Salmonella
mediated cancer therapy has achieved remarkable anti-tumor effects in experimental animal models, but the detailed mechanism remains unsolved. In this report, the active involvement of the host immune response in this process was confirmed by comparing the tumor-suppressive effects of Salmonella in immunocompetent and immunodeficient mice bearing melanoma allografts. Since flagella are key inducers of the host immune response during bacterial infection, flagella were genetically disrupted to analyse their involvement in Salmonella-mediated cancer therapy. The results showed that flagellum-deficient strains failed to induce significant anti-tumor effects, even when more bacteria were administered to offset the difference in invasion efficiency. Flagella mainly activate immune cells via Flagellin/Toll-like receptor 5 (TLR5) signalling pathway. Indeed, we showed that exogenous activation of TLR5 signalling by recombinant Flagellin and exogenous expression of TLR5 both enhanced the therapeutic efficacy of flagellum-deficient Salmonella against melanoma. Our study highlighted the therapeutic value of the interaction between Salmonella and the host immune response through Flagellin/TLR5 signalling pathway during Salmonella-mediated cancer therapy, thereby suggesting the potential application of TLR5 agonists in the cancer immune therapy.
KEY WORDS: Bacteria-mediated cancer therapy, Salmonella, VNP20009, Flagellum, Flagellin, TLR5, NF-κB, Cancer immune therapy
Abbreviations: AKT, Akt serine/threonine kinase; CFU, colony-forming units; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DN, dominant-negative; ERBB2, Erb-B2 receptor tyrosine kinase 2; ERKl, extracellular regulated protein kinase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; i.p., intraperitoneally; i.t., intratumorally; IFN-γ, interferon-γ; IL, interleukins; IκB, inhibitor of NF-κB; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; LRR, leucine-rich repeat; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; PD-1, programmed cell death protein-1; PD-L1, programmed cell death-ligand 1; PEI, polyethylenimine; TIR, Toll/Interleukin-1 receptor; TLR, Toll-like receptor; TME, tumor microenvironment; TRAF6, TNF receptor associated factor 6
Graphical abstract
Flagella play a critical role in Salmonella-mediated cancer therapy by activating host immune response through Flagellin/TLR5/NF-κB signalling pathway, indicating Flagellin's potential application in cancer immune therapy.

1. Introduction
Bacteria were recognized as a potential cure for cancer a century ago1. Many experimental therapies have been designed using Salmonella2, Bifidobacterium3, Escherichia4, Clostridium5 and Listeria6, and these approaches have achieved remarkable anti-tumor effects in various animal models. Initially, the bacterial anti-tumor activity was attributed to the lysis of tumor cells after bacterial infection, especially for pathogenic Salmonella7. Later, increasing evidence indicated the involvement of the host immune response in the process of bacteria-mediated cancer therapy8.
Salmonella infection activates a cascade of immune responses. Upon entry of Salmonella into the body, neutrophils, inflammatory monocytes and natural killer cells are recruited to eliminate the bacteria, which is an innate immune response. Then matured macrophage and dendritic cells activate T-cells to initiate an adaptive immune response, marked by the expansion of Salmonella-specific T cell9. Both lipopolysaccharide (LPS) and Flagellin are important pathogen associated molecular patterns (PAMPs) that activate the host immune response. Flagellin is recognized by Toll-like receptor 5 (TLR5) on monocytes, macrophages, dendritic cells and CD4+ T cells10, while LPS can be recognized by multiple receptors, including TLR4 and CD14, on a wider range of leukocytes11,12. Even though LPS is genetically deleted due to safety reasons, Flagellin/TLR5 recognition remains a crucial way for host immune activation by VNP20009.
Although weaker than Salmonella, tumors are also immunogenic, with various biomacromolecules released by necrotic tumor cells, creating a unique tumor microenvironment (TME) that induces the infiltration of inflammatory cells13. Natural killer cells and CD8+ T cells are able to destroy tumor cells mainly via necrosis mediated by secreted perforin and granzymes, as well as via induction of programmed cell death14,15. Meanwhile, CD4+ T cells secrete interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) to suppress the tumor growth and induce cytolysis16,17. Furthermore, IFN-γ and interleukins (ILs) secreted by CD4+ T cells can recruit a large number of macrophages, CD8+ T cells and B cells to the sites of tumors and active these cells16.
The density of intratumoral T cells is positively correlated with a good prognosis for many kinds of solid tumors, including melanoma18. However, a considerable percentage of the intratumoral T cells are dysfunctional due to the presence of regulatory T cells, M2-like macrophages and immunosuppressive cytokines, as well as the lack of costimulatory signals in the TME19. Moreover, cancer cells often express ligands for immune checkpoint receptors to suppress the activation of T cells20,21. Therefore, immunotherapies such as programmed cell death protein-1 (PD-1)/programmed cell death-Ligand 1 (PD-L1) antibodies, which aim to enhance the host immune response against malignant cells, have exhibited considerable clinical benefits in cancer patients22.
Anti-cancer bacteria could be an economical and targeted alternative for stimulating the intratumoral host immune response, since they are able to specifically colonize solid tumors and be rapidly eliminated by antibiotics after a treatment cycle1. Various attenuated Salmonella strains have been developed for the study of Salmonella-mediated cancer therapies, among which VNP20009 has shown remarkable therapeutic effects in animal models and is the only strain to be evaluated in a phase I clinical trial23,24. For unknown reasons, its anti-cancer efficacy was drastically weaker in human patients than in animal models24. Therefore, a more thorough understanding of the mechanism by which Salmonella causes tumor regression is required for the rational design of potent yet safe therapies for human cancer patients.
In this study, the efficacy of VNP20009-mediated cancer therapy was compared in wild-type mice and nude mice to investigate the importance of T cell-mediated adaptive immune response for the anti-tumor activity of Salmonella. Then, genetically engineered strains of VNP20009, in which the key components of flagellum were deleted, were generated to analyse the involvement of Flagellin/TLR5/nuclear factor kappa-B (NF-κB) pathway. Such flagellum-deficient strains exhibited a significantly impaired ability to suppress tumor growth due to poorer intratumoral colonization and the abrogation of the Flagellin/TLR5/NF-κB pathway in the lymphocytes. Our findings suggest the possibility of improving the number and the activity of intratumoral T cells by stimulating the Flagellin/TLR5/NF-κB pathway in the TME to boost the host immune response against tumor during Salmonella-mediated cancer therapy.
2. Materials and methods
2.1. Mice, cell lines and bacteria strains
6–8-week-old female C57BL/6 mice and BALB/c nude mice were purchased from Laboratory Animal Center of Yangzhou University, China. B16F10 mouse melanoma cells and Jurkat cells were purchased from American Type Culture Collection and maintained by methods described previously25. Lipid A-modified (msbB-), auxotrophic (purI-) Salmonella typhimurium VNP20009, Escherichia coli SM10λpir and TOP10 strains were obtained from American Type Culture Collection and were grown as manufacturer's instructions. All experiments involving mice were approved by Animal ethics committee of Nanjing University (Nanjing, China).
