ABSTRACT
Chronic otitis media with effusion (OME) has been associated with a shift in microbiome composition and microbial interaction in the upper respiratory tract (URT). While most studies have focused on potential pathogens, this study aimed to find bacteria that could be protective against OME through a case-control microbiome study and characterization of isolates from healthy subjects. The URT and ear microbiome profiles of 70 chronic OME patients and 53 controls were compared by 16S rRNA amplicon sequencing. Haemophilus influenzae was the most frequent classic middle ear pathobiont. However, other taxa, especially Alloiococcus otitis, were also frequently detected in the ear canal of OME patients. Streptococci of the salivarius group and Acinetobacter lwoffii were more abundant in the nasopharynx of healthy controls than in OME patients. In addition to the microbiome analysis, 142 taxa were isolated from healthy individuals, and 79 isolates of 13 different Streptococcus species were tested for their pathobiont-inhibiting potential. Of these, Streptococcus salivarius isolates showed a superior capacity to inhibit the growth of H. influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, A. otitis, and Corynebacterium otitidis. S. salivarius strains thus show potential as a probiotic for prevention or treatment of OME based on their overrepresentation in the healthy nasopharynx and their ability to inhibit the growth of respiratory pathobionts. (This study has been registered at ClinicalTrials.gov under registration no. NCT03109496.)
IMPORTANCE The majority of probiotics marketed today target gastrointestinal health. This study searched for bacteria native to the human upper respiratory tract, with a beneficial potential for respiratory and middle ear health. Comparison of the microbiomes of children with chronic otitis media with effusion (OME) and of healthy controls identified Streptococcus salivarius as a health-associated and prevalent inhabitant of the human nasopharynx. However, beneficial potential should be assessed at strain level. Here, we also isolated specific S. salivarius strains from the healthy individuals in our study. These isolates showed a beneficial safety profile and efficacy potential to inhibit OME pathogens in vitro. These properties will now have to be evaluated and confirmed in human clinical studies.
KEYWORDS: 16S rRNA, Streptococcus salivarius, ear canal, microbiome, middle ear, otitis media, otitis media with effusion, pediatric, probiotics, upper respiratory tract
INTRODUCTION
Otitis media encompasses a spectrum of disease conditions characterized by accumulation of effusion in the middle ear cavity. Otitis media with effusion (OME) is characterized by the presence of effusion behind an intact tympanic membrane in the absence of other signs or symptoms of acute inflammation (1). Chronic OME, lasting ≥3 months, is typically treated by placement of ventilation tubes into the tympanic membrane (2). Middle ear health is closely associated with upper respiratory tract (URT) health. Bacteria traditionally isolated from OME middle ear effusion are nontypeable (unencapsulated) Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis, which typically inhabit the URT (3). In addition to these classic otopathogens, multiple 16S rRNA gene sequencing studies of OME middle ear effusion also reported high levels of Alloiococcus otitis, Corynebacterium otitidis (formerly Turicella otitidis [4]), Pseudomonas spp., and Staphylococcus spp. (Staphylococcus aureus, Staphylococcus auricularis, and Staphylococcus epidermidis) (5–16). It is, however, still uncertain if these taxa, many of which are residents of the ear canal (17, 18) or the healthy URT (19), contribute to middle ear disease. Long-term perturbation of the microbiota has been associated with several chronic inflammatory diseases (20) and is also hypothesized to underlie chronic OME. Preventing such perturbation or restoring a perturbed microbiota through addition of probiotics, i.e., live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (21), could be a valuable method for OME prevention and could reduce the need for surgical intervention. Such a probiotic approach is widely used for the gastrointestinal tract but is underexplored for respiratory health or the prevention and treatment of otitis media (22). The first intervention studies testing local bacteriotherapy to prevent or cure respiratory tract infections used alpha-hemolytic streptococci (AHS) of the mitis and sanguinis groups (23, 24), as they are among the first colonizers of the human URT after birth (25). Early studies specifically targeting middle ear health also used AHS isolated from the URT of healthy individuals. A nasal spray containing 2 Streptococcus sanguinis strains, 1 Streptococcus mitis strain, and 1 Streptococcus oralis strain isolated from the opening of the Eustachian tube of healthy children reduced the rate of acute otitis media (AOM) and OME when participants were pretreated with antibiotics (26), but not when antibiotic treatment was omitted (27). One study, which compared the effect of a nasal spray containing either S. sanguinis 89a or Lacticaseibacillus rhamnosus LB21 on chronic OME, found a stronger effect for S. sanguinis, although both strains reduced effusion (28). A recent bacterial intervention study using Ligilactobacillus salivarius PS7 isolated from human milk also reported reductions in the risk of experiencing at least one episode of AOM by 49% and in the length of AOM episodes from 6 to 4 days in otitis-prone children. However, it is unclear whether this effect was achieved through local or systemic mechanisms, as the formulation was not described, and colonization of the URT was not measured (29). To date, some of the best documented commercially available probiotics targeting URT and oral health are Streptococcus salivarius strains K12, M18, and 24SMB and S. oralis 89a (30–41). S. salivarius K12 may reduce AOM by up to 71.5% and was also found to reduce adenoid and tonsil hypertrophy and cause a clearing of middle ear effusion (32–36), although one study observed no difference in AOM episodes compared to untreated controls (37). A nasal spray combining S. salivarius 24SMB and S. oralis 89a was found, by dedicated quantitative PCR (qPCR), to reduce the number and severity of AOM episodes in otitis-prone children, especially when S. salivarius 24SMB colonized the respiratory tract (38–40), and to reduce adenoid hypertrophy and middle ear effusion in children with chronic OME, with significant reduction in need for surgery from 90.9% of the children in the control group to 27.3% in the treatment group (n = 22 in each group) (41). While many of the studies described above show promising effects on AOM and OME, the selection of the tested strains was based on early cultivation results, without insights into the resident microbiome.
Here, we aimed to identify bacterial taxa with a potentially protective effect against OME starting from a microbiome-sequencing approach comparing the URT and ear microbiome of children with and without chronic OME. This comparison was used to guide the subsequent cultivation approach and phenotyping, focusing on the ability of URT isolates from healthy individuals to inhibit the growth of classic middle ear pathobionts, as well as on underexplored potential OME-associated pathobionts that were identified in the microbiome comparison.
RESULTS
Study population and sample characteristics.
URT samples were collected from 70 OME patients and two control groups with no sign of otitis media or respiratory infections, namely (i) 12 cochlear implant recipients and (ii) 41 children attending day care. Participant characteristics and locations sampled are summarized in Table 1 and shown in Fig. 1A. There was no significant difference in gender ratio (both 41% females) or age (Student’s t test, P = 0.12) between the OME group and the combined control group. Gender had no significant effect on microbiome composition, while age only influenced the anterior nares microbiome (permutational multivariate analysis of variance [PERMANOVA], P = 0.002 in the OME group and P = 0.043 in the cochlear implant group). In total, 66 OME patients received tympanostomy tubes in both ears, and 28 patients underwent adenoidectomy. Neither laterality (unilateral versus bilateral OME) nor adenoidectomy significantly influenced the microbiome composition of any of the sampled locations. Of 523 low-biomass URT samples sequenced, only 443 samples had at least twice the number of reads compared to the largest negative control after removing obvious contaminants (see Tables S1 and S2 in the supplemental material). Final library sizes ranged from 2,500 to 784,460 reads, with a mean (± standard deviation [SD]) of 36,332 ± 49,509 reads.
TABLE 1.
Participant characteristics and sampled locations by group
| Characteristic | Controls |
Chronic OME patients | ||
|---|---|---|---|---|
| Cochlear implant recipients | Children attending day care | All | ||
| No. of participants | 12 | 41 | 53 | 70 |
| Age in yrs (mean ± SD) | 9.01 ± 9.74 | 1.58 ± 8.38 | 4.56 ± 7.21 | 4.38 ± 2.42 |
| % Female | 42 | 41 | 41 | 41 |
| Sampled locationsa | AN, NP, ME | NP | AN, NP, ME | AN, NP, Ad, Ad, ME, ECa |
AN, anterior nares; NP, nasopharynx; Ad, adenoids; ME, middle ear; EC, ear canal.
FIG 1.
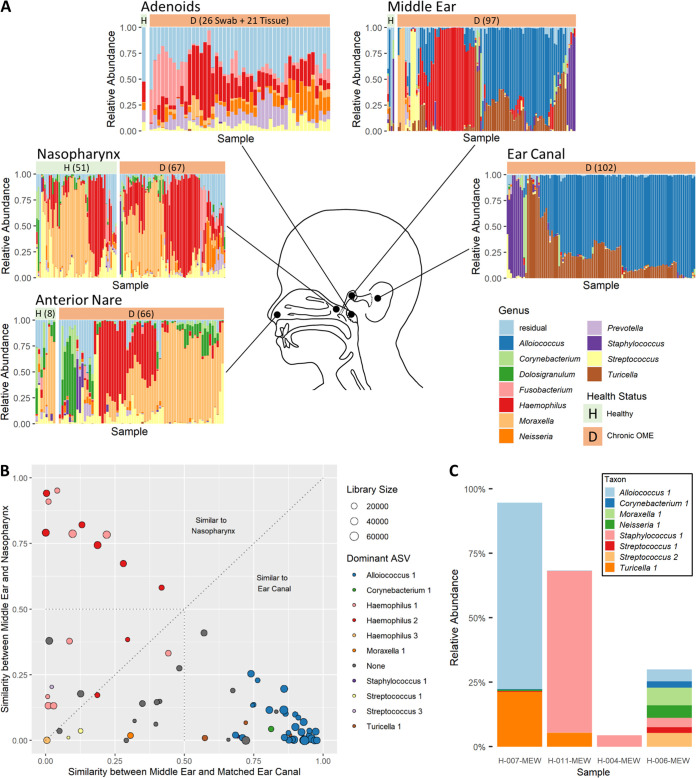
The bacterial microbiome of the human upper respiratory tract and ears. (A) Genus-level microbiome composition of multiple upper respiratory tract and ear niches in health (“H”) and during chronic otitis media with effusion (“D”). Each bar represents one sample, and the full height of a bar represents 100% of reads. The 11 most abundant genera across all samples are shown, with all other genera summarized under “Residual.” Health status is indicated by header bar color and letter, and the number of successfully sequenced samples per location and group is provided where possible. For the healthy adenoid swabs and healthy middle ear rinses, 1 and 4 samples were available for analysis, respectively. Adenoid swabs and tissue samples were grouped into one graph, as they did not differ significantly. Note that by “healthy,” we refer to the absence of inflammation and infection at the studied anatomical site. For the nasopharynx, children attending day care were included, in addition to cochlear implant recipients who were sampled as reference for the other anatomical sites. (B) Similarity (1 − Bray-Curtis dissimilarity) of middle ear effusion samples to matched nasopharynx versus side-matched ear canal samples. A high score indicates high similarity between two locations. Symbols are sized based on the number of reads remaining in the middle ear sample after contaminant filtering. Colors indicate which ASV is dominant (>50% relative abundance) in the middle ear. (C) Stacked bar charts of healthy middle ear samples. Only ASVs detected in more than one sample are shown.
