Abstract
Background
Although opioid-induced bowel dysfunction is a well-known and frequent adverse event correlated with opioids, it is scarcely investigated in patients on opioid substitution treatment (OST) and no standard of care is currently available for this population. We aimed to explore the opinion of patients on the impact of constipation on the management of OST and quality of life (QoL).
Methods
We performed a survey that was directed to opioid-dependent patients treated with OST and followed-up in a Service for Addiction Treatment in Italy. The questionnaire included questions about sociodemographic characteristics, the experience of constipation, general QoL, OST management, interference of opioid-induced constipation (OIC) with opioid management, the experience of OIC treatment in the health system, and risk factors for constipation.
Results
Constipation at the moment of the survey (n=105) was reported by 81% of patients and was the most frequent adverse event of OST; 73% of respondents reported at least one severe or very severe symptom of constipation in the last 2 weeks. OIC was reported to hinder adherence to OST by 33% of respondents and 38% of them felt that control of craving had been more difficult since initiation of constipation. Overall, 34% of patients interfered with their OST by changing the schedule on their own in an attempt to improve constipation. Patients were proactive in looking for a solution for constipation but reported poor help from the healthcare system.
Conclusion
Our patient-based survey suggests that careful and efficient management of constipation could increase adherence to OST and improve patient satisfaction and QoL.
Keywords: constipation, heroin, opioid substitution treatment, quality of life
Introduction
Given the high prevalence of risky opioid use, the number of patients on opioid substitution treatment (OST) is estimated to range from 1 to 8 per 1000 across countries.1–3 Patients on OST share the tolerability and safety issues reported by patients on opioid treatment for chronic cancer and non-cancer pain.4,5 Opioid side-effects affect the gastrointestinal tract in particular due to the marked expression of the μ-opioid receptor in the gut. Side-effects include dyspepsia, reflux, bloating, spasm, cramping, bowel movement changes, urinary obstruction or infection, and pain.4–6 These symptoms are reported by 15–90% of patients receiving opioids and were associated with reduced quality of life (QoL) and interference with a treatment regimen.4,5,7 A recent survey in Italy found that 66% of patients receiving opioids for cancer or non-cancer pain experienced opioid-induced constipation (OIC).8
Opioid-induced bowel dysfunction (OIBD) is a specific definition proposed for this symptomatology and is characterized by a change from baseline bowel habits upon initiation of opioids and by any of the following symptoms: reduced bowel movement frequency, development or worsening of straining to pass stool, a sense of incomplete rectal evacuation, or harder stool consistency.9 Contrary to the analgesic effect, bowel dysfunction does not develop tolerance so that it may reduce compliance with treatments.
Although constipation is a frequent side-effect of OST, it is an under-recognized, underdiagnosed and undertreated problem in the field of addiction. A multicentre observational study on 1057 heroin-dependent patients treated with OST found a high prevalence of constipation and reduced QoL, suggesting the need for studies evaluating optimal treatment strategies for the assessment and management of bowel dysfunction in this specific population.10
We performed a survey directed to opioid-dependent patients undergoing OST to investigate their opinion on the impact of constipation on the management of OST and QoL.
Design and methods
The survey was carried out in February 2020.
Patients
Consecutive patients followed up by a specialized Service for Addiction Treatment (SERD) in Italy were considered for inclusion in the survey. Inclusion criteria were age >18 years, current use of OST to control opioid dependence, OST initiated at least 1 month before answering the questionnaire, and experiencing constipation (the patient had new or worsening symptoms of constipation after initiating, changing or increasing opioid therapy and had fewer than two bowel movements a week, or experienced type 1 or 2 stools in the past week as defined by the Bristol Stool Chart).11 Eligible patients provided written informed consent to take part in the survey.
Setting
All patients were treated and followed up by a SERD. Physicians of participating SERDs identified all consecutive eligible patients, who were invited to answer the survey. Four SERDs (based in Lombardia, Campania, Puglia and Lazio) were invited and included in the study.
