Abstract
It has been argued that age-related changes in the neurochemical and neurophysiological properties of the GABAergic system may underlie increases in reaction time (RT) in older adults. However, the role of GABA levels within the sensorimotor cortices (SMC) in mediating interhemispheric interactions (IHi) during the processing stage of a fast motor response, as well as how both properties explain interindividual differences in RT, are not yet fully understood.
In this study, edited magnetic resonance spectroscopy (MRS) was combined with dual-site transcranial magnetic stimulation (dsTMS) for probing GABA+ levels in bilateral SMC and task-related neurophysiological modulations in corticospinal excitability (CSE), and primary motor cortex (M1)-M1 and dorsal premotor cortex (PMd)-M1 IHi, respectively. Both CSE and IHi were assessed during the preparatory and premotor period of a delayed choice RT task. Data were collected from 25 young (aged 18–33 years) and 28 older (aged 60–74 years) healthy adults.
Our results demonstrated that older as compared to younger adults exhibited a reduced bilateral CSE suppression, as well as a reduced magnitude of long latency M1-M1 and PMd-M1 disinhibition during the preparatory period, irrespective of the direction of the IHi. Importantly, in older adults, the GABA+ levels in bilateral SMC partially accounted for task-related neurophysiological modulations as well as individual differences in RT. In contrast, in young adults, neither task-related neurophysiological modulations, nor individual differences in RT were associated with SMC GABA+ levels.
In conclusion, this study contributes to a comprehensive initial understanding of how age-related differences in neurochemical properties and neurophysiological processes are related to increases in RT.
Keywords: Aging, Interhemispheric interaction, GABA, choice reaction time, MRS, TMS
1. Introduction
Healthy aging is characterized by a progressive slowing of volitional motor actions in response to external stimuli (Jordan and Rabbit 1977; Salthouse 2000; Mattay et al., 2002; Stewart et al., 2014; Woods et al., 2015). The reduced ability to quickly react to external cues may have crucial consequences for daily life when unpredictable changes in the environment require rapid adaptive behavior, such as in multiple traffic situations (Depestele et al., 2020). Therefore, unraveling the neural mechanisms underlying age-related declines in reaction speed has important implications for promoting the functional independence and quality of life of the older population.
To estimate age-related declines in reaction speed, visually cued choice reaction time (RT) tasks have been commonly used (Salthouse 2000; Yordanova et al., 2004; Falkenstein et al., 2006; Kolev et al., 2006; Roggeveen et al., 2007; Cuypers et al., 2013; Serbruyns et al., 2015; Woods et al., 2015; Boisgontier et al., 2016; Cuypers et al., 2020). A choice RT paradigm comprises different stages of central processing, including stimulus identification, action selection, and response programming. Using transcranial magnetic stimulation (TMS), it has been shown that rapid action preparation depends on multiple inhibitory processes that operate in parallel (see Bestmann and Duque 2016; Duque et al., 2017 for reviews). These processes are assumed to play a role in the accurate timing of response initiation, in assisting action selection through a competitive process, and in the modulation of background noise to improve the signal-to-noise ratio (Quoilin and Derosiere 2015; Duque et al., 2017).
By combining choice RT tasks with single-pulse TMS over the primary motor cortex (M1), modulation of corticospinal excitability (CSE) can be probed during these different central processing stages of a motor response (e.g., Leocani et al., 2000). More specifically, the magnitude of CSE is reflected in the amplitude of the motor evoked potential (MEP), recorded with electromyographic (EMG) electrodes in the contralateral target muscle. During the delayed period of pre-cued choice RT tasks, a CSE suppression has been observed in motor representations of both the selected and non-selected effectors (Kroeger et al., 2010; Duque et al., 2014; Klein et al., 2016; Hinder et al., 2018). In addition, previous work of our group demonstrated that the respective CSE suppression was less pronounced in older than in young adults, and that this was associated with longer RTs in older adults (Cuypers et al., 2013). It has indeed been argued that age-related declines in motor function, such as increases in RT, can at least in part be explained by a poorer regulation of inhibitory motor processes with increasing age (Levin et al., 2014). However, the exact neurophysiological mechanisms and the neurochemical properties associated with a dysfunctional regulation of motor inhibition in aging, as well as their role in increased RTs, are not yet fully understood.
The principal neurotransmitter for mediating cortical motor inhibition is gamma-aminobutyric acid (GABA) (Golan et al., 1996; Levin et al., 2014). GABA has a distinct affinity for two receptor subtypes, i.e., GABAA and GABAB, and the intracortical activity of each can be assessed with specific paired-pulse TMS paradigms over M1 (Kujirai et al., 1993; Di Lazzaro et al. 1998; Paulus et al., 2008). Some studies have found associations between the capacity to modulate synaptic GABAA receptor-mediated intra-cortical inhibition and performance of a unimanual go/no-go task (Fujiyama et al., 2012) or a choice RT task (Heise et al., 2013) in older adults. Besides intracortical mechanisms, modulations in interhemispheric interactions (IHi) are also crucial for preparing fast unimanual responses (Duque et al., 2007; Kroeger et al., 2010; Liuzzi et al., 2010; Hinder et al., 2012, 2018), and for avoiding mirroring movements of the other, non-moving hand (Koch et al., 2006; Duque et al., 2007; Giovannelli et al., 2009). IHi can be assessed using dual-site TMS (dsTMS) paradigms, where a test stimulus (TS) over M1 is preceded by a conditioning stimulus (CS) over a motor-related brain area in the contralateral hemisphere (Ferbert et al., 1992; Ni et al., 2009). More specifically, IHi is quantified by the average MEP amplitude in dual-site TMS (i.e., CS + TS) trials, relative to the average MEP amplitude in single-pulse TMS (i.e., TS only) trials (i.e., ). Values > 1 indicate a facilitatory IHi, whereas values < 1 indicate an inhibitory IHi. At rest, using a subthreshold CS can evoke facilitatory IHi under specific conditions (Ferbert et al., 1992; Hanajima et al., 2001; Bäumer et al., 2006), whereas using a suprathreshold CS can evoke inhibitory IHi, which may however become facilitatory under specific task-related conditions (Reis et al., 2008; Ni et al., 2009; Levin et al., 2014). IHi using a suprathreshold CS can be typically assessed using either short latencies (s-IHi; ISI ≈ 8–10 ms) or long latencies (l-IHi; ISI ≈ 40 ms), which are thought to be mediated by separate neural pathways (Reis et al., 2008; Ni et al., 2009). Regarding the mechanism at the receptor level, neurophysiological (TMS) research suggests that both s-IHi and l-IHi are mediated by post-synaptic GABAB receptor activity (Daskalakis et al., 2002; Chen et al., 2003; Chen 2004; Kukaswadia et al., 2005). Although pharmacological studies strongly support the evidence for GABAB receptor involvement in l-IHi, results for s-IHi remain rather inconclusive, with evidence for both GABAA and GABAB receptor involvement (Kawaguchi 1992; Irlbacher et al., 2007). Both s-IHi and l-IHi predominantly employ callosal pathways (Boroojerdi et al., 1996; Wahl et al., 2007; Reis et al., 2008; Ni et al., 2009), and for l-IHi these pathways might be indirect (e.g., multi-synaptic) (Ni et al., 2009; Levin et al., 2014).
Especially in choice RT tasks wherein action selection between hands is required, modulations of IHi are expected to play a pivotal role (Maes et al., 2017). Since M1 and the dorsal premotor cortex (PMd) are considered key structures during motor preparation (Schluter et al., 1998; Rushworth et al., 2003; Cisek and Kalaska 2005; Hoshi and Tanji 2007; Kroeger et al., 2010; Hinder et al., 2012), investigating task-related IHi modulations between homologue M1s (M1-M1) and PMd-M1 is of particular interest in the context of between-hands choice RT. One study reported no effect of aging on the ability to modulate M1-M1 s-IHi and l-IHi during a simple unimanual RT task, whereas older adults exhibited a significantly greater modulation of PMd-M1 l-IHi, and to a smaller extent PMd-M1 s-IHi, than young adults (Hinder et al., 2012). Moreover, greater PMd-M1 IHi modulations in older adults predicted faster RTs, suggesting that aging might be associated with an increased reliance on the PMd to compensate for age-related slowing of RTs (Hinder et al., 2012; Stewart et al., 2014). Notably, (pre)motor-motor IHi modulations during bimanual choice RT tasks have so far only been investigated in young adults (Koch et al., 2006; O’Shea et al., 2007; Kroeger et al., 2010; Hinder et al., 2018). Moreover, to the best of the authors’ knowledge, no studies so far have used a design wherein both directions of M1-M1 and PMd-M1 s-IHi and l-IHi modulations were assessed during a choice RT task.
