Abstract
The steroid hormone progesterone regulates proliferation and differentiation in the mammary gland and uterus by cell cycle phase-specific actions. The long-term effect of progestins on T-47D breast cancer cells is inhibition of cellular proliferation. This is accompanied by decreased G1 cyclin-dependent kinase (CDK) activities, redistribution of the CDK inhibitor p27Kip1 among these CDK complexes, and alterations in the elution profile of cyclin E-Cdk2 upon gel filtration chromatography, such that high-molecular-weight complexes predominate. This study aimed to determine the relative contribution of CDK inhibitors to these events. Following progestin treatment, the majority of cyclin E- and D-CDK complexes were bound to p27Kip1 and few were bound to p21Cip1. In vitro, recombinant His6-p27 could quantitatively reproduce the effects on cyclin E-Cdk2 kinase activity and the shift in molecular weight observed following progestin treatment. In contrast, cyclin D-Cdk4 was not inhibited by His6-p27 in vitro or p27Kip1 in vivo. However, an increase in the expression of the Cdk4/6 inhibitor p18INK4c and its extensive association with Cdk4 and Cdk6 were apparent following progestin treatment. Recombinant p18INK4c led to the reassortment of cyclin-CDK-CDK inhibitor complexes in vitro, with consequent decrease in cyclin E-Cdk2 activity. These results suggest a concerted model of progestin action whereby p27Kip1 and p18INK4c cooperate to inhibit cyclin E-Cdk2 and Cdk4. Since similar models have been developed for growth inhibition by transforming growth factor β and during adipogenesis, interaction between the Cip/Kip and INK4 families of inhibitors may be a common theme in physiological growth arrest and differentiation.
The steroid hormones estrogen and progesterone have diverse functions in female physiology and are necessary for the development of the reproductive organs, including the mammary gland. These functions commonly entail regulation of cell cycle progression; some of the largest physiological changes in the rates of cell proliferation are mediated by steroid hormones. The effects of progestins on cell proliferation are complex and display both cell and tissue specificity (6). Progestins have a transient stimulatory effect on breast cancer cells in tissue culture (39), but this is followed by a predominant inhibition of cell proliferation (6, 22, 63), consistent with the established use of progestins as therapy for advanced breast cancer (62). Like other effects of steroid hormones on cell cycle progression, these responses are cell cycle phase specific, such that only cells in G1 phase are sensitive. Consequently, G1 phase cyclin-dependent kinases (CDKs) have been implicated as mediators of the effects of progestins in these cells (13, 38, 40).
The rate of transit through G1 phase is regulated by the accumulation and activity of the G1 CDKs, cyclin D-Cdk4/6 and cyclin E-Cdk2. These kinases phosphorylate target substrates including the pocket proteins Rb, p107, and p130 (52, 53, 66). Their activity is governed by changes in cyclin abundance, interactions with CDK inhibitors, and regulatory phosphorylation (35, 53). Two structurally distinct classes of cellular CDK inhibitors with different mechanisms of action exist. The INK4 family, p16INK4a, p15INK4b, p18INK4c, and p19INK4d, form binary complexes with Cdk4 and Cdk6, thereby preventing assembly of active kinase holoenzymes (14–16, 21, 32). The Cip/Kip family, p21Cip1, p27Kip1 and p57Kip2, inhibit a broad range of cyclin-CDK complexes in vitro (18, 19, 29, 45, 61) but display greater selectivity in vivo (3, 4, 26). These proteins have dual functions, also acting as assembly factors for cyclin D-Cdk4/6 complexes at low stoichiometry (26). Since they bind multiple cyclin-CDK complexes with different affinities, the effect of altered abundance depends on the balance between the levels of different cyclin-CDK complexes and inhibitor levels.
Progestin-mediated growth inhibition of breast cancer cells is preceded by decreased cyclin abundance and marked decreases in CDK activity (13, 40). Following progestin treatment, Cdk4 complexes contain increased amounts of p27Kip1 (but not p21Cip1) (40). In addition, inactive cyclin E-Cdk2-p27Kip1 complexes which elute at high molecular mass (∼200 kDa) upon gel filtration chromatography preferentially form (40). These data suggest a model of progestin action in which decreased CDK activity is due to both decreased cyclin abundance and recruitment of CDK inhibitors, particularly p27Kip1, to the remaining G1 cyclin complexes. This recruitment occurs before any increase in p27Kip1 abundance is apparent (40). Cyclin D-Cdk4/6 complexes in these cells bind much of the cellular content of p27Kip1 and thus appear to act as a high-capacity, low-affinity reservoir for p27Kip1; their decreased abundance would make p27Kip1 available to bind cyclin E-Cdk2, for which it displays a higher affinity (19). This interpretation is supported by the observation that inducible expression of cyclin D1 in progestin-pretreated cells leads to restoration of cyclin E-Cdk2 activity and reappearance of the 120-kDa active form of cyclin E (40).
This study aimed to identify the role of CDK inhibitors in progestin-mediated growth arrest. Since it has been suggested that induction of p21Cip1 is important in progestin inhibition of proliferation (13, 28), the relative contributions of p21Cip1 and p27Kip1 were assessed. These experiments indicated a major contribution from p27Kip1, and thus several aspects of the role of p27Kip1 were examined. The large difference in apparent molecular mass between the active and inactive cyclin E-Cdk2 complexes (∼80 kDa) raises the possibility that p27Kip1 recruits an unknown protein to the complex, or is itself recruited, with the implication that increased p27Kip1 association may be a secondary effect. Furthermore, increasing evidence indicates that the level of p21Cip1 and p27Kip1 associated with Cdk4/6 is affected by the abundance of INK4 inhibitors and that alteration of this equilibrium can in turn affect the level of association between cyclin E-Cdk2 and p21Cip1 or p27Kip1 (32, 43, 49). Although progestin-sensitive T-47D cells do not express p16INK4a (20, 22a), it is possible that progestin regulation of the abundance of other INK4 proteins contributes to the lack of Cdk4 activity with consequent p27Kip1 redistribution. Finally, the likely contribution of increased p27Kip1 association to decreased activity of cyclin D-Cdk4/6 complexes following progestin treatment is unclear. While in some experimental models the activity of cyclin D1-Cdk4 or cyclin D3-Cdk4 is apparently inhibited by p27Kip1 (9, 24, 25, 27), in others active cyclin D-Cdk4/6-p27Kip1 complexes have been demonstrated (3, 57), likely because p27Kip1 does not reach levels sufficient to inhibit cyclin D-associated kinases (3). We have thus investigated the effects of recombinant p27Kip1 on CDK activity and apparent molecular weight in T-47D breast cancer cell lysate and tested the hypothesis that INK4 CDK inhibitors might be involved in progestin inhibition of proliferation, identifying a role for p18INK4c in this response.
MATERIALS AND METHODS
Cell culture and lysis.
T-47D human breast cancer cells (obtained from the EG & G Mason Research Institute, Worcester, Mass.) were cultured in RPMI 1640 medium supplemented with 5% fetal calf serum, insulin (10 μg/ml), and gentamicin (20 μg/ml). The synthetic progestin ORG 2058 (16α-ethoxy-21-hydroxy-19-norpregn-4-en-3,20-dione; Amersham Australia, Castle Hill, New South Wales, Australia) was dissolved in ethanol at 1,000-fold final concentration and added to cells in exponential growth. Control cultures received ethanol to the same final concentration. All cells were treated for 24 h unless otherwise stated.
Cells were lysed as follows. Cell monolayers were washed twice with ice-cold phosphate-buffered saline (PBS) and then scraped into ice-cold lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 200 μM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM NaF) or ice-cold kinase lysis buffer (50 mM HEPES [pH 7.5], 1 mM dithiothreitol [DTT], 150 mM NaCl, 10% [vol/vol] glycerol, 0.1% Tween 20, 1 mM EDTA, 2.5 mM EGTA, 10 mM β-glycerophosphate, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1.0 mM phenylmethylsulfonyl fluoride, 0.1 mM sodium orthovanadate, 1 mM NaF). To confirm the effectiveness of progestin treatment on cell cycle progression, an aliquot of cell suspension was analyzed by single-parameter flow cytometric DNA analysis, and the remainder was clarified and stored as described previously (40).
Generation and use of recombinant proteins.
The Rb fusion protein substrate for the Cdk4 activity assay was as used previously (40). Glutathione S-transferase (GST)–cyclin E and His6-Cdk2 were coexpressed by baculovirus infection of insect cells and purified as previously described (50). The GST tag was partially removed from cyclin E by proteolysis, and the mixture was purified by nickel-nitrilotriacetic acid (NTA) metal affinity chromatography (Qiagen, Clifton Hill, Victoria, Australia) to isolate cyclin E bound to His6-Cdk2.