Hypotheses
A series of hypotheses were evaluated when reviewing the questionnaire responses as reported by Table 1.
Table 1.
Hypotheses investigated by the survey.
| Topic | Hypothesis |
|---|---|
| General quality of life |
|
| OST management |
|
| Experience of OIC |
|
| OIC journey and treatments |
|
| Risk factors |
|
OIC, opioid-induced constipation; OST, opioid substitution treatment.
Questionnaire
The survey questionnaire included questions about sociodemographic characteristics, the experience of constipation, general QoL, OST management, interference of OIC with opioid management, the experience of OIC treatment in the health system and risk factors for constipation (Supplementary Material; available at: https://www.drugsincontext.com/wp-content/uploads/2021/10/dic.2021-7-2-Suppl.pdf).
The Bristol Stool Chart was used as a reference to classify stools in all questions about bowel functionality. Questions from the Patient Assessment of Constipation-Symptoms (PAC-SYM) were included to assess the severity of patient-reported symptoms of constipation.12 Questions from the Patient Assessment of Constipation Quality of Life (PAC-QOL) Questionnaire were used to measure the impact of constipation on QoL.13 Global health and QoL were measured by the Patient-Reported Outcomes Measurement Information System (PROMIS; a 10-question questionnaire investigating physical and mental health; higher scores indicate better health) Global Health Scale (omitting one question that assessed pain).14
Recruited respondents were provided a link to a 30-minute online survey. Open and closed (multiple-choice, with either single or multiple permitted answers) questions were included. Interviews were anonymous. The level of agreement was measured by a 5-point Likert scale. The English version of the survey questionnaire is presented in Additional file 1.
Statistical analysis
Descriptive statistics were used to analyse the data. In addition, Pearson correlation coefficients were run to assess relationships between certain variables within the data. A two-tailed test of significance was performed to establish the significance of these correlations.
Results
Overall, 105 patients completely responded to the survey (56% were men, age range <18–69 years) (Table 2). Respondents had initiated OST over a wide range of time (<1 month to >10 years) before the survey and had used opioids for <1 year to >20 years. Forty-five (43%) patients reported having a full-time carer. The more frequently reported previous conditions were anxiety (n=44, 42%) and depression (n=43, 41%); heart disease was reported by 8 (8%) patients, respiratory diseases by 7 (7%), diabetes and thyroid disturbance by 6 (6%), stroke and arthritis by 4 (4%), osteoporosis by 2 (2%), multiple sclerosis and lack of mobility by 1 (1%), and 60 (57%) had a digestive condition, including haemorrhoids (32, 30%), gastro-oesophageal reflux (19, 18%), irritable bowel disease (11, 10%), lactose intolerance and diverticulosis (7, 7%) and celiac disease and ulcerative colitis (1, 1%) (please note that patients could present with more than one disorder). OST had been prescribed by addiction specialists to 84 (79%) patients and by psychiatrists to 21 (20%).
Table 2.
Sociodemographic characteristics of survey respondents (n=105).