2.2. The construction of flagellum-deficient strains of VNP20009
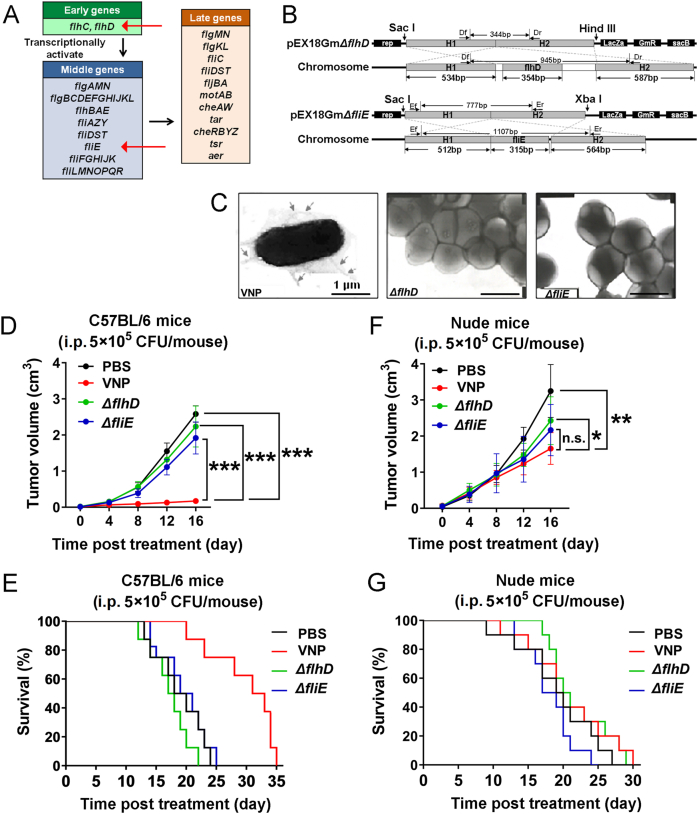
Two flagellum-deficient strains deleting flhD or fliE were constructed by amplifying 5′ flanking (primer DU1: TAGGAGCTCAAACAGCCTGTTCGATCTGTTC, DU2: GCGATGGAGTTGATTAATCTTGGCGCTCG and EU1: CATGAGCTCCAGGTGATGATTTATTTAT, EU2: CTGCGTAAAAAATCGCCAGGGCTGACAA) H1 and 3′ flanking (primers DD1: ACTCCATCGCGACGCAACTCTACTCGTC, DD2: AGCAAGCTTTCATAACTCGCTCCTTGATTGC and ED1: GATTTTTTACGCAGCAGAGATTACCAGT, ED2: TAGTCTAGAGAATTTTTCCTGATCAAGCA) H2 DNA fragments. The amplified fragments were digested and cloned into pEX18Gm to generate the deletion plasmids pEX18GmΔflhD and pEX18GmΔfliE. The plasmids were introduced into E. coli SM10-λpir for replication of these suicide plasmids and then electroporated into VNP20009 using MicroPulser Electroporator (Bio-Rad) by condition 1.6 kV, 25 μf, 400 Ω. Single crossover deletion clones with the entire plasmids integrated into genome were selected on LB agar plates containing 50 μg/mL gentamicin. Single colonies were carefully isolated from gentamicin plates using pipette tips, dissolved in 50 μL LB broth and cultivated on 5% sucrose LB agar plates for 24–36 h at 28 °C to induce the resolvation of inserted plasmids from bacteria genome. During the resolvation (also called “double-crossover”), the targeted gene would be deleted through homologous recombination in a very small percentage of clones. Single colonies were picked from sucrose LB plates by pipette tips and dissolved in 20 μL LB broth. 2 μL of LB broth containing bacteria were used for polymerase chain reaction (PCR) verification. The flhD and fliE deletion strains were confirmed by PCR using typing primers TD1, TD2 and TE1, TE2. TD1: CCCAGGTCATAAACCAGTCTGTGG, TD2: GACGTACCCCTATTCAGCAGTGTGG, TE1: TCTTGCCGCTGCCCGTTATTCA, TE2: CGTTTTGGCCCACAGCACCAT.
2.3. Electron microscopy of bacteria
Overnight culture of VNP20009 was diluted 1:100 into fresh LB and grown to OD600 ∼0.8. Bacteria were collected and washed in 0.1 mol/L NaCl, and resuspended in phosphate-buffered saline (PBS). To examine cells by electron microscopy, 10 μL of the culture was placed onto carbon-coated nickel grids (Electron Microscopy Sciences) for 1 min, washed 3 times with sterile water and then negatively stained with 0.2% uranyl acetate for 30 s. The samples were visualized using the JEM2100EX electron microscope (JEOL)26.
2.4. Green fluorescent protein (GFP) gene transfection into VNP20009
VNP20009 and its flagellum-deficient stains were grown at 37 °C to mid-logarithmic phase in liquid LB and harvested at 4 °C. Bacteria (2.0 × 108) were dissolved in 40 μL 10% glycerol and then mixed with 1 μL of peGFP (Clontech) vector for electroporation with the MicroPulser Electroporator (Bio-Rad) according to the manufacturer's instructions.
2.5. Bacteria adhesion and invasion assay
B16F10 melanoma cells were maintained and prepared for bacterial adherence and invasion assay as described before. The VNP20009 and the deletion strains were grown in liquid LB with constant shaking at 37 °C to reach OD600 = 0.8–1.0, and then added to cell monolayers (2.0 × 105 cell/well) in 24-well plates at a ratio of 50:1 (bacterial cells:tumor cells) and inoculated for 1 h. The protocol of invasion and adhesion assay has been described previously27.
In brief, for the adhesion assay, bacteria were added to the cell monolayer and incubated for 2 h. Non-adhered bacteria were washed away and the infected cells were lysed with sodium deoxycholate. Bacteria were serially diluted, spread onto modified LB agar plates (without sodium chloride) and incubated at 37 °C for 24 h before counting colony-forming units (CFU). For invasion assay, bacteria were added to the cell monolayer and incubated for 6 h. Extracellular bacteria were washed away and cleared by 1 h of treatment with 50 μg/mL gentamicin. Then the cells were lysed with sodium deoxycholate and the CFU was determined as indicated above.
Also, the melanoma cells were infected by the bacteria carrying GFP as described above. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature and rinsed with PBS for 3 times. Cell nucleus was stained with DAPI. The number of bacteria and melanoma cells were quantified by fluorescence microscopy. If brief, the cryosections of tumors were cut at 6 μm thickness and fixed with methanol for 10 s. Cell nuclei were stained with DAPI, and the slides were visualized by the FV1000 confocal microscope (Olympus).
2.6. Evaluation of antitumor effects of VNP20009 and its flagellum-deficient strains
C57BL/6 mice or BALB/c nude mice were implanted subcutaneously with 5 × 105 B16F10 cells in 0.1 mL PBS on the mid-right side. Bacteria were prepared as previously described, and treated intraperitoneally (i.p.) or intratumorally (i.t.) at indicated doses of VNP20009. The antitumor activity of treatments was evaluated as previously described. The tumor volumes were determined using Eq. (1):
| (1) |
2.7. Evaluation of peripheral and intratumoral bacteria in vivo
To evaluate the efficacy of tumor colonization and the replication ability of different bacteria strains by different methods of treatment, mice bearing melanoma allografts were i.p. administrated with 105 CFU/mouse or i.t. administrated with 107 CFU/tumor of bacteria. Peripheral blood was drawn from eyeballs after 1, 2 and 3 h. Serially diluted blood was spread onto modified LB agar plates (without sodium chloride) and incubated at 37 °C for 24 h. The titre of bacteria (CFU/mL blood) was determined by counting colonies and dividing them by the volume of blood.
