Abstract
Neurologic and psychiatric symptoms have been reported in the months following the infection with COVID-19. A low-grade inflammation has been associated both with depression and cognitive symptoms, suggesting a link between these disorders. The aim of the study is to investigate cognitive functioning 6 months following hospital discharge for COVID-19, the impact of depression, and the consequences on quality of life. Ninety-two COVID-19 survivors evaluated at 1-month follow-up, 122 evaluated at 3 months and 98 evaluated at 6 months performed neuropsychological and psychiatric evaluations and were compared with a healthy comparison group (HC) of 165 subjects and 165 patients with major depression (MDD). Cognitive performances were adjusted for age, sex, and education. Seventy-nine percent of COVID-19 survivors at 1 month and 75% at 3- and 6-month follow-up showed cognitive impairment in at least one cognitive function. No significant difference in cognitive performances was observed between 1-, 3-, and 6-months follow-up. COVID-19 patients performed worse than HC but better than MDD in psychomotor coordination and speed of information processing. No difference between COVID-19 survivors and MDD was observed for verbal fluency, and executive functions, which were lower than in HC. Finally, COVID-19 survivors performed the same as HC in working memory and verbal memory. The factor that most affected cognitive performance was depressive psychopathology which, in turn, interact with cognitive functions in determining quality of life. Our results confirm that COVID-19 sequelae include signs of cognitive impairment which persist up to 6 months after hospital discharge and affect quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-021-01346-9.
Keywords: COVID-19, Cognitive impairment, Depression
Introduction
Coronavirus outbreaks succeeded in the last 10 years; after severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS-CoV), the SARS related coronavirus-2 (SARS-CoV-2) is the third coronavirus epidemic. This novel coronavirus disease 2019 (COVID-19) was initially discovered in November 2019 in China and then rapidly became a global pandemic with 99,300,000 confirmed cases reported globally.
Inflammation plays a central role in the development of SARS manifestations and may persist after viral clearance [35]. Accordingly, several inflammatory markers have been found to be elevated in COVID-19 patients, including C-reactive protein (CRP), interleukin (IL)-1β, IL-2, IL-2 receptor, IL-4, IL-10, IL-18, interferon-γ, tumor necrosis factor-α (TNF-α) granulocyte colony stimulating factor, CXCL10, CCL2, and CCL3; also, most patients show signs of lymphopenia and T cell exhaustion. [32]. Systemic inflammation has been previously shown to promote cognitive decline [16] thus suggesting that COVID-19 survivors could experience cognitive deficits in the following years.
In the last year, several studies investigated the long-term physical and mental consequences of COVID-19 showing that although the majority of persons remit of respiratory tract symptoms, mental health consequences such as psychiatric and neurologic disturbances persist after recovery, and symptoms of anxiety and depression contribute to disability and need for treatment [6, 30, 31, 42]. At the time of discharge, one third of patients shows evidence of cognitive impairment and motor deficits [20]. Between 10 and 35 days after hospital discharge patients who required oxygen therapy and those presenting diarrhea and neurological symptoms such as headache, anosmia, dysgeusia scored lower in memory, attention and executive function compared to asymptomatic patients. In addition, patients with cognitive complaints had higher scores in anxiety and depression [2]. Within 3 months from discharge COVID-19 survivors reported impairments between 6 and 38% with more severe deficits in patients with psychiatric morbidity [33]. Finally, at 5 months from hospital discharge 42.1% of patients had deficits in processing speed and 26.3% in verbal memory. Accordingly with previous literature showing highly prevalent cognitive decline in ARDS associated to hypoxemia and severity of infection [21, 34], also in COVDI-19 deficits in verbal memory have been associated with lowest arterial oxygenation and ARDS during hospitalization (Ferrucci et al. 2021).
Whereas symptoms of post-traumatic stress disorder (PTSD), anxiety, and insomnia decrease over time after recovery from COVID-19, depressive symptoms persist [31]. Furthermore, independent of possible medical sequelae, executive functions and psychomotor coordination are impaired in about 50–60% of COVID-19 survivors, whereas 78% of patients shows a poor performance in at least one cognitive domain. Cognitive performances are influenced both by depressive symptomatology and levels of inflammation at the time of hospital admittance [31]. A similar finding was also reported by Zhou et al. who showed that an impaired performance in attention was associated with CRP levels in COVID-19 survivors [45].
The relationship between depressive psychopathology, inflammation and cognitive functioning has been previously investigated in patients affected by Major Depressive Disorder (MDD), showing that depression is associated with higher levels of inflammatory markers [24], and cognitive impairment [12], also associated with inflammation [14]. The profile of neurocognitive deficits observed in MDD is closely similar to that observed in COVID-19 survivors, persists over time and also after the remission from the acute depressive episodes, has detrimental effects on quality of life and disability of the patients, and is a target for treatment to achieve an improvement in global functioning and to prevent depressive relapse [15].
