocytes, longer disease duration with a relative increase in IgG IIS and older age with lower CSF leukocytes and less IgA and IgM IIS. Finally, OCB positivity was strongly associated with severe functional impairment. In LGI1-E, older age was associated with a less perturbed blood-CSF barrier. An opposite association was found for NMDAR-E.
Figure 6. Comprehensive Summary of Results Shows the Profound Differences in the Interdependency of Inflammatory CSF Parameters Among Each Other and Clinically Overt Disease Duration, Age at Onset, and Disease Severity in NMDAR-E and LGI1-E.
Graphical summary of all analyses. Details of the multiple analyses can be found in eTables 2–6, links.lww.com/NXI/A600. Results of the univariate (upper right halves) and multiple analyses (lower left halves) for (A) NMDAR-E and (B) LGI1-E. The variables are indicated at the top or left of the graphs. Age = age at LP; Time = time from clinical onset to LP; Cells = CSF leukocyte count; QAlb = CSF/serum albumin ratio; zIgG/A/M = z scores for IgG/A/M as a marker for quantitative intrathecal immunoglobulin synthesis. Each square summarized the results of the interaction of the 2 variables, which are color coded as indicated. Positive associations indicate significant results in at least 1 univariate analysis and a p < 0.05 in the multiple models. Putative associations were defined either as significant results with questionable relevance (LGI1-E: age vs zIgA in the absence of relevant quantitative synthesis of IgA) or as comparisons with p values <0.1 but not p < 0.05. (C) Synopsis of the results of combined univariate and multiple analyses for NMDAR-E (upper right half) and LGI1-E (lower left half). Color-coded results of the univariate analysis can be found as the uppermost and third stripe in each box for NMDAR-E and the left and third from the left strip in each box for LGI1-E. Boxes comparing 1 variable with itself are blackened. Comparison of the 3 confounders with each other or the different CSF parameters are indicated by 2 black frames. LGI1-E = leucine-rich glioma-inactivated protein 1; LP = lumbar puncture; mRS = modified Rankin Scale score at LP; NMDAR-E = NMDA receptor; OCB = oligoclonal bands restricted to the CSF.
Discussion
NMDAR- and LGI1-E are the 2 most common AE subtypes. Each is representative of 1 of 2 clusters of AE subtypes: NMDAR-E for those with prominent CSF inflammation and LGI1-E for those with little or no CSF inflammation.4 Beyond the expected quantitative differences, our detailed analysis of CSF findings in therapy-naive acute NMDAR- and LGI1-E shows that each of the 2 AE subtype exhibits a distinct pattern of CSF changes. These are characterized by differences in the interdependencies of CSF parameters and their association with age, disease duration, and disease severity.
On the quantitative level, NMDAR-E showed ∼10-fold higher CSF leukocyte counts as compared with LGI-E patients. Blood-CSF barrier dysfunction and CSF-restricted OCBs occurred ∼2- and ∼3-fold more frequently in NMDAR-E, respectively. Completely normal CSF was exceptional in patients with NMDAR-E (5%) but observed in ∼2/3 of patients with LGI1-E. This is in line with previous findings.4,12-15 We could also confirm that NMDAR-E frequently shows quantitative IIS for IgG16 and report here that IgM (35%) and IgA (15%) IIS are commonly present. In contrast, quantitatively relevant IIS is absent in LGI1-E.
