Abstract
Sepsis is an overwhelming inflammatory response to microbial infection. Sepsis management remains a clinical challenge. The role of the gut microbiome in sepsis has gained some attention. Recent evidence has demonstrated that gut microbiota regulate host physiological homeostasis mediators, including the immune system, gut barrier function and disease susceptibility pathways. Therefore, maintenance or restoration of microbiota and metabolite composition might be a therapeutic or prophylactic target against critical illness. Fecal microbiota transplantation and supplementation of probiotics are microbiota-based treatment methods that are somewhat limited in terms of evidence-based efficacy. This review focuses on the importance of the crosstalk between the gastrointestinal ecosystem and sepsis to highlight novel microbiota-targeted therapies to improve the outcomes of sepsis treatment.
Keywords: Sepsis, Microbiota, Immune, Gut barrier, Liver, Fecal microbiota transplantation, Probiotics
Highlights.
Sepsis potentially disrupts the gut ecosystem and gut dysbiosis may predispose sepsis development.
Gut barrier function and the gut–liver axis play essential roles in the host response to sepsis.
In addition to supplementation with probiotics and prebiotics, which have been shown to be clinically effective, fecal microbiota transplantation and phage therapy are promising therapeutic options for the future.
Background
Sepsis is the manifestation of a dysregulated host response, characterized by organ dysfunction, to overwhelming infection. Even with optimal management in intensive care units, sepsis is associated with high mortality rates and the World Health Organization has recognized sepsis as a global health emergency [1]. Excessive inflammation can eventually lead to dysfunction of multiple organ systems, including the pulmonary, hepatic, cardiovascular, renal and gastrointestinal systems [2]. Epidemiological studies have revealed high rates of sepsis and in-hospital mortality ranging from 25 to 30% [3]. In clinical practice, the Sequential Organ Failure Assessment (SOFA) score is used to evaluate organ dysfunction [4]. Given that sepsis is life-threatening, effective strategies to prevent, diagnose and treat it are essential.
Sepsis occurs due to impaired activation of the immune system in response to infection; therefore, its onset depends on microbial pathogen-associated molecular patterns (PAMPs) that are recognized by pattern-recognition receptors (PRRs). Meanwhile, damage-associated molecular patterns (DAMPs) derived from body tissues, such as mitochondrial DNA, heat shock proteins and high mobility group box 1 protein (HMGB1), act as potent activators of the innate immune system and initiate a systemic reaction [5].
The host immune reaction during and after sepsis onset is complicated. Hyperactivation of immune cells and overwhelming inflammation appear to be the main contributors to the pathophysiology of sepsis [6]. These processes induce the release of both proinflammatory factors and anti-inflammatory mediators through the activation of inflammatory signaling pathways. The overproduction and release of cytotoxic molecules, such as interleukin (IL)-1, IL-17 and tumor necrosis factor (TNF), which constitute the cytokine storm, are likely responsible for the excessive systemic inflammation associated with sepsis [6]. However, sepsis is also associated with immune suppression, which is mediated by both the adaptive and innate immune systems [7]. Immune suppression often increases the individual’s susceptibility to secondary infections, further increasing the risk of death [8]. Furthermore, there are several other key mechanisms, such as the complement pathway, Ca2+ homeostasis and mitochondrial dysfunction, that affect immune function during sepsis [6]. The precise series of mechanisms underlying sepsis-induced multiple organ dysfunction are not fully understood, and new insights into these processes will facilitate the development of novel therapeutics to improve sepsis outcomes.
The human gastrointestinal tract contains trillions of bacteria, the composition of which mediates the equilibrium between host wellness and disease. The gut epithelial barrier, immune system and gut microbiome are all closely interlinked to defend the body against pathogen colonization [9]. The gut microbiome has been well studied via metagenome sequencing and 16S ribosomal DNA sequencing. Gastrointestinal microbiome diversity and function have been implicated in several disorders, such as Clostridioides difficile infection (CDI), inflammatory bowel disease (IBD) and liver disease [10,11]. An imbalance of the microbiome profile has been termed dysbiosis. The gut has been recognized as the primary target site of many diseases, as gut homeostasis is among the most commonly affected physiological elements. The gut microbiome is reported to drive severe responses to sepsis and affect the outcomes of sepsis treatment [12]. Multi-omics analysis has revealed that compositional and functional alteration of the gut microbiota promotes sepsis-linked organ damage [13]. A prospective cohort study indicated that gut dysbiosis with accumulation of bacilli and their fermentation metabolites could precede late-onset sepsis [14]. The detailed pathogenetic role of gut microbiota in sepsis is incompletely understood. Here, we review the character of the gut microbiome in terms of its association with sepsis and provide an overview of potential treatments for sepsis (Figure 1).
Figure 1.
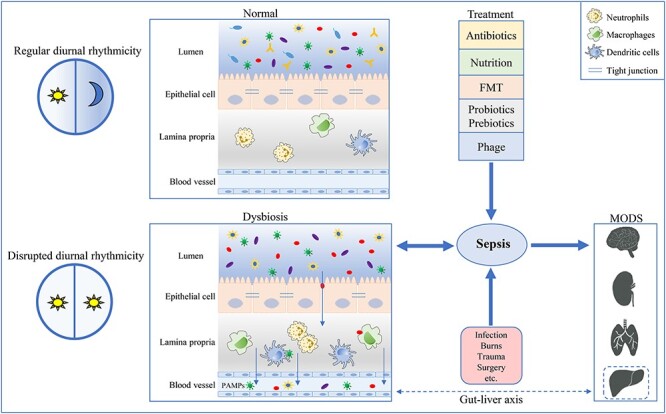
The relationship between gut microbiota and sepsis. Intestinal epithelial cells form a physical and chemical barrier to prevent bacteria and bacterial products from translocating into the lamina propria and systemic circulation. Sepsis results in gut barrier dysfunction and microbiome composition alteration, and gut bacterial dysbiosis predisposes sepsis development. Additionally, the gut–liver axis plays an important role in sepsis. Circadian disruption could cause gut microbial alterations, which possibly impact sepsis development. Promising approaches to sepsis treatment include FMT and supplementation with probiotics or prebiotics. FMT fecal microbiota transplantation, PAMPs pathogen-associated molecular patterns, MODS multiple organ dysfunction syndrome
Review
Sepsis may aggravate gut microbiome dysbiosis
It has been reported that critically ill patients who suffer sudden and severe insults present with immediate and dramatic gut microbiota alterations, along with decreased concentrations of short-chain fatty acids (SCFAs) in the gut [15]. A prospective observational study adopted shotgun metagenomic sequencing, phylogenetic profiling and microbial genome analyses to confirm that antibiotics administration in the intensive care unit markedly decreases gut microbiota diversity and that the elimination of commensal strains facilitates the transmission of hospital-acquired Enterococcus faecium infection [16]. Previous research has revealed that severe systemic inflammatory response syndrome is associated with a markedly decreased abundance of beneficial Bifidobacterium and Lactobacillus and increased levels of pathogenic Staphylococcus and Pseudomonas [17]. Clinically, the gut ecosystem may be a prognostic marker for patients with septic complications. Death of patients with systemic inflammatory response syndrome has been associated with a lower abundance of obligate anaerobes and a higher abundance of pathogenic bacteria relative to normal or baseline levels [18]. Commensal bacteria, particularly obligate anaerobes, play an important role in preventing pathogen invasion and colonization. These beneficial microbes inhibit the adherence of pathogenic bacteria to the intestinal epithelial brush border [19]. Conversely, overgrowth of pathogenic bacteria facilitates the progression of infection [17].