Persistent cognitive deficits could play a similar detrimental role in worsening the quality of life of COVID-19 survivors, in a self-reinforcing circle of depressive psychopathology, neurocognitive impairment, and reduction in global functioning. Following this line of reasoning, we aimed at investigating how COVID-19 survivors score in cognitive functions compared to a healthy comparison group (HC) and patients with major depressive disorder (MDD) and the changes in cognitive functions in 6 months following hospital discharge Furthermore, we intend to investigate the impact of depression symptomatology and clinical characteristic of the illness on cognitive performances in COVID-19 survivors and the impact on quality of life.
Methods
Sample
The sample included 312 COVID-19 survivors (92 evaluated at 1-month follow-up, 122 evaluated at 3-month follow-up, and 98 evaluated at 6-month follow-up after hospital discharge); 165 inpatients with a diagnosis of Major Depressive Disorder (MDD–DSM-V criteria, SCID-I interview) and 165 HC. Furthermore, a subsample of 60 COVID-19 patients was evaluated both at 3- and 6-month follow-up to assess changes in neurocognitive functions over time. COVID-19 survivors were recruited between May 2020 and February 2021, all the patients performing the neuropsychological evaluation have been included in the study. MDD and HC were recruited and evaluated before the COVID-19 pandemic between 2010 and 2018; MDD patients were assessed during hospital stay. Inclusion criteria for COVID-19 survivors were clinical and radiological findings suggestive of COVID-19 pneumonia at the admission to the Emergency Department. The infection was confirmed by positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasopharyngeal and/or throat swab. After evaluation at the Emergency Department, patients were either hospitalized (86% of whom 4% in ICU, hospital stay 12.68 ± 11.85 days) or treated at home (14%). After multidisciplinary clinical examination including clinical, radiologic and laboratory examination, COVID-19 survivors were excluded from the study if they presented intellectual disabilities or neurological disorders. MDD patients, basing on direct psychiatric interview and laboratory assessment, were excluded if they showed: additional diagnoses on axis I, intellectual disabilities, pregnancy, major medical and neurological disorders, history of drug or alcohol abuse or dependency. All participants were between 18 and 70 years of age. After complete description of the study to the participants, written informed consent was obtained. The study was approved by the local ethical committee in accordance with the principles in the Declaration of Helsinki.
Clinical and neuropsychological assessment
Psychiatric and neuropsychological evaluations have been performed in all patients afferent to the follow-up outpatient service in the context of COVID-BioB study at 1-, 3-, and 6-month follow-up.
Psychiatric unstructured clinical interview using the best estimation procedure was conducted to investigate the presence of a current major psychiatric disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
In COVID-19 survivors, the Zung Self-Rating Depression Scale [46], a validated self-report questionnaire sensitive to the post-COVID depressive psychopathology [30, 44], was used to assess the severity of depressive symptoms. Generally accepted standard cutoff scores were used to consider the presence of psychopathology (ZSDS index ≥ 50). Quality of life was assessed through the EQ5D [39] which includes 5 dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety depression. The dimension anxiety/depression was excluded from the analysis due to its redundancy with the ZSDS scale.
Cognitive functions were assessed through the Brief Assessment of Cognition in Schizophrenia (BACS) [23], a broad battery evaluating verbal memory, verbal fluency, working memory (digit sequencing), selective attention and processing speed (symbol coding), psychomotor coordination (token motor task), and executive functions (Tower of London). The BACS was originally developed to assess cognition in patients with schizophrenia and afterwards it has been broadly used to assess cognitive abilities in depressive disorders [11, 36–38]. To avoid practice effects, two different versions (A and B) of BACS were administered to patients evaluated both at 3- and 6-month follow-up. To control for well-known confounding factors and to deal with group differences, domain raw scores were adjusted for age, sex and education according to normative Italian scores for the BACS subtests [3]. To provide a standard metric for comparison across neurocognitive domains for each subtest an equivalent score, ranging from 0 to 4, has been obtained from adjusted scores, where scores 2, 3 or 4 reveal a good performance, while score of 0 or 1 reveals a poor performance.
Data analysis
To address statistically significant group differences for sex distribution and age in COVID-19 patients, MDD, and HC, age-, sex-, and education stratified normative data [3] were used to compute subtest for each participant on the BACS. In addition, a global cognitive index has been calculated as the mean equivalent score of all subtests of the BACS.
First, we investigated whether cognitive performances differed between subjects evaluated at 1-, 3-, and 6-month follow-up. For patients assessed both at 3- and 6-month follow-up only the 3-month performance was considered. Given the number of cognitive outcome measures, we began data analysis with an omnibus test comparing the three groups on the global cognitive index of neurocognitive performance. In the presence of significant group difference on the global neuropsychological measure, step-down domainwise analyses were undertaken. Analyses of variance have been performed to investigate the main effect of group on the global and singles cognitive measures and post hoc Tukey honestly significant difference (LSD) method for multiple comparisons has been performed to investigate differences between groups.