| Sociodemographic characteristics | Percentage of the study population |
|---|---|
| Age (years) | |
| <18 | 1% |
| 18–29 | 14% |
| 30–39 | 22% |
| 40–49 | 30% |
| 50–59 | 26% |
| 60–69 | 7% |
| 70–79 | 0% |
| 80+ | 0% |
| Employment status | |
| Working full-time | 30% |
| Working part-time | 24% |
| Self-employed | 15% |
| On long-term sick leave | 6% |
| Unemployed | 15% |
| Student/full-time training | 6% |
| Retired | 4% |
| Semi-retired | 0% |
| Sex | |
| Men | 56% |
| Women | 43% |
| Prefer not to say | 1% |
| Relationship status | |
| Single | 22% |
| In a relationship | 24% |
| Civil partnership | 14% |
| Married | 23% |
| Separated | 10% |
| Divorced | 4% |
| Widowed | 3% |
| Children | |
| Yes | 43% |
| No | 57% |
| Full-time carer | |
| Yes | 43% |
| No | 57% |
| Time since starting OST | |
| <1 month ago | 2% |
| Between 1 and 3 months ago | 13% |
| >3 months ago but <1 year ago | 20% |
| Between 1 and 3 years ago | 18% |
| Between 3 and 5 years ago | 22% |
| Between 5 and 10 years ago | 16% |
| >10 years ago | 9% |
| Time using heroin (or other street opioids) prior to OST | |
| <1 year | 9% |
| Between 1 and 3 years | 23% |
| Between 4 and 6 years | 20% |
| Between 7 and 10 years | 17% |
| Between 11 and 15 years | 20% |
| Between 16 and 20 years | 6% |
| >20 years | 6% |
Health self-rating
Based on the PROMIS Global Health Scale, 14% of respondents rated their global health as poor and 41% as fair, whilst 45% rated their health as good–excellent.
Overall, 16% of respondents rated their physical health as poor, 46% as fair and only 38% as good–excellent. In addition, 14% rated their QoL as poor and 41% as fair, whilst 45% considered their QoL as good–excellent. In particular, 51% of respondents answered that they were not able, little able or moderately able to carry out everyday physical activities, and 77% of patients reported moderate-to-severe fatigue. There was no correlation between poor QoL and duration of use of OST.
A mean mental health score of 9.8 was found. Mental health, including mood and ability to think, was rated as poor by 13% of patients, fair by 50% and good–excellent by 39%.
Experience with OST
Whilst 63% of patients reported that all items of the global health assessment had improved since the beginning of OST, 27% reported worsening of at least one domain and 10% reported no change. Incomplete satisfaction (rated as completely dissatisfied, somewhat dissatisfied, neither satisfied nor dissatisfied, somewhat satisfied) was reported by 69% of patients with regards to the management of opioid dependence and by 76% with regards to the management of heroin cravings (4% and 5% were completely dissatisfied, respectively). Nevertheless, 89% of patients felt that OST was vital for reintegration into society, 86% felt that, without OST, their QoL would be significantly worse, and 84% felt that OST had made their life better.
Side-effects of OST
Overall, 57% of respondents reported that the side-effects of OST had a significant impact on their QoL.
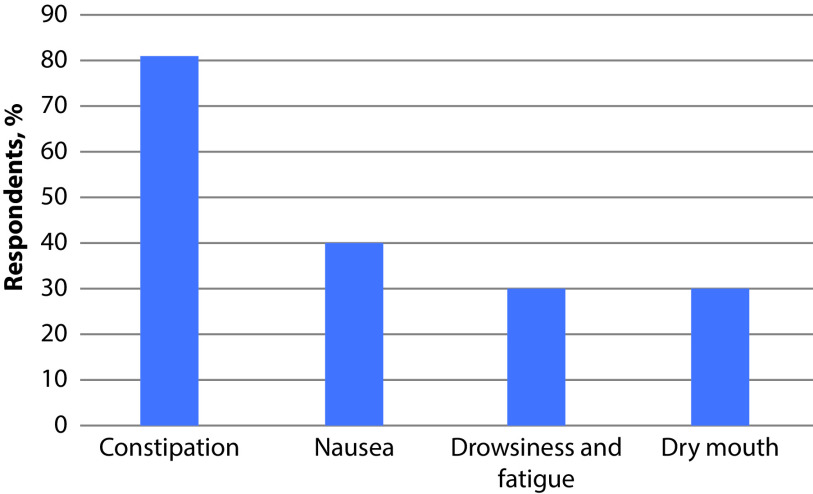
Constipation at the moment of the survey was reported by 81% of respondents and was the most frequent adverse event of OST, followed by nausea (40%), drowsiness and fatigue (30%), and dry mouth (30%) (Figure 1).
