Abstract
Objective
In this study we aimed to investigate bone–implant connections (BICs) with Ti–Al6V4 and Ti–Al6Nb7 alloys. Two types of surface morphology, resorbable blast material (RBM) and sandblasted and acid-etched surfaces (SLA), were used for implants.
Materials and methods
Thirty female Sprague Dawley rats aged 0.5–1 year were used. The rats were randomly separated into three groups: 1) Ti–Al6V4 RBM surface (n = 10), 2), Ti–Al6Nb7 RBM surface (n = 10), and 3) Ti–Al6Nb7 SLA (n = 10) surface implants were surgically integrated in femoral bones. The average roughness (Ra) values for these implants were 1–2 Ra. The rats were sacrificed four weeks after the surgical procedure. For each section, the BIC ratio (%) was determined as a percentage of the total implant surface that was in direct contact with the bone.
Results
The BIC ratio was found to be higher in the Ti–Al6Nb7 RBM and Ti–Al6Nb7 SLA groups than in the Ti–Al6V4 RBM group (p < 0.05). There was no statistically significant difference in the BIC ratios between the Ti–Al6Nb7 RBM and Ti–Al6Nb7 SLA groups (p > 0.05).
Conclusion
Ti–Al6Nb7 exhibited good biocompatibility with bone cells. Ti–Al6Nb7 alloy could be a candidate material for dental implant production.
Keywords: Ti-Al6-Nb7, Ti–Al6–V4, Resorbable blast material, Sandblasted and acid etched
1. Introduction
The interactions between bone tissue and the titanium (Ti) surface are a crucial factor in successful dental implant-supported prosthetic restorations. The chemical composition of the surface structure and the properties of an implant play a major role in determining biological compatibility. The most important properties sought in a dental implant surface are: topography, surface chemistry, surface charge, and hydrophilic characteristics.1 These properties may influence the bone cell–surface interactions, formation of the interstitial matrix, and biological processes, such as protein adsorption onto the surface, that could affect the success of the implant. All of these properties are associated with successful bone connection of the implant within the host jaw bone tissues.1, 2, 3 As implant surface properties play an important role in implant–bone cell interactions, extensive research has been conducted on the interaction between implants and surface bone tissues in recent years. These studies focused primarily on surface treatments and surface topography as a result of surface treatments. Sandblasted and acid-etched (SLA) and resorbable blast material (RBM) applications, which are implant surface-roughening technologies, have been proven to increase osteoblast differentiation in vitro and bone integration in vivo. These materials have exhibited better bone–implant connections than machine-surfaced implants.1, 2, 3, 4, 5
The chemical properties of Ti are not the only key factors in achieving successful dental implant-supported prothetic restorations; the metallurgical properties of the implant are important factors too. Because of corrosion and low fatigue resistance, the pure Ti material used in the production of dental implants offers poor protection against breakage caused by chewing forces. In addition, metal residue is released into the tissues and the circulation. Therefore, research continues in order to produce Ti implants with better mechanical and chemical properties. The most frequently used material in the manufacture of dental implants is currently the Ti–Al6V4 alloy. This alloy contains aluminum (Al) and vanadium (V), which both feature excellent mechanical strength. However, it is known that V has cytotoxic effects, can suppress cell growth, and is liable to induce oxidative stress. Recently, titanium-6-aluminum-7-niobium (Ti–Al6Nb7) has been proposed as a new alloy (biomaterial) for dental technology. Its physical properties are very similar to those of Ti–6Al–4V (titanium-6-aluminum-4-vanadium). Increased corrosion resistance has been observed; moreover, the ions in niobium (Nb) have been reported to be less toxic than those of V.6, 7, 8, 9, 10, 11
In this experimental animal study, we aimed to investigate the bone–implant connections (BICs) of Ti–Al6V4 and Ti–Al6Nb7 implants histologically. Two types of surface morphology, RBM and SLA, were used for the implants.