His6-p27 was expressed using a construct encoding hexahistidine-tagged mouse p27Kip1 generously provided by Joan Massague. Protein was expressed as previously described (46). Briefly, His6-p27 was expressed in Escherichia coli and purified by sonication and nickel-NTA chromatography. Purified protein was dialyzed against PBS and stored at −80°C. Recombinant His6-p27 of various concentrations, control protein (His6-transglutaminase II; kindly provided by Ming Jie Wu (Victor Chang Cardiac Research Institute, Sydney, Australia) or PBS control was incubated with cellular lysates for 10 min at room temperature, followed by gel filtration chromatography, kinase assay, and/or immunoprecipitation and Western blotting. An N-terminal Flag-tagged p18INK4c bacterial expression vector was constructed by PCR cloning the full-length INK4c cDNA into the pFLAG-2 vector (Eastman Kodak, New Haven, Conn.). Flag-p18 protein was expressed in E. coli and purified under nondenaturing conditions to greater than 95% purity using Flag M2-agarose beads as directed by the manufacturer (Sigma Aldrich). Recombinant Flag-p18 or Flag-heregulin (10) was incubated with 1.5 mg of whole cell extract from proliferating cells for 30 min at 37°C in 500 μl of an ATP-regenerating buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM ATP, 40 mM phosphocreatine, 0.05 mg of creative phosphokinase per ml), essentially as described (65). Aliquots of reaction mix were then diluted and immunoprecipitated for Western blot or cyclin E-dependent kinase assay.
Western blot analysis and immunoprecipitation.
For immunoprecipitation, cell lysates were precleared by incubation with protein A-Sepharose beads (Zymed, San Francisco, Calif.) (1 h, 4°C) and then immunoprecipitated by incubation (2 h, 4°C) with cyclin E (C19), Cdk4 (H22), and Cdk6 (C21) antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Affinity-purified polyclonal antibodies to INK4 family proteins were a kind gift of David Parry (DNAX Research Institute, Palo Alto, Calif.); those used for immunoprecipitation were p15INK4b (13055) and p18INK4c (13062). Antibodies were chemically cross-linked to the protein A-Sepharose beads by incubation in dimethyl pimelimidate (5 mg/ml)–sodium tetraborate (0.2 M) (pH 9.0) for 30 min at room temperature, essentially as described previously (17). The immunoprecipitated proteins were washed with lysis buffer and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (63 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 2% SDS, 5% β-mercaptoethanol). Depleted lysates were prepared for gel filtration chromatography by three rounds of incubation with protein A-Sepharose beads (mock) or immunoprecipitation with p27Kip1 antibody alone or p21Cip1 and p27Kip1 antibodies simultaneously.
Samples of immunoprecipitated or total protein in SDS-PAGE sample buffer were heated to 95°C for 3 min and then separated by SDS-PAGE and transferred to nitrocellulose or polyvinylidene difluoride. The membranes were incubated for 1 to 2 h at room temperature or overnight at 4°C with the following primary antibodies: cyclin E (HE12), Cdk4 (C-22), and Cdk6 (C21) antibodies from Santa Cruz Biotechnology; cyclin D1 antibody (DCS6) from Novocastra, Newcastle-upon-Tyne, United Kingdom; p21Cip1 (C24420) and p27Kip1 (K25020) antibodies from Transduction Laboratories, Lexington, Ky.; Flag (M2) antibody from Eastman Kodak. Affinity-purified polyclonal antibodies to p15INK4b (13054), p18INK4c (13063), and p19INK4d (13067) were a gift of David Parry. Following incubation (1 h at room temperature) with horseradish peroxidase-conjugated sheep anti-mouse or donkey anti-rabbit secondary antibody (Amersham Australia), specific proteins were visualized by chemiluminescence (Dupont NEN, Boston, Mass.). Where the proteins of interest were of sufficiently different mobility, membranes were incubated either sequentially or simultaneously with several primary antibodies. If required, membranes were stripped as described (40).
Kinase assays.
The histone H1 kinase activity of cyclin E immunoprecipitates from 100 μg of cellular protein was measured as previously described (47), using 10 μg of histone H1 as substrate. For Cdk4 assays, cells were harvested and lysed using kinase lysis buffer as described above. Kinase activity of Cdk4 immunoprecipitates from 400 μg of cellular protein from these lysates was measured using 10 μg of GST-Rb773-928 fusion protein substrate as previously described (47). The degree of background phosphorylation in Rb phosphorylation assays was estimated from parallel control samples immunoprecipitated following blocking of specific antibody binding with the appropriate antigenic peptide. Following termination of kinase reactions samples were incubated at 95°C for 2 min in SDS-PAGE sample buffer and separated by SDS-PAGE (10% gel).
mRNA isolation and analysis.
Total RNA was extracted (using a guanidinium isothiocyanate-cesium chloride procedure), and the level of selected CDK inhibitor mRNA transcripts was quantitated by RNase protection assays following manufacturer's instructions (Riboquant in vitro transcription kit with h-CC2 template kit; PharMingen, San Diego, Calif.). Riboprobes were radiolabeled with [α-32P]UTP.
Two-dimensional gel electrophoresis.
Whole cell lysate was precipitated with trichloroacetic acid, and the total protein pellet or immunoprecipitated proteins were redissolved in IPG buffer, composed of 8 M urea, 100 mM DTT, 4% CHAPS (Sigma), 0.5% (wt/vol) carrier ampholyte pH 3 to 10 (Pharmacia Biotech, Uppsala, Sweden), 0.001% orange G, 10 mM NaF, 10 mM sodium pyrophosphate, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, and 200 μM sodium orthovanadate. Samples in buffer were absorbed overnight into immobilized pH gradient polyacrylamide gel strips (Immobiline Drystrip, pH 3 to 10, 11 cm; Pharmacia). Proteins were focused at 300 V for 5 h, then 1,000 V for 5 h, and then 3,500 V for 14 h. The polyacrylamide strip was then treated with 2% (wt/vol) DTT in equilibration buffer (0.025 M Tris [pH 8.9], 6 M urea, 2% [wt/vol] SDS, 20% [vol/vol] glycerol, 0.2 M glycine) for 10 min followed by 2.5% (wt/vol) iodoacetamide in equilibration buffer for 10 min. Proteins were then separated in the second dimension by SDS-PAGE on a 6 to 12% gradient gel, transferred to nitrocellulose, and Western blotted.
Gel filtration.
Cell lysates prepared in kinase lysis buffer were filtered and fractionated on a Hiload 16/60 Superdex 200 column/FPLC system (Pharmacia), using 20 mM HEPES (pH 7.5)–250 mM NaCl–1 mM EDTA–0.1% (vol/vol) β-mercaptoethanol–0.01% Tween 20 at a flow rate of 1.2 ml/min. The void volume of the column was 45 ml. Fractions of 2 ml were collected between elution volumes of 50 and 95 ml. Column calibration was performed under the same conditions using ferritin (440 kDa), aldolase (158 kDa), and ovalbumin (43 kDa). Eluted protein was concentrated prior to Western blotting: 500 μl of each fraction was placed at −70°C for 3 h with 2.5 ml of acetone and 10 μg of carrier bovine serum albumin protein; pellets were collected by centrifugation (15,000 × g, 10 min, 4°C), and then resuspended by boiling for 4 min in 30 μl of SDS sample buffer.
Image and data analysis.
Images captured by PhosphorImager (Molecular Dynamics 445 SI) or, for chemiluminescence, by densitometer scanning of X-ray film (Molecular Dynamics PDSI), were quantitated using IP Lab Gel H software (Signal Analytics, Vienna, Va.). Quantitation of protein levels by this method was linear over the range of intensities measured. All figures were compiled using Deneba Canvas 5.0 software.
RESULTS
Cyclin E is inactive and predominantly p27Kip1 bound after ORG 2058 treatment.
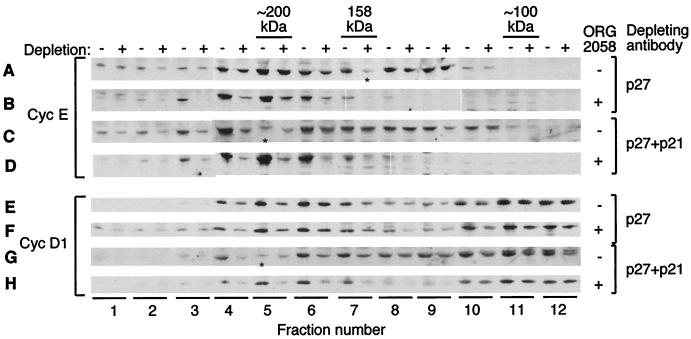
Inactivation of both cyclin E-Cdk2 and cyclin D-Cdk4 complexes by progestins is accompanied by increased p27Kip1 binding (40). However, p21Cip1 is induced by progestins (13, 40). To assess the relative levels of these inhibitors associated with the major G1 cyclins, D1 and E, lysates from vehicle- or ORG 2058-treated cells were depleted of p27Kip1 alone, p21Cip1 alone, or both p27Kip1 and p21Cip1 by immunoprecipitation, then fractionated by gel filtration chromatography, Western blotted, and compared to mock-depleted samples. Three rounds of immunoprecipitation were performed to give >90% depletion of p21Cip1 and/or p27Kip1 in all fractions. As described previously (40), cyclin E from untreated cell lysate eluted as two peaks of ∼120 kDa (kinase active) and ∼200 kDa (inactive), and following treatment only the peak of ∼200 kDa remained (Fig. 1A to D). Immunoprecipitation of p21Cip1 plus p27Kip1 depleted little of the ∼120-kDa cyclin E complexes from control lysates (Fig. 1C, fractions 8 to 10) compared to mock-depleted samples, while the complexes of ∼200 kDa (fractions 4 to 6) were partially depleted. In contrast, immunoprecipitation of p27Kip1 alone was sufficient to deplete the majority of cyclin E complexes following ORG 2058 treatment (Fig. 1B). Additional depletion of p21Cip1 from treated lysates had a minor effect (Fig. 1D), consistent with the modest effect of depletion of p21Cip1 alone. These results imply that following ORG 2058 treatment, the majority of cyclin E is complexed with p27Kip1, and that the transient induction of p21Cip1 accompanying progestin treatment (13, 40) has little effect on cyclin E-Cdk2 activity.
