Abstract
Background
Considering the relatively high 5-yr survival rate (76.9%) for bladder cancer (BC), its overall prevalence will probably continue to increase. Therefore, it is important to understand the effects of BC diagnosis and management, including psychological sequelae.
Objective
To determine the prevalence of depression among elderly patients with BC and identify patient characteristics associated with depression.
Design, setting, and participants
Survey responses from a population-based sample of 5787 patients older than 65 yr with a history of BC were retrieved from the Surveillance, Epidemiology and End Results-Medicare Health Outcomes Survey registry, spanning 1999–2014.
Outcome measurements and statistical analysis
The primary outcome measured is the prevalence of a positive depression screen. Cancer characteristics and demographic, socioeconomic, health-related, and activities of daily living (ADL)-related data were reviewed. Univariate analysis was conducted to identify correlation between a positive depression screen and patient characteristics. Multivariate analysis was performed to identify independent predictors of depression.
Results and limitations
The prevalence of a positive depression screen was 14.0%. Poor general health (p < 0.001), impairment of ADL (p < 0.001), greater number of comorbidities (p < 0.001), and income <$30 000 (p < 0.001) were identified as correlates of depression. Univariate analysis found no association between a positive depression screen and time since the initial cancer diagnosis (p = 0.858) or cancer stage (p = 0.90). Multivariate analysis showed higher levels of education (p = 0.0097), increasing age (p = 0.0027), and marriage (p < 0.0001) were protective against the development of depression. Limitations include the lack of consideration of treatment outcomes and whether patients have active disease or only a history of cancer.
Conclusions
Depression affects a substantial percentage (14%) of elderly patients with BC. Poor general health and impaired ability to complete ADL were the greatest risk factors for depression. Acknowledgment of sociodemographic factors may improve awareness of depression in patients with BC and a potential need for psychosocial support.
Patient summary
Depression affects a significant proportion of patients with bladder cancer. Social and demographic factors influence a patient’s risk of depression. Acknowledgment of these factors may improve the detection of depression and a possible need for intervention.
Keywords: Bladder cancer, Depression, Patient-reported outcomes, Urologic cancer
Take Home Message
Depression affects a significant percentage of elderly patients with bladder cancer. Poor general health and impaired ability to complete activities of daily living were the greatest risk factors. Acknowledgement of sociodemographic factors may improve awareness of depression and any potential need for psychosocial support.
1. Introduction
Bladder cancer (BC) is the sixth most prevalent cancer in the USA, affecting approximately 700 000 individuals [1]. In 2020 there were an estimated 81 400 new cases and 17 980 deaths due to BC, representing 4.5% of new cancer diagnoses and 3.0% of all cancer deaths [1], [2]. Considering the relatively high 5-yr survival of 76.9% [1], the overall prevalence of BC will probably only continue to increase. Thus, it is imperative to understand the extensive effects of BC diagnosis and management, including both physical and psychological sequelae.
The impact of a cancer diagnosis on a patient’s health is profound and multifaceted. Irrespective of cancer type, a patient’s physical and psychological health are adversely affected, leading to impaired physical and social functioning, reduced ability to carry out activities of daily living (ADL), and an overall diminished quality of life [3], [4]. The rate of depression among cancer survivors is 13.7%, and these individuals, including patients with BC, are also at higher risk of death from suicide than the general population [5], [6]. Despite these facts, 45–90% of psychological symptoms are not addressed during the routine care of a cancer patient [7].
Prior studies have evaluated the psychological distress associated with BC diagnosis and treatment [8], [9], [10], [11], [12]. The prevalence of depression in these study populations was high, ranging from 4.7% to 71.3% across all cancer stages and statuses, with variation depending on the geographic region and culture studied [5]. There is currently an incomplete understanding of the overall prevalence of depression among patients with BC in the USA, and of patient characteristics associated with higher risk of depression. Better understanding and recognition of depression in patients with BC will guide physicians in future management.
Using data from the Surveillance, Epidemiology and End Results (SEER) registry linked with the Medicare Health Outcomes Survey (MHOS), we conducted this study to examine the prevalence of depression among patients with BC older than 65 yr. A secondary aim was to identify correlates of depressive symptoms in this population.
