Abstract
Background
Dopamine D2 receptor agonists, bromocriptine and cabergoline, are notable medications in the treatment of Parkinsonism, hyperprolactinemia, and hyperglycemia. An affiliation was found between the initiation of myocardial injury ailment and long term treatment with dopamine D2 agonist drugs identified with the partial activation of 5-hydroxytryptamine receptor 2 A (5-HT2A). The investigation aimed to examine the activity of sarpogrelate (a 5-HT2A receptor blocker) in reducing myocardial injury prompted by extended haul utilisation of D2 receptor agonists in rats with alloxan-induced diabetes.
Methods
Both bromocriptine and cabergoline were managed independently and combined with sarpogrelate for about a month in diabetic nephropathy rats. Both tail-cuff blood pressure and the BGL were recorded weekly. For all animals, the kidney hypertrophy index, serum creatinine, blood urea nitrogen, alanine transaminase, and aspartate transaminase levels were measured after one month of treatment. The severity of the cardiac injury was assessed by the estimation of lactate dehydrogenase-1 (LDH-1), cardiac troponin I, and tumor necrosis factor alpha 1 (TNF1). The triphenyltetrazolium chloride (TTC) staining method was used to determine the experimental myocardial infarction (MI) size.
Results
Bromocriptine and cabergoline created a significant reduction in BGL, BP, and kidney hypertrophy index in diabetic nephropathy rats. Administration of bromocriptine and cabergoline, alone, or in combination with sarpogrelate fundamentally diminished the blood concentrations of alkaline phosphatase (ALP), Aspartate aminotransferase (AST), urea, and creatinine. Bromocriptine and cabergoline alone showed a noteworthy increase in the LDH-1, Troponin I, and TNF1 levels in the serum (p < 0.05). Paradoxically, utilising bromocriptine or cabergoline with sarpogrelate treatment altogether decreased the levels of the myocardial biomarkers in the serum. A mix of bromocriptine or cabergoline with sarpogrelate diminished the level of the myocardial infarct size in the heart assessed through the TTC staining method.
Conclusions
The examination demonstrated that the combined use of sarpogrelate with bromocriptine or cabergoline decreased the potential adverse effects of these two drugs on the myocardial tissues.
Keywords: Cardiovascular, Diabetic nephropathy, Myocardial injury, TNFα1, Dopamine receptors
Background
Among the various diabetes progress complications, diabetic nephropathy is the foremost well-known renal concern and the ultimate source of end-stage renal disease. The later phases of diabetic nephropathy define glomerulosclerosis. It is represented by transforming growth factor-ß1 overexpression, extracellular matrix deposition, and glomerular structure loss [1]. Hyperglycemia is the primary factor for diabetic nephropathy progression [2]. Several in vitro studies of streptozotocin-induced diabetic nephropathy demonstrated that renal damage is claimed to occur due to the excessive production of reactive oxygen species (ROS) under hyperglycemic conditions [3–5]. Bromocriptine is a sympatholytic dopamine D2 agonist that has been approved for treating type 2 diabetes without the risk of hypoglycemia because there is no insulin secretion. Supported previous studies stated bromocriptine administration is believed to strengthen low hypothalamic dopamine levels and inhibit excessive sympathetic tone within the central system, leading to a reduction in the post-meal plasma glucose levels because of the enhanced suppression of hepatic glucose production [6, 7]. Agonists working on dopamine D2 receptors can lower hypertension signs by the inhibition of sodium–potassium adenosine triphosphatase (Na/k ATPase) activity, vasodilation, and sympathetic nerve activity inhibition. Bromocriptine may protect against the ischemia-reperfusion (I/R) injury of the kidney utilising p44/42 mitogen-initiated protein kinase actuation [8] and prevent chronic nephropathy [9].
Conversely, the bromocriptine-actuated hypotension was wholly nullified by pretreatment with metoclopramide, a dopamine D2 receptor antagonist that crosses the blood brain barrier [10, 11]. However, the use of D2 agonists is often related to cardiovascular complications, including hypotension and myocardial disorder. There is an identified relationship between the utilisation of D2-like R agonists in patients with Parkinson’s disease and cardiopathy, especially in the early phase of therapy [12]. D2 receptors are pleiotropic receptors that have multiple effects, in that, activating them inhibit adenylyl cyclase, resulting in the inhibition of voltage-gated calcium ions and activation of potassium conductance [13]. Early studies suggested that the use of D2 receptor agonists results in a reduction in pituitary tumour mass [14].
