ABSTRACT
Chemosensory pathways are among the most abundant prokaryotic signal transduction systems, allowing bacteria to sense and respond to environmental stimuli. Signaling is typically initiated by the binding of specific molecules to the ligand binding domain (LBD) of chemoreceptor proteins (CRs). Although CRs play a central role in plant-microbiome interactions such as colonization and infection, little is known about their phylogenetic and ecological specificity. Here, we analyzed 82,277 CR sequences from 11,806 representative microbial species covering the whole prokaryotic phylogeny, and we classified them according to their LBD type using a de novo homology clustering method. Through phylogenomic analysis, we identified hundreds of LBDs that are found predominantly in plant-associated bacteria, including several LBDs specific to phytopathogens and plant symbionts. Functional annotation of our catalogue showed that many of the LBD clusters identified might constitute unknown types of LBDs. Moreover, we found that the taxonomic distribution of most LBD types that are specific to plant-associated bacteria is only partially explained by phylogeny, suggesting that lifestyle and niche adaptation are important factors in their selection. Finally, our results show that the profile of LBD types in a given genome is related to the lifestyle specialization, with plant symbionts and phytopathogens showing the highest number of niche-specific LBDs. The LBD catalogue and information on how to profile novel genomes are available at https://github.com/compgenomicslab/CRs.
IMPORTANCE Considering the enormous variety of LBDs at sensor proteins, an important question resides in establishing the forces that have driven their evolution and selection. We present here the first clear demonstration that environmental factors play an important role in the selection and evolution of LBDs. We were able to demonstrate the existence of LBD families that are highly enriched in plant-associated bacteria but show a wide phylogenetic spread. These findings offer a number of research opportunities in the field of single transduction, such as the exploration of similar relationships in chemoreceptors of bacteria with a different lifestyle, like those inhabiting or infecting the human intestine. Similarly, our results raise the question whether similar LBD types might be shared by members of different sensor protein families. Lastly, we provide a comprehensive catalogue of CRs classified by their LBD region that includes a large number of putative new LBD types.
KEYWORDS: MCP, chemoreceptor, chemotaxis, methyl-accepting chemotaxis protein, plant-associated bacteria
INTRODUCTION
To ensure cell survival, bacteria have to adapt to changing environmental conditions (1). For this, bacterial cells are equipped with an array of different signal transduction systems that sense different environmental stimuli, such as osmolarity, oxygen tension, temperature, pH, light, nutrients, toxins, and other chemicals (2). Chemosensory pathways represent one of the primary bacterial signal transduction mechanisms, and more than half of all the bacterial genomes contain signaling genes (3). Most chemosensory pathways appear to mediate chemotaxis (3), whereas others have been associated with type IV pilus-based motility (4) or alternative cellular functions such as the control of second messenger levels (4, 5).
In a canonical chemosensory pathway, signals are perceived by binding specific molecules to the ligand binding domain (LBD) of chemoreceptors (CRs), which modulates the activity of the CheA autokinase and the subsequent transphosphorylation to the CheY response regulator. In canonical CRs, the extracytosolic LBD is flanked by two transmembrane (TM) regions, a cytosolic HAMP domain, and a signaling domain (MCPsignal). While the CR signaling domain (MCPsignal) is highly conserved, LBDs are rapidly evolving domains (6), which reflects the wide variety of chemoeffectors to be sensed. To date, more than 80 different LBD families have been identified (7, 8), and new types of LBDs continue to be discovered (9). The thermodynamic parameters for ligand binding to the individual CRs are very similar to those for binding to specific LBDs (10, 11), supporting the idea that the molecular determinants for signal recognition by CRs are located in the LBD. Further evidence of this came from the construction of chimeric receptors recombining LBDs with other signaling domains (e.g., autokinase domains), where the LBD was proved to define the function of the chimera (12, 13). Thus, while the conserved MCPsignal domain can be used to identify CRs, their LBDs allow them to be classified on the basis of their function (7, 8).
On the other hand, there is evidence suggesting that the genomic repertory of CRs is related to bacterial lifestyle (14, 15). For instance, it has been shown that plant-associated bacteria (PAB) possess a particularly large number of CRs (8, 16), indicating that chemosensory signaling is indeed an important requisite for plant-bacterium interactions. This is of particular relevance for plant pathogens and symbionts, for which it has been shown that flagellum-mediated chemotaxis is required for optimal virulence or symbiosis establishment (17–25). Plants represent complex habitats for colonization by different kinds of microorganisms, and PAB species can colonize the plant rhizosphere, phyllosphere, or endosphere (26). Motile sensory behavior has been shown to play a key role in the establishment of plant-microbe interactions, since bacteria that can sense and rapidly navigate toward niches optimal for growth and survival will have a clear competitive advantage (27–29). These considerations are valid for both pathogenic and nonpathogenic relationships between microorganisms and plants (8, 16). Similarly, microbial inhabitants of the phyllosphere, comprising the aerial part of plants, have to deal with the challenges of life on leaf surfaces, where flagellar motility confers advantages in terms of epiphytic fitness (30). The epiphytic lifestyle also represents the initial stage of foliar colonization by many bacterial phytopathogens, preceding entry into the leaf apoplast via wounds or natural plant openings (e.g., stomata) (30). However, despite their biological significance, the function and cognate signal have been determined for only a limited number of CRs from PAB, and very little information exists on their phylogenetic and ecological specificity.
In order to study those LBD types most tightly coupled to the plant-associated lifestyle, here we comprehensively identified the CR genes in all known bacterial lineages and classified them according to their LBDs, with a particular focus on the LBD types linked to a plant-associated lifestyle. As such, we employed a novel de novo methodology to extract putative LBD regions from all CR sequences and group them into homology-based clusters (i.e., putative LBD types). This analysis allowed us to identify hundreds of LBD types highly specific for PAB species, many of them unknown. We further found that the taxonomic distribution of the majority of PAB-specific LBD clusters is only partially explained by phylogeny, suggesting that niche and host adaptation might have played relevant roles for their selection. Together, these results form a solid basis for the design of experiments aimed at identifying CRs that are essential for plant-microbe interactions and virulence.
RESULTS
Towards a global catalogue of chemoreceptors in plant-associated bacteria.
In order to maximize the coverage of our analysis, we first built a comprehensive catalogue of CRs detected across the entire prokaryotic phylogeny (Fig. 1). Species genomes were retrieved from the proGenomes v2 databases (31). Unlike the NCBI Taxonomy database, which is not an authoritative source for nomenclature or classification (32), proGenomes2 data do not rely on taxonomic names to identify species. Instead, each species-representative genome in proGenomes is delineated based on the evolutionary distances calculated between universally conserved genes present in nearly all organisms (32, 33). To establish links between CRs and the plant-associated lifestyle, we compiled three manually curated databases of PAB (see Materials and Methods): (i) PAB-broad, a reference database of 960 organisms found in multiple plant environments including leaves, roots, and rhizospheric soil; (ii) PAB-phyto, a subset database of 119 species including only known phytopathogens; and (iii) PAB-symb, which groups 192 plant symbionts. Using HMM-based searches, we then mined all the sequences containing the MCPsignal domain in the 11,806 species-representative genomes from the proGenomes database, compiling a global catalogue of 82,277 CR sequences from 5,546 genomes (see Data Set S3 in the supplemental material). This confirms the broad distribution of CRs, with 47% of the representative genomes containing at least one chemotactic receptor.
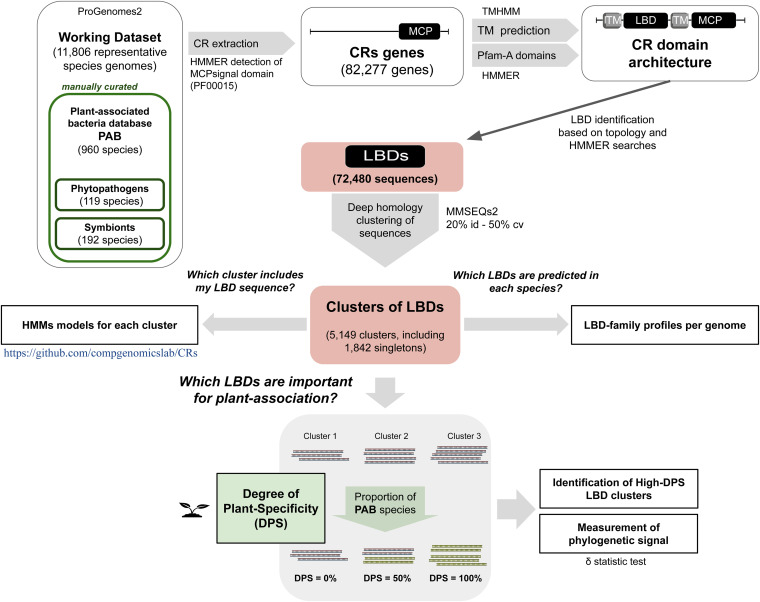
FIG 1.