On Day 2 post-treatment, mice were sacrificed. Tumors were harvested aseptically homogenized with PBS at a ratio of 5:1 [PBS volume (mL):tumor weight (g)]. Serially diluted homogenates were spread onto modified LB agar plates (without sodium chloride) and incubated at 37 °C for 24 h. The titre of bacteria (CFU/g tissue) was determined by counting colonies and dividing them by the weight of the tissue.
2.8. Evaluation of intracellular bacteria number in vivo
To evaluate bacteria internalization in vivo, tumor masses were injected with 108 VNP20009 or its flagellum-deficient strains (all strains carrying GPF plasmids) and then processed for confocal analysis and gentamicin protection assays.
Gentamicin protection assays were conducted on tumors resected 30 min after the administration of VNP20009, ΔflhD and ΔfliE strains. After resection, tumors were dissected, digested and smashed through 70 μm cell strainers (Corning), and depleted of RBC by incubation with red blood cell lysis buffer on ice for 5 min. After that, the tumor cells were treated with 50 μg/mL gentamicin for 2 h at 37 °C to eliminate the extracellular bacteria. Then the cells were counted and lysed with sodium deoxycholate to release the intracellular bacteria. The number of bacteria was measured by colony formation assay as described above.
2.9. Evaluation of antitumor effect of VNP20009 and ΔfliE strains with the same intracellular bacteria number
To check if the intracellular infection ability by different bacteria determined their antitumor effects, we tested different doses of VNP20009, ΔflhD or ΔfliE strains to ensure equivalent intracellular bacteria in tumor. As a result, 2 × 108 CFU of ΔfliE intratumoral treatment resulted in equivalent intracellular bacteria compared to 105 CFU of VNP20009 intratumoral treatment on the next day post-treatment, but even to 1011 CFU/tumor ΔflhD treatment still could not reach similar intracellular bacteria number as VNP20009 or ΔfliE treatment. The evaluation of antitumor effect under equivalent intracellular bacteria was conducted as described above.
2.10. Flow-cytometry analysis of splenocytes and intratumoral leukocytes
Mice bearing melanoma allografts were i.p. injected with PBS, VNP20009 and two flagellum-deficient strains. The tumors and spleens were resected after 6 days. Half of the tumor mass was dissected and incubated in PBS containing collagenase I (Gibco), collagenase IV (Sigma–Aldrich), DNase I (Sigma–Aldrich) and hyaluronidase (Worthington) for 1 h at 37 °C. Then the cell suspension was filtered through a 70 μm cell strainer to form single cell suspension. Then red blood cells were removed by 5 min of incubation with red blood cell lysis buffer on ice. The mice spleens were smashed thoroughly between frosted glass slides and filtered through a 70 μm cell strainer to prepare single cell suspension. Then red blood cells were removed by 5 min of incubation with red blood cell lysis buffer on ice.
After that, cells were stained with following antibodies: anti-CD4-PE (#553652, BD Pharmingen), anti-CD8-FITC (#561966, BD Pharmingen), anti-F4/80-PE (#565410, BD Pharmingen) and were processed for flow cytometry analysis by Cytomic FC 500MCL flow cytometer (Beckman Coulter).
2.11. The extraction of total RNA and RT-PCR
Total RNA was purified using a Trizol Plus kit (Invitrogen). First-strand cDNA synthesis was performed on 5 μg of total RNA using the QuantiTect Reverse Transcription kit (Qiagen). Gene expression levels were determined by PCR analysis. The following primers are used: Il4-Forward: CCATATCCACGGATGCGACA; Il4-Reverse: AAGCCCGAAAGAGTCTCTGC; Il5-Forward: CGTGGGGGTACTGTGGAAAT; Il5-Reverse: CTCAGCCTCAGCCTTCCATT; Il13-Forward: CACACAAGACCAGACTCCCC; Il13-Reverse: CTCATTAGAAGGGGCCGTGG; Il17a-Forward: ACTACCTCAACCGTTCCACG; Il17a-Reverse: GGACCAGGATCTCTTGCTGG; Il21-Forward: GGAGACTCAGTTCTGGTGGC; Il21-Reverse: TCTGTGGGAACGAGAGCCTA; Il22-Forward: TGCGATCTCTGATGGCTGTC; Il22-Reverse: CCTCGGAACAGTTTCTCCCC; Ifng-Forward: GCTACACACTGCATCTTGGC; Ifng-Reverse: GCATCCTTTTTCGCCTTGCT; Gapdh-Forward: CCCTTAAGAGGGATGCTGCC; Gapdh-Reverse: ACTGTGCCGTTGAATTTGCC.
2.12. Purification of T cells from tumors
Tumors were injected with PBS, VNP20009 and two flagellum-deficient strains, and were resected after 6 days. The tumor mass was dissected and incubated in PBS containing collagenase I (Gibco), collagenase IV (Sigma–Aldrich), DNase I (Sigma–Aldrich) and hyaluronidase (Worthington) for 1 h at 37 °C. Then the cell suspension was filtered through a 70 μm cell strainer (Corning). Tumor-infiltrating T cells were enriched using CD3 Dynabeads (Thermo Fisher Scientific) according to manufacturer's instructions. In brief, 1 × 107 total tumor cells were suspended in 1 mL isolation buffer, and incubated with 25 μL CD3 Dynabeads for 20 min at 4 °C. The tube was placed in a magnet for 2 min. Then the supernatant containing unwanted tumor cells were discarded. The magnetic beads were washed 3 times with cold PBS. The enriched CD3+ T cells were lysed for RNA extraction by adding cell extraction buffer of Trizol Plus kit (Invitrogen) to the magnetic beads.
2.13. Western blots and immunohistochemistry
Western blots were performed as depicted previously28 and the following antibodies were used: NF-κB P65 (sc-8008, Santa Cruz Biotechnology), phospho-P65 (#3039, Cell Signaling Technology), P38 (sc-398305, Santa Cruz Biotechnology), phospho-P38 (sc-166182, Santa Cruz Biotechnology), AKT serine/threonine kinases (AKT, sc-81434, Santa Cruz Biotechnology), extracellular regulated protein kinase 1 (ERK1, sc-271269, Santa Cruz Biotechnology), phospho-ERK1/2 (sc-81492, Santa Cruz Biotechnology), c-Jun N-terminal kinase (JNK, sc-7345, Santa Cruz Biotechnology), phospho-JNK (sc-6254, Santa Cruz Biotechnology), Flag (#F1804, Sigma–Aldrich), inhibitor of NF-κB (IκB, #9242, Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, #2118, Cell Signaling Technology), and Flagellin (ab93713, abcam).
For immunohistochemistry, the resected tumor tissues were fixed with 4% formaldehyde, embedded in paraffin and sectioned. Immunohistochemistry staining was performed with CD45 antibody (ab10558, Abcam) according to standard histological procedures.