Number of patients enrolled and numbers of samples which could have been collected, were collected, and passed quality control (QC) after sequencing. CI, cochlear implant recipients; DC, children attending day care. Download Table S1, DOCX file, 0.02 MB (24.6KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxa detected in negative extraction and PCR controls and action taken. Download Table S2, PDF file, 0.2 MB (223.2KB, pdf) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Identification of bacteria dominant in OME middle ear effusion.
To identify potential bacterial pathogens for chronic OME in children resident in Flanders, Belgium, we analyzed 97 OME middle ear effusion aspirates of 59 children. Of these, 80% were dominated (≥50% relative abundance) by a single amplicon sequence variant (ASV) (Table 2). In just 33% of effusions, the dominant ASV belonged to one of the classic otopathogen genera Haemophilus, Moraxella, or Streptococcus, but these genera showed high prevalence, with detection in 75%, 53% and 56% of middle ear samples, respectively. Other dominant ASVs were Alloiococcus 1 (39%), Turicella 1 (4%), Staphylococcus 1 (3%), and Corynebacterium 1 (1%) (Table 2).
TABLE 2.
Prevalence of ASVs dominant in at least one middle ear effusion
| ASV | Species | Middle ear |
Ear canal |
||
|---|---|---|---|---|---|
| Prevalence (%) | Dominance (%)a | Prevalence (%) | Dominance (%)a | ||
| Alloiococcus 1 | Alloiococcus otitis | 87.6 | 39.2 | 90.2 | 75.5 |
| Haemophilus 1 | Haemophilus influenzae | 56.7 | 14.4 | 46.1 | 0 |
| Haemophilus 2 | Haemophilus aegyptius | 42.3 | 10.3 | 22.5 | 0 |
| Streptococcus 3 | Streptococcus pyogenes | 4.1 | 1.0 | 2.9 | 0 |
| Streptococcus 1 | Streptococcus pneumoniae / Streptococcus pseudopneumoniae b | 14.4 | 2.1 | 4.9 | 0 |
| Corynebacterium 1 | Corynebacterium pseudodiphtheriticum / Corynebacterium propinquum | 8.2 | 1.0 | 13.7 | 1.0 |
| Haemophilus 3 | Haemophilus quentini/H. influenzae | 10.3 | 1.0 | 1.0 | 0 |
| Moraxella 1 | Moraxella catarrhalis/Moraxella nonliquefaciens | 47.4 | 4.1 | 43.1 | 0 |
| Staphylococcus 1 | Not resolved to species level | 51.5 | 3.1 | 69.6 | 4.9 |
| Turicella 1 | Corynebacterium otitidis | 71.1 | 4.1 | 81.4 | 7.8 |
Dominance was defined as the percentage of samples with a relative abundance of ≥50%.
ASV represents both listed species.
Origin of bacteria dominant in OME middle ear effusion.
For 73 effusion samples of 49 patients, matched nasopharynx and ear canal swabs were sequenced successfully. To explore body site continuity, we plotted the similarity (1 − Bray-Curtis dissimilarity) between the effusion and nasopharynx microbiome against the similarity between the effusion and the side-matched ear canal microbiome (Fig. 1B). All effusion samples dominated by Alloiococcus, Turicella, Staphylococcus, or Corynebacterium were more similar to the ear canal (similarity score, ≥0.574) than to the nasopharynx (similarity score, ≤0.254). These taxa were also frequently dominant in the ear canals of all patients (Fig. 1A), indicating that they probably originated from the ear canal.
The microbiome of the healthy middle ear cavity.
Twelve middle ear rinses collected from microbiologically healthy cochlear implant recipients were sequenced to identify bacteria associated with middle ear health. However, only four of these were retained after quality filtering. In addition, of the 107 ASVs detected in the remaining four samples, only seven were present in multiple samples (Fig. 1C), and of these, only Streptococcus 1 and Corynebacterium 1 (Table 2) were absent from the negative controls.
Comparison of the nasopharynx microbiome in health and during chronic OME.
Because of the high risk of bias by contaminants and the low number of successfully sequenced samples of the middle ear, we decided to focus on the nasopharynx. This enabled us to compare the URT microbiomes of OME patients and of healthy controls and to identify health-associated bacteria. For this analysis, data from the nasopharynx swabs from 67 chronic OME patients, 10 microbiologically healthy cochlear implant recipients, and 41 healthy children attending day care were included. The nasopharynx microbiome differed significantly between OME patients and healthy controls (PERMANOVA, P = 0.014), a difference which was more pronounced than that observed between the cochlear implant and the day care control groups (PERMANOVA, P = 0.049). A total of 134 taxa were shared between both control groups and the OME group (see Table S3 in the supplemental material). Differential abundance analysis (Analysis of Composition of Microbiomes [ANCOM] [42]) with stringent correction for multiple testing identified Acinetobacter 1 (Acinetobacter lwoffii or Acinetobacter pseudolwoffii) and Streptococcus 5 (S. salivarius, S. thermophilus, or Streptococcus vestibularis) as health associated, since the Aitchison’s log ratio of the abundance of these ASVs to other ASVs was significantly higher in the healthy group than in the OME group (Fig. 2; see also Fig. S1 in the supplemental material). When searching for taxa specific for OME, no taxon was significantly more abundant in the nasopharynx of OME patients compared to controls, suggesting that we should focus on health-associated rather than OME-specific taxa for further functional analyses.
FIG 2.
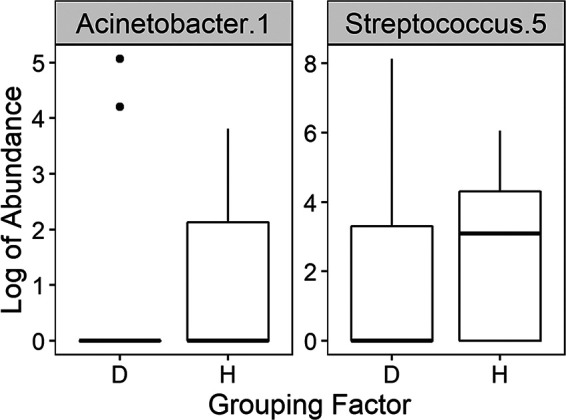
ASVs significantly differentially abundant in the nasopharynx of chronic OME (“D”) versus controls (“H”) by Analysis of Composition of Microbiomes (ANCOM) analysis with stringent correction for multiple testing (see Fig. S1 in the supplemental material for an alternative analysis approach).
Prevalence and mean relative abundance of nasopharynx amplicon sequence variants (ASVs) shared between otitis media with effusion (OME) patients and healthy controls. The healthy controls were considered cumulatively (HNPAll) and individually (HNPCI, cochlear implant recipients; HNPDC, children attending day care). To calculate the mean relative abundance, only samples in which the taxon was present was considered. Download Table S3, PDF file, 0.3 MB (271.7KB, pdf) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Compositional differential abundance of nasopharynx amplicon sequence variants (ASVs) between chronic otitis media with effusion (OME) patients and healthy controls. Each tile shows an estimate of the differential abundance of an ASV on the y axis relative to an ASV on the x axis. A plus/minus sign means that the ASV is significantly more/less abundant in healthy controls compared with OME patients, with a false discovery rate of 10% (capped with the method of Benjamini and Yekutieli). The data set was rarefied to the size of the smallest sample (2,820 reads) before performing a Wilcoxon rank-sum test, and only taxa that were differentially abundant in comparison to at least one other taxon are shown. Download FIG S1, TIF file, 0.5 MB (497.6KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Isolation of bacteria from the healthy human URT.
We next aimed to characterize the potentially beneficial properties of bacteria isolated from healthy controls in more detail, especially with their potential to control the growth of middle ear pathobionts. We isolated 142 bacterial isolates belonging to 11 different genera from anterior nare (n = 8), nasopharynx (n = 9), and adenoid swabs (n = 1) from 9 microbiologically healthy cochlear implant recipients (see Fig. S2 in the supplemental material). Streptococcus spp. were most frequently isolated (n = 66), especially from the nasopharynx, while A. lwoffii and A. pseudolwoffii, which we identified as potential beneficial members based on ANCOM analysis, were not cultivated, likely due to their low relative abundance (0.1%) in cochlear implant controls (Table S3). All Streptococcus isolates from healthy children belonged to either the mitis group (n = 28), the salivarius group (n = 32), or the sanguinis group (n = 5).
Overview of bacterial isolates obtained from the upper respiratory tract of healthy children. (A) Distribution of isolated species by anatomic location, colored by genus. (B) Number of strains per species isolated from each patient. (C) Distribution of genera by anatomic location. Download FIG S2, TIF file, 0.8 MB (795.1KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Antimicrobial activity of streptococci against classic otopathogens.