2. Materials and methods
2.1. Animals and experimental design
All the experimental and surgical procedures in this study were performed at the Firat University Experimental Research Center, Elazig, Turkiye, and ethical consent was obtained from Firat University's Animal Experiments Local Ethics Committee, Elazig, Turkiye (Protocol Number: 2017/37). The animals that were used in this study were provided by Firat University's Experimental Research Center. The study was in full compliance with the World Medical Association Declaration of Helsinki recommendations for the conservation of experimental animals. Thirty (30) Sprague Dawley rats (weight 280–320 g; age 0.5–1 year; female) were used. All the rats were housed in temperature-controlled plastic cages in a 12 h–12 h light–dark cycle, with free access to food and water during the four-week experimental period.
The rats were randomly divided into three groups, as follows (all implants were obtained from Implance Dental Implant System, AGS Implant Corporation, Istanbul, Turkiye).
-
1)
Ti–Al6V4 RBM (n = 10): Ti–Al6V4 RBM surface implants were surgically integrated in the rat femur bones. The average roughness (Ra) values for these implants were 1–2 Ra.3,4
-
2)
Ti–Al6Nb7 RBM (n = 10): Ti–Al6Nb7 RBM surface implants were surgically integrated in the rat femur bones. The Ra values for these implants were 1–2 Ra.3,4
-
3)
Ti–Al6Nb7 SLA (n = 10): Ti–Al6Nb7 SLA surface implants were surgically integrated. The Ra values for these implants were 1–2 Ra.3,4
2.1.1. Surgical procedures
All the surgical applications were done under general anesthesia, which was administered by an intramuscular injection of 5 mg/kg xylazine with 40 mg/kg ketamine hydrochloride. Care was taken to perform all surgical procedures under sterile conditions. Following the administration of anesthesia, the right femoral skins were shaved and then washed with povidone iodine before surgery. After general anesthesia was achieved, a linear incision (2–2.5 cm) was made on the skin on the right femoral bone in each rat. After completion of the skin incision and dissection of the muscles, we used a periosteal elevator to reach the metaphyseal part of the femur that makes a joint with the tibia bone. Implant sockets were created by appropriate drills (point drill, 1.8 mm drill, and 2.2 mm drill) under sterile serum physiological perfusion.12 Next, through primary stabilization, the Ti implants (4 mm in length and 2.5 mm in diameter) were integrated into the femoral bone.12 The subcutaneous tissues and skin were then moved back to their original positions. They were then sutured with 4–0 polyglactin absorbable sutures. After all the surgical procedures, an analgesic (0.1 mg/kg tramadol hydrochloride) and an antibiotic (50 mg/kg penicillin) were administered intramuscularly for three days to prevent pain and infection. In total, 30 implants were integrated into the corticocancellous part of the right femoral bones of the rats. All the surgical procedures were performed by the same researcher.
2.2. Analyzes
At the end of the four-week experimental period, all the control and test rats were sacrificed, and the implants and surrounding tissue were taken out after removing the muscles and soft tissues. The samples were fixed in a solution (10% formaldehyde) for seven days. After fixation, all samples were embedded in 2-hydroxymethylmethacrylate resin to allow the non-calcified bone and Ti to be cut in half with a hard tissue saw (EXAKT®, Germany). For histological analysis, the implants and surrounding tissue were milled with grinders, starting from thick to thin, respectively (EXAKT®, Germany). For the light microscope analysis, 50 μm-thick sections were obtained and then stained with toluidine blue.1,12 All the procedures were performed at the University of Erciyes, Faculty of Dentistry research laboratory in Kayseri, Turkiye. The histological analyses were performed with a light microscope (Nikon, Japan) that was available at the Department of Medical Microbiology (Faculty of Medicine, Firat University). For each non-decalcified section, the BIC ratio (%) was measured as a percentage of the total length of the implant surface that had direct contact with the surrounding bone tissues.1,12 In the analysis of bone–implant connections, the implant circumference of each sample was measured with a microscope, after which the length of the part of the implant in contact with the bone was measured. The percentage of BIC (%) for each implant was determined by the ratio of the length of the implant in contact with the bone to its entire circumference.12
2.3. Statistical analyzes
IBM, SPSS Statistics for Windows, Version 23.0 (USA), was used for the statistical analysis. Data are given as mean and standard deviation. Tukey's honestly significant difference (HSD) test and one-way analysis of variance (ANOVA) were used for the BIC ratio (%) analysis. A p value of <0.05 was considered sufficient to indicate statistical significance.