Second, the same analyses have been performed to investigate differences in cognitive measures between HC, COVID-19 survivors, and MDD. To establish how COVID-19 patients scored, compared to HC and MDD, all patients were considered independently of the month of assessment. A profile analysis was conducted using a multivariate analysis of variance (MANOVA), with groups as between factor and subtests as within factor, and with the group-by-subtest interaction as the main effect of interest.
Third, to explore the effect of clinical and psychopathological factors known to affect cognitive performances we entered saturation at follow-up, glycaemia at follow-up, duration of hospitalization, admittance to ICU, duration of ICU stay, non-invasive ventilation, comorbidity (i.e., hypertension, coronaropathy, diabetes mellitus, chronic obstructive pulmonary disease, chronic renal insufficiency), and depression (ZSDS) as predictors in an elastic net penalized regression. (For a detailed description see supplementary material).
Fourth, to explore the influence of cognitive functions and depressive symptomatology on quality of life, and considering the a priori expected significant interaction between the two independent factors, independent variables were entered into a General Linear Model analysis of homogeneity of variances with the 4 dimensions of the EQ5D as dependent variables. The significance of the effects was calculated with Wilks’ λ, which indicates the proportion of generalized variance in the dependent variables that is accounted for by the predictors.
Finally, to investigate whether cognitive performances changed over time we performed a repeated measure ANOVA only in the subgroup of patients evaluated both at 3- and 6-month follow-up. To test the effect of having depressive symptoms at 3 months on changes in cognition, and considering the a priori expected significant interaction with other independent factors (age, sex) independent variables were entered into a Generalized Linear Model (GLZM) analysis of homogeneity of variances with an identity link function.
Results
Clinical and demographic characteristics and neuropsychological performances of COVID-19 survivors are summarized in Table 1.
Table 1.
Clinical, demographic characteristics of the sample and cognitive performances
| COVID-19 (n = 312) | T-F/p | |||
|---|---|---|---|---|
| 1 month (n = 92) | 3 months (n = 122) | 6 months (n = 98) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 53.44 ± 7.48 | 53.47 ± 10.35 | 54.95 ± 9.81 | 1.24/0.292 |
| Education (years) | 13.09 ± 4.13 | 12.56 ± 3.54 | 12.74 ± 3.66 | 0.55/0.580 |
| Sex | 72F 93M | 17F 44M | 104F 61M | χ 25.77/ < 0.001 |
| ZSDS index | 40.83 ± 11.45 | 43.27 ± 11.45 | 12.59 ± 11.65 | 1.07/0.343 |
| Verbal memory | 39.87 ± 8.29 | 41 ± 10.21 | 39.23 ± 10.48 | 1.23/0.294 |
| Verbal fluency | 49.08 ± 12.63 | 45.61 ± 12.25 | 48.98 ± 14.09 | 2.98/0.052 |
| Working memory | 20.64 ± 6.61 | 20.31 ± 4.87 | 20.16 ± 6.06 | 0.20/0.819 |
| Attention and speed of information processing | 48.64 ± 10 | 46.81 ± 11.59 | 47.93 ± 12.56 | 0.72/0.489 |
| Executive functions | 14.79 ± 3.80 | 14.03 ± 4.54 | 14.39 ± 6.13 | 0.60/0.550 |
| Psychomotor coordination | 72.91 ± 10.52 | 71.67 ± 22.41 | 70.12 ± 12.36 | 0.97/0.380 |
| EQ5D—mobility | 1.08 ± 0.27 | 1.11 ± 0.32 | 1.15 ± 0.39 | 0.99/0.36 |
| EQ5D—self-care | 1.01 ± 0.11 | 1.02 ± 0.16 | 1.05 ± 0.23 | 1.20/0.30 |
| EQ5D—usual activities | 1.28 ± 0.49 | 1.23 ± 0.44 | 1.25 ± 0.46 | 0.30/0.74 |
| EQ5D—pain/discomfort | 1.31 ± 0.49 | 1.37 ± 0.55 | 1.37 ± 0.51 | 0.42/0.65 |
| EQ5D—anxiety/depression | 1.25 ± 0.43 | 1.35 ± 0.51 | 1.40 ± 0.53 | 1.95/0.14 |
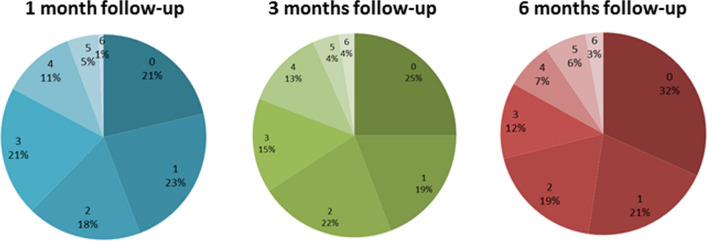
Seventy-nine percent of patients at 1 month and 75% at 3- and 6-month follow-up showed cognitive impairment in at least one cognitive function (Percentage of impaired cognitive functions are showed in Fig. 1). The global cognitive index showed no significant difference in cognitive performances between 1-, 3-, and 6-month follow-up (F = 2.59, p = 0.077). Twenty-three percent of patients at 1-month follow-up, 26% at 3-month follow-up, and 27% at 6 month follow-up showed depressive symptomatology as measured by the ZSDS scale.