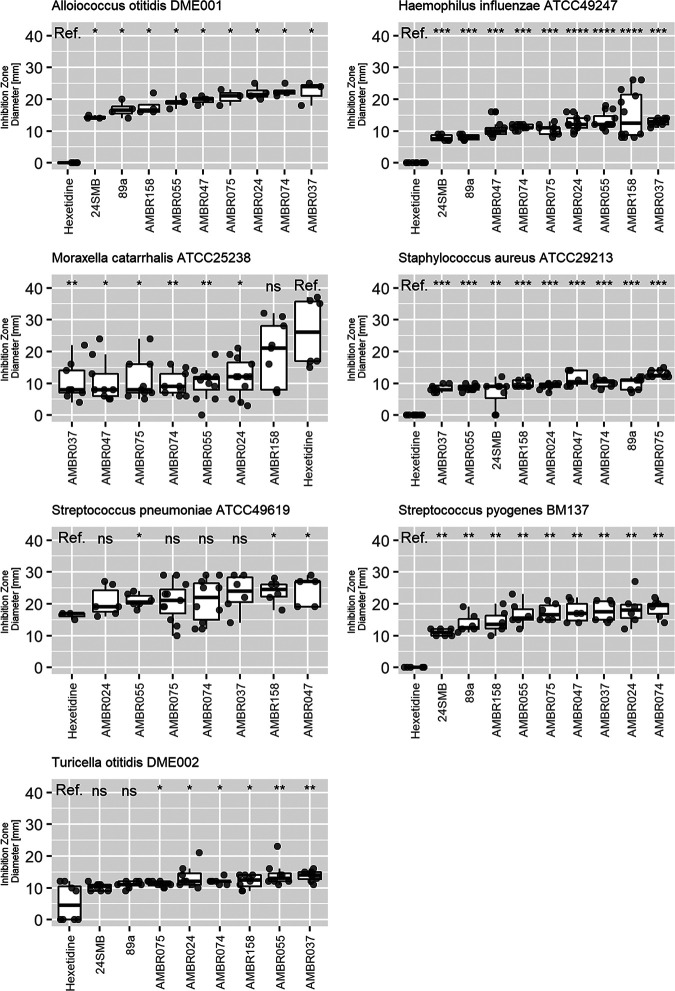
In the present study, salivarius group streptococci were identified as health associated, but other research groups have also associated other commensal streptococci with respiratory and oral health and confirmed that they are safe in human clinical trials (26, 28, 43–45). We therefore screened 78 commensal streptococci (53 from this study and 25 isolated from healthy adults [19, 46]) for their antimicrobial activity against the three classic otopathogens. Based on results obtained with spot assays, in which the pathogen and the potentially beneficial bacteria were cocultured, all tested species could inhibit the growth of H. influenzae, with Streptococcus anginosus, Streptococcus pseudopneumoniae, and S. salivarius isolates showing the largest inhibition zones on average (17 ± 5 mm, 16 ± 4 mm, and 13 ± 4 mm, respectively [mean ± SD]), although two S. salivarius isolates had no effect (Fig. 3A). S. anginosus (9 ± 4 mm) and S. salivarius (9 ± 6 mm) could also inhibit M. catarrhalis, but this effect was strain dependent (Fig. 3B). S. salivarius (19 ± 5 mm) and S. vestibularis (19 ± 3 mm) were most effective against S. pneumoniae, with S. anginosus in third place (Fig. 3C). Based on the spot assays and the results of the differential abundance analysis, we selected seven S. salivarius isolates (AMBR024, AMBR037, AMBR047, AMBR055, AMBR074, AMBR075, and AMBR158) for more detailed antimicrobial screening. These isolates were additionally tested against the URT pathobionts Streptococcus pyogenes BM137 and Staphylococcus aureus ATCC 29213 and the suspected middle ear pathobionts A. otitis AMBR153 and C. otitidis AMBR154 isolated from OME middle ear effusion during this study. S. salivarius 24SMB and S. oralis 89a isolated from the probiotic nasal spray Rinogermina (DMG Italia) were used as references. All isolates could inhibit all tested pathobionts in spot assays, whereby AMBR158 was the most effective isolate against the classic otopathogen genera H. influenzae, M. catarrhalis, and S. pneumoniae (Fig. 4). Screening of the genomes against the secondary metabolite databases AntiSMASH 5.0 (47) and BAGEL4 (48) revealed that all seven in-house isolates harbored a bacteriocin-like peptide (blp) cassette with different predicted bacteriocins, ABC transporters, and immunity proteins (Table 3) (49, 50). AMBR037, AMBR074, and AMBR075 additionally encoded a lactococcin 972 family bacteriocin, including an ABC transporter and an immunity mechanism (49). Lantipeptide loci were detected in the genomes of AMBR055 (related to salivaricin A2 [50] and salivaricin 9 [51]) and AMBR074 (related to streptococcin A M49 [52] and macedocin [53]). Lasso peptides were detected in AMBR024 (undetermined) and AMBR158 (related to streptomonomicin [54]). In addition to bacteriocins, antiSMASH (18) also predicted a gramidicin nonribosomal peptide synthetase (NRPS) locus in AMBR024 (Table 3).
FIG 3.
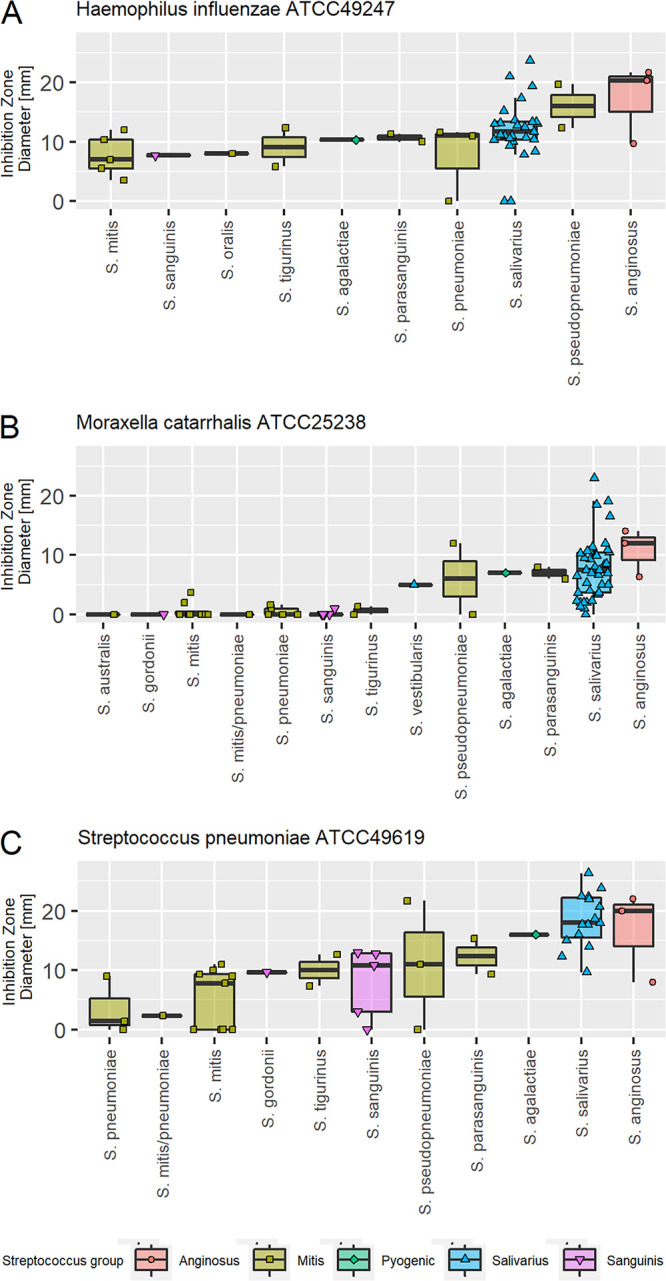
Mean inhibition of classic otopathogens by Streptococcus species isolated from the URT of healthy children and adults, as measured through the spot assay method. Each point represents a different isolate.
FIG 4.
Inhibition of upper respiratory tract and classic and suspected otopathogens by 7 S. salivarius isolates, as measured through the spot assay method. Isolates were compared to S. salivarius 24SMB and S. oralis 89a, isolated from the Rinogermina probiotic nasal spray, and Hextril mouthwash (0.1% hexetidine) served as a positive control. For each pathobiont, the tested isolates are sorted from smallest (left) to largest (right) mean inhibition zone diameter. Statistical comparison of inhibition zones was performed with 0.1% hexetidine as a reference. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
TABLE 3.
Genetic loci encoding potentially antimicrobial secondary metabolites
| Isolate | Most closely related bacteriocin type |
||||
|---|---|---|---|---|---|
| Class I |
Class II |
NRPSa | |||
| Lantipeptides (Ia) | Lasso peptides (If) | IIc (blp locus) | IId | ||
| AMBR024 | Lasso peptide | Mutacin IV | Gramicidin | ||
| AMBR037 | Lactococcin | Lactococcin 972 | |||
| AMBR047 | Lactococcin | ||||
| AMBR055 | Salivaricin A2/salivaricin 9 | Lactococcin and gassericin | |||
| AMBR074 | Streptococcin A | Lactococcin and Thermophilin A | Lactococcin 972 | ||
| M49/macedocin | |||||
| AMBR075 | Lactococcin | Lactococcin 972 | |||
| AMBR158 | Streptomonomicin | Thermophilin A | |||
NRPS, nonribosomal peptide synthetase.
Initial evaluation of antibiotic resistance and virulence genes.
Potential probiotics should not carry transferable antibiotic resistance markers (55), as these could be transmitted to pathogens, complicating their treatment. We therefore screened the isolate genomes for transferable antibiotic resistance markers (see Table S4 in the supplemental material) and tested their susceptibility to key antibiotic classes in vitro. S. salivarius AMBR055 and AMBR047 were predicted to harbor the adjacent genes mef(A) (100% coverage with 96% identity) and mel [also known as msr(D); 100% coverage with 100% identity], which encode efflux pumps for macrolide class antibiotics. These genes are part of the mobile genetic element mega (macrolide efflux genetic assembly), which has been shown to be transferable to S. pneumoniae via transformation (56–58). Phenotypic testing indicated only resistance for AMBR047 against the macrolide erythromycin (MIC of 4 to 16 mg/liter with a cutoff of 2 mg/liter) and against chloramphenicol (MIC of 8 mg/liter with a cutoff 4 mg/liter). Based on this finding, AMBR055 and AMBR047 were excluded from further analysis.