With regard to the disease-specific mutual interactions of CSF parameters, in NMDAR-E, but not in LGI1-E, CSF leukocytes were higher when OCBs were present. In the early phase of the disease, higher CSF leukocytes were associated with more pronounced quantitative IIS, especially with IgM. These associations were absent in LGI1-E. In NMDAR-E, CSF pleocytosis might thus be a surrogate marker for a factor that, at least in the acute phase, boosts IIS, e.g., like the B cell–attracting chemokine C-X-C motif chemokine 13, which is associated with the intrathecal synthesis of NMDAR-specific IgG in NMDAR-E.17 However, even in infectious diseases of the CNS, only 10–20% of intrathecally synthesized IgG is pathogen-specific.18 Thus, only a fraction of the total IgG quantitative IIS described herein can be expected to be NMDAR-specific IgG. The extent of intrathecal anti-NMDAR-IgG synthesis neither correlated with the extent of intrathecal synthesis of total IgG nor had NMDAR-E patients with positive OCB more NMDAR-specific IIS than those without. This indicates that total and the NMDAR-specific IgG synthesis in the CSF compartment are regulated quite differently in NMDAR-E. Our results also show that later in the course of NMDAR-E, IIS becomes independent of CSF leukocytes. In NMDAR-E brains, antibody-secreting cells reside in perivascular, interstitial, and Virchow-Robin spaces,19 which may be the source of the continuously synthesized Ig. In contrast to NMDAR-E, CSF leukocytes were strongly associated with blood-CSF barrier dysfunction in LGI1-E. It has been hypothesized that in LGI1-E, systemically synthesized anti–LGI1-IgG and complement from plasma enter the brain at sites of blood-barrier dysfunction, where both will induce focal inflammation.20,21 Although the blood-CSF barrier is not completely identical to the blood-brain barrier, we find that a relative increase in a major plasma protein, albumin, in the LGI1-E CSF is associated with cellular signs of inflammation. Thus, our findings might reflect and support the presumed pathophysiology of LGI-E with proteins from the systemic circulation that enter the intrathecal space playing an important role in the induction of inflammation.
Patient-specific confounders such as age, disease duration, and severity also differentially affect the CSF findings in NMDAR- and LGI1-E. In NMDAR-E, but not in LGI1-E, CSF leukocyte counts become lower with longer disease duration and older age. Higher CSF leukocytes in NMDAR-E very early after onset as compared with later time points have been reported previously.15 Blood-CSF barrier dysfunction became more prominent in older patients with NMDAR-E but was less prominent in older patients with LGI1-E. Although OCB occurred ∼2-fold less frequently in mild NMDAR-E, clinical severity in LGI1-E was independent of the presence or absence of OCB. In NMDAR-E, older age seems to dampen the IIS for IgM and even more so for IgA.
We have already reported that AE subtypes that typically manifest at older age show less CSF inflammation.4 Here, we show that even in patients with the same antibody-defined AE subtype, NMDAR-E, advanced age decreases the likelihood of inflammatory CSF findings, possibly a result of immune senescence.22 Thus, normal routine CSF findings should not preclude testing for antineuronal antibodies in the elderly when AE is suspected.
Our findings are in contrast to previous reports that OCBs rarely occur in early NMDAR-E and become more frequent with time.15 In our cohort, OCBs were positive in almost 2/3 of patients with NMDAR-E, even within the first week, compared with <10% at first LP in the previous study.15 Moreover, positive OCBs did not become significantly more frequent at later time points in both AE subtypes studied. The reason for these discrepancies remains unknown, and they might result from different methods applied for OCB detection. However, our results of frequently positive OCBs in very early NMDAR-E are confirmed by quantitative IIS in 40% of patients within the first 3 days after clinical onset. In Herpes simplex virus encephalitis, quantitative IIS was found in only 10% of patients within the first week after onset.23 Considering the slow pace at which quantitative IIS develops in pathogen-induced encephalitis, we conclude that the presence of quantitative IIS in NMDAR-E most likely precedes clinical onset of AE in a relevant proportion of cases.
As the pattern of quantitative IIS (IgG > IgM >> IgA) in NMDAR-E resembled MS,8,24 we tested for polyspecific immune activation (aka MRZ reaction) in NMDAR-E CSF. The MRZ reaction is positive in ∼75% of patients with MS.25,26 In addition, the MRZ reaction is regarded as highly specific for MS.25,26 The MRZ reaction is believed to evolve in a chronically inflamed CNS when circulating plasmablasts/plasma cells are unspecifically recruited to niches for survival and expansion over time.27,28 MRZ positivity in our subcohort of patients with NMDAR-E (>1/3) proved to be the highest among any neurologic disease besides MS.26 In addition to the robust early quantitative IIS, this finding supports the hypothesis of a preexisting MS-like inflammatory CNS process in some NMDAR-E cases. Of note, white matter lesions in patients with NMDAR-E suggestive of demyelination are frequently found in NMDAR-E.1,29 Even symptomatic episodes of demyelination have been reported. However, those were mostly associated with aquaporin-4 (AQP4) and myelin oligodendrocyte protein (MOG) antibodies.30 Regardless of symptomatic demyelination, AQP4 and MOG antibodies occur in 4% of patients with NMDAR-E.31 Of note, the MRZ reaction is typically negative in AQP4 and MOG antibody–associated demyelinating diseases.25,26 In our NMDAR-E subcohort, the frequency of MRZ positivity was 10-fold higher that the reported frequency of AQP4 and MOG antibodies.32 In addition, single cases of NMDAR-E coexisting with MS have been reported.33-35 Thus, the immunologic and clinical commonalities of NMDAR-E and MS may be more relevant than those with AQP4 and MOG antibody-associated demyelinating diseases and require further investigation.