FIG. 1.
Association of p21Cip1 and p27Kip1 with cyclins and CDKs. Exponentially growing T-47D human breast cancer cells were treated with 10 nM ORG 2058 or ethanol vehicle, and lysates were collected 24 h after treatment. Lysates were depleted of p27Kip1 or p27Kip1 plus p21Cip1 with the relevant antibodies and protein A-Sepharose beads or mock depleted with beads alone. Depleted lysates were fractionated by gel filtration chromatography on a 16/60 Superdex 200 column, and protein complexes eluting in the range of 50 to 100 ml were collected into 2-ml fractions. Aliquots of fractions across the range of 58 to 81 ml (labeled fractions 1 to 12) were precipitated, analyzed by SDS-PAGE, and Western blotted with the indicated antibodies. Depleted samples (+) were loaded with the matching mock-depleted sample (−) for comparison. Molecular weight estimates are made using proteins of known mass, including aldolase (158 kDa), which eluted in fraction 7. Lysates treated and depleted as indicated were fractionated and blotted with a cyclin E antibody (A to D) or with an antibody to cyclin D1 (E to H). Fractions that failed to precipitate as determined by protein staining are indicated by ∗.
Cyclin D1 eluted in two main peaks of ∼160 and ∼80 kDa (Fig. 1E to H). The elution of the lower-molecular-weight form of cyclin D1 was not affected by progestin treatment, and it contained no kinase activity. Little was depleted by immunoprecipitation of p21Cip1 and p27Kip1, and further immunoprecipitation experiments suggest it is not associated with Cdk4 or Cdk6 (Fig. 1E to H, fractions 10 to 12, and data not shown). In contrast, cyclin D1 of ∼160 kDa eluted coincident with the peak of complexed Cdk4 and Cdk6 protein and with Cdk4 activity from proliferating cells and is putatively bound to Cdk4 (Fig. 1E to H, fractions 4 to 8) (40). The majority of ∼160-kDa cyclin D1 from proliferating cells was depleted by p27Kip1, and additional depletion of p21Cip1 had a minor effect (Fig. 1E and G), as did depletion of p21Cip1 alone (not shown). Treatment of cells with progestin resulted in a small increase in the proportion of ∼160-kDa cyclin D1 depleted by p27Kip1 and p21Cip1 (compare Fig. 1E and G with Fig. 1F and H). Furthermore, Western blotting of similar experiments suggested that cyclin D3 in complex with Cdk4/6 was highly depleted by p27Kip1 immunoprecipitation both before and after treatment (data not shown).
Given that the majority of cyclin D-Cdk4/6 complexes were bound to p27Kip1, these data imply that active cyclin D1-Cdk4/6 complexes contain p27Kip1 and perhaps, to a lesser extent, p21Cip1. This assertion is corroborated by our observation that p21Cip1 and p27Kip1 immunoprecipitates from proliferating cells contain an Rb kinase similar in activity to Cdk4 (data not shown). Given the small change in this association following treatment, regulation of Cdk4/6 activity by progestins is unlikely to be mediated by altered association with p21Cip1 or p27Kip1.
Addition of p27Kip1 to control lysates is sufficient to mimic the effects of ORG 2058 on cyclin E but not Cdk4 complexes.
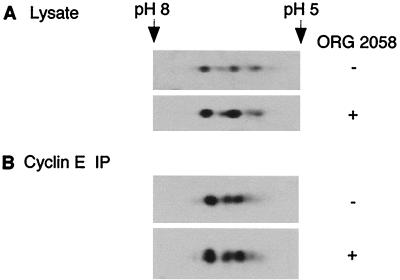
The immunodepletion studies indicated that ORG 2058 treatment increased association of p27Kip1 with cyclin E. Since binding and inactivation of cyclin-CDK complexes by CDK inhibitors can be regulated by posttranslational modification or sequestration of the inhibitor (37, 56) as well as by changes in the ratio of inhibitor to CDK, progestin treatment may induce a change in the ability of p27Kip1 to bind cyclin E complexes. The possibility that p27Kip1 was being covalently modified was investigated using two-dimensional gel electrophoresis of cell lysates followed by Western blotting. This revealed multiple p27Kip1 isoforms in control lysates (Fig. 2A), consistent with previous data (37). However, there was no change in the position or relative intensity of the four p27Kip1 isoforms following treatment (Fig. 2A). Cyclin E-associated p27Kip1 represents only a minority of total p27Kip1, and so to confirm that the finding with whole cell extract also applied to this fraction of p27Kip1, cyclin E immunoprecipitates from control and treated lysates were analyzed in the same fashion. Western blotting of p27Kip1 revealed a profile similar to whole cell lysate that was unchanged by treatment (Fig. 2B). This result implies that modifications to p27Kip1 itself do not account for the effects of ORG 2058 on cyclin-CDK complexes.
FIG. 2.
Two-dimensional electrophoretic mobility of p27Kip1. T-47D cells were treated and cellular extracts were collected as described for Fig. 1. (A) Extracts from control and treated cells were precipitated, resuspended in IPG buffer, separated by two-dimensional electrophoresis, and Western blotted with a p27Kip1 antibody. (B) Cyclin E-associated p27Kip1 was immunoprecipitated from control and treated lysates using a cyclin E antibody bound to protein A-Sepharose beads. Immunoprecipitated proteins were resuspended in IPG buffer, separated, and analyzed as for panel A. Approximate isoelectric points are indicated.
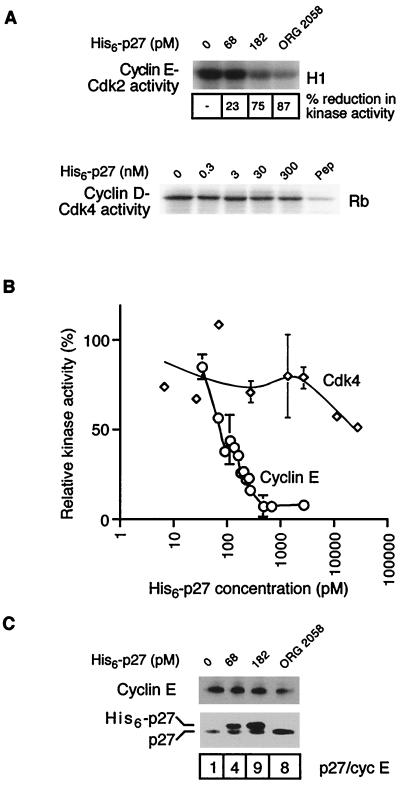
To test the hypothesis that alterations in the stoichiometry of p27Kip1 binding to cyclin E complexes might account for the altered cyclin E elution profile and kinase activity, recombinant His6-p27 was expressed in E. coli and used to determine if addition of p27Kip1 to control lysates in vitro could recapitulate the effects of progestins on cyclin E complexes. Addition of His6-p27 but not the same concentration of a control protein (His6-transglutaminase II [41]) caused a concentration-dependent decrease in cyclin E-associated kinase activity to a minimum of ∼8% of untreated samples, identical to the level of inactivation caused by ORG 2058 (Fig. 3A and B). A small increase in the concentration of His6-p27 from ∼34 to 182 pM was sufficient to bring about a majority of this decrease. Similar concentrations of recombinant p27Kip1 inhibited recombinant cyclin A-Cdk2 complexes in vitro (3). In contrast, incubation of control lysates with high concentrations (up to 300 nM) of His6-p27 led to only a limited inactivation of Cdk4-associated kinase activity (Fig. 3A). Taken together with the results shown in Fig. 1, these data suggest that while p21Cip1 and p27Kip1 bind a majority of cyclin D-Cdk4/6 complexes, p27Kip1 is unable to inhibit Cdk4 activity in our system.
FIG. 3.
Effect of His6-p27 addition on cyclin-CDK complex activity and composition. Recombinant His6-p27 was expressed in E. coli, purified on Ni-NTA beads, and incubated with extract from proliferating T-47D cells in vitro over a range of concentrations of His6-p27. (A) Cyclin E and Cdk4 were immunoprecipitated following incubation with His6-p27, the immune complexes were resuspended in kinase buffer, and associated kinase activity was determined by the transfer of [32P]phosphate to histone H1 (cyclin E) or GST-Rb (Cdk4) as described previously (40). To indicate the level of nonspecific Rb kinase activity in Cdk4 assays, a sample was immunoprecipitated in the presence of the antigenic peptide (Pep). (B) Kinase activity associated with cyclin E (○) or Cdk4 (◊) from multiple experiments was quantitated and graphed as percent control in the absence of added His6-p27. Points with error bars are representative of at least three independent experiments. (C) Cyclin E was immunoprecipitated from the lysates incubated with His6-p27 and from ORG 2058-treated cell lysate; the immune complexes were separated by SDS-PAGE and Western blotted with the indicated antibodies. The ratio of intensities of total p27Kip1 to cyclin E in the immunoprecipitate is shown.
