Abstract
Cardiovascular reactivity has been proposed as a biomarker linking childhood adversity and poorer health. The current study examined the association of childhood adversity, cardiovascular reactivity, and health in the Dunedin (n=922) and MIDUS studies (n=1,015). In both studies, participants who experienced more childhood adversity had lower cardiovascular reactivity. In addition, people with lower cardiovascular reactivity had poorer self-reported health and greater inflammation. Dunedin participants with lower cardiovascular reactivity were aging biologically faster, and MIDUS participants with lower heart rate reactivity had increased risk of early mortality. Cardiovascular reactivity was not associated with hypertension in either study. Results were partially accounted for by greater reactivity among more conscientious, less depressed, and higher-functioning participants. These results suggest that people who experienced childhood adversity have a blunted physiological response, which is associated with poorer health. The findings highlight the importance of accounting for individual differences when assessing cardiovascular reactivity using cognitive stressor tasks.
Keywords: Cardiovascular reactivity, blood pressure, heart rate, health, wellbeing, cognition
To better understand why some people are more likely than others to develop physical health problems, clinical psychological scientists often use physiological biomarkers to try to capture psychological vulnerabilities. In particular, there is a long tradition of using measures of cardiovascular function in clinical psychology to assist in the dual purposes of inferring psychological states and linking these states with health outcomes. The assessment of people’s cardiovascular physiological response to a stressor—termed cardiovascular reactivity (Allen & Psychosocial Working Group, 2000)—has been used productively for both purposes.
The cardiovascular reactivity hypothesis (Blascovich & Katkin; Manuck, 1994; Treiber et al., 2003) arose from the observation that people with hypertension seemed to evidence greater increases in their blood pressure during cold pressor tasks compared to people without hypertension (Manuck, 1994). If people with higher levels of physiological reactivity in response to lab stressors were more likely to respond in an exaggerated way to stressors in their environment, it was thought that this could result in poorer cardiovascular health over time through atherosclerotic processes and damage to the cardiovascular system broadly (Manuck, 1994; Treiber et al., 2003). Empirical evidence began to provide support for the cardiovascular reactivity hypothesis, which was summarized in a 2010 meta-analysis of 36 studies that found people with higher cardiovascular reactivity were more likely to have cardiovascular risk factors in the form of high blood pressure and hypertension (Chida & Steptoe, 2010), particularly in the case of reactivity to cognitive task stressors. People with higher cardiovascular reactivity were also shown to have greater risk of mortality (Carroll et al., 2012).
During the early 2000’s, life-course researchers proposed an extension of the cardiovascular reactivity hypothesis as a potential mechanism to explain the well-established association between childhood adversity and poorer health in adulthood (Allen 2000 & Psychosocial Working Group; Cohen, Janicki-Deverts, Chen, & Matthews, 2010). Children who experience adverse childhood experiences (ACEs) and socioeconomic disadvantage are more likely to have poorer health in later life (Cohen et al., 2010; Galobardes, Lynch, & Smith, 2004; Moffitt, 2013). Alterations in cardiovascular reactivity were proposed as one way by which adversity could become biologically embedded and result in poorer health. The MacArthur SES & Health Network’s psychosocial notebook, for example, highlighted the possibility that “exposure to a more threatening or challenging environment by lower SES individuals results in greater reactivity in various organ systems in response to this exposure (Allen & Psychosocial Working Group, 2000).” Over time, these exaggerated responses could then translate to poorer health outcomes, particularly in the cardiovascular system. This possibility, in part, prompted the adoption of cardiovascular reactivity as a physiological biomarker that might help explain how childhood adversity could affect health in longitudinal cohort studies, such as the Dunedin Longitudinal Study (Poulton et al., 2015).
As the cardiovascular reactivity hypothesis was applied more broadly to areas of health outside the cardiovascular system, a new set of findings began to challenge the traditional paradigm linking increased reactivity to poorer health. The aptly titled article “Are Large Physiological Reactions to Acute Stress Always Bad for Health?” (Carroll, Lovallo, & Phillips, 2009) reviewed empirical evidence that lower cardiovascular reactivity was associated with poorer health outcomes outside the cardiovascular system, including obesity (Carroll, Philips, Hunt, & Der, 2007) and poorer self-reported health (Phillips, 2011). Concurrently, a number of studies also found that trauma and childhood adversity were associated with lower levels of cardiovascular reactivity (McLaughlin, Sheridan, Alves, & Mendes, 2014 McLaughlin et al., 2015). These results were organized by researchers into a model suggesting that lower reactivity indexed dysregulation in the fronto-limbic system responsible for marshaling resources for motivated behavior (Carroll, Ginty, Whittaker, Lovallo, & de Rooij, 2017; Ginty, Gianaros, Derbyshire, Phillips, & Carroll, 2013). This motivational dysregulation was posited to result in poorer health due to differences in health behaviors, such as increased rates of smoking and obesity (Phillips, Ginty, & Hughes, 2013), rather than—or perhaps in addition to—direct cardiovascular pathophysiological mechanisms, such as atherosclerosis. These findings became the basis of the blunting hypothesis of cardiovascular reactivity. These competing models raise important questions: is lower cardiovascular reactivity in the lab a marker of people’s psychological characteristics, specifically fronto-limbic dysregulation related to motivated behavior, or does greater reactivity hold direct relevance to cardiovascular physiological functioning and later health?
Cardiovascular reactivity has been measured in response to a wide array of tasks in the lab and it seems unlikely that reactivity to different stressful tasks—e.g., the Trier Social Stress Test, trauma recalls, cold pressors, and cognitive stressors, such as serial subtraction and Stroop tasks—all reflect the same “reactivity” (Manuck, 1994). Such measurement issues would be particularly relevant for stressors that might be more or less engaging or stressful for people based on the individual characteristics they bring into the lab while performing cardiovascular lab protocols. For example, lower cardiovascular reactivity is associated with less conscientiousness (Sesker, 2019) and lower cognitive ability (Carroll et al., 2017), as well as depression (Salomon, Clift, Kalsdottir, & Rottenberg, 2009). People who are more conscientious or have better cognitive ability might engage more fully with cognitive tasks, which could impact their cardiovascular reactivity to such tasks. Similarly, people who are depressed might be less willing or able to fully engage with cognitive tasks. Accounting for participants’ individual differences in such measures could provide additional context important to interpreting the associations between childhood adversity, cardiovascular reactivity, and health.
Present Study
The present study reports the associations between childhood adversity, health, and cardiovascular reactivity to two commonly studied cognitive stressors: the Stroop test and a mental arithmetic task. This study evolved over several stages that are relevant to interpreting the findings. In 2005, we introduced a cardiovascular reactivity protocol into the Dunedin Longitudinal Study (Poulton et al., 2015) when participants were 32 years old to investigate prospectively-measured childhood adversity and adult cardiovascular reactivity. Resulting data yielded associations between childhood adversity and cardiovascular reactivity in the opposite direction of what was expected: People with more adverse childhoods had lower levels of cardiovascular reactivity at age 32. At the time, we did not publish these results because they ran counter to the contemporary understanding of the cardiovascular reactivity hypothesis and we were uncertain what to make of them. Now, 13 years later, we have collected health outcome data in the Dunedin Study that enabled us to investigate the association between cardiovascular reactivity at age 32 and health at age 45. In addition, we identified data from the MIDUS study (Brim et al., 1994; Ryff et al. 2004) from a cardiovascular reactivity protocol administered in 2004-2006 that used the same cognitive stressors as those in the Dunedin Study. This allowed us to replicate our results in a second dataset and ensure robustness of findings that had initially puzzled us in the mid-2000’s.
This process resulted in the present report, which used cardiovascular reactivity data from the Dunedin Study (n = 922) and MIDUS Study (n = 1,015) to examine the association of childhood adversity, cardiovascular reactivity, and health in two longitudinal studies. In particular, we were interested in examining reactivity in blood pressure and heart rate, as indicators of cardiovascular reactivity. The original grant proposal in the Dunedin Study hypothesized—in line with the cardiovascular reactivity hypothesis—that people with more adversity in childhood would have greater cardiovascular reactivity and this would explain their poorer adult health. In the years following the data collection in Dunedin, new evidence regarding cardiovascular blunting and health presented an alternative possibility, that lower cardiovascular reactivity, rather than higher, might be associated with poorer health. To provide additional context to our primary results related to childhood adversity, cardiovascular reactivity, and health, we conducted secondary analyses examining whether individual differences in psychological characteristics—specifically conscientiousness, depression, and cognitive ability—could help explain responses to the two cognitive stressful tasks.
Method
Participants and Study Design
Participants were drawn from two longitudinal studies, the Dunedin Study (Poulton et al., 2015) and the MIDUS Study (Brim et al., 1994; Ryff et al. 2004).
Dunedin study.