Nevertheless, our results should be interpreted with caution because of the retrospective and cross-sectional nature of the present study and the limited number of patients included, especially for testing the MRZ reaction. However, we could not detect any relevant selection bias of the patients characterized in detail compared with the total population in GENERATE. We estimate biases due to laboratory imprecisions as a result of including local laboratory analyses to be minor because no OCB-negative cases with quantitative IIS according to the Reiber formulas occurred. Still, at low QAlbs, the corresponding Z scores had to be adjusted. However, alternatively to analytical imprecision at low CSF IgG/M values, this might hint at a more general phenomenon, as similar observations have been reported.36 Taken together, these observations suggest the possibility that at low QAlbs, the Reiber formulas tend to result in slightly too low Qmeans for IgG and IgM. However, studies with larger cohorts are needed to confirm this assumption. Still, the Reiber formulas are much better than other formulas for IIS reference values because those only define upper normal limits36-38 and are thus not suitable to calculate Z scores.
In summary, we show that NMDAR-E and LGI1-E show very different CSF patterns on detailed analysis, indicating divergent immunopathogeneses. We present evidence for a more chronic inflammatory response in NMDAR-E with robust quantitative IIS and frequently positive polyspecific immune activation, presumably preceding clinical onset. In addition, our finding that higher CSF leukocytes are associated with more pronounced quantitative IIS in the hyperacute phase of encephalitis but not at later stages when CSF leukocytes decrease while IIS persists indicates that the acute inflammation during onset of encephalitis transiently triggers the more long-lasting quantitative IIS before or soon after the clinical manifestation of encephalitis. CSF leukocyte counts and blood-CSF barrier dysfunction have been reported to be of prognostic value in NMDAR-E.39,40 Future studies are needed to investigate whether including quantitative IgG, IgA, and IgM IIS may refine the prognostic relevance of CSF in NMDAR-E. With regard to diagnostic decisions, we show that the presence of quantitative IIS and/or pleocytosis >50 cells/µL makes that diagnosis of LGI-E highly unlikely. Finally, our study shows that in the 2 most common AE subtypes, inflammatory CSF becomes less frequent in elderly patients. This is explained by not only the older age of patients with LGI-E with their frequently normal CSF but also a decreased frequency of inflammatory CSF in older patients with NMDAR-E. This important information warrants that noninflammatory CSF in elderly patients with clinically possible AE should not lead to an omission of antibody testing.
Acknowledgment
This work was supported by members of the GENERATE network, who contributed to patient recruitment and data acquisition and entry. All members of the GENERATE network as of September 2020 are indicated in the Appendix. The authors are indebted to all active and associated members of the GENERATE network and especially to patients and relatives willing to support this research. They thank Pamela Maher, PhD, for the critical reading of the manuscript.
Glossary
- AE
autoimmune encephalitis
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AI
antibody index
- AQP4
aquaporin-4
- CASPR2
contactin-associated protein-like 2
- DPPX
Dipeptidyl-peptidase-like protein-6
- GABA
γ-aminobutyric acid
- GENERATE
German Network of Research on Autoimmune Encephalitis
- IIS
immunoglobulin synthesis
- IQR
interquartile range
- LGI1-E
leucine-rich glioma-inactivated protein 1
- LP
lumbar puncture
- MOG
myelin oligodendrocyte protein
- mRS
modified Rankin Scale
- MRZ reaction
measles-rubella-zoster reaction
- NMDAR-E
NMDA receptor
- OCB
oligoclonal band
- VZV
varicella zoster
Appendix 1. Authors
Appendix 2. Coinvestigators

Contributor Information
Marc Dürr, Email: duerrmarc@live.de.
Gunnar Nissen, Email: gunnarn@web.de.