Patients with sepsis have been shown to have lower levels of fecal SCFAs than healthy volunteers, and this reduced concentration has been shown to last for 6 weeks [20]. SCFA absorption is lower in association with critical illness, and this could aggravate gut barrier dysfunction, facilitate pathogen colonization and affect the systemic immune response [21]. SCFAs also provide energy for intestinal epithelial cells and influence the proliferation and differentiation of these cells [21]. Low SCFA concentrations may negatively impact host recovery from critical illness. By employing multi-omics analysis, we previously found septic patients to exhibit enteric dysbiosis at both the compositional and functional levels [13]. Importantly, such disrupted ecosystems could promote septic organ damage progression, further demonstrating that sepsis-associated dysbiosis may serve as an upstream contributor to sepsis development.
Gut microbiota modulate sepsis progression
Gut microbiota influence the immune response during sepsis
The intestinal microbiome has been recognized as an important modulator of the host immune system. Commensal microorganisms act as essential factors in response to bacterial infection in distal tissues [22]; for example, translocation of peptidoglycan from the gut can directly enhance neutrophil function and rapidly primes the systemic immunomodulatory response to pneumococcal sepsis [23]. The clearance of gut bacteria by antibiotic treatment or the use of germ-free animals has been reported to influence inflammation and organ injury in sepsis [24,25]. Mechanistically, gut microbiota clearance impairs immune cell function. Macrophages obtained from gut microbe-depleted mice have been shown to express diminished phagocytic ability with increased bacterial dissemination and organ failure. Importantly, transcriptome analysis of alveolar macrophages has shown that the gut microbiome influences metabolic pathways, especially those responsible for responsiveness toward bacterial virulence factors and phagocytosis capacity [26]. The microbiome regulates the number of aged neutrophils, and a study of mice treated with antibiotics and injected with lipopolysaccharide (LPS) showed that the antibiotics significantly reduced the abundance of neutrophil extracellular traps [25]. Clinically, antibiotic use can alleviate pathobiont virulence and infection, but antibiotic-resistant bacteria are still a challenge associated with antimicrobial interventions [27]. Under some conditions, the adverse effects of broad-spectrum antibiotics will disrupt the host immune response against infection. In one study, mice exposed to antibiotics combined with dextran sodium sulfate (DSS) co-treatment developed a sepsis-like disease and not colitis. This process involved a decrease in beneficial bacterial species and primarily the expansion of pathobionts into the systemic sites [28]. However, a prior study demonstrated that antibiotic pre-treatment rendered mice more susceptible to DSS treatment, but the disease onset was not associated with the expansion of pathobionts into systemic sites; rather, it was due to a failure of gut epithelial injury repair via toll-like receptor (TLR)-dependent signals [29]. Normally, the recognition of TLR ligands on commensals by TLRs expressed on the intestinal epithelium results in a protective effect to maintain gut homeostasis [30]. Zeng et al. reported that gut symbionts can regulate systemic immunity. They demonstrated that the antigens highly expressed on the outer membrane of gram-negative bacteria induce systemic serum immunoglobulin (Ig) G production and that the microbiome-specific IgG plays a protective role in systemic infection [31]. Thus, gut microbiota and the derived products have systemic influences on extraintestinal tissues and organs. These findings suggest potential mechanisms by which gut commensals combat systemic infection.
Numerous studies have highlighted the pivotal roles of microbiota and their metabolic products in maintaining host immune and metabolic homeostasis [32,33]. Dietary fiber alters the composition of gut microbiota, such as Akkermansia and Lachnospiraceae, reducing systemic inflammation and improving survival [34]. Bacterial fermentation of dietary fiber can produce SCFAs, which have been observed to exhibit anti-inflammatory effects [21]. During Klebsiella pneumoniae infection, gut microbiome-derived SCFAs promote macrophage bacterial clearance by upregulating late endosomal/lysosomal adaptor, mitogen-activated protein kinase (MAPK) and MTOR activator 2 (LAMTOR2) [35]. Granisetron, which is derived from gut microbial metabolism, has anti-inflammatory effects, modulates the host immune system and mediates susceptibility to sepsis in mice [36]. Bacterial diversity influences immune regulation and disease outcomes; accordingly, one study found that mice obtained from different vendors had significantly different immunophenotypes and microbiome profiles; they even exhibited different responses in the context of sepsis. After cohousing, the mice had similar gut microbiome compositions and all immunophenotypical differences disappeared [37]. Interestingly, the gut microbiome also influences serum IgA, which depends on T-cell abundance, to protect against sepsis [38]. IgA-mediated crosslinking enchains bacteria in the gut to protect against pathogenic infection [39]. Research on the precise mechanism underlying the microbiome-mediated shaping of the immune system will be of good clinical translational value.
