Abstract
Background:
Gestational diabetes mellitus (GDM) and type 2 diabetes mellitus (T2DM) represent two different components of the spectrum of diabetes mellitus (DM). Women with GDM have a high chance of developing T2DM in later life and this relative risk depends on a number of factors including ethnicity.
Aim:
To compare and estimate the risk of developing T2DM in South Asian women with a history of GDM compared to those without a history of GDM.
Methods:
This is a systematic review of PubMed and MEDLINE articles reporting the progression of GDM to T2DM that were published in English from 2000 to 2020. We performed meta-analysis to calculate risk ratios (RR).
Results:
We selected 6 studies considering the inclusion and exclusion criteria after sorting 25 full-text articles. Of the 44165 South Asian women assessed, 3095 had GDM and 41070 were without GDM. 995 women in GDM group and 1525 women in non-GDM group had developed T2DM. The RR of women with GDM over non-GDM in developing T2DM was 10.81 (95% confidence interval (CI): 7.61–15.35) suggesting that women with GDM are at 10.81 times more risk of developing T2DM than non-GDM. The cumulative incidence of T2DM in GDM group was 17.34% at 5 years of follow-up and 33% at more than 10 years of follow-up.
Conclusion:
The risk of developing T2DM in later life is higher in South Asian women with GDM than without GDM. Therefore, lifestyle and pharmacological interventions, patient communication, timely screening, and long-term follow-up of GDM patients are important to reduce the risk.
Keywords: GDM, pregnancy, South Asian women, systematic review, type 2 diabetes mellitus
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is high and is a major public health issue in South Asia[1] (India, Pakistan, Bangladesh, Afghanistan, Sri Lanka, Nepal, Bhutan, and Maldives). The report of International Diabetes Federation (IDF) in 2019 showed the number of people with diabetes in India, Bangladesh, Sri Lanka, and Nepal was 77 million, 8.4 million, 1.2 million, and 0.7 million, respectively.[2] The prevalence of diabetes in India is reported to be within 4.65% to 14% in urban areas and 1.7% to 13.2% in rural areas and an estimated 4 million women live with gestational diabetes mellitus (GDM) at any point of time.[3] GDM, defined as diabetes first diagnosed during second or third trimester of pregnancy, has already become a global health issue.[4,5] GDM is an established risk factor for developing various morbidity in later life including type 2 diabetes mellitus (T2DM)[6] and cardiovascular diseases.[7] Several studies and meta-analyses in the past reported that women with a history of GDM had several-fold higher risks of developing T2DM later in their life.[6,8,9,10] Considering the vast population with diabetes in South Asia, we undertook this study to estimate the risk of developing type 2 diabetes mellitus in women of South Asian Ethnicity with a history of GDM compared to those without a history of GDM.
METHODS
We conducted the study according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.[11] The protocol for this systematic review and meta-analysis is registered with PROSPERO as CRD42020199808.
Data source and search strategy
We searched electronic databases, i.e. PubMed and MEDLINE to find studies based on the progression of GDM to T2DM. We used the MeSH terms “gestational diabetes,” “GDM,” “gestational diabetes mellitus,” “type 2 diabetes mellitus,” “T2DM,” “Type 2 diabetes,” and “South Asia,” “South Asian,” “South Asian countries,” “India,” “Pakistan,” “Bangladesh,” “Afghanistan,” “Sri Lanka,” “Nepal,” “Bhutan,” “Maldives,” as keywords for our search. We also searched the reference section of the selected studies manually published in English language from the year 2000 to 2020 and considered for further evaluation.
Study selection
After selecting studies from our initial search, we first reviewed the abstracts and subsequently examined the full text of relevant studies in detail.
Inclusion criteria
Studies with gestational diabetes mellitus (GDM) patients of South Asian ethnicity and post-partum follow-up of at least 1 year to diagnose the development of T2DM.
Studies with GDM group and control group (non-GDM group) with data of women subsequently developing T2DM in both groups.
Exclusion criteria
Studies with a sample population outside the target population, studies with follow-up of less than 1 year.
Studies without a control group.