2. Patients and methods
2.1. Study population
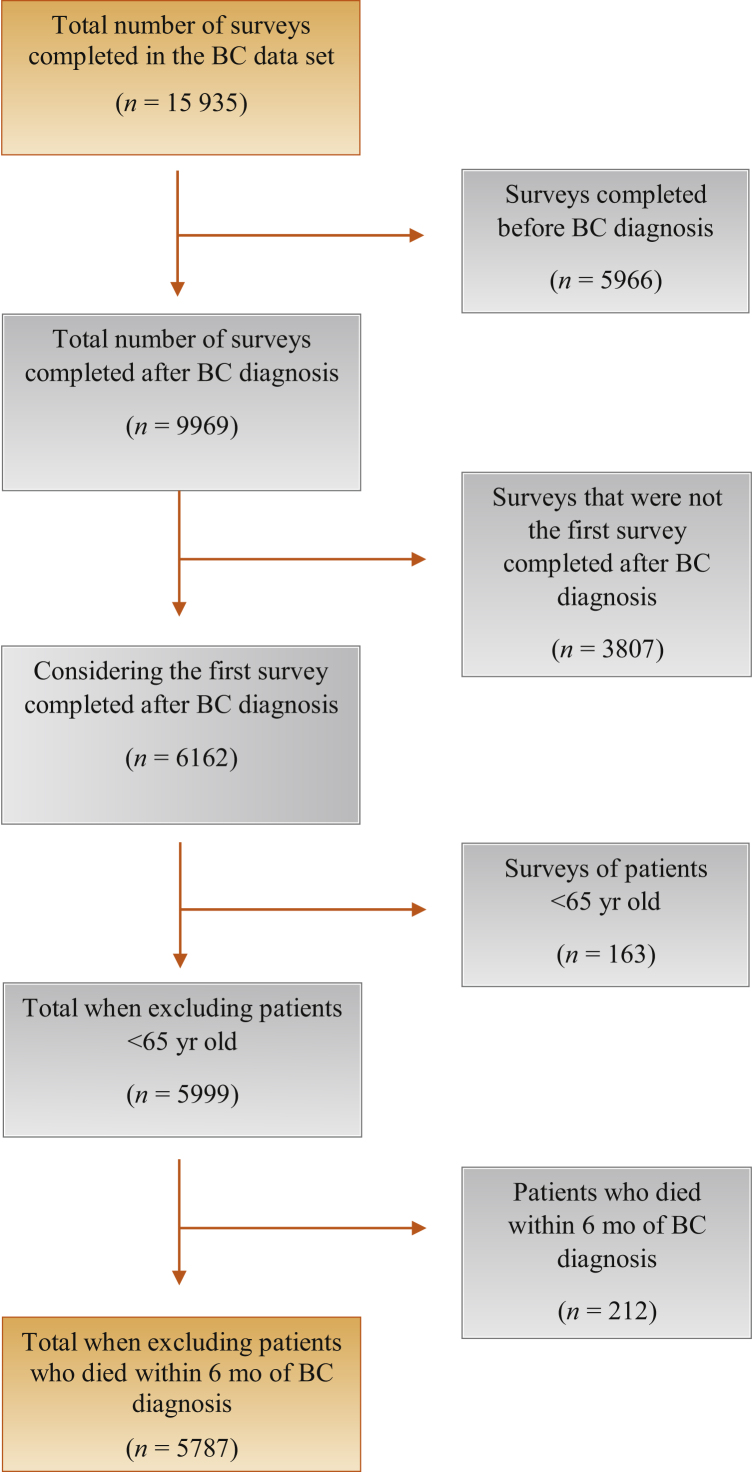
An exemption was obtained from our institutional review board allowing us to perform a retrospective cohort study using deidentified data provided by the SEER-MHOS linked data set. The SEER program of registries collects clinical, demographic, and cause-of-death information for individuals with cancer and the MHOS provides information on the health-related quality of life of Medicare Advantage Organization enrollees [13]. This dataset encompasses 15 cohorts spanning 1999–2014. Each cohort was created annually throughout compilation of this dataset, and each cohort is distinct according to the year of survey completion by patients. Each cohort consists of patients who responded to a baseline survey and a 2-yr follow-up survey. Patients older than 65 yr with a diagnosis of BC were included in our study. We used 65 yr as the age threshold for the study because MHOS specifically surveyed Medicare Advantage Organization enrollees, for which age ≥65 yr is an eligibility requirement for the program. For all patients, we considered only the first survey completed after BC diagnosis, because some patients were surveyed at multiple times throughout their disease course and were therefore initially included in multiple cohorts. We excluded patients who died within 6 mo of their BC diagnosis. The patient flow diagram in Figure 1 describes the patient selection process.