Schematic view of the bioinformatics pipeline used to identify CRs that are potentially relevant for plant association. From a set of 11,806 representative prokaryotic genomes, 82,277 protein sequences were mined using HMM-based searches against the MCPsignal Pfam domain (PF00015). CR topology was analyzed by predicting transmembrane regions (TMs) and Pfam domains. Based on the topological analysis, LBD regions were predicted and a set of 72,480 LBD sequences was obtained. Clustering of LBDs based on sequence homology (20% minimum sequence identity with at least 50% sequence coverage) resulted in 5,149 clusters or subfamilies of LBDs, of which 1,842 contained a single sequence. To study a possible link between the LBD profiles and plant-associated lifestyle, a manually curated subset of 960 representative species of plant-associated bacteria (PAB) was generated, including phytopathogen (119) and symbiont (192) subsets. The determination of the proportion of PAB LBDs present in each cluster allowed us to assign the degree of plant specificity (DPS) value for each LBD subfamily. Subsequent analysis of high-DPS clusters identified LBD clusters that are potentially important for bacterium-plant associations. Furthermore, the validation of the high-DPS clusters as good ecological indicators was corroborated by measuring their phylogenetic signal. A detailed step-by-step description of the process can be found in Materials and Methods.
List of the 5,546 species-representative genomes in the proGenomes2 database having at least one CR. Download Data Set S3, XLSX file, 0.4 MB (374.5KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PAB species possessed almost twice as many CRs per genome (22.86) as those species not classified as plant associated (12.94), with the subset of phytopathogens showing the highest number (27.29). No CRs were predicted in 178 out of the 960 PAB genomes, indicating that more than 81% of PABs possess at least one CR gene, a percentage largely superior to the bacterial average (47%). From all species considered in this study, 36 PAB genomes stood out by their high content of CRs (Table 1), most notably the following: (i) 14 genomes from the Pseudomonas genus (49 to 60 CRs), including the well-known plant pathogens P. syringae and P. savastanoi (34), and (ii) 9 genomes from the Herbaspirillum genus (52 to 67 CRs), a group of betaproteobacteria that endophytically colonize gramineous species, thereby promoting their growth (35).
TABLE 1.
List of PAB with the highest number of predicted CRs
| TaxId | Biosample | Representative species | No. of CRs | PAB-phytoa | PAB-symbb |
|---|---|---|---|---|---|
| 1078773 | SAMN04334956 | Herbaspirillum rubrisubalbicans M1 | 67 | ||
| 1144319 | SAMN00839627 | Herbaspirillum sp. CF444 | 67 | ||
| 964 | SAMN03779333 | Herbaspirillum seropedicae | 65 | ||
| 193 | SAMN02982994 | Azospirillum lipoferum | 65 | ||
| 286727 | SAMN02982917 | Azospirillum oryzae | 64 | ||
| 346179 | SAMN03785417 | Herbaspirillum rhizosphaerae | 62 | ||
| 864073 | SAMN02471292 | Herbaspirillum frisingense GSF30 | 62 | ||
| 237610 | SAMN05860868 | Pseudomonas psychrotolerans | 60 | ||
| 288000 | SAMN02598359 | Bradyrhizobium sp. BTAi1 | 60 | S | |
| 92645 | SAMN06130964 | Herbaspirillum frisingense | 59 | ||
| 1175306 | SAMN02469572 | Herbaspirillum sp. GW103 | 59 | ||
| 1121033 | SAMN02440867 | Azospirillum halopraeferens DSM 3675 | 58 | ||
| 169679 | SAMN05170519 | Clostridium saccharobutylicum | 58 | ||
| 29438 | SAMN03837775 | Pseudomonas savastanoi | 57 | P | |
| 1262470 | SAMN03010392 | Herbaspirillum hiltneri N3 | 55 | ||
| 582667 | SAMN05192568 | Methylobacterium pseudosasicola | 54 | ||
| 50340 | SAMN05216581 | Pseudomonas fuscovaginae | 54 | ||
| 1001585 | SAMN02603190 | Pseudomonas mendocina NK-01 | 54 | ||
| 1749078 | SAMN04216969 | Pseudomonas sp. EpS/L25 | 53 | ||
| 1190415 | SAMN05216593 | Pseudomonas asturiensis | 53 | ||
| 50340 | SAMN03100370 | Pseudomonas fuscovaginae | 53 | ||
| 129140 | SAMN03976254 | Pseudomonas syringae pv. tagetis | 52 | P | |
| 294 | SAMN04992557 | Pseudomonas fluorescens | 52 | ||
| 1855289 | SAMN05216319 | Duganella sp. CF402 | 52 | ||
| 1144342 | SAMN00839653 | Herbaspirillum sp. YR522 | 52 | ||
| 47885 | SAMN03365871 | Pseudomonas oryzihabitans | 51 | ||
| 205918 | SAMN02604347 | Pseudomonas syringae pv. syringae B728a | 51 | P | |
| 1907416 | SAMN05880558 | Aeromonas sp. RU39B | 51 | ||
| 693986 | SAMN03075686 | Methylobacterium oryzae CBMB20 | 50 | ||
| 1736267 | SAMN04151647 | Pseudomonas sp. Leaf127 | 50 | ||
| 114615 | SAMEA3138227 | Bradyrhizobium sp. ORS 278 | 50 | S | |
| 1028989 | SAMD00019511 | Pseudomonas sp. StFLB209 | 50 | ||
| 80867 | SAMN04009978 | Acidovorax avenae | 50 | P | |
| 1122963 | SAMN02440654 | Pleomorphomonas oryzae DSM 16300 | 50 | ||
| 223283 | SAMN02604017 | Pseudomonas syringae pv. tomato DC3000 | 49 | P | |
| 1245469 | SAMD00061052 | Bradyrhizobium oligotrophicum S58 | 49 | S |
P, phytopathogen.
S, plant symbiont.
Classifying chemoreceptors according to their ligand binding domain.
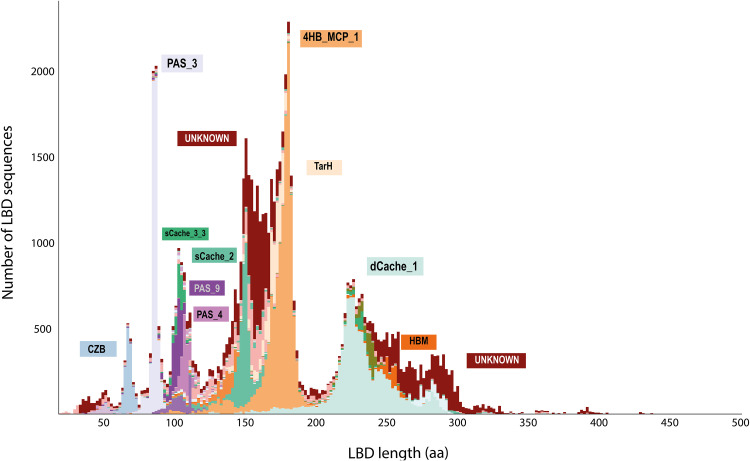
As the ecological relevance of CRs is mostly defined by their LBD region, we explored whether sequence segments corresponding to the LBD, rather than the full-length CR sequences, were related to a plant-associated lifestyle. To maximize the number of LBD sequences included in our analysis and not limit this to known LBD types from the Pfam database (7), we inferred LBDs based on the domain architecture of each CR. First, we extracted LBD sequences from the whole set of 82,277 CRs. Next, and given the high variability in the domains that could be considered LBDs, we identified putative LBDs using three different strategies: (i) detecting sequence regions matching any known domain other than the MCPsignal or HAMP, (ii) locating sequence regions flanked by two TM regions, and (iii) taking domains between the N-terminus and a single TM region. In total, we retrieved 72,480 putative LBD sequences, which could be fitted into three main groups based on their length (Fig. 2). The first group includes LBDs with a size between 60 and 110 amino acids, containing 21% of all the LBDs detected. The most abundant LBD family within this size range was PAS_3. The second group, comprising LBDs from 130 to 200 amino acids, contained over 45% of all LBDs and included 4HB_MCP_1 as the predominant family. The third group, comprising LBD lengths between 220 and 299 amino acids, covers 26% of all LBDs and has dCache_1 as the most abundant LBD family. Only 8% of all the LBDs detected fell outside these three size ranges, and the three most abundant LBDs were 4HB_MCP_1 (17.6%), dCache_1 (15.5%), and PAS_3 (9.2%).