Studies with no original data (meetings, editorials, letters, and commentaries).
Study quality assessment
We assessed the risk of bias and quality of the selected studies by using Newcastle-Ottawa (NOS) quality assessment scale.[12] Evaluation of the studies was under the categories of selection, comparability, and outcome and a maximum of 9 stars could be awarded to each study. Estimation of publication bias was done using funnel plot as asymmetry graph and Begg's and Egger's statistical tests.[13]
Data extraction and statistical analysis
Three authors (Sharvil Gadve, Sneha Chavanda, and Aridita Datta Mukherjee) extracted data independently. Any disagreement was settled by consensus among authors. Data was extracted using Cochrane handbook for systematic reviews of interventions. Risk ratio (RR) was calculated to assess the relative risk of developing T2DM in the GDM group. Heterogeneity was assessed statistically using I2 test and graphically represented using forest plot diagram. I2% >50% was considered as the presence of significant heterogeneity. Results were pooled using random effects model considering it is unlikely to have a common effect size for different selected studies. P value of <0.05 was considered to be statistically significant. RevMan Review Manager 5.3 software was used for the meta-analysis. Meta-regression models were used to study the effects of heterogeneity of study and cumulative risks of developing T2DM by mean age of participants at the beginning of study, length of follow-up, and publication year.
RESULTS
The initial search resulted 1276 studies addressing the research question. After careful screening of the abstracts of these studies, we sorted 25 studies for further evaluation and the full text of these selected studies was gone through one by one and analyzed. Three studies were systematic review and meta-analysis, 5 studies were not relevant to the research question, 5 studies had sample population from outside the target population, 2 studies did not explore the outcome of GDM, 3 studies did not have a control group, and 1 was questionnaire-based study. After excluding these 19 studies, 6 studies[14,15,16,17,18,19] fulfilled all the inclusion criteria and were included in our study for systematic review and meta-analysis [Figure 1]. A total of 44165 women were included in our study of which 3095 represented the GDM group and 41070 represented the non-GDM group.
Figure 1.
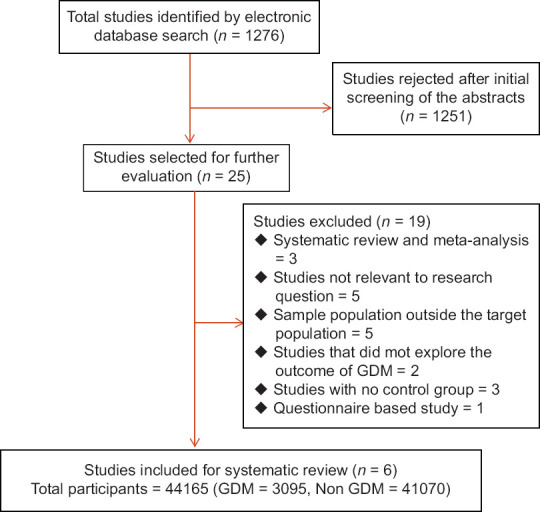
Flow chart of literature search
Study quality assessment
Quality assessment was done using NOS quality assessment scale. Three studies (Krishnaveni et al. 2007,[14] Mukerji et al. 2012,[15] and Herath et al. 2017[17]) had scored 8 stars each out of 9. Sreelakshmi et al. 2015[16] scored 7 stars, whereas Gadgil et al. 2017[18] scored a total of 6 stars [Table 1].
Table 1.
Quality assessment of selected studies according to NOS
| Studies | Selection | Comparability | Outcome | Total Score | Average Score |
|---|---|---|---|---|---|
| Krishnaveni et al. 2007 | **** | * | *** | 8 stars | 7.4 stars |
| Mukerji et al. 2012 | **** | * | *** | 8 stars | |
| Shreelakshmi et al. 2015 | *** | * | *** | 7 stars | |
| Herath et al. 2017 | **** | * | *** | 8 stars | |
| Gadgil et al. 2017 | *** | * | ** | 6 stars | |
| Aziz et al. 2018 | Quality could not be assessed due to follow-up study design. | ||||
We could not award score using NOS scale to Aziz et al. due to their study design. Average score of all the included studies was 7.4 stars thus suggesting that the risk of bias is low. Publication bias was assessed using funnel plot diagram and Begg's and Egger's test. No publication bias was detected among the selected studies [Figure 2: Funnel plot, Table 2: Begg's and Egger's test].
