Abstract
Post-Traumatic Stress Disorder (PTSD) is not solely a psychiatric disorder; it also includes significant medical morbidity. Although there is evidence of increased risk of metabolic syndrome (MetS) in PTSD, the interpretation of previous studies is confounded by inclusion of people on antipsychotic medications, which independently cause increased MetS. In this study we investigated whether Veterans with PTSD not treated with antipsychotic medications (n=115) demonstrate increased MetS compared to an age-comparable group of people from the U.S. National Health and Nutrition Examination Survey (NHNES; n=1005). Using standardized criteria (abnormal values in 3 out of the 5 domains of obesity, hypertension, high density lipoprotein, triglyceride and fasting glucose concentrations) we compared the prevalence of MetS across groups. Relative to the NHNES group, a significantly higher proportion of the Veteran PTSD group met criteria for MetS (26.9% vs. 41.7%) with a higher proportion of abnormal values in four out of five MetS domains (excepting glucose). Our results suggest that the elevation of MetS associated with PTSD cannot be fully explained by iatrogenic effects of antipsychotic medication. We suggest that extra attention be devoted to the clinical management of metabolic risk factors for morbidity in patients with PTSD.
Keywords: Diabetes, cardiovascular, accelerated aging, obesity, hypertension, lipids
INTRODUCTION
Post-Traumatic Stress Disorder (PTSD) is one of the leading psychiatric disorders in terms of VA mental health service utilization, representing 40–60% of the OEF/OIF Veterans treated in VA mental health clinics (Seal, Bertenthal, Miner, Sen, & Marmar, 2007; Seal et al., 2009). Although access to and provision of evidenced-based mental health services are critical to meeting the treatment needs for these Veterans, PTSD is frequently associated with comorbid medical conditions including type 2 diabetes and cardiovascular disease, as well as early mortality, and these warrant coordinated care among mental health and physical health care providers (Babic et al., 2007; Boscarino, 2008; Dirkzwager, van der Velden, Grievink, & Yzermans, 2007; Edmondson & Cohen, 2013; Edmondson, Kronish, Shaffer, Falzon, & Burg, 2013; Farr, Sloan, Keane, & Mantzoros, 2014; Glaesmer, Brahler, Gundel, & Riedel-Heller, 2011; Heppner et al., 2009; Jin et al., 2009; Levine, Levine, & Levine, 2014; Lohr et al., 2015; Michopoulos, Vester, & Neigh, 2016). One of the major risks for increased morbidity and death in this population is Metabolic Syndrome (MetS) (Brown, 1997; Colton & Manderscheid, 2006; Osby, Correia, Brandt, Ekbom, & Sparen, 2000).
The concept of MetS was introduced in 1988 by Reaven (originally called “syndrome x” or “insulin resistance syndrome”), and has since come to be widely acknowledged as a significant risk factor for development of type 2 diabetes and cardiovascular disease (Ford, 2005; Lorenzo, Williams, Hunt, & Haffner, 2007; Reaven, 1988). A number of specific criteria for MetS have been proposed since Reaven’s article, with variation in terms of specific requirements - e.g. whether or not a person diagnosed with MetS must exhibit obesity or, if instead, the signs constitute a “fuzzy set” with no specific sign/symptom necessary or sufficient. However the defining components have consistently involved several co-occurring signs including obesity, hypertension (HTN), insulin resistance, and dyslipidemia (Alberti et al., 2009; Alberti, Zimmet, & Shaw, 2005; Grundy et al., 2005) For example, per the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria (Expert Panel on Detection & Adults, 2001), MetS is defined by presence of any three or more of the following five signs: (1) obesity; (2) elevated systolic or diastolic blood pressure or treatment with antihypertensive medications; (3) elevated fasting blood glucose concentration or treatment with hypoglycemic medications; (4) elevated high-density lipoprotein (HDL) cholesterol level or medical treatment for elevated cholesterol; and (5) elevated triglyceride level or medical treatment for elevated triglycerides. Combined presence of three or more of any of the above five factors is associated with an increased risk of type 2 diabetes, cardiovascular disease (heart attack or stroke), and early mortality (Lorenzo et al., 2007).
There have been several previous studies showing an increased prevalence of MetS in PTSD – however none of these, to our knowledge, has taken into account treatment with antipsychotic medications (Babic et al., 2007; Babic, Maslov, Babic, & Vasilj, 2013; Blessing et al., 2017; Heppner et al., 2009; Jakovljevic, Saric, Nad, Topic, & Vuksan-Cusa, 2006; Jin et al., 2009; Linnville, Hoyt, Moore, Segovia, & Hain, 2011; Takahashi et al., 2020; Violanti et al., 2006; Weiss et al., 2011). Antipsychotic, and particularly second generation antipsychotic, medications have been considered to carry with them serious increased metabolic and cardiovascular risk (Daumit et al., 2008; Doménech-Matamoros, 2020; Goff et al., 2005; Jin, Meyer, & Jeste, 2004; Kameg & Champion, 2021; Li et al., 2021; Wirshing et al., 2002).