2.14. The construction of expression plasmids
Full length mouse Tlr5 gene was cloned (primer TLR5-1: GATGGCATGTCAACTTGACTTGC; TLR5-2: TATGCGGCCGCCTAGGAAATGGTTGCTATGG) from B16F10 melanoma cell and ligated into modified pRK5-Flag vector (Addgene). The mouse dominant-negative (DN) plasmids were generated with pRK5-Flag vector by amplifying the sequence as following: myeloid differentiation factor 88 DN (Myd88-DN) (Δ1-435, with primers MyD-1: ATTGGATCCGGCATCACCACCCTTGATGACC, MyD-2: TATCTCGAGTCAGGGCAGGGACAAAGCCTTGG), TNF receptor associated factor 6 DN (Traf6-DN) (Δ1-864, with primers TRAF-1: ATTGGATCCGCCGCCTCTCCATCCCGGGGAT, TRAF-2: CTCCTCGAGTCACTGGAAACCCTCCTTCCGAA), Tlr5 leucine-rich repeat domain DN (LRR-DN) (Δ907-1707, with primers LRR-1: GATGGCATGTCAACTTGACTTGC; LRR-2: AAAACACGAAGCGAAGAGAAGATAAAGCCGTGCG; LRR-3: CGCACGGCTTTATCTTCTCTTCGCTTCGTGTTTT; LRR-4: GGCAAGCTTTCAGGAAATGGTTGCTATGGTT) and Tlr5 Toll/Interleukin-1 receptor domain DN (TIR-DN) (Δ2041-2575, with primers TIR-1: GATGGCATGTCAACTTGACTTGC; TIR-2: TTAGCGGCCGCTCAGTCCTTGAACACCAGCTTC).
2.15. NF-κB luciferase reporter assay
Jurkat cells were plated in 24-well plates with the density of 5 × 104 cells/well, and transfected with polyethylenimine (PEI, Yeasen) and expression plasmids at the ratio of 5 μL PEI: 1 μg plasmid. 0.5 μg mouse NF-κB-P65 firefly luciferase reporter plasmid was transfected with 0.1 μg Renilla luciferase reporter vector as an internal control. After 24 h, cells were treated with 10 ng/mL recombinant Flagellin (Sigma–Aldrich) for 10 min to 4 h, and harvested for luciferase reporter assays with the Dual-Luciferase system (Promega) following the manufacturer's instructions. The NF-κB-P65 promoter activity was quantified by calculating the ratio of Firefly luciferase signal and Renilla luciferase signal, and then the basal promoter activity level of the control group without the Flagellin treatment was adjusted to 1. The data were presented as the mean ± standard deviation (SD) of triplicate experiments.
2.16. The intratumoral injection of lentivirus overexpressing TLR5 and its dominant-negative isoforms
Tlr5, Tlr5-LRR-DN and Tlr5-TIR-DN were subcloned from pRK5-Flag vector to pLenti-GIII-CMV-GFP-2A-Puro vector (Applied Biological Materials) using the following primers: Flag-5′: AGACGAGCTAGCATGGACTACAAAGAC. TLR5-3′: AGCCAGTCTAGACTAGGAAATGGTTGCTATGG. LRR-3′: AGCCAGTCTAGATCAGGAAATGGTTGCTATGGTT. TIR-3′: AGCCAGTCTAGATCAGTCCTTGAACACCAGCTTC.
1 × 107 HEK293T cells were seeded in a 10 cm dish one day ahead and transfected with 4 μg pLenti-GIII-CMV-GFP-2A-Puro expression vector and 4 μg Third Generation Packaging Mix (Applied Biological Materials) by mixing with PEI at the ratio of 1 μg plasmid:3 μL PEI to produce lentivirus. The medium containing virus were collected at 48 and 72 h after the transfection, and passed through 0.45 μm membranes to remove cell debris. Virus was concentrated by PEG8000-precipitation method and titrated as previously described29.
C57BL/6 mice were inoculated with melanoma allografts as described above. When tumors could be detected, the mice were randomly grouped as 9 mice/group. On Days 7, 9 and 11 post inoculation, 2 × 106 U of lentivirus overexpressing TLR5 or DN proteins were injected into tumors with 20 μL of DMEM. On Day 10, a low dose (1000 CFU/mouse) of VNP20009 was injected into tumors. On Day 16, mice in pTIR-DN and pTIR-DN + VNP20009 groups were randomly divided into two subgroups and injected with lentivirus expressing TLR5 or the empty vehicle at the same dosage as the first treatment. Then the antitumor effects were evaluated.
2.17. Bio-Plex Multiplex Suspension Array
Tumor tissue was weighed and homogenized for 1 h on ice in 50 mmol/L HEPES (pH 7.4), 100 mmol/L NaCl, 50 mmol/L NaF, 2 mmol/L EDTA, 1% Triton-100 and 100 μg/mL phenylmethanesulfonylfluoride. Cytokines were measured using the Bio-Plex Suspension Array System (Bio-Rad) according to the manufacturer's protocols as described previously25.
2.18. Statistical analysis
Unpaired Student's t test analysis was carried out on data using the SPSS software to assess statistical significance. Differences between experimental groups were considered significant when P < 0.05. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001.
3. Results
3.1. VNP20009 showed weakened anti-tumor activity in immunodeficient mice
To test whether the host immune response has a crucial role in VNP20009-mediated cancer therapy, subcutaneous melanoma allografts were established on both immunocompetent and immunodeficient mice using murine B16F10 cells. A single intraperitoneal administration of VNP20009 could achieve a 93.3% reduction in tumor volume in the immunocompetent mice, while the reduction rate fell to 48.9% in the immunodeficient mice (Fig. 1A–D). A similar trend was also observed following intratumoral treatment with VNP20009 (Supporting Information Fig. S1A–S1D). Additionally, strong immunohistochemical staining for CD45 in tumor tissues resected from VNP20009-inoculated immunocompetent mice indicated a high infiltration of inflammatory cells after Salmonella infection (Fig. 1E). In addition, the level of IL-2, mainly secreted by activated CD4+ T cells, was significantly increased in tumors resected from immunocompetent mice (Fig. S1E). These results suggest that the host immune response is an important factor influencing the anti-tumor activity of VNP20009.
Figure 1.
VNP20009 exhibited stronger anti-tumor activity in immunocompetent mice, compared with immunodeficient mice. Immunocompetent (A) and immunodeficient (B) mice carrying melanoma allografts were intraperitoneally administrated with phosphate-buffered saline (PBS) or VNP20009 (labelled as VNP in the figure). The tumor volumes were recorded and plotted as means ± SD, n = 8 for immunocompetent mice, n = 10 for immunodeficient mice. (C) The treatment schedule for mice bearing B16F10 melanoma allografts. (D) The tumor volumes at the end of experiments (Day 16). Data are mean ± SD, n = 8 for immunocompetent mice, n = 10 for immunodeficient mice. (E) The representative immunohistochemical staining of CD45+ cells of tumors resected from immunocompetent mice bearing melanoma allografts on Day 6 after VNP20009 treatment. Red arrow indicated CD45+ cells. Images in large green squares were the enlargement of images within small green squares. ∗∗P < 0.01; ∗∗∗P < 0.001; n.s. stands for nonsignificant. i.p. stands for intraperitoneally.
3.2. Flagella deletion of VNP20009 reduced its anti-tumor activity in immunocompetent mice, but not in immunodeficient mice
VNP20009 carries a deletion of the msbB gene to reduce systemic toxicity associated with LPS23. However, the structure of flagellum remains intact and is still able to activate monocytes, macrophages, dendritic cells and CD4+ T cells. Moreover, flagella are also the locomotory organelles of Salmonella. To test the importance of flagella in VNP20009-mediated cancer therapy, the flagella deletion strains ΔflhD (one of the master regulatory genes in flagella organization) and ΔfliE (a basal body structure protein) were constructed by double-crossover gene replacement (Fig. 2A and B, and Supporting Information Fig. S2A)30. Neither ΔflhD or ΔfliE strains expressed Flagellin, nor could they form flagella (Fig. 2C and Fig. S2B). In immunocompetent mice, the flagellum-deficient strains failed to reduce the volume of tumors (Fig. 2D) or extend the survival of mice (Fig. 2E), indicating that the anti-tumor activity was mostly lost in the flagellum-deficient strains. ΔfliE had a slightly better anti-tumor effect than ΔflhD. However, this remarkable difference in the anti-tumor activity between the wild-type VNP20009 and the flagellum-deficient strains was substantially diminished in immunodeficient mice (Fig. 2F and G).