FIG 2.
Length distribution of the LBDs. The analysis was conducted on 72,480 LBDs, and the predominant LBD types within each of the main peaks are indicated. Only LBDs shorter than 500 amino acids (aa) are represented.
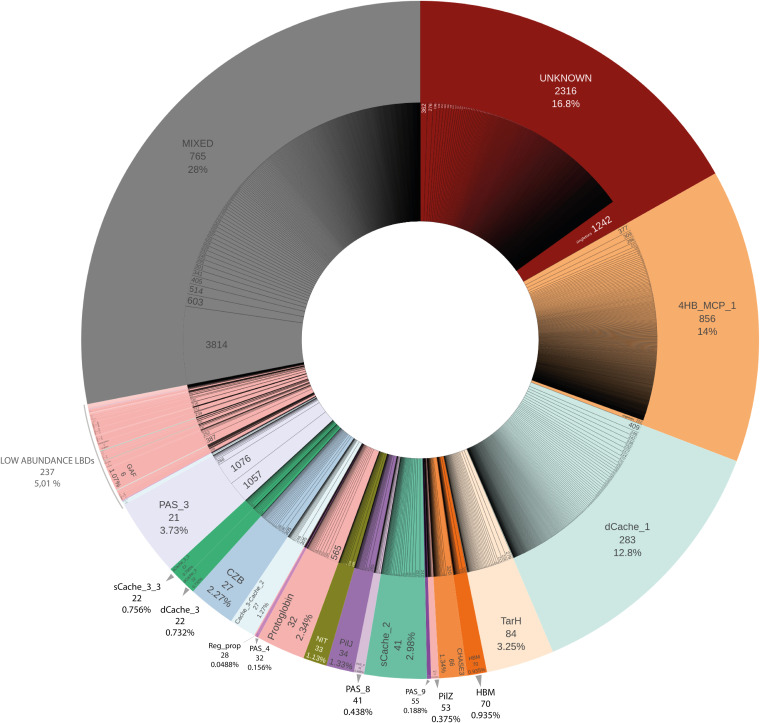
We next investigated whether LBDs could be classified into broader sequence homology clusters, each representing a group of LBD sequences sharing a common evolutionary origin. Using relaxed homology thresholds (E value ≤10−3, 50% coverage, 20% amino acid identity), we grouped all 72,480 LBD sequences into 5,149 family clusters (Data Set S4), of which 3,307 contain more than 1 sequence. This de novo clustering approach might not be adequate for a detailed functional characterization of LBDs, as single residue changes have been shown to modify LBD ligand affinities (36–38). Nevertheless, each of our LBD clusters could be interpreted as an independent LBD type, with implicit levels of functional and ecological conservation. In fact, our approach consistently recovered all known LBD types and distributed them into 2,068 compact clusters where 90% of their members belonged to the same Pfam domain family (Table S1). Moreover, our clustering strategy allowed us to split large LBD families into finely grained subcategories (Fig. 3). For example, despite 4HB_MCP and Cache-like being present at similar levels in the initial CR sequence database, the number of derived clusters differs significantly, namely, 20.9% for 4HB_MCP compared to 8.3% for Cache-like. In the case of 4HB_MCP_1, the 10,034 sequences group into 856 different clusters compared to the 283 clusters for the 9,162 dCache_1 sequences, indicating higher sequence conservation in the latter. The situation is even more drastic in the case of PAS_3 LBDs, where 2,675 sequences group into just 21 clusters (Table S1), indicating a very low degree of diversity.
FIG 3.
Visual representation of the abundance of the LBD families. The outer donut of the chart represents the distribution of each LBD type and its relative abundance (in percentage of sequences), and the number of clusters with at least 90% of their sequences sharing the same LBD type, as defined by the Pfam signature. The LBDs are sorted according to the number of clusters within each LBD type. The inner donut of the chart represents all the clusters included within each LBD category, indicating the number of sequences contained in each subfamily. All singletons are merged in the last section of each LBD type (e.g., LBDs classified as “Unknown” have 1,242 singletons, that is, clusters containing only one sequence). “Mixed clusters” are those that do not reach the 90% threshold of sequences with the same Pfam model per cluster. “Low-abundance LBDs” include those LBD types that group into fewer than 12 “compact clusters.”
Distribution of LBD types among “compact” LBD clusters (at least 90% of their sequences sharing the same LBD type). Download Table S1, PDF file, 0.06 MB (61.5KB, pdf) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of all CRs identified in 5,546 species-representative genomes obtained from the proGenomes2 database indicating their cluster classification and the amino acid coordinates for their predicted domains. Download Data Set S4, XLSX file, 6.9 MB (6.9MB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Notably, an important fraction (45%) of the LBD clusters inferred could not be confidently associated with any previous family of Pfam domains, since more than 90% of their LBD sequences did not match to any known domain signature, suggesting the existence of a large number of unknown LBD types.
Identifying PAB-specific ligand binding domains.
To identify LBD families specific to a plant-associated lifestyle, we analyzed each LBD cluster and calculated the corresponding percentage of PAB species therein, which we referred to as the degree of plant specificity (DPS; see Materials and Methods). For each LBD cluster, we calculated three DPS values, based on three databases of PAB species: (i) DPS-broad, calculated based on the PAB-broad reference database; (ii) DPS-phyto, based on the PAB-phyto subset; and (iii) DPS-symb, using the PAB-symb subgroup as a reference. In all cases, the DPS values ranged from 0% (no LBD family observed in the corresponding PAB database) to 100% (the LBD cluster includes only species from a given reference PAB database). From the 3,307 LBD nonsingleton clusters, we identified 419 and 139 clusters with a DPS-broad score of ≥50%, and ≥80%, respectively. Similarly, many LBD clusters showed high specificity in the stricter PAB reference databases (Data Set S5).
List of the 5,149 clusters of LBDs indicating their DPS-broad, DPS-phyto, and DPS-symb. Download Data Set S5, XLSX file, 0.3 MB (276.2KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To further validate our findings, we cross-linked our predictions with experimental data from previous studies (39–43). In particular, we found that CRs with increased expression in planta, and particularly those required for full bacterial virulence, belonged to high-DPS clusters (Table 2). This list includes CRs that are upregulated in Dickeya dadantii 3937 and Pectobacterium carotovorum WPP14, two soft-rot bacterial strains (39); Dickeya dianthicola RNS04.9, which grows on macerated potato tubers (40); and Xanthomonas fragariae, which grows on strawberry leaves (41). Similarly, we found several CRs with very high DPS values (80%) that were shown to be relevant in Xanthomonas citri virulence (42) or required for fitness of Pseudomonas savastanoi pv. savastanoi in olive knots (43). Taken together, these data support the validity of our approach to identify CRs that are relevant for a plant-associated lifestyle.
TABLE 2.