It was demonstrated that long term use of bromocriptine therapy was associated with an increased risk of developing heart disease, which occurred in a cumulative dose-dependent treatment [15]. Dopamine D2 receptor agonists are classified as ergot dopamine D2 agonists and non-ergot D2 agonists. Bromocriptine and cabergoline (ergot derivative) are related to valvularcardiopathy since these two drugs have agonistic activities against both dopamine D2 and serotonin 5-HT2A receptors. Pramipexole (non-ergot derivative) shows few incidences of the onset of heart disease since it does not affect 5-HT2B receptors [16–18]. Both sarpogrelate and ketanserin (selective 5-HT2A/2B antagonists) reduce cardiac fracture, infarct size, and changes within the electrocardiogram resulting from myocardial injury. Likewise, these outcomes bolster the view that serotonin and 5-HT2A may augment the harmful impacts of ischemic injury within the heart [19, 20]. Sarpogrelate was found to possess beneficial impacts in peripheral vascular sickness, restenosis after coronary stenting, pneumonic hypertension, intense and relentless myocardial localised necrosis [21]. The current study aimed to identify the possible protective effect of sarpogrelate, a 5-HT2A receptor blocker, associated with the cumulative use of D2 receptor agonist drug therapy on cardiac and renal functions in a rat model of diabetic nephropathy.
Methods
Materials
Sarpogrelate was obtained from Shanghai Linebon Ltd., Shanghai, China. Bromocriptine was sourced from Novartis, Italy. Cabergoline was purchased from Pfizer, Italy. Domperidone was purchased from Jamjoom Pharmaceuticals Co., KSA. Alloxan was obtained from Sigma-Aldrich (St Louis, MO, USA). Urea, creatinine, alkaline phosphatase, aspartate aminotransferase activity, LDH-1 assay kits, and the TNF alpha ELISA kit were obtained from Abcam, USA. The troponin I test kit was obtained from Encode Medical Engineering Company, Jeddah, KSA. 2,3,5-Triphenyltetrazolium chloride was obtained from Gold Biotechnology, USA.
Animals
Forty-two Wistar albino male rats 5–7 weeks of age weighing 150–200 g were obtained from the animal house of Batterjee Medical College, Jeddah, KSA. Three rats were housed per cage under controlled standard laboratory conditions in monitored ventilated cages and spontaneously given food and water. The ethical committee of research at Batterjee Medical College approved the steps of the investigation. Tutelage was taken, particularly with relevant housing conditions, to avoid or minimise the animals’ discomfort. The animals were kept on solid floored cages with a deep layer of sawdust, and the cages were changed daily. Data were coded before analysis so that the treatment group could not be identified before the analysis was completed. At the end of the study, all animals were euthanised by thiopental (intravenous injection, 150 mg/kg) for tissue collection.
Induction of diabetes by alloxan
Alloxan monohydrate was dissolved in sterile normal saline. Diabetes was induced in 30 rats (150–200 g) by a single intraperitoneal injection of alloxan (5 %) 150 mg/kg b.w. The rats were kept in a fasted state for 12 h before being injected with alloxan. The fasting plasma glucose was measured in blood samples collected from the tail vein. The rats that showed a plasma glucose level of 200 mg/dl or more were considered diabetic and taken in the study [22].
Experimental design
Tests took place four weeks after the induction of diabetes. The diabetic rats were divided randomly into six diabetic groups and one healthy control group, each group comprising six rats. The samples were calculated based on the resource equation method [23]. Once there was a stable rise in the urea and creatinine levels in the blood, drugs were injected once daily for one month.
The normal control group (Saline, IP).
Diabetic control group (Saline, IP).
Diabetic group treated with bromocriptine (4 mg/kg b.w, IP) [24].
Diabetic group treated with cabergoline (0.6 mg/kg b.w ,IP) [25].
Diabetic group treated with sarpogrelate (50 mg/kg b.w, IP) [26].
Diabetic group treated with a combination of bromocriptine and sarpogrelate at the same doses.
Diabetic group treated with a combination of cabergoline and sarpogrelate at the same doses.
Dopamine D2 agonists’ bromocriptine and cabergolin were used to induce myocardial infarction in the rats. The drugs were administered daily doses at 4 mg/kg and 0.6 mg/kg IP respectively in two groups of rats for one month. 50 mg/kg IP of sarpogrelate in combination with bromocriptine and cabergoline respectively was administered daily in two groups of rats for one month for testing its potential protective effect on the heart.
Determination of blood glucose level
Blood glucose levels were tested on the7th, 14th, 21th, and 28th days from the beginning of the treatment. One millimetre of tail end was cut and a drop of blood was used for blood glucose tests using an advanced glucometer (Roche, USA). The accuracy of the glucometer was checked with the O-toluidine method [27].
Blood pressure recording
Basal blood pressure was measured by employing the tail-cuff non-invasive blood pressure recording apparatus (Ugobasile instruments, Italy). The mean systolic blood pressure was measured for each group of rats once time weekly for one month.
Each rat was placed in an exceeding restrainer, and an appropriate cuff sensor was mounted on its tail and warmed to about 33–35 °C. The tail cuff was inflated to a pressure above 200 mmHg. Systolic blood pressure and diastolic blood pressure was measured directly by the tail-cuff and pulse sensor two hours after treatment with drugs [28].