Antibiotic resistance and virulence factor genes. Download Table S4, DOCX file, 0.02 MB (23KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
As an additional safety check, we also verified that the potential probiotics did not carry any known virulence factors. No virulence genes of concern were observed, although two genes showed a hit with the Virulence Factor Database (VFDB) (14) (Table S4) for all isolates, namely, psaA, encoding a putative adhesin (with 87.85% to 88.92% coverage of and 76.24% to 76.96% identity to the pneumococcal surface adhesion gene of S. pneumoniae TIGR4), and hasC, encoding UDP-glucose pyrophosphorylase (91.26% to 91.85% coverage and 76.94% to 77.67% identity to the gene of S. pyogenes M1 GAS), which is probably involved in capsular polysaccharide biosynthesis. Sufficient adhesion is considered a desired property for most probiotic applications because it can promote persistence in the niche (59). S. salivarius produces a levan or dextran capsule instead of the known virulence factor hyaluronic acid capsule found in pathogenic S. pyogenes that mimics human connective tissue (60, 61). These two conserved genes were therefore not considered virulence factors, but rather adaptation factors, reflecting adaptation of S. salivarius to the URT rather than pathogenicity.
Ability of S. salivarius to adhere to respiratory epithelium.
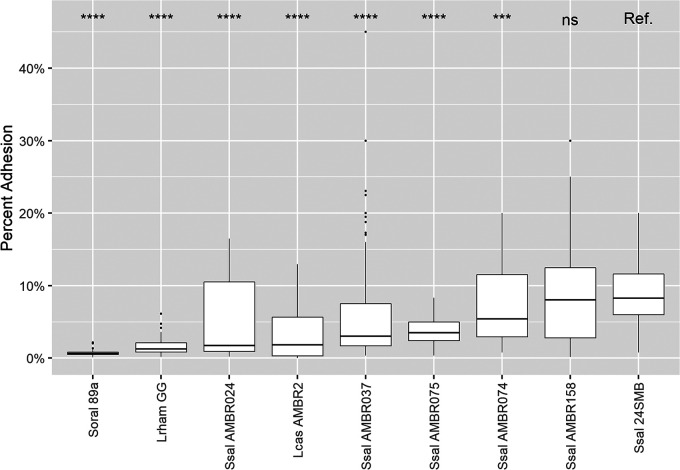
Since sufficient adhesion to the host mucosa or epithelial cells is known to increase a probiotic’s opportunity to interact with its host and mediate displacement of already adhered bacteria and competitive exclude pathogens that bind to the same receptor (62, 63), we also phenotypically characterized the interaction of the five remaining isolates with the respiratory epithelial Calu-3 cells. As examples of taxa with a higher and lower ability to adhere to respiratory epithelium, we also included the gastrointestinal probiotic Lacticaseibacillus rhamnosus GG (median adhesion of 1.2%) and the URT-derived investigational new probiotic Lacticaseibacillus casei AMBR2 (1.9%) (64). All five S. salivarius isolates could adhere to the cells, with median adhesion values between 1.8% (AMBR024) and 8.1% (AMBR158), the latter of which was statistically (Wilcoxon rank sum test, P > 0.05) as adherent as the commercial URT probiotic S. salivarius 24SMB (8.3%) (Fig. 5). In contrast, the commercial URT probiotic S. oralis 89a adhered with a median adhesion value of 0.6%, even lower than that of L. rhamnosus GG.
FIG 5.
Adhesion of bacterial isolates to respiratory epithelial cells (Calu-3). The adhesion of Streptococcus salivarius (Ssal) isolates obtained in the present study was statistically compared to that of S. salivarius 24SMB (38–40) of the Rinogermina probiotic nasal spray using an unpaired Wilcoxon rank sum test. S. oralis (Soral) 89a was also isolated from Rinogermina, but it showed a lower adhesion capability than all S. salivarius strains. Lacticaseibacillus casei (Lcas) AMBR2 and Lacticaseibacillus rhamnosus (Lrham) GG were included as examples of lactobacilli with a higher and a lower ability to adhere to respiratory epithelium, respectively (64). Isolates are sorted from lowest to highest median adhesion percentage. ns, P > 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. P values are adjusted by the Holm method.
To investigate the observed differences in adhesion, we manually screened the five S. salivarius isolate genomes annotated by The PATRIC Comprehensive Genome Analysis pipeline (65) and by Operon-mapper (66) for genes associated with adhesion. Apart from the psaA gene mentioned above, all isolates also harbored two copies of the peb1A gene, which is annotated as major cell-binding factor in the Gram-negative bacterium Campylobacter jejuni (67). All of our isolates also encoded homologues of SalivA_1472 and SalivA_1475, known genes for adhesion of S. salivarius JIM87777 (68, 69), although the SalivA_1475 homologue of AMBR047 appeared to be truncated. AMBR055, AMBR024, and AMBR047 also encoded a homologue of SalivA_1473 (zinc metalloprotease ZmpB precursor) (68, 69), which was absent from the other isolates. No isolate encoded a full set of the srpA, srpB, and srpC genes encoding accessory Sec-dependent serine-rich glycoprotein adhesins (SalivA_1458 to SalivA_1456). Indeed, AMBR055 encoded srpA and srpC, while AMBR024 and AMBR047 both encoded srpB an and srpC. Of note, srpB is the only gene associated with adhesion by both studies (68, 69) (Fig. S3). Although differences between isolates are thus observed, no clear conclusions about the various adhesion capacities can be made based on the isolate genomes, and the gene-function relations remain to be further substantiated in follow-up work.
Alignment of a genomic region with 6 genes associated with adhesion in S. salivarius JIM8777 (each highlighted with a square) using the PATRIC Compare Region Viewer tool. Homologues are indicated by the same color, with the exception of the AMBR055 region (marked with an asterisk (*). The original Compare Region Viewer alignment stopped after the homologue of SalivA_1458, as the homologue of Saliva_1457 is missing. A new alignment was started using the SalivA_1456, of which the homologue is present in AMBR055 (here indicated in red) caused a fresh assignment of colors. SALIVA_1456 to SALIVA_1458 = accessory Sec-dependent serine-rich glycoprotein adhesin genes srpC, srpB and srpA. SALIVA_1472 and SALIVA_1475 = YSIRK signal domain/LPXTG anchor domain surface protein. SALIVA_1473 = zinc metalloprotease ZmpB precursor. Download FIG S3, TIF file, 0.9 MB (959.3KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
This study aimed to characterize the microbiome of the URT and ear during chronic OME as a representative chronic inflammatory childhood disease versus healthy control samples of children attending day care (nasopharynx) or receiving a cochlear implant (access to the middle ear). For this microbiome comparison, special attention was given to the identification and characterization of taxa that were more prevalent and abundant in healthy control samples in order to identify potential novel probiotic species and strains.
First, we identified bacteria associated with middle ear disease. Of the classic otopathogen genera, H. influenzae was most frequently dominant, with few effusion samples harboring high levels of M. catarrhalis or S. pneumoniae. Surprisingly, the microbiome of almost half of all effusion samples consisted of typical ear canal bacteria, with A. otitis most frequently dominant, despite the great care that was taken not to touch the ear canal during sampling. Other authors who sequenced the middle ear effusion microbiome of OME (6–8, 13) or recurrent acute otitis media (rAOM) (70) reported similar findings. OME occurs by definition behind an intact tympanic membrane (1), but patients may have had unidentified perforations in the past or inflammation that caused microlesions that could allow bacteria to translocate into the middle ear (7, 8).
Attempts to sequence the microbiome of the healthy middle ear were considered unsuccessful, indicating that very few or even no bacteria were present in this body site under healthy conditions. This is in accordance with a recent study which argued that bacterial signals detected in this body site under healthy conditions are likely due to contamination (71). However, typical middle ear pathobionts colonize the nasopharynx before ascending via the Eustachian tube into the middle ear. Therefore, we hypothesize that URT probiotics targeting the nasopharynx could be used to reduce the incidence or severity of otitis media. Differential abundance analysis identified two taxa (ASVs) as significantly more abundant in the healthy nasopharynx than in the nasopharynx of chronic OME patients, A. lwoffii and salivarius group streptococci.
A. lwoffii (recently split into A. lwoffii and A. pseudolwoffii [72]) has been shown to decrease allergic reactions in human dendritic cells and mouse models by inducing a weak TH1-type immune response. The mice also produced less mucus-producing goblet cells in their lungs upon sensitization with ovalbumin (73). Allergies are also a risk factor for OME (74). Furthermore, goblet cells and mucous glands are responsible for mucin production in the middle ear, with high densities observed during chronic OME (75). Protection from allergies could decrease the risk of developing OME, and reduction of mucin production could decrease its severity. Whether A. lwoffii has a similar effect on the human middle ear as on mouse lungs remains to be determined. Here, we decided to focus on S. salivarius isolates as potential URT probiotics due to their high prevalence and safety history. The Streptococcus salivarius group encompasses the species S. salivarius, S. vestibularis, and S. thermophilus. The latter is an industrially used dairy fermenter with generally recognized as safe (GRAS) and qualified presumption of safety (QPS) status and which is also marketed as a gastrointestinal probiotic (76). In contrast, S. vestibularis and S. salivarius are mainly found in the human oral cavity (77). Since the oral cavity is in direct contact with the URT, it can be rationalized that translocation from the oral cavity to the nasopharynx can occur, as our research group recently also suggested for members of the Lactobacillaceae (64). Similarly to the present study, Walker et al. (78) identified a taxon related to S. thermophilus as significantly more abundant in the anterior nares of healthy controls than in those of chronic OME patients through differential abundance analysis with DESeq2 (79). At least three isolates of S. salivarius are already marketed as probiotics, namely (i) S. salivarius K12, which mainly targets pharyngotonsillitis caused by Streptococcus pyogenes but has also shown some effect on AOM and oral malodor (reviewed in reference 80); (ii) S. salivarius M18, which targets dental and gingival health (30); and (iii) S. salivarius 24SMB, which targets rAOM and other recurrent URT infections (31) and has recently also been shown to reduce adenoid hypertrophy and OME when used in conjunction with Streptococcus oralis 89a (41). Thus, the literature also points to the potential for S. salivarius as a probiotic, but not many probiotic strains have yet been described, indicating the importance of further characterization and screening.
One of the most important ways in which probiotics can benefit their host is by limiting the growth or the establishment of pathogens. Therefore, we characterized the capacity of seven S. salivarius isolates to inhibit the growth of OME pathogens in more detail. They inhibited the growth of all tested pathobionts in the direct interaction assay, but not when using filter-sterilized supernatant of an overnight culture (data not shown). This points toward the importance of close interaction between the potential beneficial bacteria and pathobionts in a living state (81), growth-phase-specific gene expression (82), or the requirement of a high culture density to activate the synthesis of antimicrobial molecules (83), as occurs on solid media. However, it is also possible that S. salivarius might need a different experimental setup to produce antimicrobial molecules in a broth culture. Genome analysis showed that each isolate harbored multiple secondary metabolites that were potentially involved in the observed inhibition (Table 3), but their activity and target(s) still need to be confirmed through peptide mass spectrometry analysis or in vitro knockout studies.