Gut barrier function in sepsis
The gut tract is colonized by trillions of microorganisms, and gut barrier integrity is crucial for preventing bacteria and bacterial products from translocating into the systemic circulation. Intestinal epithelial cells form a physical and chemical barrier that maintains intestinal homeostasis [40]. Claudins, occludin, junctional adhesion molecules and cytosolic proteins [e.g. zonula occludens (ZO)-1, ZO-2 and ZO-3] are essential tight junction proteins for barrier function [41]. Peyer’s patches, the lamina propria, mesenteric lymph nodes and intraepithelial lymphocytes constitute the largest ‘immune organ’ of the body. Some specialized epithelial cells that secrete mucins and antimicrobial proteins also regulate the immunological environment [40]. Critical illness could markedly induce gut barrier dysfunction and result in a leaky gut [42]. Severe burn injury is at high risk of developing sepsis and leading to multiple organ failure, and gut barrier dysfunction may play an essential role in this progression [43]. Moreover, burn injury has been reported to alter the gut microbial composition and decrease the concentration of SCFAs, which may disrupt gut barrier function [44]. Fecal microbiota transplantation has been demonstrated as an effective way to restore microbiome diversity and increase gut barrier integrity in a murine model of burn injury [45]. During the development of polymicrobial sepsis, the redistribution of colonic tight junction proteins, including claudins 1, 3, 4, 5 and 8, leads to gut barrier dysfunction, meanwhile protein expression of claudin-2 is markedly upregulated [46]. Tight junction alterations appear as early as 1 h after sepsis onset, then microbial pathogens translocate from the intestine into the bloodstream and then pass through the liver via the portal vein [47]. Sepsis results in intestinal hyperpermeability through the upregulation of inflammation, epithelial cell apoptosis and the alteration of microbiome composition [48,49]. Proinflammatory mediators, such as TNF-α, IL-1β, IL-6, are recognized as important factors for modulating the intestinal barrier function and increasing gut permeability [50–52]. Myosin light chain kinase (MLCK) is commonly associated with increased cytokine levels, which, in turn, activates MLCK and causes intestinal hyperpermeability [53]. Previous research has shown that gut hyperpermeability was prevented and the survival time was significantly increased in MLCK−/− mice following cecum ligation and puncture (CLP) [54]. Intestinal epithelial cell (IEC) apoptosis is another phenomenon that occurs during sepsis [55]. When gut integrity is disrupted, the apoptotic response appears to be activated and IEC apoptosis may augment gut permeability [56]. The gut-specific overexpression of the anti-apoptotic protein, Bcl-2, has been shown to result in enhanced tight junction protein expression following sepsis-induced hyperpermeability [57]. Epidermal growth factor is a cytoprotective polypeptide, and mice administered with systemic epidermal growth factor, as well as those with enterocyte-specific overexpression of epidermal growth factor, display improved intestinal barrier function and survival following sepsis [58,59]. Alteration of gut microbial communities disrupts tight junctions and increases gut permeability [60], and reversal of gut barrier dysfunction can effectively decrease the mortality associated with sepsis [61].
Gut microenvironment changes have been reported to increase susceptibility to sepsis development. Translocating endotoxins and bacteria could activate systemic inflammation and promote organ dysfunction and failure [62]. Once barrier dysfunction has occurred, some other gut microbiome products, such as fecal-derived extracellular vesicles, are likely absorbed into remote organs via the lymphatic vessels and bloodstream [63]. Translocating bacteria can be phagocytosed by mesenteric lymph nodes in normal circumstances. But host deficiencies and immunosuppression are probably among the risk factors leading to bacterial translocation-associated complications. Human studies have recognized that bacterial translocation appears to be associated with significantly higher rates of postoperative sepsis among surgical patients [64]. A healthy intestine would preclude the translocation of PAMPs to the systemic circulation and inhibit immune reactions. Additionally, the gut–lymph hypothesis suggests that gut-derived factors could translocate through the mesenteric lymph to distal organs and induce systemic inflammation [65]. Viable bacteria have been detected in sterile tissue, such as mesenteric lymph nodes, of gnotobiotic mice intragastrically administered with the whole cecal microflora of specific-pathogen-free (SPF) mice [66]. Using a ligated mesenteric lymph duct murine model, Badami et al. confirmed that factors derived from the gut and carried by the mesenteric lymph duct might contribute to death associated with critical illness [67]. Moreover, mesenteric lymph collected from animals after trauma and hemorrhagic shock was sufficient to induce acute lung injury when injected into rats [68], which confirmed that the gut–lymph pathway is a driving factor for host immune system activation. The details of the molecular mechanisms that contribute to sepsis-induced barrier function are complicated. Directly or indirectly targeting tight junctions is effective for preventing bacterial translocating into the bloodstream and improving survival following sepsis.
Gut–liver axis in sepsis
Gastrointestinal and liver function is intricately connected via the portal vein, and the gut–liver axis appears to have critical responses to sepsis [69]. The liver plays key roles in preventing and ameliorating the effects of systemic infections through bacterial clearance and production of inflammatory mediators [70]. Additionally, intestinal bacteria can convert liver-derived bile acids and modulate host metabolism and immunity [71,72]. Hence, the interaction between hepatic function and microbial function may provide an important defense mechanism against pathogens via immune activation. Tissue-resident macrophages, Kupffer cells, are the first-line immune cells in the liver that defend against blood-borne bacteria. Quick blood clearance can prevent bacteremia and ensure a sterile bloodstream [73]. Kupffer cells can efficiently clear circulating microbiome-derived products depending on the macrophage complement receptor immunoglobulin (CRIg) [74]. Additionally, CRIg also recognizes and binds to the lipoteichoic acid of gram-positive bacteria to capture circulating pathogens [75]. The commensal metabolite, D-lactate, reaches the liver through the portal vein and promotes Kupffer cells to capture and kill pathogens from the circulation [76]. Liver-mediated bacterial capture is critical for limiting the dissemination of bacteria into the systemic circulation and other susceptible organs during infection. Listeria monocytogenes infections could induce the early necroptotic death of Kupffer cells, which induces hepatocytes to release IL-33, which promotes IL-4 production by basophils. Importantly, these, in turn, cause monocyte-derived macrophage recruitment to the liver with the activation of antibacterial inflammatory reactions, eventually restoring hepatic immune homeostasis [77]. As polarization of Kupffer cells plays an essential role in modulating their immune function in inflammation, enhancing the M2 (alternative) polarization phenotype could protect against liver injury [78]. Therefore, it is clear that sepsis results in liver injury, which further leads to host immune reaction disruption and increases susceptibility to infection. Future investigations will be required to explain the detailed turnover mechanism of liver macrophages upon infection leading to sepsis. More importantly, hepatocytes, hepatic stellate cells and sinusoidal endothelial cells may all be involved in immune activation during sepsis [79].