Fig. 1.
Population flow diagram illustrating patient selection for the study.
BC = bladder cancer.
2.2. Study variables
Demographic and socioeconomic data including age, race, sex, education, income, marital status, smoking status, and homeownership were examined. Cancer-related factors including cancer stage were considered. Patient-reported outcomes such as ADL, self-reported comorbidities, and overall general health were also included in the analysis.
2.3. Outcomes and definitions
Patient-reported outcomes of depression from the survey were analyzed. We used a positive depression screen as the primary outcome. A positive depression screen was defined as an affirmative response to at least one of the three depression screening questions listed below, and a mental component score of <42 on the Veterans RAND 12-item Health Survey (VR-12), which is a standard used in previous studies assessing similar outcomes [14], [15], [16]. The three depression screening questions were as follows:
-
1
In the past year, have you had 2 weeks or more during which you felt sad, blue, or depressed; or when you lost interest or pleasure in things that you usually cared about or enjoyed?
-
2
In the past year, have you felt depressed or sad much of the time?
-
3
Have you ever had 2 years or more in your life when you felt depressed or sad most days, even if you felt okay sometimes?
The presence of patient-reported depression symptoms was also analyzed to determine the prevalence of depression symptoms in this patient population. Having a depression symptom was defined as an affirmative response to at least one of the three depression questions listed above. The VR-12 mental component score was not considered for determination of the presence of depression symptoms. Major depressive disorder (MDD) is defined in brief as at least five of the following symptoms present during the same 2-wk period: depressed mood, anhedonia, changes in weight, insomnia/hypersomnia, psychomotor slowing or agitation, fatigue, feelings of worthlessness/ guilt, difficulty concentrating, or suicidal ideations (with at least one of the symptoms as either depressed mood or anhedonia). Multiple factors create challenges in the diagnosis of cancer patients with MDD, so we focused our analysis on the presence of a positive depression screen.
2.4. Statistical analysis
Statistical analyses were performed using SAS (SAS Institute, Cary, NC, USA) and statistical significance was set at p = 0.05. Univariate analysis was performed to identify any correlation between the patient characteristics and a positive depression screen. Categorical variables were compared using the χ2 test of independence and continuous variables using t tests. Results for continuous variables are reported as the mean ± standard deviation. Multivariable analysis of data was performed using logistic regression modeling to control for potential confounding variables. A separate model for the outcome of a positive depression screen was used to identify independent predictors of the outcome variable. Multivariable analysis results are reported as the odds ratio (OR) with 95% confidence interval (CI).
3. Results
A total of 5787 patients met the inclusion criteria. The mean patient age was 77.4 ± 6.8 yr and the mean time from diagnosis to survey was 92.7 ± 81.3 mo. Overall patient-related factors are listed in Table 1. The overall prevalence of a positive depression screen was 14.0% (812/5787) and the prevalence of depressive symptoms was 27.2% (1576/5787). Results for univariate analysis between patients with and without depression revealed many patient and cancer-related factors associated with a positive depression screen, including female gender and current tobacco consumption (Table 2).
Table 1.
Sociodemographic and cancer characteristics of the patient cohort (n = 5787)
| Variable | Result |
|---|---|
| Mean age, yr (standard deviation) | 77.4 (6.8) |
| Gender, n (%) | |
| Male | 4398 (76.0) |
| Female | 1389 (24.0) |
| Race, n (%) | |
| White | 4886 (84.4) |
| Non-white | 901 (15.6) |
| Marital status, n (%) | |
| Married | 3649 (63.1) |
| Unmarried | 2138 (36.9) |
| Education, n (%) (missing = 152) | |
| High school or higher | 4079 (72.4) |
| Less than high school | 1556 (27.6) |
| Income, n (%) (missing = 594) | |
| <$30 000 | 2670 (51.4) |
| >$30 000 | 2523 (48.6) |
| Homeowner, n (%) (missing = 214) | |
| Yes | 4499 (80.7) |
| No | 1074 (19.3) |
| Current smoker, n (%) (missing = 503) | |
| Yes | 837 (15.8) |
| No | 4447 (84.2) |
| General health, n (%) | |
| Excellent to good | 3871 (66.9) |
| Fair to poor | 1916 (33.1) |
| Mean time from diagnosis to survey, mo (standard deviation) | 92.7 (81.5) |
| Cancer stage, n (%) (missing = 2297) | |
| Stage 0 or 1 | 2866 (82.1) |
| Stage 2 | 327 (9.3) |
| Stage 3 or 4 | 297 (8.5) |
| Positive depression screen, n (%) | 812 (14.0) |
Table 2.