Whereas TMS determines phasic, synaptic GABA receptor-mediated activity, magnetic resonance spectroscopy (MRS) can reliably quantify total GABA+ (i.e., GABA with the contribution of co-edited macro-molecules) concentrations within specific brain regions in vivo (Puts and Edden 2012; Yasen et al., 2017; de Graaf 2019). Up to now, studies that aimed to identify age-related GABA+ level changes revealed mixed results. While several studies showed age-related reductions in GABA+ levels in multiple brain areas (Gao et al., 2013; Porges et al., 2017a; Chalavi et al., 2018; Hermans et al., 2018; Cassady et al., 2019; Cuypers et al., 2020), other studies failed to do so (Mooney et al., 2017; Hermans et al., 2018; Ferland et al., 2019; Cuypers et al., 2020). Furthermore, associations between GABA+ levels in motor-related areas and behavior were identified (Boy et al., 2010; Sumner et al., 2010; Stagg et al., 2011; Hermans et al., 2018). Nevertheless, whether and how local GABA+ levels and task-related phasic neurotransmission interact is still poorly understood (Levin et al., 2014). Overall, past research mainly focused on mapping associations between TMS-derived measures of intracortical synaptic GABA transmission and MRS-derived total GABA+ levels within SMC and reported either a poor or no relationship (Stagg et al., 2011; Tremblay et al., 2013; Dyke et al., 2017; Mooney et al., 2017; Hermans et al., 2018; Ferland et al., 2019; Cuypers et al., 2020, et al. 2020). Remarkably, only Hermans and colleagues investigated the association between resting-state M1-M1 IHi and GABA+ levels in the originating SMC but reported no relationship (Hermans et al., 2018). In this respect, it is noteworthy that IHi is thought to be mediated by excitatory glutamatergic projections that cross the corpus callosum and synapse with local GABAergic interneurons in the contralateral target M1 (Daskalakis et al., 2002; Chen 2004; Irlbacher et al., 2007; Lee et al., 2007; Reis et al., 2008; Palmer et al., 2012). However, to the best of our knowledge, the association between ‘task-related’ IHi modulations and GABA+ levels in the target SMC has not been investigated to date. In sum, a bimodal approach combining both MRS and TMS-derived measures for IHi during planning/performance of a motor task should deliver a complementary and more comprehensive understanding of how these neurochemical properties and neurophysiological processes predict human motor behavior (Maes et al., 2017; Cuypers et al., 2018; Cuypers and Marsman 2021).
The goals of the present study were threefold: (1) to investigate how TMS-assessed CSE modulations and bidirectional M1-M1 and PMd-M1 IHi modulations during a bimanual choice RT as well as MRS-assessed GABA+ levels in SMC are affected by aging; (2) to explore whether and how task-related TMS measures are related to SMC GABA+ levels; and (3) to identify to what extent SMC GABA+ levels, CSE modulations, and (pre)motor-motor IHi modulations can predict RTs. We hypothesized reduced SMC GABA+ levels in older as compared to young adults, which might be related to altered task-related CSE and (pre)motor-motor IHi modulations as well as increases in RT during a bimanual choice RT task. We also expected that, within the older adults group, an increase in PMd-M1 IHi modulation would predict faster RTs.
2. Methods and materials
2.1. Participants
Twenty-five healthy young adults [aged 18–33 years, 22.08 ± 4.40 (mean ± SD); 12 males] and twenty-eight healthy older adults [aged 60–74 years, 67.29 ± 4.16 (mean ± SD); 12 males] participated in this study. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971) [laterality quotient: 91.16 ± 12.39 (mean ± SD)], reported no history of neurological, psychiatric, cardio-vascular, or neuromuscular disorders, were free of psychoactive (e.g., anti-depressants, -psychotics, -epileptics, sedatives, etc.) medications, and had normal or corrected-to-normal vision. The experimental protocol was approved by the Ethics Committee Research UZ/KU Leuven (project S58333) and was conducted according to the Declaration of Helsinki (1964) and its amendments (World Medical Association 2013). Prior to participation, all participants provided written informed consent and were screened for TMS and magnetic resonance imaging contraindications.
2.2. Experimental design
The study consisted of one MRS scanning session, followed by two TMS sessions. The time between the two TMS sessions was 7 days (median) with an interquartile range of 4 to 11 days. During the MRS session, a structural T1-weighted image was obtained from each participant and GABA+ levels were measured in bilateral SMC using GABA-edited MRS. In the two subsequent sessions, a choice RT task was combined with a dsTMS procedure (Ferbert et al., 1992; Mochizuki et al., 2004; Koch et al., 2006; Ni et al., 2009; Fujiyama et al., 2016a), to assess task-related modulations in M1-M1 and PMd-M1 IHi. Within one TMS session, either M1-M1 or PMd-M1 IHi modulations were examined both from left to right and right to left hemisphere. The two TMS sessions were performed on two different days with a minimum of 48 h between them and their order was counterbalanced across participants.
2.3. Magnetic resonance spectroscopy (MRS)
Scanning was performed on a Philips 3T Achieva MR scanner (Philips Healthcare, The Netherlands) with a 32-channel receiver head coil. The imaging protocol consisted of a high-resolution 3D magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted structural image (TR = 9.6 ms; TE = 4.6 ms; voxel size = 0.98 × 0.98 × 1.20 mm3; field of view = 256 × 256 mm2; 160 sagittal slices; flip angle = 8°) that was obtained from each participant prior to the acquisition of MRS data. GABA-edited MRS data were acquired using the MEGA-PRESS spectral editing method (Mescher et al., 1998): 14-ms editing pulses at 1.9 (edit-ON) and 7.46 (edit-OFF) ppm; TR = 2000 ms; TE = 68 ms; 320 averages; 2048 points; 2 kHz spectral width; MOIST water suppression. Sixteen water-unsuppressed averages were also automatically acquired with each voxel acquisition. The water signal was used as an internal concentration reference. The left and right SMC voxels (30 × 30 × 30 mm3) were placed over the hand knob area (Yousry et al., 1997), parallel to the anterior-posterior axis. The MATLAB-based (R2019b, The MathWorks Inc., Natick, MA, 2000) Gannet (version 3.1.5) software toolkit (https://markmikkelsen.github.io/Gannet-docs/index.html) (Edden et al., 2014) was used for offline data processing and GABA+ quantification. Processing steps and parameter selection were described in detail in (Cuypers et al., 2020). Tissue fractions (grey matter, white matter and cerebrospinal fluid) were obtained for each SMC voxel using SPM12 (Ashburner and Friston 2005). These tissue fractions were used to determine tissue-corrected GABA+ levels (in institutional units, i.u.) for each voxel. The alpha correction from Harris et al. (2015) was applied to account for the fact that there is about twice as much GABA in grey matter than in white matter (Mikkelsen et al., 2016). Additionally, the alpha-corrected GABA+ levels were normalised to the average voxel tissue composition of each age group (see Harris et al., 2015, Eq. 6). The group averages of tissue compositions and the fit error of the fitted GABA+ model, for both SMC voxels in both young and older adults are reported in Table 1.
Table 1.
Tissue fractions and fit error (%) of the fitted GABA+ model (mean ± SD) of the left (dominant) and right (non-dominant) SMC voxels for both young and older adults. Significant p-values (group comparison) are printed in bold. A parametric unpaired t-test was used when the data of each group were normally distributed, whereas a non-parametric Wilcoxon Rank Sum test was used when the respective normality assumption was not met.
| DOMINANT (LEFT) SMC | NON-DOMINANT (RIGHT) SMC | |||||
|---|---|---|---|---|---|---|
| YOUNG | OLDER | p-value | YOUNG | OLDER | p-value | |
| GREY MATTER | 0.337 ± 0.028 | 0.256 ± 0.030 | <0.001 | 0.356 ± 0.032 | 0.268 ± 0.031 | <0.001 |
| WHITE MATTER | 0.603 ± 0.036 | 0.625 ± 0.041 | 0.022 | 0.572 ± 0.034 | 0.600 ± 0.039 | 0.010 |
| CEREBROSPINAL FLUID | 0.060 ± 0.016 | 0.119 ± 0.034 | <0.001 | 0.072 ± 0.019 | 0.131 ± 0.031 | <0.001 |
| FIT ERROR (%) | 4.146 ± 0.764 | 4.470 ± 1.393 | 0.901 | 4.437 ± 1.316 | 4.732 ± 1.299 | 0.392 |
2.4. Transcranial magnetic stimulation (TMS) and electromyographic (EMG) recording
2.4.1. TMS conditions
Five different TMS measures were acquired: CSE, M1-M1 s-IHi, M1-M1 l-IHi, PMd-M1 s-IHi and PMd-M1 l-IHi. These measures were assessed at three different time points during a choice RT trial (see Fig. 1 A and section “Experimental protocol” for a detailed description of these time points). CSE was assessed using single-pulse TMS (i.e., TS only) over M1 in both hemispheres. For the assessment of M1-M1 IHi and PMd-M1 IHi, a dsTMS procedure was used, wherein a CS on M1 or PMd in the originating hemisphere preceded a TS on M1 in the target hemisphere at three different ISIs: 10 ms (M1-M1 s-IHi), 8 ms (PMd-M1 s-IHi) and 40 ms (M1-M1 l-IHi and PMd-M1 l-IHi) (Mochizuki et al., 2004; Koch et al., 2006; O’Shea et al., 2007; Ni et al., 2009; Kroeger et al., 2010; Hinder et al., 2012; Fujiyama et al., 2016a). The CS and TS were delivered with two figure-of-eight coils (50 mm inner diameter of each wing), connected to a Magstim BiStim2 stimulator (Whitland, Dyfed, UK). IHi was examined in both directions: non-dominant M1 to dominant M1 (M1ND − M1D IHi); dominant M1 to non-dominant M1 (M1D−M1ND IHi); non-dominant PMd to dominant M1 (PMdND−M1D IHi); or dominant PMd to non-dominant M1 (PMdD−M1ND IHi); see Fig. 1 B.