Figure 2.
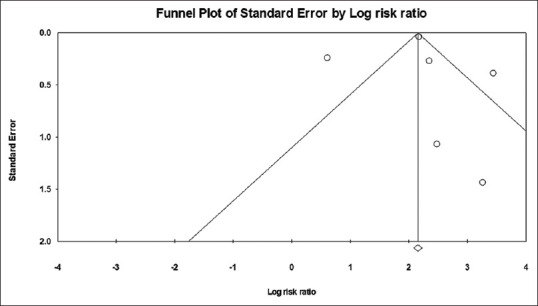
Funnel plot diagram (Publication bias assessment)
Table 2.
Publication bias assessment (Begg’s and Egger’s test)
| Begg’s | Egger’s test | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Kendall’s tau | P | Intercept (95% CI) | t | df | P |
| 0.0 | 0.5 | −0.1829 (−5.28503,4.91923) | 0.09953 | 4 | 0.46 |
Study characteristics
Five studies were cohort studies and one study was a follow-up study. Two studies were retrospective cohort studies,[16,17] one study was prospective cohort study,[18] and two studies were only described as cohort study.[14,15] Two studies were conducted in India,[14,16] while 1 study each were conducted in Sri Lanka[17] and Pakistan.[19] The studies of Mukerji et al.[15] 2012 and Gadgil et al.[18] 2014 were conducted in the South Asian community living in Canada and the United States, respectively. The average follow-up of the studies was 6.3 years. Table 3 shows the detailed characteristics of the included studies and Table 4 shows demographic characteristics of the included studies.
Table 3.
Characteristics of the included studies
| Included study | Study design | Country | GDM* criteria | T2DM criteria | Follow-up number | Follow-up years | T2DM‡/GDM* | T2DM‡/Non-GDM* |
|---|---|---|---|---|---|---|---|---|
| Krishnaveni et al. 2007[14] | Cohort study | India | Carpenter Coustan Criteria | WHO criteria (assessed in 2006) | GDM*=35 NGDM†=489 |
5 years | 13/35 | 8/489 |
| Mukerji et al. 2012[15] | Cohort study | Canada | - | - | GDM*=2763 NGDM†=39758 |
15 years (Median 7.6 years) | 878/2763 | 1431/39758 |
| Sreelakshmi et al. 2015[16] | Retrospective cohort study | India | - | - | GDM*=60 NGDM†=120 |
4 years | 6/60 | 1/120 |
| Herath et al. 2017[17] | Retrospective cohort study | Sri Lanka | WHO§ 1999 | WHO§ 1999 | GDM*=119 NGDM†=240 |
10.8 year | 73/119 | 14/240 |
| Gadgil et al. 2017[18] | Prospective cohort study | USA | - | - | GDM*=40 NGDM†=374 |
- | 14/40 | 71/374 |
| Aziz et al. 2018[19] | Follow-up study | Pakistan | IADPSG|| | - | GDM*=78 NGDM†=89 |
2 years | 11/78 | 0/89 |
*GDM=Gestational diabetes mellitus; †NGDM=Non-gestational diabetes mellitus; ‡T2DM=Type 2 diabetes mellitus; §WHO=World Health Organization; ||IADPSG=International Association of the Diabetes and Pregnancy Study Groups
Table 4.