Estimation of liver and kidney functions
At the end of the study, a blood sample was withdrawn through the retro-orbital venous plexus method, kept at 37 °C for 30 min, and centrifuged at 4 °C, 3000 rpm for 10 min. Then, the separated serum was stored at -20 °C for various biochemical analyses. Alkaline phosphatase and aspartate aminotransferase activities were determined using the method described by King and King [29]. The procedure of Tietz et al. [30] was used to determine the serum creatinine concentration, while the serum urea concentration was determined by the method given by Kaplan [31]. At the end of the study, all the rats were euthanized. The kidney samples were collected, washed with normal saline, and weighed. The kidney hypertrophy index was calculated by determining the kidney weight and body weight ratio (g/g) x103.
Estimation of serum biomarkers of myocardial injury
The severity of the cardiac injury was assessed by estimating the lactate dehydrogenase (LDH-1) and cardiac troponin I (cTnI) levels in serum. The LDH-1 and cTnI levels were analysed by spectrophotometric methods using commercially available diagnostic kits, consistent with the methods of Nieland [32].
Serum TNF-αconcentration
The serum TNF-alpha cytokines level was identified by the ELISA technique using a quantitative sandwich enzyme immunoassay technique (Abcam Company). The test was done according to the company’s instructions. The ELISA reader’s optical density at 405 nm was immediately calculated and applied on a standard curve to sort out the cytokine concentration.
Evaluation of myocardial infarct size by TTC
For assessment of the myocardial infarcted area, the hearts were removed, washed in phosphate-buffered saline, frozen, and stored at -20 °C. The frozen hearts were sliced into 1 mm sections along the long axis, from apex to base. Triphenyltetrazolium chloride (TTC) staining was used to assess the myocardial tissue viability and determine the myocardial infarction size. The tissue slices were incubated in 1 % TTC phosphate-buffered saline solution, pH 7.4, at 37 °C for 20 min. Tissues were fixed in 10 % PBS-buffered formalin overnight at 2–8 °C. Each side of every TTC-stained tissue slice was photographed with the photographic Optika camera (Digi, Italy) to differentiate the red-stained viable and white-unstained necrotic tissues [33]. Digital photographs were downloaded to a computer. Areas stained in white and red were measured using the SigmaScan software (SPSS Science) in trace-measurement mode. That mode was used to measure either the ischemic or infarcted areas, which may be a sum of calibrated pixels during a defined region. This was done manually by drawing an image layer on the photograph [34]. The infarction size percentage was calculated using the following equation:
Statistical analysis
The data and statistical analysis done complied with the recommendations for experimental design and analysis in pharmacology [35]. The results were expressed as mean ± SE. The significance of the differences between the values was performed by a one-way ANOVA test and Tukey Kramer’s Multiple Comparison Test using the GraphPad Prism 9 software. P < 0.05 was considered to be a significant difference.
Results
Estimation of blood glucose levels
The diabetic control group showed a significant increase in the BGL compared to the healthy control group. On repeated administration of the bromocriptine (4 mg/kg) and cabergoline (0.6 mg/kg) individually or in combination with sarbogrelate, a significant (p < 0.05) decrease was observed in blood glucose by time, compared to the diabetic control group throughout the four weeks of treatment (Table 1).
Table 1.
Effect of the tested drugs on BGL in alloxan-induced diabetic rats
| BGL mg/dL | ||||
|---|---|---|---|---|
| Groups | Week 1 | Week 2 | Week 3 | Week 4 |
| Normal control group | 136.5 ± 18.52 | 121.33 ± 12.08 | 119.25 ± 11.35 | 109 ± 10.92 |
| Diabetic control group | 359.75 ± 45.13* | 328 ± 141.34* | 428.5 ± 121.17* | 396.5 ± 80.60* |
| Diabetic group treated with bromocriptine | 191 ± 16.89# | 199.25 ± 65.05# | 238.25 ± 56.88# | 284.25 ± 148.00# |
| Diabetic group treated with Cabergoline | 213.75 ± 37.74# | 211.75 ± 38.62# | 293 ± 213.15# | 280.25 ± 220.48# |
| Diabetic group treated with sarpogrelate | 326.25 ± 93.45 | 298.25 ± 122.37 | 393.75 ± 118.77 | 369.75 ± 43.15 |
| Diabetic group treated with bromocriptine + sarpogrelate | 247.75 ± 50.35# | 215.5 ± 33.20# | 310.25 ± 70.59# | 288.25 ± 109.41# |
| Diabetic group treated with cabergoline + sarpogrelate | 196 ± 54.20# | 158.75 ± 35.01# | 235.5 ± 182.22# | 215.75 ± 141.46# |
Values shown are means ± SEM; n = 6 rats per group. * P < 0.05, significantly different from the normal control group; # P < 0.05, significantly different from the diabetic control group
Kidney hypertrophy index
The results showed that the kidney hypertrophy index significantly increased in the diabetic group of rats compared with the normal control rats. However, the index was markedly reduced by both bromocriptine and cabergoline treatments, individually or mixed with sarpogrelate (as seen in Table 2). There was no effect of using sarpogrelate on the diabetic kidney index.
Table 2.