CRs predicted to be involved in plant-bacterium interactions
| CR gene ID (in the original source) | LBD type | DPS (%) | DPS-phyto (%) | DPS-symb (%) | Cluster no. | Amino acid identity (%) to representative LBDs from the database | Bacterial species and strain | Reference |
|---|---|---|---|---|---|---|---|---|
| ABF-0014824 | TarH | 100 | 100 | 0 | 932 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0015168 | TarH | 68.09 | 40.43 | 0 | 179 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0016115 | HBM | 63.64 | 48.48 | 0 | 409 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0016585 | TarH | 51.09 | 14.60 | 0.73 | 42 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0017097 | Unknown | 80.00 | 80.00 | 0 | 838 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0017674 | 4HB_MCP_1 | 100 | 87.50 | 0 | 630 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0019851 | TarH | 61.22 | 42.86 | 0 | 123 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0019855 | TarH | 61.22 | 42.86 | 0 | 123 | 100 | Dickeya dadantii 3937 | 39 |
| ABF-0020431 | sCache_2 | 34.55 | 12.73 | 0 | 233 | 100 | Dickeya dadantii 3937 | 39 |
| DDI_0843 | dCache_1 | 38.61 | 10.13 | 1.27 | 51 | 100 | Dickeya dianthicola RNS04.9 | 40 |
| DDI_0932 | sCache_2 | 34.55 | 12.73 | 0 | 233 | 100 | Dickeya dianthicola RNS04.9 | 40 |
| DDI_1647 | TarH | 61.22 | 42.86 | 0 | 123 | 88.97 | Dickeya dianthicola RNS04.9 | 40 |
| DDI_1649 | TarH | 61.22 | 42.86 | 0 | 123 | 100 | Dickeya dianthicola RNS04.9 | 40 |
| DDI_2258 | HBM | 100 | 100 | 0 | 792 | 100 | Dickeya dianthicola RNS04.9 | 40 |
| DDI_4092 | 4HB_MCP_1 | 100 | 87.50 | 0 | 630 | 100 | Dickeya dianthicola RNS04.9 | 40 |
| ADT-0000027 | HBM | 63.64 | 48.48 | 0 | 409 | 96.03 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0000661 | sCache_2 | 38.46 | 12.82 | 0 | 160 | 99.30 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0001320 | TarH | 51.09 | 14.60 | 0.73 | 42 | 98.84 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0001602 | TarH | 56.99 | 29.03 | 0 | 116 | 94.15 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0001887 | TarH | 61.22 | 42.86 | 0 | 123 | 97.59 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0002104 | TarH | 100 | 100 | 0 | 932 | 100 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0003152 | TarH | 68.09 | 40.43 | 0 | 179 | 91.15 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0003245 | 4HB_MCP_1 | 100 | 87.50 | 0 | 630 | 97.40 | Pectobacterium carotovorum WPP14 | 39 |
| ADT-0003418 | Unknown | 80.00 | 80.00 | 0 | 838 | 95.60 | Pectobacterium carotovorum WPP14 | 39 |
| PSA3335_17610 | Unknown | 87.50 | 50.00 | 0 | 835 | 100 | Pseudomonas savastanoi NCPPB3335 | 43 |
| XAC1892 | Unknown | 100 | 100 | 0 | 846 | 86.77 | Xanthomonas citri subsp. citri XHG3 | 42 |
| XAC2448 | 4HB_MCP_1 | 39.23 | 14.62 | 0 | 77 | 98.88 | Xanthomonas citri subsp. citri XHG3 | 42 |
| NBC2815_01024 | 4HB_MCP_1 | 92.00 | 84.00 | 0 | 549 | 100 | Xanthomonas fragariae IPO 3485 | 41 |
| NBC2815_02005 | 4HB_MCP_1 | 60.53 | 42.11 | 0 | 353 | 100 | Xanthomonas fragariae IPO 3485 | 41 |
| NBC2815_02008 | 4HB_MCP_1 | 88.46 | 88.46 | 0 | 273 | 100 | Xanthomonas fragariae IPO 3485 | 41 |
| NBC2815_02009 | 4HB_MCP_1 | 82.14 | 75.00 | 0 | 340 | 100 | Xanthomonas fragariae IPO 3485 | 41 |
Interestingly, we also found that many PAB-specific clusters (41.75%) are formed by proteins of unknown LBD type, suggesting the presence of a significant number of uncharacterized LBD types. Excluding unknown LBD families, the most common domains among high-DPS clusters are 4HB_MCP_1 (26%), TarH (4.5%), and HBM (4%) (Table 3). It is remarkable that the three domain families form four-helix bundle structures (37, 38). The case of the HBM and TarH domains is particularly interesting, as the majority of sequences that belonged to these categories concentrated in very few high-DPS clusters: 57.0% (516/906) of all HBM sequences are grouped into 23 high-DPS clusters, and 36.7% (1,042/2,840) of all TarH sequences are grouped into 26 high-DPS clusters. This indicates a strong association of the TarH and HBM domains with the plant-associated lifestyle. In contrast, despite being the second most abundant LBD in bacteria (Table S2), the dCache_1 domain was not very abundant in PAB.
TABLE 3.
Distribution of LBD types among clusters with high DPS (≥50%)
| LBD type | No. of clusters | % of clusters over totala | No. of LBD sequences with the indicated domainb | Avg no. of LBD sequences per cluster |
|---|---|---|---|---|
| Unknown | 243 | 41.75 | 2,053 | 8.45 |
| 4HB_MCP_1 | 151 | 25.95 | 2,642 | 17.50 |
| TarH | 26 | 4.47 | 1,042 | 40.08 |
| HBM | 23 | 3.95 | 516 | 22.43 |
| CHASE3 | 9 | 1.55 | 142 | 15.78 |
| PilZ | 7 | 1.20 | 39 | 5.57 |
| PAS_9 | 6 | 1.03 | 7 | 1.17 |
| sCache_2 | 4 | 0.69 | 245 | 61.25 |
| NIT | 4 | 0.69 | 79 | 19.75 |
| Protoglobin | 3 | 0.52 | 153 | 51 |
| PAS_8 | 3 | 0.52 | 9 | 3 |
| PAS_3 | 3 | 0.52 | 82 | 27.33 |
| dCache_1 | 3 | 0.52 | 151 | 50.33 |
| Cache_3-Cache_2 | 3 | 0.52 | 79 | 26.33 |
| PAS_4 | 2 | 0.34 | 4 | 2 |
| CHASE4 | 2 | 0.34 | 3 | 1.50 |
| Usher | 1 | 0.17 | 1 | 1 |
| Tox-URI2 | 1 | 0.17 | 1 | 1 |
| SURF1 | 1 | 0.17 | 1 | 1 |
| SOR_SNZ | 1 | 0.17 | 1 | 1 |
| sCache_3_3 | 1 | 0.17 | 2 | 2 |
| Porin_4 | 1 | 0.17 | 1 | 1 |
| Peripla_BP_5 | 1 | 0.17 | 1 | 1 |
| PAS_7 | 1 | 0.17 | 19 | 19 |
| PapC_N | 1 | 0.17 | 1 | 1 |
| Glyco_hydro_2_N | 1 | 0.17 | 1 | 1 |
| Glyco_hydro_106 | 1 | 0.17 | 1 | 1 |
| FHIPEP | 1 | 0.17 | 1 | 1 |
| DUF4077 | 1 | 0.17 | 5 | 5 |
| dCache_3 | 1 | 0.17 | 71 | 71 |
| CBS | 1 | 0.17 | 2 | 2 |
| Asparaginase | 1 | 0.17 | 1 | 1 |
| ABC_tran | 1 | 0.17 | 1 | 1 |
| 5TM-5TMR_LYT | 1 | 0.17 | 20 | 20 |
| Total | 510 | 7,377 |
Percentages are calculated over the total number of LBD clusters with at least 90% of their sequences sharing the same LBD type. These total clusters comprise more than 88% of the total number of clusters in this work.
Sum of the total number of sequences sharing the same domain type found in the indicated clusters.
Distribution of LBD types and domain models in the total LBD data set. Download Table S2, PDF file, 0.04 MB (42.9KB, pdf) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic versus ecological signal in PAB-specific ligand binding domains.