Fig. 1.
Percentages of impaired cognitive functions in COVID-19 survivors at 1-, 3-, and 6-month follow-up
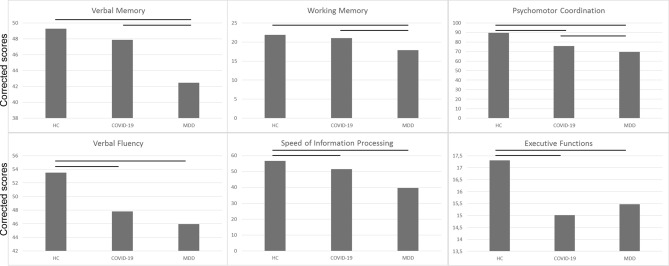
Clinical and demographic characteristics and neuropsychological performances of COVID-19 survivors, HC and MDD patients, are summarized in Table 2 and Fig. 2. The three groups significantly differed for age (F = 75.02, p < 0.001) and sex (χ2 = 31.63, p < 0.001). HC were the youngest (HSD HC vs MDD p < 0.001; HC vs COVID-19 p < 0.001) and COVID-19 survivors were the oldest (HSD COVID-19 vs MDD p = 0.003); also, MDD were mainly females (F = 63%), whereas COVID-19 survivors and HC were mainly males (HC, F = 43%; COVID-19, F = 36%). Results on the mean equivalent score showed a significant effect of diagnosis (F = 43.20, p < 0.001). Tukey HSD post hoc indicated that COVID-19 survivors performed worse than HC (p < 0.001) and better than MDD (p < 0.001).
Table 2.
Clinical, demographic characteristics of the sample and cognitive performances corrected for age, sex, and education
| Controls (n = 165) | COVID-19 (n = 312) | MDD (n = 165) |
T-F/p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 40.57 ± 11.79 | 52.63 ± 8.81 | 49.41 ± 11.19 | 75.02/ < 0.001 |
| Education (years) | 13.45 ± 3.79 | 12.94 ± 3.76 | 12.59 ± 3.96 | 2.50/0.082 |
| Sex | 72F 93M | 17F 44M | 104 F 61M | χ 39.02/ < 0.001 |
| Verbal memory | 49.25 ± 9.06 | 47.86 ± 9.35 | 42.46 ± 10.78 | 23.17/ < 0.001 |
| Verbal fluency | 53.52 ± 13.62 | 47.82 ± 12.73 | 45.95 ± 15.12 | 14.11/ < 0.001 |
| Working memory | 21.88 ± 4.31 | 20.98 ± 6.09 | 17.84 ± 5.14 | 25.67/ < 0.001 |
| Attention and speed of information processing | 56.78 ± 9.93 | 51.59 ± 10.83 | 39.64 ± 13.37 | 100.16/ < 0.001 |
| Executive functions | 17.30 ± 2.90 | 15.01 ± 4.99 | 15.46 ± 4.22 | 14.74/ < 0.001 |
| Psychomotor coordination | 89.60 ± 12.04 | 75.83 ± 16.12 | 69.49 ± 18.97 | 61.79/ < 0.001 |
Fig. 2.
Mean adjusted scores of cognitive performances in COVID-19 survivors at 1-, 3-, and 6-month follow-up. Scores are adjusted for age, sex and education
Given the observed significant global deficit, cognitive performances in each neuropsychological domain have been investigated. Results indicated a significant effect of diagnosis in all six domains, with HC showing better performances compared to COVID-19 patients which, in turn, performed better than MDD in psychomotor coordination (token motor task: F = 48.93, p < 0.001; HSD HC vs COVID-19 p < 0.001; HSD MDD vs COVID-19 p < 0.001), attention and speed of information processing (symbol coding: F = 88.93, p < 0.001; HSD HC vs COVID-19 p < 0.001; HSD MDD vs COVID-19 p < 0.001). No difference between COVID-19 survivors and MDD was observed for verbal fluency (F = 8.02, p < 0.001; HSD HC vs COVID-19 p < 0.001), and executive functions (Tower of London: F = 7.27, p < 0.001; HSD HC vs COVID-19 p < 0.001). Finally, COVID-19 survivors performed the same as HC, and significantly better than MDD in working memory (digit sequencing: F = 21.17, p < 0.001; HSD MDD vs COVID-19 p < 0.001) and verbal memory (F = 30.09, p < 0.001; HSD MDD vs COVID-19 p < 0.001).