3. Results
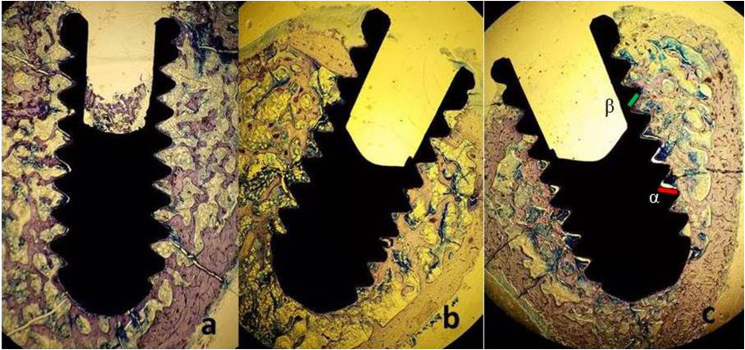
No fatal or non-fatal complications, such as wound dehiscence and infection, were detected during the experimental period. Two specimens were removed from each group because of improper histological preparation. The results of the histomorphometric analyses of the BICs in each group are presented in Table 1. The BIC ratio was found to be higher in the Ti–Al6Nb7 RBM and Ti–Al6Nb7 SLA groups than in the Ti–Al6V4 RBM group (p < 0.05). There was no statistically significant difference in the BIC ratios between the Ti–Al6Nb7 RBM and Ti–Al6Nb7 SLA groups (Fig. 1 a,b,c) (p > 0.05).
Table 1.
Bone implant contact ratio of the groups.
|
Groups |
Bone Implant Contact (%) (mean ± sd) | P |
|---|---|---|
| TiAl6Va4_RBM(n = 8) | 37.8 | <0.05a |
| TiAl6Nb7_RBM (n = 8) | 54.98α | |
| TiAl6Nb7_SLA (n = 8) | 60.71α |
α Statistically significant different compared with TiAl6Va4_RBM (Tukey HSD, P < 0.05).
One Way Anowa Test.
Fig. 1.
a, b, c: Non-decalcified histologic images of the Ti–6Al–4V_RBM (a), Ti–6Al–7Nb_RBM (b), and Ti–6Al–7Nb_SLA (c) implants (4X) (X: 10 Times Magnification, staining with methilen blue). Implant surface not contacting bone (α) (It is shown with the green line on the figure), Implant surface in contacting with bone (β) (It is shown with the red line on the figure), Total implant surface: £, Bone Implant Contact Ratio (%): £-α(β)/£.