We then evaluated adhesion capacity to the respiratory epithelial cell line Calu-3. These immortalized cells were chosen over primary cells to increase reproducibility. All tested isolates showed an adherence percentage above 1%, in agreement with the fact that they express adhesion factors, with AMBR158 being the best-performing strain. Screening the genomes for adhesion-related genes did not provide a clear explanation for the range of adhesion ability observed for the five S. salivarius isolates, indicating that more research into the interaction of S. salivarius with human respiratory epithelium is required.
Probiotics “confer,” by definition, “a health benefit to the host” (21) and should thus not be able to cause disease or contribute to the survival of pathogens, e.g., by spreading antibiotic resistance genes. Our isolates did not harbor any known virulence genes. However, it must be stated that genotyping for virulence factors is challenging and limited because nonpathogenic species possess “virulence” genes in common with pathogenic ones. In our isolates, we rationalized that the two genes flagged by VFDB could be considered adaptation factors, used for good function in the URT. Indeed, sometimes these “virulence factors” could be useful to combat a pathogenic species. Two isolates, AMBR055 and AMBR047, on the other hand, harbored acquired genes for macrolide efflux pumps, and AMBR047 additionally showed decreased sensitivity to chloramphenicol in vitro, making these isolates unsuitable as probiotic candidates. As an additional safety check, it must be confirmed in future work that the selected S. salivarius strains do not have a disruptive effect on the (beneficial members) of the URT microbiome, given their inherent antimicrobial capacity.
We acknowledge that this study has some potential drawbacks. First, in this study, bacteria with a potentially beneficial effect on middle ear health were identified at the nasopharynx level, as healthy middle ear samples did not yield trustworthy microbiome results. The nasopharynx is the natural habitat of the classic middle ear pathobionts, making it a suitable and accessible location for probiotics targeting middle ear health. In addition, our study cannot answer conclusively if ear canal commensals are involved in OME pathogenesis, as it is not possible to completely exclude touching the ear canal or ear drum when sampling middle ear effusion. However, our S. salivarius isolates were able to also inhibit the growth of these taxa (A. otitis and C. otitidis). Finally, we used samples from two control groups with different extraction methods to make up for the unexpectedly low number of suitable cochlear implant controls, but care was taken during the data analysis to ensure that the observed microbiome differences between health and OME were not due to differences in sample processing.
Conclusions.
Our study confirms findings from previous work undertaken in the URT area with a robust sequencing and molecular approach. We identified H. influenzae as the most frequent classic middle ear pathobiont for chronic OME in children resident in Flanders, Belgium, with ear canal taxa, especially A. otitis, as potential additional etiologic agents. At the nasopharynx level, we identified A. lwoffii and salivarius group streptococci as health associated. Phenotypic tests confirmed that five of our S. salivarius isolates could inhibit typical respiratory and middle ear pathogens while lacking known virulence and antibiotic resistance mechanisms. Our findings will now have to be confirmed in more elaborate safety and probiotic efficacy studies in humans.
MATERIALS AND METHODS
More details are provided in Text S1 in the supplemental material.
Supplemental Materials and Methods. Download Text S1, DOCX file, 0.06 MB (64.3KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sample collection.
Ethical approval was obtained from the ethical committee of the Antwerp University Hospital (ClinicalTrials.gov identifier NCT03109496, registered 12 April 2017), and informed consent was obtained from a parent or legal guardian before sampling. OME patients (n = 70) were recruited from a group of children aged 1 to 10 years who were receiving unilateral or bilateral tympanostomy tubes with or without concurrent adenoidectomy to relieve symptoms of persistent (≥3 months) OME. One control group consisted of cochlear implant recipients aged 1 to 45 years (n = 12), and a second control group consisted of children aged 6 to 30 months who were healthy enough to attend day care (originally sampled for the NPcarriage study [84]; n = 41). Exclusion criteria were comorbidities affecting the URT anatomy, immune system, or mucociliary system; acute or chronic URT infection; and use of antibiotics or steroids up to 1 week before surgery. Swabs of the anterior nares, nasopharynx, and ear canal, middle ear effusion aspirates, and, in the case of simultaneous adenoidectomy, both tissue and swabs of the adenoids were collected from OME patients. Cochlear implant controls provided anterior nare and nasopharynx swabs and a middle ear wash, while children attending day care provided a nasopharynx swab. The mean age of cases and controls was compared using Student’s t test (ggpubr version 0.2.5 [85]).
16S rRNA amplicon sequencing, quality control, and data analysis.
DNA of samples from cochlear implant controls and OME patients was extracted with the QIAamp PowerFecal DNA kit and quantified with a Qubit 3.0 fluorometer (Thermo Fisher Scientific). NPcarriage study samples were received as NucliSens easyMag (bioMérieux) extracted DNA. All samples were further processed and sequenced on an Illumina MiSeq desktop sequencer (M00984; Illumina) as described previously (19). After sequencing of the V4 region of the 16S rRNA gene, trimming, error correction, chimera removal, and classification of paired reads against the EzBioCloud 16S database version 19.01.2018 (86) were all performed in DADA2 version 1.6.0 (87). This workflow resulted in an ASV table with a single-nucleotide difference resolution. Sequenced extraction and PCR controls served as indicators of background contamination. For contaminant filtering (see Table S2 in the supplemental material) and data visualization, packages included in tidyverse 1.2.1 and the tidyamplicons package (https://github.com/SWittouck/tidyamplicons) were used. ASVs of interest were further classified using the online EzBioCloud 16S-based ID web application (update 2020.05.13) (86).
The differential abundance of ASVs between the nasopharynx of OME patients and of controls was calculated using the Analysis of Composition of Microbiomes (ANCOM) R tool (version 1.1.2 [42] with default tuning parameters and stringent correction for multiple testing). Permutational multivariate analysis of variance (PERMANOVA) (vegan version 2.2.5 [88]) was used to determine the effect of metadata on microbiome composition and to compare the same anatomic location between cases and controls.
Isolation of lactic acid bacteria.
Samples from cochlear implant controls were plated out on three different agar media (see Table S5 in the supplemental material). From each plate, one colony per morphology was selected and identified by Sanger sequencing of the 16S rRNA genes (primers 27F and 1492R) (89, 90).
Cultivation conditions. Download Table S5, DOCX file, 0.02 MB (21.6KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Whole-genome sequencing, assembly, and analysis.
Bacterial DNA was extracted for whole-genome sequencing following protocol P3 by Alimolaei and Golchin (91) and sequenced on an Illumina MiSeq platform. The resulting reads were assembled de novo with SPAdes-based Shovill (https://github.com/tseemann/shovill), followed by quality control with CheckM (92) and annotation with Prokka (93). Assembled contigs were screened against the ResFinder 3.2 database for the presence of transferable antibiotic resistance genes (94), and against the Virulence Factor Database (VFDB) for virulence factors (95) using ABRicate (https://github.com/tseemann/abricate). Secondary metabolites were identified by antiSMASH 5.0 and BAGEL4. Genes of interest were further characterized using NCBI-BLAST (96). In addition, the genomes were submitted to the Pathosystems Resource Integration Center (PATRIC) Comprehensive Genome Analysis pipeline (65) and to Operon-mapper (66). Annotations from these programs were considered in addition to Prokka annotation when looking for toxin and adhesion genes. Starting from the locus tag or gene annotation of S. salivarius JIM877, the presence of genes previously associated with adhesion in S. salivarius (68, 69) was confirmed with the PATRIC Compare Region Viewer function set to a region size of 100,000 bp, 50 public and private genomes, and using the PLfams method. As PATRIC does not allow setting of which genomes are used for this alignment, we extracted the visualization and aligned the genomic regions of JIM8777 and our own isolates manually. The Compare Region Viewer was also used to visualize the genomic region upstream and downstream of the mef(A) gene in AMBR047 and AMBR055 after screening of these genomes with primers for mef(A) or mef(E) and mega (57) could only confirm presence of the mega element for AMBR047.
Antimicrobial screening.
The ability of isolates to inhibit the growth of URT and middle ear pathobionts was tested (i) by overlaying 48-h 2-μl spots of isolate with pathobiont-containing soft agar (spot assay) and (ii) by inoculating 30 μl spent filter-sterilized culture supernatant into wells punched into pathobiont-containing agar (radial diffusion assay) (97). Hexetidine (0.1%, Hextril; Johnson & Johnson) and Todd Hewitt (TH) broth served as positive and negative controls, respectively. For some repetitions, the pH of the TH broth was reduced to 5. Growth conditions are summarized in Table S5. Inhibition zone sizes were compared to the reference hexetidine using an unpaired Wilcoxon rank sum test (ggpubr version 0.2.5 [85]).
Antibiotic susceptibility assay.
Minimum inhibitory concentrations (MIC) of antibiotics (ampicillin, chloramphenicol, clindamycin, erythromycin, gentamicin, tetracycline, streptomycin, and vancomycin) were determined using a broth microdilution assay with 2-fold serial dilutions between 0.5 μg/ml and 128 μg/ml and evaluation of presence/absence of growth after 24 h of incubation (98). Cutoff values for S. thermophilus were used based on the guidelines of the European Food Safety Authority (EFSA) (55).
Adherence assay to airway epithelial cells.
The human airway epithelial cell line Calu-3 ATCC HTB-55TM (purchased from ATCC, Molsheim Cedex, France) was cultured in 75 cm2 flasks containing 20 ml minimum essential medium (MEM; Life Technologies, Erembodegem, Belgium) supplemented with heat-inactivated fetal bovine serum (Thermo Fisher, Asse, Belgium) and penicillin-streptomycin (100 U/ml; Life Technologies) and maintained in a humidified 5% CO2 incubator at 37°C. The culture medium was changed every 3 to 4 days, and the cells were passaged weekly at a 1:2 split ratio using a 0.25% trypsin-EDTA solution (Life Technologies). To test the ability to adhere to human respiratory epithelium, 2 × 108 CFU of bacteria were added for 1 h to fully grown Calu-3 cultures seeded at a density of 3 × 105 cells/cm2. The CFU/ml of bacteria added and bacteria retrieved after incubation and washing with phosphate-buffered saline (PBS) were compared, as previously described (99). Adhesion percentages were compared to a reference using an unpaired Wilcoxon rank sum test (ggpubr version 0.2.5 [85]).