Effect of the tested drugs on Kidney Hypertrophic Index in alloxan-induced diabetic rats
| Kidney Hypertrophic Index | |
|---|---|
| Groups | KHI ( g/g * 1000 ) |
| Normal control group | 4.555 ± 0.423 |
| Diabetic control group | 6.6125 ± 0.57* |
| Diabetic group treated with bromocriptine | 4.7475 ± 0.33# |
| Diabetic group treated with cabergoline | 4.81 ± 0.49# |
| Diabetic group treated with sarpogrelate | 5.90 ± 0.27 |
| Diabetic group treated with bromocriptine + sarpogrelate | 4.715 ± 0.61# |
| Diabetic group treated with cabergoline + sarpogrelate | 4.46 ± 0.48# |
Values shown are means ± SEM; n = 6 rats per group. * P < 0.05, significantly different from the normal control group; # P < 0.05, significantly different from the diabetic control group
Hemodynamic parameter (Antihypertensive activity)
Alloxan-induced diabetes in rats showed a significant rise in blood pressure after three weeks from the induction of diabetes. Daily oral administration of bromocriptine and cabergoline, individually or in combination with sarpogrelate showed a significant decrease in the systolic blood pressure in the third and fourth weeks of treatment (Table 3). There was no effect of sarpogrelate on the BP in diabetic rats.
Table 3.
Effect of the tested drugs on mean blood pressure in alloxan-induced diabetic rats
| Mean Blood Pressure MBP (mmHg) | ||||
|---|---|---|---|---|
| Groups | Week 1 | Week 2 | Week 3 | Week 4 |
| Normal control group | 110 ± 4.01 | 118.25 ± 8.99 | 108.75 ± 4.57 | 111.5 ± 4.20 |
| Diabetic control group | 112.5 ± 9.14 | 121.75 ± 11.35 | 150.75 ± 5.25* | 151.25 ± 5.12* |
| Diabetic group treated with bromocriptine | 115 ± 9.12 | 102.75 ± 6.8 | 109 ± 10.23# | 121.75 ± 15.26# |
| Diabetic group treated with cabergoline | 103.75 ± 7.0 | 105.5 ± 9.14 | 123 ± 7.87# | 107.75 ± 107.75# |
| Diabetic group treated with sarpogrelate | 118.75 ± 10.87 | 114.75 ± 8.53 | 137.75 ± 8.53 | 139 ± 18.56 |
| Diabetic group treated with bromocriptine + sarpogrelate | 104.5 ± 7.59 | 104.75 ± 4.99 | 106.25 ± 11.08# | 118 ± 13.03# |
| Diabetic group treated with cabergoline + sarpogrelate | 109.25 ± 11.58 | 105 ± 9.05 | 106 ± 18.3# | 104.25 ± 5.5# |
Values shown are means ± SEM; n = 6 rats per group. * P < 0.05, significantly different from the normal control group; # P < 0.05, significantly different from the diabetic control group
Estimation of liver and kidney functions
The serum levels of AST, ALP, urea, and creatinine were recorded as the indicators of liver and kidney functions, as presented in Table 4. Data revealed that the diabetic control group had a significant increase in the previous biomarkers’ serum concentrations compared to the healthy control rats. The daily administration of bromocriptine and cabergoline, both alone or in combination with sarbogrelate, significantly decreased the serum concentrations of the previous biomarkers compared to the positive control of diabetic rats. There was no marked effect of sarpogrelate treatment on the previous biochemical indicators in the diabetic rats.
Table 4.
Effect of the tested drugs on the liver and kidney functions in alloxan-induced diabetic rats after 4 weeks
| Biochemicals | ||||
|---|---|---|---|---|
| Group | ALP(IU/L) | AST(IU/L) | Urea (mmol/L) | Creatinine (mmol/L) |
| Normal control group | 60.85 ± 8.77 | 25.04 ± 4.96 | 43.38 ± 4.77 | 0.46 ± 0.07 |
| Diabetic control group | 89.05 ± 10.44* | 45.86 ± 7.57* | 75.81 ± 18.85* | 1.89 ± 0.19* |
| Diabetic group treated with Bromocriptine | 68.83 ± 7.27# | 34.18 ± 5.78# | 60.41 ± 6.22# | 1.04 ± 0.26# |
| Diabetic group treated with Cabergoline | 73.27 ± 7.93# | 21.37 ± 10.05# | 52.09 ± 2.23# | 0.79 ± 0.11# |
| Diabetic group treated with Sarpogrelate | 82.16 ± 23.83 | 41.47 ± 10.02 | 69.41 ± 7.65 | 1.68 ± 0.25 |
| Diabetic group treated with Bromocriptine + sarpogrelate | 64.8 ± 7.21# | 31.03 ± 6.44# | 44.4 ± 8.23# | 1.26 ± 0.25# |
| Diabetic group treated with cabergoline + sarpogrelate | 73.44 ± 6.68# | 33.79 ± 11.18# | 51.16 ± 10.43# | 1.33 ± 0.21# |
Values shown are means ± SEM; n = 6 rats per group. * P < 0.05, significantly different from the normal control group; # P < 0.05, significantly different from the diabetic control group
Myocardial biomarkers
Animals treated with bromocriptine and cabergoline for one month in doses 10 mg/kg and 0.6 mg/kg, respectively, displayed significant elevation of the LDH-1 activity in the serum. In contrast, it was observed that using a combination of bromocriptine and cabergoline with sarpogrelate treatment significantly decreased the level of the biomarkers in the serum. The results in Table 5 indicate that the quantitative test of the troponin I reagent kit showed only positive results with the bromocriptine and cabergoline treated groups. The results of the ELISA test suggest that the expression levels of TNF-alpha 1 in the diabetic rat groups treated with bromocriptine or cabergoline individually were significantly higher compared to the diabetic control rats. The groups administered a combination of bromocriptine and cabergoline with sarbogrelate showed lower TNF-alpha 1 expression levels than the groups treated with bromocriptine or cabergoline individually (Table 5).