Figure 2.
The disruption of flagella of VNP20009 significantly diminished its anti-tumor activity, especially in immunocompetent mice. (A) The critical genes regulating the production and assembling of flagella in Salmonella. Among these genes, Early genes are the master regulators which directly activate the expression of Middle genes and indirectly regulate Late genes. The temporal sequence of gene expression was shown by black arrows. The genes of interests were highlighted by red arrows. (B) The strategy for deleting flhD and fliE by double-crossover gene replacement. H1 and H2 represented homologous sequences. (C) The electron microscopic images showing the disruption of flagella in ΔflhD and ΔfliE strains. Immunocompetent (D) and immunodeficient (F) mice carrying melanoma allografts were intraperitoneally administered with phosphate-buffered saline (PBS) or an equal dose of wild-type VNP20009 (labelled as VNP in the figure), ΔflhD and ΔfliE strains. The tumor volumes were recorded and plotted as mean ± SD, n = 8 for immunocompetent mice, n = 10 for immunodeficient mice. The Kaplan–Meier survival curves of immunocompetent (E) and immunodeficient (G) mice bearing melanoma allografts after intraperitoneal treatment of PBS or an equal dose of wild-type VNP20009, ΔflhD and ΔfliE strains. n = 8 for immunocompetent mice, n = 10 for immunodeficient mice. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; n.s. stands for nonsignificant. i.p. stands for intraperitoneally.
These results indicate that flagella play a crucial role in VNP20009-mediated cancer therapy, partially in a host immune response dependent manner.
3.3. Weakening of infectivity partially explained the loss of anti-tumor activity of the flagellum-deficient strains
For the host immune system, flagellum are pattern recognition ligands, while they are also important locomotive organelles for microorganisms. The destruction of flagella would affect the mobility and infectivity of VNP20009, having minimal effects on the bacterial growth (Fig. S2C). ΔflhD exhibited weakened adhesion to cell monolayers (Fig. 3A), and both ΔflhD and ΔfliE showed significantly impaired invasive tendency in vitro, with ΔflhD exhibiting almost complete loss of the invasive ability (Fig. 3B, Supporting Information Fig. S3A and S3B).
Figure 3.
The flagellum-deficient strains exhibited reduced infectivity, which partially contributed to the loss of anti-tumor activity. The results of adhesion (A) and invasion (B) assays to compare the infectivity of wild-type VNP20009 (labelled as VNP in the figure), ΔflhD and ΔfliE strains on B16F10 monolayers in vitro. Data are mean ± SD, n = 3. (C) The representative fluorescent image of melanoma infected with eGFP-VNP20009. N, necrotic area. The bacterial titres in the peripheral blood (D) and tumors (E) after intraperitoneal treatment of wild-type VNP20009, ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. (F) The intracellular bacteria number was evaluated 30 min after the intratumoral treatment of an equal dose of wild-type VNP20009, ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. (G) The intracellular bacteria number was evaluated 30 min after the intratumoral treatment of various doses of wild-type VNP20009, ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. (H) The anti-tumor activity of wild-type VNP20009 and ΔfliE was evaluated by monitering the tumor volume in the condition of an equal intracellular bacteria number by the intratumoral treatment of different doses of wild-type VNP20009 and ΔfliE. Data are mean ± SD, n = 8. (I) The infection-independent anti-tumor activity of wild-type VNP20009 and ΔfliE was evaluated by monitering the tumor volume after the intratumoral treatment of heat-inactivated bacteria. Data are mean ± SD, n = 8. ∗∗P < 0.01; ∗∗∗P < 0.001; n.s. stands for nonsignificant. PBS: phosphate-buffered saline; i.p. stands for intraperitoneally; i.t. stands for intratumorally.
Fluorescent microscopy of tumor tissues infected with GFP-labelled VNP20009 showed that bacteria invaded tumors cells during Salmonella-mediated cancer therapy (Fig. 3C), suggesting that adhesion and invasion are two important steps for successful intratumoral colonization. Our results also showed that the destruction of flagella had a fundamental influence on bacterial behaviour during cancer therapy in vivo. First, the flagellum-deficient strains were cleared from the peripheral blood much more rapidly than the wild-type strain. By 2 h after intraperitoneal administration, most flagellum-deficient VNP20009 had been eliminated, while wild-type VNP20009 still persisted in the peripheral circulation (Fig. 3D).
Moreover, bacterial colonization in the tumors was also significantly poorer for the flagellum-deficient strains, especially when they were administered i.p. (Fig. 3E). Direct intratumoral injection narrowed the gap in the intracellular bacteria number between the wild-type and ΔfliE strains (Fig. 3F and Fig. S3C), which could be further reduced to a nonsignificant difference if a higher dose of ΔfliE was used (Fig. 3G). By performing intratumoral injection of unequal doses of VNP20009 and ΔfliE, their anti-tumor activity could be compared on the premise of equal infectivity. However, with equal initial intracellular colonization, the flagellum-deficient strain ΔfliE still exhibited significantly weakened anti-tumor activity (Fig. 3H). Moreover, intratumoral administration of heat-inactivated wild-type bacteria could still achieve a moderate suppressive effect on tumor growth in immunocompetent mice, which was still significantly weakened if the flagellum-deficient strain ΔfliE was used (Fig. 3I). In this experiment, the dead wild-type bacteria had completely lost infectivity, but their anti-tumor activity partially remained.
These results suggest that the reduced infectivity does not fully explain why the destruction of flagella has such an adverse effect on the anti-tumor activity of VNP20009. Other mechanisms must contribute to this process parallelly. In addition to functioning as a locomotory organelle, the flagellum is also an important PAMP that strongly activates the host immune response31. Considering the emerging evidence showing how immune response can influence the development of cancer, we speculated that the flagellum-induced host immune response probably also plays an important role in the anti-tumor activity of Salmonella.
3.4. The activation of the host immune response was significantly weakened when flagellum-deficient bacteria were used
To test whether the flagellum-mediated host immune response has a role during VNP20009-mediated tumor therapy, both the systemic immune response and the intratumoral immune response were analysed after intraperitoneal administration of wild-type or flagellum-deficient strains in mice bearing melanoma allografts. Drastic enlargement of the spleen, which mainly contained highly activated T cells, was found only in mice that received wild-type VNP20009 (Fig. 4A–C and Fig. S4), suggesting that the destruction of flagella weakened the activation of the systemic immune response against Salmonella infection.
Figure 4.