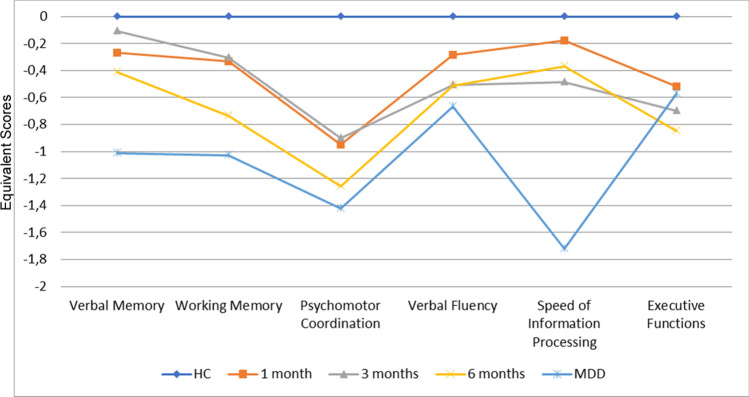
A MANCOVA testing for profile differences indicated a significant group-by subtest interaction (F = 6.34, p < 0.001). A cognitive profile of the three diagnostic groups is showed in Fig. 3.
Fig. 3.
Neuropsychological Profiles on the Brief Assessment of Cognition in Schizophrenia (BACS) for COVID-19 survivors compared to healthy comparisons and major depression
Results of elastic net penalized regression (Supplementary Tables 1–6) showed that the factor that most affect cognitive performance was depression as measured by the ZSDS. Depression negatively influenced cognitive performances in working memory, verbal fluency, speed of information processing, and executive functions. Other factors surviving the VIP threshold of 75% were glycaemia at follow-up and previous coronaropathy which associated with verbal memory.
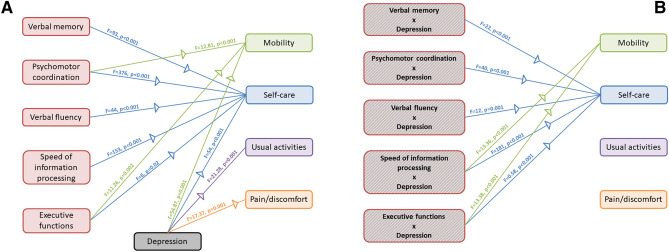
Both cognitive functions and depressive symptomatology predicted quality of life (Fig. 4). We observed a significant main effect of verbal memory (λ = 0.31, p < 0.001), psychomotor coordination (λ = 0.10, p < 0.001), verbal fluency (λ = 0.47, p < 0.001), speed of information processing (λ = 0.21, p < 0.001), executive functions (λ = 0.65, p = 0.002), and depressive symptoms (λ = 0.25, p < 0.001). Univariate analyses showed a significant effect of verbal memory on the self-care dimension (F = 93, p < 0.001), of psychomotor coordination on mobility (F = 12.81, p < 0.001) and self-care (F = 376, p < 0.001), of verbal fluency on self-care (F = 44, p < 0.001), speed of information processing on self-care (F = 153, p < 0.001), executive functions on mobility (F = 11.26, p = 0.002) and self-care (F = 6, p = 0.02), of depressive symptoms on all subscales (mobility: F = 54.87, p < 0.001; self-care: 54, p < 0.001; usual activities: F = 21.28, p < 0.001; pain/discomfort: F = 17.37, p < 0.001). Furthermore, we observed a significant interaction between cognitive functions and depressive symptoms on quality of life: verbal memory (λ = 0.58, p < 0.001), working memory (λ = 0.17, p < 0.001), psychomotor coordination (λ = 0.47, p < 0.001), verbal fluency (λ = 0.73, p < 0.001), executive functions (λ = 0.50, p < 0.001). Univariate statistic showed that the dimension of mobility was predicted by the interaction between depressive symptoms and working memory (F = 13.36, p < 0.001) and executive functions (F = 13.38, p < 0.001), whereas self-care was predicted by the interaction between depressive symptoms and verbal memory (F = 22, p < 0.001), working memory (F = 181, p < 0.001), psychomotor coordination (F = 40, p < 0.001), verbal fluency (F = 12, p = 0.001), executive functions (F = 0.58, p < 0.001).
Fig. 4.