Figure 1.
Frequency of side-effects during OST (n=105).
The frequency of warning about each possible adverse event by healthcare professionals did not correspond to the frequency of the event itself; only 58% of patients were warned about the possibility of constipation, whilst 40% were warned about nausea and 23% of patients were not informed about possible adverse events. As a consequence of this discrepancy, 23% of patients experienced constipation without being informed about the effects of OST on bowel functionality.
The mean PAC-SYM score was 22.6; 73% of respondents reported at least one severe or very severe symptom of constipation in the last 2 weeks before the survey. Severe and very severe symptoms were pain and bowel movement-related symptoms in 50% of respondents.
Impact of constipation on QoL
The average of the final PAC-QOL scores was 89.73. Physical discomfort was a frequent complaint: 42% of respondents felt bloated to the point of bursting and 52% felt heavy because of their constipation during the last 2 weeks before the survey.
Some factors were correlated with an increased risk of experiencing a greater impact of constipation on QoL: older age (r=0.332, p≤0.01), prolonged use of heroin (r=0.325, p≤0.01), presence of a carer (r=0.298, p≤0.01) and full-time employment (r=0.260, p≤0.01). Increasing severity of the following symptoms was correlated with a greater impact on QoL (all; p≤0.01): feeling like you have to pass a bowel movement but you could not (r=0.632, p≤0.01), discomfort in your abdomen (r=0.62, p≤0.01), straining or squeezing to try to pass bowel movements (r=0.580, p≤0.01), pain in your abdomen (r=0.576, p≤0.01), painful bowel movements (r=0.570, p≤0.01), bowel movements that were too small (r=0.567, p≤0.01), rectal burning during or after a bowel movement (r=0.563), bowel movements that were too hard (r=0.523), incomplete bowel movement, like you did not ‘finish’ (r=0.508, p≤0.01), and stomach cramps (r=0.456, p≤0.01).
Constipation was reported to be present before use of any opioid by 8% of respondents, during use of heroin by 29% and during OST by 81% of participants. Bowel movements occurred less than twice a week in 6% of participants before the use of any opioid, in 15% whilst taking heroin and in 24% during OST. Only one bowel movement in a week was reported whilst taking heroin by 8% of respondents and during OST by 24%. Regular daily movements were present in 30% of patients before the use of opioids, in 12% during heroin use and only in 3% during OST. Only 7% of patients reported that their caregiver helped a great deal in managing constipation and 64% received moderate help from their caregiver. Finally, 36% of patients discussed constipation with an addiction specialist, 30% with a general practitioner, 16% with a gastroenterologist, 5% with a psychiatrist and 3% with a pharmacist.
Impact of constipation on OST management
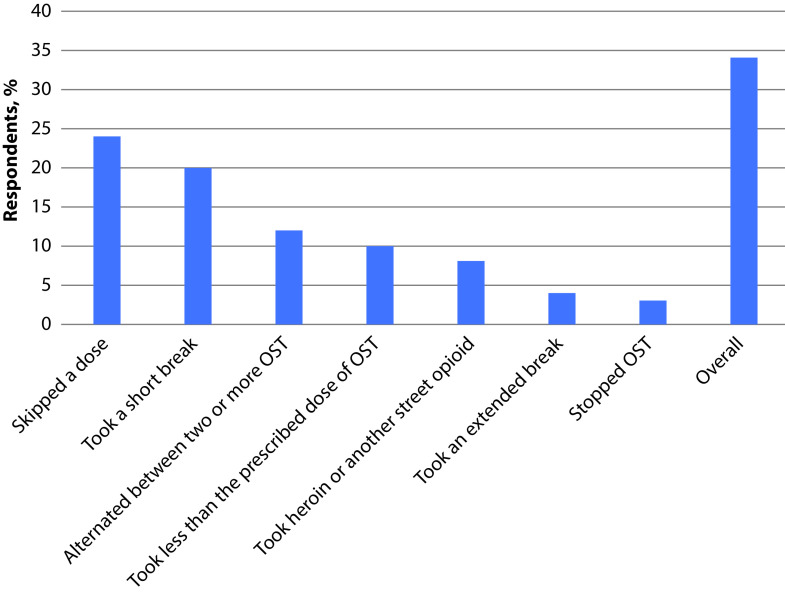
Opioid-dependent constipation was reported to hinder adherence to OST by 33% of respondents and 38% of them felt that control of craving had been more difficult since initiation of constipation. Overall, 34% of patients interfered with their OST in an attempt to improve constipation; specifically, 24% skipped a dose, 20% took a short break, 12% alternated between two or more OSTs, 10% took less than the prescribed OST dose, 8% took heroin or another street opioid, 4% took an extended break and 3% stopped OST (Figure 2) (please note that some patients chose more than one answer).