Demographic characteristics of the included studies
| Study | Length of follow-up (Years) | Age (years) | Mean BMI‡ (kg/m2) (At Follow-up) | Family history of T2DM† (%) |
|---|---|---|---|---|
| Sreelakshmi et al. 2015[16] | 4 | Age at follow-up: GDM* + non-GDM: 32±7.8 GDM* developing T2DM†: 37±7.2 |
GDM*: 24.6±3.9 Non-GDM: 24.8±2.98 |
GDM* developing T2DM†: 48.3 |
| Krishnaveni et al. 2007[14] | 5 | Age at follow-up: GDM*: 33.25 Non-GDM: 28.6 GDM*developing T2DM†: 33.5 (29.5 to 38.5) Non-GDM developing T2DM†: 28.6 (27.3 to 30) |
GDM* developing T2DM†: 26.7±4.6 Non-GDM developing T2DM†: 28.9±4.9 |
GDM* developing T2DM†: 92 Non-GDM developing T2DM†: 63 |
| Mukerji et al. 2012[15] | 15 (median 7.6) | Median age at pregnancy: 29 (26-32 interquartile range) | - | - |
| Herath et al. 2017[17] | 10.8 | GDM*: 42.7±5.37 Non-GDM: 38.7±5.36 |
- | GDM*: 47.1 Non-GDM: 21.7 |
| Gadgil et al. 2017[18] | - | Age at follow-up: GDM*: 51.1±7 Non-GDM: 54.7±8.7 |
GDM*: 26.7±3.8 Non-GDM: 26±4.3 |
GDM*: 12.2 Non-GDM: 6.2 |
| Aziz et al. 2018[19] | 2 | Antenatal data GDM*: 28.9±2.84 Non-GDM: 25.68±3.01 |
- | - |
*GDM=Gestational diabetes mellitus;†T2DM=Type 2 diabetes mellitus; ‡BMI=Body Mass Index
Risk review and meta-analysis
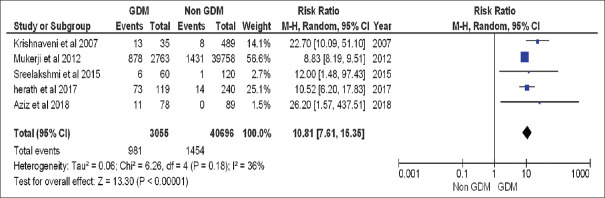
In our review, 44165 women were included. 3095 women had history of GDM during their pregnancy and were included in the GDM group and 41070 women were included in the non-GDM group. Nine ninety-five women from the GDM group had subsequently developed T2DM during their follow-up, while 1525 women from the non-GDM group had developed T2DM during their follow-up. We had to exclude Gadgil et al. 2017 from the meta-analysis as this study was responsible for introducing significant heterogeneity. Finally, we had included 3055 women from GDM group and 40696 women in the non-GDM group. 981 and 1454 women from both groups developed T2DM subsequently. The individual risk ratios of each study showed higher risk of development of T2DM among women having history of GDM. The pooled risk ratio of developing T2DM in the GDM group was 10.81 (95% CI: 7.61–15.35) suggesting that women with GDM history are at 10.81 times more risk of developing T2DM than the non-GDM counterparts. There was no significant heterogeneity (I2 = 36%) among the included studies. Heterogeneity was plotted graphically using forest plot diagram. [Figure 3] shows the results of meta-analysis and forest plot diagram. Meta-regression analyses showed that the study effect size was significantly associated with mean age of patients and length of follow-up [Table 5].
Figure 3.
Meta-analysis and forest plot of chance of having T2DM in women with history of GDM
Table 5.
Meta-regression analysis
| Covariate | Coefficient (95% CI*) | Standard error | Z | P |
|---|---|---|---|---|
| Mean age | −0.0865 (−0.167, −0.0059) | 0.0411 | −2.1 | 0.0354 |
| Length of follow-up | −0.0956 (−0.1555, −0.0357) | 0.0306 | −3.13 | 0.0018 |
| Publication year | −0.0822 (−0.2394, 0.075) | 0.0802 | −1.02 | 0.3055 |
*CI=Confidence interval
In South Asian women with history of GDM, when the follow-up was done for up to 5 years, the cumulative incidence of T2DM was 17.34% (95% CI: 12.02–23.82) and when follow-up done for more than 10 years, the cumulative incidence of T2DM was 33.00% (95% CI: 31.28–34.75) [Table 6].
Table 6.