Graphical representation of the effects of cognitive functions and depression on quality of life. A Main effect of cognitive functions and depression. B Interaction between depression and cognitive functions
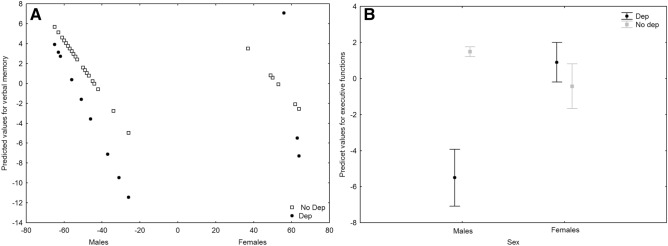
Compared to the 3-month follow-up performance, at 6-month COVID-19 survivors showed improved performances in verbal fluency (F = 14.27, p < 0.001) and attention and speed of information processing (F = 11.53, p = 0.001). A GLZM analysis of the effects of depressive symptoms at 3 months on the changes in cognitive functions showed a significant interaction of depression and age and sex on verbal memory (χ2 = 4.944, p = 0.0262) (Fig. 5a), a significant interaction of depression and sex on executive functions (χ2 = 13.335, p < 0.001) (Fig. 5b), and a significant negative effect of age on attention and speed of information processing (χ2 = 4.172, p = 0.041).
Fig. 5.
A Interaction effect of sex*depression on verbal memory. B Interaction effect of sex*depression on executive functions
Discussion
This is the first study to investigate cognitive functioning in COVID-19 survivors at three time points, through a face-to-face approach in a sample of mainly hospitalized patients not needing ICU admission and comparing COVID-19 survivors cognitive functioning with a sample of MDD and HC.
Here we showed that: (1) about 75% of patients recovered from COVID-19 show impairments in at least 1 cognitive function; (2) there is no difference in cognitive performances in three different cohorts of patients assessed, respectively, at 1-, 3-, and 6-month follow-up; (3) COVID-19 survivors scored lower than HC in psychomotor coordination and attention and speed of information processing, performed worse than HC and similar to MDD patients in verbal fluency and executive functions, but did not differ from HC in working memory and verbal memory; (4) among several demographic and clinical predictors the factor that most affected cognitive performance is depression; and (5) cognitive functions interact with depressive symptoms in determining quality of life in COVID-19 survivors.
Our findings are in agreement with previous studies showing cognitive impairment both following discharge [20] and in the following 1–3 months especially in the domains of information processing, verbal fluency and executive functions [2, 33]. According to our results, cognitive impairment seems to be stable at 1-, 3-, and 6-month follow-up, although an improvement in verbal fluency and speed of information processing was observed between 3 and 6 months in association with mood improvement. Depression seems to be the factor that best predicts cognitive functions, more that clinical parameters associated with COVID-19 symptomatology. Furthermore, depression interact with cognitive function in affecting two aspects of the quality of life of these patients: the mobility and the self-care dimensions. In particular, almost all cognitive functions contribute to the ability to take care on one-self. This last finding in is agreement with previous literature in depressed subjects, where executive functions predict disability in quality of life, social, occupational, and global functioning as well as self-perceived daily functioning and longitudinal psychosocial functioning [9].
Several mechanisms could underpin the observed deficits with different pathways, which might act in different individuals with accumulation and interaction effects. First, cognitive deficits could follow depressive symptomatology. Depressive mood has been consistently associated with cognitive impairment [5] and depressive symptoms have been reported in COVID-19 survivors [29] and have been shown to persist months after hospital discharge [31]. In agreement with this hypothesis, we recently showed that depression predicts cognitive performances in attention and speed of information processing [31] and here we showed that depression is the variable which best predicts cognitive performances, particularly working memory, verbal fluency, speed of information processing, and executive functions. Furthermore, performances in attention and speed of information processing improve between 3 and 6 months from hospital discharge. The ability to pay attention to and manipulate information, in turn, may affect the overall performance leading to more widespread cognitive deficits.
Depression, however, is associated with several neurobiological effects, which can also be observed in COVID-19 survivors, such as increased cortisol levels, glutamatergic neurotoxicity and increased pro-inflammatory factors which can also impact cognitive functioning. Furthermore, coronaviruses are considered to be potentially neurotrophic as suggested by their presence in the cerebrospinal fluid after less than a week from infection [8]. This hypothesis has been confirmed by the presence of SARS-CoV-1 genome sequences throughout the cortex and hypothalamus [17] and by brain lesion in white matter and gray matter in frontal, temporal and parietal regions of patients infected by MERS-CoV [4].
Inflammation can compromise the BBB through the upregulation of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α and inflammatory mediators that increase BBB permeability [27]. The disruption of the BBB enables cytokines to enter the CNS and activate microglia and oxidative stress, that can promote cognitive decline [1].
Several studies have confirmed the link between inflammation activation and cognitive dysfunction [10]. Older demented patients with worse cognitive function show higher levels of inflammation [13] and inflammation plays a critical role in the pathological process of mild cognitive impairment [41]. In addition, cytokines and chemokines have been previously demonstrated to play a role in cognitive development [28]. In agreement with this hypothesis, in a previous study we observed that the systemic inflammation index influence verbal memory, verbal fluency, speed of information processing, and psychomotor coordination [31]. This finding was confirmed in the present study, where verbal fluency, speed of information processing, and psychomotor coordination are impaired in COVID-19 survivors compared to HC.