Figure 2.
Interference with OST due to constipation (n=105).
The total PAC-QOL score was significantly and directly correlated with interference with OST due to constipation (r=0.25, p≤0.01) and was inversely correlated with the satisfaction that OST had helped manage opioid dependence (r=−0.2, p≤0.05) and satisfaction of craving control (r=−0.28, p≤0.01). Conversely, the total PAC-SYM total score was not significantly correlated with either of these factors.
Interference with OST regimen (skipped a dose, a short break, took more than the prescribed dose or frequency, skipped several doses, took less than the prescribed dose, an extended break) was reported to improve constipation in 59% of cases but, as expected, it worsened craving experience in 44% of cases.
Several factors pertaining to worse symptoms of constipation and greater impact of constipation on QoL were identified as risk factors for interference with OST due to constipation (Table 3).
Table 3.
Factors associated with interference with OST (skipped a dose, a short break, took more than the prescribed dose or frequency, skipped several doses, took less than the prescribed dose, an extended break) due to constipation.
| Factor | R | p value |
|---|---|---|
| Pain in your abdomen | 0.216 | 0.05 |
| Rectal burning during or after a bowel movement | 0.345 | 0.01 |
| Felt less self-confident because of your condition | 0.355 | 0.01 |
| Felt irritable because of your condition | 0.325 | 0.01 |
| Been worried that your condition will become worse | 0.325 | 0.01 |
| Been worried about not being able to choose what you eat (e.g. at a friend’s house) | 0.293 | 0.01 |
| Felt stressed by your condition | 0.281 | 0.01 |
| Been worried about having to change your daily routine (e.g. travelling, being away from home) | 0.279 | 0.01 |
| Had to be careful about what you eat | 0.272 | 0.01 |
| Felt obsessed by your condition | 0.268 | 0.01 |
| Been embarrassed about staying in the bathroom for so long when you were away from home | 0.254 | 0.01 |
| Been embarrassed to be with other people | 0.214 | 0.05 |
Management of opioid-dependent constipation
More than half of the respondents reported having discussed constipation experienced during OST with a healthcare practitioner (58% after the first prescription of OST and 64% at the repeated prescription of OST). Nevertheless, only 33% had received a formal diagnosis of OIC. Amongst those who had reported constipation to a healthcare practitioner, 59% were recommended to change diet and lifestyle and 39% were prescribed treatment for constipation after the first OST (61% and 49%, respectively, after repeated OST).
Only 21% of patients reporting constipation to a healthcare practitioner felt that they had fully understood the cause of constipation and how to deal with it, whilst 44% felt that they fully understood the cause of constipation but were not clear how to deal with it.