Fig. 1.
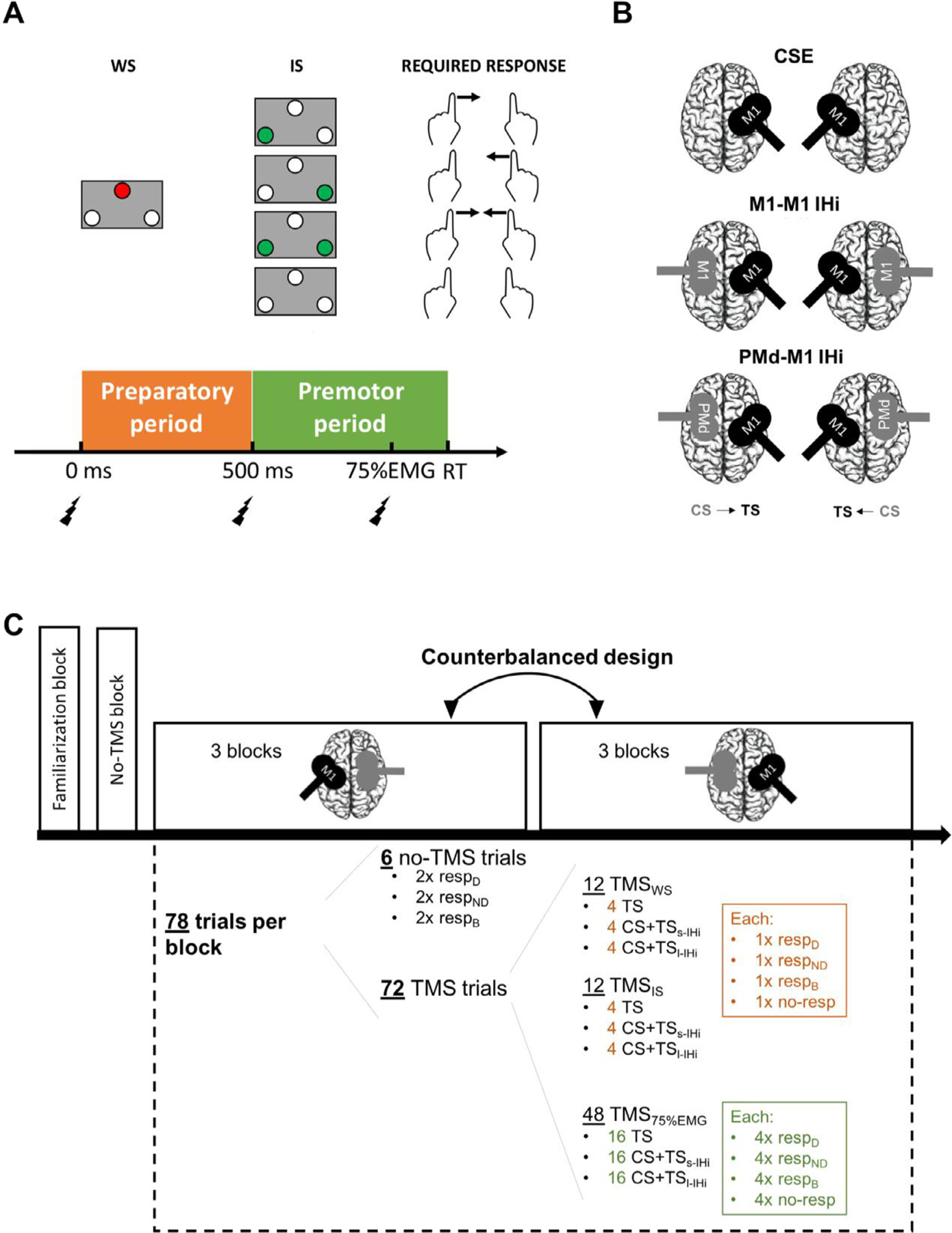
Experimental TMS protocol for investigating corticospinal excitability (CSE), and interhemispheric interactions (IHi) between homologous primary motor cortices (M1-M1) and the dorsal premotor cortex (PMd) and M1 during a choice reaction time (RT) task: (A) Trial design. At the start of each trial, a warning signal (WS, 500 ms duration) was displayed, followed by an imperative signal (IS, 1000 ms duration) indicating that either a left, right, bimanual, or no response [indicated by the green LED(s) and the corresponding arrow(s)] was required. The test stimulus (TS) was delivered either at the WS, IS, or at 75% of the time between the IS and the EMG onset (75%EMG); (B) Coil positioning and orientation for the different TMS conditions. The conditioning stimulus (CS) coil is visualized in grey and the TS coil in black; (C) Experimental protocol during a TMS session. In one session, the CS was applied on either the PMd or M1. Following the no-TMS block, there were six blocks with TMS. In three consecutive TMS blocks, IHi was measured from the dominant to the non-dominant hemisphere, whereas in the other three consecutive TMS blocks, the other direction was measured. Each block consisted of 78 trials, from which 72 with TMS. The order of all trials within a block was randomised. Abbreviations: s-IHi, short latency interhemispheric interaction; l-IHi, long latency interhemispheric interaction; resp, response; D, dominant hand; ND, non-dominant hand; B, bilateral hand.
To define the M1 “hotspot ” for TS delivery for each participant, an orthogonal 1 × 1 cm2 coordinate grid was marked on a swimming cap with references to anatomical landmarks (i.e., left and right external auditory meatus, nasion, inion, and vertex). The hotspot was then defined as the scalp location from which 5 consecutive stimuli produced the highest and most consistent mean motor evoked potential (MEP) in the contralateral relaxed first dorsal interosseus (FDI) muscle. The stimulation intensity for the TS was set to the stimulator output necessary to evoke a MEP of ~1 mV peak-to-peak amplitude in the resting FDI muscle. The coil was rotated 45° lateral from the midline with the handle pointing posteriorly (Fig. 1 B) to induce a posterior-anterior electrical current in M1. The CS over M1 was delivered over the M1 hotspot, whereas PMd localization was defined based on a method similar to the one used in Bäumer et al., 2006; Koch et al., 2006; and Kroeger et al., 2010. Specifically, PMd was located at 8% of the nasion-inion distance anterior to the ipsilateral M1 hotspot. For the CS over either M1 or PMd, the coil was orientated perpendicular to the midline with the handle pointing laterally, to induce a latero-medial current in the brain (Fig. 1 B) (Ni et al., 2009). For all dsTMS conditions, CS intensity was set to 110% of the individual resting motor threshold (rMT) (Koch et al., 2006; Kroeger et al., 2010; Hinder et al., 2012; Fujiyama et al., 2016a). The rMT was defined as the lowest stimulation intensity required to elicit MEPs with an amplitude larger than 50 μV peak-to-peak in the resting FDI muscle in at least 5 of 10 consecutive trials when stimulating the hotspot (Rossini et al., 1994). Mean rMTs for both TMS sessions, hemispheres and age groups are summarized in Table 2.
Table 2.
Average resting motor threshold (rMT) (mean ± SD) of the left (dominant) and right (non-dominant) hemisphere for each age group (young and older adults), for each TMS session (1 and 2). Values are expressed in % of maximal stimulator output (MSO).
| rMT (% MSO) | DOMINANT (LEFT) | NON-DOMINANT (RIGHT) | ||
|---|---|---|---|---|
| YOUNG | OLDER | YOUNG | OLDER | |
| SESSION 1 | 52.48 ± 7.59 | 52.96 ± 9.91 | 52.84 ± 8.26 | 53.72 ± 9.66 |
| SESSION 2 | 51.72 ± 8.01 | 53.72 ± 11.22 | 53.08 ± 8.60 | 55.64 ± 9.80 |
To enable a precise online monitoring of the stimulated areas, the high resolution T1-weighted anatomical image obtained from each participant in the MRI session was entered in a neuronavigation system to guide TMS (Brainsight, Rogue Research Inc, Montreal, Quebec, Canada). The mean MNI (Montreal Neurological Institute) coordinates corresponding to the stimulation sites on the scalp are summarized in Table 3.