Table 5.
Effect of the tested drugs on the Lactate dehydrogenase-1, Troponin I, and TNFα1 in alloxan-induced diabetic rats
| Myocardial biomarkers | |||
|---|---|---|---|
| Groups | LDH-1 (IU/L) | Troponin I (pg/ml) | TNFα1 (pg/mL) |
| Normal control group | 17.42 ± 0.69 | 92.12 ± 9.19 | 06.12 ± 0.45 |
| Diabetic control group | 18.50 ± 1.53 | 95.73 ± 8.23 | 08.32 ± 0.56 |
| Diabetic group treated with bromocriptine | 40.50 ± 10.69# | 313.43 ± 29.43# | 36.56 ± 2.32# |
| Diabetic group treated with cabergoline | 36.86 ± 12.47# | 143.23 ± 12.93# | 31.20 ± 4.23# |
| Diabetic group treated with carpogrelate | 19.55 ± 2.20 | 101.17 ± 8.45 | 09.74 ± 0.98 |
| Diabetic group treated with bromocriptine + sarpogrelate | 27.75 ± 3.91 a | 241.34 ± 17.83a | 14.84 ± 1.23 a |
| Diabetic group treated with cabergoline + sarpogrelate | 34.57 ± 6.60 b | 128.83 ± 13.53 | 08.59 ± 0.76 b |
Values shown are means ± SEM; n = 6 rats per group. # P < 0.05, significantly different from the diabetic control group. a P < 0.05, significantly different from the diabetic group treated with bromocriptine. b P < 0.05, significantly different from the diabetic group treated with cabergoline
Evaluation of myocardial injury
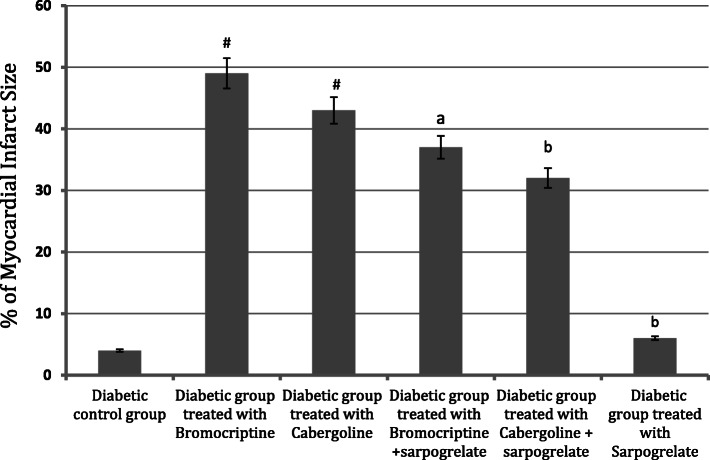
The myocardial infarction size was used as an indicator of the progress of the myocardial injury. As demonstrated in Fig. 1, TTC unstained areas correlated closely with areas of infarction, the infarction areas of the myocardial sections for the treated groups were compared to the diabetic control group. The hearts of rats treated with bromocriptine (4 mg/kg) and cabergoline (0.6 mg/kg) showed a significant increase in risk area infarction. In contrast, the combination of bromocriptine or cabergoline with sarpogrelate (50 mg/kg) reduced the myocardial infarct size (Fig. 1). The infarction sizes in bromocriptine and cabergoline treated groups 48.80±6.15%, and 42.21±5.21% resp. were larger than bromocriptine and cabergoline in combination with sarpogrelate treated groups 36.23±4.90%, and 31.22±3.46% resp. (Fig. 2).
Fig. 1.
Effect of the tested drugs on the hearts in alloxan-induced diabetic rats after four weeks of treatment. Triphenyl tetrazolium chloride stained cross-section of the heart of A Diabetic control rat showing uniform staining pattern. Both B bromocriptine treated group, and C cabergoline treated group showing a relative large pale, TTC-negative area of infarct. Both D Bromocriptine + sarbogrelate treated group, and E Cabergoline + sarbogrelate treated group showing a relative small pale, TTC-negative area of infarct
Fig. 2.