Comparison of bladder cancer patients aged ≥ 65 yr with and without a positive depression screen (n = 5787)
| Variable | No depression (n = 4975) | Depression (n = 812) | p value |
|---|---|---|---|
| Mean age, yr (SD) | 77.3 (6.8) | 77.8 (7.1) | 0.0708 |
| Gender, n (%) | |||
| Male | 3805 (76.5) | 593 (73.0) | 0.0327 |
| Female | 1170 (23.5) | 219 (27.0) | |
| Race, n (%) | |||
| White | 4254 (85.5) | 632 (77.8) | <0.0001 |
| Non-White | 721 (14.5) | 180 (22.2) | |
| Marital status, n (%) | |||
| Married | 3217 (64.7) | 432 (53.2) | <0.0001 |
| Unmarried | 1758 (35.3) | 380 (46.8) | |
| Education, n (%) (missing = 152) | |||
| High school or higher | 3612 (74.5) | 467 (59.4) | <0.0001 |
| Less than high school | 1237 (25.5) | 319 (40.6) | |
| Income, n (%) (missing = 594) | |||
| <$30 000 | 2181 (49.1) | 489 (65.0) | <0.0001 |
| >$30 000 | 2260 (50.9) | 263 (35.0) | |
| Homeowner, n (%) (missing = 214) | |||
| Yes | 3925 (81.9) | 574 (73.8) | <0.0001 |
| No | 870 (18.1) | 204 (26.2) | |
| Current smoker, n (%), (missing = 503) | |||
| Yes | 683 (13.7) | 154 (19.0) | 0.0004 |
| No | 3855 (86.3) | 592 (81.0) | |
| General health, n (%) | |||
| Excellent to good | 3604 (72.4) | 267 (32.9) | |
| Fair to poor | 1371 (27.6) | 545 (67.1) | <0.0001 |
| Mean time from diagnosis to survey, mo (SD) | 92.7 (81.3) | 93.1 (82.6) | 0.8965 |
| Cancer stage, n (%) (missing = 2297) | |||
| Stage 0 or 1 | 2439 (82.1) | 427 (82.4) | 0.90 |
| Stage 2 | 281 (9.5) | 46 (8.9) | |
| Stage 3 or 4 | 252 (8.5) | 45 (8.7) |
SD = standard deviation.
Univariate analysis found no association between a positive depression screen and age (p = 0.071), time since the initial cancer diagnosis (p = 0.858), or cancer stage (p = 0.90; Table 2).
The presence and number of comorbidities were also examined for potential association with depression in the patient cohort. The comorbidities examined include hypertension, cardiovascular disease, chronic obstructive pulmonary disease, diabetes, prior stroke, gastrointestinal disease, musculoskeletal disease including arthritis, urinary difficulties, and the presence of an additional primary malignancy. Except for another primary malignancy, each of these comorbidities was associated with a positive depression screen (all p < 0.001). For patients with multiple comorbidities, the risk of a positive depression screen increased (p < 0.001) with the number of comorbidities. Impairment of any ADL was also associated with higher risk of a positive depression screen (all p < 0.001) on univariate analysis (Table 3).
Table 3.