Table 3.
Average XYZ coordinates in MNI space (mean ± SD) corresponding to the TMS locations on the scalp over M1 and PMd in the left (dominant) and right (non-dominant) hemisphere for each age group (young and older adults).
| M1 | DOMINANT (LEFT) | NON-DOMINANT (RIGHT) | ||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| YOUNG | −48.30 ± 4.74 | −3.00 ± 7.71 | 71.55 ± 5.76 | 45.17 ± 5.91 | −8.05 ± 8.19 | 72.62 ± 5.30 |
| OLDER | −46.10 ± 7.57 | 1.13 ± 11.66 | 70.81 ± 5.31 | 43.34 ± 5.51 | −3.58 ± 12.99 | 71.62 ± 6.74 |
| PMd | X | Y | Z | X | Y | Z |
| YOUNG | −44.60 ± 4.70 | 24.21 ± 7.16 | 64.49 ± 6.20 | 45.28 ± 5.52 | 21.38 ± 9.74 | 63.77 ± 6.34 |
| OLDER | −43.50 ± 7.30 | 28.66 ± 10.17 | 61.48 ± 6.46 | 43.30 ± 5.14 | 26.03 ± 10.79 | 62.18 ± 6.41 |
2.4.2. EMG recording
EMG signals from the left and right FDI muscles were continuously monitored using EMG (Bagnoli-16, Delsys Inc, Boston, USA). To further ensure that the hand musculature was relaxed, the abductor digiti minimi muscle of both hands was monitored as well. The raw EMG signals were amplified (gain = 1000), filtered (band-pass 4–1500 Hz), eliminated for 50/60 Hz noise (Humbug, Quest Scientific, North Vancouver, Canada), digitized at 5000 Hz (CED Signal Version 4.03, Cambridge Electronic Design, Cambridge, UK), and stored on a laboratory computer for offline analysis.
2.5. Choice RT task
A pre-cued, bimanual choice RT task was used (Cuypers et al., 2020). Participants were seated on a chair with both forearms pronated on a platform, consisting of two pairs of contact switches: i.e., two home button switches (Honeywell V-7–2B17D8–162, operating force 0.10 N, Honeywell, Charlotte, USA) and two target switches (Omron Electronic Components D2FS-FL-N-T, operating force 0.25 N, Omron, Osaka, Japan). A signaling apparatus was positioned at eye level 1 m in front of the participant and consisted of a red light-emitting diode (LED) centrally at the top and two green LEDs at the bottom right and left corner, as illustrated in Fig. 1A. The red LED was used to display a neutral warning signal (WS), whereas the right and left green LEDs were used to display the imperative signal (IS) for either a dominant (right) index finger response; a non-dominant (left) index finger response; a bilateral index finger response; or no response.
Participants had their index fingers resting on their respective home button switch. At the beginning of each trial, the WS was displayed for 500 ms (“preparatory period ”). Then, the WS was switched off and the IS was presented immediately for 1000 ms. According to the displayed IS, participants were instructed to react as fast as possible by abducting their dominant and/or non-dominant index finger(s) towards their respective target switch(es). After each trial, participants were instructed to reposition their finger(s) on the home button(s). In “no-response ” trials, during which the WS was not followed by any green light, participants were instructed to refrain from moving their index fingers. RT was defined as the time between IS onset and release of the home button switch. This period is referred to as the “premotor period ”. The intertrial interval (i.e., time between two WSs) varied randomly between 4 and 6 s.
2.6. Experimental protocol
The course of a TMS session is illustrated in Fig. 1C. In the first TMS session only, a familiarization block of 40 choice RT trials without TMS (12 dominant response trials, 12 non-dominant response trials, 12 bimanual response trials, and 4 no-response trials in random order) was implemented. Before starting the main experiment in both TMS sessions, all participants performed an identical “no-TMS block ”, to calculate the average time between the IS and EMG onset in the responding muscle for each participant, required to set the TMS timing for stimulating at approximately 75% of the EMG onset.
After this no-TMS block, the main experiment started in which the choice RT task was combined with TMS to examine task-related modulations in CSE and IHi. The main experiment of each session consisted of six blocks. In three consecutive blocks, IHi was measured from the dominant to the non-dominant hemisphere, while in the three other consecutive blocks, IHi was measured from the non-dominant to the dominant hemisphere. The order of the direction of IHi measurement was counterbalanced across participants of each age group.
Each experimental block consisted of 78 choice RT trials that were presented in a random order, of which 72 trials were performed with TMS. During a trial with TMS, the time point of TS delivery was either at the onset of WS, at the onset of the IS, or at 75% of the expected time for voluntary EMG onset in the responding FDI muscle (referred to as time point 75%EMG); see Fig. 1 A. The first time point (WS) served as a baseline measurement. The second time point (IS) was chosen because CSE suppression is expected to become most prominent in anticipation of the IS (e.g., Bestmann and Duque 2016; Lebon et al., 2016). Finally, the third time point (75%EMG) was selected since it was expected that CSE facilitation (in case that the targeted FDI was selected for movement, either unimanually or bimanually) or inhibition (in case that the targeted FDI was not selected for movement, either during a unimanual response of the non-targeted FDI or during no response) would reach its peak around this time point (e.g., Cuypers et al., 2013). Specifically, the 72 trials with TMS consisted of 12 TMS trials delivered at time point WS; 12 TMS trials at time point IS; and 48 trials at time point 75%EMG. The 12 TMS trials at time point WS and IS each consisted of 4 (always consisting of a dominant, non-dominant, bimanual, and no-response trial) TS only trials; 4 CS + TS trials for s-IHi assessment; and 4 CS + TS trials for l-IHi assessment. The 48 trials at time point 75%EMG consisted of 16 (always consisting of 4 dominant, 4 non-dominant, 4 bimanual, and 4 no-response trials) TS only trials; 16 CS + TS trials for s-IHi assessment; and 16 CS + TS trials for l-IHi assessment. Finally, the remaining 6 trials of each experimental block (i.e., 2 dominant, 2 non-dominant, and 2 bimanual response trials) were performed without TMS delivery. Trials without TMS were included in order to obtain an unbiased measure for RT as it has been shown that TMS delivery prior to the onset of voluntary movement can influence RT (Pascual-Leone et al., 1992; Leocani et al., 2000).
2.7. Data processing
From the total of 28 included older adults in this study, two older adults did not start the TMS sessions since they had a rMT > 80%. Therefore, RT data were collected from 26 older adults [aged 60–74 years, 67.42 ± 4.29 (mean ± SD); 11 males]. RTs were calculated from the trials without TMS, as the TMS pulse can influence RT (Pascual-Leone et al., 1992). Trials with erroneous or premature responses, and trials with RTs exceeding 1400 ms were discarded. These criteria resulted in an elimination of 6.72 ± 11.37% (mean ± SD) and 10.56 ± 8.43% (mean ± SD) of the RT data for young and older adults, respectively.
For TMS analysis, one older adult exhibited excessive muscle activity prior to TS delivery in more than 80% of the choice RT trials and was therefore excluded from TMS data analysis. Therefore, the sample of older adults for the TMS data analysis comprised 25 participants [aged 60–74 years, 67.48 ± 4.37 (mean ± SD); 11 males]. MEPs were excluded from analysis in case of (1) either premature or incorrect response to the choice RT task; (2) if they occurred after the onset of voluntary EMG activity in the FDI or ADM muscle; (3) if they did not appear in a window 10–50 ms after the onset of TMS; (4) if the root mean square of the EMG signal in at least one of the four monitored muscles exceeded 20 μV during the 50 ms period immediately preceding the onset of the TS (i.e., high background EMG) (see also Cuypers et al., 2020). In total, 8.67 ± 10.98% (mean ± SD) of all MEPs were excluded for the young adults and 13.72 ± 8.64% (mean ± SD) for older adults (p = 0.004, Wilcoxon Rank Sum test).
CSE was assessed in terms of the average peak-to-peak MEP amplitude in the target FDI muscle in TS only trials. MEPs at WS and IS were averaged across all response trials and for each participant. MEPs at the expected time point of 75%EMG in the premotor period were averaged for each participant across all trials of the same required response (i.e., dominant response, non-dominant response, bimanual response, or no response). CSE modulations in the preparatory and the premotor period were calculated using the following equations: (1) for CSE modulation in the preparatory period; and (2) for CSE modulation in the premotor period. Here, values > 1 indicate an increase of CSE from WS to IS or from IS to the end of the premotor period (i.e., a relative CSE facilitation of the FDI muscle) whereas values < 1 indicate a decrease of CSE from WS to IS or from IS to the end of premotor period (i.e., a relative CSE suppression).