The Dunedin Multidisciplinary Health and Development Study is a longitudinal investigation of health and behavior in a representative birth cohort. The 1037 Study members (91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible on the basis of residence in the province and who participated in the first assessment at age 3 years (Poulton, Moffitt, & Silva, 2015). The cohort represented the full range of socioeconomic status (SES) in the general population of New Zealand’s South Island. As adults, the cohort matches the results from the New Zealand National Health and Nutrition Survey on key adult health indicators (Poulton et al. 2015). The cohort also matches the distribution of educational attainment of citizens of the same age from the New Zealand Census (Richmond-Rakerd et al. 2020). The cohort is predominantly white (93%), matching South Island demographic characteristics (Poulton et al., 2015). Fourteen assessments were performed from birth to age 45 years, when 938 of the 997 participants (94.1%) still alive participated. Supplemental Figure 1 presents the results of an attrition analysis of those who participated in the study at age 45 showing that they were representative of the sample as a whole based on childhood SES and IQ. During data-collection assessments, each participant was brought to the research unit for interviews and examinations. Written informed consent was obtained from cohort participants, and study protocols were approved by the appropriate institutional ethical review boards. Research reported here was conducted in accordance with the provisions of the World Medical Association Declaration of Helsinki. The assessments at age 32 included the collection of heart rate and blood pressure reactivity during an experimental protocol. The study sample included participants who had heart rate and blood pressure reactivity scores from these laboratory tasks (n = 922, 49.1% women).
MIDUS Study.
The original MIDUS sample (Brim et al., 1994) included 7,108 participants. The MIDUS study used a random-digit dialing procedure to recruit non-institutionalized, English-speaking people between the ages of 25 and 74 in the United States in 1995 and 1996. Participants provided informed consent and completed a variety of measures via telephone. This sample was assessed 10 years later (n = 3,487) for the MIDUS 2 assessments (Ryff et al., 2004), which repeated much of the original MIDUS assessment. This second wave of data collection also included a subsample of participants (n = 1,255) who completed a lab visit that provided biomarkers and measures of cardiovascular reactivity (Ryff, Seeman, & Weinstein, 2004). Project staff obtained indicators of heart rate and blood pressure reactivity during the experimental protocol. The present study included MIDUS participants who had heart rate or blood pressure scores from the laboratory tasks (n = 1,015). The sample was 53.0 years old (SD = 11.1) years old, on average, and 56.2% were women.
Measures
Cardiovascular reactivity.
Cardiovascular reactivity was measured in the Dunedin Study and MIDUS using cardiovascular responses to two stressful tasks—a computerized Stroop task and a mental arithmetic task. This protocol was adapted from exportable technology developed as part of an NHLBI Cooperative Agreement (HL41340), which aimed to create standardized tasks to evoke cardiovascular reactivity in population-based epidemiological investigations (Kamarck et al., 1992). The principles from this work formed that basis of these two tasks, which have been widely used since.
In the Dunedin study, data were collected in four tasks in the order: 10-min “vanilla” baseline (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992), 4-min Stroop stressor, 10-min “vanilla” baseline, and 4-min mental arithmetic stressor. During the baseline conditions, participants watched a colored rectangle. This changed color every few seconds, and participants were asked to count the number of occurrences of a certain color, determined randomly. During the Stroop condition, participants were presented with a word, the name of one of the colors red, green, blue, or yellow, in the middle of the computer monitor. They were asked to indicate what color the word was printed in by selecting one of four options with a keypad operated by their dominant hand. The four options named colors and were also printed in color on the computer screen. The combinations of colored print and color names were randomized. Furthermore, a recorded voice recited the names of the colors in a random order. Participants’ responses were timed, and software tracked the response time window, adjusting it to become shorter when the participants made correct responses, and longer when participants made incorrect responses. Feedback was provided about the correctness and timeliness of each response.
In the mental arithmetic task, participants were presented with either an addition or subtraction problem on the computer monitor. The problem disappeared, and the word ‘equals’ appeared, then a number, the answer, was presented with the words ‘yes’ and ‘no’ printed beneath it. Participants were required to select either yes or no using the keypad mentioned above depending on whether they thought the answer presented was correct or incorrect. Difficulty was varied by using three-digit, two-digit, or single-digit numbers in the problems. In this condition, the number of digits in the problems was increased in response to more correct responses, and decreased in response to more incorrect responses. There was a time-limit on responding, and feedback was given on response correctness and timeliness. During all tasks, participants wore a Dinamap blood-pressure cuff on their non-dominant arm, and a Polar heart-rate monitor. Blood pressure and pulse rate were taken automatically seven times during the baseline condition, and four times during the stressor conditions at 17s, 1m47s, 3m17s, 4m47s in all conditions, plus 6m17s, 7m47s, 9m17s in the baseline conditions. Heart rate was monitored continuously from the beginning to end of each condition.
The protocol described above was largely followed in the same way for data collection during the MIDUS 2 Biomarker Sample psychophysiological assessment (Ryff et al. 2004). Briefly, participants completed the same tasks—two 10-minute baseline conditions and the two 6-minute stressor conditions—with two differences: (1) the baseline in MIDUS was a simple resting baseline (for a discussion of simple resting and “vanilla” baselines, see Jennings et al., 1992) that did not include viewing colored rectangles and (2) the order of the two stressors in MIDUS was randomized. In addition, blood pressure was measured continuously using a Finometer blood pressure cuff placed on the middle finger of the non-dominant hand and spectral analysis of the BP waveform was used to process the data. More specific details of the protocol are provided in the MIDUS Study documentation (Ryff et al., 2004).
In both studies, baseline heart rate and blood pressure were calculated using the mean of the two baseline periods. For both heart rate and blood pressure, cardiovascular reactivity was calculated using difference scores between the appropriate baseline level and subsequent stressful task. If one baseline was missing, the available task baseline was used for both scores. The reactivity scores for each task were then averaged to create a single mean reactivity score for heart rate, systolic blood pressure, and diastolic blood pressure. Full reactivity scores are reported in Supplemental Table 1. Mean arterial pressure was calculated using the standard formula: ([2*diastolic blood pressure] + systolic blood pressure)/3. Mean arterial pressure was used as the measure of blood pressure reactivity to simplify the reporting of results in the main text; however, all substantive results reported in the study replicated when using systolic or diastolic blood pressure as the predictor or outcome (see Supplemental Table 2). Heart rate reactivity (mean = 3.5, SD = 5.4) and blood pressure reactivity (mean = 6.0, SD = 4.2) correlated in the Dunedin Study, r = 0.50, p < .001. Heart rate reactivity (mean = 3.6, SD = 3.7) and blood pressure reactivity (mean = 8.2, SD = 6.4) also correlated in the MIDUS Study, r = 0.31, p < .001.
Childhood predictors.
We examined two measures of early childhood experiences that were hypothesized to be associated with later cardiovascular reactivity—adverse childhood experiences and childhood SES. The measures were assessed prospectively in Dunedin and retrospectively in MIDUS.
Adverse childhood experiences.
In Dunedin, adverse childhood experiences were generated from archival Dunedin Study records gathered during study assessments from ages birth to 15 years. As previously described (Reuben et al., 2016), archival study data were reviewed by four independent raters to determine whether study members experienced 10 types of events that best matched current guidelines, including five types of child harm (physical abuse, emotional abuse, physical neglect, emotional neglect, and sexual abuse) and five types of household dysfunction (incarceration of a family member, household substance abuse, household mental illness, loss of a parent, and household partner violence). Counts greater than four were recoded to four, in line with the Center for Disease Control procedure (CDC, 2016). The distribution of adverse childhood experiences in Dunedin (mean = 1.0, SD = 1.2) matched the distribution observed in the CDC study (Reuben et al., 2016).
In MIDUS, adverse childhood experiences were measured using the Childhood Trauma Questionnaire (Bernstein & Fink, 1998), which assessed retrospective reports of abuse and neglect that participants experienced in childhood. The five subscales in the measure (emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect) were summed, with higher scores representing higher levels of adversity (mean = 7.8, SD = 3.0).
Childhood SES.
In Dunedin, the childhood SES of participants’ families was measured using the 6-point Elley-Irving Socioeconomic Index for New Zealand (Elley & Irving, 1976). Childhood SES represented the highest SES level of either parent averaged across the interviews of the Dunedin study from the study member’s birth through age 15 (mean = 3.8, SD = 1.1).
In MIDUS, childhood SES was assessed using study members’ retrospective reports of their parents’ occupations. The maximum value of the occupational Socioeconomic Index (SEI) score between the male and female head of household at MIDUS 1 assessment was used as the measure of childhood SES, with higher scores representing higher SES (mean = 39.8, SD = 14.1).
Health outcomes.
Five health outcomes across Dunedin and MIDUS were studied, three of which were measured in both studies (self-reported health, inflammation, and hypertension) and two of which were measured in only one study (biological aging in Dunedin and mortality in MIDUS).
Self-reported health.