In total, 69% of respondents were proactive in looking for a solution to constipation within 2 weeks since experiencing symptoms; as a first attempt, 68% of them used a treatment, 36% used laxatives, 17% changed their diet and 11% had fibre supplementation (please note that some patients chose more than one answer). Overall, many methods were reported as ever used: drinking more fluids (83%), diet changes (82%), suppository or enema (72%), laxatives (71%), over-the-counter methods (65%), a fibre supplement (54%), change to exercise regimen (49%) and manual methods (39%). Only 40% of those using suppository or enema, 42% of those using laxatives and 50% of those using fibre supplement were satisfied with the results. In addition, 55% of those who used laxatives reported at least one negative physical effect.
The level of satisfaction with the way constipation was dealt with by the healthcare system was low in 20% of patients.
Discussion
Herein, we report the results on a survey of opioid-dependent patients receiving OST and affected by constipation to assess the level of awareness about this issue and the way it is managed in Italy. This study addressed a rarely studied aspect from the patient’s perspective and may provide a reliable report on patient opinion, satisfaction level and unanswered necessities.
Overall, the experience with OST was deemed useful and effective as it improved the global health of most patients; only 4% reported complete dissatisfaction and over 80% felt that OST had made their life better. Interestingly, although some patients had a poor QoL, this was not related to their time on OST.
Nevertheless, adverse events were reported. We found that constipation is a frequent complaint during OST, starting during the use of heroin or street opioid and frequently worsening after OST initiation. Constipation was the most frequent adverse event during OST. Constipation heavily impacted QoL and several factors increased such impact: older age, prolonged use of heroin, presence of a carer, full-time employment and high severity of constipation symptoms. In addition, constipation negatively impacted OST management; adherence to OST was more difficult, with poorer control of craving, and many patients had interfered with OST in an attempt to improve constipation.
Interestingly, it was found that the impact of constipation on QoL interfered with OST more heavily than constipation symptom severity, indicating that it is important for physicians to understand the holistic nature of the condition, rather than just the physical symptoms, when contemplating the risk of interference. Patients were proactive in looking for a solution for constipation but reported poor help from the healthcare system. We found that many patients addressed their discomfort with constipation without medical advice. Healthcare practitioners were not always consulted and they were not often ready to prescribe a treatment. Similar observations were drawn from a survey of patients with pain.8 About half of the respondents (both patients on OST and those with pain) reported not having discussed constipation with a healthcare practitioner, whilst dissatisfaction with the way constipation was dealt with was expressed by similar proportions of patients in both surveys.8 As our patients seeking a solution to constipation too often interfered with OST without medical advice, skilled guidance would be necessary but healthcare practitioners were not always consulted and they were not often ready to prescribe a treatment. These findings confirm previous reports of inadequate counselling for the management of OIC to patients on opioids, independently of the pathology.8,15,16 Patients’ willingness to address the problem was also shown by Epstein et al., who reported that patients were motivated to discuss OIC with their healthcare provider.17 On the other hand, a low attention of healthcare providers for constipation was previously found and was considered a barrier to correct management of patients.18–20 Indications for a stepwise approach to OIC have been provided by expert panels in Italy and in Europe, and it may be supposed that increased discussion on the issue and widespread awareness amongst healthcare providers will lead to better approaches to the problem.20,21
Although OIBD is a well-known and frequent adverse event correlated with opioids, it has been scarcely investigated in patients receiving OST and no standard of care is currently available for this population. An observational study in Italy found that over 90% of patients on OST were affected by constipation, with higher severity and impact on QoL in women compared to men.10 Previous studies have found a prevalence of constipation secondary to OST ranging from 17% to 45%.22–26 Such a high variability could be secondary to the lack of standardized methods and specific trials to assess OIBD in these patients.
Herein, the incidence of severe symptoms of constipation was intermediate (73%) to that found in patients with cancer pain (83%) and in patients with non-cancer pain (58%).8 In addition, older age was associated with a greater impact of OIC on QoL, whilst this association was found with younger age in patients on opioids for cancer or non-cancer pain.8 Interference with the opioid regimen was declared by 48% non-cancer pain patients, 34% of patients on OST and 6% of cancer pain patients, suggesting that the risk of interference could be related to the perception of the importance of opioids administration.