Data availability.
The 16S rRNA gene sequencing data generated for this study were deposited in the European Nucleotide Archive under accession number PRJEB33591. The whole-genome sequence data have been added to project PRJEB32716 under assembly numbers GCA_905071825 (isolate AMBR024), GCA_905071875 (AMBR037), GCA_905071915 (AMBR047), GCA_905071845 (AMBR055), GCA_905071895 (AMBR074), GCA_905071855 (AMBR075), and GCA_905071905 (AMBR158).
ACKNOWLEDGMENTS
This work was supported by the Agentschap Innoveren en Ondernemen (grant IWT150052); the University of Antwerp (grants DOC PRO FFB130135 and IOF-POC FFI 170288 to M.V.D.B., and a Dehousse scholarship to W.V.B.); the Fonds Wetenschappelijk Onderzoek (grants FWO 1S17916N to S.W., FWO 11A0618N to I.D.B., and FWO 1150017N to I.W.); the Antwerp Study Centre for Infectious Diseases; and an investigator-initiated research grant from Pfizer.
We are grateful to Katrien Broes, Klara Van Gool, Berina Ihtijarevic, Chloé Kastoer, Camille Levie, Nicolien A. van der Poel, Diane Van Rompaey, Anneclaire V. Vroegop, and all supporting staff at the ENT department of the Antwerp University Hospital for their help in patient recruitment and sample collection. We further thank the entire ENdEMIC group, particularly Eline F. M. Oerlemans and Sander Wuyts for developing the in house MiSeq sample preparation standard operating procedure (SOP) on which our sample processing was based, Ines Tuyaerts and Sarah Ahannach for loading the MiSeq instrument, Leen van Ham for lab management and organization, and Irina Spacova for her feedback on the manuscript. Sander Wuyts also wrote the whole-genome sequence analysis pipeline. We also thank Hatice Kurban, who helped with the laboratory analysis in the framework of her master’s thesis. In addition, we thank Abbas Rahman for his help with obtaining ethical permission to analyze the microbiome of NPcarriage study samples and Esra Ekinci for looking up background data of these samples.
A.B., I.D.B., J.J., M.V.D.B., P.V.H., V.V.R., V.T., O.V., and S.L. contributed to the design of the study. S.L. and O.V. were responsible for the coordination and supervision of the project, including funding acquisition. O.V. and A.B. also performed surgeries (see previous paragraph for other surgeons who contributed samples). J.J. processed the samples under the close guidance of I.D.B., who optimized the sample preparation SOP for low-biomass samples. I.D.B. also loaded and monitored the first MiSeq run. J.J., S.W., W.V.B., S.L., and O.V. performed different parts of the data analysis and interpretation. W.V.B. and S.W. also submitted the data to a repository. I.W., S.D., L.V.H., J.V., O.V.D., S.M.-K., and H.T. were involved in the setup, execution and sample processing of the NPcarriage study, and H.T. organized the sharing of NPcarriage study samples, including obtaining ethical committee permission for 16S sequencing. J.J. drafted the first version of the manuscript, and S.L., M.V.D.B., W.V.B., S.W., I.D.B., and H.T. contributed to manuscript revisions. All authors read and approved the submitted version.
Contributor Information
Sarah Lebeer, Email: sarah.lebeer@uantwerpen.be.
Paul D. Cotter, Teagasc Food Research Centre
REFERENCES
- 1.Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. 2016. Otitis media. Nat Rev Dis Primer 2:16063. doi: 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djurhuus BD, Skytthe A, Christensen K, Faber CE. 2014. Increasing rate of middle ear ventilation tube insertion in children in Denmark. Int J Pediatr Otorhinolaryngol 78:1541–1544. doi: 10.1016/j.ijporl.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Ngo CC, Massa HM, Thornton RB, Cripps AW. 2016. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One 11:e0150949. doi: 10.1371/journal.pone.0150949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek I, Kim M, Lee I, Na S-I, Goodfellow M, Chun J. 2018. Phylogeny trumps chemotaxonomy: a case study involving Turicella otitidis. Front Microbiol 9:834. doi: 10.3389/fmicb.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CM, Cosetti MK, Aziz M, Buchhagen JL, Contente-Cuomo YL, Price LB, Keim PS, Lalwani AK. 2011. The otologic microbiome: a study of the bacterial microbiota in a pediatric patient with chronic serous otitis media using 16SrRNA gene-based pyrosequencing. Arch Otolaryngol Head Neck Surg 137:664–668. doi: 10.1001/archoto.2011.116. [DOI] [PubMed] [Google Scholar]
- 6.Jervis-Bardy J, Rogers GB, Morris PS, Smith-Vaughan HC, Nosworthy E, Leong LEX, Smith RJ, Weyrich LS, De Haan J, Carney AS, Leach AJ, O’Leary S, Marsh RL. 2015. The microbiome of otitis media with effusion in Indigenous Australian children. Int J Pediatr Otorhinolaryngol 79:1548–1555. doi: 10.1016/j.ijporl.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Chan CL, Wabnitz D, Jervis-Bardy J, Bassiouni A, Wormald P-J, Vreugde S, Psaltis AJ. 2016. The microbiome of otitis media with effusion. The Laryngoscope 126:2844–2851. doi: 10.1002/lary.26128. [DOI] [PubMed] [Google Scholar]
- 8.Chan CL, Wabnitz D, Bassiouni A, Wormald P-J, Vreugde S, Psaltis AJ. 2017. Identification of the bacterial reservoirs for the middle ear using phylogenic analysis. JAMA Otolaryngol Head Neck Surg 143:155–161. doi: 10.1001/jamaoto.2016.3105. [DOI] [PubMed] [Google Scholar]
- 9.Krueger A, Val S, Pérez-Losada M, Panchapakesan K, Devaney J, Duah V, DeMason C, Poley M, Rose M, Preciado D. 2017. Relationship of the middle ear effusion microbiome to secretory mucin production in pediatric patients with chronic otitis media. Pediatr Infect Dis J 36:635–640. doi: 10.1097/INF.0000000000001493. [DOI] [PubMed] [Google Scholar]
- 10.Boers SA, de Zeeuw M, Jansen R, van der Schroeff MP, van Rossum AMC, Hays JP, Verhaegh SJC. 2018. Characterization of the nasopharyngeal and middle ear microbiota in gastroesophageal reflux-prone versus gastroesophageal reflux non-prone children. Eur J Clin Microbiol Infect Dis 37:851–857. doi: 10.1007/s10096-017-3178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Val S, Poley M, Anna K, Nino G, Brown K, Pérez-Losada M, Gordish-Dressman H, Preciado D. 2018. Characterization of mucoid and serous middle ear effusions from patients with chronic otitis media: implication of different biological mechanisms? Pediatr Res 84:296–305. doi: 10.1038/s41390-018-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ari O, Karabudak S, Kalcioglu MT, Gunduz AY, Durmaz R. 2019. The bacteriome of otitis media with effusion: does it originate from the adenoid? Int J Pediatr Otorhinolaryngol 126. doi: 10.1016/j.ijporl.2019.109624. [DOI] [PubMed] [Google Scholar]
- 13.Johnston J, Hoggard M, Biswas K, Astudillo-García C, Radcliff FJ, Mahadevan M, Douglas RG. 2019. Pathogen reservoir hypothesis investigated by analyses of the adenotonsillar and middle ear microbiota. Int J Pediatr Otorhinolaryngol 118:103–109. doi: 10.1016/j.ijporl.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Kolbe AR, Castro-Nallar E, Preciado D, Pérez-Losada M. 2019. Altered middle ear microbiome in children with chronic otitis media with effusion and respiratory illnesses. Front Cell Infect Microbiol 9:339. doi: 10.3389/fcimb.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Dai W, Liang Q, Ren D. 2020. The microbiomes of adenoid and middle ear in children with otitis media with effusion and hypertrophy from a tertiary hospital in China. Int J Pediatr Otorhinolaryngol 134:110058. doi: 10.1016/j.ijporl.2020.110058. [DOI] [PubMed] [Google Scholar]
- 16.Enoksson F, Rodriguez AR, Peno C, Lopez CB, Tjernström F, Bogaert D, Hakansson AP, Bergenfelz C. 2020. Niche- and gender-dependent immune reactions in relation to the microbiota profile in pediatric patients with otitis media with effusion. Infect Immun 88:19. doi: 10.1128/IAI.00147-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroman DW, Roland PS, Dohar J, Burt W. 2001. Microbiology of normal external auditory canal. Laryngoscope 111:2054–2059. doi: 10.1097/00005537-200111000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Frank DN, Spiegelman GB, Davis W, Wagner E, Lyons E, Pace NR. 2003. Culture-independent molecular analysis of microbial constituents of the healthy human outer ear. J Clin Microbiol 41:295–303. doi: 10.1128/jcm.41.1.295-303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Boeck I, Wittouck S, Wuyts S, Oerlemans EFM, van den Broek MFL, Vandenheuvel D, Vanderveken O, Lebeer S. 2017. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front Microbiol 8:2372. doi: 10.3389/fmicb.2017.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Zeng MY, Núñez G. 2017. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med 49:e339. doi: 10.1038/emm.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 22.van den Broek MFL, De Boeck I, Kiekens F, Boudewyns A, Vanderveken OM, Lebeer S. 2019. Translating recent microbiome insights in otitis media into probiotic strategies. Clin Microbiol Rev 32:e00010-18. doi: 10.1128/CMR.00010-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos K, Holm SE, Grahn E, Lind L. 1993. Alpha-streptococci as supplementary treatment of recurrent streptococcal tonsillitis: a randomized placebo-controlled study. Scand J Infect Dis 25:31–35. doi: 10.1080/00365549309169666. [DOI] [PubMed] [Google Scholar]
- 24.Roos K, Grahn E, Holm SE, Johansson H, Lind L. 1993. Interfering α-streptococci as a protection against recurrent streptococcal tonsillitis in children. Int J Pediatr Otorhinolaryngol 25:141–148. doi: 10.1016/0165-5876(93)90047-7. [DOI] [PubMed] [Google Scholar]
- 25.Könönen E, Jousimies-Somer H, Bryk A, Kilpi T, Kilian M. 2002. Establishment of streptococci in the upper respiratory tract: longitudinal changes in the mouth and nasopharynx up to 2 years of age. J Med Microbiol 51:723–730. doi: 10.1099/0022-1317-51-9-723. [DOI] [PubMed] [Google Scholar]
- 26.Roos K, Håkansson EG, Holm S. 2001. Effect of recolonisation with “interfering” α streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ 322:210–210. doi: 10.1136/bmj.322.7280.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tano K, Grahn Håkansson E, Holm SE, Hellström S. 2002. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int J Pediatr Otorhinolaryngol 62:17–23. doi: 10.1016/s0165-5876(01)00581-x. [DOI] [PubMed] [Google Scholar]