In Dunedin and MIDUS, self-reported health was assessed using an item that asked participants, “In general, would you say your health is?” Responses were on a 5-point scale and included excellent, very good, good, fair, and poor. Scores were coded so that higher scores represented better health. In Dunedin, self-reported health was assessed at age 45, 13 years following the cardiovascular reactivity assessment (mean = 3.7, SD = 0.9), whereas in MIDUS self-reported health was assessed at the MIDUS 2 assessment (Ryff et al., 2004), approximately concurrent with the cardiovascular reactivity assessment (mean = 3.7, SD = 0.9).
Inflammation.
In Dunedin and MIDUS, systemic inflammation was assessed using blood samples. In Dunedin, samples were collected at age 45. The collection was performed between 4:15 and 4:45 PM for all participants. Serum high-sensitivity CRP (CRP, mg/I) was measured on a Modular P analyzer (Roche Diagnostics GmbH, Mannheim, Germany) using a particle-enhanced immunoturbidimetric assay. In MIDUS, participants who completed the biomarker lab visit (Ryff, Seeman, & Weinstein, 2004) provided fasting blood samples between 6:30 and 7:00 AM, which were collected and processed using procedures described by Ryff and colleagues (2004). CRP was measured using a BNII nephelometer from Dade Behring utilizing a particle enhanced immunonepholometric assay. Samples falling below the assay range for CRP by this method were re-assayed by immunoelectrochemiluminescence using a high-sensitivity assay kit (Ryff et al., 2004). CRP in Dunedin and MIDUS was not normally distributed and was log-transformed for further analyses, as is commonly done in the literature (The Emerging Risk Factors Collaboration, 2012). Values above 10 mg/I were recoded as missing following standard practice (Pearson et al., 2003), as such values often represent acute infection rather than systemic inflammation. Mean values were 0.9 (SD = 0.8) in Dunedin and 0.9 (SD = 0.6) in MIDUS.
Hypertensive status.
In Dunedin and MIDUS, hypertensive status was assessed using assessments of resting systolic and diastolic blood pressure. In Dunedin, resting blood pressure was assessed at age 45 and measured using the average of three reading taken at 5 minute intervals. In MIDUS, resting blood pressure was assessed as part of the biomarker assessment (Ryff et al., 2004). In both studies, participants were coded as having hypertension if their systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg, matching current definitions of hypertension from the American Heart Association (Whelton et al., 2018). The proportion of participants in the hypertensive range was 50.5% in Dunedin and 35.7% in MIDUS.
Biological aging.
Biological aging was assessed using a previously validated approach called the Pace of Aging (Belsky et al., 2015). The Pace of Aging was measured for each participant using repeated assessments of a 19 biomarker panel taken at ages 26, 32, 38, and 45 years. Biomarkers included body mass index, waist-to-hip ratio, glycated hemoglobin level, leptin level, blood pressure, cardiorespiratory fitness, forced expiratory volume in 1 second, ratio of forced expiratory volume in 1 second to forced vital capacity, total cholesterol level, triglyceride level, high-density lipoprotein cholesterol level, ratio of apolipoprotein B100 to apolipoprotein A1, lipoprotein(a) level, creatinine clearance, blood urea nitrogen level, C-reactive protein level, white blood cell count, gum health, and caries-affected tooth surfaces. Change over time in each biomarker was modeled with a mixed-effects growth model, and these 19 rates of change were combined into a single index scaled (within sex) in years of physiological change occurring per 1 chronological year (SD = 0.3). Participants’ Pace of Aging ranged from approximately 0.4 years to 2.4 years of biological aging per chronological year.
Mortality.
MIDUS 3 data collection included information on all known MIDUS and Milwaukee decedents as of November, 2017. This date was combined with the date of physiological assessment to create a time variable for survival analyses. Mortality tracing was conducted by the University of Wisconsin Survey Center. Methods included NDI searches and ongoing longitudinal sample maintenance (Ryff et al., 2016). Among the MIDUS sample included in this study, 61 deaths were recorded up to the censor date (6.0%).
Individual difference variables.
Three individual-difference variables were included as covariates to help contextualize the main study findings.
Conscientiousness.
In Dunedin, study members’ personalities were assessed using reports by co-informants at ages 26, 32, and 38. At each age, Dunedin study members nominated three people “who knew them well.” Informants were mailed questionnaires asking them to describe the study member using a brief, 25-item version of the Big 5 Inventory (Benet-Martínez & John, 1998). Complete Big 5 data were obtained for 946 (96%) participating study members at age 26; 935 (96%) at age 32; and 933 (97%) at age 38. Conscientiousness was assessed to index the degree to which study members were “responsible, attentive, careful, persistent, orderly, planful, and future-oriented (Benet-Martínez & John, 1998).” Scores from each informant were averaged across informants and across study occasions to provide an average measure of conscientiousness, with higher score reflecting greater conscientiousness (mean = 7.2, SD = 1.5).
In MIDUS 2, participants responded to 31 self-descriptive adjectives designed to assess the Big 5 personality traits (Ryff et al., 2004). The adjectives used to create the measure of conscientiousness included Organized, Responsible, Hardworking, Careless (reverse coded), and Thorough. Self-reports were completed on a four-point scale ranging from “a lot” to “not at all (4)”. Higher scores corresponded to relatively higher levels of conscientiousness (mean = 3.5, SD = 0.4).
Cognitive ability.
In Dunedin, cognitive ability in adulthood was assessed using the Wechsler Adult Intelligence Scale–IV (WAIS-IV) at age 38 (Wechsler, 2008). The WAIS-IV generates the Intelligence Quotient (IQ), with a standardized mean = 100, SD = 15.
In MIDUS, cognitive ability was assessed using the Brief Test of Adult Cognition by Telephone (Lachman & Tun, 2008; Tun & Lachman, 2008), which was collected as part of the MIDUS 2 Cognitive Project (Ryff & Lachman, 2006). Tasks included word recall (immediate and delayed), digit span backward, category fluency, number series, and backward counting. The variable was calculated as the mean of the z-scores of the five subtests (mean = 0.2, SD = 0.9).
Depressive symptoms.
In Dunedin, symptoms of depression were assessed as part of the Diagnostic Interview Schedule (Robins, Helzer, Crottler, & Goldring, 1989). The scale represented a sum of the criteria for major depressive disorder according to DSM-IV. Scores ranged from 0 to 9, with higher scores representing relatively greater depression (mean = 1.5, SD = 2.9).
In MIDUS, the Center for Epidemiological Studies Depression Scale (Radloff, 1977) was used to assess depressive symptoms. The scale consists of 20-items that are summed together, with greater scores representing relatively more depressive symptoms (mean = 7.8, SD = 12.0).
Data Analysis
We used a series of multiple regression models to test whether childhood adversity was associated with later cardiovascular reactivity, as well as whether cardiovascular reactivity was associated with later health outcomes in each study. The associations were first tested among the Dunedin study members, then replicated in the MIDUS study using the best available matching variables. All models used multiple regression with the exception of models predicting hypertension and mortality in MIDUS, which instead used logistic regression and a survival model predicting time-to-death controlling for the date of cardiovascular reactivity assessment, respectively. All analyses in both Dunedin and MIDUS were adjusted for sex and baseline cardiovascular activity. MIDUS analyses were also adjusted for age (the Dunedin participants were all the same age, i.e., born in a 1-year period).
After testing these models, we conducted five additional sets of analyses. The first set of analyses examined whether childhood adversity was indirectly associated with health outcomes via cardiovascular reactivity using mediation models. Second, we examined whether there might be a non-linear association between cardiovascular reactivity and the health outcomes, which we tested by including a quadratic term in our models. Third, we tested whether windsorizing cardiovascular reactivity scores greater than two standard deviations from the mean within each sample attenuated the associations. Fourth, we examined sex differences in the primary results using moderation in a multiple regression framework. Fifth, we controlled for three individual-difference variables—conscientiousness, cognitive ability, and depression—to test whether including these measures as covariates attenuated the primary results. To account for missing data in our models, we used full information maximum likelihood (Graham, 2009) in MPLUS version 8.3 (Muthén & Muthén, 2012).
Results
Childhood Predictors of Cardiovascular Reactivity
Adverse Childhood Experiences.
Dunedin study members with more adverse childhood experiences had lower heart rate reactivity, β = −0.16, 95% CI [−0.22, −0.09], p < .001, and blood pressure reactivity, β = −0.12, 95% CI [−0.18, −0.06], p < .001. These results replicated in the MIDUS study; participants who retrospectively reported more abuse and neglect in childhood had lower heart rate reactivity, β = −0.08, 95% CI [−0.18, −0.02], p = .012, and lower blood pressure reactivity, β = −0.11, 95% CI [−0.17, −0.05], p = .001.
Childhood SES.