Effect of the tested drugs on the percentage of myocardial infarct size in alloxan-induced diabetic rats. Values shown are mean ± SEM; n = 6 rats per group. # P < 0.05, significantly different from the diabetic control group. (a) P < 0.05, significantly different from the diabetic group treated with bromocriptine. (b) P < 0.05, significantly different from the diabetic group treated with cabergoline
Discussion
Both bromocriptine and cabergoline induced hypoglycemic activity, which may have been due to enhanced suppressive hepatic glucose production [36]. The exact mechanism of their action as antidiabetic substances is not entirely identified. Bromocriptine decreases the hepatic production of glucose, increases glucose transporter production, or increases or mimics glucagon-like peptide-1 activity [37]. Its contribution to hypoglycaemia may be because it modulates the neurotransmitter action in the brain and has been shown to improve glucose tolerance and insulin resistance in animal models of obesity and diabetes [38]. Bromocriptine was approved by the Food and Drug Administration (FDA) in May 2009 for the treatment of type 2 diabetes.
In the present study, diabetic nephropathy was the most common cause of renal complication and the leading of hypertension. Both bromocriptine and cabergoline showed a marked antihypertensive activity; this result agrees with some previous reports [39, 40]. The antihypertensive activity is related to the activation of dopamine D2 receptors, which can lower the blood pressure by inhibition of Na/k ATPase activity, vasodilation, and inhibition of the sympathetic nerve activity. Both bromocriptine and cabergoline induced a marked improvement in kidney function by decreasing urea and creatinine serum levels. The pharmacological pathways that explain this effect have not been sufficiently determined. The result agrees with some of the previous studies, suggesting that the development of therapies directed to increase renal D2 receptor expression and function may provide novel and practical approaches to treating renal injury [41].
The noted severe adverse effect of bromocriptine and cabergoline on the heart was represented in myocardial injury and infarction, approved in the present study by using relative overdoses for a month of treatment. The action may be related to the agonistic properties of dopamine D2 and serotonin 5-HT2A receptors, increasing the heart pumping rate. It was reported that both bromocriptine and cabergoline (ergot derivative) have been associated with heart disease since the two drugs have both dopamine D2 and serotonin 5-HT2A receptors agonistic properties [17, 18]. It is hypothesised that the 5-HT2A receptor, which is a common contributing factor underlying aspects of vasoconstriction and cardiovascular disorders [42] and vasodilation through activating nitric oxide (NO) synthase (NOS) via serotonin 5-HT1B receptors in endothelial cells, possesses different effects on regulating vascular tone. These facts lead to the consideration that sarpogrelate, a 5-HT2A receptor blocker may increase coronary blood flow via either attenuation of vasoconstriction through the 5-HT2A receptor blockade or of vasodilation by the relative stimulation of NOS through 5-HT1B receptor [43].
According to the current observed data, the combination of bromocriptine and cabergoline with the new chemical agent sarpogrelate (selective 5-HT2A/2B antagonists) decreased the adverse effects of these two drugs on the heart. The protective effects of sarpogrelate on myocardial tissue were approved by its ability to decrease the secretion of myocardial biomarkers as shown, by decreasing LDH-1, Troponin I, and TNF alpha 1 during the treatment of bromocriptine and cabergoline. Sarpogrelate attenuates cardiac dysfunction and infarct size; these results support the view that serotonin and 5-HT2A might contribute to the harmful effects of ischemic injury in the heart. Regarding the biochemical study, sarpogrelate can be considered a safe drug for liver and kidney functions.
Conclusions
According to the given study, the dopamine D2 receptor agonists bromocriptine and cabergoline induced myocardial infarction. However, using sarpogrelate in combination with bromocriptine or cabergoline decreased their potential adverse effects on the myocardial tissues. Thus, we suggest sarpogrelate deserves additional experimental and clinical research related to cardiovascular diseases.