To determine whether the level of associated p27Kip1 required to inactivate cyclin E-Cdk2 in vitro was comparable with that observed following progestin treatment in culture, cyclin E was immunoprecipitated from progestin-treated lysates and from control lysates that had been incubated with a range of His6-p27 concentrations as described above. SDS-PAGE and Western blotting for p27Kip1 revealed two bands consisting of endogenous p27Kip1 and a slower-migrating band of exogenous His6-p27 (Fig. 3C). When the ratio of total p27Kip1 bound to cyclin E was compared for samples maximally inactivated by ORG 2058 or by addition of His6-p27, it was apparent that the relative amount of p27Kip1 binding to cyclin E under both conditions was equivalent (Fig. 3A and C) although the ORG 2058-treated sample contained ∼40% less cyclin E than the comparable His6-p27-treated sample and hence had correspondingly lower kinase activity (Fig. 3A). Incubation with lower concentrations of His6-p27 led to partial kinase inactivation and lower amounts of p27Kip1 bound to cyclin E complexes. However, incubation with a control protein had no effect on p27Kip1-cyclin E association. Given the correspondence between the amount of p27Kip1 bound and cyclin E-Cdk2 activity of His6-p27-inactivated samples compared with ORG 2058-treated samples, it appears that binding of p27Kip1 contributes to the inactivation of cyclin E-Cdk2 observed following progestin treatment.
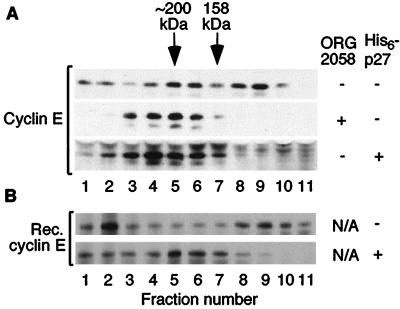
Progestin treatment causes a dramatic change in the elution of cyclin E in gel filtration chromatography. To determine the role of p27Kip1 in this change in elution profile, lysates from control cells incubated with an excess of His6-p27 were analyzed by gel filtration chromatography and Western blotting and compared to lysates from ORG 2058-treated cells. Addition of His6-p27 was sufficient to completely shift the ∼120-kDa cyclin E complexes to ∼200 kDa, indistinguishable from the shift resulting from ORG 2058 treatment (Fig. 4A).
FIG. 4.
Effect of His6-p27 on the elution profile of cyclin E-Cdk2 complexes. (A) Lysates from exponentially growing T-47D cells were incubated (as described for Fig. 3) with >1 nM His6-p27. Lysates with or without added His6-p27 and lysates from ORG 2058-treated cells were fractionated in parallel by gel filtration chromatography/SDS-PAGE and Western blotted with a cyclin E antibody as described for Fig. 1. (B) Reproduction of cyclin E gel filtration shift using recombinant components. Excess His6-p27 or buffer only was incubated with a purified preparation composed of recombinant (GST-cyclin E)-(His6-Cdk2) complexes which were partially proteolysed to cleave the GST epitope tag from cyclin E. The resulting complexes were purified by Ni-NTA affinity chromatography to remove non-Cdk2-bound cyclin E, analyzed by gel filtration chromatography, and Western blotted for cyclin E as described for Fig. 1.
These results suggest that p27Kip1 binding increases the apparent molecular mass of cyclin E complexes by ∼80 kDa, a larger shift than predicted from its molecular weight. To test whether p27Kip1 alone is sufficient to cause this shift or if other factors present in cell extract are required, excess His6-p27 was incubated with purified recombinant cyclin E-Cdk2 complexes. The resulting complexes were isolated, analyzed by gel filtration chromatography, Western blotted for cyclin E, and compared to recombinant cyclin E-Cdk2 alone. In the absence of His6-p27, a peak of cyclin E protein composed of cyclin E-Cdk2 complexes was observed at ∼120 kDa (Fig. 4B, fractions 8 to 10). Following addition of His6-p27, this peak shifted to ∼200 kDa, as was observed for cellular extracts (Fig. 4, fractions 4 to 7). Immunoprecipitation of cyclin E from fractions 5 and 9 confirmed the presence of p27Kip1 in the ∼200-kDa complexes but not in the ∼120-kDa complexes (data not shown).
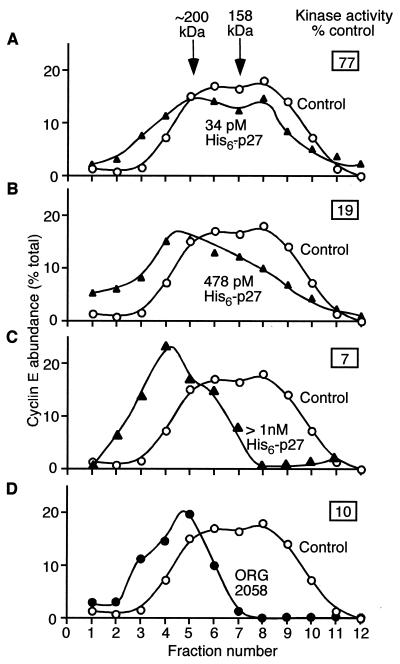
To further investigate the relationship between changes in cyclin E-Cdk2 kinase activity and gel filtration profile, concentrations of His6-p27 sufficient to partially inhibit kinase activity were incubated with extract from proliferating cells, analyzed by kinase assay and gel filtration as above, and compared to ORG 2058-treated lysate. Addition of 34 pM His6-p27 had only a small effect on kinase activity and cyclin E-Cdk2 elution profile (Fig. 5A). However, addition of 478 pM His6-p27 caused significant loss of kinase activity and a corresponding decrease in the abundance of cyclin E in fractions 8 and 9 (Fig. 5B), which account for the majority of cyclin E-Cdk2 activity in T-47D cells (40, 58). Higher concentrations of His6-p27 led to ∼90% inhibition of kinase activity and essentially complete loss of cyclin E protein in these fractions, comparable to the effects of ORG 2058 (Fig. 5C and D). Thus, across the range of His6-p27 concentrations used there was a clear relationship between the level of cyclin E kinase inactivation and the proportion of cyclin E complexes in the low (fractions 8 and 9)- and high (fractions 4 to 6)-molecular-weight fractions. These data demonstrate that the composition, activity, and stoichiometry of cyclin E-Cdk2 complexes in ORG 2058-treated lysates can be reproduced following the addition of His6-p27 to control lysates. This provides strong evidence that p27Kip1 alone is responsible for the shift in gel filtration profile of cyclin E and that it inactivates the cyclin E remaining after progestin treatment.
FIG. 5.
Quantitation of the effect of His6-p27 on the elution profile of cyclin E complexes. Lysate from exponentially growing cells was incubated with the indicated concentrations of His6-p27 (▴) as described for Fig. 3 and together with lysate from ORG 2058-treated cells (●) was fractionated by gel filtration/SDS-PAGE and Western blotted as described for Fig. 1. Densitometric analysis of the Western blot data is displayed for each treatment compared to an untreated control sample without added His6-p27 (○). The relative cyclin E kinase activity obtained from an aliquot of each incubation is also displayed.
Progestins increase p18INK4c levels and Cdk4/6 association with consequent inhibition of cyclin E-Cdk2.
In addition to the effects on cyclin E complexes and activity, treatment of T-47D cells with ORG 2058 results in ablation of Cdk4-associated kinase activity within 24 h. We have previously ruled out involvement of changes in the posttranslational modification of Cdk4 by CDK-activating kinase (CAK) following progestin treatment and have shown that inactivation is accompanied by decreased cyclin D binding to Cdk4/6 (40). The failure of His6-p27 to inhibit cellular Cdk4 in vitro (Fig. 3A) argues that p27Kip1 does not play a significant role in the inhibition of Cdk4 by ORG 2058 treatment. However, since the total levels of cyclins D1 and D3 decrease by only ∼50% following ORG 2058 treatment, it also seems unlikely that decreased Cdk4 and cyclin D abundance can wholly explain the complete loss of Cdk4-associated kinase activity. Thus, the potential involvement of the INK4 family of inhibitors was investigated.