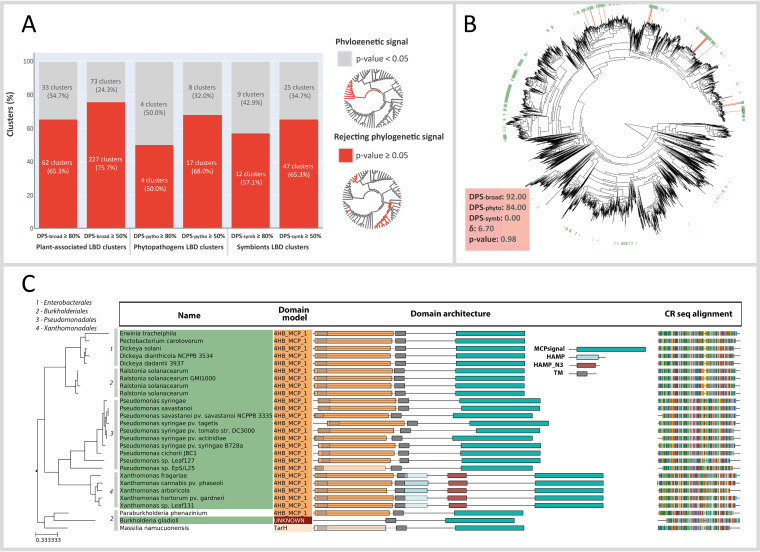
Intrigued by the potential ecological significance of PAB-specific LBD clusters, we further tested whether their taxonomic distribution is due to the phylogenetic signal of the underlying species, or if it might be driven by additional ecological factors. To address this issue, we reconstructed the complete phylogeny of the 11,806 species considered here (see Materials and Methods) and used it to assess the taxonomic distribution of each individual LBD cluster. Using the δ-approach (44), we found that the majority (75.7%) of plant-associated LBD types (DPS ≥50%) did not follow the expected phylogenetic signal. In contrast, the taxonomic distribution of most PAB-specific LBDs was scattered over the global bacterial phylogeny (Fig. 4). This observation was consistent for the three PAB reference databases considered in this study, using stricter DPS cutoffs, and even when the species lacking CR genes were excluded from the analysis (Fig. S1).
FIG 4.
Phylogenetic signal detection in plant-associated LBD clusters. (A) Proportion of the significant phylogenetic signal among LBD clusters enriched in PAB-broad, PAB-phyto, and PAB-symb species within two thresholds (≥50%, ≥80%). The significance through a P value test with 100 iterations, P value ≥0.05 rejects the null hypothesis of a phylogenetic signal (see Materials and Methods). (B) LBD cluster 549 (in red) distributed according to the chemotactic species phylogeny (5,763 representative species). The green leaves of the tree represent the PAB species. DPS, δ, and P values for this LBD cluster are represented in the lower left box. (C) Phylogeny representation of LBD cluster 549, containing 27 LBD sequences distributed across 4 orders (numbered 1 to 4 in the tree). The domain architecture prediction is shown for each of the CRs.
Phylogenetic signal detection in plant-associated LBD clusters using the phylogeny of chemotactic species tree. Proportion of significant phylogenetic signal among LBD clusters enriched in PAB-broad, PAB-phyto, and PAB-symb species within two thresholds (≥50%, ≥80%). The reference phylogeny used in this analysis excluded any species without CRs in their genome. Download FIG S1, TIF file, 0.5 MB (551.3KB, tif) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overall, the lack of phylogenetic signal for most of the LBD clusters, together with the fact that the LBDs tested are enriched in PAB species, suggests that the evolution of the sensory machinery of bacterial species might be at least partially driven by ecological pressures. This should allow the use of particular LBD clusters, even if functionally undefined, as lifestyle biomarkers. This issue is best illustrated by the LBD cluster 549 (Fig. 4B and C), which contains 27 CRs from broadly distributed bacterial families and orders, while retaining a high plant-association signal (DPS-broad >80%).
LBD profiles per genome.
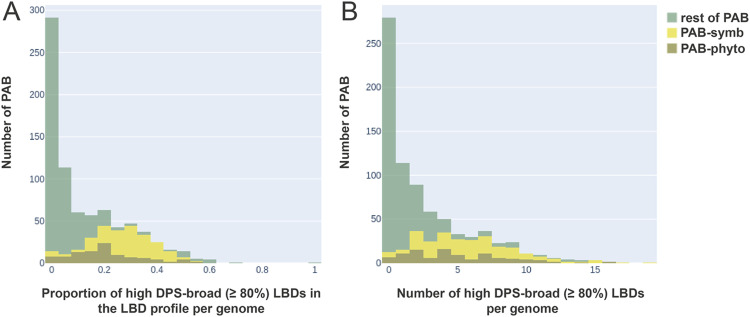
To investigate whether the profile of LBD clusters per genome could be informative about the plant-associated bacterial lifestyle, we studied the full repertoire of CRs among different PAB species. The genomes from the PAB species not only contained more CRs than those of non-PAB species, but also, many of their CRs could be considered highly specific to plant-related environments. In fact, assessing the LBD profiles per genome showed that microorganisms with a pronounced plant-associated lifestyle (i.e., PAB-symb and PAB-phyto) harbor more specific CRs than other PAB species (Fig. 5). On average, 28% and 20% of plant-symbiont and plant-phytopathogen CRs, respectively, are highly specific (DPS-broad >80%). In contrast, other PAB with a less pronounced plant-associated lifestyle, like nonsymbiont and nonphytopathogen plant-associated species, contained significantly fewer specific CRs (6%) (Fig. 5). Taken together, this information reinforces the idea that the repertoire of CRs has been partially shaped by niche adaptation, with more specialized adaptations leading to more specific CRs.
FIG 5.
Distribution of the high-plant-specificity LBDs in the PAB species profiles per genome. (A) Calculation of the proportion of high-DPS-broad (≥80%) LBDs in the total number of LBDs present in each species. The graph illustrates the distribution of the number of species according to the proportional ranges, plotting the species count as PAB-symb (yellow), PAB-phyto (brown), and the rest of PAB (green). (B) Absolute number of LBDs with a DPS-broad value of ≥80% in each PAB genome. The graph illustrates the distribution of the number of species as an absolute count of high-DPS LBD ranges. The species count is plotted as PAB-symb (yellow), PAB-phyto (brown), and the rest of PAB (green).
DISCUSSION
In the present study, we carried out a comprehensive phylogenomic analysis of the full repertoire of CRs from a wide collection of microbial genomes, classifying them according to their LBDs. To maximize the representativeness of our study, we used more than 82,000 species-level CR sequences from 11,000 species-representative genomes, significantly expanding the scope of previous works (7, 15, 45), in terms of both the number of sequences examined and the phylogenetic coverage. To achieve this, we developed a novel method to extract LBDs and classified them based on a de novo homology-based clustering approach, departing from the traditional classification of CRs centered around their general protein topology (15, 45–47) or on known LBD domain searches (7). This approach allowed us to identify many new potential LBD types, suggesting that the chemosensing landscape remains largely unexplored. Additionally, we believe that our strategy delineating large LBD families into finely grained subcategories could provide further information (Fig. 3). Moreover, by classifying CRs based on their putative LBD type, for the first time we were able to quantify to what extent the chemosensory activity of PAB is linked to lifestyle.
Considering the enormous variety of LBDs at sensor proteins, establishing the forces that have driven their evolution is an important question that was never specifically addressed. To our knowledge, we present here the first clear demonstration showing that environmental factors play an important role in the selection and evolution of LBDs. We found that the specificity of LBDs to a plant-associated lifestyle could not be explained by just a phylogenetic signal, since the taxonomic distribution of most PAB-specific LBD types was scattered over the microbial phylogeny, which at times covered different orders and phyla. This indicates that the selection of the certain CRs might indeed be guided by ecological factors, opening the possibility of identifying lifestyle biomarkers.
We also found that bacterial species more tightly associated with plant environments (such as plant symbionts and phytopathogens) tend to have stronger lifestyle specificity signals in their CR repertory. For instance, plant symbionts had the largest number of PAB-specific LBDs per genome, followed by phytopathogens, with both showing significantly higher ratios than generic soil microbiota. It appears likely that even stronger links between the chemosensory capabilities of bacteria and their lifestyle will be detected in the future as more data become available on new organisms (e.g., via metagenomics sequencing) and on their niche adaptation (i.e., plant-tissue specificity).