Dunedin study members with lower SES in childhood had lower heart rate reactivity, β = 0.12, 95% CI [0.06, 0.18], p < .001, and lower blood pressure reactivity, β = 0.10, 95% CI [0.04, 0.16], p = .002. MIDUS participants’ retrospectively-reported childhood SES was not significantly associated with heart rate reactivity, β = 0.03, 95% CI [−0.04, 0.10], p = .430, or blood pressure reactivity, β = 0.06, 95% CI [−0.01, 0.13], p = .088, though both associations were in the same direction as those in Dunedin. The full results of the associations between childhood predictors and cardiovascular reactivity are presented in Table 1.
Table 1.
The Association of Childhood Adversity and Health Outcomes with Cardiovascular Reactivity in the Dunedin and MIDUS Studies
| Dunedin (n = 922) | MIDUS (n = 1,015) | |||
|---|---|---|---|---|
|
| ||||
| HR reactivity | BP reactivity | HR reactivity | BP reactivity | |
| Childhood adversity | ||||
| Adverse childhood experiences | −0.16* [−0.22, −0.09] | −0.12* [−0.18, −0.06] | −0.08* [−0.18, −0.02] | −0.11* [−0.17, −0.05] |
| Childhood SES | 0.12* [0.06, 0.18] | 0.10* [0.04, 0.16] | 0.03 [−0.04, 0.10] | 0.06 [−0.01, 0.13] |
| Health outcomes | ||||
| Self-reported health | 0.10* [0.04, 0.17] | 0.19* [0.12, 0.25] | 0.10* [0.04, 0.17] | 0.13* [0.06, 0.20] |
| Inflammation | −0.09* [−0.16, −0.01] | −0.13* [−0.20, −0.06] | −0.06* [−0.13, −0.00] | −0.09* [−0.15, −0.02] |
| Hypertensive status† | −0.00 [−0.03, 0.02] | −0.02 [−0.06, 0.01] | 0.07* [0.04, 0.11] | −0.02 [−0.05, 0.02] |
| Pace of aging | −0.17* [−0.23, −0.11] | −0.22* [−0.28, −0.15] | — | — |
| Mortality† | — | — | −0.08* [−0.16, −0.01] | −0.03 [−0.07, 0.01] |
Dunedin Longitudinal Study models are adjusted for sex and baseline cardiovascular activity, and MIDUS Study models are adjusted for sex, age, and baseline cardiovascular activity. MIDUS childhood predictors were retrospective reports. HR = heart rate, BP = blood pressure.
All statistics are β values except results for hypertension and mortality, which are log survival odds.
= p < .05
Health Outcomes and Cardiovascular Reactivity
Self-rated Physical Health.
Among Dunedin study participants, lower cardiovascular reactivity at age 32 years was associated with worse self-reported health 13 years later—for heart rate reactivity, β = 0.10, 95% CI [0.04, 0.17], p = .002; for blood pressure reactivity, β = 0.19, 95% CI [0.12, 0.25], p < .001. These associations replicated in the MIDUS study: lower cardiovascular reactivity during the MIDUS 2 biomarker assessment was associated with worse self-reported health assessed at MIDUS 2—for heart rate reactivity, β = 0.10, 95% CI [0.04, 0.17], p = .002; for blood pressure reactivity, β = 0.13, 95% CI [0.06, 0.20], p < .001.
Inflammation.
Among Dunedin study participants, lower cardiovascular reactivity at age 32 years was associated with higher inflammation levels 13 years later—for heart rate reactivity, β = −0.09, 95% CI [−0.16, −0.01], p = .018; for blood pressure reactivity, β = −0.13, 95% CI [−0.20, −0.06], p < .001. These associations replicated in the MIDUS Study: lower cardiovascular reactivity was associated with higher inflammation levels at the MIDUS 2 biomarker assessment—for heart rate reactivity, β = −0.06, 95% CI [−0.13, −0.00], p = .044; and blood pressure reactivity, β = −0.09, 95% CI [−0.15, −0.02], p = .008.
Hypertension.
Among Dunedin study participants, cardiovascular reactivity at age 32 years was not significantly associated with hypertensive status 13 years later—for heart rate reactivity, HR = 1.00 95% CI [0.97, 1.03], p = .845; for blood pressure reactivity, HR = 0.98 95% CI [0.94, 1.01], p = .192. In the MIDUS Study, blood pressure reactivity was not significantly associated with hypertensive status at the MIDUS 2 biomarker assessment, HR = 0.98, 95% CI [0.95, 1.02], p = .537, although higher heart rate reactivity was associated with a higher likelihood of hypertension, HR = 1.08, 95% CI [1.04, 1.12], p < .001. Given that hypertension is dependent on a cutoff for resting blood pressure, we also tested these associations between blood pressure reactivity and hypertension without controlling for resting blood pressure. Neither study evidenced a significant association—Dunedin, HR = 1.00, 95% CI [0.97, 1.03], p = .971; MIDUS, β = 0.99, 95% CI [0.97, 1.01], p = .245.
Biological Aging.
Dunedin study participants with lower cardiovascular reactivity had a faster pace of biological aging—for heart rate reactivity, β = −0.17, 95% CI [−0.23, −0.11], p < .001; for blood pressure reactivity, β = −0.22, 95% CI [−0.28, −0.15], p < .001).
Mortality.
MIDUS study participants with lower heart rate reactivity were at greater risk of early mortality, HR = 1.09, 95% CI [1.01, 1.17], p = .026, although there was not a significant association between blood pressure reactivity and early mortality, HR = 1.03, 95% CI [0.99, 1.07], p = .139. Notably, the sample only included 61 deaths (6.0%), which likely limited the power to detect associations in MIDUS. The full results of the association between cardiovascular reactivity and health outcomes are presented in Table 1.
Secondary Analyses: Indirect Effects of Childhood Adversity on Health Via Cardiovascular Reactivity
Having established direct effects between childhood adversity, cardiovascular reactivity, and health in both samples, we next tested whether there were significant indirect effects of childhood adversity on health via cardiovascular reactivity using a series of mediation models. We ran all possible models independently for the two measures of childhood adversity, two measures of cardiovascular reactivity, and four health outcomes. All mediation models were fully saturated, run using bootstrapping (n = 1,000), and controlled for baseline cardiovascular activity.
A limited number of significant indirect effects linked childhood adversity (childhood ACEs and SES) to health outcomes consistently across the two samples. ACEs were significantly associated with later self-reported health and inflammation via blood pressure reactivity in both MIDUS and Dunedin (Table 2). There were no other significant indirect effects between childhood adversity and health via cardiovascular reactivity that replicated between the two samples. Of the measures available in only one sample, ACEs and childhood SES were indirectly associated with biological aging as assessed in the Dunedin study via both measures of cardiovascular reactivity. The full results of the mediation models are presented in Table 2. Broadly, we observed nonsignificant indirect effects, or significant indirect effects that were small in size. Direct effects between childhood adversity and health were generally larger in size and these effects remained significant when accounting for cardiovascular reactivity.
Table 2.
Indirect Effects from Childhood Adversity to Health Via Cardiovascular Reactivity
| Dunedin (n = 922 | MIDUS (n = 1,015) | |||
|---|---|---|---|---|
|
| ||||
| HR reactivity | BP reactivity | HR reactivity | BP reactivity | |
| ACEs → Self-reported health: Direct effect | −0.12* [−0.19, −0.06] | −0.12* [−0.19, −0.05] | −0.25* [−0.32, −0.17] | −0.25* [−0.32, −0.17] |
| Indirect effect | −0.01* [−0.02, −0.00] | −0.02* [−0.03, −0.01] | −0.01 [−0.02, −0.00] | −0.01* [−0.02, −0.00] |
| ACEs → Inflammation: Direct effect | 0.09* [0.02, 0.15] | 0.10* [0.03, 0.17] | 0.08* [0.01, 0.14] | 0.08* [0.02, 0.15] |
| Indirect effect | 0.01 [−0.00, 0.02] | 0.02* [0.00, 0.03] | 0.01 [−0.00, 0.01] | 0.01* [0.00, 0.02] |
| ACEs → Hypertensive status†: Direct effect | −0.01 [−0.13, 0.12] | 0.06 [−0.07, 0.18] | −0.04 [−0.09, 0.01] | −0.02 [−0.13, 0.04] |
| Indirect effect | 0.00 [−0.02, 0.02] | 0.01 [−0.01, 0.03] | −0.01* [−0.01, −0.00] | 0.00 [−0.01, 0.01] |
| ACEs → Pace of aging: Direct effect | 0.18* [0.11, 0.25] | 0.18* [0.11, 0.25] | — | — |
| Indirect effect | 0.02* [0.01, 0.03] | 0.02* [0.01, 0.04] | — | — |
| ACEs → Mortality†: Direct effect | — | — | 0.12* [0.04, 0.20] | 0.12* [0.04, 0.20] |
| Indirect effect | — | — | 0.01 [−0.00, 0.02] | 0.01 [−0.01, 0.02] |
| Childhood SES → Self-reported health: Direct effect | 0.15* [0.09, 0.22] | 0.15* [0.10, 0.24] | 0.12* [0.06, 0.19] | 0.12* [0.06, 0.19] |
| Indirect effect | 0.01* [0.00, 0.02] | 0.02* [0.01, 0.03] | 0.00 [−0.00, 0.01] | 0.01 [−0.00, 0.02] |
| Childhood SES → Inflammation: Direct effect | −0.13* [−0.19, −0.06] | −0.13* [−0.19, −0.06] | −0.08* [−0.15, −0.01] | −0.08* [−0.15, −0.02] |
| Indirect effect | −0.01 [−0.02, 0.00] | −0.01* [−0.02, −0.00] | −0.00 [−0.01, 0.00] | −0.01 [−0.00, 0.00] |
| Childhood SES → Hypertensive status†: Direct effect | −0.14* [−0.26, −0.01] | −0.17* [−0.31, −0.04] | 0.00 [−0.01, 0.01] | 0.00 [−0.02, 0.02] |
| Indirect effect | 0.00 [−0.02, 0.02] | −0.01 [−0.02, 0.01] | 0.00 [−0.00, 0.00] | −0.00 [−0.00, 0.00] |
| Childhood SES → Pace of aging: Direct effect | −0.21* [−0.29, −0.17] | −0.22* [−0.27, −0.16] | — | — |
| Indirect effect | −0.02* [−0.03, −0.01] | −0.02* [−0.03, −0.01] | — | — |
| Childhood SES → Mortality†: Direct effect | — | — | −0.00 [−0.03, 0.03] | 0.00 [−0.03, 0.03] |
| Indirect effect | — | — | −0.00 [−0.01, 0.01] | −0.00 [−0.01, 0.01] |
Models are adjusted for sex and baseline cardiovascular activity. MIDUS models are also adjusted for age. ACEs = Adverse childhood experiences, HR = heart rate, BP = blood pressure.