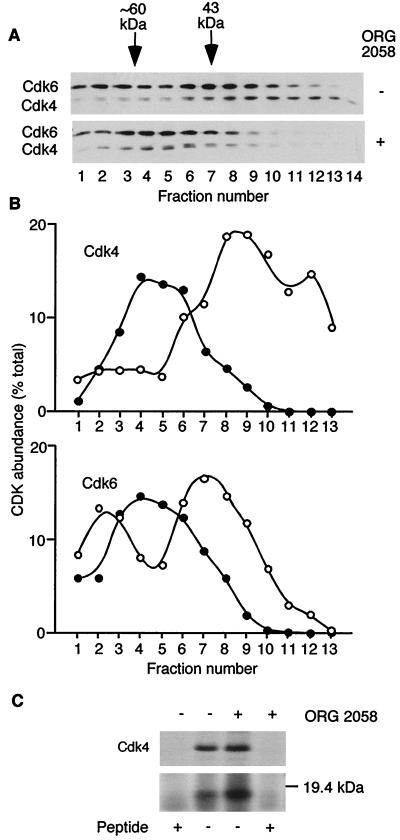
Previous reports indicate that in vivo, INK4 proteins form binary complexes with Cdk4/6 (14–16, 21, 32). These complexes elute at apparent molecular weights distinct from those of free CDK molecules upon gel filtration (8, 43). In proliferating T-47D cells, there appears to be no shortage of low-molecular-mass Cdk4, as typically ∼50% of Cdk4 is found in fractions of less than ∼80 kDa. As a first step toward identifying the role of INK4 family CDK inhibitors, high-resolution gel filtration chromatography was performed to determine whether binary Cdk4/6-INK4 complexes might be present. Lysates from control and treated cells were fractionated, and fractions corresponding to the ∼20- to 80-kDa range were analyzed by Western blotting for Cdk4 and Cdk6. Prior to treatment, most of the Cdk4 and ∼50% of the Cdk6 in this molecular mass range eluted in fractions corresponding to their native molecular masses of 33 and 53 kDa, respectively (Fig. 6A and B). Following treatment with ORG 2058, all of the low-molecular-weight Cdk4 and Cdk6 eluted at apparent molecular mass of ∼20 kDa greater than expected for the native protein, suggesting extensive association with a ∼20-kDa protein (Fig. 6A and B).
FIG. 6.
Binding of Cdk4 to a low-molecular-weight protein. (A) Lysates from control and treated cells were fractionated by gel filtration as described for Fig. 1 and collected as 1-ml fractions. Aliquots of fractions corresponding to 79 to 92 ml (labeled fractions 1 to 14) were precipitated, resuspended in SDS sample buffer, separated by SDS-PAGE, and Western blotted with antibodies to Cdk4 and Cdk6. Molecular weight estimates are made using proteins of known mass, including albumin (43 kDa), which eluted in fraction 7. (B) Densitometric data from Cdk4 and Cdk6 Western blots in panel A for untreated (○) and ORG 2058-treated (●) samples. (C) T-47D cells treated for 24 h with ORG 2058 or ethanol vehicle were metabolically labeled with [35S]methionine 4 h prior to the collection of lysates. Cdk4 was immunoprecipitated from the labeled lysates in the presence and absence of antigenic peptide, and precipitated proteins were detected by SDS-PAGE and autoradiography.
To confirm the presence of a ∼20-kDa protein in Cdk4/6 complexes, T-47D cells were metabolically labeled with [35S]methionine during treatment with ORG 2058 or vehicle, Cdk4 was immunoprecipitated, and the immune complexes were analyzed by SDS-PAGE and autoradiography. An 18-kDa band which coimmunoprecipitated with Cdk4 more than doubled in intensity following treatment (Fig. 6C) and was not apparent following preincubation of the Cdk4 antibody with antigenic peptide.
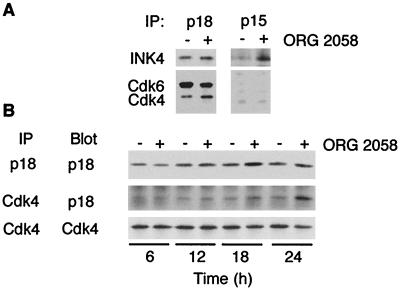
The molecular weight of the coimmunoprecipitating band suggested it was most likely a member of the INK4 family. The INK4 family consists of four structurally and functionally-related proteins, p15INK4b, p16INK4a, p18INK4c, and p19INK4d. The p16INK4a protein is not expressed in T-47D cells due to methylation of the corresponding gene promoter (20, 22a). To identify which of the INK4 proteins may be binding Cdk4 and to measure the total INK4 protein levels, INK4 family members were immunoprecipitated from T-47D cell lysate and Western blotted. Both p15INK4b and p18INK4c were detected in T-47D lysates, and in both cases, the total abundance increased following progestin treatment (Fig. 7A). In contrast, p19INK4d was not detected. Cdk4 and Cdk6 could not be detected in p15INK4b immunoprecipitates but were readily detected in parallel p18INK4c immunoprecipitates (Fig. 7A). These data suggest that p18INK4c is the only functionally relevant INK4 family member in our system.
FIG. 7.
Association of Cdk4 and INK4 inhibitors. (A) Lysates were prepared as described for Fig. 1, and p15INK4b and p18INK4c immunoprecipitates were Western blotted with the cognate antibodies and with antibodies to Cdk4 and Cdk6 as indicated. (B) Lysates were prepared from T-47D cells treated for 6, 12, 18, or 24 h with ORG 2058 or ethanol vehicle; Cdk4 and p18INK4c complexes were immunoprecipitated in parallel and Western blotted using the cognate antibodies.
To investigate the temporal regulation of p18INK4c levels and association with Cdk4 and Cdk6, lysates treated for various times with ORG 2058 or ethanol were immunoprecipitated using antibodies to p18INK4c and Cdk4 and Western blotted. Total p18INK4c levels increased following treatment, reaching a maximum of more than twofold induction over control by 18 h after treatment (Fig. 7B). The absolute level of Cdk4-associated p18INK4c was clearly increased from 18 h and by 24 h was more than three times greater than control levels (Fig. 7B). Over the same time interval, total Cdk4 levels declined by ∼40% (Fig. 7B) and the proportion of total cellular Cdk4 bound to p18INK4c following treatment increased so that approximately 50% of Cdk4 was bound to p18INK4c. Immunoprecipitation of p18INK4c from ORG 2058-treated gel filtration fractions corresponding to the peak of Cdk4 at ∼60 kDa depleted a majority of Cdk4 (data not shown), demonstrating that p18INK4c-associated Cdk4 constitutes a large proportion of the Cdk4 below 100 kDa following ORG 2058 treatment.
Consistent with the view that p18INK4c preferentially forms complexes with Cdk6 (14), when the level of p18INK4c-bound Cdk6 was compared to the level in whole cell extract, an even higher proportion of total Cdk6 was p18INK4c bound than for Cdk4, suggesting association of a majority of Cdk6 with p18INK4c following treatment. This increase in Cdk4- and Cdk6-bound p18INK4c is likely responsible for the ∼20-kDa increase in apparent molecular mass of Cdk4 and Cdk6 following gel filtration chromatography (Fig. 6A and B).
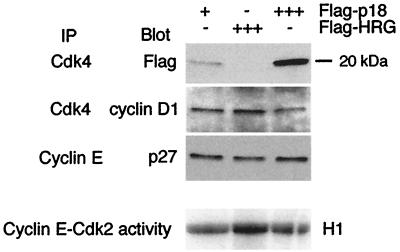
While INK4 proteins were initially identified as inhibitors of Cdk4/6, recent evidence suggests that an equally important mode of action for INK4 proteins in cell cycle arrest is inhibition of Cdk2 activity. This is proposed to occur by preventing Cip/Kip inhibitors from binding Cdk4/6, hence making inhibitors available to bind Cdk2 (32, 43, 48). Thus, increased binding of p18INK4c to Cdk4 may contribute to the accumulation of p27Kip1 in cyclin E complexes and consequent inactivation of cyclin E-Cdk2 complexes. An in vitro system was used to evaluate the ability of exogenous p18INK4c to cause such a redistribution of p27Kip1 between CDK complexes. Purified recombinant Flag-p18 or a control protein, Flag-heregulin (10), was incubated with lysates from proliferating cells, and the compositions and kinase activities of relevant cyclin-CDK complexes were determined. Incubation of lysates with Flag-p18 led to the formation of Flag-p18-Cdk4 complexes and a reduction in the binding of cyclin D1 to Cdk4 compared to the control protein (Fig. 8). Furthermore, binding of Cdk4 to Flag-p18, but not Flag-heregulin, coincided with increased p27Kip1 binding to cyclin E (Fig. 8). Most importantly, incubation of lysates with Flag-p18 led to inactivation of cyclin E kinase activity by ∼50% compared to the Flag-heregulin control (Fig. 8). Thus, increased p18INK4c levels are sufficient to trigger redistribution of cyclin-CDK complexes with consequent effects on cyclin E-Cdk2 activity.
FIG. 8.
Effect of Flag-p18 on the composition and activity of CDK complexes. Whole cell extract (1.5 mg) from proliferating cells was incubated with 1 (+) or 25 (+++) μg of Flag-p18 or 25 μg of Flag-heregulin (Flag-HRG; +++). Cdk4 and cyclin E immunoprecipitates were assayed by Western blotting and/or kinase assay as indicated.
Progestins induce INK4c gene expression.