Percentage of patients with and without a positive depression screen who reported difficulty with activities of daily living (n = 5787)
| Activity | Overall, n (%) | No depression, n (%) | Depression, n (%) | p value |
|---|---|---|---|---|
| Bathing | 881 (15.5) | 560 (11.4) | 321 (40.6) | <0.0001 |
| Dressing | 732 (12.9) | 451 (9.2) | 281 (35.7) | <0.0001 |
| Eating | 309 (5.4) | 167 (3.4) | 142 (18.0) | <0.0001 |
| Rising from a chair | 1463 (25.8) | 1048 (21.5) | 415 (52.5) | <0.0001 |
| Walking | 2069 (36.4) | 1548 (31.7) | 521 (65.7) | <0.0001 |
| Using the toilet | 543 (9.6) | 330 (6.8) | 213 (27.0) | <0.0001 |
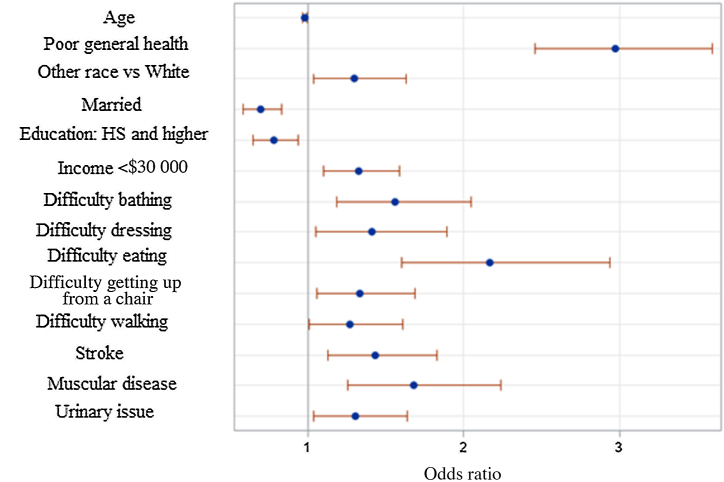
Multivariable analysis was also performed to identify independent predictors of a positive depression screen (Table 4). The backward elimination technique was used to build a parsimonious logistic regression model of outcomes from the significant effects (Fig. 2). Increasing age (OR 0.981, 95% CI 0.968–0.993), being married (OR 0.697, 95% CI 0.583–0.833), and higher education level (OR 0.78, 95% CI 0.646–0.942) were inversely associated with a positive depression screen (Table 4). Independent predictors of a positive depression screen included poor general health, non-White race, income <$30 000, difficulties with ADL, and comorbidities including stroke, muscular disease, and urinary issues (Table 4).
Table 4.
Multivariate analysis for predictors of a positive depression screen in elderly patients with bladder cancer
| Effect | OR (95% CI) | p value |
|---|---|---|
| Age | 0.981 (0.968–0.993) | 0.0027 |
| Poor general health | 2.971 (2.455–3.596) | <0.0001 |
| Non-White race | 1.299 (1.036–1.628) | 0.0233 |
| Married | 0.697 (0.583–0.833) | <0.0001 |
| Education: high school and higher | 0.78 (0.646–0.942) | 0.0097 |
| Income <$30 000 | 1.325 (1.103–1.592) | 0.0026 |
| Difficulty bathing | 1.557 (1.185–2.047) | 0.0015 |
| Difficulty dressing | 1.41 (1.051–1.893) | 0.0221 |
| Difficulty eating | 2.168 (1.601–2.936) | <0.0001 |
| Difficulty rising from a chair | 1.335 (1.056–1.687) | 0.0155 |
| Difficulty walking | 1.271 (1.006–1.607) | 0.0446 |
| Stroke | 1.436 (1.126–1.832) | 0.0035 |
| Muscular disease | 1.679 (1.258–2.241) | 0.0004 |
| Urinary issue | 1.304 (1.04–1.635) | 0.0214 |
OR = odds ratio; CI = confidence interval.
Fig. 2.
Logistic regression modeling of the positive depression screen. Bars denote the Wald 95% confidence limits.
HS = high school.
4. Discussion
Despite the knowledge that cancer patients have higher rates of depression and suicidality than the general population, psychological symptoms often go unaddressed for these patients [7]. Previous studies have evaluated the psychological distress associated with BC, but to the best of our knowledge this is the largest population-based study to date investigating the overall prevalence of depression and depressive symptoms, as well as factors associated with higher rates of depression.
We found that 14% of patients screened positive for depression and 27% reported depressive symptoms, consistent with results from previous studies [5], [17], [18]. A systematic review of patients with BC reported that the prevalence of depression ranged from 4.7% to 71.3%, with variation depending on the geographic region and culture studied [5]. The prevalence of major depressive disorders in the general population on the basis of community samples of adults aged ≥65 yr is estimated to range from 1% to 5% in the USA and internationally [19]. Our study includes 15 different cohorts spanning a duration of 15 yr through the SEER-MHOS linked data set, which offers greater generalizability to practicing urologists.