These findings thus offer a number of research opportunities in the field of signal transduction. First, it can be explored whether similar relationships can be observed in CRs of bacteria with a different lifestyle, such as for example those that inhabit or infect the human intestine. Another interesting issue that needs to be addressed is the question whether similar LBD types are shared by members of different sensor protein families. Major families of these receptors are sensor histidine kinases; chemoreceptors; adenylate, diadenylate, and diguanylate cyclases; and certain cAMP, c-di-AMP, and c-di-GMP phosphodiesterases, as well as Ser/Thr/Tyr protein kinases and phosphoprotein phosphatases (48). As the different sensor proteins of a given strain are exposed to the same signals, it appears plausible that the same LBD types might be present in members of different sensor protein families. Several examples have been reported in this direction, such as the specific sensing of nitrate by PilJ-type LBDs of the NarQ-type sensor kinases (49), the McpN chemoreceptor (50), and the PAS domain, universally found in different signal transduction systems (48). It would be of interest to estimate the global occurrence of such cases.
Overall, we believe that our study provides a comprehensive resource for future studies on bacterial chemoreception and that it sets the basis for the identification of novel CRs relevant for bacterium-plant interactions.
MATERIALS AND METHODS
Chemoreceptor (CR) sequence retrieval.
From the genomes of 11,806 representative species in the proGenomes2 database (31), 82,277 CR sequences were obtained. The representative species in proGenomes2 are the result of a phylogeny-based classification of all RefSeq (51) genomes, where species delineation is based on a systematic phylogenetic threshold (i.e., <95% divergence in 40 universal marker genes) rather than relying on the NCBI taxonomic names. Although this might lead to inconsistencies with the current NCBI Taxonomy names for strains and species, it better represents the genomic definition of species, as well as providing a standardized classification system (33, 52). To identify CRs in our set of representative genomes, all the sequences matching the MCPsignal Pfam domain signature (PF00015) were retrieved using HMMER 3.1b2 (53), Pfam-A 31.0 (54), and the specific gathering threshold provided for the MCPsignal HMM Pfam model. Multiple hits were resolved by retaining the match with the highest bit score. In analogy to previous studies (7, 55, 56), the presence of an MCPsignal domain in the sequence was the only criterion used for CR identification.
Ligand binding domain (LBD) extraction.
For each CR sequence, transmembrane regions (TMs) were predicted using TMHMM2 (57). The position of the TM region(s) was used to infer the putative extracellular LBD regions, which were subsequently annotated using the Pfam domain database. When no significant Pfam matches were found, LBD sequences were labeled as “unknown.” Two different topologies of extracellular LBDs were considered: (i) sequence regions flanked by two TM regions and (ii) sequence regions located between one TM and the N-terminal sequence. In both cases, sequences shorter than 30 amino acids were discarded. Intracellular LBD regions, as well as potentially overlooked extracellular LBDs (e.g., due to undetected TMs), were inferred based on the detection of Pfam domains other than the MCPsignal and HAMP domains. Pfam mappings were performed using HMMER (53) searches as implemented in eggNOG-mapper v.2.0.5 (53, 58). When more than two domains mapped to the same region, the best hit was selected. The final data set contained 72,480 LBD sequences.
Clustering of LBD sequences.
De novo homology-based clustering of the 72,480 LBD sequences was inferred using MMseqs2 (59) with an E value threshold of 0.01, 20% minimum identity, and 50% minimum query coverage. These parameters were chosen to maximize remote homology detection and to infer LBD clusters with broad phylogenetic divergence (i.e., distant homologues) while still grouping sequences with a common evolutionary origin. The MMseqs2 command used was “mmseqs cluster -c 0.2 –min-seq-id 0.5 –cov-mode 2”.
Construction of the databases for plant-associated bacteria (PAB).
A curated list of PAB was manually curated from the 11,806 representative species. As a first filter, we used the habitat information (i.e., “host plant-associated” label) provided by proGenomes2, which is based on the PATRIC database (31). The resulting list was reviewed manually to exclude uncertain or incorrectly annotated entries by checking their metadata and associated literature. Additionally, we included other known plant-associated species on the list that were missed by the PATRIC database but that were considered PAB based on published data. In total, we identified 960 reference species (PAB-broad) that could be considered related to the plant environment. From this list, we extracted two subdatabases (see Data Set S1 in the supplemental material): phytopathogens (PAB-phyto, 119 members) and plant symbionts (PAB-symb, 192 members).
List of the bacterial TaxIds included in the plant-associated bacteria (PAB) database. Download Data Set S1, XLSX file, 0.1 MB (117KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Degree of plant specificity (DPS).
A specificity score for plant association was calculated for each LBD type based on the proportion of PAB species present in each LBD cluster. We calculated three score values, which we refer to as the degree of plant specificity (DPS), depending on the PAB reference database used: DPS-broad, the proportion of PAB-broad species in each LBD cluster; DPS-phyto, the proportion of PAB-phyto species; and DPS-symb, the proportion of PAB-symb species.
Phylogenetic tree reconstruction and visualization.
Multiple sequence alignments were built for each cluster using Clustal Omega v1.2.4 (60), and phylogeny was inferred by IQ-Tree v1.6.12 using the default parameters (61). The trees were further analyzed and visualized using ETE3 v3.0 (62), with custom Python scripts integrating the annotations of each sequence for its taxonomy, domain architecture, sequence alignment, and plant-specificity prediction (DPS).
Phylogenetic signal tests.
The phylogenetic signal tests were performed using the δ-approach (44), a phylogenetic analogue of the Shannon entropy that measures the degree of phylogenetic signal between a categorical trait (trait vector) and a phylogeny (metric-tree). We used the δ-approach to specifically test the null hypothesis that a given taxonomic distribution of an LBD follows the phylogenetic signal of the underlying species, which provided us with a P value for each LBD cluster. We applied 100 iterations per test and set the P value threshold at 0.05.
The species phylogeny used as a reference in all the tests was reconstructed using the ETE3 (62) supermatrix-based workflow and a concatenated alignment of 40 universal marker genes (63) extracted from the 11,806 species-representative genomes using the FetchMG tool (64). Multiple sequence alignments were inferred using Clustal Omega v1.2.4 (60), and phylogenetic reconstruction was performed with FastTree v2.1 (65). Moreover, an alternative species phylogeny including only genomes with at least one CR was reconstructed using the same methodology. As the δ-statistic has poor sensitivity in detecting the phylogenetic signal for small taxon sample sizes (<20 taxa), LBD clusters mapping to reference phylogenetic tree nodes smaller than 20 leaves were discarded from the analysis (Data Set S2).
Delta-statistic values to calculate the phylogenetic signal of the LBD clusters. Download Data Set S2, XLSX file, 0.08 MB (86.5KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
This research has been supported by grants PGC2018-098073-A-I00 MCIU/AEI/FEDER, UE (to J.H.-C.), BIO2016-76779-P (to T.K.), AGL2017-82492-C2-1-R (to C.R.), and RTI2018-095222-B-I00 (to E.L.-S.) from the Ministerio de Ciencia, Innovación y Universidades, Spain, as well as grant P18-FR-1621 (to T.K.) from the Junta de Andalucía. C.S.-L. was supported by the FPU program (FPU19/06635, MICINN-Spain), and J.P.C.-V. by the FPI program (BES-2016-076452, MINECO-Spain).
Contributor Information
Jaime Huerta-Cepas, Email: huerta.jaime@inia.es.
José J. Rodríguez-Herva, Email: jj.rodriguez@upm.es.
Marnix Medema, Wageningen University.