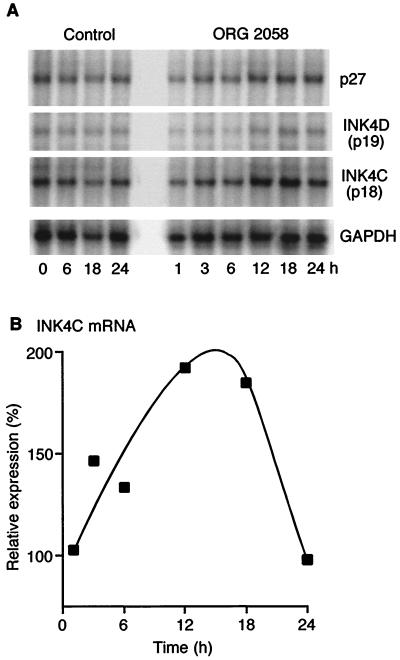
Since the data presented above indicate that both p18INK4c and p27Kip1 contribute to progestin inhibition, mRNA levels were examined to determine whether their increased abundance was mediated by transcriptional activation of the corresponding gene. RNase protection was used to simultaneously examine the expression of the INK4 family and p27Kip1 in proliferating and progestin-treated T-47D cells. Single bands were detected in samples from proliferating cells at the expected fragment sizes, with INK4c mRNA significantly more abundant than that of the remaining INK4 family members (Fig. 9A). INK4b mRNA was faint or undetectable, suggesting very low levels of expression which may account for our inability to detect p15INK4b-associated Cdk4 or Cdk6 proteins. Similarly, INK4d mRNA was present only at low levels, consistent with the lack of detectable p19INK4d protein. Following progestin treatment, the level of INK4c mRNA increased by 6 h and when normalized for GAPDH expression peaked at an ∼2-fold increase at 12 and 18 h, returning to baseline levels by 24 h (Fig. 9B). This change was similar in magnitude to the increase in p18INK4c protein, and preceded it by several hours, consistent with the interpretation that this response is largely mediated by transcriptional induction of p18INK4c.
FIG. 9.
Transcriptional regulation of INK4 family genes by progestins. Ten micrograms of total RNA from T-47D cells treated with ORG 2058 or vehicle at various time points was subjected to RNase protection analysis using a template set focused on cell cycle inhibitors according to the manufacturer's instructions (hCC-2; Pharmingen). Protected fragments corresponding to mRNA encoding p18INK4c, p27Kip1, and p19INK4d are shown in comparison with the fragment encoding the metabolic enzyme GAPDH as a control for RNA loading. (B) Densitometric values for levels of mRNA encoding p18INK4c are expressed as a percentage of averaged control values. The values for 6, 12, 18, and 24 h represent averages of two independent experiments.
The level of p27Kip1 mRNA following treatment was also determined. Treatment of cells with ORG 2058 resulted in little change in p27Kip1 mRNA, with at most ∼50% induction over control at 12 h (Fig. 9A). This suggests that the induction of p27Kip1 protein apparent at 30 h (40) is due to a posttranscriptional mechanism.
DISCUSSION
This study investigates the role of Cip/Kip and INK4 family CDK inhibitors in progestin inhibition of proliferation. We show that the predominant Cip/Kip CDK inhibitor involved in progestin inhibition of cell cycle progression is p27Kip1, and that addition of p27Kip1 to breast cancer cell lysate is sufficient to recapitulate the effects of progestin treatment on cyclin E-Cdk2 activity and complex formation. Despite marked effects on cyclin E-Cdk2, added p27Kip1 had little effect on cyclin D-Cdk4/6 activity. Rather, Cdk4 and Cdk6 are extensively bound by the INK4 family inhibitor p18INK4c following progestin treatment. Addition of p18INK4c to breast cancer cell lysate displaced cyclin D1 from Cdk4, making p27Kip1 available to bind cyclin E-Cdk2. Thus, the redistribution of p27Kip1 and consequent inhibition of cyclin E-Cdk2 activity following progestin treatment apparently result at least in part from increased p18INK4c levels.
Addition of p27Kip1 recapitulates the effects of progestin on cyclin E-Cdk2.
Progestin treatment leads to decreased cyclin E-Cdk2 activity, accompanied by preferential formation of a high-molecular-weight, p27Kip1-bound form of cyclin E-Cdk2. Immunodepletion of p27Kip1 removed essentially all of the high-molecular-weight, inactive form of cyclin E-Cdk2 following progestin treatment, consistent with the hypothesis that increased p27Kip1 association with cyclin E-Cdk2 is causally related to the shift in cyclin E-Cdk2 elution profile and loss of kinase activity. In contrast, little cyclin E was associated with p21Cip1 either before or after progestin treatment, arguing against a role for p21Cip1 in inhibition of cyclin E-Cdk2. Unexpectedly, in lysates from exponentially proliferating cells a minority of high-molecular-weight cyclin E-Cdk2 was p27Kip1 associated, and a significant fraction was not bound to either p21Cip1 or p27Kip1. These data suggest that rather than altering the equilibrium between the two predominant forms of cyclin E-Cdk2 in T-47D cells, progestin treatment results in the preferential formation of a p27Kip1-bound species of cyclin E-Cdk2 at the expense of the existing species present in untreated cells. Given their apparent high molecular weight, these cyclin E-Cdk2 complexes in proliferating cells may contain proteins in addition to cyclin E and Cdk2, an interpretation supported by the presence of an ∼110-kDa protein in cyclin E immunoprecipitates of the 200-kDa peak in [35S]methionine-labeled control but not progestin-treated lysates (A. Swarbrick, unpublished data). This protein remains unidentified but does not appear to be one of the pocket proteins known to bind to cyclin E-Cdk2 (2, 8) since preliminary experiments failed to detect them in cyclin E immunoprecipitates.
When increasing amounts of His6-p27 were added to T-47D lysates, cyclin E-Cdk2 activity decreased over a narrow range of His6-p27 concentration. Over the same concentration range, the 120-kDa peak of cyclin E-Cdk2 was converted to a 200-kDa form, demonstrating that both the shift in elution profile and decrease in kinase activity can result from p27Kip1 association with cyclin E-Cdk2. These responses appeared to be closely linked, such that there was an inverse correlation between the proportion of cyclin E-Cdk2 in the 120-kDa peak and cyclin E-Cdk2 activity. The ∼80-kDa difference in apparent molecular mass between the p27Kip1-bound and p27Kip1-free, active cyclin E-Cdk2 raised the possibility that p27Kip1 binding alone did not account for the alteration in elution profile. However, recombinant cyclin E-Cdk2 coeluted with the ∼120-kDa active form of cellular cyclin E and increased in molecular mass to ∼200 kDa after incubation with purified recombinant His6-p27, coincident with the high-molecular-weight form of cyclin E-Cdk2 present after progestin treatment. p27Kip1 is therefore sufficient to shift the profile of preexisting cyclin E-Cdk2 complexes, rather than requiring the degradation of ∼120-kDa complexes or formation of ∼200-kDa complexes de novo.
Preliminary data suggest that cyclin E-Cdk2 complexes containing His6-p27 do not also contain endogenous p27Kip1, consistent with the interpretation that binding of a single p27Kip1 molecule is sufficient to inhibit kinase activity (Swarbrick, unpublished data). Furthermore, essentially complete inhibition of kinase activity occurred at levels of p27Kip1 which were not sufficient to saturate the binding capacity of cyclin E-Cdk2. This finding supports the interpretation that binding of a single p27Kip1 molecule to cyclin E-Cdk2 is sufficient to both inhibit kinase activity and alter the apparent molecular mass to ∼200 kDa.
Regulation of p27Kip1 has been suggested to be primarily by posttranslational mechanisms involving obligatory phosphorylation, nuclear export, and subsequent degradation of p27Kip1 protein (51, 55, 64). Consistent with this view, analysis of mRNA levels indicated that progestins do not significantly upregulate the levels of p27Kip1 mRNA. However, examination of p27Kip1 protein half-life following labeling with [35S]methionine did not provide evidence for early alterations in p27Kip1 stability (C. S. L. Lee, unpublished data). Two-dimensional gel electrophoresis revealed no change in the phosphorylation status of p27Kip1 following progestin treatment, nor was there an effect on its subcellular compartmentalization (Swarbrick, unpublished data). Cdk2 can phosphorylate p27Kip1, and this step is sufficient, and perhaps necessary, for ubiquitination and degradation of p27Kip1 by the proteosome (34, 42). Accumulation of p27Kip1 occurs >30 h after progestin treatment, likely due to stabilization of the p27Kip1 protein resulting from a lack of Cdk2 activity. The reassortment of p27Kip1 among cyclin-CDK complexes thus apparently reflects alterations in the levels of other components of these complexes, with its late increase in abundance a consequence rather than a cause of decreased CDK activity.
Inhibition of cyclin D-Cdk4 activity is not due to association with p27Kip1.
In T-47D breast cancer cells, Cdk4 is the major cyclin D-associated CDK (58), and we have thus focused on regulation of Cdk4 activity as a marker of cyclin D action. Since addition of recombinant His6-p27 inhibited Cdk4 activity by only <30% at concentrations well in excess of those required to wholly inhibit cyclin E-Cdk2, p27Kip1 appears unlikely to make a major contribution to decreases in Cdk4 activity in T-47D cells. This observation is consistent with the demonstration that both in vivo and in vivo p27Kip1 can inhibit Cdk2 at concentrations where Cdk4 is unaffected (3).