Most of the available data on prescribing patterns of antipsychotic medications in the treatment of PTSD are from U.S. Veterans returning from the Iraq/Afghanistan conflicts up through 2013. Estimates of antipsychotic treatment among such Veterans range from 11.8% (Nobles et al., 2017), 13.9% (Bernardy, Lund, Alexander, & Friedman, 2012), 18.9%, (Cohen, Shi, Neylan, Maguen, & Seal, 2015), and 21.8% (Krystal et al., 2017). Use of antipsychotics in the treatment of PTSD appears to have dropped. Some data suggest a decrease in such prescriptions. The VA data reported by Holder et al. (2021) showed such prevalence of prescriptions changed for from 12.4% in 2009 to 7.0% in 2018. In an examination of administrative data from active duty U.S. military personnel with PTSD, Loeffler, Coller, Tracy, & Derderian (2018) found use of antipsychotics decreased from 22.6% in 2007 to 14.6% in 2013. Additional data have been reported in two studies from Germany. Bachmann Czwikla, Jacobs, Fegert, & Hoffmann (2021) found 14.1% percent of Germans with PTSD in 2017 were prescribed antipsychotic medications. The above rates were for predominantly outpatient samples. The acute use of antipsychotic medications for inpatient treatment of PTSD may be substantially higher as a dataset of inpatients with PTSD in German-speaking countries, 58.4% received an antipsychotic while an inpatient in 2017 (Reinhard et al., 2020).
Although patients with PTSD may be treated with antipsychotic medications, there are reasons to believe that antipsychotic medications may not completely account for earlier findings of increased metabolic syndrome in PTSD. A previous study from our laboratory found that PTSD severity among Veterans continued to be a significant and unique predictor of risk for metabolic syndrome, after taking into account antipsychotic medications - but this study did not compare prevalence of metabolic syndrome in PTSD with a control sample (Heppner et al., 2012). Additionally, a study of obesity in Veterans with PTSD found that antipsychotic medications could not explain the increase in obesity seen in these Veterans (Vieweg et al., 2006). We are unaware of any previous studies which directly analyzed the prevalence of MetS in patients with PTSD who were not being treated with antipsychotic medications, so it is unclear exactly to what extent having a diagnosis of PTSD is in itself associated with increased metabolic risks.
In the present study, we sought to examine the prevalence of MetS in a clinical sample of Veterans with PTSD who were not treated with antipsychotic medications. We compared these rates to those in a subsample of respondents from the general U.S. population using data from the National Health and Nutrition Examination Survey (NHNES). We hypothesized that prevalence of MetS in Veterans with PTSD would be greater than an age-comparable normative population sample.
METHODS
This project involved retrospective combination and comparison of existing data from two systems. Data for the Veteran PTSD group was collected with an IRB-approved HIPAA-consent waiver through review of the VASDHS Computerized Patient Record System (CPRS) charts, with pre-selected variables entered into a password-protected Excel spreadsheet in de-identified form (with no personally identifiable information). In order to compare rates of MetS in the PTSD group relative to the general population, comparison data was used from the public, de-identified datasets from the 2015–2016 release of the National Health and Nutrition Examination Survey (NHNES) (National Center for Health Statistics, 2019). The NHANES is an ongoing study of the health and nutrition of the non-institutionalized United States (U.S.) civilian population. For the overall NHANES A form of sequenced stratified sampling was used, with oversampling of select demographic subgroups (to increase precision of subgroup parameter estimates) (Chen, Clark, Riddles, Mohadjer, & Fakhouri, 2020).
The data for PTSD subjects were obtained by review of existing clinical records for Veterans ages 25 to 54 years being psychiatrically treated for PTSD by any of five psychiatrists during the preceding year. Inclusion criteria included: a) DMS-5 diagnosis of PTSD (as established by treating clinician), b) age of 25–54 years old, c) seen in the VASDHS PTSD clinic within a 2-month time period of data extraction, and d) vital signs and laboratory results recorded within ±1 year of the index clinic visit where BMI was obtained. Exclusion criteria included: (a) current or past documented treatment with antipsychotic medications, or (b) absence of data for any of the five MetS criteria within the previous year.
Data for respondents in the NHNES datasets were combined to provide information on age, gender, race/ethnicity, medications, as well as data on the above five MetS criteria. Data from NHNES respondents were excluded if the respondent was younger than 25 or older than 54 years, or if the respondent was on antipsychotic medication or receiving treatment for type 1 diabetes (for comparability to the Veteran group because individuals with type 1 diabetes are excluded from entering the military).
Data extraction:
PTSD Sample:
Data were extracted from the CPRS database by two of the co-authors (CS and MM) under the supervision of a VA Staff Psychiatrist co-investigator (HJ). All measures extracted were anchored on date of BMI information, with the closest value to that BMI assessment being selected, with a maximum range of ±12 months. Data on glucose were corrected using the conversion formula from the NHNES to address discrepancies in machine calculation (https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/GLU_I.htm). Data were initially entered in an Excel spreadsheet, but subsequently imported into an SPSS dataset using the import function of SPSS 27.0.