Bacterial outer membrane vesicles in sepsis
Bacterial outer membrane vesicles (OMVs) are naturally derived from bacteria, mainly from gram-negative bacteria [80]. OMVs contain many microorganism-associated molecular patterns (MAMPs), such as proteins, lipids, DNA and RNA, as well as abundant LPS. These MAMPs can recognize host pattern recognition receptors, leading to an immune response [81]. LPS, as a key outer membrane component of gram-negative bacteria, can be delivered via OMVs. OMVs isolated from Escherichia coli could trigger a sepsis response [82]. Moreover, OMVs activate the cytosolic LPS-sensing pathway during infection [83]. OMVs can act as a vehicle for delivering cargo into cells. They facilitate host–microbial crosstalk and enable bacteria to modulate intracellular communication within the host. Increased intestinal permeability also influences the delivery of OMVs from the gut into the circulatory system and remote organs, eventually causing systemic inflammation and sepsis [63]. Fecal-derived extracellular vesicles, which are secreted by multiple gut microbes, have the potential to induce peritonitis and sepsis-like systemic inflammation [63]. However, some OMVs also have beneficial effects on the host. Immunization with Acinetobacter baumannii-derived OMVs increased survival in a sepsis model [84]. Moreover, immunization with Burkholderia pseudomallei-derived OMVs can also provide protection against lethal sepsis, probably because OMVs may serve as a potent ‘vaccine’ [85]. Additionally, through the activation of Th1 and Th17 cells, E. coli-derived OMVs may prevent bacteria-induced mortality [86]. During infection, OMVs have many other effects on host cells, such as immune cells, endothelial cells and platelets [81]. However, the detailed mechanisms underlying the various effects of OMVs in sepsis have not been thoroughly studied. OMVs also need to be investigated for their therapeutic potential in the clinical treatment of sepsis.
Bacterial circadian rhythms in sepsis
Modern lifestyles, including jet lag and shift work, often cause circadian disruptions, which strongly influence host–microbial interactions with increased susceptibility to metabolic syndrome and gastrointestinal pathology [87]. Although there is no direct evidence demonstrating that the circadian rhythm of the microbiome could directly impact sepsis patients, several clues suggest that such an association might be possible. First, human gut microbiota display circadian oscillations, and such diurnal oscillations indeed directly influence host pathophysiology. For example, Thaiss et al. reported that dysbiosis induced by jet lag could disrupt glucose metabolism [88]. Second, in mice, Salmonella enterica infection with onset in the morning was shown to cause greater inflammation and pathogen colonization compared with night-onset infection [89]. This rhythmic difference may result from the crosstalk among the gut microbiome, host circadian system and immune responses [90]. Third, at the molecular level, gut microbiota-derived MAMPs could directly regulate host immune responses, especially in inflammatory disease development. All of these observations suggest that the gut microbiome may directly impact host immune responses during infection, and disruption of gut microbial circadian oscillation may, in turn, impact sepsis development.
Both the compositional and functional components of the gut microbiota exhibit robust circadian rhythms [91], and this has been recognized as a key modulator of host circadian rhythms [92]. Host sensitivity to a variety of infections follows circadian features such that inflammation levels are increased and susceptibility to pathogens is higher at the beginning of the resting phase [93]. Circadian disruption impacts the immune system and mediates the higher susceptibility to inflammatory disease [94]. It has been reported that dynamic changes in microbiome compositions have been observed in intensive care unit patients during the acute phase [95]. A light/dark cycle improved survival after CLP compared with a dark/dark cycle in a murine model [96]. Through the gut microbiota–subdiaphragmatic vagus nerve axis, short-term sleep after LPS administration has been shown to augment the systemic inflammatory reaction and organ injury associated with sepsis [97]. Importantly, host circadian disruption also affects the diurnal microbial fluctuations that accompany bacterial dysbiosis [88]. Rodents with disrupted circadian rhythms have been shown to exhibit dysbiosis with decreased levels of butyrate producers, such as Eubacterium plexicaudatum and Subdoligranulum [98]. Circadian rhythm disturbances decrease host radioresistance, and this may be partly mediated by gut microbiome composition [99]. Sleep deprivation could affect immune cell abundance and function, indicating that people with sleep loss may be more susceptible to infections [100]. These phenomena reveal the potential crosstalk between bacterial circadian rhythms and critical illness. Dysbiosis disrupts gut homeostasis, impacts the sensing pathway of commensal bacterial products and influences the circadian clock guiding epithelial cells and systemic metabolism [30]. Based on the circadian interactions of the host and microbiome, developing specific pharmacologic compounds aimed at regulating daily oscillations might be beneficial to host immune response modulation. Understanding the tight regulation between circadian rhythms, gut microbiota and the immune system will facilitate the development of more precise therapies to treat infection.
Targeting the microbiome in sepsis therapy
Current clinical treatments for sepsis include the administration of antibiotics and nutritional support. However, antibiotics produce a sustained effect on the gut microbiome and may cause antibiotic resistance. Antibiotic resistance is still a challenge affecting the treatment of critically ill patients worldwide. Direct or indirect modulation of the gut microbiota may yield new potential therapeutic approaches to improve sepsis management.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is a therapeutic method for restoring a normal intestinal environment via the administration of healthy donor feces. Living microorganisms from healthy donors are transferred to the recipients to rescue the disordered microbial ecosystem, suppress the activation of inflammatory reactions and prevent pathogen colonization [101]. FMT has been applied in the treatment of many diseases, such as IBD, cancer and CDI [102–104]. In the treatment of antibiotic-resistant CDI, FMT has been demonstrated as more effective than vancomycin [105]. FMT has been successfully used to treat sepsis and improve clinical outcomes following microbiota composition and immune system modifications [106,107]. FMT restores the specific bacterial populations, drives the clearance of systemic pathogens and increases survival in sepsis. Importantly, the expression of interferon regulatory factor 3, which is necessary for TLR signaling pathway activation, has been shown to be altered by FMT [108]. Additionally, FMT induces the expression of gut tight junctions and improves the survival of rats with sepsis [61]. Using a murine model, researchers have shown that FMT modulates the intestinal microbiome composition; specifically, FMT has been shown to increase the abundance of commensals and reduce the abundance of opportunistic gut pathogens, alleviating septic encephalopathy via anti-inflammatory effects [109]. The clinical potential of FMT is promising. However, due to the complexity and variability of donor feces, the detailed mechanisms by which bacterial strains or metabolites are responsible for FMT’s beneficial effects are still not clear. Specialized methods, including capturing detailed donor medical data as well as standardized stool collection and preparation procedures, are required to reduce the risks associated with FMT. Collectively, the aim of FMT is to reconstitute a normal intestinal ecosystem to facilitate improved immune function against pathogens.