Our study found no significant association between time from diagnosis and the prevalence of depression. This runs counter to previous studies of patients with breast, gynecologic, and colon cancers that found that the prevalence of depression decreased over time after cancer diagnosis [15], [20]. The results of the current study suggest that there might be more difficulty in adapting to a diagnosis and management of BC than for other cancers. Potential reasons for this include the substantial effect of BC on quality of life, frequent invasive surveillance, and greater impairment of ADL over time after diagnosis [4].
While we suspect that the high morbidity of treatment plays a role, especially with cystectomy for muscle-invasive disease [18], cancer stage did not have a significant association with depressive symptoms or a positive depression screen. These results are in contrast to a previous study that revealed a higher risk of post-treatment psychiatric disorders for patients with more advanced disease [18], which was attributable to more intensive treatment and follow-up and greater morbidity. As we were unable to identify which treatment method each patient received in our data set, further analysis and differentiation by stage and treatment (chemotherapy, cystectomy) might provide a clearer understanding of these effects on the risk of depression.
The strongest associations with a positive depression screen were reporting of poor general health and impairment of ADL. It has been demonstrated that functional status, based on ADL completion, significantly declines after major surgery for BC, such as radical cystectomy [21]. Considering the association we found between depression and impaired ADL performance, it is important for clinicians to recognize the potential interplay between these factors in patients with BC, especially those undergoing major surgery.
Previous studies have also concluded that a greater number of comorbidities has a detrimental impact on health-related quality of life for patients with cancer, across both physical and mental health [22]. Our study echoes this finding. As comorbidity is also a significant independent predictor of overall survival for patients with BC [23], it is worth considering the potential underlying effects of depression in those with comorbidities, and how depression may affect treatment adherence and follow-up in this setting.
The relationship between the income of patients and parameters affecting psychological health has been studied. The income of patients with cancer was associated with quality of life [24], and inversely associated with the risk of depression [15]. Our study demonstrates similar findings, as it reveals a significant association between lower income (<$30 000 per year) and the risk of depression. Demographic and other socioeconomic factors also played a role. Factors not associated with the development of depression in this study included White race, being married, high school education or higher, and being a homeowner. These findings are consistent with those of Jazzar et al [18], who found that White married patients had a lower risk of developing a post-treatment psychiatric condition. This is a notable finding highlighting the need to consider the interaction between psychological outcomes and survival outcomes, as better survival outcomes have been observed for married patients with cancer [25].
The current study found that older age is significantly associated with a slightly lower risk of a positive depression screen. This is consistent with a recent study that found that younger patients (<65 yr) were more likely to experience psychological distress than older patients at BC diagnosis and after surgical treatment with radical cystectomy [26]. Jazzar et al [18] also noted that younger patients with muscle-invasive BC are more likely to be diagnosed with a post-treatment psychiatric disorder potentially because of the substantial morbidity and mortality of treatment. This underscores the important consideration of patient age when addressing not only potential complications and mortality but also depression risk and a potential need for future psychological management. Furthermore, the prevalence of frailty in several studies in the USA ranges from 4% to 16% among community-dwelling men and women aged ≥65 yr [27]. It has been shown that frailty is associated with age, sex, income, education, number of chronic diseases, ADL disability, and instrumental ADL (IADL) disability. Wong et al [27] demonstrated that among those classified as frail, 29.1% had ADL disabilities, 92.7% had IADL disabilities, and 81.8% had comorbidity. The relationship of frailty to sociodemographic variables and comorbidities warrants further analysis regarding its role in the development of depression in the elderly population with BC.
While our study investigates the prevalence of depression among patients diagnosed with BC, the true prevalence of MDD was not identified. Multiple factors create challenges in the diagnosis of MDD in patients with cancer, as mood changes are difficult to evaluate in patients who may have comorbidities such as dementia, or who may be fatigued, undergoing chemotherapy or radiation treatment, or experiencing pain [17]. Importantly, because of expanding knowledge of the detrimental effects of depression on cancer mortality, the National Comprehensive Cancer Network Survivorship Guidelines and recommendations from the American Society of Clinical Oncology include an assessment of the late psychological effects of cancer and intervention for potential consequences of disease and treatment [28], [29]. Further study should be undertaken to more precisely identify the prevalence of MDD among patients with BC and to consider the influence of pretreatment psychological distress on the overall prevalence of depression after diagnosis.