The activation of the host immune response was significantly weakened for the flagellum-deficient bacteria. (A) The weight of spleens of immunocompetent mice bearing melanoma allografts 6 days after the intraperitoneal treatment of wild-type VNP20009 (labelled as VNP in the figure), ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. Representative images of resected spleens were shown below the columns. The percentage of CD69+ cells in total CD4+ T cells (B) and total CD8+ T cells (C) were measured by flow-cytometry 6 days after the intraperitoneal treatment of wild-type VNP20009, ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. The percentage of CD4+ T cells (D), CD8+ T cells (E) and F4/80+ macrophages (F) infiltrating in tumors were measured by flow-cytometry 6 days after the intraperitoneally treatment of wild-type VNP20009, ΔflhD and ΔfliE strains. Data are mean ± SD, n = 3. (G) The intratumoral concentrations of various inflammatory cytokines (Ca) were measured by Bio-Plex Multiplex Suspension Array 6 days after the intraperitoneal treatment of wild-type VNP20009, ΔflhD and ΔfliE strains. The fold change of the cytokine concentration (CCa) relative to the mean of all mice (Cmean) was calculated by the formula: CCa=(CCa–Cmean)/Cmean × 100%. The values were converted into colors and plotted. Green indicates reductions, and red indicates increase. (H) The RNA levels of Il4, Il5, Il13, Il17α, Il21, Il22 and Ifng in the tumor-infiltrating T cells were compared by RT-PCR 6 days after the intraperitoneal treatment of wild-type VNP20009, ΔflhD and ΔfliE strains. (I) The activation status of ERK/JNK/MAPK and NF-κB signaling pathways was evaluated by Western blots. ∗∗∗P < 0.001; n.s. stands for nonsignificant. PBS: phosphate-buffered saline; i.p. stands for intraperitoneally.
Similarly, significant increases in the levels of tumor-infiltrating CD4+ T cells (Fig. 4D), CD8+ T cells (Fig. 4E) and macrophages (Fig. 4F) were also observed only in mice that received wild-type bacteria. Consistently, a comprehensive analysis of the protein levels of cytokines showed that the flagellum-deficient strains induced much weaker inflammatory cytokine production in the tumor, than did the wild-type strains (Fig. 4G). T cells isolated from tumors treated with wild-type VNP20009 exhibited significantly higher RNA expression levels of key inflammatory cytokines including IL-4, IL-5, IL-13, IL-17, IL-21, IL-22 and IFN-γ, than did T cells extracted from tumors receiving flagellum-deficient strains (Fig. 4H). Indeed, Western blot analysis of the lysates of these tumor-infiltrating T cells showed higher phosphorylation levels for P65, P38, JNK and ERK, and a lower protein level of IκB in the T cells extracted from tumors receiving wild-type VNP20009, indicating that two key downstream pathways of TLR signalling, the mitogen-activated protein kinase (MAPK) pathway and NF-κB pathway32, were strongly activated by only wild-type VNP20009 (Fig. 4I).
All these results suggest that the host immune response is specifically activated by the flagella of VNP20009, inducing the production of inflammatory factors, which may have a critical role in VNP20009-mediated cancer therapy.
3.5. Activation of the Flagellin/TLR5/NF-κB pathway in the TME is crucial for the anti-tumor activity of VNP20009
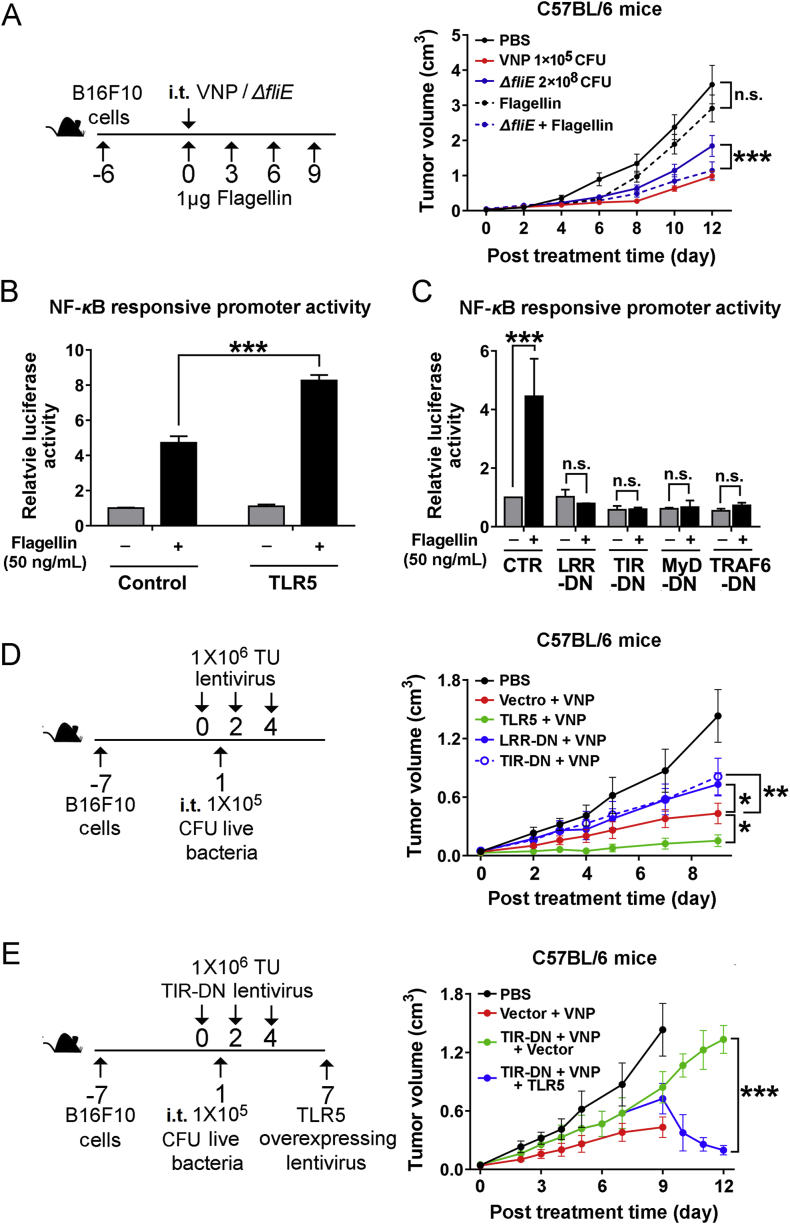
Flagellin on the tip of flagella specifically binds and activates the receptor TLR510, which is widely expressed on monocytes, macrophages, dendritic cells and CD4+ T cells. The binding between Flagellin and TLR5 initiates the activation of TLR5/NF-κB pathway and promotes the expression of various inflammatory cytokines. Theoretically, the combination of isolated Flagellin and flagellum-deficient VNP20009 should restore the anti-tumor activity of flagellum-deficient strains if the host immune response truly has a crucial role in VNP20009-mediated cancer therapy. To test this hypothesis, wild-type VNP20009, the ΔfliE mutant or a combination of Flagellin and the ΔfliE mutant were administered i.t. to mice bearing melanoma. Flagellin alone exhibited a weak suppressive effect on tumor growth; while the combination of Flagellin and the ΔfliE mutant achieved remarkable anti-tumor activity that was comparable to the therapeutic effect of VNP20009, suggesting that the lack of Flagellin/TLR5/NF-κB signalling could be an important factor causing the loss of anti-tumor activity foreseen with flagellum-deficient strains.