The majority of cyclin D1 coelutes with the major peak of complexed, active Cdk4 and Cdk6 at ∼160 kDa upon gel filtration chromatography (30, 40). Both before and after progestin treatment, essentially all of the cyclin D1 and D3 of this molecular mass was associated with either p21Cip1 or p27Kip1. While cyclin D1 can bind p27Kip1 independently of association with a CDK (61), this suggests that p27Kip1 and p21Cip1 are present in most of the cyclin D-CDK complexes, consistent with their role as assembly factors (5, 26), and that the active cyclin D-CDK complexes in control cells contain p21Cip1 or p27Kip1. In support of this interpretation, p27Kip1 and p21Cip1 immunoprecipitates from T-47D cell lysate phosphorylated Rb in vitro.
Role of INK4 family CDK inhibitors in progestin action.
Several lines of evidence demonstrate that p18INK4c plays an important role in the inhibition of CDK activity following progestin treatment. Gel filtration chromatography and Cdk4 immunoprecipitates from [35S]methionine-labeled cells indicated that Cdk4 bound an ∼20-kDa protein which increased in abundance after progestin treatment. Immunoblotting with specific antibodies indicated that this protein was p18INK4c. The abundance of p15INK4b and its association with Cdk4 also increased, but immunoblotting of parallel p15INK4b and p18INK4c immunoprecipitates indicated that p18INK4c-Cdk4/6 complexes are present in much greater abundance than p15INK4b-Cdk4/6 complexes. Since p19INK4d and p16INK4a are apparently not expressed, p18INK4c appears to be the major INK4 family member in these cells.
In contrast with Cdk4, where the low-molecular-weight peak was largely monomeric in control cells, the elution profile of Cdk6 suggested that a significant proportion was bound to p18INK4c even in untreated cells. This observation is consistent with the finding that p18INK4c displays preference for stable complex formation with Cdk6 over Cdk4 (14, 43) and perhaps accounts for the relative lack of cyclin D-Cdk6 complex formation in these cells (58). Although data from some cell lines suggest that stable p18INK4c-Cdk4 complexes do not form in exponentially growing cells (14, 43), p18INK4c was clearly present in Cdk4 immunoprecipitates from control cells, and the elution profile of Cdk4 in the low-molecular-weight range indicates that this complex accounts for 20% of Cdk4 prior to treatment. The increased p18INK4c-Cdk4 interaction following progestin treatment is likely favored not only by increased p18INK4c abundance but also by the decreased levels of competing D-type cyclins.
Unlike Cip/Kip inhibitors, p18INK4c displays a restricted expression pattern and thus may function in a tissue-specific manner (14, 21). During adipogenesis, myogenesis, and B-cell terminal differentiation, p18INK4c accumulates to high levels, suggesting a role in the initiation or maintenance of growth arrest during differentiation (12, 36, 44). The progestin regulation of p18INK4c in breast cancer cells demonstrated here is to our knowledge the first demonstration of steroidal regulation of INK4 family CDK inhibitors and suggests that p18INK4c may be involved in the induction of differentiation in progestin target tissues in vivo.
Mechanisms of CDK inactivation by progestins: cooperation between p27Kip1 and p18INK4c.
The data presented here and in previous publications (13, 40) suggest a model for progestin inhibition of proliferation in which p27Kip1 inhibition of cyclin E-Cdk2 and p18INK4c inhibition of Cdk4/6 cooperate with reduced cyclin D and E abundance to bring about growth inhibition. A model involving cooperation between p18INK4c and p27Kip1 shares many features with the proposed mechanisms for transforming growth factor β inhibition of proliferation, in which p15INK4b acts jointly with p27Kip1 to inhibit the activity of the major G1 cyclin complexes (48, 49). Interestingly, recent data indicate that p18INK4c and p27Kip1 lie on separate but functionally linked pathways during pituitary tumorigenesis (11) and that they interact to cause growth arrest during adipogenesis (44). Furthermore, p19INK4d and p21Cip1 are implicated in the growth arrest of macrophages after alpha interferon treatment (31). Thus, cooperation between INK4 and Kip/Cip family CDK inhibitors appears to be a potent mechanism for physiological inhibition of cell proliferation and perhaps the induction of differentiation, since p18INK4c and p19INK4d have been implicated in this process (1, 12, 36, 44).
The interaction between INK4 inhibitors and p27Kip1 or p21Cip1 can occur at several levels. They target complementary subsets of G1 cyclin-CDK complexes, and in addition the balance between them is a determinant of the formation of different cyclin-CDK-inhibitor complexes. INK4-Cdk4 complexes increase in cells lacking p21Cip1 and p27Kip1 (5), and conversely, induction of ectopic p16INK4a or p15INK4b favors the interaction of p27Kip1 and p21Cip1 with Cdk2 over Cdk4, thereby leading to the inhibition of both Cdk4 and Cdk2 (23, 32, 33, 48, 49, 65). Similarly, addition of recombinant p18INK4c to breast cancer cell lysate led to a decrease in cyclin D1-Cdk4 complexes, releasing sufficient p27 to decrease cyclin E-Cdk2 activity by ∼50%. Thus the ability to induce reassortment of cyclin-CDK complexes with consequent inhibition of cyclin E-Cdk4 as well as Cdk4/6 (32, 43, 48, 49) appears to be a property shared by all the INK4 family inhibitors. In progestin-treated breast cancer cells, increased p18INK4c may complement decreased cyclin D1 in making p27Kip1 available for binding to cyclin E-Cdk2, thus accounting for elevated p27Kip1 binding to this complex before substantial changes in p27Kip1 abundance are observed (40). The maximal induction of p18INK4c mRNA at 12 to 18 h is one of the earliest responses implicated in progestin inhibition of proliferation, preceding the changes in cyclin D1 and cyclin E mRNA by ∼6 h, thus suggesting that this induction is one of the events triggering subsequent decreases in CDK activity.
The combination of reduced cyclin D levels and increased p18INK4c levels is expected to significantly diminish the association of Cdk4/6 with D-type cyclins. The increasing predominance of p18INK4c-Cdk4 complexes over cyclin D-Cdk4 is illustrated by the increased abundance of low-molecular-weight forms of Cdk4 and the increased fraction of these bound by p18INK4c. An approximately 2-fold increase in p18INK4c together with a ∼50% decrease in Cdk4 (40) would lead to an expected ∼4-fold increase in p18INK4c association with Cdk4, consistent with data presented in Fig. 7 and with the substantial shift in the elution profile of low-molecular-weight Cdk4. This is, however not sufficient to prevent formation of cyclin D1-Cdk4 complexes, since cyclin D-Cdk4 complexes are present following progestin treatment, as indicated by the coimmunoprecipitation of cyclins D1 and D3 with Cdk4 in the ∼150- to 200-kDa molecular size range (40). These complexes lack activity for reasons which remain unclear.
The model for progestin inhibition outlined here is based on data from breast cancer cells in tissue culture. Progestins do, however, inhibit proliferation in several different physiological settings, notably their inhibition of estrogen-induced proliferation in uterine epithelium (6), raising the question of the generality of these mechanisms. Recent data have demonstrated that in the mouse uterus progestin inhibition of the mitogenic response to estrogen is accompanied by lack of cyclin D1-Cdk4 nuclear translocation and inhibition of cyclin E-Cdk2 activation (60). With the exception of p27Kip1, CDK inhibitors were present at low levels and not significantly regulated by hormone treatment. Progesterone did not prevent the downregulation of p27Kip1 following estrogen treatment (60), in contrast with data indicating induction of p27Kip1 in the human endometrium following progestin treatment (54). Furthermore, in the uterus of p27−/− mice progestins are able to inhibit estrogen activation of cyclin E-Cdk2 activity (59, 60), perhaps due to inhibition of cyclin E induction. A more quantitative analysis will be necessary to define the relative roles of p27Kip1 and cyclin E in progestin action in the uterus. In breast cancer cells and uterine epithelium, progestins apparently utilize different mechanisms for inhibition of cyclin D1-Cdk4, since translocation of cyclin D1 was not observed in T-47D cells following progestin treatment (Swarbrick, unpublished data). Further experimentation will be required to identify whether this relates to differences in tissue type, altered control mechanisms accompanying oncogenesis, or simply a different balance between similar components. However, despite differences in the precise mechanisms, in both cell types cyclin D- and cyclin E-dependent kinases are targeted by multiple, interdependent pathways.
ACKNOWLEDGMENTS
We thank David Parry and Emma Lees for the INK4 antibodies, Owen Prall for helpful discussion and critical evaluation of the manuscript, and the following colleagues for reagents and advice: Boris Sarcevic, Amanda Mawson, Bev Warner, Grant Macarthur, Roger Daly, and Steve Coats.
This work was supported by grants from the New South Wales Cancer Council and the National Health and Medical Research Council of Australia. A.S. is the recipient of an Australian Postgraduate Award.