NHNES data:
Files downloaded and merged from the NHNES website included those containing the information for demographics (DEMO_I.XTP), systolic and diastolic blood pressure (BPX_I.XPT), BMI (BMX_I.XPT), HDL (HDL_I.XPT), triglycerides (TRIGLY.XPT), plasma fasting glucose (GLU_I.XPT), prescription medications and reported reason for the medication (R_RX_I.XPT). The publicly available datasets are in SAS format, but for analysis all files were converted using the import function of SPSS 27.0. Files were merged using the sequence identifier variable (SEQN) which uniquely identifies each person for merging records across files.
Determination of MetS:
For the measurement of obesity we chose to use a BMI cutoff of 30, and this was used for both the Veterans and the control subjects from NHNES. (Although often defined in reference to waist-circumference, the VA system does not routinely measure waist circumference). For the remainder of the domains, we used the following National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) (Expert Panel on Detection & Adults, 2001). Thus, the criteria were:
obesity - BMI ≥ 30
systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg, or active antihypertension treatment
fasting blood glucose concentration ≥ 100 mg/dL or active pharmacologic treatment for elevated glucose
blood high-density lipoprotein cholesterol (HDL) concentration < 40 mg/dL or active pharmacologic treatment for abnormal cholesterol
blood triglyceride concentration ≥ 150 mg/dL or active pharmacologic treatment for elevated triglycerides.
For both groups, MetS was then determined to be present if the individual scored outside of cutoff ranges for at least 3 out of the 5 metabolic markers. [Defining MetS as having 3 out of 5 markers is the general standard in current literature (Aguilar, Bhuket, Torres, Liu, & Wong, 2015; Alexander et al., 2003; Beltran-Sanchez, Harhay, Harhay, & McElligott, 2013; Saklayen, 2018).]
Statistical Analysis:
The sample sizes for present analyses included 115 Veterans with PTSD, and 1,005 respondents from the NHNES. All analyses were conducted with SPSS 27.0. The demographic characteristics and each component of the MetS were summarized with descriptive statistics (Mean and SD, or proportion). Between-group comparisons of categorical variables were made using Pearson’s Chi-square tests, and those involving continuous variables were compared via independent t-tests. We also conducted exploratory analyses restricting the comparison to males (due to higher proportion of male Veterans) and to a subsample of the NHNES stratified to the PTSD group in terms of race/ ethnicity. Significance was defined as p<.05 (two-tailed).
RESULTS
Sample description:
The mean ages (and SD) of the NHNES and PTSD groups were 39.3 (8.3) vs. 41.7 (8.0) years. Although statistically significant, t (1018) = 2.83, p=.005, this equates to a small effect size difference (Cohen’s d = 0.29). The NHNES sample had a lower proportion of males relative to the PTSD group, 47.7% vs. 90.8%, X2[1, N=1114] = 73.4, p<.001. The latter is consistent with the general focus of the two samples (general population survey vs. Veteran/VA PTSD clinic patients). The PTSD group also had a larger proportion of Caucasians, relative to the NHNES sample, 41.1% vs. 28.8%, X2[1, N=1117] = 7.28, p = .007.
Metabolic syndrome components:
As shown in Table 1, relative to the NHNES group, the PTSD sample had significantly worse mean values on three of the five MetS measures (BMI, blood pressure, HDL cholesterol, and triglycerides, all t-values > 2.26, all p-values<.05), and were more likely to be receiving medication to control HTN and glucose (both X2-values > 5.80, both p-values<.05). There were no significant group differences in fasting glucose levels, t(1118) = .891, p=.929, or proportion of individuals receiving medication to control cholesterol or triglyceride levels, X2[1, N=1120] = 0.76, p = .385.
Table 1.
Metabolic syndrome values and medication status for NHNES vs. PTSD
| NHNES | PTSD | ||||
|---|---|---|---|---|---|
| Variables | (n=1005) | (n=115) | t or X2 | df | p |
| Age (years) | 39.3 (8.6) | 41.7 (8.0) | 2.83 | 1118.0 | .005 |
| Gender (% Male) | 47.4%% | 90.8% | 73.39 | 1.0 | <.001 |
| % non-Latinx Caucasian | 28.8% | 41.1% | 7.28 | 1.0 | .007 |
| Race/Ethnicity | 31.23a | 5 | <.001 | ||
| non-Latinx Caucasian | 28.8% | 41.1% | |||
| African-American | 19.9% | 10.7% | |||
| Latinx | 31.7% | 23.2% | |||
| Asian/Pacific Islander | 15.5% | 17.9% | |||
| Other | 4.1% | 3.6% | |||
| Decline to state | 0.0% | 3.6% | |||
| BMI | 29.6 (7.3) | 31.0 (5.4) | 2.55 | 167.5 | .012 |
| Systolic BP (mm Hg) | 120.3 (15.7) | 123.0 (11.6) | 2.27 | 166.2 | .025 |
| Diastolic BP (mm Hg) | 71.8 (11.3) | 78.2 (8.8) | 7.05 | 160.1 | <.001 |
| Fasting glucose (mg/dL) | 105.9 (32.0) | 105.6 (35.9) | 0.89 | 1118.0 | .929 |
| HDL cholesterol (mg/dL) | 54.5 (16.4) | 46.0 (12.0) | 6.81 | 166.5 | <.001 |
| Triglycerides (mg/dL) | 113.2 (85.7) | 145.3 (109.8) | 3.03 | 130.4 | .003 |
| ↓HTN medication | 10.8% | 26.1% | 22.05 | 1.0 | <.001 |
| ↓Glucose medication | 3.3% | 7.8% | 5.90 | 1.0 | .015 |
| ↓Cholesterol or triglyceride medication | 5.4% | 3.5% | 0.76 | 1.0 | .385 |
Likelihood Ration presented as three cells had expected counts less than 5.