Acknowledgements
I would like to express my sincere gratitude to my advisor, Prof. Hekma Abdul Latif, for her continuous support of my research. I appreciate her patience, motivation, and immense knowledge. I would like to thank the rest of my thesis committee, Assistant Prof. May Galal and Assistant Prof. Mohamed El yammany, for their insightful comments. I also sincerely thank Dr Iklas Sindi, Batterjee medical college, Jeddah, KSA for providing me with the opportunity to work on this project in the college laboratories.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- TNF
Tumour necrosis factor
- TTC
Triphenyltetrazolium chloride
- LDH
Lactate dehydrogenase
- TTC
Triphenyltetrazolium chloride
- 5-HT
5-hydroxytryptamine
- ELISA
Enzyme-linked immunosorbent assay
Authors’ contributions
All authors conceived and planned the experiments. MF carried out the implementation of the experiments. RM planned and carried out the statistical analyses and contributed to sample preparation. MF took the lead in writing the manuscript. HA, MY, MG, SK and IS contributed to the interpretation of the results, provided critical feedback, and supervised and helped shape the research, analysis, and manuscript. The author(s) read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved in December 2019 by the Research Ethical Committee at Batterjee Medical College, Jeddah, KSA, under the reference number RES-2019-0020. Besides, the research experiments on animals followed the guidelines of the Experimental Animals’ Research and Ethics Committee (EAREC), King Abdul-Aziz University, Jeddah, KSA.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:250–3. doi: 10.1097/01.ASN.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 2.Debnam ES, Unwin RJ. Hyperglycemia and intestinal and renal glucose transport: implications for diabetic renal injury. Kidney Int. 1996;50:1101–9. doi: 10.1038/ki.1996.416. [DOI] [PubMed] [Google Scholar]
- 3.Fridlyand LE, Philipson LH. Oxidative reactive species in cell injury: mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci. 2005;1066:136–15. doi: 10.1196/annals.1363.019. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes SM, Cordeiro PM, Watanabe M, Fonseca CD, Vattimo MF. The role of oxidative stress in streptozotocin-induced diabetic nephropathy in rats. Arch Endocrinol Metab. 2016;60:443–9. doi: 10.1590/2359-3997000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariee AD, Abd-Allah GM, El-Yamany MF. Renal oxidative stress and nitric oxide production in streptozotocin-induced diabetic nephropathy in rats: the possible modulatory effects of garlic (Allium sativum L.) Biotechnol Appl Biochem. 2009;52:227–32. doi: 10.1042/BA20080086. [DOI] [PubMed] [Google Scholar]
- 6.Defronzo RA, Bromocriptine A sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes [published correction appears in Diabetes Care. 2011; 34:1442. Dosage error in article text] Diabetes Care. 2011;34:789–94. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shivaprasad C, Kalra S. Bromocriptine in type 2 diabetes mellitus. Indian J Endocrinol Metab. 2011;15:17–24. doi: 10.4103/2230-8210.83058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narkar V, Kunduzova O, Hussain T, Cambon C, Parini A, Lokhandwala M. Dopamine D2-like receptor agonist bromocriptine protects against ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;66:633–40. doi: 10.1111/j.1523-1755.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- 9.Mejía-Rodríguez O, Herrera-Abarca JE, Ceballos-Reyes G, Avila-Diaz M, Prado-Uribe C, Belio-Caro F, Salinas-González A, Vega-Gomez H, Alvarez-Aguilar C, Lindholm B, García-López E, Paniagua R. Cardiovascular and renal effects of bromocriptine in diabetic patients with stage 4 chronic kidney disease. BioMed Res Int. 2013;2013:104059. [DOI] [PMC free article] [PubMed]
- 10.Lahlou S, Duarte GP. Hypotensive action of bromocriptine in the DOCA-salt hypertensive rat: contribution of spinal dopamine receptors. Fundam Clin Pharmacol. 1998;12:599–606. doi: 10.1111/j.1472-8206.1998.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 11.Luchsinger A, Grilli M, Velasco M. Metoclopramide, and domperidone block the antihypertensive effect of bromocriptine in hypertensive patients. Am J Ther. 1998;5:81–8. doi: 10.1097/00045391-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mokhles M. Dopamine agonist and heart failure in patients with Parkinson’s disease: a nested case-control study on multiple health care databases. In: Proceedings of the European Society of Cardiology Congress, Stockholm, Sweden. 2010.
- 13.Bordet R. Central dopamine receptors: general considerations (Part 1). Rev Neurol (Paris). 2004;160:862–870. [Article in French]. [DOI] [PubMed]
- 14.Melmed S, Braunstein GD, Chang RJ, Becker DP. Pituitary tumors secreting growth hormone and prolactin. Ann Intern Med. 1986;105:238–53. doi: 10.7326/0003-4819-105-2-238. [DOI] [PubMed] [Google Scholar]
- 15.Louis CS, Tan KKC, Ng W-L, Au RKK, Lee Y-H, Chan, Nigel CK. Tan.Bromocriptine use and the risk of valvular heart disease. J Mov Disord. 2009;24:344–9. doi: 10.1002/mds.22228. [DOI] [PubMed] [Google Scholar]
- 16.Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL. Low dose cabergoline for hyperprolactinaemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol. 2008;159:R11–4. doi: 10.1530/EJE-08-0365. [DOI] [PubMed] [Google Scholar]
- 17.Kekewska A, Hübner H, Gmeiner P, Pertz HH. The bulky N6 substituent of cabergoline is responsible for agonism of this drug at 5-hydroxytryptamine 5-HT2A and 5-HT2B receptors and thus is a determinant of valvular heart disease. J Pharmacol Exp Ther. 2011;338:381–91. doi: 10.1124/jpet.111.181255. [DOI] [PubMed] [Google Scholar]