4. Discussion
Titanium (Ti) and titanium alloys, which are biocompatible, have become commonly used basic biomaterials in the production of dental, orthopedic, neurological, and cardiovascular implants. Ti has been used extensively since the 1950s as a biomedical material. Additionally, tit alloys such as Ti–Al6V4 have been developed to improve the mechanical properties of commercially pure Ti. However, owing to the cytotoxicity of vanadium (V) and its ability to trigger a tissue reaction, its clinical use is limited. Consequently, V-free Ti alloys have been recently developed to overcome the potential cytotoxic effects of V and adverse reactions in body tissues. Many of these alloys use non-toxic but heavy alloy elements, such as Nb, zirconium, and tantalum. Because of these properties of Nb, Ti–Al6Nb7 is often the first choice for the successful reconstruction of jaw or facial bone defects.6–2 Semlitsch et al.13 Developed in the 1980s, the Ti–Al6Nb7 alloy is a typical Nb alloy manufactured by cast material forging for hip replacement. This alloy is widely used in the production of medical devices, especially joint prostheses, as well as plates, rods, and nails for the fixation of a fracture, and spinal cord devices, screws, and wires.13, 14, 15, 16, 17
The shape, surface structure, and morphology of intraosseous Ti implants are key factors for achieving long-term mechanical stability and for increasing bone–implant integration after surgical placement. Consequently, the choice of coating materials for the treatment of the implant surface can contribute significantly to the attachment of bone cells to the implant surface and the integration of the implant and the bone. Surface modifications have been developed to improve the clinical performance of Ti implants. The surface modifications include machining, sand blasting, etching with acid, porous sintering, oxidizing, anodising, plasma spraying, and coating with hydroxyapatite, as well as combinations of these procedures. Currently, many types of implants, with variations in form, material, size, surface properties, and surface geometry, are being produced. The best surface for achieving osseointegration and the clinical success of dental implants is still the subject of research.1, 2, 3, 4, 5 The histological results of Dundar et al.1 indicated that the five surfaces (RBM, anodized, SLA, blasted, and microarc) investigated in their experimental study were biocompatible and osseconductive. Osseointegration percentages for the dental implants were not statistically different between the groups. Dundar et al.1 reported that the RBM and SLA surface implants exhibited similar BIC connection ratios with 1–2 Ra. As in Dundar et al., the present study found no statistically significant difference between the RBM and SLA groups. The average roughness of all the implants in the present study was 1–2 Ra. The BIC results indicated no statistically significant difference among the two groups, the Ra values for the groups being similar.1
In their in vivo study, Johansson and Albrektsson examined the osseointegration of commercially pure Nb implants into rabbit bone. They compared the bone–implant contact with this method and that with pure Ti implants. Researchers have reported that, after three months of recovery, the torque values for Nb implants are significantly higher than those for Ti implants. In the histological analysis of the implants, Johansson and Albrektsson found no significant difference in the BIC rates between Nb and Ti materials.6 They reported a statistically significant difference in the removal torque values of Nb and Ti implants as a result of the differences in the two implant surfaces.6 The statistically significant difference in the torque values between the Nb and Ti implants has been attributed to the more irregular surface topography of Nb implants. In addition, Nb is a softer metal than Ti. That the Nb metal has higher biocompatibility properties than Ti is another possible reason for the observed differences. The results of the present study confirm this previous research. The BIC ratios for the RBM and SLA surfaces in the Nb groups had high statistical significance when compared with commercial Ti–Al6V4 metals.6