Note: values represent mean (and SD) or proportion.
Key to abbreviations: National Health and Nutrition Examination Survey = NHNES, Post-traumatic Stress Disorder = PTSD, Body Mass Index = BMI, blood pressure = BP, millimeters mercury = MM Hg, milligrams per deciliter = mg/dL, for prescribed medications ↓ = prescribed medication to control the specified sign.
Observed rates of metabolic syndrome (MetS) and component signs:
As shown in Table 2, relative to the NHNES group, the Veteran PTSD group had significantly higher rates of MetS, 26.9% vs. 41.7%, respectively, X2[1, N=1120] = 11.23, p < .001. This pattern remained largely similar when restricted to male subjects (33.4% vs. 43.4%, respectively), and when using a random subsample of the NHNES (n=570) that was stratified by race/ethnicity to the match the proportions in the PTSD sample (28.6% vs. 417%).
Table 2.
Prevalence of positive metabolic syndrome (MetS) signs and overall prevalence of MetS
| NHNES | PTSD | ||||
|---|---|---|---|---|---|
| MetS sign | (n=1005) | (n=115) | X2 | df | p |
| BMI ≥ 30 | 39.2% | 50.4% | 5.41 | 1 | .020 |
| HTN or HTN medication | 30.0% | 42.6% | 7.70 | 1 | .006 |
| Fasting glucose ≥ 100 mg/dL or medication | 51.0% | 44.3% | 1.85 | 1 | .174 |
| HDL < 40 mg/dL or medication | 21.6% | 34.8% | 10.16 | 1 | .001 |
| Triglycerides ≥ 150 mg/dL or medication | 25.4% | 37.4% | 7.63 | 1 | .006 |
| Metabolic Syndrome (3 or more of 5 signs) | 26.9% | 41.7% | 11.23 | 1 | <.001 |
Association of age and MetS:
As a secondary exploratory analysis, we also examined the association between age and presence of MetS. As illustrated in Figure 1 in terms of the rates of MetS among each of three decade-long age bins (25–34, 35–44, and 45–54), there appeared to be increasing rates of MetS with age, which was most strongly evident among those with PTSD in the 45–54 year age group versus the 35–44 age group (58.8% vs. 32.5%, respectively). The parallel increase within the NHNES sample was 28.8% at age 35–44 years vs. 34.4% age 45–54 years. A differential age association was also seen when the data were examined using point-biserial correlations between MetS status and age, i.e. for NHNES and PTSD groups, r = .171 vs. r = .383 respectively (both p-values <.001).
Figure 1.