REFERENCES
- 1.Miller LD, Russell MH, Alexandre G. 2009. Diversity in bacterial chemotactic responses and niche adaptation. Adv Appl Microbiol 66:53–75. doi: 10.1016/S0065-2164(08)00803-4. [DOI] [PubMed] [Google Scholar]
- 2.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 3.Wuichet K, Zhulin IB. 2010. Origins and diversification of a complex signal transduction system in prokaryotes. Sci Signal 3:ra50. doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitchurch CB, Leech AJ, Young MD, Kennedy D, Sargent JL, Bertrand JJ, Semmler ABT, Mellick AS, Martin PR, Alm RA, Hobbs M, Beatson SA, Huang B, Nguyen L, Commolli JC, Engel JN, Darzins A, Mattick JS. 2004. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol Microbiol 52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 5.Hickman JW, Tifrea DF, Harwood CS. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA 102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavira JA, Gumerov VM, Rico-Jiménez M, Petukh M, Upadhyay AA, Ortega A, Matilla MA, Zhulin IB, Krell T. 2020. How bacterial chemoreceptors evolve novel ligand specificities. mBio 11:e03066-19. doi: 10.1128/mBio.03066-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortega Á, Zhulin IB, Krell T. 2017. Sensory repertoire of bacterial chemoreceptors. Microbiol Mol Biol Rev 81:e00033-17. doi: 10.1128/MMBR.00033-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matilla MA, Krell T. 2018. The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol Rev 42:fux052. doi: 10.1093/femsre/fux052. [DOI] [PubMed] [Google Scholar]
- 9.Elgamoudi BA, Andrianova EP, Shewell LK, Day CJ, King RM, Taha Rahman H, Hartley-Tassell LE, Zhulin IB, Korolik V. 2021. The Campylobacter jejuni chemoreceptor Tlp10 has a bimodal ligand-binding domain and specificity for multiple classes of chemoeffectors. Sci Signal 16:eabc8521. doi: 10.1126/scisignal.abc8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milligan DL, Koshland DE, Jr.. 1993. Purification and characterization of the periplasmic domain of the aspartate chemoreceptor. J Biol Chem 268:19991–19997. doi: 10.1016/S0021-9258(20)80684-X. [DOI] [PubMed] [Google Scholar]
- 11.Clarke S, Koshland DE, Jr.. 1979. Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem 254:9695–9702. doi: 10.1016/S0021-9258(19)83572-X. [DOI] [PubMed] [Google Scholar]
- 12.Bi S, Pollard AM, Yang Y, Jin F, Sourjik V. 2016. Engineering hybrid chemotaxis receptors in bacteria. ACS Synth Biol 5:989–1001. doi: 10.1021/acssynbio.6b00053. [DOI] [PubMed] [Google Scholar]
- 13.Reyes-Darias JA, Yang Y, Sourjik V, Krell T. 2015. Correlation between signal input and output in PctA and PctB amino acid chemoreceptor of Pseudomonas aeruginosa. Mol Microbiol 96:513–525. doi: 10.1111/mmi.12953. [DOI] [PubMed] [Google Scholar]
- 14.Alexandre G, Greer-Phillips S, Zhulin IB. 2004. Ecological role of energy taxis in microorganisms. FEMS Microbiol Rev 28:113–126. doi: 10.1016/j.femsre.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lacal J, García-Fontana C, Muñoz-Martínez F, Ramos J-L, Krell T. 2010. Sensing of environmental signals: classification of chemoreceptors according to the size of their ligand binding regions. Environ Microbiol 12:2873–2884. doi: 10.1111/j.1462-2920.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 16.Scharf BE, Hynes MF, Alexandre GM. 2016. Chemotaxis signaling systems in model beneficial plant–bacteria associations. Plant Mol Biol 90:549–559. doi: 10.1007/s11103-016-0432-4. [DOI] [PubMed] [Google Scholar]
- 17.Antunez-Lamas M, Cabrera E, Lopez-Solanilla E, Solano R, González-Melendi P, Chico JM, Toth I, Birch P, Pritchard L, Liu H, Rodriguez-Palenzuela P. 2009. Bacterial chemoattraction towards jasmonate plays a role in the entry of Dickeya dadantii through wounded tissues. Mol Microbiol 74:662–671. doi: 10.1111/j.1365-2958.2009.06888.x. [DOI] [PubMed] [Google Scholar]
- 18.Cerna-Vargas JP, Santamaría-Hernando S, Matilla MA, Rodríguez-Herva JJ, Daddaoua A, Rodríguez-Palenzuela P, Krell T, López-Solanilla E. 2019. Chemoperception of specific amino acids controls phytopathogenicity in Pseudomonas syringae pv. tomato. mBio 10:e01968-19. doi: 10.1128/mBio.01868-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida A, Oku S, Kawasaki T, Nakashimada Y, Tajima T, Kato J. 2015. Identification of the mcpA and mcpM genes, encoding methyl-accepting proteins involved in amino acid and L-malate chemotaxis, and involvement of McpM-mediated chemotaxis in plant infection by Ralstonia pseudosolanacearum (formerly Ralstonia solanacearum phylotypes I and III). Appl Environ Microbiol 81:7420–7430. doi: 10.1128/AEM.01870-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar Verma R, Samal B, Chatterjee S. 2018. Xanthomonas oryzae pv. oryzae chemotaxis components and chemoreceptor Mcp2 are involved in the sensing of constituents of xylem sap and contribute to the regulation of virulence-associated functions and entry into rice. Mol Plant Pathol 19:2397–2415. doi: 10.1111/mpp.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J, Allen C. 2006. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J Bacteriol 188:3697–3708. doi: 10.1128/JB.188.10.3697-3708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antúnez-Lamas M, Cabrera-Ordóñez E, López-Solanilla E, Raposo R, Trelles-Salazar O, Rodríguez-Moreno A, Rodríguez-Palenzuela P. 2009. Role of motility and chemotaxis in the pathogenesis of Dickeya dadantii 3937 (ex Erwinia chrysanthemi 3937). Microbiology 155:434–442. doi: 10.1099/mic.0.022244-0. [DOI] [PubMed] [Google Scholar]
- 23.Río-Álvarez I, Muñoz-Gómez C, Navas-Vásquez M, Martínez-García PM, Antúnez-Lamas M, Rodríguez-Palenzuela P, López-Solanilla E. 2015. Role of Dickeya dadantii 3937 chemoreceptors in the entry to Arabidopsis leaves through wounds. Mol Plant Pathol 16:685–698. doi: 10.1111/mpp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raina J-B, Fernandez V, Lambert B, Stocker R, Seymour JR. 2019. The role of microbial motility and chemotaxis in symbiosis. Nat Rev Microbiol 17:284–294. doi: 10.1038/s41579-019-0182-9. [DOI] [PubMed] [Google Scholar]
- 25.Kamoun S, Kado CI. 1990. Phenotypic switching affecting chemotaxis, xanthan production, and virulence in Xanthomonas campestris. Appl Environ Microbiol 56:3855–3860. doi: 10.1128/aem.56.12.3855-3860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones P, Garcia BJ, Furches A, Tuskan GA, Jacobson D. 2019. Plant host-associated mechanisms for microbial selection. Front Plant Sci 10:862. doi: 10.3389/fpls.2019.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Zhang N, Huang Q, Raza W, Li R, Vivanco JM, Shen Q. 2015. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens. Sci Rep 5:13438. doi: 10.1038/srep13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta Sood S. 2003. Chemotactic response of plant-growth-promoting bacteria towards roots of vesicular-arbuscular mycorrhizal tomato plants. FEMS Microbiol Ecol 45:219–227. doi: 10.1016/S0168-6496(03)00155-7. [DOI] [PubMed] [Google Scholar]
- 29.Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 30.Vorholt JA. 2012. Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 31.Mende DR, Letunic I, Maistrenko OM, Schmidt TSB, Milanese A, Paoli L, Hernández-Plaza A, Orakov AN, Forslund SK, Sunagawa S, Zeller G, Huerta-Cepas J, Coelho LP, Bork P. 2020. proGenomes2: an improved database for accurate and consistent habitat, taxonomic and functional annotations of prokaryotic genomes. Nucleic Acids Res 48(D1):D621–D625. doi: 10.1093/nar/gkz1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I. 2020. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020:baaa062. doi: 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mende DR, Sunagawa S, Zeller G, Bork P. 2013. Accurate and universal delineation of prokaryotic species. Nat Methods 10:881–884. doi: 10.1038/nmeth.2575. [DOI] [PubMed] [Google Scholar]
- 34.Silby MW, Winstanley C, Godfrey SAC, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 35.Baldani JI, Pot B, Kirchhof G, Falsen E, Baldani VLD, Olivares FL, Hoste B, Kersters K, Hartmann A, Gillis M, Dobereiner J. 1996. Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a mild plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF Group 1) as Herbaspirillum species 3. Int Syst Bacteriol 46:802–810. doi: 10.1099/00207713-46-3-802. [DOI] [PubMed] [Google Scholar]