- 28.Skovbjerg S, Roos K, Holm SE, Grahn Hakansson E, Nowrouzian F, Ivarsson M, Adlerberth I, Wold AE. 2008. Spray bacteriotherapy decreases middle ear fluid in children with secretory otitis media. Arch Dis Child 94:92–98. doi: 10.1136/adc.2008.137414. [DOI] [PubMed] [Google Scholar]
- 29.Cárdenas N, Martín V, Arroyo R, López M, Carrera M, Badiola C, Jiménez E, Rodríguez JM. 2019. Prevention of recurrent acute otitis media in children through the use of Lactobacillus salivarius PS7, a target-specific probiotic strain. Nutrients 11:376. doi: 10.3390/nu11020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JDF, Wescombe PA. 2013. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol 62:875–884. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 31.Passali D, Passali GC, Vesperini E, Cocca S, Visconti IC, Ralli M, Bellussi LM. 2019. The efficacy and tolerability of Streptococcus salivarius 24SMB and Streptococcus oralis 89a administered as nasal spray in the treatment of recurrent upper respiratory tract infections in children. Eur Rev Med Pharmacol Sci 23:67–72. doi: 10.26355/eurrev_201903_17352. [DOI] [PubMed] [Google Scholar]
- 32.Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, Albera R. 2012. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med 5:991–997. doi: 10.2147/IJGM.S38859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pierro F, Di Pasquale D, Di Cicco M. 2015. Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study. Int J Gen Med 8:303–308. doi: 10.2147/IJGM.S92488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Pierro F, Colombo M, Zanvit A, Rottoli AS. 2016. Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: a pilot investigation. Drug Healthc Patient Saf 8:77–81. doi: 10.2147/DHPS.S117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Pierro F, Colombo M, Giuliani MG, Danza ML, Basile I, Bollani T, Conti AM, Zanvit A, Rottoli AS. 2016. Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children. Eur Rev Med Pharmacol Sci 20:4601–4606. [PubMed] [Google Scholar]
- 36.Di Pierro F, Risso P, Poggi E, Timitilli A, Bolloli S, Bruno M, Caneva E, Campus R, Giannattasio A. 2018. Use of Streptococcus salivarius K12 to reduce the incidence of pharyngo-tonsillitis and acute otitis media in children: a retrospective analysis in not-recurrent pediatric subjects. Minerva Pediatr 70:240–245. [DOI] [PubMed] [Google Scholar]
- 37.Gregori G, Righi O, Risso P, Boiardi G, Demuru G, Ferzetti A, Galli A, Ghisoni M, Lenzini S, Marenghi C, Mura C, Sacchetti R, Suzzani L. 2016. Reduction of group A beta-hemolytic Streptococcus pharyngo-tonsillar infections associated with use of the oral probiotic Streptococcus salivarius K12: a retrospective observational study. Ther Clin Risk Manag 12:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchisio P, Santagati M, Scillato M, Baggi E, Fattizzo M, Rosazza C, Stefani S, Esposito S, Principi N. 2015. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur J Clin Microbiol Infect Dis 34:2377–2383. doi: 10.1007/s10096-015-2491-x. [DOI] [PubMed] [Google Scholar]
- 39.La Mantia I, Varricchio A, Ciprandi G. 2017. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for preventing recurrent acute otitis media in children: a real-life clinical experience. Int J Gen Med 10:171–175. doi: 10.2147/IJGM.S137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantarutti A, Rea F, Donà D, Cantarutti L, Passarella A, Scamarcia A, Lundin R, Damiani V, Giaquinto C, Corrao G. 2020. Preventing recurrent acute otitis media with Streptococcus salivarius 24SMB and Streptococcus oralis 89a five months intermittent treatment: an observational prospective cohort study. Int J Pediatr Otorhinolaryngol 132:109921. doi: 10.1016/j.ijporl.2020.109921. [DOI] [PubMed] [Google Scholar]
- 41.La Mantia I, Varricchio A, Di Girolamo S, Minni A, Passali GC, Ciprandi G. 2019. The role of bacteriotherapy in the prevention of adenoidectomy. Eur Rev Med Pharmacol Sci 23:44–47. [DOI] [PubMed] [Google Scholar]
- 42.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. 2015. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprunt K, Leidy G. 1988. The use of bacterial interference to prevent infection. Can J Microbiol 34:332–338. doi: 10.1139/m88-061. [DOI] [PubMed] [Google Scholar]
- 44.Roos K, Holm SE, Grahn-Håkansson E, Lagergren L. 1996. Recolonization with selected α-streptococci for prophylaxis of recurrent streptococcal pharyngotonsillitis—a randomized placebo-controlled multicentre study. Scand J Infect Dis 28:459–462. doi: 10.3109/00365549609037940. [DOI] [PubMed] [Google Scholar]
- 45.Falck G, Grahn-Håkansson E, Holm SE, Roos K, Lagergren L. 1999. Tolerance and efficacy of interfering alpha-streptococci in recurrence of streptococcal pharyngotonsillitis: a placebo-controlled study. Acta Otolaryngol (Stockh) 119:944–948. [DOI] [PubMed] [Google Scholar]
- 46.De Boeck I, Wittouck S, Martens K, Claes J, Jorissen M, Steelant B, van den Broek MFL, Seys SF, Hellings PW, Vanderveken OM, Lebeer S. 2019. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere 4:e00532-19. doi: 10.1128/mSphere.00532-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jong A, van Hijum SAFT, Bijlsma JJE, Kok J, Kuipers OP. 2006. BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res 34:W273–W279. doi: 10.1093/nar/gkl237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campelo AB, Roces C, Mohedano ML, López P, Rodríguez A, Martínez B. 2014. A bacteriocin gene cluster able to enhance plasmid maintenance in Lactococcus lactis. Microb Cell Fact 13:77. doi: 10.1186/1475-2859-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng M, Austin F, Shin R, Smith L. 2017. Covalent structure and bioactivity of the type aii lantibiotic salivaricin A2. Appl Environ Microbiol 84:e02528-17. doi: 10.1128/AEM.02528-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbour A, Philip K, Muniandy S. 2013. Enhanced production, purification, characterization and mechanism of action of salivaricin 9 lantibiotic produced by Streptococcus salivarius NU10. PLoS One 8:e77751. doi: 10.1371/journal.pone.0077751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hynes WL, Friend VL, Ferretti JJ. 1994. Duplication of the lantibiotic structural gene in M-type 49 group A Streptococcus strains producing streptococcin A-M49. Appl Environ Microbiol 60:4207–4209. doi: 10.1128/AEM.60.11.4207-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papadelli M, Karsioti A, Anastasiou R, Georgalaki M, Tsakalidou E. 2007. Characterization of the gene cluster involved in the biosynthesis of macedocin, the lantibiotic produced by Streptococcus macedonicus. FEMS Microbiol Lett 272:75–82. doi: 10.1111/j.1574-6968.2007.00740.x. [DOI] [PubMed] [Google Scholar]
- 54.Metelev M, Tietz JI, Melby JO, Blair PM, Zhu L, Livnat I, Severinov K, Mitchell DA. 2015. Structure, bioactivity, and resistance mechanism of streptomonomicin, an unusual lasso peptide from an understudied halophilic actinomycete. Chem Biol 22:241–250. doi: 10.1016/j.chembiol.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos MDL, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López-Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R, Galobart J, EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J 16:e05206. doi: 10.2903/j.efsa.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stadler C, Teuber M. 2002. The macrolide efflux genetic assembly of Streptococcus pneumoniae is present in erythromycin-resistant Streptococcus salivarius. Antimicrob Agents Chemother 46:3690–3691. doi: 10.1128/aac.46.11.3690-3691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaffanel F, Charron-Bourgoin F, Libante V, Leblond-Bourget N, Payot S. 2015. Resistance genes and genetic elements associated with antibiotic resistance in clinical and commensal isolates of Streptococcus salivarius. Appl Environ Microbiol 81:4155–4163. doi: 10.1128/AEM.00415-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varaldo PE, Montanari MP, Giovanetti E. 2009. Genetic elements responsible for erythromycin resistance in streptococci. Antimicrob Agents Chemother 53:343–353. doi: 10.1128/AAC.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashbaugh CD, Alberti S, Wessels MR. 1998. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol 180:4955–4959. doi: 10.1128/JB.180.18.4955-4959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelstrup J. 1981. Extracellular polysaccharides of smooth and rough variants of Streptococcus salivarius. Scand J Dent Res 89:374–383. doi: 10.1111/j.1600-0722.1981.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 62.Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. 2018. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol 49:207–216. doi: 10.1016/j.copbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Spacova I, O’Neill C, Lebeer S. 2020. Lacticaseibacillus rhamnosus GG inhibits infection of human keratinocytes by Staphylococcus aureus through mechanisms involving cell surface molecules and pH reduction. Benef Microbes 11:703–715. doi: 10.3920/BM2020.0075. [DOI] [PubMed] [Google Scholar]
- 64.De Boeck I, van den Broek MFL, Allonsius CN, Spacova I, Wittouck S, Martens K, Wuyts S, Cauwenberghs E, Jokicevic K, Vandenheuvel D, Eilers T, Lemarcq M, De Rudder C, Thys S, Timmermans J-P, Vroegop AV, Verplaetse A, Van de Wiele T, Kiekens F, Hellings PW, Vanderveken OM, Lebeer S. 2020. Lactobacilli have a niche in the human nose. Cell Rep 31:107674. doi: 10.1016/j.celrep.2020.107674. [DOI] [PubMed] [Google Scholar]
- 65.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taboada B, Estrada K, Ciria R, Merino E. 2018. Operon-mapper: a web server for precise operon identification in bacterial and archaeal genomes. Bioinformatics 34:4118–4120. doi: 10.1093/bioinformatics/bty496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leon‐Kempis MDR, Guccione E, Mulholland F, Williamson MP, Kelly DJ. 2006. The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol Microbiol 60:1262–1275. doi: 10.1111/j.1365-2958.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- 68.Chaffanel F, Charron-Bourgoin F, Soligot C, Kebouchi M, Bertin S, Payot S, Le Roux Y, Leblond-Bourget N. 2018. Surface proteins involved in the adhesion of Streptococcus salivarius to human intestinal epithelial cells. Appl Microbiol Biotechnol 102:2851–2865. doi: 10.1007/s00253-018-8794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Couvigny B, Lapaque N, Rigottier-Gois L, Guillot A, Chat S, Meylheuc T, Kulakauskas S, Rohde M, Mistou M-Y, Renault P, Doré J, Briandet R, Serror P, Guédon E. 2017. Three glycosylated serine-rich repeat proteins play a pivotal role in adhesion and colonization of the pioneer commensal bacterium, Streptococcus salivarius: serine-rich repeat glycoproteins of S. salivarius. Environ Microbiol 19:3579–3594. doi: 10.1111/1462-2920.13853. [DOI] [PubMed] [Google Scholar]