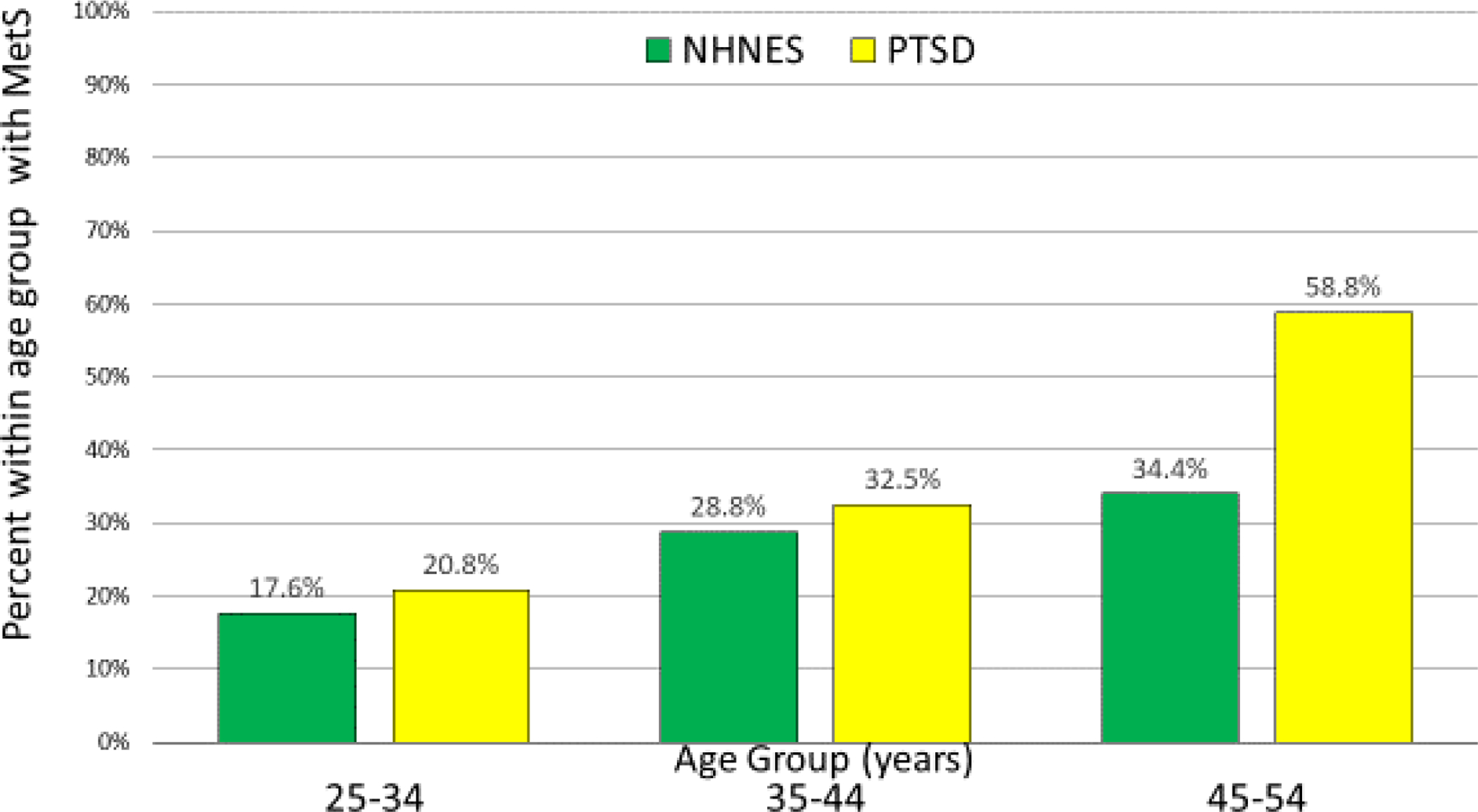
Prevalence of Metabolic Syndrome by sample and age group.
DISCUSSION
Our study indicates that Metabolic Syndrome (MetS) prevalence was significantly higher among Veterans with PTSD untreated with antipsychotic medications compared to the rates seen in a comparison sample of age-comparable respondents in the National Health and Nutrition Examination Survey (NHNES). This pattern was also found when the analyses were restricted to data from male Veterans with PTSD and male NHNES respondents. This finding of elevated MetS rates is consistent with previous studies of MetS in PTSD (Heppner et al., 2009; Jin et al., 2009), but to our knowledge this is the first study to examine the prevalence within a PTSD sample that excluded patients receiving antipsychotic medications (which are themselves a significant risk factor for MetS). In short, it appears that the elevation in MetS associated with PTSD cannot be fully explained by iatrogenic effects of antipsychotic medication.
Although we found that four of the five metabolic domains showed greater impairment in the PTSD group, in the case of glucose there was a non-statistically significant group difference, and the pattern was actually for higher rates of elevated glucose in the NHNES sample relative to the PTSD group. The reason for the relatively higher rates of elevated fasting glucose in the NHNES sample is not clear. It is possible that a larger proportion of the latter sample had not actually fasted, but this seems unlikely because the self-report data indicated that all participants had fasted at least 8 hours (data not shown), and there is little reason to expect differential problems with self-reported fasting in the NHNES vs. PTSD groups. Another possibility is that MetS in PTSD may not involve elevated glucose levels to the same extent as in the control population, although it is not clear why this should be the case.
There are some caveats/limitations in drawing definitive interpretations from the present study alone. Foremost, this study represents a retrospective analysis of data originally generated from two groups for distinct purposes (clinical care vs. population survey research). Also, the PTSD group was a convenience sample of patients of five specific providers in our local VA clinic, and was comprised of predominantly male Veterans, and may not be representative of other populations of patients with PTSD. However, because this is a general psychiatric clinic at VA San Diego, there was no preferential enrollment into the clinic for any particular clinical reason. We considered utilizing a demographically matched subsample of participants from the NHNE sample, but decided against doing so because the demographic differences in the non-stratified sample were in the conservative direction (minimizing Type 1 error) in testing the hypothesis that PTSD would be associated with higher rates of MetS. Due to the convenience sampling, we were limited by the data available for the PTSD. Thus, we measured obesity in reference to BMI, which has been employed in earlier MetS criteria, but waist circumference has been the preferred measure in recent formulations (Saklayen, 2018). The findings are also limited to those ages 25 to 54. This range was select in part due to the relatively limited numbers outside that range in this general clinic. However, another consideration was prior evidence of a substantial upswing in the prevalence of MetS with advancing age, as well as substantial increases in other medical comorbidities which could have obscured the PTSD-MetS association (Rigamonti et al., 2021; Shin, Kongpakpaisarn, & Bohra, 2018).