Probiotics, prebiotics and synbiotics
Restoration of microbial diversity is a complementary method for rebuilding the normal gut microbial ecology. Probiotic (‘good’ bacteria) supplementation has been shown to modulate the gut microbiome composition and yield beneficial effects on the host [110]. The beneficial roles of probiotics include immune modulation, pathogen prevention, gut barrier function improvements and some metabolic effects [110]. Lactobacillus, Bifidobacterium and Streptococcus are commonly used probiotics [111]. Prebiotics, as non-digestible nutrients, can promote commensal bacterial growth. Additionally, the administration of synbiotics, which are combinations of probiotics and prebiotics, regulates the host gut microbiome [112].
There are several published case reports detailing the clinical effects and outcomes of probiotics and prebiotics administration [113–118] (Table 1). In a cohort of elderly volunteers, administration of galacto-oligosaccharides as prebiotics for 10 weeks regulated fecal bacterial populations and natural killer cell activity [113]. Probiotics modulate gene expression in inflammatory pathways and the immune system in epithelial and immune cells, including nuclear factor of activated B cells protein kinase (NF-κB), MAPKs, IL-6, IL-8 and TNF-α. [119]. Administration of a synbiotic containing Lactobacillus plantarum has been associated with significantly decreased rates of neonatal sepsis and death [114]. A meta-analysis showed that supplementation of probiotics significantly reduces the risk of late-onset sepsis from 16.3% among placebo recipients to 13.9% among probiotic recipients [120]. Administration of probiotics effectively decreased mortality by regulating the microbiome composition and metabolites in a CLP mouse model [121,122]. A randomized controlled pilot trial revealed that probiotic intervention successfully increased stool bacterial diversity in early sepsis [118]. Singer et al. revealed that maternal antibiotic exposure affects neonatal susceptibility to late-onset sepsis, but some Lactobacilli strains can act as effective probiotics to prevent late-onset sepsis in susceptible individuals [123]. Furthermore, prophylactic Lactobacillus rhamnosus GG treatment improves gut barrier integrity and attenuates inflammatory responses in sepsis [124].
Table 1.
Some selected probiotics/prebiotics usage in clinic
| Probiotics/prebiotics | Study population and intervention | Functions | References |
|---|---|---|---|
| Galacto-oligosaccharide mixture |
• 65-80 years old volunteers • 10 weeks with daily doses of 5.5 g/d with a 4-week washout period in between |
• Increase bacteroides and bifidobacteria • Affect immune function associated with ageing |
[113] |
| Lactobacillus plantarum plus fructooligosaccharide |
• Newborns • Beginning on day 2–4 of life for 7 days |
• 40% reduction in the primary combined outcome of death and neonatal sepsis |
[114] |
|
Bifidobacterium breve strain Yakult and Lactobacillus casei strain Shirota plus galactooligosaccharides Lactobacillus acidophilus, Enterococcus faecium and Bifidobacterium infantum Lactobacillus reuteri DSM 17938 Multispecies probiotica |
• Ventilated patients with sepsis • Initiated within 3 days after admission • Preterm infants • twice daily until discharge • Preterm infants • once daily until discharge • Early sepsis patients • Twice daily for 4 weeks |
• Increase the levels of beneficial bacteria and SCFAs • Decrease the incidence of enteritis and ventilator-associated pneumonia • Decrease the frequency of late-onset sepsis and infections • Improve feeding tolerance, promote growth • Increase daily defecations • Shorten the length of hospitalstay • Enrich the gut microbiome with probiotic strains • Increase gut functional diversity |
[115] [116] [117] [118] |
SCFAs: short-chain fatty acids
aMultispecies probiotic contains Lactobacillus plantarum W1, Lactobacillus paracasei W20, Bifidobacterium bifidum W23, Lactobacillus salivarius W24, Lactobacillus acidophilus W37, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W55, Lactobacillus plantarum W62, Lactobacillus rhamnosusW71
However, the efficacy and safety of probiotics are still controversial in clinical settings. In one study, translocation of probiotics from the mucosal epithelium to extraintestinal sites was rarely observed in healthy individuals, and even if it occurred, it hardly caused detrimental effects [125]. Such translocation may be limited by mesenteric lymph nodes or mediated by the macrophage killing function. However, probiotic administration is associated with an increased risk of bacteremia via probiotic bacteria transmission to the bloodstream among intensive care unit patients [126]. Cancer, diabetes and transplantation could increase the patients’ susceptibility to Lactobacillus bacteremia, mostly due to immunocompromise [127]. McNaught et al. conducted a prospective study of surgical patients and found that the mortality rate associated with sepsis was slightly higher in the probiotic group than the control group, although this difference was not statistically significant [128]. In another study, patients with non-severe sepsis undergoing probiotic treatment exhibited higher mortality risk [129], and another study showed that probiotics administration increased bacterial translocation in patients with organ failure [130]. These observations highlight the need to determine the specificity and safety of various strains used in sepsis treatment.
Phage therapy
Bacteriophages, also known as phages, are viruses within bacteria and archaea. Phages have anti-inflammatory and immunomodulatory effects [131,132]. Phages containing antibacterial agents are considered superior to antibiotics [133,134]. Bacteriophage therapeutics have been emerging as a novel approach for treating bacterial infections and their efficacy has been demonstrated in mouse models of sepsis [135,136]. In one study, a phage cocktail was tested against bacterial species isolated from neonates with sepsis [137]. In a single-arm, non-comparative trial, treatment with bacteriophages (AB-SA01) was safe for treating septic shock caused by Staphylococcus aureus infections [138]. Interestingly, lytic phages have been reported to knock down their targeted bacteria, affect other non-targeted bacterial communities and eventually modulate the gut metabolome [139]. Characterizing the interplay between phage and commensal bacteria will provide more precise approaches for infectious disease therapy. Understanding phage biology and acquiring the highly purified phages will pave the way for the development of phage therapeutics for sepsis. However, virus treatment still has potential risks and more reliable safety assessments are urgently needed.