As for CSE, IHi measured at WS and IS was averaged across all trials and for each participant; and IHi at time point 75%EMG was averaged for each participant across all trials of the same required responses. IHi modulations in the preparatory and premotor period were calculated using the following equations: (1) for IHi modulation in the preparatory period; and (2) for IHi modulation in the premotor period. Values > 1 indicate a relative disinhibitory/facilitatory IHi modulation from WS to IS or from IS to the end of the premotor period, whereas values < 1 indicate a relative inhibitory IHi modulation from WS to IS or from IS to the end of premotor period.
2.8. Statistical analyses
The statistical software RStudio (version 1.3.959, RStudio Team 2020) was used to perform all statistical analyses.
2.8.1. Aging effects
Aging effects were analysed using linear mixed effects models (nlme package, version 3.1–131, Pinheiro et al., 2017), with SUBJECT added in each model as a random intercept, to account for repeated measures within one subject. The original models included all 2-way and 3-way interaction effects, which were then simplified by stepwise model building (i.e., removing stepwise non-significant interaction effects). Significant interaction and/or main effects in the final models were further explored using Tukey HSD post hoc pairwise comparisons, which control for multiple comparisons (emmeans package, version 1.3.0, Lenth 2018). The level of significance was set at α < 0.05.
2.8.1.1. Choice RT performance.
RT was analysed by a 2 [AGE: Young vs. Older] × 3 [RESPONSE: non-dominant vs. dominant vs. bimanual] linear mixed model, with AGE and RESPONSE as fixed effects.
2.8.1.2. Task-related TMS measures.
For analysing task-related modulations in CSE, M1-M1 and PMd-M1 IHi, the factor TARGET HEMISPHERE was added. TARGET HEMISPHERE has two levels (“dominant ” versus “non-dominant ”) and is defined as the hemisphere of TS delivery. For CSE, there was no difference in task-related MEP sizes between the two TMS sessions (p > 0.42). Therefore, the CSE data of both sessions were collapsed to increase the statistical power of the further analyses.
CSENORM(prep) was analysed by a 2 [AGE: Young vs. Older] × 2 [TARGET HEMISPHERE: dominant vs. non-dominant] linear mixed model, with AGE and TARGET HEMISPHERE as fixed effects. For analysing M1-M1 IHiNORM(prep) and PMd-M1 IHiNORM(prep), we additionally added the fixed effect IHi TYPE (2 levels: s-IHi vs. l-IHi) to differentiate between the two types of IHi assessment.
CSENORM(prem) was analysed by a 2 [AGE: Young vs. Older] × 2 [TARGET HEMISPHERE: dominant vs. non-dominant] × 4 [HAND ACTION: selectedunimanual response vs. non-selectedunimanual response vs. bimanual vs. no response] linear mixed model, with AGE, TARGET HEMISPHERE and HAND ACTION added as fixed effects. Similarly, we analysed M1-M1 IHiNORM(prem) and PMd-M1 IHiNORM(prem), with the fixed effect IHi TYPE [s-IHi vs. l-IHi] added into the model.
Direct comparisons of the absolute levels of CSE, M1-M1 s-IHi, M1-M1 l-IHi, PMd-M1 s-IHi, and PMd-M1 l-IHi at WS and IS between age groups are provided in the appendix (see Tables A.1 and A.2).
2.8.1.3. GABA+ levels.
GABA+ levels in bilateral SMC were analysed using a 2 [AGE: Young vs. Older] × 2 [VOXEL: SMCD vs. SMCND] linear mixed model, with AGE and VOXEL implemented as fixed effects.
2.8.2. Associations between SMC GABA+ levels and task-related TMS measures
Exploratory correlational analyses were performed to investigate the correlation between task-related TMS measures and GABA+ levels in the target SMC. If the assumption of normality of the data (Shapiro-Wilk test) was met, Pearson’s r correlations were calculated. Otherwise, Spearman’s ρ correlations were used. All correlations were clustered per family of tests (i.e., by age group and TMS measure). A false discovery rate (FDR) correction method (Benjamini and Hochberg 1995) was applied to correct for multiple testing within each cluster.
2.8.3. Predicting RT
Multiple linear regression models (stats package, version 3.4.1, R Core Team 2017) were built for predicting RT of a unimanual dominant hand and non-dominant hand response, for young and older adults separately. A step-wise selection procedure was used, employing a combined forward-backward F–test-based selection criterion. For controlling multi-collinearity, Variance Inflation Factor (VIF) scores were calculated and were considered problematic if > 10. For the dominant hand RT model, the included predictor candidates were TMS-derived CSENORM(prep),D, M1ND−M1D s-IHiNORM(prep), M1ND−M1D l-IHiNORM(prep), PMdND−M1D s-IHiNORM(prep) and PMdND−M1D l-IHiNORM(prep) during the preparatory period; CSENORM(prem),D, M1ND−M1D s-IHiNORM(prem), M1ND−M1D l-IHiNORM(prem), PMdND−M1D s-IHiNORM(prem) and PMdND−M1D l-IHiNORM(prem) during the premotor period in the trials with a unimanual selection of the dominant FDI; and MRS-derived SMCD GABA+ and SMCND GABA+. For non-dominant hand RT prediction, the respective predictor candidates were CSENORM(prep),ND, M1D−M1ND s-IHiNORM(prep), M1D−M1ND l-IHiNORM(prep), PMdD−M1ND s-IHiNORM(prep) and PMdD−M1ND l-IHiNORM(prep) during the preparatory period; CSENORM(prem),ND, M1D−M1ND s-IHiNORM(prem), M1D−M1ND l-IHiNORM(prem), PMdD−M1ND s-IHiNORM(prem) and PMdD−M1ND l-IHiNORM(prem) during the premotor period in the trials with a unimanual selection of the non-dominant FDI; SMCND GABA+ and SMCD GABA+.
3. Results
3.1. Aging effects
3.1.1. Choice RT performance
Fig. 2 displays the performance by age group and response condition. There was a significant AGE × RESPONSE interaction effect (F(2,98) = 3.23, p = 0.04), indicating that the effect of RESPONSE on RT differed between age groups. Post hoc Tukey contrasts yielded that for all response conditions, older adults (mean RT ± SD = 419 ± 63 ms) were slower than young adults (mean RT ± SD = 334 ± 67 ms, all p < 0.004). Only in older adults, RTs for a bimanual response (mean RT ± SD = 407 ± 58 ms) were faster than RTs for a non-dominant hand response (mean RT ± SD = 431 ± 69 ms; t(96) = −3.45, p = 0.01). All other pairwise comparisons between response conditions within an age group yielded no significant differences (all p > 0.51).
Fig. 2.