All statistics are β values except results for hypertension and mortality, which are log survival odds.
= p < .05
Secondary Analyses: Probing the Robustness of the Study Findings
Having established that more adverse childhoods were associated with less cardiovascular reactivity and less cardiovascular reactivity was associated with poorer midlife health outcomes, we conducted additional analyses to verify and better understand the study findings.
Testing for Non-linearity.
We first tested whether there might be non-linear associations between cardiovascular reactivity and the health outcomes used in the study. Prior research has suggested that it might be detrimental to health to be on either extreme in terms of cardiovascular reactivity (Phillips, 2016), and one method to test this possibility is to test if there are non-linear associations in our data. To do so, we created quadratic transformations of our cardiovascular reactivity variables by standardizing and squaring them (e.g., standardized heart rate reactivity * standardized heart rate reactivity). We then ran our primary models of interest including both the linear and quadratic forms of the cardiovascular reactivity scores as predictors. Neither cardiovascular reactivity measure evidenced a consistent non-linear association with any of the health outcomes in the primary models. One exception was the association of heart rate reactivity with inflammation in MIDUS, β = −0.08, 95% CI [−0.15, −0.01], p = .043. This effect did not replicate within sample using the second measure of reactivity, nor did it replicate for either measure of reactivity in the other study cohort. All other associations were non-significant and relatively small in terms of effect size. Full results are reported in Supplemental Table 3. Figure 1 illustrates mean levels among the continuously measured health outcomes for participants grouped into high, low, and average cardiovascular reactivity.
Figure 1.
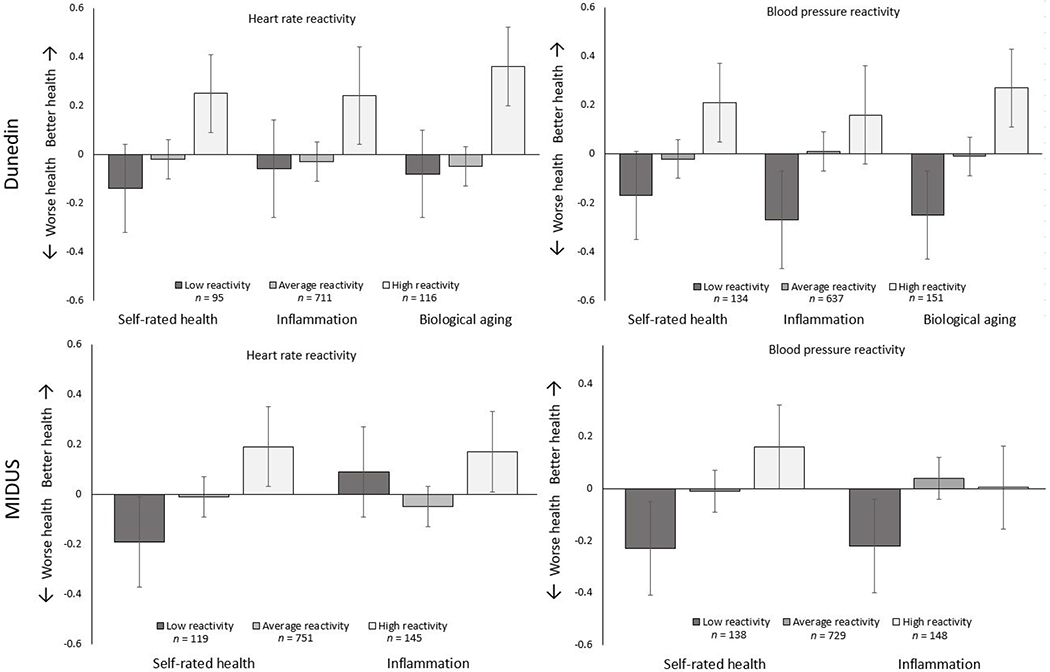
Health outcomes illustrating the mean levels by heart rate and blood pressure reactivity levels. Low reactivity = participants more than one SD below the mean, average reactivity = participants between −1 and 1 SDs, and high reactivity = participants above 1 SD. Outcomes were standardized and scaled so that higher scores equaled better health. Ns for CRP excluded participants missing data due to values > 10 mg/L.
Cardiovascular Reactivity Outliers.
We next tested whether these associations were due to outliers influencing the associations. We identified participants within each study who had heart rate and blood pressure reactivity scores greater or lesser than two standard deviations around the mean and windsorized these scores to ±2 SD from the mean. In Dunedin, this included 43 heart rate (4.7%) and 39 blood pressure scores (4.2%). In MIDUS, this included 43 heart rate scores (4.2%) and 53 blood pressure scores (5.2%). When windsorizing these outlier scores, all effect sizes changed a negligible amount when compared to the primary analyses, |Δβs| ≤ 0.03 (see Supplemental Table 4 for full results), providing evidence that the main findings were not due to outliers in cardiovascular reactivity.
Testing for Sex Differences.
We also tested whether the associations might evidence sex differences. To do so, we ran the models for our primary results while adding the appropriate interaction term to the predictors. The results did not indicate any consistent patterns of sex differences that replicated across the cohorts. There was one significant interaction term, β = 0.08, [0.01, 0.15], p = .027, such that lower blood pressure reactivity was associated with greater risk for early mortality among women in MIDUS, but not men. This result was not replicated for heart rate reactivity. Full results are presented in Supplemental Table 5.
Evidence from Individual Difference Variables.
Given the findings supported a “blunting” model of cardiovascular reactivity (Carroll et al., 2009; Carroll et al., 2017; Ginty et al., 2013), we tested whether three individual differences—conscientiousness, cognitive ability, and depressive symptoms— might help explain these results. First, we tested whether the lab stressors elicited greater reactivity among highly conscientious research participants. People who are more conscientious are also generally healthier (Roberts, Walton, & Bogg, 2005), and conscientious participants may have been more likely to fully engage with the computerized cognitive stressor tasks. Indeed, conscientious participants in both Dunedin and MIDUS were generally more reactive to the stressor tasks (in Dunedin: for heart rate reactivity, β = 0.13, 95% CI [0.07, 0.19], p < .001, for blood pressure reactivity, β = 0.18, 95% CI [0.11, 0.24], p < .001; in MIDUS: for blood pressure reactivity, β = 0.11, 95% CI [0.04, 0.18], p = .002, but not heart rate reactivity, β = 0.04, 95% CI [−0.02, 0.11], p = .189;). Second, we tested whether the lab stressors elicited greater reactivity among high-functioning participants. People with greater cognitive ability are generally healthier (Batty, 2009), and these participants may have more fully engaged with the computerized cognitive stressor tasks as well. High-functioning participants in both Dunedin and MIDUS were more reactive to the cognitive stressor tasks (in Dunedin: for heart rate reactivity, β = 0.20, 95% CI [0.13, 0.26], p < .001, for blood pressure reactivity, β = 0.26, 95% CI [0.20, 0.32], p < .001; in MIDUS: for heart rate reactivity, β = 0.10, 95% CI [0.04, 0.17], p = .002, for blood pressure reactivity, β = 0.17, 95% CI [0.11, 0.23], p < .001). Finally, we tested whether the lab stressors elicited less reactivity among participants with higher levels of depressive symptoms. Depression is cited as a possible explanation for why blunted reactivity is associated with poorer health outcomes (Carroll et al., 2017; Ginty et al., 2013; Phillips et al., 2013) and might attenuate the observed associations. Similar to the other individual differences, participants with more depressive symptoms were less reactive to the cognitive stressor tasks in both studies (in Dunedin: for heart rate reactivity, β = −0.08, 95% CI [−0.14, − 0.02], p = .015, for blood pressure reactivity, β = −0.17, 95% CI [−0.24, −0.11], p < .001; in MIDUS: for heart rate reactivity, β = −0.14, 95% CI [−0.20, −0.08], p < .001, for blood pressure reactivity, β = −0.21, 95% CI [−0.27, −0.15], p < .001).