- 36.Bi S, Yu D, Si G, Luo C, Li T, Ouyang Q, Jakovljevic V, Sourjik V, Tu Y, Lai L. 2013. Discovery of novel chemoeffectors and rational design of Escherichia coli chemoreceptor specificity. Proc Natl Acad Sci USA 110:16814–16819. doi: 10.1073/pnas.1306811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goers Sweeney E, Henderson JN, Goers J, Wreden C, Hicks KG, Foster JK, Parthasarathy R, Remington SJ, Guillemin K. 2012. Structure and proposed mechanism for the pH-sensing Helicobacter pylori chemoreceptor TlpB. Structure 20:1177–1188. doi: 10.1016/j.str.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb BA, Hildreth S, Helm RF, Scharf BE. 2014. Sinorhizobium meliloti chemoreceptor McpU mediates chemotaxis toward host plant exudates through direct proline sensing. Appl Environ Microbiol 80:3404–3415. doi: 10.1128/AEM.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma B, Charkowski AO, Glasner JD, Perna NT. 2014. Identification of host-microbe interaction factors in the genomes of soft rot-associated pathogens Dickeya dadantii 3937 and Pectobacterium carotovorum WPP14 with supervised machine learning. BMC Genomics 15:508. doi: 10.1186/1471-2164-15-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raoul des Essarts Y, Pédron J, Blin P, Van Dijk E, Faure D, Van Gijsegem F. 2019. Common and distinctive adaptive traits expressed in Dickeya dianthicola and Dickeya solani pathogens when exploiting potato plant host. Environ Microbiol 21:1004–1018. doi: 10.1111/1462-2920.14519. [DOI] [PubMed] [Google Scholar]
- 41.Puławska J, Kałużna M, Warabieda W, Pothier JF, Gétaz M, van der Wolf JM. 2020. Transcriptome analysis of Xanthomonas fragariae in strawberry leaves. Sci Rep 10:20582. doi: 10.1038/s41598-020-77612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei C, Ding T, Chang C, Yu C, Li X, Liu Q. 2019. Global regulator PhoP is necessary for motility, biofilm formation, exoenzyme production, and virulence of Xanthomonas citri subsp. citri on citrus plants. Genes (Basel) 10:340. doi: 10.3390/genes10050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matas IM, Lambertsen L, Rodríguez-Moreno L, Ramos C. 2012. Identification of novel virulence genes and metabolic pathways required for full fitness of Pseudomonas savastanoi pv. savastanoi in olive (Olea europaea) knots. New Phytol 196:1182–1196. doi: 10.1111/j.1469-8137.2012.04357.x. [DOI] [PubMed] [Google Scholar]
- 44.Borges R, Machado JP, Gomes C, Rocha AP, Antunes A. 2019. Measuring phylogenetic signal between categorical traits and phylogenies. Bioinformatics 35:1862–1869. doi: 10.1093/bioinformatics/bty800. [DOI] [PubMed] [Google Scholar]
- 45.Salah Ud-Din AIM, Roujeinikova A. 2017. Methyl-accepting chemotaxis proteins: a core sensing element in prokaryotes and archaea. Cell Mol Life Sci 74:3293–3303. doi: 10.1007/s00018-017-2514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wuichet K, Alexander RP, Zhulin IB. 2007. Comparative genomic and protein sequence analyses of a complex system controlling bacterial chemotaxis. Methods Enzymol 422:1–31. doi: 10.1016/S0076-6879(06)22001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhulin IB. 2001. The superfamily of chemotaxis transducers: from physiology to genomics and back. Adv Microb Physiol 45:157–198. doi: 10.1016/s0065-2911(01)45004-1. [DOI] [PubMed] [Google Scholar]
- 48.Galperin MY. 2018. What bacteria want. Environ Microbiol 20:4221–4229. doi: 10.1111/1462-2920.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gushchin I, Melnikov I, Polovinkin V, Ishchenko A, Yuzhakova A, Buslaev P, Bourenkov G, Grudinin S, Round E, Balandin T, Borshchevskiy V, Willbold D, Leonard G, Büldt G, Popov A, Gordeliy V. 2017. Mechanism of transmembrane signaling by sensor histidine kinases. Science 356:eaah6345. doi: 10.1126/science.aah6345. [DOI] [PubMed] [Google Scholar]
- 50.Martín-Mora D, Ortega Á, Matilla MA, Martínez-Rodríguez S, Gavira JA, Krell T. 2019. The molecular mechanism of nitrate chemotaxis via direct ligand binding to the PilJ domain of McpN. mBio 10:e02334-18. doi: 10.1128/mBio.02334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, O’Neill KR, Haft DH, DiCuccio M, Chetvernin V, Badretdin A, Coulouris G, Chitsaz F, Derbyshire MK, Durkin AS, Gonzales NR, Gwadz M, Lanczycki CJ, Song JS, Thanki N, Wang J, Yamashita RA, Yang M, Zheng C, Marchler-Bauer A, Thibaud-Nissen F. 2021. RefSeq: expanding the prokaryotic genome annotation pipeline reach with protein family model curation. Nucleic Acids Res 49:D1020–D1028. doi: 10.1093/nar/gkaa1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 53.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD. 2019. The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexander RP, Zhulin IB. 2007. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc Natl Acad Sci USA 104:2885–2890. doi: 10.1073/pnas.0609359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gumerov VM, Ortega DR, Adebali O, Ulrich LE, Zhulin IB. 2020. MiST 3.0: an updated microbial signal transduction database with an emphasis on chemosensory systems. Nucleic Acids Res 48:D459–D464. doi: 10.1093/nar/gkz988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 58.Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P. 2017. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol Biol Evol 34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinegger M, Söding J. 2017. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol 35:1026–1028. doi: 10.1038/nbt.3988. [DOI] [PubMed] [Google Scholar]
- 60.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huerta-Cepas J, Serra F, Bork P. 2016. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33:1635–1638. doi: 10.1093/molbev/msw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P. 2006. Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
- 64.Milanese A, Mende DR, Paoli L, Salazar G, Ruscheweyh H-J, Cuenca M, Hingamp P, Alves R, Costea PI, Coelho LP, Schmidt TSB, Almeida A, Mitchell AL, Finn RD, Huerta-Cepas J, Bork P, Zeller G, Sunagawa S. 2019. Microbial abundance, activity and population genomic profiling with mOTUs2. Nat Commun 10:1014. doi: 10.1038/s41467-019-08844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the 5,546 species-representative genomes in the proGenomes2 database having at least one CR. Download Data Set S3, XLSX file, 0.4 MB (374.5KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Distribution of LBD types among “compact” LBD clusters (at least 90% of their sequences sharing the same LBD type). Download Table S1, PDF file, 0.06 MB (61.5KB, pdf) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of all CRs identified in 5,546 species-representative genomes obtained from the proGenomes2 database indicating their cluster classification and the amino acid coordinates for their predicted domains. Download Data Set S4, XLSX file, 6.9 MB (6.9MB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of the 5,149 clusters of LBDs indicating their DPS-broad, DPS-phyto, and DPS-symb. Download Data Set S5, XLSX file, 0.3 MB (276.2KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Distribution of LBD types and domain models in the total LBD data set. Download Table S2, PDF file, 0.04 MB (42.9KB, pdf) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogenetic signal detection in plant-associated LBD clusters using the phylogeny of chemotactic species tree. Proportion of significant phylogenetic signal among LBD clusters enriched in PAB-broad, PAB-phyto, and PAB-symb species within two thresholds (≥50%, ≥80%). The reference phylogeny used in this analysis excluded any species without CRs in their genome. Download FIG S1, TIF file, 0.5 MB (551.3KB, tif) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of the bacterial TaxIds included in the plant-associated bacteria (PAB) database. Download Data Set S1, XLSX file, 0.1 MB (117KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Delta-statistic values to calculate the phylogenetic signal of the LBD clusters. Download Data Set S2, XLSX file, 0.08 MB (86.5KB, xlsx) .
Copyright © 2021 Sanchis-López et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.