The burden of OIC may be high in patients in OST compared with patients on opioids for cancer or serious pain; any barrier to OST should be removed to prevent drop-out, which is not expected in patients with serious pain. In addition, interference with an active life is especially relevant as patients on OST are frequently young people not rarely responsible for children and work. To promote better management of OIC in patients with OST, education amongst both healthcare providers and patients is needed as well as the widespread use of a validated diagnostic assessment (i.e. use of Rome IV criteria and Bristol Stool Chart).27 The physicians who were more frequently consulted for constipation were addiction specialists and general practitioners, who seem to be the usual point of reference for these patients. Indeed, 24 (57%) of patients reported that they talked about constipation to the doctor responsible for OST and only one patient talked to a doctor he had never seen before. Effective treatment should rely on interventions aiming to improve bowel function (i.e. fluid and fibre intake, exercise) and on pharmacological treatments, including laxatives in the first line and peripherally acting μ-opioid receptor antagonists in the second line.21
Finally, our survey provided useful information on a little-studied issue, though the study had some limitations such as lack of identification of the drug used in OST and difficulty in the quantification of the event weight. In addition, the study was multicentre but based only in Italy; findings may be difficult to be generalized to other countries with different healthcare system models and with a different cultural approach to opioid dependence and, as a consequence, to OIBD. In addition, although the drug used in OST may be relevant to the occurrence of constipation, this information could not be obtained from patients.
Conclusion
Our patient-based survey suggests that constipation is a frequent issue for patients undergoing OST and that it interferes with wellbeing and compliance to therapy. Careful and efficient management of constipation could increase adherence to OST and improve patient satisfaction and QoL.
Acknowledgements
The authors thank Domenico Alvaro for his critical revision of the manuscript. Editorial assistance was provided by Aashni Shah and Laura Brogelli (Polistudium Srl, Milan, Italy). This assistance was supported by Molteni.
Footnotes
Contributions: FL generated the questionnaire. GH and VB produced the survey. All authors analysed results, revised and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. The information supporting the findings of this study is available from Insight Dojo.
Disclosure and potential conflicts of interest: FL is a member of the Italian Board on Opioid-Induced Constipation, supported by Molteni. Insight Dojo received payment from Molteni and Shionogi for conducting the research, and GH and VB were employed in Insight Dojo at the time of the research. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/09/dic.2021-7-2-COI.pdf
Funding declaration: This study has been possible thanks to the unconditional support of Molteni Farmaceutici and Shionogi Srl.
Correct attribution: Copyright © 2021 Lugoboni F, Hall G, Banerji V. https://doi.org/10.7573/dic.2021-7-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Quaglio G, Lugoboni F, Pattaro C, et al. Erectile dysfunction in male heroin users, receiving methadone and buprenorphine maintenance treatment. Drug Alcohol Depend. 2008;94:12–18. doi: 10.1016/j.drugalcdep.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Quaglio G, Pattaro C, Gerra G, et al. Buprenorphine in maintenance treatment: experience among Italian physicians in drug addiction centers. Am J Addict. 2010;19:222–230. doi: 10.1111/j.1521-0391.2010.00040.x. [DOI] [PubMed] [Google Scholar]
- 3.European Drug Report 2015 Trends and developments. Lisbon: EMCDDA; 2015. [Accessed 20 December 2020]. https://www.emcdda.europa.eu/system/files/publications/974/TDAT15001ENN.pdf. [Google Scholar]
- 4.Coyne KS, Margolis MK, Yeomans K, et al. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: laxative use, response, and symptom burden over time. Pain Med. 2015;16:1551–1565. doi: 10.1111/pme.12724. [DOI] [PubMed] [Google Scholar]