We repeated our primary analyses controlling for conscientiousness, cognitive ability, and depression to test whether these factors may have helped explain the associations between childhood adversity, cardiovascular reactivity, and health. Controlling for these individual differences attenuated the associations observed in the Dunedin and MIDUS studies, although effects were less attenuated in MIDUS. In Dunedin, controlling for these measures attenuated the primary associations, on average, by 0.06 in terms of absolute standardized effect size for heart rate (52.3% of original effect sizes), and by 0.08 for blood pressure (67.8% of original effect sizes). In MIDUS, controlling for these measures attenuated the primary associations, on average, by 0.03 in absolute effect size for heart rate (48.2% of original effect sizes), and 0.03 for blood pressure (47.4% of original effect sizes). In total, two-thirds of the significant associations observed in the primary results were no longer significant (12 of 18 associations) when accounting for these variables. These results suggest that some participants’ conscientiousness, cognitive ability, or depression may have influenced their engagement during the CVR tasks. Figure 2 illustrates the attenuation of effects and Supplemental Table 6 provides full results.
Figure 2.
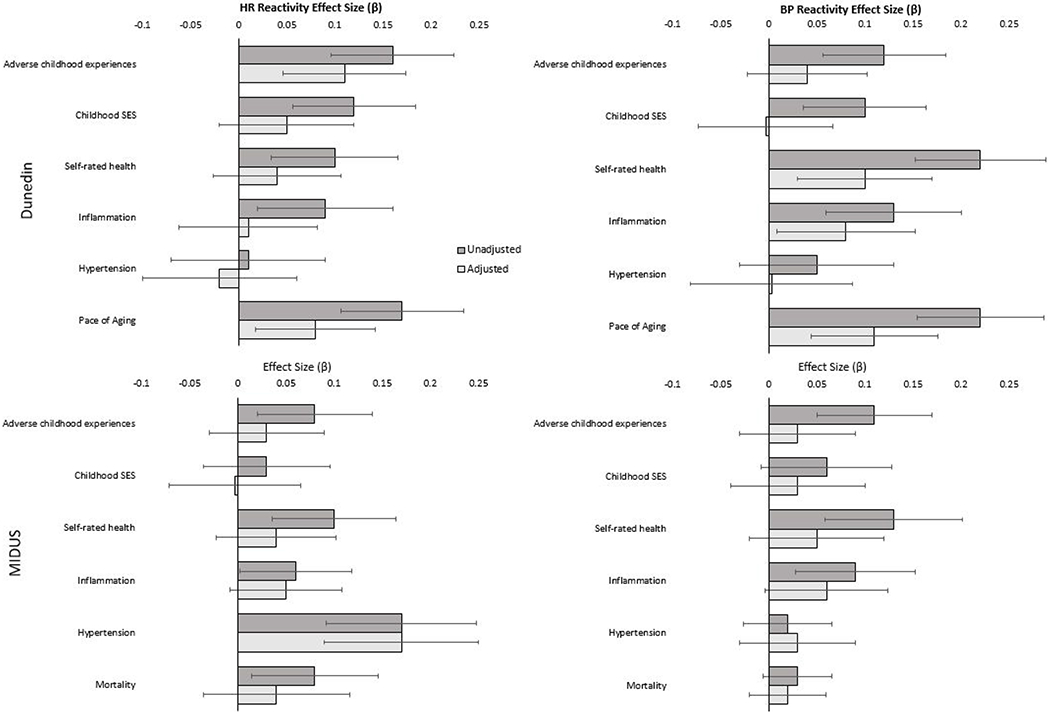
Associations of childhood predictors and health outcomes with cardiovascular reactivity when unadjusted and adjusted for conscientiousness, cognitive ability, and depression.
Discussion
The current study used two longitudinal cohorts, the Dunedin (n = 922) and the MIDUS Study (n = 1,015), to examine the links between early life adversity, cardiovascular reactivity, and health outcomes in adulthood. The primary results showed that people with more adversity in childhood consistently had lower levels of later cardiovascular reactivity, and lower cardiovascular reactivity was consistently associated with poorer health—with the exception of hypertensive status. These findings largely replicated across the two cohorts. Although not empirically tested, descriptively when the two cohorts differed on how constructs were measured, associations were stronger in the cohort that used more proximal or reliable measurement (e.g., prospectively-measured childhood SES and ACEs in Dunedin compared to retrospective reports in MIDUS; full scale IQ in Dunedin compared to a shorter cognitive battery in MIDUS).
The secondary results revealed two main findings. First, when testing indirect effects of childhood adversity on health via cardiovascular reactivity, significant effects were observed across the two cohorts linking ACEs to poorer self-rated health and greater inflammation via lower blood pressure reactivity. Greater adversity in childhood was also indirectly associated with faster biological aging in Dunedin via lower cardiovascular reactivity. Second, our models showed that cardiovascular reactivity was associated with individual differences in conscientiousness, cognitive ability, and depressive symptoms and that including these constructs as covariates in our models partially attenuated the association of cardiovascular reactivity with childhood adversity and health. The results suggest these individual differences account for the observed results, at least in part.
How should these results be understood? We propose two interpretations. First, these results may reflect the specific tasks and methods used to assess cardiovascular reactivity in the Dunedin and MIDUS studies. Both studies used cognitive stressors that were administered through computerized prompts, and both assessed participants who had completed a number of previous study assessments. Social evaluation plays an important role in eliciting cardiovascular reactivity (Bosch et al., 2009; Smith, Nealey, Kircher, & Limon, 1997), and the computerized administration of these tasks may have presented participants with less social evaluation than is present when an experimenter asks participants to complete math questions. Second, participants in both cohorts had long-standing familiarity with research studies. They may have habituated to the experience of participating in study tasks, which may have resulted in lower levels of social evaluation during the Stroop and math stressors than might be the case among people participating in a research study for the first time.
Reactivity to these computerized, cognitive-stressor tasks may instead have varied systematically based on participants’ individual differences. For example, people with higher levels of intelligence and/or who are more conscientious may have placed more value on performing well on these tasks and evidenced higher reactivity as a result. Similarly, more depressed participants may engage less fully in the tasks. The associations between reactivity and the individual difference variables provide some evidence for this conclusion. This interpretation would suggest that future studies of cardiovascular reactivity carefully choose the types of stressors used. Although cognitive stressors like the Stroop task or mental arithmetic task are assumed to be stressful for all people, they could evoke differences in cardiovascular activity based on individual differences in how people appraise or respond to such stressors—which may be correlated with the relevant predictors, outcomes, or both in any given study—resulting in spurious findings.
Notably, the current study assessed these covariates at a similar point in time as cardiovascular reactivity and alternative explanations are also possible. For example, rather than reducing engagement to result in blunting, these individual differences might also be consequences of blunted cardiovascular reactivity, or both could be affected by an unmeasured third variable. Future studies would benefit from also using stressful tasks that do not appear to reflect individual differences strongly, such as stressors like the cold pressor (Brindle, Whittaker, Bibbey, Carroll, & Ginty, 2017). Alternatively, tasks that are high in emotional salience, such as a trauma recall or anger recall, might result in more consistent task engagement. Additional empirical study using multiple methods of eliciting reactivity (e.g., cognitive, physiological, and emotionally salient stressful tasks) would be needed to provide evidence to this end.
A second interpretation is that the life-course conceptualization of cardiovascular reactivity linking early adversity and later health through greater reactivity needs to be revised. Our results found associations in the opposite direction of what was expected based on extending the cardiovascular reactivity hypothesis. Greater cardiovascular reactivity being associated with better health stands in opposition to research across a variety of contexts, tasks, and populations (see Chida & Steptoe, 2010; Jennings et al., 2004; Karmarck et al., 1997; Manuck, 1994; Treiber et al, 2003). However, much of the research linking cardiovascular reactivity with poorer health is focused on hypertension and disease states. For example, although a 2010 meta-analysis (Chida & Steptoe, 2010) found an overall association between cardiovascular reactivity and cardiovascular risk status (r = 0.09), this effect was restricted to prospective associations with higher blood pressure—there was a null association when examining the pooled correlation between reactivity and subclinical markers of atherosclerosis. Notably, we did not observe a consistent association between cardiovascular reactivity and hypertension in the Dunedin and MIDUS cohorts. Some of the included studies actually found that greater reactivity was associated with improved markers of disease (Heponiemi et al., 2007). More recent studies have found similar associations; for example, people with higher diastolic blood pressure reactivity had lower risk for subsequent early mortality (Kupper, Denollet, Widdershoven, & Kop, 2015).