Cumulative incidence of type 2 diabetes by length of follow-up
| Study follow-up length (years) | No of contributing studies | GDM* % (95% CI) | Controls (% (95% CI†) |
|---|---|---|---|
| 1-5 | 3 | 17.34 (12.02-23.82) | 1.29 (0.59-2.43) |
| >10 | 2 | 33.00 (31.28-34.75) | 3.61 (3.43-3.80) |
| All studies | 6# | 32.15 (30.50-33.83) | 3.71 (3.53-3.90) |
*GDM=Gestational diabetes mellitus; †CI=Confidence interval. #One study did not report follow-up duration; hence, it was only considered in all studies
DISCUSSION
Summary of findings
This systematic review and meta-analysis included 44165 participants of South Asian ethnicity from 6 studies. 3095 women had the previous history of GDM, while 41070 women had no history of GDM during their pregnancy. Herath et al. 2017 used WHO 1999 criteria for diagnosis of both GDM and T2DM, while Sreelakshmi et al. 2007 used Carpenter Coustan Criteria for diagnosis of GDM and WHO criteria for diagnosis of T2DM (assessed in 2006). Aziz et al. diagnosed GDM using International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria. Shreelakshmi et al. 2015, Gadgil et al. 2017, and Mukerji et al. 2012 did not specify diagnostic criteria used in their respective studies. Gadgil et al. 2017 also did not specify the actual length of follow-up of the patients in their study. The overall NOS score of all the included studies was 7.4. This is suggestive of the inclusion of good quality studies being included in the meta-analysis. There was no publication bias detected. However, Gadgil et al. 2017 scored 6 in NOS scale, lowest among all. Gadgil et al. 2017 were also responsible for the introduction of significant heterogeneity among the studies (I2 = 90% after inclusion of Gadgil et al. 2017 in the meta-analysis). The average length of follow-up of participants in our meta-analysis was 6.3 years. 995 women from the GDM group and 1525 women from the non-GDM group subsequently developed T2DM during this time. The RR of developing T2DM in the GDM group was 10.81 (95% CI: 7.61–15.35) suggesting 10.81-fold higher risk of developing T2DM in GDM group compared to the non-GDM group.
Comparison with existing literature
Our meta-analysis is the first such meta-analysis specifically of South Asian population and found a 10.81 times risk of developing T2DM in GDM patients. Vounzoulaki et al. 2020[6] in their systematic review and meta-analysis found women (included multiple different ethnicities) with history of GDM are at 9.51-fold higher risk of developing T2DM. Li et al. 2020[8] found in their meta-analysis that an estimated risk in women (included multiple different ethnicities) for developing T2DM after GDM was 19.72% at 10 years. Our meta-analysis shows a risk of 33% at more than 10 years of follow-up. Li et al. 2020[8] have further found the estimated risks for T2DM as 29.36% at 20 years, 39.00% at 30 years, 48.64% at 40 years, and 58.27% at 50 years, respectively. Our meta-analysis has also found increasing risk of T2DM with longer duration of follow-up. Rayanagoudar et al. 2016[9] in their meta-analysis (included GDM women of multiple different ethnicities) found that BMI (RR 1.95 [95% CI: 1.60, 2.31]), family history of diabetes (RR 1.70 [95% CI: 1.47, 1.97]), non-white ethnicity (RR 1.49 [95% CI: 1.14, 1.94]), and advanced maternal age (RR 1.20 [95% CI: 1.09, 1.34]) were associated with future risk of type 2 diabetes. Our meta-analysis has also found advanced maternal age as a risk factor for future T2DM in GDM patients. Girgis et al. 2012[20] in a prospective study found women of South Asian ethnicity with history of GDM had significantly higher risk of developing T2DM compared to other ethnic groups. A study conducted in the Indian state of Uttar Pradesh by Rajesh Jain et al. 2019[21] had found that GDM women with lower blood glucose level (140 mg% - <160 mg%) had significantly lower risk of developing T2DM than those having higher blood glucose (>160 mg% - >200 mg%). They concluded that better blood sugar control during GDM can reduce the risk of developing future diabetes mellitus. Mahalakshmi et al. 2014[22] in a study of south Indian women with GDM have found that progression to type 2 diabetes mellitus (T2DM) in Indian women with GDM is rapid.