Conclusions
With the use of sequencing approaches, we can better comprehend the symbiotic crosstalk between microbiota and the host. Gut microbiota and associated products regulate key host functions. As highlighted in this review, sepsis induces gut microbial dysbiosis. Moreover, the gut microbiome participates in the development of sepsis and affects host susceptibility to sepsis. Additionally, gut–liver crosstalk may supply a basis for the treatment of sepsis-induced organ injury. Strengthening gut barrier function is a good method for minimizing translocation of bacteria and bacterial metabolites and alleviating sepsis-induced organ injury. The gut microbiome includes bacteria, fungi, viruses and archaea. The detailed roles of these microorganisms in the pathophysiology of sepsis need further investigation. Current limitations relate to whether gut microbiome composition can be used as a biomarker for sepsis outcomes. Hopefully, a more detailed understanding of the mechanistic role of gut microbiota in sepsis will allow for the development of original and effective approaches to alleviating sepsis morbidity and mortality.
Abbreviations
CDI: Clostridioides difficile infection; CLP: cecum ligation and puncture; CRIg: complement receptor immunoglobulin; DAMPs: damage-associated molecular patterns; DSS: dextran sodium sulfate; FMT: fecal microbiota transplantation; HMGB1: high mobility group box 1 protein; IBD: inflammatory bowel disease; IECs: intestinal epithelial cells; Ig: immunoglobulin; IL: interleukin; LAMTOR2: late endosomal/lysosomal adaptor, MAPK and MTOR activator 2; MAPK: mitogen-activated protein kinase; MLCK: myosin light chain kinase; LPS: lipopolysaccharide; MAMPs: microorganism-associated molecular patterns; NF-κB: nuclear factor of activated B cells protein kinase; OMVs: outer membrane vesicles; PAMPs: pathogen-associated molecular patterns; PRRs: pattern-recognition receptors; SCFAs: short-chain fatty acids; SOFA: Sequential (or Sepsis-related) Organ Failure Assessment; SPF: specific-pathogen-free; TNF: tumor necrosis factor; TLRs: toll-like receptors; ZO: zonula occludens.
Authors’ contributions
M.N. drafted the manuscript, P.C. supervised and edited the review.
Funding
This study was supported by National Natural Science Foundation of China (81 873 926) toPC.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Mengwei Niu, Department of Pathophysiology, Guangdong Provincial Key Laboratory of Proteomics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
Peng Chen, Department of Pathophysiology, Guangdong Provincial Key Laboratory of Proteomics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, China.
References
- 1. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a Global Health priority - a WHO resolution. N Engl J Med. 2017;377:414–7. [DOI] [PubMed] [Google Scholar]
- 2. Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417–27. [DOI] [PubMed] [Google Scholar]
- 3. Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. [DOI] [PubMed] [Google Scholar]
- 4. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajaee A, Barnett R, Cheadle WG. Pathogen- and danger-associated molecular patterns and the cytokine response in sepsis. Surg Infect (Larchmt). 2018;19:107–16. [DOI] [PubMed] [Google Scholar]
- 6. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315:1469–79. [DOI] [PubMed] [Google Scholar]
- 9. Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez C, Romero E, Garrido-Sanchez L, Alcaín-Martínez G, Andrade RJ, Taminiau B, et al. Microbiota insights in clostridium difficile infection and inflammatory bowel disease. Gut Microbes. 2020;12:1725220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–44. [DOI] [PubMed] [Google Scholar]
- 12. Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135–43. [DOI] [PubMed] [Google Scholar]
- 13. Liu Z, Li N, Fang H, Chen X, Guo Y, Gong S, et al. Enteric dysbiosis is associated with sepsis in patients. FASEB J. 2019;33:12299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graspeuntner S, Waschina S, Künzel S, Twisselmann N, Rausch TK, Cloppenborg-Schmidt K, et al. Gut Dysbiosis with bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin Infect Dis. 2019;69:268–77. [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56:2361–5. [DOI] [PubMed] [Google Scholar]
- 16. Ravi A, Halstead FD, Bamford A, Casey A, Thomson NM, van Schaik W, et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb Genom. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126–33. [DOI] [PubMed] [Google Scholar]
- 18. Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. [DOI] [PubMed] [Google Scholar]
- 20. Yamada T, Shimizu K, Ogura H, Asahara T, Nomoto K, Yamakawa K, et al. Rapid and sustained Long-term decrease of Fecal short-chain fatty acids in critically ill patients with systemic inflammatory response syndrome. JPEN J Parenter Enteral Nutr. 2015;39:569–77. [DOI] [PubMed] [Google Scholar]
- 21. Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80:37–49. [DOI] [PubMed] [Google Scholar]
- 22. Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun. 2014;82:4596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD. Et al., The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oteo J, Pérez-Vázquez M, Campos J. Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23:320–6. [DOI] [PubMed] [Google Scholar]
- 28. Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- 30. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27. [DOI] [PubMed] [Google Scholar]
- 31. Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016;44:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morowitz MJ, Di Caro V, Pang D, Cummings J, Firek B, Rogers MB, et al. Dietary supplementation with nonfermentable Fiber alters the gut microbiota and confers protection in murine models of sepsis. Crit Care Med. 2017;45:e516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu T, Li H, Su C, Xu F, Yang G, Sun K, et al. Microbiota-derived short-chain fatty acids promote LAMTOR2-mediated immune responses in macrophages. mSystems. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gong S, Yan Z, Liu Z, Niu M, Fang H, Li N, et al. Intestinal microbiota mediates the susceptibility to Polymicrobial sepsis-induced liver injury by Granisetron generation in mice. Hepatology. 2019;69:1751–67. [DOI] [PubMed] [Google Scholar]
- 37. Fay KT, Klingensmith NJ, Chen CW, Zhang W, Sun Y, Morrow KN, et al. The gut microbiome alters immunophenotype and survival from sepsis. FASEB J. 2019;33:11258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, et al. Commensal microbes induce serum IgA responses that protect against Polymicrobial sepsis. Cell Host Microbe. 2018;23:302–311.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moor K, Diard M, Sellin ME, Felmy B, Wotzka SY, Toska A, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498–502. [DOI] [PubMed] [Google Scholar]
- 40. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. [DOI] [PubMed] [Google Scholar]