The emerging research from the past decade and a half examining blunted cardiovascular reactivity and health (Phillips, 2016) provides important context to these results. Over the last decade, low levels of cardiovascular reactivity (i.e., a blunted response) to cognitive stressors have been linked to poorer health outcomes (Allen, 2013; Carroll, Lovallo & Phillips, 2009; Carroll, Phillips, & Lovallo, 2012), leading to the theory that particularly low levels of reactivity—in addition to higher reactivity—may be detrimental to health due to associated dysregulation in behavioral motivation (Carroll et al., 2017; Ginty et al., 2013; Phillips et al., 2013). Within this model, one could expect that both high and low reactivity would be associated with poorer health. However, we did not find evidence of nonlinearity in our results—participants with the highest cardiovascular reactivity were the healthiest and vice versa.
Studies of post-traumatic stress disorder and developmental psychopathology have also found that trauma and childhood adversity are associated with lower cardiovascular reactivity (Ginty, Masters, Nelson, Kaye, & Conklin, 2017; McLaughlin et al., 2014; Murali & Chen, 2005; Voellmin et al., 2015), which may be relevant to understanding the mixed evidence linking cardiovascular reactivity and health broadly. Such work theorizes that adversity and trauma, particularly severe or chronic exposure to such stressors, can result in dysregulation of the stress response system and a “freezing” response to stress (McLaughlin et al., 2014). A freezing response would result in decreased cardiovascular reactivity when faced with threatening tasks (Heleniak, McLaughlin, Ormel, & Riese, 2016). The current results could support a blunted cardiovascular reactivity hypothesis and suggest that people who experience early life adversity develop lower later cardiovascular reactivity as a result of changes to the stress response system. These lower levels of cardiovascular reactivity, in turn, could be associated with poorer health by indexing individual differences in behavioral motivation (Carroll et al., 2017). The specific mechanisms linking motivated behavior to health were not explicitly tested in this study, however, which limits the degree to which these findings support such theoretical speculation.
This study has notable strengths. The samples are large compared to many studies of cardiovascular reactivity and include a variety of measures that are not available in some of the larger, population-based cohort studies. In addition, we were able to replicate the majority of our results across two independent cohorts using multiple measures of cardiovascular reactivity to the same cognitive stressors, providing evidence that the results are not simply due to type I error. These strengths speak to the reliability of the results, but speak less to which interpretation of the results is more appropriate. Determining the best interpretation would require, at minimum, examining associations between childhood adversity, cardiovascular reactivity, and health outcomes using reactivity to other types of stressful tasks, particularly as people with blunted responses to cognitive stressors do not evidence this same blunting to cold pressor and exercise tasks (Brindle et al. 2017). Regardless of the correct interpretation, publication of these results is particularly important given concerns about file-drawer effects in the study of cardiovascular reactivity. As stated by Allen (2013), “Indeed, one wonders how many “file cabinet” data sets are out there that were not published or even submitted for publication due to not getting the expected exaggerated reactivity to be associated with a variable of interest.” Of the 313 publications using MIDUS biomarker data from 2005 to 2020, to our knowledge only three studies (Coyle et al., 2020; Creaven, Higgins, Ginty, & Gallagher, 2020; Lin, Heffner, Mapstone, Chen, & Porsteisson, 2016) used cardiovascular reactivity. Similarly, the Dunedin Longitudinal Study (Poulton, Moffitt, & Silva, 2015) collected cardiovascular reactivity at age 32, but—as described in the Introduction—has not published any empirical articles on these data to date.
This study also has limitations. First, although both cohort studies included longitudinal assessments, neither included multiple assessments of cardiovascular reactivity. Assessments of cardiovascular reactivity in childhood would be necessary to test if reactivity had been “blunted’ from a previously normal level among people who experienced adversity in childhood. Future studies would benefit from multiple measurements of cardiovascular reactivity, which might provide temporal ordering when examining its associations with childhood adversity, individual difference variables (e.g., conscientiousness, depressive symptoms, cognitive ability), and health. Second, both studies relied on cognitive stressors to assess cardiovascular reactivity, and it is unknown whether different stressors (e.g., a cold pressor task) would yield different results. Third, there was significant overlap in the constructs measured in the two cohorts, but differences in the timing and type of measures of childhood adversity (e.g., retrospective compared to prospective) or health outcomes (e.g., mortality compared to biological aging) limited our ability to replicate findings across cohorts.
Conclusion
This study used two longitudinal cohorts to investigate the association between early life adversity, cardiovascular reactivity, and health outcomes. The results were in the opposite direction to what would be predicted based on the traditional cardiovascular reactivity hypothesis. Early life adversity was associated with lower levels of later cardiovascular reactivity, and lower cardiovascular reactivity was associated with worse health outcomes in adulthood. These results were somewhat attenuated when controlling for individual differences in personality, intelligence, and depression that may have affected participants’ reactions to the stressors used in the study. These results highlight the importance of accounting for individual differences when assessing cardiovascular reactivity using computerized cognitive stressors. In addition, the results may support links between early life adversity, a lower cardiovascular response to stress, and poorer health.
Supplementary Material
Acknowledgements:
The first author received support from National Institute on Aging Training Grant T32-AG000029. The study’s preregistration materials and addendum can be accessed online at https://sites.google.com/site/moffittcaspiprojects/home/projectlist/bourassa_2019b. Correspondence can be directed to Kyle Bourassa, Ph.D., kyle.bourassa@duke.edu. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council and New Zealand Ministry of Business, Innovation and Employment (MBIE). This research received support from the US-National Institute of Aging grant R01AG032282, and the UK Medical Research Council grant MR/P005918/1. We thank the Dunedin Study members, unit research staff, and Dunedin Study founder Phil Silva, PhD, University of Otago. Since 1995 the MIDUS study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network, National Institute on Aging (P01-AG020166), and National institute on Aging (U19-AG051426). Biomarker data collection was further supported by the NIH National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program as follows: UL1TR001409 (Georgetown), UL1TR001881 (UCLA), 1UL1RR025011 (UW).