Implication on public health
GDM has an adverse impact on immediate maternal and neonatal outcomes during pregnancy.[3] In the long term, GDM increases the risk of T2DM and metabolic syndrome in later years.[23,24] Shriraam et al. 2013[25] found low awareness of GDM among antenatal women from rural area in South India. Knowledge on risk factors of GDM and subsequent risk of developing T2DM was also low among the antenatal women. Another study by Koning et al. 2016[26] observed low rates of longer-term follow-up regarding postpartum glucose testing and suboptimal adherence to a healthy lifestyle for women with a history of GDM. Considering these facts, our study has important implications on public health. Increased risk of T2DM in GDM women necessitates proper postpartum screening and follow-up.[27] India being the country with the highest population in South Asia, this meta-analysis shows that the health care policy should include an emphasis on early detection as well as efforts of prevention of T2DM in all women with history of GDM.
Strengths of the present study
Our study is one of the first systematic review and meta-analysis studies to explore the nature of association between GDM and future T2DM in South Asian women. Multiple studies were included in our systematic review with total participants of 44165 women with follow-up ranging from 1 to 15 years.
Limitations of the present study
The authors acknowledge that there are number of important caveats regarding the present meta-analysis. We included fewer studies for our meta-analysis due to the limited availability of research articles on the target population. This had resulted in inclusion of a relatively small sample size. Different studies had used different diagnostic criteria for GDM and T2DM and two studies did not specify the diagnostic criteria in their studies. Moreover, we could not perform subanalysis to assess the effects of other factors (age, body mass index, family history, or country of origin) in the development of T2DM among women with previous GDM due to lack of information. Person years of follow-up were not published for every study included in the meta-analysis, and so we were unable to measure incidence rate ratios consistently. The risk of T2DM development in women with previous GDM was estimated using relative risks. We estimated the cumulative incidence by study length of follow-up but could not derive the timing of T2DM onset using study-level data, as the cumulative incidence was not known when the events occurred. It will be possible to assess more accurately the cumulative incidence if the individual patient data in a cohort with regular screening is available.
CONCLUSION
Our systematic review and meta-analysis showed a 10.8-fold higher risk of T2DM among previous GDM women in the South Asian region. The cumulative incidence of T2DM in GDM group was 17.34% at 5 years of follow-up and 33% at more than 10 years of follow-up. There is a lack of awareness about this risk and, hence, proper communication, timely screening for glucose intolerance, and long-term follow-up are necessary among pregnant women. Therefore, clinicians should encourage lifestyle modification and pharmacological intervention for women at risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Dr. Padma Rekha Jirge, Gynecologist, Shreyas Hospital, Kolhapur for her valuable advice and suggestions in our systematic review.
PROSPERO Registration number: CRD42020199808.
REFERENCES
- 1.Cheema A, Adeloye D, Sidhu S, Sridhar D, Chan KY. Urbanization and prevalence of type 2 diabetes in Southern Asia: A systematic analysis. J Glob Health. 2014;4:010404. doi: 10.7189/jogh.04.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes in South East Asia. International Diabetes Federation. [Last accessed on on 2020 May 22]. Available from: https://idf.org/our-network/regions-members/south-east-asia/diabetes-in-sea.html .
- 3.Mithal A, Bansal B, Kalra S. Gestational diabetes in India: Science and society. Indian J Endocr Metab. 2015;19:701–4. doi: 10.4103/2230-8210.164031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra S, Bhadoria AS, Kishore S, Kumar R. Gestational diabetes mellitus 2018 guidelines: An update. J Fam Med Prim Care. 2018;7:1169–72. doi: 10.4103/jfmpc.jfmpc_178_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: A systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:494. doi: 10.1186/s12884-018-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia. 2019;62:905–14. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Cheng Y, Wang D, Chen H, Chen H, Ming W, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review and meta-analysis of 170,139 women? J Diabetes Res. 2020;2020:3076463. doi: 10.1155/2020/3076463. doi: 10.1155/2020/3076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59:1403–11. doi: 10.1007/s00125-016-3927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Zhang S, Wang L, Leng J, Li W, Li N, et al. Fasting and 2-hour plasma glucose, and HbA1c in pregnancy and the postpartum risk of diabetes among Chinese women with gestational diabetes. Diabetes Res Clin Pract. 2016;112:30–6. doi: 10.1016/j.diabres.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Clin Res Ed) 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in metaanalysis. [Last accessed on 2020 May 22]. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 13.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. [Last accessed on 2020 Dec 20]. Available from: www.handbook.cochrane.org .