- 18.Oana F, Onozuka H, Tsuchioka A, et al. Function and expression differences between ergot and non-ergot dopamine D2 agonists on heart valve interstitial cells. J Heart Valve Dis. 2014;23:246–52. [PubMed] [Google Scholar]
- 19.Brasil D, Temsah RM, Kumar K, Kumamoto H, Takeda N, Dhalla NS. Blockade of 5-HT(2A) receptors by sarpogrelate protects the heart against myocardial infarction in rats. J Cardiovasc Pharmacol Ther. 2002;7:53–9. doi: 10.1177/107424840200700i108. [DOI] [PubMed] [Google Scholar]
- 20.Satomura K, Takase B, Hamabe A, Ashida K, Hosaka H, Ohsuzu F, Kurita A. Sarpogrelate, a specific 5HT2-receptor antagonist, improves the coronary microcirculation in coronary artery disease. Clin Cardiol. 2002;25:28–32. doi: 10.1002/clc.4950250108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini HK, Takeda N, Goyal RK, Kumamoto H, Arneja AS, Dhalla NS. Therapeutic potentials of sarpogrelate in cardiovascular disease. Cardiovasc Drug Rev. 2004;22:27–54. doi: 10.1111/j.1527-3466.2004.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanter M, Coskun O, Korkmaz A, Oter S. Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:685–91. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- 23.Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43:244–58. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro-de-Oliveira A, Jr, Guerra RM, Fóscolo RB, Marubayashi U, Reis AM, Coimbra CC. Effects of chronic bromocriptine (CB-154) treatment on the plasma glucose and insulin secretion response to neurocytoglucopenia in rats. J Endocrinol. 1999;162:237–42. doi: 10.1677/joe.0.1620237. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim N, Zidan RA, Karam R. Does green tea have an ameliorative effect against cabergoline-induced cardiotoxicity in adult male albino rats? A histological and biochemical study. Egy J Hist. 2012;35:13–22. [Google Scholar]
- 26.Kim DH, Choi BH, Ku SK, Park JH, Oh E, Kwak MK. Beneficial effects of sarpogrelate and rosuvastatin in high fat diet/streptozotocin-induced nephropathy in mice. PLoS One. 2016;11(4):e0153965. doi: 10.1371/journal.pone.0153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee KI. Medical laboratory technology. Tata McGraw Hill. 1988;3:991–3. [Google Scholar]
- 28.Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28:1064–9. doi: 10.1161/01.HYP.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 29.Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez J, Carl A, Burtis ER, Ashwood, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnosis (5th edition): Elsevier, St. Louis, USA, 2012, 2238 pp. 909 illustrations. ISBN: 978-1-4160-6164-9. Indian J Clin Biochem. 2013;28:104–105.
- 31.Kaplan A. Urea nitrogen and urinary ammonia. In: Meites S, editor. Standard method of clinical chemistry. New York: Academic Press Inc.; 1965. pp. 245–56. [Google Scholar]
- 32.Nielands JB. In Methods in Enzymology. Ed. by Colowick, S. P. & Kaplan, N. 0. New York: Academic Press Inc.; 1955. p. 449.
- 33.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyltetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593. doi: 10.1016/0002-8703(81)90226-X. [DOI] [PubMed] [Google Scholar]
- 34.Nachlas MM, Schnitka TK. Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity. Am J Pathol. 1963;42:379–406. [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA, Gilchrist A, Hoyer D, Insel PA, Izzo AA, Lawrence AJ, MacEwan DJ, Moon LD, Wonnacott S, Weston AH, McGrath JC. Experimental design and analysis and their reporting: new guidance for publication in BJP [published correction appears in Br J Pharmacol. 2015 Sep; 172(18):4600]. Br J Pharmacol. 2015;172:3461–3471. [DOI] [PMC free article] [PubMed]
- 36.Defronzo RA. Bromocriptine: a sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34:789–94. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scranton R, Cincotta A. Bromocriptine–unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmaco. 2010;11:269–79. doi: 10.1517/14656560903501544. [DOI] [PubMed] [Google Scholar]
- 38.Aminorroaya A, Janghorbani M, Ramezani M, Haghighi S, Amini M. Does bromocriptine improve glycemic control of obese type-2 diabetics? Horm Res. 2004;62:55–9. doi: 10.1159/000078932. [DOI] [PubMed] [Google Scholar]
- 39.Zeng C, Zhang M, Asico LD, Eisner GM, Jose PA. The dopaminergic system in hypertension. Clin Sci (Lond) 2007;112:583–97. doi: 10.1042/CS20070018. [DOI] [PubMed] [Google Scholar]
- 40.McCoy CE, Douglas FL, Goldberg LI. Selective antagonism of the hypotensive effects of dopamine agonists in spontaneously hypertensive rats. Hypertension. 1986;8:298–302. doi: 10.1161/01.HYP.8.4.298. [DOI] [PubMed] [Google Scholar]
- 41.Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight. 2016;1:e85888. doi: 10.1172/jci.insight.85888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols CD. Serotonin 5-HT(2A) Receptor function as a contributing factor to both neuropsychiatric and cardiovascular diseases. Cardiovasc Psychiatry Neurol. 2009;2009:475108. [DOI] [PMC free article] [PubMed]
- 43.Fujita M, Minamino T, Sanada S, Asanuma H, Hirata A, Ogita H, Okada K, Tsukamoto O, Takashima S, Tomoike H, Node K, Hori M, Kitakaze M. Selective blockade of serotonin 5-HT2A receptor increases coronary blood flow via augmented cardiac nitric oxide release through 5-HT1B receptor in hypoperfused canine hearts. J Mol Cell Cardiol. 2004;37:1219–23. doi: 10.1016/j.yjmcc.2004.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.