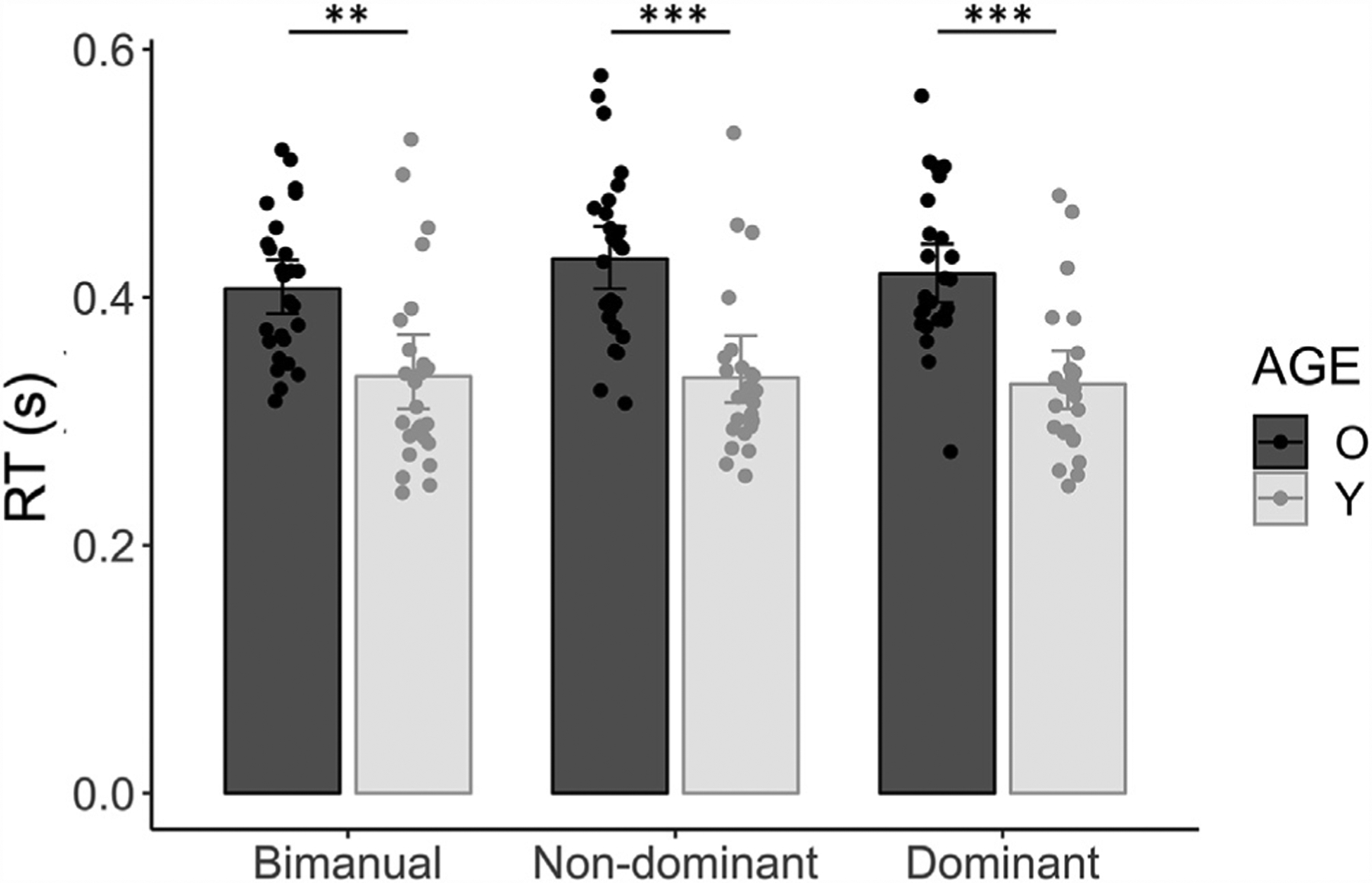
Choice RT task performance by AGE and RESPONSE. Mean RTs (s) are plotted for the bimanual, the non-dominant, and the dominant FDI response, for younger (light grey bars) and older adults (dark grey bars). Error bars indicate 95% CIs. Significant Tukey-corrected pairwise comparisons between age groups within each response condition are indicated by asterisks. Abbreviations: RT, Reaction Time; O, Older adults; Y, Young adults; **, p < 0.01; ***, p < 0.001.
3.1.2. Task-related TMS measures
3.1.2.1. CSE modulations.
Results for CSENORM during the preparatory and premotor period are illustrated in Fig. 3 A.
Fig. 3.
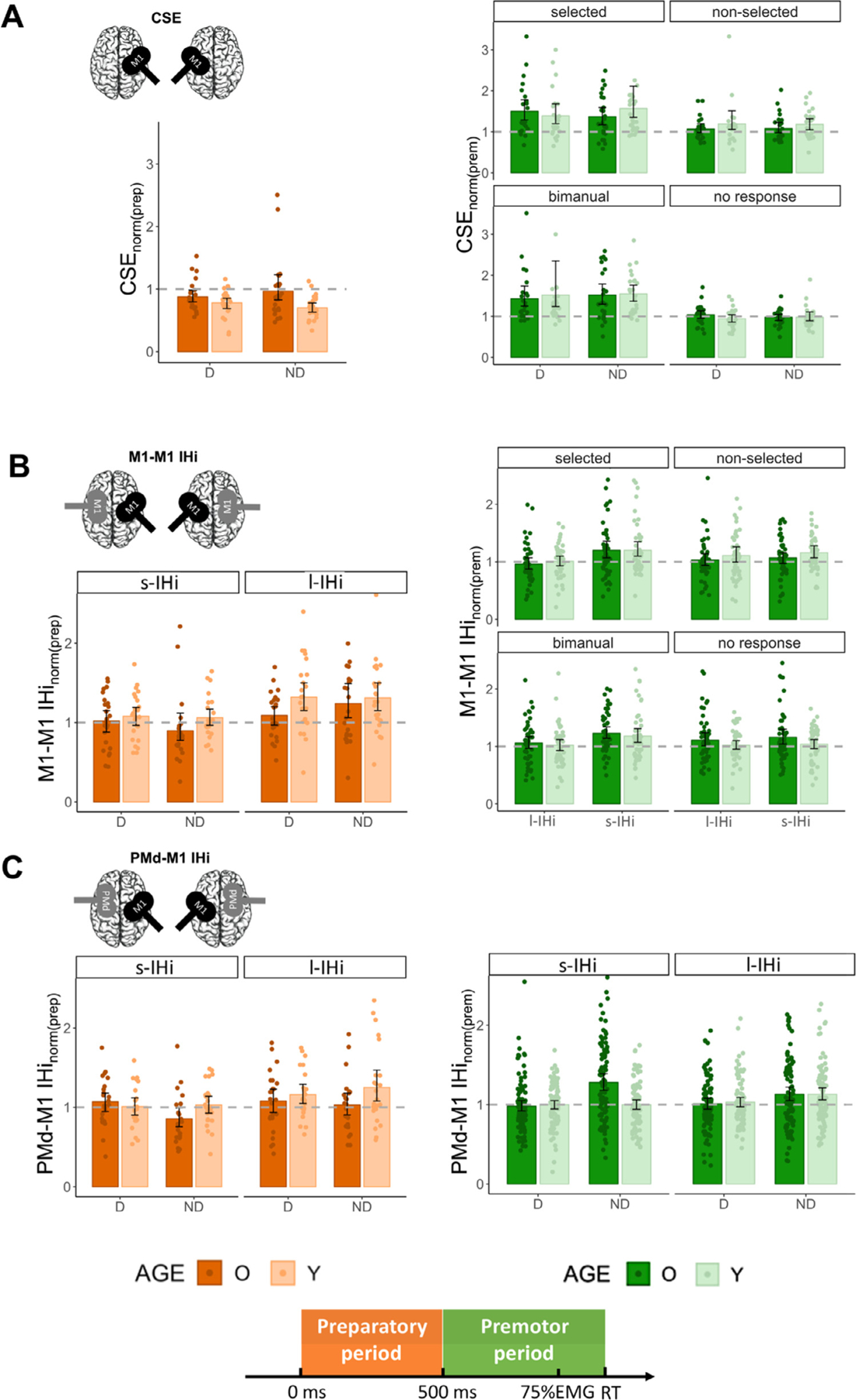
Aging effects on task-related TMS measures: (A) Corticospinal excitability modulation (CSENORM); (B) M1-M1 IHi modulation (M1-M1 IHiNORM); and (C) PMd-M1 IHi modulation (PMd-M1 IHiNORM). The orange bars and green bars represent modulation during the preparatory period and premotor period , respectively. For the preparatory period, results are presented for each target hemisphere and type of IHi (B, C). For the premotor period, an additional distinction is made between hand actions. Note that for M1-M1 IHiNORM(prem), data are collapsed for the factor TARGET HEMISPHERE, since there were no significant (interaction) effects with/of this factor. Note that for the PMd-M1 IHiNORM(prem), the data are collapsed for the factor HAND ACTION, since there were no significant (interaction) effects with/of this factor. Values below the dashed line indicate an inhibitory modulation relative to TMSWS (for modulation during the preparatory period) or TMSIS (for modulation during the premotor period), whereas values above the dashed line indicate a relative facilitatory/disinhibitory modulation. Error bars represent 95% CIs. Abbreviations: D, Dominant; ND, Non-Dominant; O, Older, Y, Young; PMd; dorsal premotor cortex; M1; primary motor cortex; IHi, interhemispheric interaction; s-IHi, short latency interhemispheric interaction; l-IHi, long latency interhemispheric interaction.
For CSE modulation during the preparatory period, the AGE × TARGET HEMISPHERE interaction just failed to reach significance (F(1,48) = 3.76, p = 0.06). There was a significant main effect of AGE (F(1,48) = 6.08, p = 0.02), with significantly lower CSENORM(prep) in young adults (mean ± SD = 0.74 ± 0.20) than in older adults (mean ± SD = 0.92 ± 0.38). An exploratory post hoc test of the AGE × TARGET HEMISPHERE interaction factor suggested that the main effect of AGE was mainly driven by age differences of CSENORM in the non-dominant hemisphere (t(48) = 3.11, p = 0.003), rather than in the dominant hemisphere (t(48) = 1.12, p = 0.27, and see Fig. 3 A). There was no significant main effect of TARGET HEMISPHERE (F(1,49) = 0.03, p = 0.87).