In Jurkat cells (an immortalized line of human T lymphocytes), overexpression of TLR5 enhanced the activation of the NF-κB pathway upon Flagellin stimulation (Fig. 5B, Supporting Information Fig. S5A and S5B), which could be blocked by dominant-negative forms of TLR5, which consisted of either the extracellular domain with LRR motif or the intracellular TIR domain33, as well as DN forms of MyD88 and TRAF634 (Fig. 5C and Fig. S5C). Overexpression of TLR5 or its DN forms showed little effect on the growth of B16F10 cells in vitro (Fig. S5D). However, intratumoral administration of TLR5 by a lentivirus could significantly enhance the anti-tumor activity of VNP20009, while the dominant-negative forms of TLR5 weakened the therapeutic effects of VNP20009 (Fig. 5D); this weakening could be rescued by reintroducing full-length TLR5 (Fig. 5E), indicating that the status of the Flagellin/TLR5/NF-κB pathway in the TME, most likely in inflammatory cells, is critical for Salmonella-mediated cancer therapy.
Figure 5.
The activation of the Flagellin/TLR5/NF-κB pathway in TME was crucial for the anti-tumor activity of VNP20009. (A) The immunocompetent mice were inoculated with melanoma allografts and intratumoral treated with wild-type VNP20009 (labelled as VNP in the figure) or a combination of wild-type VNP20009 and ΔfliE. The doses of bacteria were adjusted to achieve a similar intracellular bacteria number for wild-type VNP20009 and ΔfliE in the tumor. The treatment schedule was illustrated in the schematic diagram. The tumor volume was monitored and means ± SD were plotted, n = 8. The activity of NF-κB responsive promoter was evaluated in Jurkat cells after the treatment of Flagellin for 1 h. Jurkat cells were also transfected with either full-length TLR5 (B) or the dominant-negative (DN) truncates of TLR5 (LLR-DN, TIR-DN) and downstream transducers of TLR5-NF-κB signaling pathway (MyD-DN, TRAF6-DN) (C). Data are mean ± SD, n = 3. (D) The immunocompetent mice were inoculated with melanoma allografts and intratumorally treated with the combination of wild-type VNP20009 and lentivirus overexpressing full-length TLR5 or its dominant-negative truncates (LLR-DN, TIR-DN). The treatment schedule was illustrated in the schematic diagram. The tumor volume was monitored and the means ± SD were plotted, n = 8. (E) The immunocompetent mice were inoculated with melanoma allografts and intratumorally treated with the combination of wild-type VNP20009 and lentivirus overexpressing dominant-negative truncates of TLR5 (TIR-DN). Seven days post the TIR-DN lentivirus treatment, lentivirus overexpressing full-length TLR5 were administered to rescue the effects of TIR-DN. The treatment schedule was illustrated in the schematic diagram. The tumor volume was monitored and the means ± SD were plotted, n = 8. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; n.s. stands for nonsignificant. PBS: phosphate-buffered saline; i.p. stands for intraperitoneally; i.t. stands for intratumorally.
Taken together, our study shows that flagella contribute to the anti-tumor activity of Salmonella both as locomotory organelles and as PAMPs activating the host immune response in the TME (Fig. 6). Decreasing the infectivity and immunogenicity of Salmonella by destroying flagella impairs its anti-tumor activity, while enhancing the host immune response by activating the Flagellin/TLR5/NF-κB pathway can improve the efficacy of Salmonella-mediated cancer therapy.
Figure 6.
The schematic diagram illustrates the roles of flagella in Salmonella-mediated cancer therapy. Flagella are actively involved in Salmonella-mediated cancer therapy both as major locomotory organelles which increase the infectivity, as well as PAMPs activating the host immune response both systemically and intratumorally. The Flagellin/TLR5/NF-κB signalling pathway plays an important role during this process.
4. Discussion
Our study showed that the flagellum of Salmonella is critical for the activation of the host immune response, turning the immunosuppressive TME into an immune-active TME during Salmonella-mediated cancer therapy. Recently, Min et al.35 reported that engineered Salmonella typhimurium inducibly secreting Vibrio vulniificus Flagellin B could promote M1-like macrophage polarization in the TME to strongly suppress the growth of tumors in a host TLR signalling pathway-dependent manner. Our observation reconfirmed the importance of flagella in Salmonella-mediated cancer therapy in an independent experimental setting.
There are still debates about whether bacteria must enter tumor cells before killing them. Moreover, it is not clear whether VPN20009 enters tumor cells via active infection or passive endocytosis. Even though we measured the intracellular bacteria number with a gentamicin protection assay in this study, it is worth noting that this method might lead to an incorrect conclusion, since bacteria temporarily surviving within phagosomes would contaminate the final count of live bacteria colonizing tumor cells. This study mainly focused on the immunogenic role of Flagellin during Salmonella-mediated cancer therapy, and these effects could be achieved in the absence of intracellular bacteria. More experiments precisely analysing the processes and consequences of bacteria entering tumor cells should be conducted to establish a solid causal relationship between bacteria entering tumor cells and the death of tumor cells.
In addition to the activation of the TLR signalling pathway by LPS and Flagellin, cancer-specific antigens also contribute to Salmonella-mediated cancer therapy. The drastic tumor cell lysis caused by Salmonella infection provides a substantial pool of antigens for antigen-presenting cells, potentiating the generation of tumor-specific cytotoxic T cells and memory T cells and leading to long-term protection against metastasis and recurrence36, similar to the effects of cancer vaccination or adoptive T cell therapy. However, the response rate of peptide-based cancer vaccination is generally unsatisfactory probably due to the heterogeneity of cancer37, while personalized adoptive T cell therapy sometimes leads to a lethal autoimmune response due to the similarities between cancer cells and healthy cells. For example, transferring T cells targeting ERB-B2 receptor tyrosine kinase 2 (ERBB2), a cell-surface protein overexpressed by various kinds of cancer, can cause serious adverse effects due to T cells mistakenly attacking the lung epithelium, which exhibits a low level of ERBB2 expression38. The limitation of stringently cancer-specific antigens and the possibility for engineered T cells to cross-react to an endogenous antigen with a similar structure hamper the safety of such therapies for general applications39. In contrast, Salmonella-mediated cancer therapies utilize autologous T cells that have undergone thymic selection, preventing severe systemic autoimmune reactions.
Cancer immunotherapies based on immune checkpoint inhibitors are also developing rapidly. Monoclonal antibodies against PD-1/PD-L1 (pembrolizumab and nivolumab) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (ipilimumab) have been approved for the treatment of Hodgkin lymphoma40 and melanoma41,42, but their effects are hampered in tumors with a low level of T cell infiltration43. Therefore, Salmonella-mediated cancer therapy holds promise for improving the efficacy of immune checkpoint inhibitors in such circumstances by actively attracting more lymphocytes into the hypoxic centre of tumors, which Salmonella preferentially colonizes. Combinations of Salmonella with PD-1 antibody, CTLA-4 antibody and adoptive T cell therapy have been evaluated in tumor-bearing mice, which achieved encouraging improvement in tumor burden reduction and relapse prevention44,45.
To date, many compounds of different chemical constitutions have been designed for the specific activation of TLRs, such as, polyAU for TLR346, and synthetic small-molecule compounds targeting TLR7/847. However, since the expression of TLRs is not strictly restricted to immune cells48, more investigations must be conducted to evaluate the biological effects of these compounds on antigen presenting cells, lymphocytes and tumor cells to obtain practical guidance for the safe application of TLR agonists in cancer immunotherapy.
5. Conclusions
Our findings regarding the importance of the Flagellin/TLR5/NF-κB pathway in Salmonella-mediated cancer therapy suggest that agonists of TLRs can also be used in cancer immunotherapies.