Osathanon et al.7 compared the early response of human osteoblast-like cells to pure Ti and the Ti–Al6Nb7 alloy in vitro. No statistically significant difference was found between Ti and the Ti–Al6Nb7 alloy in terms of cell attachment. In addition, the electron microscopy screening assays indicated that the cells on the Ti–Al6Nb7 exhibited better spreading after 4 h. After 48 h, two different tests (the reverse transcription polymerase chain reaction assays and the western blot analysis) showed that the cells cultured in the Ti–Al6Nb7 synthesized bigger amounts of osteopontin and fibronectin than the cells seeded on the commercially pure Ti or glass slide. These results demonstrated that Ti–Al6Nb7 has the potential to support cell activity and osteopontin and fibronectin synthesis in osteoblast-like cells. Therefore, it is an appropriate material for use in the manufacture of implants.7 Rotaru et al.10 evaluated the biological compatibility of the Ti–Al6Nb7 alloy. They compared the growth viability of various osteoblast-like cells on Ti–Al6Nb7 alloy samples implanted into the cranial bones of Wistar rats. No inflammatory reactions were detected after the integration of the Ti–Al6Nb7 alloy10; however, the Ti–Al6Nb7 implants and the Ti–Al6Nb7 coated with hydroxyapatite produced better results. This suggests that Nb metal has potential as an implant material. In their in vitro cell culture study, Shapira et al. found that the highest proliferation rate for the osteoblast cells was on the machined-surface disks containing Nb. In addition, alkaline phosphatase, osteocalcin, and tissue growth factor-β activity was determined to be higher with the Ti–Al6Nb7 alloy than with the Ti–Al6V4 alloy. On the basis of their cell culture preclinical model, Shapira et al.8 suggested that Ti–Al6Nb7 could replace the Ti–Al6V4 alloy as a dental implant material. Shapira et al. reported that the negative effects of the V resulted in the higher BIC ratios with the Ti–Al6Nb7 implants than with the Ti–Al6V4 implants. Additionally, in their recent biocompatibility study, Yolun et al.18 evaluated the biocompatibility of Ti Nb implants in vivo. They reported that Nb increased the mechanical properties of the implant and had no toxic effects, thereby demonstrating that Nb implants could be used orthopedically. In another in vitro study, Kuroda et al.19 reported good results from their tests of the biomechanical properties of Ti and Nb alloy, which did not exhibit cytotoxic effects. Balbinot et al.20 conducted a study to evaluate the bone healing ability of bioactive glasses containing Nb in a rat femur model with X-ray computed microtomography, and reported that bioactive glasses containing Ni promoted bone formation similar to autogenous bone.
5. Conclusion
Within the limitations and parameters of this study, Ti–Al6Nb7 exhibited good biocompatibility on bone cells, indicating that it could be a candidate material for implant dentistry. Further studies are needed to explain the biocompatible features of Nb metals for use in dental implantation.
Declaration of competing interest
The authors declerate there is no conflict of interest.
Acknowledgment
The authors wish to thanks Implance Dental Implant Corporation, AGS Medical Corporation, Istanbul, Turkiye for the supplying the implants and supporting the nondecalcified histological analyzes of the implants.
References
- 1.Dundar S., Yaman F., Bozoglan A. Comparison of osseointegration of five different surfaced titanium implants. J Craniofac Surg. 2018;29(7):1991–1995. doi: 10.1097/SCS.0000000000004572. [DOI] [PubMed] [Google Scholar]
- 2.Le Guehennec L., Goyenvalle E., Lopez-Heredia M.-A., Weiss P., Amouriq Y., Layrolle P. Histomorphometric analysis of the osseointegration of four different implant surfaces in the femoral epiphyses of rabbits. Clin Oral Implants Res. 2008;19:1103–1110. doi: 10.1111/j.1600-0501.2008.01547.x. [DOI] [PubMed] [Google Scholar]
- 3.Le Guehennec L., Soueidan A., Layrolle P., Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T., Wennerberg A. Oral implant surfaces: part 2 – review focusing on clinical knowledge of different surfaces. Int J Prosthod. 2004;17:544–564. [PubMed] [Google Scholar]