Limitations of the study include a lack of consideration of treatment outcomes, such as how many patients receive transurethral resection of bladder tumor, intravesical treatment, cystectomy, or chemotherapy, and a lack of understanding of cancer progression (as cancer stage was not tracked over time) or whether patients in this study had active disease or only a history of BC. In addition, the study does not address prior psychiatric diagnoses or use of psychiatric medications that could confound the study outcomes. Financial distress placed on the patient due to treatment costs were also not addressed. Further research would be beneficial to determine the impact of BC treatment costs on patient psychological health, especially as BC carries the highest lifetime treatment cost for patients [30].
Despite its limitations, our study is the largest of its kind to describe the prevalence of depression in patients with BC. It also identifies important factors associated with depression in these patients, which may be considered in daily urologic practice to better assess for and detect depression in patients with BC and to optimize individual patient care. It is our aim that greater clinician awareness of these sociodemographic factors would manifest as an increase in the use of standardized, validated questionnaires in clinics to recognize the need for psychosocial support, such as the PHQ-2, PHQ-9, or Geriatric Depression Scale when appropriate. The finding that patient characteristics, rather than disease-related factors, have the strongest association with depression is an eye-opening finding and a reminder that all patients with BC are at risk, regardless of their stage and treatment course.
5. Conclusions
Our study demonstrated that depression affects a substantial percentage of patients with BC. Poor general health and impaired ability to complete ADLs were the greatest risk factors for depression. The most significant protective factors were marital status, higher education, and being a homeowner. Depression prevalence does not decline with prolonged time after BC diagnosis, underscoring the importance of continued assessment for depression in these patients, even after diagnosis and treatment. Acknowledgment of sociodemographic factors can improve clinician awareness of depression in patients with BC and any potential need for psychosocial support throughout a patient’s disease course.
Author contributions: Katie Murray had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Murray, Anwar, Golzy.
Acquisition of data: Murray.
Analysis and interpretation of data: Murray, Anwar, Golzy, Oserowsky.
Drafting of the manuscript: Oserowsky, Anwar, Lough.
Critical revision of the manuscript for important intellectual content: Lough, Murray.
Statistical analysis: Golzy.
Obtaining funding: Murray.
Administrative, technical, or material support: Murray, Golzy.
Supervision: Murray, Golzy.
Other: None.
Financial disclosures: Katie Murray certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: Funding for this study was provided by a University of Missouri Richard Wallace Faculty Incentive Grant. The sponsor played a role in data collection.
Associate Editor: Guillaume Ploussard
References
- 1.National Cancer Institute. Cancer stat facts: bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html.
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Fung C., Pandya C., Guancial E. Impact of bladder cancer on health related quality of life in 1,476 older Americans: a cross-sectional study. J Urol. 2014;192:690–695. doi: 10.1016/j.juro.2014.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungerer G., Anwar T., Golzy M., Murray K.S. Living with bladder cancer: self-reported changes in patients’ functional and overall health status following diagnosis. Eur Urol Open Sci. 2020;20:14–19. doi: 10.1016/j.euros.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vartolomei L., Ferro M., Mirone V., Shariat S.F., Vartolomei M.D. Systematic review: depression and anxiety prevalence in bladder cancer patients. Bladder Cancer. 2018;4:319–326. doi: 10.3233/BLC-180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham H., Torres H., Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol. 2019;37:97–107. doi: 10.1016/j.urolonc.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 7.van den Beuken-van Everdingen M.H., de Rijke J.M., Kessels A.G., Schouten H.C., van Kleef M., Patijn J. Quality of life and non-pain symptoms in patients with cancer. J Pain Symptom Manage. 2009;38:216–233. doi: 10.1016/j.jpainsymman.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Kulaksizoglu H., Toktas G., Kulaksizoglu I.B., Aglamis E., Unlüer E. When should quality of life be measured after radical cystectomy? Eur Urol. 2002;42:350–355. doi: 10.1016/s0302-2838(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 9.Palapattu G.S., Haisfield-Wolfe M.E., Walker J.M. Assessment of perioperative psychological distress in patients undergoing radical cystectomy for bladder cancer. J Urol. 2004;172:1814–1817. doi: 10.1097/01.ju.0000141245.08456.1a. [DOI] [PubMed] [Google Scholar]
- 10.Månsson A., Al Amin M., Malmström P.U., Wijkström H., Abol Enein H., Månsson W. Patient-assessed outcomes in Swedish and Egyptian men undergoing radical cystectomy and orthotopic bladder substitution—a prospective comparative study. Urology. 2007;70:1086–1090. doi: 10.1016/j.urology.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 11.Benner C., Greenberg M., Shepard N., Meng M.V., Rabow M.W. The natural history of symptoms and distress in patients and families following cystectomy for treatment of muscle invasive bladder cancer. J Urol. 2014;191:937–942. doi: 10.1016/j.juro.2013.10.101. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y.L., Liu L., Li M.Y., Shi M., Wang L. Psychological disorders and psychosocial resources of patients with newly diagnosed bladder and kidney cancer: a cross-sectional study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Brief description of the SEER-MHOS data resource. https://healthcaredelivery.cancer.gov/seer-mhos/overview/.