- 70.Lappan R, Imbrogno K, Sikazwe C, Anderson D, Mok D, Coates H, Vijayasekaran S, Bumbak P, Blyth CC, Jamieson SE, Peacock CS. 2018. A microbiome case-control study of recurrent acute otitis media identified potentially protective bacterial genera. BMC Microbiol 18. doi: 10.1186/s12866-018-1154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jervis-Bardy J, Leong LEX, Papanicolas LE, Ivey KL, Chawla S, Woods CM, Frauenfelder C, Ooi EH, Rogers GB. 2019. Examining the evidence for an adult healthy middle ear microbiome. mSphere 4:e00456-19. doi: 10.1128/mSphere.00456-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nemec A, Radolfová-Křížová L, Maixnerová M, Nemec M, Clermont D, Bzdil J, Ježek P, Španělová P. 2019. Revising the taxonomy of the Acinetobacter lwoffii group: the description of Acinetobacter pseudolwoffii sp. nov. and emended description of Acinetobacter lwoffii. Syst Appl Microbiol 42:159–167. doi: 10.1016/j.syapm.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, von Mutius E, Bufe A, Gatermann S, Renz H, Holst O, Heine H. 2007. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol 119:1514–1521. doi: 10.1016/j.jaci.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 74.Cheng X, Sheng H, Ma R, Gao Z, Han Z, Chi F, Cong N, Wang J, Liu X, Luo X, Yu J, Ra Y. 2017. Allergic rhinitis and allergy are risk factors for otitis media with effusion: a meta-analysis. Allergol Immunopathol (Madr) 45:25–32. doi: 10.1016/j.aller.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Tos M, Caye-Thomasen P. 2002. Mucous glands in the middle ear—what is known and what is not. ORL J Otorhinolaryngol Relat Spec 64:86–94. doi: 10.1159/000057786. [DOI] [PubMed] [Google Scholar]
- 76.Delorme C. 2008. Safety assessment of dairy microorganisms: Streptococcus thermophilus. Int J Food Microbiol 126:274–277. doi: 10.1016/j.ijfoodmicro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Delorme C, Poyart C, Ehrlich SD, Renault P. 2007. Extent of horizontal gene transfer in evolution of streptococci of the salivarius group. J Bacteriol 189:1330–1341. doi: 10.1128/JB.01058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker RE, Walker CG, Camargo CA, Bartley J, Flint D, Thompson JMD, Mitchell EA. 2019. Nasal microbial composition and chronic otitis media with effusion: a case-control study. PLoS One 14:e0212473. doi: 10.1371/journal.pone.0212473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zupancic K, Kriksic V, Kovacevic I, Kovacevic D. 2017. Influence of oral probiotic Streptococcus salivarius K12 on ear and oral cavity health in humans: systematic review. Probiotics Antimicrob Proteins 9:102–110. doi: 10.1007/s12602-017-9261-2. [DOI] [PubMed] [Google Scholar]
- 81.Chanos P, Mygind T. 2016. Co-culture-inducible bacteriocin production in lactic acid bacteria. Appl Microbiol Biotechnol 100:4297–4308. doi: 10.1007/s00253-016-7486-8. [DOI] [PubMed] [Google Scholar]
- 82.Fontaine L, Boutry C, de Frahan MH, Delplace B, Fremaux C, Horvath P, Boyaval P, Hols P. 2010. A Novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J Bacteriol 192:1444–1454. doi: 10.1128/JB.01251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mignolet J, Fontaine L, Sass A, Nannan C, Mahillon J, Coenye T, Hols P. 2018. Circuitry rewiring directly couples competence to predation in the gut dweller Streptococcus salivarius. Cell Rep 22:1627–1638. doi: 10.1016/j.celrep.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 84.Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, Malhotra-Kumar S, Theeten H, NPcarriage Study Group. 2019. Follow-up of serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine 37:1080–1086. doi: 10.1016/j.vaccine.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 85.Kassambara A. 2018. ggpubr: “ggplot2” based publication ready plots.
- 86.Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H, Chun J. 2017. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara R, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2019. vegan: community ecology package.
- 89.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, NY. [Google Scholar]
- 90.Turner S, Pryer KM, Miao VPW, Palmer JD. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 91.Alimolaei M, Golchin M. 2017. A comparison of methods for extracting plasmids from a difficult to lyse bacterium: Lactobacillus casei. Biologicals 45:47–51. doi: 10.1016/j.biologicals.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 94.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Zheng D, Liu B, Yang J, Jin Q. 2016. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res 44:D694–D697. doi: 10.1093/nar/gkv1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van den Broek MFL, De Boeck I, Claes IJJ, Nizet V, Lebeer S. 2018. Multifactorial inhibition of lactobacilli against the respiratory tract pathogen Moraxella catarrhalis. Benef Microbes 9:429–439. doi: 10.3920/BM2017.0101. [DOI] [PubMed] [Google Scholar]
- 98.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 99.Lebeer S, Claes I, Tytgat HLP, Verhoeven TLA, Marien E, von Ossowski I, Reunanen J, Palva A, de Vos WM, De Keersmaecker SCJ, Vanderleyden J. 2012. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78:185–193. doi: 10.1128/AEM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santagati M, Scillato M, Stefani S. 2018. Genetic organization of Streptococcus salivarius 24SMBc blp-like bacteriocin locus. Front Biosci (Schol Ed) 10:238–247. doi: 10.2741/s512. [DOI] [PubMed] [Google Scholar]
- 101.Dawid S, Roche AM, Weiser JN. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun 75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of patients enrolled and numbers of samples which could have been collected, were collected, and passed quality control (QC) after sequencing. CI, cochlear implant recipients; DC, children attending day care. Download Table S1, DOCX file, 0.02 MB (24.6KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxa detected in negative extraction and PCR controls and action taken. Download Table S2, PDF file, 0.2 MB (223.2KB, pdf) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Prevalence and mean relative abundance of nasopharynx amplicon sequence variants (ASVs) shared between otitis media with effusion (OME) patients and healthy controls. The healthy controls were considered cumulatively (HNPAll) and individually (HNPCI, cochlear implant recipients; HNPDC, children attending day care). To calculate the mean relative abundance, only samples in which the taxon was present was considered. Download Table S3, PDF file, 0.3 MB (271.7KB, pdf) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Compositional differential abundance of nasopharynx amplicon sequence variants (ASVs) between chronic otitis media with effusion (OME) patients and healthy controls. Each tile shows an estimate of the differential abundance of an ASV on the y axis relative to an ASV on the x axis. A plus/minus sign means that the ASV is significantly more/less abundant in healthy controls compared with OME patients, with a false discovery rate of 10% (capped with the method of Benjamini and Yekutieli). The data set was rarefied to the size of the smallest sample (2,820 reads) before performing a Wilcoxon rank-sum test, and only taxa that were differentially abundant in comparison to at least one other taxon are shown. Download FIG S1, TIF file, 0.5 MB (497.6KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overview of bacterial isolates obtained from the upper respiratory tract of healthy children. (A) Distribution of isolated species by anatomic location, colored by genus. (B) Number of strains per species isolated from each patient. (C) Distribution of genera by anatomic location. Download FIG S2, TIF file, 0.8 MB (795.1KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Antibiotic resistance and virulence factor genes. Download Table S4, DOCX file, 0.02 MB (23KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alignment of a genomic region with 6 genes associated with adhesion in S. salivarius JIM8777 (each highlighted with a square) using the PATRIC Compare Region Viewer tool. Homologues are indicated by the same color, with the exception of the AMBR055 region (marked with an asterisk (*). The original Compare Region Viewer alignment stopped after the homologue of SalivA_1458, as the homologue of Saliva_1457 is missing. A new alignment was started using the SalivA_1456, of which the homologue is present in AMBR055 (here indicated in red) caused a fresh assignment of colors. SALIVA_1456 to SALIVA_1458 = accessory Sec-dependent serine-rich glycoprotein adhesin genes srpC, srpB and srpA. SALIVA_1472 and SALIVA_1475 = YSIRK signal domain/LPXTG anchor domain surface protein. SALIVA_1473 = zinc metalloprotease ZmpB precursor. Download FIG S3, TIF file, 0.9 MB (959.3KB, tif) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental Materials and Methods. Download Text S1, DOCX file, 0.06 MB (64.3KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cultivation conditions. Download Table S5, DOCX file, 0.02 MB (21.6KB, docx) .
Copyright © 2021 Jörissen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The 16S rRNA gene sequencing data generated for this study were deposited in the European Nucleotide Archive under accession number PRJEB33591. The whole-genome sequence data have been added to project PRJEB32716 under assembly numbers GCA_905071825 (isolate AMBR024), GCA_905071875 (AMBR037), GCA_905071915 (AMBR047), GCA_905071845 (AMBR055), GCA_905071895 (AMBR074), GCA_905071855 (AMBR075), and GCA_905071905 (AMBR158).