- 14.Krishnaveni GV, Hill JC, Veena SR, Geetha S, Jayakumar MN, Karat CL, et al. Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res Clin Pract. 2007;78:398–404. doi: 10.1016/j.diabres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukerji G, Chiu M, Shah BR. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia. 2012;55:2148–53. doi: 10.1007/s00125-012-2549-6. [DOI] [PubMed] [Google Scholar]
- 16.Sreelakshmi PR, Nair S, Soman B, Alex R, Vijayakumar K, Kutty VR. Maternal and neonatal outcomes of gestational diabetes: A retrospective cohort study from Southern India. J Family Med Prim Care. 2015;4:395–8. doi: 10.4103/2249-4863.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herath H, Herath R, Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—A community based retrospective cohort study. PLoS One. 2017;12:e0179647. doi: 10.1371/journal.pone.0179647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadgil MD, Oza-Frank R, Kandula NR, Kanaya AM. Type 2 diabetes after gestational diabetes mellitus in South Asian women in the United States. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2891. doi: 10.1002/dmrr. 2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz S, Munim TF, Fatima SS. Post-partum follow-up of women with gestational diabetes mellitus: Effectiveness, determinants, and barriers. J Matern Fetal Neonatal Med. 2018;31:1607–12. doi: 10.1080/14767058.2017.1321630. [DOI] [PubMed] [Google Scholar]
- 20.Girgis CM, Gunton JE, Cheung NW. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: A prospective study and review of the literature. ISRN Endocrinol. 2012;2012:341638. doi: 10.5402/2012/341638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain R, Davey S, Olejas S, Jain R. A prospective study on impact of gestational diabetes mellitus (GDM) management on burden of diabetes mellitus (DM) in Uttar Pradesh India. Arch Community Med Public Health. 2019;5:35–9. [Google Scholar]
- 22.Mahalakshmi MM, Bhavadharini B, Kumar M, Anjana RM, Shah SS, Bridgette A, et al. Clinical profile, outcomes, and progression to type 2 diabetes among Indian women with gestational diabetes mellitus seen at a diabetes center in south India. Indian J Endocrinol Metab. 2014;18:400–6. doi: 10.4103/2230-8210.131205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sodhi NK, Nelson AL. Prevalence of glucose intolerance and metabolic syndrome within one year following delivery of a pregnancy complicated by gestational diabetes. Contracept Reprod Med. 2018;3:27. doi: 10.1186/s40834-018-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X. Metabolic syndrome risk after gestational diabetes: A systematic review and meta-analysis. PLoS One. 2014;9:e87863. doi: 10.1371/journal.pone.0087863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shriraam V, Rani MA, Sathiyasekaran BW, Mahadevan S. Awareness of gestational diabetes mellitus among antenatal women in a primary health center in South India. Indian J Endocrinol Metab. 2013;17:146–8. doi: 10.4103/2230-8210.107861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koning SH, Lutgers HL, Hoogenberg K, Trompert CA, van den Berg PP, Wolffenbuttel BH. Postpartum glucose follow-up and lifestyle management after gestational diabetes mellitus: General practitioner and patient perspectives. J Diab Metab Disord. 2016;15:56. doi: 10.1186/s40200-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray LJ, Tringham JR, Davies MJ, Webb DR, Jarvis J, Skinner TC, et al. Screening for type 2 diabetes in a multiethnic setting using known risk factors to identify those at high risk: A cross-sectional study. Vasc Health Risk Manag. 2010;6:837–42. doi: 10.2147/VHRM.S12504. [DOI] [PMC free article] [PubMed] [Google Scholar]