- 14.Rost K., Burnam M.A., Smith G.R. Development of screeners for depressive disorders and substance disorder history. Med Care. 1993;31:189–200. doi: 10.1097/00005650-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Clark C.J., Fino N.F., Liang J.H., Hiller D., Bohl J. Depressive symptoms in older long-term colorectal cancer survivors: a population-based analysis using the SEER-Medicare healthcare outcomes survey. Support Care Cancer. 2016;24:3907–3914. doi: 10.1007/s00520-016-3227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health Services Advisory Group . Medicare Health Outcomes Survey. HSAG; Phoenix, AZ: 2007. Report on the health status of managed care smokers and nonsmokers. [Google Scholar]
- 17.Massie M.J. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 18.Jazzar U., Yong S., Klaassen Z. Impact of psychiatric illness on decreased survival in elderly patients with bladder cancer in the United States. Cancer. 2018;124:3127–3135. doi: 10.1002/cncr.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasin D.S., Goodwin R.D., Stinson F.S., Grant B.F. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 20.Stafford L., Judd F., Gibson P., Komiti A., Mann G.B., Quinn M. Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support Care Cancer. 2015;23:2215–2224. doi: 10.1007/s00520-014-2571-y. [DOI] [PubMed] [Google Scholar]
- 21.Murray K.S., Prunty M., Henderson A. Functional status in patients requiring nursing home stay after radical cystectomy. Urology. 2018;121:39–43. doi: 10.1016/j.urology.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith A.W., Reeve B.B., Bellizzi K.M. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Megwalu I.I., Vlahiotis A., Radwan M., Piccirillo J.F., Kibel A.S. Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. 2008;53:581–589. doi: 10.1016/j.eururo.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krupski T.L., Fink A., Kwan L. Health-related quality-of-life in low-income, uninsured men with prostate cancer. J Health Care Poor Underserved. 2005;16:375–390. doi: 10.1353/hpu.2005.0037. [DOI] [PubMed] [Google Scholar]
- 25.Mahal B.A., Inverso G., Aizer A.A. Incidence and determinants of 1-month mortality after cancer-directed surgery. Ann Oncol. 2015;26:399–406. doi: 10.1093/annonc/mdu534. [DOI] [PubMed] [Google Scholar]
- 26.Ajaj R., Berlin A., Klaassen Z. Age differences in patient-reported psychological and physical distress symptoms in bladder cancer patients — a cross sectional study. Urology. 2019;134:154–162. doi: 10.1016/j.urology.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Wong C.H., Weiss D., Sourial N. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22:54–62. doi: 10.1007/BF03324816. [DOI] [PubMed] [Google Scholar]
- 28.Denlinger C.S., Ligibel J.A., Are M. NCCN guidelines insights: survivorship, version 1.2016. J Natl Compr Cancer Netw. 2016;14:715–724. doi: 10.6004/jnccn.2016.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen B.L., DeRubeis R.J., Berman B.S. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung C., Dinh T., Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32:1093–1104. doi: 10.1007/s40273-014-0194-2. [DOI] [PubMed] [Google Scholar]