Finally, COVID-19 has also been found to invade endothelial cells, leading to vascular inflammation and to affect small and large vessels in the brain [43] and brain alteration of vascular origin have been previously associated with memory, executive functions, and processing speed in patients with depression [25, 40]. Furthermore, a recent brain imaging study showed areas of hypometabolism in the rectal/orbital gyrus (including the olfactory gyrus), the amygdala, the hippocampus, the thalamus, and the cerebellum. Interestingly, younger patients which are supposed to develop a more benign form of the disease, showed, instead, persistent cognitive impairment [18]. Brain alteration have been observed also in gray and white matter with COVID-19 patients reporting higher gray matter volumes in several regions including olfactory cortices, hippocampi, insula, and cingulate gyrus and alterations of white matter microstructure with increased fractional anisotropy and reduced radial, axial, and mean diffusivity. Furthermore, gray matter alterations have been associated with memory loss [26].
Limitation of the present study include a cross-sectional nature that does not allow interpretation for causality, the presence of only a small group of patients with a longitudinal assessment at different follow-ups, and differences in age and sex distribution between COVID-19 survivors, MDD patients and HC. As expected by the epidemiology, MDD patients were younger and mainly females [19] when compared with COVID-19 survivors that notably have a more severe course needing hospitalization in older males [22]. Furthermore, HC subjects were the youngest. To account for differences between groups the analyses have been performed on scores corrected for age, sex, and education. Furthermore, the presence of cognitive deficits prior to COVID-19 cannot be excluded. In the present study, we did not used a neuropsychological battery specific for affective disorders; therefore, further studies are needed to investigate whether COVID-19 might affect also the affective component of cognition. Finally, apart from sex, age, and education, also intelligence quotient could affect neuropsychological scores [7]; however, considering the limited health care resources and patient’s compliance related to the outpatients clinical setting, we were not able to routinely assess baseline IQ with a standardized test.
Conclusions
Our results confirm that neuropsychiatric consequences of COVID-19 such as cognitive impairment persist in the months following hospital discharge with only partial improvement at 6-month follow-up, and that cognitive impairment, in association with depression, has a detrimental impact on the quality of life, especially on the ability to take care of one-self. Furthermore, we confirmed the central role of depression, which is the best predictor of cognitive performance and of its improvement. Treatment strategies should take into account the persistent effects of COVID-19 on mood and cognition and novel therapeutic approaches should be developed, specifically targeting cognitive impairments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The COVID-19 BioB Outpatient Clinic Study group also includes: Bollettini Irene, Bosio Sara, Bravi Beatrice, Bussolari Ceciclio, Calvisi Stefania, Canti Valentina, Caselani Elisa, Castellani Jacopo, Cilla Marta, Cinel Elena, Colombo Federica, Damanti Sarah, D’Orsi Greta, Di Pasquasio Camilla, Ferrante Marica, Fiore Paola, Fumagalli Anna, Magnaghi Cristiano, Martinenghi Sabina, Mazza Elena Beatrice, Melloni Elisa Maria Teresa, Merolla Aurora, Pomaranzi Chiara, Santini Chiara, Vai Benedetta, Vitali Giordano
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Availability of data and materials
Data will be available upon reasonable request.
Declarations
Conflicts of interest
Not applicable.
Ethics approval
The authors assert that all activities have been approved by the local ethical committee and that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Contributor Information
Sara Poletti, Email: poletti.sara@hsr.it.