- 5.Webster LR. Opioid-induced constipation. Pain Med. 2015;16(Suppl 1):S16–S21. doi: 10.1111/pme.12911. [DOI] [PubMed] [Google Scholar]
- 6.Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16:2324–2337. doi: 10.1111/pme.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol. 2014;2:31–37. doi: 10.1038/ajgsup.2014.7. [DOI] [PubMed] [Google Scholar]
- 8.Varrassi G, Banerji V, Gianni W, et al. Impact and consequences of opioid-induced constipation: a survey of patients. Pain Ther. 2021 doi: 10.1007/s40122-021-00271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26:1386–1395. doi: 10.1111/nmo.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugoboni F, Mirijello A, Zamboni L, et al. High prevalence of constipation and reduced quality of life in opioid-dependent patients treated with opioid substitution treatments. Expert Opin Pharmacother. 2016;17:2135–2141. doi: 10.1080/14656566.2016.1232391. [DOI] [PubMed] [Google Scholar]
- 11.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 12.Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. doi: 10.1080/003655299750025327. [DOI] [PubMed] [Google Scholar]
- 13.Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540–551. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andresen V, Banerji V, Hall G, et al. The patient burden of opioid-induced constipation: new insights from a large, multinational survey in five European countries. United European Gastroenterol J. 2018;6:1254–1266. doi: 10.1177/2050640618786145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallerand AH, Hendry S, Baldys E, et al. Analysis of patient-provider interactions regarding the burden and treatment of opioid-induced constipation in adults with chronic noncancer pain. Pain Med. 2019;20:889–896. doi: 10.1093/pm/pny151. [DOI] [PubMed] [Google Scholar]
- 17.Epstein RS, Teagarden JR, Cimen A, et al. When people with opioid-induced constipation speak: a patient survey. Adv Ther. 2017;34:725–731. doi: 10.1007/s12325-017-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller MS, Jusufagic A, Spiegel BMR. Patient and provider differences in the treatment of opioid-induced constipation: a qualitative study. BMC Gastroenterol. 2019;19:182. doi: 10.1186/s12876-019-1097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoCasale RJ, Datto C, Margolis MK, et al. Satisfaction with therapy among patients with chronic noncancer pain with opioid-induced constipation. J Manag Care Spec Pharm. 2016;22:246–253. doi: 10.18553/jmcp.2016.22.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer AD, Drewes AM, Chiarioni G, et al. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United European Gastroenterol J. 2019;7:7–20. doi: 10.1177/2050640618818305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian Multidisciplinary Panel. Adv Ther. 2021;38(7):3589–3621. doi: 10.1007/s12325-021-01766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973;223:665–668. doi: 10.1001/jama.1973.03220060039009. [DOI] [PubMed] [Google Scholar]
- 23.Langrod J, Lowinson J, Ruiz P. Methadone treatment and physical complaints: a clinical analysis. Int J Addict. 1981;16:947–952. doi: 10.3109/10826088109038903. [DOI] [PubMed] [Google Scholar]
- 24.Winstock AR, Lea T, Sheridan J. Patients’ help-seeking behaviours for health problems associated with methadone and buprenorphine treatment. Drug Alcohol Rev. 2008;27:393–397. doi: 10.1080/09595230802093745. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan J, Wheeler A, Walters C. Health problems and help-seeking activities of methadone maintenance clients at Auckland Methadone Service (AMS): potential for community pharmacy service expansion? Harm Reduct J. 2005;2:25. doi: 10.1186/1477-7517-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haber PS, Elsayed M, Espinoza D, et al. Constipation and other common symptoms reported by women and men in methadone and buprenorphine maintenance treatment. Drug Alcohol Depend. 2017;181:132–139. doi: 10.1016/j.drugalcdep.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Alvaro D, Caraceni AT, Coluzzi F, et al. What to do and what not to do in the management of opioid-induced constipation: a choosing wisely report. Pain Ther. 2020;9(2):657–667. doi: 10.1007/s40122-020-00195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]