References
- Allen MT (2013). Integrative commentary: Implications of blunted reactivity. International Journal of Psychophysiology, 90(2), 95–98. 10.1016/j.ijpsycho.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Allen MT, & The Psychosocial Working Group. (2000). Cardiovascular Reactivity. MacArthur Research Network on SES & Health. https://macses.ucsf.edu/research/psychosocial/default.php [Google Scholar]
- Batty GD, Wennerstad KM, Smith GD, Gunnell D, Deary IJ, Tynelius P, & Rasmussen F (2009). IQ in early adulthood and mortality by middle age: cohort study of 1 million Swedish men. Epidemiology, 100–109. 10.1097/EDE.0b013e31818ba076. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, … & Sugden K (2015). Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences, 112(30), E4104–E4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet-Martínez V, & John OP (1998). Los Cinco Grandes across cultures and ethnic groups: Multitrait-multimethod analyses of the Big Five in Spanish and English. Journal of Personality and Social Psychology, 75(3), 729–750. 10.1037//0022-3514.75.3.729 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, & Foote J (1998). Childhood trauma questionnaire. Assessment of family violence: A handbook for researchers and practitioners. 10.1037/t02080-000 [DOI] [Google Scholar]
- Blascovich JJ, & Katkin ES (Eds.). (1993). APA Science Vols. Cardiovascular reactivity to psychological stress & disease. American Psychological Association. [Google Scholar]
- Bosch JA, De Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJV, … & Edwards KM (2009). A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosomatic Medicine, 71(8), 877–885. 10.1097/PSY.0b013e3181baef05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, … Shweder RA (1994). Midlife in the United States (MIDUS 1), 1995-1996. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-09-09. 10.3886/ICPSR02760.v18 [DOI] [Google Scholar]
- Brindle RC, Whittaker AC, Bibbey A, Carroll D, & Ginty AT (2017). Exploring the possible mechanisms of blunted cardiac reactivity to acute psychological stress. International Journal of Psychophysiology, 113, 1–7. 10.1016/j.ijpsycho.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, & Phillips AC (2012). Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology, 49(10), 1444–1448. 10.1111/j.1469-8986.2012.01463.x [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, & de Rooij SR (2017). The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience & Biobehavioral Reviews, 77, 74–86. 10.1016/j.neubiorev.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Lovallo WR, & Phillips AC (2009). Are large physiological reactions to acute psychological stress always bad for health?. Social and Personality Psychology Compass, 3(5), 725–743. 10.1111/j.1751-9004.2009.00205.x [DOI] [Google Scholar]
- Carroll D, Phillips AC, & Lovallo WR (2012). The behavioral and health corollaries of blunted physiological reactions to acute psychological stress: Revising the reactivity hypothesis. In Wright RA & Gendolla GHE (Eds.), How motivation affects cardiovascular response: Mechanisms and applications (p. 243–263). American Psychological Association. 10.1037/13090-012 [DOI] [Google Scholar]
- Carroll D, Phillips AC, Hunt K, & Der G (2007). Symptoms of depression and cardiovascular reactions to acute psychological stress: Evidence from a population study. Biological Psychology, 75, 68–74. 10.1016/j.biopsycho.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). About the CDC-Kaiser ACE study. Centers for Disease Control and Prevention, 8. Accessed via: https://www.cdc.gov/violenceprevention/childabuseandneglect/acestudy/about.html [Google Scholar]
- Chida Y, & Steptoe A (2010). Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension, 55(4), 1026–1032. 10.1161/HYPERTENSIONAHA.109.146621 [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, & Matthews KA (2010). Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences, 1186(1), 37–55. 10.1111/j.1749-6632.2009.05334.x [DOI] [PubMed] [Google Scholar]
- Coyle DKT, Howard S, Bibbey A, Gallagher S, Whittaker AC, & Creaven A-M (2020). Personality, cardiovascular, and cortisol reactions to acute psychological stress in the Midlife in the United States (MIDUS) study. International Journal of Psychophysiology, 148, 67–74. 10.1016/j.ijpsycho.2019.11.014 [DOI] [PubMed] [Google Scholar]
- Creaven AM, Higgins NM, Ginty AT, & Gallagher S (2020). Social support, social participation, and cardiovascular reactivity to stress in the Midlife in the United States (MIDUS) study. Biological Psychology, 155, 107921. 10.1016/j.biopsycho.2020.107921 [DOI] [PubMed] [Google Scholar]
- Elley WB, & Irving JC (1976). Revised socioeconomic index for New-Zealand. New Zealand Journal of Educational Studies, 11(1), 25–36. 10.1111/obr.12073 [DOI] [Google Scholar]
- Galobardes B, Lynch JW, & Smith GD (2004). Childhood socioeconomic circumstances and causespecific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews, 26(1), 7–21. 10.1093/epirev/mxh008 [DOI] [PubMed] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Gianaros PJ, Derbyshire SW, Phillips AC, & Carroll D (2013). Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology, 50(3), 219–229. 10.1111/psyp.12017 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Masters NA, Nelson EB, Kaye KT, & Conklin SM (2017). Cardiovascular reactions to psychological stress and abuse history: The role of occurrence, frequency, and type of abuse. Anxiety, Stress, & Coping, 30(2), 155–162. 10.1080/10615806.2016.1210791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleniak C, McLaughlin KA, Ormel J, & Riese H (2016). Cardiovascular reactivity as a mechanism linking child trauma to adolescent psychopathology. Biological psychology, 120, 108–119. 10.1016/j.biopsycho.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Pulkki L, Puttonen S, Raitakari O, & Keltikangas-Järvinen L (2007). Cardiac autonomic reactivity and recovery in predicting carotid atherosclerosis: the cardiovascular risk in young Finns study. Health Psychology, 26(1), 13–21. 10.1037/0278-6133.26.1.13 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, & Salonen JT (2004). Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation, 110(15), 2198–2203. 10.1161/01.CIR.0000143840.77061.E9 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, & Manuck SB (1992). Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology, 29(1), 17–28. 10.1111/j.1469-8986.1992.tb02006.x [DOI] [PubMed] [Google Scholar]
- Kupper N, Denollet J, Widdershoven J, & Kop WJ (2015). Cardiovascular reactivity to mental stress and mortality in patients with heart failure. JACC: Heart Failure, 3(5), 373–382. 10.1016/j.jchf.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Lachman ME, & Tun PA (2008). Cognitive testing in large-scale surveys: Assessment by telephone. In Hofer SM & Alwin DF (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives (pp. 506–523). Thousand Oaks, CA, US: Sage Publications, Inc. 10.4135/9781412976589.n30 [DOI] [Google Scholar]
- Lin F, Heffner K, Mapstone M, Chen DGD, & Porsteisson A (2014). Frequency of mentally stimulating activities modifies the relationship between cardiovascular reactivity and executive function in old age. The American Journal of Geriatric Psychiatry, 22(11), 1210–1221. 10.1016/j.jagp.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB (1994). Cardiovascular reactivity in cardiovascular disease: “Once more unto the breach”. International Journal of Behavioral Medicine, 1(1), 4–31. 10.1207/s15327558ijbm0101_2 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, & Mendes WB (2014). Child maltreatment and autonomic nervous system reactivity: Identifying dysregulated stress reactivity patterns using the biopsychosocial model of challenge and threat. Psychosomatic Medicine, 76(7), 538–546. 10.1097/PSY.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on stress response system development in children. Proceedings of the National Academy of Sciences, 112, 5637–5642. 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE (2013). Childhood exposure to violence and lifelong health: Clinical intervention science and stress-biology research join forces. Development and Psychopathology, 25, 1619–1634. 10.1017/S0954579413000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali R, & Chen E (2005). Exposure to violence and cardiovascular and neuroendocrine measures in adolescents. Annals of Behavioral Medicine, 30(2), 155–163. 10.1207/s15324796abm3002_8 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon III RO, Criqui M, … & Rifai N (2003). Markers of inflammation and cardiovascular disease. Circulation, 107(3), 499–511. 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- Phillips AC (2011). Blunted cardiovascular reactivity relates to depression, obesity, and self-reported health. Biological Psychology, 86(2), 106–113. 10.1016/j.biopsycho.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Phillips AC (2016). Stress and cardiovascular reactivity. In Alvarenga ME & Byrne D (Eds.), Handbook of Psychocardiology (p. 163–177). Springer Science + Business Media. 10.1007/978-981-287-206-7_16 [DOI] [Google Scholar]
- Phillips AC, Ginty AT, & Hughes BM (2013). The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology, 90(1), 1–7. 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Poulton R, Moffitt TE, & Silva PA (2015). The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Social Psychiatry and Psychiatric Epidemiology, 50(5), 679–693. 10.1007/s00127-015-1048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, … & Danese A (2016). Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. 10.1111/jcpp.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, D’Souza S, Andersen SH, Hogan S, Houts RM, Poulton R, … & Moffitt TE. (2020). Clustering of health, crime and social-welfare inequality in 4 million citizens from two nations. Nature Human Behaviour, 4(3), 255–264. 10.1038/s41562-019-0810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, & Bogg T (2005). Conscientiousness and health across the life course. Review of General Psychology, 9(2), 156–168. 10.1037/1089-2680.9.2.156 [DOI] [Google Scholar]
- Robins LH, Helzer JE, Crottler L, Goldring E (1989). Diagnostic Interview Schedule, Version III-R. St Louis, Washington University. [Google Scholar]
- Ryff C, Almeida D, Ayanian J, Binkley N, Carr DS, Coe C, … Williams DR (2016). Midlife in the United States: Mortality Data, 2016. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-09-12. 10.3886/ICPSR37237.v2 [DOI] [Google Scholar]
- Ryff C, Almeida DM, Ayanian J, Carr DS, Cleary PD, Coe C, … Williams D (2004). Midlife in the United States (MIDUS 2), 2004-2006. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2017-11-20. 10.3886/ICPSR04652.v7 [DOI] [Google Scholar]
- Ryff CD, & Lachman ME (2006) Midlife in the United States (MIDUS 2): Cognitive Project, 2004-2006. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2017-11-17. 10.3886/ICPSR25281.v6 [DOI] [Google Scholar]
- Ryff CD, Seeman T, & Weinstein M (2004). Midlife in the United States (MIDUS 2): Biomarker Project, 2004-2009 Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2019-03-27. 10.3886/ICPSR29282.v9 [DOI] [Google Scholar]
- Salomon K, Clift A, Karlsdóttir M, & Rottenberg J (2009). Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychology, 28(2), 157–165. 10.1037/a0013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesker A (2019). Conscientiousness and cardiovascular reactivity to recurrent acute psychological stress. (Doctoral dissertation). National University of Ireland, Galway. Retrieved from Access to Research at NUI Galway. http://hdl.handle.net/10379/15001 [Google Scholar]
- Smith TW, Nealey JB, Kircher JC, & Limon JP (1997). Social determinants of cardiovascular reactivity: Effects of incentive to exert influence and evaluative threat. Psychophysiology, 34(1), 65–73. 10.1111/j.1469-8986.1997.tb02417.x [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, & Taylor T (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65(1), 46–62. 10.1097/00006842-200301000-00007 [DOI] [PubMed] [Google Scholar]
- Tun PA, & Lachman ME (2008). Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Developmental Psychology, 44(5), 1421. 10.1037/a0012845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, … & Bader K (2015). Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology, 51, 58–67. 10.1016/j.psyneuen.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008) Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Pearson Assessment. [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, … & MacLaughlin EJ (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71(19), e127–e248. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