Kurt-Wolfram Sühs, Email: suehs.kurt-wolfram@mh-hannover.de.
Philipp Schwenkenbecher, Email: schwenkenbecher.philipp@mh-hannover.de.
Christian Geis, Email: christian.geis@med.uni-jena.de.
Marius Ringelstein, Email: marius.ringelstein@med.uni-duesseldorf.de.
Hans-Peter Hartung, Email: hans-peter.hartung@uni-duesseldorf.de.
Manuel A. Friese, Email: manuel.friese@zmnh.uni-hamburg.de.
Max Kaufmann, Email: m.kaufmann@uke.de.
Michael P. Malter, Email: michael.malter@uk-koeln.de.
Marie Madlener, Email: marie.madlener@uk-koeln.de.
Franziska S. Thaler, Email: franziska.thaler@med.uni-muenchen.de.
Tania Kümpfel, Email: tania.kuempfel@med.uni-muenchen.de.
Makbule Senel, Email: makbule.senel@uni-ulm.de.
Martin G. Häusler, Email: haeusler@rwth-aachen.de.
Hauke Schneider, Email: hauke.schneider@uk-augsburg.de.
Florian Then Bergh, Email: thenberf@medizin.uni-leipzig.de.
Christoph Kellinghaus, Email: christoph.kellinghaus@klinikum-os.de.
Uwe K. Zettl, Email: uwe.zettl@med.uni-rostock.de.
Klaus-Peter Wandinger, Email: klaus-peter.wandinger@uksh.de.
Nico Melzer, Email: nico.melzer@med.uni-duesseldorf.de.
Catharina C. Gross, Email: catharina.gross@ukmuenster.de.
Peter Lange, Email: peter-la@web.de.
Jens Dreyhaupt, Email: jens.dreyhaupt@uni-ulm.de.
Hayrettin Tumani, Email: hayrettin.tumani@uni-ulm.de.
Frank Leypoldt, Email: frank.leypoldt@uksh.de.
Study Funding
This study was supported by the German Federal Ministry of Education and Research (BMBF) through a grant Forschungsverbund CONNECT-GENERATE, grant codes 01GM1908A and 01GM1908C.
Disclosure
M. Dürr, G. Nissen, M. Kaufmann, M. Madlener, K.-P. Wandinger, C. Kellinghaus, J. Dreyhaupt, M.G. Häusler, and H. Schneider and P. Lange report no conflict of interest. K.-W. Sühs received honoraria from Merck, Celgene, UCB Pharma, and Alexion, all outside the submitted work. P. Schwenkenbecher received travel compensation and congress fee from Merck-Serono. C. Geis received speaker honoraria from Roche and Alexion and travel reimbursement from Merck. His research is funded by German Federal Ministry of Education and Research (BMBF) through a grant Forschungsverbund CONNECT-GENERATE, grant codes 01GM1908B and 01GM1908E, the Schilling Foundation, and Deutsche Forschungsgemeinschaft (DFG, GE2519/9-1, GE2519/8-1). M. Ringelstein received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, and Merck, none related to this study. H.-P. Hartung has received honoraria for serving on steering and data monitoring committees from Bayer, Biogen, GeNeuro, Merck, Novartis, Roche, Sanofi Genzyme, and TG Therapeutics, with approval by the Rector of HHU. M.A. Friese received speaker honoraria from Biogen, Merck, Novartis, and Roche, none related to this study. His research is funded by the German Federal Ministry of Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Hamburg Ministry of Science and Research, Fritz Thyssen Stiftung, Gemeinnützige Hertie-Stiftung, Else Kröner-Fresenius-Stiftung, Werner Otto Stiftung, and Stifterverband, outside the submitted work. M. P. Malter has received honoraria for lectures and consultancies from UCB Pharma and EISAI GmbH. FT received grant support from Novartis. T. Kümpfel has received speaker honoraria and/or personal fees for advisory boards from Bayer HealthCare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche Pharma, and Biogen and grant support from Novartis and Chugai Pharma in the past. MS has received consulting and/or speaker honoraria from Alexion, Bayer, Biogen, Merck, Roche, and Sanofi Genzyme. She has received travel support from Celgene and Teva. She has received research funding from the Hertha-Nathorff-Program. F.T. Bergh received speaker honoraria from Actelion, Alexion, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; travel reimbursement to attend scientific meetings from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; research support for investigator-initiated studies, through his institution, from the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), Actelion, Bayer, Merck-Serono, Novartis, and Teva; none of these funds were related to this study. U. K. Zettl received speaker fees, travel compensation and/or his section received support from Alexion, Almirall, Bayer, Biogen, Celgene, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), German