Another limitation is that we did not study a group of Veterans without PTSD, so it is unclear whether there may be other issues relating to being a Veteran that could put one at increased risk for MetS. Such studies are difficult to perform, however, because there are many confounds that need to be addressed when considering Veterans who have developed PTSD vs those who do not, including type of deployment and combat exposure, lack of reporting of PTSD symptoms, partial PTSD syndromes, and differences in resilience factors.
In regard to gender, when we analyzed only the data from males from both groups, we still found an increase in the percentage of individuals with MetS. Other interpretative caveats are the small sample size of the PTSD group (n=115) relative to that drawn from the NHNES database (n=1005), and that the diagnosis for the PTSD sample were based on clinical assessment rather than on a structured interview such as the Structured Clinical Interview for DSM-5 or SCID.(Structured Clinical Interview for DSM-5 (R) Disorders -- Clinician Version (SCID-5-CV), 2015) Finally, although our PTSD and control subject samples had not received antipsychotic medications for at least one to two years, it was not possible to determine with complete confidence whether they had ever received such medications in the past, although we had no documented evidence that this was the case.
The above caveats and limitations noted, the present results are of substantial importance as they suggest that, independent of any deleterious effects of antipsychotic medications, Veterans with PTSD have an elevated risk of MetS, which is in turn known to be a significant factor for type 2 diabetes, cardiovascular disease, and early mortality. Further studies are warranted, particularly studies using identical methods for the assessment of metabolic indices in Veterans with and without PTSD and including larger proportions of women. Studies should also look at the morbidity outcomes of such data, including cardiovascular and metabolic outcomes, to determine the clinical significance of these findings. Further prospective research is also needed to better elucidate the underlying mechanisms of this association of PTSD with MetS.
A variety of different hypotheses have been proposed for an association between PTSD and MetS (Jin et al., 2009; Rosenbaum et al., 2015). These have included chronic effects of stress on cortisol and a related increase in insulin resistance (Agyemang, Goosen, Anujuo, & Ogedegbe, 2012; Blessing et al., 2017), an increase in catecholamines (Pervanidou & Chrousos, 2012), and increase in inflammatory processes including increased cytokines (Farr et al., 2014; Michopoulos et al., 2016) and an increase in unhealthy lifestyle among patients with PTSD, particularly including smoking (Agyemang et al., 2012; Calhoun et al., 2011; Dennis et al., 2014; Dirkzwager et al., 2007). Based on our prior systematic review we have considered that the increase in metabolic abnormalities may be a component of a larger issue in which there is an acceleration of aging processes in PTSD (Lohr et al., 2015). This may tie into a variety of different pathophysiological processes, including increased oxidative damage and inflammatory processes in PTSD in general. However, at this time, the underlying mechanisms for any relationship between PTSD and MetS remain unknown.
The present data strongly indicate the need for clinicians to pay close attention to the high risks of metabolic morbidity in Veterans with PTSD, and not solely among those patients treated with antipsychotic medications. This is critical for the development of comprehensive personalized clinical care and effective coordination among mental health and other health care providers.
Acknowledgements:
The authors wish to thank Lisa Camball, M.D. Jenny Yi, M.D., Abigail Angkaw, Ph.D., and Nicholas Mellos, M.D.
Conflicts of Interests and Source of Funding:
The authors have no conflicts of interest to declare. This study was supported, in part by VA Merit Award 1I01 CX001351, and the Department of Veterans Affairs.