- 41. Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–51. [DOI] [PubMed] [Google Scholar]
- 43. He W, Wang Y, Wang P, Wang F. Intestinal barrier dysfunction in severe burn injury. Burns Trauma. 2019;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng Y, Huang Y, Wang Y, Wang P, Wang F. Severe burn injury alters intestinal microbiota composition and impairs intestinal barrier in mice. Burns Trauma. 2019;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuethe JW, Armocida SM, Midura EF, Rice TC, Hildeman DA, Healy DP, et al. Fecal microbiota transplant restores mucosal integrity in a murine model of burn injury. Shock. 2016;45:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Q, Zhang Q, Wang C, Liu X, Li N, Li J. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol. 2009;218:210–21. [DOI] [PubMed] [Google Scholar]
- 47. Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haussner F, Chakraborty S, Halbgebauer R, Huber-Lang M. Challenge to the intestinal mucosa during sepsis. Front Immunol. 2019;10:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-α on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G970–9. [DOI] [PubMed] [Google Scholar]
- 51. Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–9. [DOI] [PubMed] [Google Scholar]
- 52. Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 54. Lorentz CA, Liang Z, Meng M, Chen CW, Yoseph BP, Breed ER, et al. Myosin light chain kinase knockout improves gut barrier function and confers a survival advantage in polymicrobial sepsis. Mol Med. 2017;23:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. [DOI] [PubMed] [Google Scholar]
- 56. Chen WY, Wang M, Zhang J, Barve SS, McClain CJ, Joshi-Barve S. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am J Pathol. 2017;187:2686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Otani S, Oami T, Yoseph BP, Klingensmith NJ, Chen CW, Liang Z, et al. Overexpression of BCL-2 in the intestinal epithelium prevents sepsis-induced gut barrier dysfunction via altering tight junction protein expression. Shock. 2020;54:330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hu J, Luo H, Wang J, Tang W, Lu J, Wu S, et al. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp Mol Med. 2017;49:e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Assimakopoulos SF, Papadopoulou I, Bantouna D, de Lastic AL, Rodi M, Mouzaki A, et al. Fecal microbiota transplantation and hydrocortisone ameliorate intestinal barrier dysfunction and improve survival in a rat model of Cecal ligation and puncture-induced sepsis. Shock. 2020;55:666–75. [DOI] [PubMed] [Google Scholar]
- 62. Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334–42. [DOI] [PubMed] [Google Scholar]
- 63. Park KS, Lee J, Lee C, Park HT, Kim JW, Kim OY, et al. Sepsis-like systemic inflammation induced by Nano-sized extracellular vesicles from Feces. Front Microbiol. 2018;9:1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Edwin A, DEITCH DA, Zhong XU, Qi LU. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction syndrome: a new look at gut origin sepsis. Journal of Organ Dysfunction. 2006;2:70–9. [Google Scholar]
- 66. Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Badami CD, Senthil M, Caputo FJ, Rupani BJ, Doucet D, Pisarenko V, et al. Mesenteric lymph duct ligation improves survival in a lethal shock model. Shock. 2008;30:680–5. [DOI] [PubMed] [Google Scholar]
- 68. Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, et al. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822–30. [DOI] [PubMed] [Google Scholar]
- 69. Sun J, Zhang J, Wang X, Ji F, Ronco C, Tian J, et al. Gut-liver crosstalk in sepsis-induced liver injury. Crit Care. 2020;24:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–13. [DOI] [PubMed] [Google Scholar]
- 71. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 72. Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Broadley SP, Plaumann A, Coletti R, Lehmann C, Wanisch A, Seidlmeier A, et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20:36–48. [DOI] [PubMed] [Google Scholar]
- 74. Luo Z, Ji Y, Gao H, Gomes Dos Reis FC, Bandyopadhyay G, Jin Z. Et al, CRIg(+) macrophages prevent gut microbial DNA-containing extracellular vesicle-induced tissue inflammation and insulin resistance. Gastroenterology. 2021;160:863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeng Z, Surewaard BG, Wong CH, Geoghegan JA, Jenne CN, Kubes P. CRIg functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe. 2016;20:99–106. [DOI] [PubMed] [Google Scholar]
- 76. McDonald B, Zucoloto AZ, Yu IL, Burkhard R, Brown K, Geuking MB, et al. Programing of an intravascular immune firewall by the gut microbiota protects against pathogen dissemination during infection. Cell Host Microbe. 2020;28:660–668.e4. [DOI] [PubMed] [Google Scholar]
- 77. Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–58. [DOI] [PubMed] [Google Scholar]
- 78. Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–42. [DOI] [PubMed] [Google Scholar]
- 79. Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. [DOI] [PubMed] [Google Scholar]
- 80. Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87. [DOI] [PubMed] [Google Scholar]
- 82. Shah B, Sullivan CJ, Lonergan NE, Stanley S, Soult MC, Britt LD. Circulating bacterial membrane vesicles cause sepsis in rats. Shock. 2012;37:621–8. [DOI] [PubMed] [Google Scholar]
- 83. Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and Caspase-11 activation. Cell. 2016;165:1106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang W, Yao Y, Long Q, Yang X, Sun W, Liu C, et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PLoS One. 2014;9:e100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, et al. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol. 2014;21:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, Lee WH, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013;190:4092–102. [DOI] [PubMed] [Google Scholar]
- 87. Mortaş H, Bilici S, Karakan T. The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chronobiol Int. 2020;37:1067–81. [DOI] [PubMed] [Google Scholar]
- 88. Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–29. [DOI] [PubMed] [Google Scholar]
- 89. Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, et al. Circadian clock regulates the host response to salmonella. Proc Natl Acad Sci U S A. 2013;110:9897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rosselot AE, Hong CI, Moore SR. Rhythm and bugs: circadian clocks, gut microbiota, and enteric infections. Curr Opin Gastroenterol. 2016;32:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu G, Tang W, He Y, Hu J, Gong S, He Z, et al. Light exposure influences the diurnal oscillation of gut microbiota in mice. Biochem Biophys Res Commun. 2018;501:16–23. [DOI] [PubMed] [Google Scholar]