Ministry of Economy (BMWi), Deutsche Forschungsgesellschaft (DFG), and European Union (EU), outside the submitted work. N. Melzer received honoraria for lecturing and travel expenses for attending meetings from Biogen Idec, GlaxoSmithKline, Teva, Novartis Pharma, Bayer HealthCare, Genzyme, Alexion Pharmaceuticals, Fresenius Medical Care, Diamed, and BIAL and has received financial research support from Euroimmun, Fresenius Medical Care, Diamed, Alexion Pharamceuticals, and Novartis Pharma. C.C. Gross received speaker honoraria from Mylan, Bayer HealthCare, and Sanofi-Genzyme and travel/accommodation/meeting expenses from Bayer HealthCare, Biogen, Euroimmun, Novartis, and Sanofi-Genzyme. She also received research support from Biogen and Novartis. H. Tumani reports funding for research projects, lectures, and travel from Alexion, Bayer, Biogen, Celgene, Genzyme, Fresenius, Merck, Mylan, Novartis, Roche, Siemens Health Diagnostics, and Teva and received research support from DMSG and German Federal Ministry of Education and Research (BMBF). F. Leypoldt received speaker fees and travel compensation and serves on advisory boards for/from Alexion, Bayer, Biogen, Fresenius, Merck-Serono, Novartis, Roche, and Teva. His research is funded by the German Federal Ministry of Education and Research (BMBF) and Deutsche Forschungsgesellschaft (DFG) and European Union (EU), outside the submitted work. He works for an academic institution, which also offers commercial antibody testing. J. Lewerenz received speaker fees or travel compensation from UCB, Bayer, Roche, Teva, and the Cure Huntington's Disease Initiative (CHDI). His institution has been reimbursed for his role as a principal investigator in trials for UCB and CHDI. His research is funded by the European Huntington's Disease Initiative and Ministry for Education and Research Baden-Württemberg, outside the submitted work, and the German Federal Ministry of Education and Research (BMBF). He works for an academic institution, which also offers commercial antibody testing.
References
- 1.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. [DOI] [PubMed] [Google Scholar]
- 2.van Sonderen A, Schreurs MW, Wirtz PW, Sillevis Smitt PA, Titulaer MJ. From VGKC to LGI1 and Caspr2 encephalitis: the evolution of a disease entity over time. Autoimmun Rev. 2016;15(10):970-974. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blinder T, Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-A systematic analysis. Front Neurol. 2019;10:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2(5):200-215. [DOI] [PubMed] [Google Scholar]
- 7.Reiber H. Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122(2):189-203. [DOI] [PubMed] [Google Scholar]
- 8.Reiber H. Cerebrospinal fluid—physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler. 1998;4(3):99-107. [DOI] [PubMed] [Google Scholar]
- 9.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37(7):1153-1160. [PubMed] [Google Scholar]
- 10.Schwenkenbecher P, Skripuletz T, Lange P, et al. Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariño H, Armangué T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87(8):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69(5):582-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malter MP, Frisch C, Schoene-Bake JC, et al. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261(9):1695-1705. [DOI] [PubMed] [Google Scholar]
- 14.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Guan HZ, Ren HT, Wang W, Hong Z, Zhou D. CSF findings in patients with anti-N-methyl-D-aspartate receptor-encephalitis. Seizure. 2015;29:137-142. [DOI] [PubMed] [Google Scholar]
- 17.Leypoldt F, Höftberger R, Titulaer MJ, et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol. 2015;72(2):180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobi C, Lange P, Reiber H. Quantitation of intrathecal antibodies in cerebrospinal fluid of subacute sclerosing panencephalitis, herpes simplex encephalitis and multiple sclerosis: discrimination between microorganism-driven and polyspecific immune response. J Neuroimmunol. 2007;187(1-2):139-146. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77(6):589-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tröscher AR, Klang A, Franch M, et al. Selective limbic blood–brain barrier breakdown in a feline model of limbic encephalitis with LGI1 antibodies. Front Immunol. 2017;8:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klang A, Schmidt P, Kneissl S, et al. IgG and complement deposition and neuronal loss in cats and humans with epilepsy and voltage-gated potassium channel complex antibodies. J Neuropathol Exp Neurol. 2014;73(5):403-413. [DOI] [PubMed] [Google Scholar]