Data availability statement:
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- Aguilar M, Bhuket T, Torres S, Liu B, & Wong RJ (2015). Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama, 313(19), 1973–1974. [DOI] [PubMed] [Google Scholar]
- Agyemang C, Goosen S, Anujuo K, & Ogedegbe G (2012). Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. Eur J Public Health, 22(5), 658–662. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, … Smith SC Jr. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120(16), 1640–1645. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, & Shaw J (2005). The metabolic syndrome--a new worldwide definition. Lancet, 366(9491), 1059–1062. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National, H., Nutrition Examination, S., & National Cholesterol Education, P. (2003). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes, 52(5), 1210–1214. [DOI] [PubMed] [Google Scholar]
- Babic D, Jakovljevic M, Martinac M, Saric M, Topic R, & Maslov B (2007). Metabolic syndrome and combat post-traumatic stress disorder intensity: preliminary findings. Psychiatr Danub, 19(1–2), 68–75. [PubMed] [Google Scholar]
- Babic R, Maslov B, Babic D, & Vasilj I (2013). The prevalence of metabolic syndrome in patient with posttraumatic stress disorder. Psychiatr Danub, 25 Suppl 1, 45–50. [PubMed] [Google Scholar]
- Bachmann CJ, Czwikla J, Jacobs H, Fegert JM, & Hoffmann F (2021). [Prevalence and Treatment of Posttraumatic Stress Disorder in Germany: An Analysis of Nationwide Health Insurance Data, 2008 vs 2017 [English-language abstract of German-language journal article]. Psychiatr Prax. [DOI] [PubMed] [Google Scholar]
- Beltran-Sanchez H, Harhay MO, Harhay MM, & McElligott S (2013). Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol, 62(8), 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy NC, Lund BC, Alexander B, & Friedman MJ (2012). Prescribing trends in veterans with posttraumatic stress disorder. The Journal of clinical psychiatry, 73(3), 297–303. [DOI] [PubMed] [Google Scholar]
- Blessing EM, Reus V, Mellon SH, Wolkowitz OM, Flory JD, Bierer L, … Marmar CR (2017). Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology, 82, 91–97. [DOI] [PubMed] [Google Scholar]
- Boscarino JA (2008). A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosomatic Medicine, 70(6), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S (1997). Excess mortality of schizophrenia. A meta-analysis. British Journal of Psychiatry, 171, 502–508. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Levin HF, Dedert EA, Johnson Y, Va Mid-Atlantic Mental Illness Research, E. C. C. R. W., & Beckham JC (2011). The relationship between posttraumatic stress disorder and smoking outcome expectancies among U.S. military veterans who served since September 11, 2001. Journal of traumatic stress, 24(3), 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Clark J, Riddles MK, Mohadjer LK, & Fakhouri THI (2020). National Health and Nutrition Examination Survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat, 2(184). [PubMed] [Google Scholar]
- Cohen BE, Shi Y, Neylan TC, Maguen S, & Seal KH (2015). Antipsychotic prescriptions in Iraq and Afghanistan veterans with posttraumatic stress disorder in Department of Veterans Affairs healthcare, 2007–2012. J Clin Psychiatry, 76(4), 406–412. [DOI] [PubMed] [Google Scholar]
- Colton CW, & Manderscheid RW (2006). Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis, 3(2), A42. [PMC free article] [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, … Lieberman JA (2008). Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Research, 105(1–3), 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Ulmer CS, Calhoun PS, Sherwood A, Watkins LL, Dennis MF, & Beckham JC (2014). Behavioral health mediators of the link between posttraumatic stress disorder and dyslipidemia. J Psychosom Res, 77(1), 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkzwager AJ, van der Velden PG, Grievink L, & Yzermans CJ (2007). Disaster-related posttraumatic stress disorder and physical health. Psychosomatic Medicine, 69(5), 435–440. [DOI] [PubMed] [Google Scholar]
- Doménech-Matamoros P (2020). Influence of the use of atypical antipsychotics in metabolic syndrome. Rev Esp Sanid Penit, 22(2), 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, & Cohen BE (2013). Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis, 55(6), 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, & Burg MM (2013). Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J, 166(5), 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection, E., & Adults, T. o. H. B. C. i. (2001). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA, 285(19), 2486–2497. [DOI] [PubMed] [Google Scholar]
- Farr OM, Sloan DM, Keane TM, & Mantzoros CS (2014). Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metabolism, 63(12), 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES (2005). Risks for All-Cause Mortality, Cardiovascular Disease, and Diabetes Associated With the Metabolic Syndrome. A summary of the evidence, 28(7), 1769–1778. [DOI] [PubMed] [Google Scholar]
- Glaesmer H, Brahler E, Gundel H, & Riedel-Heller SG (2011). The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosomatic Medicine, 73(5), 401–406. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, … Lieberman JA (2005). A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res, 80(1), 45–53. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, … Costa F (2005). Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. [DOI] [PubMed] [Google Scholar]
- Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, … Baker DG (2009). The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner PS, Lohr JB, Kash TP, Jin H, Wang H, & Baker DG (2012). Metabolic syndrome: relative risk associated with post-traumatic stress disorder (PTSD) severity and antipsychotic medication use. Psychosomatics, 53(6), 550–558. [DOI] [PubMed] [Google Scholar]
- Holder N, Woods A, Neylan TC, Maguen S, Seal KH, Bernardy N, … Cohen BE (2021). Trends in Medication Prescribing in Patients With PTSD From 2009 to 2018: A National Veterans Administration Study. The Journal of clinical psychiatry, 82(3), 0–0. [DOI] [PubMed] [Google Scholar]