REFERENCES
- 1.Adachi M, Roussel M F, Hevenith K, Sherr C J. Features of macrophage differentiation induced by p19INK4d, a specific inhibitor of cyclin D-dependent kinases. Blood. 1997;90:126–137. [PubMed] [Google Scholar]
- 2.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blain S W, Montalvo E, Massagué J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential for the function of p21Cip1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng M, Olivier O, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21Cip1 and p27Kip1 CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke C L, Sutherland R L. Progestin regulation of cellular proliferation. Endocrine Rev. 1990;11:266–302. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 7.Della Ragione F, Russo G L, Oliva A, Mercurio C, Mastropietro S, Della Peitra V, Zappia V. Biochemical characterisation of p16INK4a- and p18-containing complexes in human cell lines. J Biol Chem. 1996;271:15942–15949. doi: 10.1074/jbc.271.27.15942. [DOI] [PubMed] [Google Scholar]
- 8.De Luca A, Maclachlan T K, Bagella L, Dean C, Howard C M, Paulo Claudio P, Baldi A, Khalili K, Giordano A. A unique domain of pRb2/p130 acts as an inhibitor of Cdk2 kinase activity. J Biol Chem. 1997;272:20971–20974. doi: 10.1074/jbc.272.34.20971. [DOI] [PubMed] [Google Scholar]
- 9.Dong F, Agrawal D, Bagui T, Pledger W J. Cyclin D3-associated kinase activity is regulated by p27kip1 in BALB/c 3T3 cells. Mol Biol Cell. 1998;9:2081–2092. doi: 10.1091/mbc.9.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiddes R J, Janes P W, Sanderson G M, Sivertsen S P, Sutherland R L, Daly R J. Heregulin (HRG)-induced mitogenic signaling and cytotoxic activity of a HRG/PE40 ligand toxin in human breast cancer cells. Cell Growth Differ. 1995;6:1567–1577. [PubMed] [Google Scholar]
- 11.Franklin D S, Godfrey V L, Lee H, Kovalev G I, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. CDK inhibitors p18INK4c and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin D S, Xiong Y. Induction of p18INK4c and its predominant association with CDK4 and CDK6 during myogenic differentiation. Mol Biol Cell. 1996;7:1587–1599. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groshong S D, Owen G I, Grimison B, Schauer I E, Todd M C, Langan T A, Sclafani R A, Lange C A, Horwitz K B. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27Kip1. Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 14.Guan K-L, Jenkins C W, Li Y, Nichols M A, Wu X, O'Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 15.Guan K-L, Jenkins C W, Li Y, O'Keefe C L, Noh S, Wu X, Zariwala M, Matera A G, Xiong Y. Isolation and characterisation of p19INK4d, a p16-related inhibitor specific to CDK4 and CDK6. Mol Biol Cell. 1996;7:57–70. doi: 10.1091/mbc.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall M, Bates S, Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995;11:1581–1588. [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 18.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 19.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L-H, Zhang P, Dobrowlski S, Bai C, Connell-Crowley L, Swindell E, Fox M P, Wei N. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman J G, Merlo A, Mao L, Lapidus R G, Issa J-P J, Davidson N E, Sidransky D, Baylin S B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 21.Hirai H, Roussel M F, Kato J-Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz K B, Freidenberg G R. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45:167–173. [PubMed] [Google Scholar]
- 22a.Hui, R., D. Macmillan, F. S. Kenny, E. A. Musgrove, R. W. Blarney, R. I. Nicholson, J. F. R. Robertson, and R. L. Sutherland. INK4a gene expression and methylation in primary breast cancer: overexpression of p16INK4a mRNA is a marker of poor prognosis. Clin. Cancer Res., in press. [PubMed]
- 23.Jiang H, Chou H S, Zhu L. Requirement of cyclin E-Cdk2 inhibition in p16INK4a-mediated growth suppression. Mol Cell Biol. 1998;18:5284–5290. doi: 10.1128/mcb.18.9.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A, Takahashi H, Takahashi Y, Matsushime H. Inactivation of the cyclin-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J Biol Chem. 1997;272:8065–8070. doi: 10.1074/jbc.272.12.8065. [DOI] [PubMed] [Google Scholar]
- 25.Kato J-Y, Matsuoka M, Polyak K, Massagué J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 26.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 27.Ladha M H, Lee K Y, Upton T M, Reed M F, Ewen M E. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lange C A, Richer J K, Horwitz K B. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 29.Lee M H, Reynisdottir I, Massagué J. Cloning of p57kip2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 30.Mahony D, Parry D A, Lees E. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene. 1998;16:603–611. doi: 10.1038/sj.onc.1201570. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka M, Tani K, Asano S. Interferon-α-induced G1 phase arrest through up-regulated expression of CDK inhibitors, p19Ink4D and p21Cip1 in mouse macrophages. Oncogene. 1998;16:2075–2086. doi: 10.1038/sj.onc.1201745. [DOI] [PubMed] [Google Scholar]
- 32.McConnell B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitra J, Dai C Y, Somasundaram K, El-Diery W, Satamoorthy K, Herlyn M, Enders G H. Induction of p21WAF1/Cip1 and inhibition of Cdk2 mediated by the tumor suppressor p16INK4a. Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 36.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Induction of cell cycle arrest and B cell terminal differentiation by CDK inhibitor p18(INK4c) and IL-6. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 37.Müller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge W, Huttner W, Eilers M. Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene. 1997;15:2561–2576. doi: 10.1038/sj.onc.1201440. [DOI] [PubMed] [Google Scholar]
- 38.Musgrove E A, Hamilton J A, Lee C S L, Sweeney K J E, Watts C K W, Sutherland R L. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musgrove E A, Lee C S L, Sutherland R L. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor α, epidermal growth factor receptor, c-fos and c-myc genes. Mol Cell Biol. 1991;11:5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musgrove E A, Swarbrick A, Lee C S L, Cornish A L, Sutherland R L. Mechanisms of CDK inactivation by progestins. Mol Cell Biol. 1998;18:1812–1825. doi: 10.1128/mcb.18.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaoka H, Perez D, Baek K J, Das T, Husain A, Misono K, Im M-J, Graham R J. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen H, Gitig D M, Koff A. Cell-free degradation of p27kip1, a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteosome. Mol Cell Biol. 1999;19:1190–1201. doi: 10.1128/mcb.19.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parry D, Mahony D, Wills K, Lees E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phelps D E, Xiong Y. Regulation of cyclin-dependent kinase 4 during adipogenesis involves switching of cyclin D subunits and concurrent binding of p18INK4c and p27Kip1. Cell Growth Differ. 1998;9:595–610. [PubMed] [Google Scholar]
- 45.Polyak K, Kato J-Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 46.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 47.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 48.Reynisdóttir I, Massagué J. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 49.Reynisdóttir I, Polyak K, Iavarone A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 50.Sarcevic B, Lilischkis R, Sutherland R L. Differential phosphorylation of T-47D human breast cancer cell substrates by D1-, D3-, E-, and A-cyclin-CDK complexes. J Biol Chem. 1997;272:33327–33337. doi: 10.1074/jbc.272.52.33327. [DOI] [PubMed] [Google Scholar]
- 51.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 52.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 53.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 54.Shiozawa T, Nikaido T, Nakayama K, Lu X, Fujii S. Involvement of cyclin-dependent kinase inhibitor p27Kip1 in growth inhibition of endometrium in the secretory phase and of hyperplastic endometrium treated with progesterone. Mol Hum Reprod. 1998;4:899–905. doi: 10.1093/molehr/4.9.899. [DOI] [PubMed] [Google Scholar]
- 55.Shirane M, Harumiya Y, Noriko I, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M. Down-regulation of p27kip1 by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem. 1999;274:13886–13893. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- 56.Singh S P, Lipman J, Goldman H, Ellis F H, Jr, Aizenman L, Cangi M G, Signoretti S, Chiaur D S, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 57.Soos T J, Kiyokawa H, Yan J S, Rubin M S, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- 58.Sweeney K J, Swarbrick A, Sutherland R L, Musgrove E A. Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene. 1998;16:2865–2878. doi: 10.1038/sj.onc.1201814. [DOI] [PubMed] [Google Scholar]
- 59.Tong W, Kiyokawa H, Soos T J, Park M S, Soares V C, Manova K, Pollard J W, Koff A. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa-luteal transition. Cell Growth Differ. 1998;9:787–794. [PubMed] [Google Scholar]
- 60.Tong W, Pollard J W. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 62.Veronesi U, Goldhirsch A, Yarnold J. Breast cancer. In: Peckham M, Pinedo H M, Veronesi U, editors. Oxford textbook of oncology. Oxford, England: Oxford University Press; 1995. pp. 1243–1289. [Google Scholar]
- 63.Vignon F, Bardon S, Chalbos D, Rochefort H. Antiestrogenic effect of R5020, a synthetic progestin in human breast cancer cells in culture. J Clin Endocrinol Metab. 1983;56:1124–1130. doi: 10.1210/jcem-56-6-1124. [DOI] [PubMed] [Google Scholar]
- 64.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 65.Warner B J, Blain S W, Seoane J, Massagué J. Myc downregulation by transforming growth factor β required for activation of the p15Ink4b G1 arrest pathway. Mol Cell Biol. 1999;19:5913–5922. doi: 10.1128/mcb.19.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]