The model for CSE modulation during the premotor period only yielded a main effect of HAND ACTION (F(3346) = 29.72, p < 0.001). Post hoc Tukey contrasts yielded significantly higher CSENORM(prem) when the target FDI was selected for movement either during a unimanual (mean ± SD = 1.46 ± 0.65) or bimanual response (mean ± SD = 1.50 ± 0.74), than when the target FDI was required not to move either in the unimanual (“non-selectedunimanual response”, mean ± SD = 1.13 ± 0.37) or “no response ” condition (mean ± SD = 0.99 ± 0.24, all p < 0.001), irrespective of age group and targeted hemisphere. The remaining two contrasts yielded no significant differences (both p > 0.12). All other main and interaction effects were not significant (all p > 0.36).
In sum, the results indicated a reduced bilateral CSE suppression in older, as compared to young adults during the preparatory period. In the premotor period, the facilitatory CSE modulation in anticipation of a unimanual (selected) or bimanual response did not differ between age groups and hemispheres.
3.1.2.2. M1-M1 IHi modulations.
M1-M1 IHiNORM results during the preparatory and premotor period are presented in Fig. 3 B.
The model for M1-M1 IHi modulation during the preparatory period yielded two significant main effects. The main effect of AGE (F(1,48) = 5.03, p = 0.03) indicated higher IHiNORM(prep) in young adults (mean ± SD = 1.19 ± 0.39) as compared to older adults (mean ± SD = 1.06 ± 0.41), irrespective of TARGET HEMISPHERE and IHi TYPE. An additional exploratory analysis revealed that age differences in M1-M1 IHiNORM were the largest for M1ND−M1D l-IHi (t(48) = −2.15, p = 0.03, and see Fig. 3B). The main effect of IHi TYPE (F(1140) = 17.21, p < 0.001) showed higher IHiNORM(prep) values for l-IHi (mean ± SD = 1.24 ± 0.44) as compared to s-IHi (mean ± SD = 1.01 ± 0.33). Neither the main effect of TARGET HEMISPHERE, nor the interaction effects were significant (all p > 0.19).
The model for M1-M1 IHi modulation during the premotor period yielded two significant interaction effects. The AGE × HAND ACTION interaction effect (F(3704) = 2.78, p = 0.04) showed that the age effect was task-dependent. However, all post hoc pairwise Tukey-corrected comparisons yielded no further differences between the combined (AGE × HAND ACTION) groups (all p > 0.45). Post hoc tests for the significant HAND ACTION × IHi TYPE interaction effect (F(3704) = 2.99, p = 0.03) indicated, irrespective of AGE and TARGET HEMISPHERE, higher IHiNORM(prem) values for s-IHi (mean ± SD = 1.20 ± 0.48) than for l-IHi (mean ± SD = 0.99 ± 0.32) when the targeted FDI was selected for unimanual movement (t(704) = −4.15, p = 0.001). Similarly, higher IHiNORM(prem) values for s-IHi (mean ± SD = 1.21 ± 0.39) than for l-IHi (mean ± SD = 1.04 ± 0.36) were observed during a bimanual response (t(704) = −3.12, p = 0.04). All other interaction effects and the main effect of TARGET HEMISPHERE were not significant (all p > 0.08).
To summarize, during the preparatory period, bidirectional disinhibitory M1-M1 IHi modulations were more prominent for l-IHi as compared to s-IHi. In older adults, the magnitude of this disinhibitory modulation was lower than in young adults. In contrast, irrespective of age, bidirectional disinhibitory IHi modulations during the premotor period were more prominent for s-IHi, as compared to l-IHi, when a unimanual (selected) or bimanual response was required.
3.1.2.3. PMd-M1 IHi modulations.
Fig. 3 C illustrates the task-related PMd-M1 IHi modulations during the preparatory and premotor period.
For PMd-M1 IHi modulation during the preparatory period, the main effect of IHi TYPE (F(1140) = 9.61, p = 0.002) showed significantly higher IHiNORM(prep) values for l-IHi (mean ± SD = 1.13 ± 0.39) than for s-IHi (mean ± SD = 0.99 ± 0.29), irrespective of AGE and TARGET HEMISPHERE. Age differences in PMd-M1 IHiNORM(prep) were significantly dependent on which M1 was targeted (AGE × TARGET HEMISPHERE interaction effect: F(1140) = 4.18, p = 0.04). A post hoc test suggested that, irrespective of IHi TYPE, in young adults PMdD−M1ND IHiNORM(prep) was higher than in older adults, but this result just failed to reach significance (t(48) = −2.58, p = 0.06). An additional exploratory analysis revealed that this AGE × TARGET HEMISPHERE interaction effect was mainly driven by age differences in PMdD−M1ND l-IHiNORM (t(48) = −2.22, p = 0.03, and see Fig. 3 C). All other interaction effects were not significant (all p > 0.21).
The model for PMd-M1 IHi modulation in the premotor period yielded a significant AGE × TARGET HEMISPHERE × IHi TYPE interaction effect (F(1699) = 8.14, p = 0.005). Tukey-corrected post hoc tests indicated significantly higher IHiNORM(prem) values for PMdD−M1ND s-IHi in older adults (mean ± SD = 1.28 ± 0.51) than young adults (mean ± SD = 1.00 ± 0.29, t(48) = 4.97, p < 0.001). In older adults, IHiNORM(prem) for s-IHi was significantly greater for PMdD−M1ND than for PMdND−M1D (mean ± SD = 0.98 ± 0.34, t(699) = −6.10, p < 0.001), and IHiNORM(prem) for PMdD−M1ND s-IHi was greater than for PMdD−M1ND l-IHi (mean ± SD = 1.13 ± 0.43, t(699) = 3.13, p = 0.04). Interestingly, this pattern was independent of HAND ACTION (4-way interaction effect: F(6687) = 0.37, p = 0.90). The remaining interaction effects and the main effect of HAND ACTION were not significant (all p > 0.09).
In sum, as for M1-M1 IHi modulations during the preparatory period, bidirectional disinhibitory PMd-M1 IHi modulations were more prominent for l-IHi as compared to s-IHi. In older adults, the magnitude of the disinhibitory PMdD−M1ND IHi modulation tended to be lower than in younger adults. During the premotor period, older adults showed significantly greater disinhibitory PMdD−M1ND s-IHi modulation than young adults, irrespective of the upcoming response.
3.1.3. GABA+ levels
Individual GABA+ spectra, exemplary voxel positions and GABA+ levels by AGE and VOXEL are illustrated in Fig. 4. The linear mixed model yielded a main effect of AGE (F(1,48) = 14.32, p < 0.001) indicating that, irrespective of voxel, GABA+ levels in young adults (mean ± SD = 2.42 ± 0.23 i.u.) were on average significantly higher than in older adults (mean ± SD = 2.20 ± 0.27 i.u., t(48) = −3.78, p < 0.001). The main effect of VOXEL and the AGE × VOXEL interaction effect were not significant (F(1,46) = 0.08, p = 0.76 and F(1,45) = 0.04, p = 0.83, respectively).
Fig. 4.