The COVID-19 BioB Outpatient Clinic Study group:
Bollettini Irene, Bosio Sara, Bravi Beatrice, Bussolari Ceciclio, Calvisi Stefania, Canti Valentina, Caselani Elisa, Castellani Jacopo, Cilla Marta, Cinel Elena, Colombo Federica, Damanti Sarah, D’Orsi Greta, Di Pasquasio Camilla, Ferrante Marica, Fiore Paola, Fumagalli Anna, Magnaghi Cristiano, Martinenghi Sabina, Mazza Elena Beatrice, Melloni Elisa Maria Teresa, Merolla Aurora, Pomaranzi Chiara, Santini Chiara, Vai Benedetta, and Vitali Giordano
References
- 1.Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anselmetti S, Poletti S, Ermoli E, Bechi M, Cappa S, Venneri A, Smeraldi E, Cavallaro R. The brief assessment of cognition in schizophrenia. normative data for the Italian population. Neurol Sci. 2008;29:85–92. doi: 10.1007/s10072-008-0866-9. [DOI] [PubMed] [Google Scholar]
- 4.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, Saeed BT, Wahbi A, Saedy A, AlDabbagh T, Okaili R, Sadat M, Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atique-Ur-Rehman H, Neill JC. Cognitive dysfunction in major depression: From assessment to novel therapies. Pharmacol Ther. 2019;202:53–71. doi: 10.1016/j.pharmthera.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Mazza M, Cavalli G, Ciceri F, Dagna L, Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J Neuroimmune Pharmacol. 2021;16:1–3. doi: 10.1007/s11481-020-09966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder LM, Iverson GL, Brooks BL. To err is human: "abnormal" neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- 8.Bohmwald K, Galvez NMS, Rios M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157–171. doi: 10.1016/j.psychres.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarty T, Torres IJ, Bond DJ, Yatham LN. Inflammatory cytokines and cognitive functioning in early-stage bipolar I disorder. J Affect Disord. 2019;245:679–685. doi: 10.1016/j.jad.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Cholet J, Sauvaget A, Vanelle JM, Hommet C, Mondon K, Mamet JP, Camus V. Using the Brief Assessment of Cognition in Schizophrenia (BACS) to assess cognitive impairment in older patients with schizophrenia and bipolar disorder. Bipolar Disord. 2014;16:326–336. doi: 10.1111/bdi.12171. [DOI] [PubMed] [Google Scholar]
- 12.Douglas KM, Gallagher P, Robinson LJ, Carter JD, McIntosh VV, Frampton CM, Watson S, Young AH, Ferrier IN, Porter RJ. Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disord. 2018;20:260–274. doi: 10.1111/bdi.12602. [DOI] [PubMed] [Google Scholar]
- 13.Duarte PO, Duarte MGF, Pelichek A, Pfrimer K, Ferriolli E, Moriguti JC, Lima NKC. Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clin Exp Res. 2017;29:411–417. doi: 10.1007/s40520-016-0603-9. [DOI] [PubMed] [Google Scholar]
- 14.Fourrier C, Singhal G, Baune BT. Neuroinflammation and cognition across psychiatric conditions. CNS Spectr. 2019;24:4–15. doi: 10.1017/S1092852918001499. [DOI] [PubMed] [Google Scholar]
- 15.Gonda X, Pompili M, Serafini G, Carvalho AF, Rihmer Z, Dome P. The role of cognitive dysfunction in the symptoms and remission from depression. Ann Gen Psychiatry. 2015;14:27. doi: 10.1186/s12991-015-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, Million M, Raoult D, Cammilleri S, Eldin C. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 20.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of Acute Respiratory Distress Syndrome. Brain Inj. 2006;20:263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- 22.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 25.Kohler S, Thomas AJ, Lloyd A, Barber R, Almeida OP, O'Brien JT. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br J Psychiatry. 2010;196:143–149. doi: 10.1192/bjp.bp.109.071399. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral micro-structural changes in COVID-19 patients—an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010;30:1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 28.Magalhaes RC, Pimenta LP, Barbosa IG, Moreira JM, de Barros J, Teixeira AL, Simoes ESAC. Inflammatory molecules and neurotrophic factors as biomarkers of neuropsychomotor development in preterm neonates: a systematic review. Int J Dev Neurosci. 2018;65:29–37. doi: 10.1016/j.ijdevneu.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, group C-BOCS. Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, Ciceri F, Rovere Querini P, Benedetti B. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021 doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Hlh Across Speciality Collaboration UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez R, Balanza-Martinez V, Luperdi SC, Estrada I, Latorre A, Gonzalez-Jimenez P, Feced L, Bouzas L, Yepez K, Ferrando A, Hervas D, Zaldivar E, Reyes S, Berk M, Menendez R. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;323:2052. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, Group HUSS Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poletti S, Aggio V, Brioschi S, Dallaspezia S, Colombo C, Benedetti F. Multidimensional cognitive impairment in unipolar and bipolar depression and the moderator effect of adverse childhood experiences. Psychiatry Clin Neurosci. 2017;71:309–317. doi: 10.1111/pcn.12497. [DOI] [PubMed] [Google Scholar]
- 37.Pu S, Setoyama S, Noda T. Association between cognitive deficits and suicidal ideation in patients with major depressive disorder. Sci Rep. 2017;7:11637. doi: 10.1038/s41598-017-12142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quek YF, Yang Z, Dauwels J, Lee J. The impact of negative symptoms and neurocognition on functioning in MDD and schizophrenia. Front Psychiatry. 2021;12:648108. doi: 10.3389/fpsyt.2021.648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 40.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/S0006-3223(00)00994-X. [DOI] [PubMed] [Google Scholar]
- 41.Shen XN, Niu LD, Wang YJ, Cao XP, Liu Q, Tan L, Zhang C, Yu JT. Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry. 2019;90:590–598. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- 42.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F, Wang RR, Huang HP, Du CL, Wu CM, Qian XM, Li WL, Wang JL, Jiang LY, Jiang HJ, Yu WJ, Cheng KB. A randomized trial in the investigation of anxiety and depression in patients with coronavirus disease 2019 (COVID-19) Ann Palliat Med. 2021;10:2167–2174. doi: 10.21037/apm-21-212. [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, Shi C, Hu S. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon reasonable request.