Acknowledgments
This study was supported by grants from the Jiangsu Provincial Nature Science Foundation (BK20192005, China), National Natural Science Foundation of China (81630092, 81903143, 81802338, and 82072646), Zhejiang Provincial Natural Science Foundation of China for Distinguished Young Scholars (LR21H160001) and Start-up Grant of HZNU (4125C5021820470, China). We thank Dr. Wenhui Jiang for her assistance for the immunohistochemical staining during the experiments and thank Dr. Dongping Wei for his encouragement on the setup of this project. We are grateful to the mice for their contributions to this study.
Author contributions
Zichun Hua and Jianxiang Chen designed experiment. Jianxiang Chen and Yiting Qiao performed most assays and wrote the manuscript. Guo Chen, Cunjie Chang and Heng Dong contributed to the genetic modifications of bacteria and in vitro experiments. Bo Tang, Xiawei Cheng and Xiufeng Liu helped with animal experiments.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.04.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Forbes N.S. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawelek J.M., Low K.B., Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 3.Kohwi Y., Imai K., Tamura Z., Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gan. 1978;69:613–618. [PubMed] [Google Scholar]
- 4.Yu Y.A., Shabahang S., Timiryasova T.M., Zhang Q., Beltz R., Gentschev I. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 5.Parker R.C., Plummer H.C. Effect of histolyticus infection and toxin on transplantable mouse tumors. Proc Soc Exp Biol Med. 1947;66:461–467. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 6.Malmgren R.A., Flanigan C.C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 7.Mroczenski-Wildey M.J., Di Fabio J.L., Cabello F.C. Invasion and lysis of HeLa cell monolayers by Salmonella typhi: the role of lipopolysaccharide. Microb Pathog. 1989;6:143–152. doi: 10.1016/0882-4010(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 8.Stern C., Kasnitz N., Kocijancic D., Trittel S., Riese P., Guzman C.A. Induction of CD4+ and CD8+ anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer. 2015;137:2019–2028. doi: 10.1002/ijc.29567. [DOI] [PubMed] [Google Scholar]
- 9.Mittrucker H.W., Kaufmann S.H. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi F., Smith K.D., Ozinsky A., Hawn T.R., Yi E.C., Goodlett D.R. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 11.Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 12.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 14.Smyth M.J., Hayakawa Y., Takeda K., Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 15.Rubio V., Stuge T.B., Singh N., Betts M.R., Weber J.S., Roederer M. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 16.Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci U S A. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon T., Coulie P.G., Van den Eynde B.J., van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski T.F., Meng Y., Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Liu X., Zhang N., Yin M., Dong J., Zeng Q. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Kang W., Li O., Qi F., Wang J., You Y. Abrogation of USP7 is an alternative strategy to downregulate PD-L1 and sensitize gastric cancer cells to T cells killing. Acta Pharm Sin B. 2021;11:694–707. doi: 10.1016/j.apsb.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low K.B., Ittensohn M., Luo X., Zheng L.M., King I., Pawelek J.M. Construction of VNP20009: a novel, genetically stable antibiotic-sensitive strain of tumor-targeting Salmonella for parenteral administration in humans. Methods Mol Med. 2004;90:47–60. [PubMed] [Google Scholar]
- 24.Toso J.F., Gill V.J., Hwu P., Marincola F.M., Restifo N.P., Schwartzentruber D.J. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., Qiao Y., Tang B., Chen G., Liu X., Yang B. Modulation of Salmonella tumor-colonization and intratumoral anti-angiogenesis by triptolide and its mechanism. Theranostics. 2017;7:2250–2260. doi: 10.7150/thno.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y., Evans C.R., Ling J. Reduced protein synthesis fidelity inhibits flagellar biosynthesis and motility. Sci Rep. 2016;6:30960. doi: 10.1038/srep30960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkelstroter L.K., De Martinis E.C. In vitro protective effect of lactic acid bacteria on Listeria monocytogenes adhesion and invasion of Caco-2 cells. Benef Microbes. 2015;6:535–542. doi: 10.3920/BM2013.0091. [DOI] [PubMed] [Google Scholar]
- 28.Qiao Y., Chen J., Lim Y.B., Finch-Edmondson M.L., Seshachalam V.P., Qin L. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 2017;19:1495–1502. doi: 10.1016/j.celrep.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 29.Chang L.J., Zaiss A.K. Self-inactivating lentiviral vectors and a sensitive Cre-loxP reporter system. Methods Mol Med. 2003;76:367–382. doi: 10.1385/1-59259-304-6:367. [DOI] [PubMed] [Google Scholar]
- 30.Chevance F.F., Hughes K.T. Coupling of flagellar gene expression with assembly in Salmonella enterica. Methods Mol Biol. 2017;1593:47–71. doi: 10.1007/978-1-4939-6927-2_4. [DOI] [PubMed] [Google Scholar]
- 31.Feuillet V., Medjane S., Mondor I., Demaria O., Pagni P.P., Galan J.E. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda K., Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Ivison S.M., Khan M.A., Graham N.R., Bernales C.Q., Kaleem A., Tirling C.O. A phosphorylation site in the Toll-like receptor 5 TIR domain is required for inflammatory signalling in response to flagellin. Biochem Biophys Res Commun. 2007;352:936–941. doi: 10.1016/j.bbrc.2006.11.132. [DOI] [PubMed] [Google Scholar]
- 34.Burns K., Martinon F., Esslinger C., Pahl H., Schneider P., Bodmer J.L. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J.H., Nguyen V.H., Jiang S.N., Park S.H., Tan W., Hong S.H. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 36.Avogadri F., Martinoli C., Petrovska L., Chiodoni C., Transidico P., Bronte V. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caspi R.R. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol. 2008;8:970–976. doi: 10.1038/nri2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasamon Y.L., de Claro R.A., Wang Y., Shen Y.L., Farrell A.T., Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncol. 2017;22:585–591. doi: 10.1634/theoncologist.2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barone A., Hazarika M., Theoret M.R., Mishra-Kalyani P., Chen H., He K. FDA approval summary: pembrolizumab for the treatment of patients with unresectable or metastatic melanoma. Clin Cancer Res. 2017;23:5661–5665. doi: 10.1158/1078-0432.CCR-16-0664. [DOI] [PubMed] [Google Scholar]
- 42.Camacho L.H. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4:661–672. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang H., Wang Y., Chlewicki L.K., Zhang Y., Guo J., Liang W. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–296. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebelt N.D., Zuniga E., Marzagalli M., Zamloot V., Blazar B.R., Salgia R. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase restructures the immune contexture to improve checkpoint blockade efficacy. Biomedicines. 2020;8:617. doi: 10.3390/biomedicines8120617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binder D.C., Arina A., Wen F., Tu T., Zhao M., Hoffman R.M. Tumor relapse prevented by combining adoptive T cell therapy with Salmonella typhimurium. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y.S., Xu F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol Ther. 2010;10:1219–1223. doi: 10.4161/cbt.10.12.13450. [DOI] [PubMed] [Google Scholar]
- 47.Weeratna R.D., Makinen S.R., McCluskie M.J., Davis H.L. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005;23:5263–5270. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 48.So E.Y., Ouchi T. The application of Toll like receptors for cancer therapy. Int J Biol Sci. 2010;6:675–681. doi: 10.7150/ijbs.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.