- 5.Esposito M., Coulthard P., Thomsen P., Worthington H.V. The role of implant surface modifications, shape and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur J Prosthodont Restor Dent. 2005;13:15–31. [PubMed] [Google Scholar]
- 6.Johansson C.B., Albrektsson T.A. Removal torque and histomorphometric study of commercially pure niobium and titaniumimplants in rabbit bone. Clin Oral Implants Res. 1991;2(1):24–29. doi: 10.1034/j.1600-0501.1991.020103.x. [DOI] [PubMed] [Google Scholar]
- 7.Osathanon T., Bespinyowong K., Arksornnukit M., Takahashi H., Pavasant P. Ti-6Al-7Nb promotes cell spreading and fibronectin and osteopontin synthesis in osteoblast-like cells. J Mater Sci Mater Med. 2006;17(7):619–625. doi: 10.1007/s10856-006-9224-8. [DOI] [PubMed] [Google Scholar]
- 8.Shapira L., Klinger A., Tadir A., Wilensky A., Halabi A. Effect of a niobium-containing titanium alloy on osteoblast behavior in culture. Clin Oral Implants Res. 2009;20(6):578–582. [PubMed] [Google Scholar]
- 9.Spriano S., Bosetti M., Bronzoni M. Surface properties and cell response of low metal ion release Ti-6Al-7Nb alloy after multi-step chemical and thermal treatments. Biomaterials. 2005;26(11):1219–1229. doi: 10.1016/j.biomaterials.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Rotaru H., Armencea G., Spîrchez D. In vivo behavior of surface modified Ti6Al7Nb alloys used in selective laser melting for custom-made implants. A preliminary study. Rom J Morphol Embryol. 2013;54(3 Suppl):791–796. [PubMed] [Google Scholar]
- 11.Olesova V.N., Shashmurina V.R., Shugailov I.A., Olesov E.E., Mirgazizov M.Z. Study of the biocompatibility of titanium-niobium implants by the parameters of their osseointegration under experimental conditions. Bull Exp Biol Med. 2019;166(5):686–688. doi: 10.1007/s10517-019-04418-y. [DOI] [PubMed] [Google Scholar]
- 12.Bozoglan A., Dundar S., Yildirim T.T. Effects of different levels of restraint stress on bone-implant contact. J Craniofac Surg. 2019;30(4):1294–1297. doi: 10.1097/SCS.0000000000005104. [DOI] [PubMed] [Google Scholar]
- 13.Semlitsch M.F., Weber H., Streicher R.M., Schön R. Joint replacement components made of hot-forged and surface-treated Ti-6Al-7Nb alloy. Biomaterials. 1992;13(11):781–788. doi: 10.1016/0142-9612(92)90018-j. [DOI] [PubMed] [Google Scholar]
- 14.Semlitsch M., Weber H., Streicher R.M., Schön R. Joint prostheses components of warm-forged and surface treated Ti-6Al-7Nb alloy. Biomed Tech. 1991;36(5):112–129. doi: 10.1515/bmte.1991.36.5.112. [DOI] [PubMed] [Google Scholar]
- 15.Semlitsch M., Weber H., Steger R. 15 years experience with the Ti-6Al-7Nb alloy for joint prostheses. Biomed Tech. 1995;40(12):347–355. doi: 10.1515/bmte.1995.40.12.347. [DOI] [PubMed] [Google Scholar]
- 16.Wang X.J., Li Y.C., Lin J.G., Yamada Y., Hodgson P.D., Wen C.E. In vitro bioactivity evaluation of titanium and niobium metals with different surface morphologies. Acta Biomater. 2008;4:1530–1535. doi: 10.1016/j.actbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Avelar-Batista Wilson J.C., Banfield S., Housden J., Olivero C., Chapon P. Surface & coatings technology. On the response of Ti–6Al–4V and Ti–6Al–7Nb alloys to a nitron-100 treatment. Surf Coating Technol. 2014;260:335–346. [Google Scholar]
- 18.Yolun A., Şimşek M., Kaya M., Annaç E.E., Köm M., Ö Çakmak. Fabrication, characterization, and in vivo biocompatibility evaluation of titanium-niobium implants. Proc Inst Mech Eng H. 2021 Jan;235(1):99–108. doi: 10.1177/0954411920960854. Epub 2020 Sep 28. PMID: 32988330. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda P.A.B., da Silva L.M., Sousa K.D.S.J., Donato T.A.G., Grandini C.R. Preparation, structural, microstructural, mechanical, and cytotoxic characterization of Ti-15Nb alloy for biomedical applications. Artif Organs. 2020 Aug;44(8):811–817. doi: 10.1111/aor.13624. Epub 2020 Jan 31. PMID: 31876963. [DOI] [PubMed] [Google Scholar]
- 20.Balbinot G.S., Leitune V.C.B., Ponzoni D., Collares F.M. Bone healing with niobium-containing bioactive glass composition in rat femur model: a micro-CT study. Dent Mater. 2019 Oct;35(10):1490–1497. doi: 10.1016/j.dental.2019.07.012. Epub 2019 Aug 9. PMID: 31402134. [DOI] [PubMed] [Google Scholar]