- 22.Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC aging and senescent immune remodeling. Trends Immunol. 2015;36(12):815-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fomsgaard A, Kirkby N, Jensen IP, Vestergaard BF. Routine diagnosis of herpes simplex virus (HSV) encephalitis by an internal DNA controlled HSV PCR and an IgG-capture assay for intrathecal synthesis of HSV antibodies. Clin Diagn Virol. 1998;9(1):45-56. [DOI] [PubMed] [Google Scholar]
- 24.Deisenhammer F, Zetterberg H, Fitzner B, Zettl UK. The cerebrospinal fluid in multiple sclerosis. Front Immunol. 2019;10:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hottenrott T, Dersch R, Berger B, et al. The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS. 2015;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarius S, Eichhorn P, Franciotta D, et al. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol. 2017;264(3):453-466. [DOI] [PubMed] [Google Scholar]
- 27.Bonnan M. Does disease-irrelevant intrathecal synthesis in multiple sclerosis make sense in the light of tertiary lymphoid organs? Front Neurol. 2014;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto C, Hofmann J, Ruprecht K. Antibody producing B lineage cells invade the central nervous system predominantly at the time of and triggered by acute Epstein-Barr virus infection: a hypothesis on the origin of intrathecal immunoglobulin synthesis in multiple sclerosis. Med Hypotheses. 2016;91:109-113. [DOI] [PubMed] [Google Scholar]
- 29.Wang RJ, Chen BD, Qi D. Anti-N-methyl-D-aspartate receptor encephalitis concomitant with multifocal subcortical white matter lesions on magnetic resonance imaging: a case report and review of the literature. BMC Neurol. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Hernandez E, Guasp M, Garcia-Serra A, et al. Clinical significance of anti-NMDAR concurrent with glial or neuronal surface antibodies. Neurology. 2020;94(22):e2302-e2310. [DOI] [PubMed] [Google Scholar]
- 32.Baheerathan A, Brownlee WJ, Chard DT, Shields K, Gregory R, Trip SA. Antecedent anti-NMDA receptor encephalitis in two patients with multiple sclerosis. Mult Scler Relat Disord. 2017;12:20-22. [DOI] [PubMed] [Google Scholar]
- 33.Fleischmann R, Prüss H, Rosche B, et al. Severe cognitive impairment associated with intrathecal antibodies to the NR1 subunit of the N-methyl-D-aspartate receptor in a patient with multiple sclerosis. JAMA Neurol. 2015;72(1):96-99. [DOI] [PubMed] [Google Scholar]
- 34.Gulec B, Kurucu H, Bozbay S, et al. Co-existence of multiple sclerosis and anti-NMDA receptor encephalitis: a case report and review of literature. Mult Scler Relat Disord. 2020;42:102075. [DOI] [PubMed] [Google Scholar]
- 35.Uzawa A, Mori M, Takahashi Y, Ogawa Y, Uchiyama T, Kuwabara S. Anti-N-methyl D-aspartate-type glutamate receptor antibody-positive limbic encephalitis in a patient with multiple sclerosis. Clin Neurol Neurosurg. 2012;114(4):402-404. [DOI] [PubMed] [Google Scholar]
- 36.Auer M, Hegen H, Zeileis A, Deisenhammer F. Quantitation of intrathecal immunoglobulin synthesis—a new empirical formula. Eur J Neurol. 2016;23(4):713-721. [DOI] [PubMed] [Google Scholar]
- 37.Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37(5):397-401. [DOI] [PubMed] [Google Scholar]
- 38.Blennow K, Fredman P, Wallin A, et al. Formulas for the quantitation of intrathecal IgG production. Their validity in the presence of blood-brain barrier damage and their utility in multiple sclerosis. J Neurol Sci. 1994;121(1):90-96. [DOI] [PubMed] [Google Scholar]
- 39.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92(3):e244-e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47(1):102604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available but can be obtained by qualified researchers from the corresponding author on reasonable request.