- 92. Parkar SG, Kalsbeek A, Cheeseman JF. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 2019;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tognini P, Thaiss CA, Elinav E, Sassone-Corsi P. Circadian coordination of antimicrobial responses. Cell Host Microbe. 2017;22:185–92. [DOI] [PubMed] [Google Scholar]
- 94. Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. 2016;61:1628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–32. [DOI] [PubMed] [Google Scholar]
- 97. Zhang Y, Xie B, Chen X, Zhang J, Yuan S. A key role of gut microbiota-vagus nerve/spleen axis in sleep deprivation-mediated aggravation of systemic inflammation after LPS administration. Life Sci. 2021;265:118736. [DOI] [PubMed] [Google Scholar]
- 98. Deaver JA, Eum SY, Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front Microbiol. 2018;9:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cui M, Xiao H, Luo D, Zhang X, Zhao S, Zheng Q, et al. Circadian rhythm shapes the gut microbiota affecting host Radiosensitivity. Int J Mol Sci. 2016;17:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Christoffersson G, Vågesjö E, Pettersson US, Massena S, Nilsson EK, Broman JE, et al. Acute sleep deprivation in healthy young men: impact on population diversity and function of circulating neutrophils. Brain Behav Immun. 2014;41:162–72. [DOI] [PubMed] [Google Scholar]
- 101. Keskey R, Cone JT, DeFazio JR, Alverdy JC. The use of fecal microbiota transplant in sepsis. Transl Res. 2020;226:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Haifer C, Leong RW, Paramsothy S. The role of faecal microbiota transplantation in the treatment of inflammatory bowel disease. Curr Opin Pharmacol. 2020;55:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer. 2019;145:2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306:G310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. [DOI] [PubMed] [Google Scholar]
- 106. Li Q, Wang C, Tang C, He Q, Zhao X, Li N, et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care. 2015;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016;20:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim SM, DeFazio JR, Hyoju SK, Sangani K, Keskey R, Krezalek MA, et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat Commun. 2020;11:2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li S, Lv J, Li J, Zhao Z, Guo H, Zhang Y, et al. Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci Lett. 2018;662:98–104. [DOI] [PubMed] [Google Scholar]
- 110. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–29. [DOI] [PubMed] [Google Scholar]
- 111. Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, et al. Are there any different effects of Bifidobacterium, lactobacillus and streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9:e90153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and Synbiotics on human health. Nutrients. 2017;9:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015;114:586–95. [DOI] [PubMed] [Google Scholar]
- 114. Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407–12. [DOI] [PubMed] [Google Scholar]
- 115. Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kanic Z, Micetic Turk D, Burja S, Kanic V, Dinevski D. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr. 2015;127:S210–5. [DOI] [PubMed] [Google Scholar]
- 117. Cui X, Shi Y, Gao S, Xue X, Fu J. Effects of lactobacillus reuteri DSM 17938 in preterm infants: a double-blinded randomized controlled study. Ital J Pediatr. 2019;45:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stadlbauer V, Horvath A, Komarova I, Schmerboeck B, Feldbacher N, Klymiuk I, et al. Dysbiosis in early sepsis can be modulated by a multispecies probiotic: a randomised controlled pilot trial. Benef Microbes. 2019;10:265–78. [DOI] [PubMed] [Google Scholar]
- 119. Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684. [DOI] [PubMed] [Google Scholar]
- 121. Chen L, Xu K, Gui Q, Chen Y, Chen D, Yang Y. Probiotic pre-administration reduces mortality in a mouse model of cecal ligation and puncture-induced sepsis. Exp Ther Med. 2016;12:1836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen L, Li H, Chen Y, Yang Y. Probiotic lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition. 2020;78:110863. [DOI] [PubMed] [Google Scholar]
- 123. Singer JR, Blosser EG, Zindl CL, Silberger DJ, Conlan S, Laufer VA, et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat Med. 2019;25:1772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen L, Li H, Li J, Chen Y, Yang Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int J Mol Med. 2019;43:1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhou JS, Shu Q, Rutherfurd KJ, Prasad J, Birtles MJ, Gopal PK, et al. Safety assessment of potential probiotic lactic acid bacterial strains lactobacillus rhamnosus HN001, lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int J Food Microbiol. 2000;56:87–96. [DOI] [PubMed] [Google Scholar]
- 126. Yelin I, Flett KB, Merakou C, Mehrotra P, Stam J, Snesrud E, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cannon JP, Lee TA, Bolanos JT, Danziger LH. Pathogenic relevance of lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis. 2005;24:31–40. [DOI] [PubMed] [Google Scholar]
- 128. McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. A prospective randomised study of the probiotic lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut. 2002;51:827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Barraud D, Blard C, Hein F, Marçon O, Cravoisy A, Nace L, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36:1540–7. [DOI] [PubMed] [Google Scholar]
- 130. Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250:712–9. [DOI] [PubMed] [Google Scholar]
- 131. Górski A, Dąbrowska K, Międzybrodzki R, Weber-Dąbrowska B, Łusiak-Szelachowska M, Jończyk-Matysiak E, et al. Phages and immunomodulation. Future Microbiol. 2017;12:905–14. [DOI] [PubMed] [Google Scholar]
- 132. Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep. 2017;7:8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pirisi A. Phage therapy--advantages over antibiotics? Lancet. 2000;356:1418. [DOI] [PubMed] [Google Scholar]
- 134. Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010;28:591–5. [DOI] [PubMed] [Google Scholar]
- 135. Wu M, Hu K, Xie Y, Liu Y, Mu D, Guo H, et al. A novel phage PD-6A3, and its Endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front Microbiol. 2018;9:3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gorski A, Jonczyk-Matysiak E, Lusiak-Szelachowska M, Miedzybrodzki R, Weber-Dabrowska B, Borysowski J. The potential of phage therapy in sepsis. Front Immunol. 2017;8:1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gatea Kaabi SA, Musafer HK. New phage cocktail against infantile sepsis bacteria. Microb Pathog. 2020;148:104447. [DOI] [PubMed] [Google Scholar]
- 138. Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5:465–72. [DOI] [PubMed] [Google Scholar]
- 139. Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25:803–814.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