- Jakovljevic M, Saric M, Nad S, Topic R, & Vuksan-Cusa B (2006). Metabolic syndrome, somatic and psychiatric comorbidity in war veterans with post-traumatic stress disorder: Preliminary findings. Psychiatr Danub, 18(3–4), 169–176. [PubMed] [Google Scholar]
- Jin H, Lanouette NM, Mudaliar S, Henry R, Folsom DP, Khandrika S, … Jeste DV (2009). Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol, 29(3), 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Meyer JM, & Jeste DV (2004). Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophrenia Research, 71(2–3), 195–212. [DOI] [PubMed] [Google Scholar]
- Kameg B, & Champion C (2021). Atypical antipsychotics: Managing adverse effects. Perspect Psychiatr Care. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Davis LL, Neylan TC, Raskind MA, Schnurr PP, Stein MB, … Huang GD (2017). It Is Time to Address the Crisis in the Pharmacotherapy of Posttraumatic Stress Disorder: A Consensus Statement of the PTSD Psychopharmacology Working Group. Biological Psychiatry, 82(7), e51–e59. [DOI] [PubMed] [Google Scholar]
- Levine AB, Levine LM, & Levine TB (2014). Posttraumatic stress disorder and cardiometabolic disease. Cardiology, 127(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Li L, Yoo ES, Li X, Wyler SC, Chen X, Wan R, … Liu C (2021). The atypical antipsychotic risperidone targets hypothalamic melanocortin 4 receptors to cause weight gain. Journal of Experimental Medicine, 218(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnville S, Hoyt RE, Moore JL, Segovia F, & Hain RE (2011). Posttraumatic stress disorder and metabolic syndrome: retrospective study of repatriated prisoners of war. Military Medicine, 176(4), 369–374. [DOI] [PubMed] [Google Scholar]
- Loeffler G, Coller R, Tracy L, & Derderian BR (2018). Prescribing Trends in US Active Duty Service Members With Posttraumatic Stress Disorder: A Population-Based Study From 2007–2013. J Clin Psychiatry, 79(4). [DOI] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, … Jeste DV (2015). Is Post-Traumatic Stress Disorder associated with premature senescence? A review of the literature. American Journal of Geriatric Psychiatry, 23(7), 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo C, Williams K, Hunt KJ, & Haffner SM (2007). The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care, 30(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Vester A, & Neigh G (2016). Posttraumatic stress disorder: A metabolic disorder in disguise? Experimental Neurology, 284(Pt B), 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2019). National Health and Nutrition Examination Survey. Retrieved from https://www.cdc.gov/nchs/nhanes/index.htm
- Nobles CJ, Valentine SE, Zepeda ED, Ahles EM, Shtasel DL, & Marques L (2017). Usual course of treatment and predictors of treatment utilization for patients with posttraumatic stress disorder. The Journal of clinical psychiatry, 78(5), 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, & Sparen P (2000). Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. Bmj, 321(7259), 483–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, & Chrousos GP (2012). Metabolic consequences of stress during childhood and adolescence. Metabolism, 61(5), 611–619. [DOI] [PubMed] [Google Scholar]
- Reaven GM (1988). Role of Insulin Resistance in Human Disease. Diabetes, 37(12), 1595–1607. [DOI] [PubMed] [Google Scholar]
- Reinhard MA, Seifert J, Greiner T, Toto S, Bleich S, & Grohmann R (2020). Pharmacotherapy of 1,044 inpatients with posttraumatic stress disorder: current status and trends in German-speaking countries. European Archives of Psychiatry and Clinical Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti AE, Cicolini S, Tamini S, Caroli D, Cella SG, & Sartorio A (2021). The Age-Dependent Increase of Metabolic Syndrome Requires More Extensive and Aggressive Non-Pharmacological and Pharmacological Interventions: A Cross-Sectional Study in an Italian Cohort of Obese Women. International journal of endocrinology, 2021, 5576286–5576286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, & Vancampfort D (2015). The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism, 64(8), 926–933. [DOI] [PubMed] [Google Scholar]
- Saklayen MG (2018). The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep, 20(2), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Miner CR, Sen S, & Marmar C (2007). Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med, 167(5), 476–482. [DOI] [PubMed] [Google Scholar]
- Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, & Marmar CR (2009). Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. American Journal of Public Health, 99(9), 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Kongpakpaisarn K, & Bohra C (2018). Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. International Journal of Cardiology, 259, 216–219. [DOI] [PubMed] [Google Scholar]
- Structured Clinical Interview for DSM-5 (R) Disorders -- Clinician Version (SCID-5-CV). (2015). [DOI] [PMC free article] [PubMed]
- Takahashi A, Ohira T, Okazaki K, Yasumura S, Sakai A, Maeda M, … Ohira H (2020). Effects of Psychological and Lifestyle Factors on Metabolic Syndrome Following the Fukushima Daiichi Nuclear Power Plant Accident: The Fukushima Health Management Survey. J Atheroscler Thromb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg WV, Julius DA, Fernandez A, Tassone DM, Narla SN, & Pandurangi AK (2006). Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry, 8(1), 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violanti JM, Fekedulegn D, Hartley TA, Andrew ME, Charles LE, Mnatsakanova A, & Burchfiel CM (2006). Police trauma and cardiovascular disease: association between PTSD symptoms and metabolic syndrome. Int J Emerg Ment Health, 8(4), 227–237. [PubMed] [Google Scholar]
- Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, … Ressler KJ (2011). Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry, 33(2), 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, & Wirshing WC (2002). The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry, 63(10), 856–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.