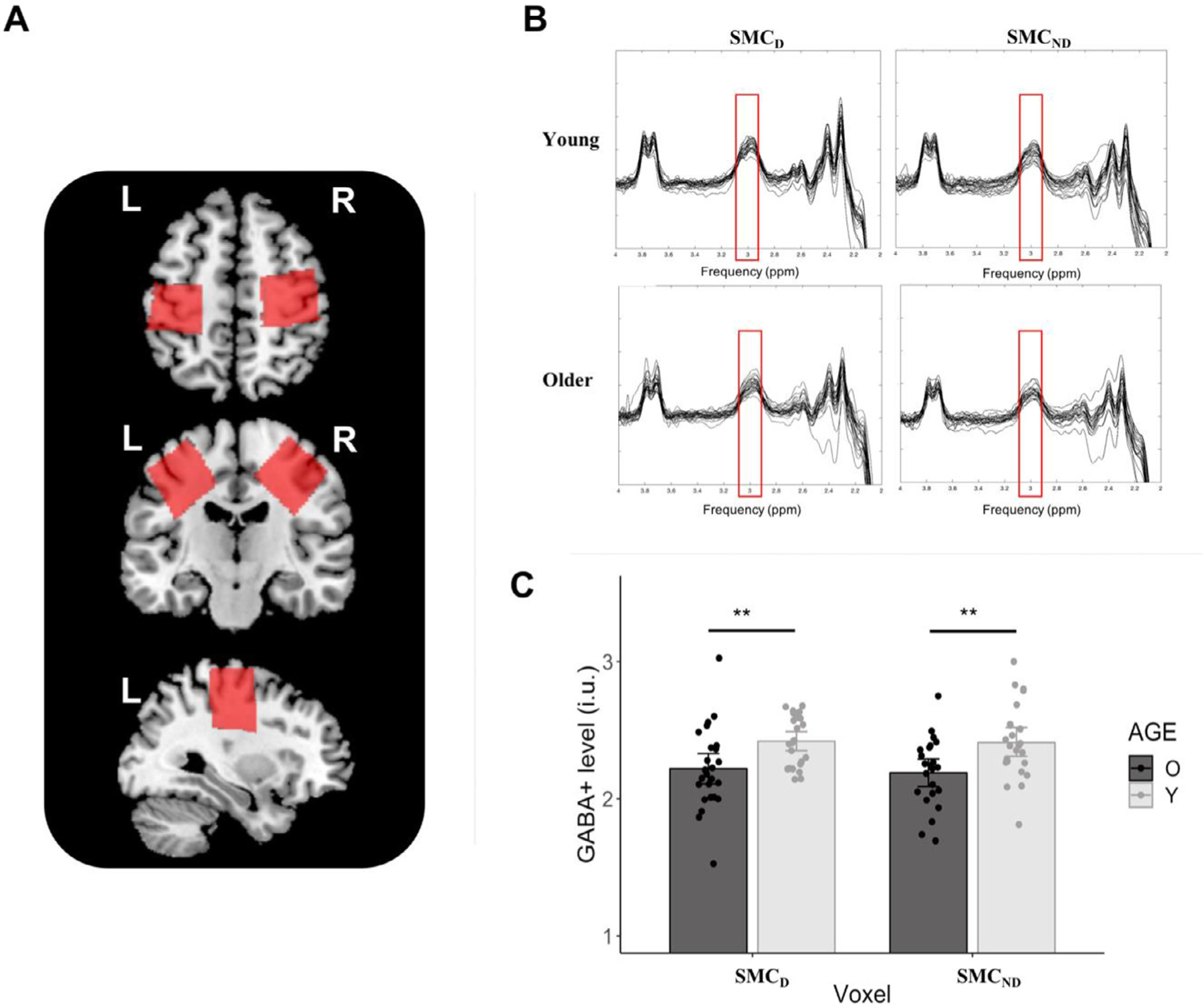
GABA+ results by AGE and VOXEL: (A) Exemplary voxel positions in the axial, coronal and sagittal plane; (B) Individual difference-edited spectra, with red frames highlighting the GABA+ peak at 3 ppm; (C) Mean GABA+ levels in young and older adults are plotted for SMCD, SMCND. Error bars indicate 95% CIs. Significant exploratory Tukey-corrected pairwise comparisons between age groups within each voxel are indicated by asterisks. Abbreviations: O, Older adults; Y, Young adults; SMC, sensorimotor cortex; D, Dominant hemisphere; ND, Non-Dominant hemisphere; i.u., institutional units; **, p < 0.01.
3.2. Associations between SMC GABA+ levels and task-related TMS measures
Older adults with higher target SMC GABA+ levels exhibited significantly lower IHiNORM(prem) values for PMdND−M1D l-IHi when the dominant FDI was required not to move during a unimanual response of the non-dominant FDI (Pearson’s r = −0.62, FDR-corrected p = 0.02, Fig. 5).
Fig. 5.
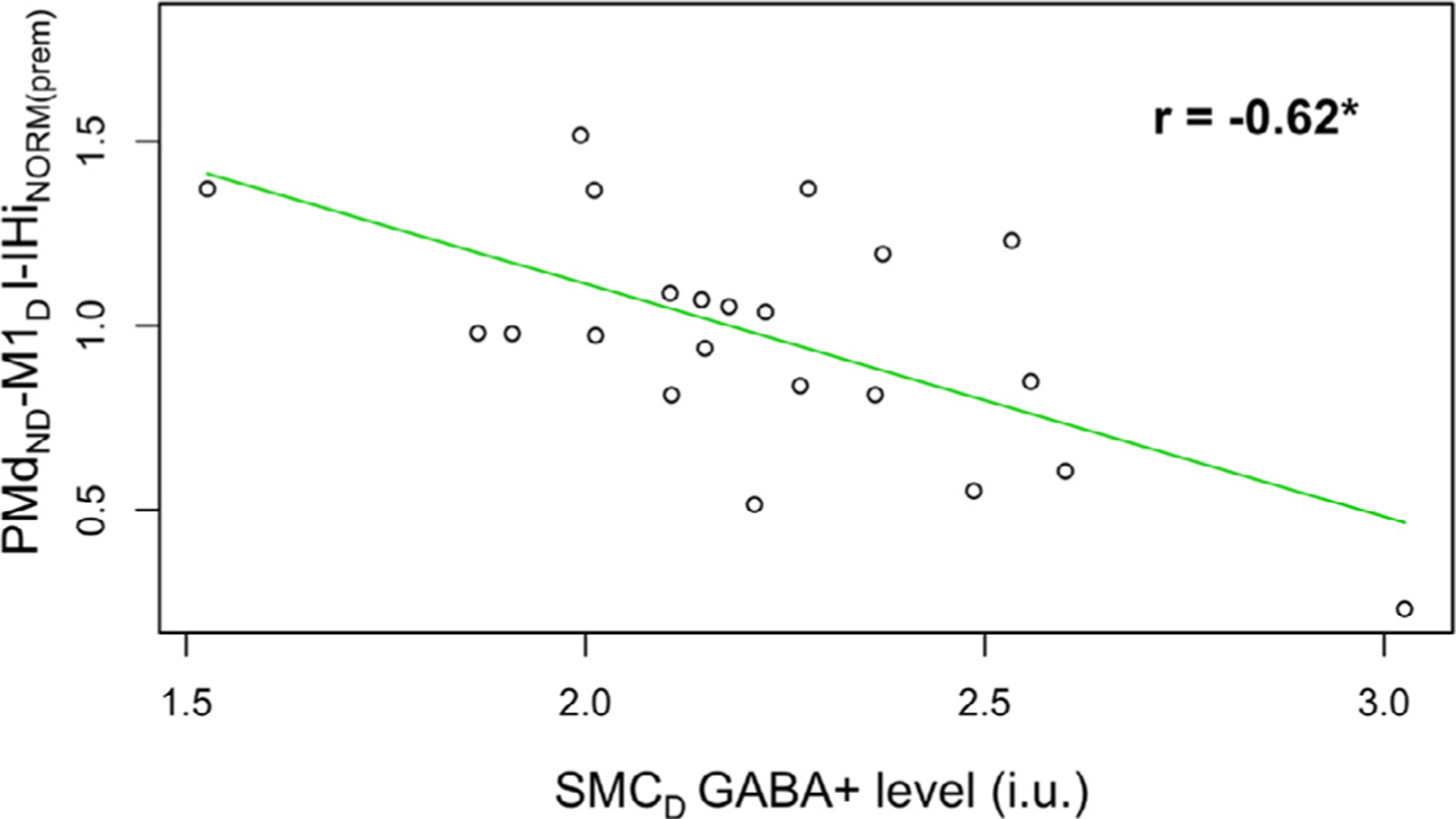
Relationship among older adults between (1) non-dominant dorsal premotor cortex and dominant primary motor cortex (PMdND−M1D) l-IHiNORM(prem) when the target (dominant) FDI was required not to move during a unimanual response of the non-targeted (non-dominant) FDI and (2) dominant SMC (SMCD) GABA+ levels. Abbreviations: SMC, sensorimotor cortex; l-IHiNORM(prem), modulation of long latency interhemispheric interaction during the premotor period; i.u., institutional units; *, p < 0.05.
There were no other significant associations between target SMC GABA+ levels and task-related CSE, M1-M1 IHi and PMd-M1 IHi modulations after FDR correction for multiple testing (see Tables A.3–A.7 in the appendix for uncorrected p-values).
3.3. Predicting RT
In young adults, model building for RT of the dominant FDI resulted in one significant predictor, which was M1ND−M1D s-IHi modulation during the premotor period of a response with the dominant FDI (F(1,17) = 6.34, β = 0.07, p = 0.02, Adj. R2 = 0.23), indicating more disinhibitory M1ND−M1D s-IHi modulation was related to longer RTs; see Table 4. For RT of the non-dominant FDI, none of the predictor candidates were significant in younger adults (all p > 0.09).
Table 4.
Linear regression model for young adults for predicting RT of the dominant FDI.
| Y = Dominant FDI RT | |||||
|---|---|---|---|---|---|
| Coefficients | Estimate (β) | Std. Error | t-value | p-value | Delta R 2 |
| (Intercept) | 0.251 | 0.032 | 7.729 | 0.000 *** | |
| M1ND–M1D s-IHiNORM(prem) | 0.065 | 0.030 | 2.518 | 0.022 * | 0.272 |
| F(1,17)-statistic | 6.341 | ||||
| p-value | 0.022* | ||||
| Residual Std. Error | 0.05 | ||||
| Adj. R 2 | 0.229 | ||||
p < 0.05;
p < 0.01;
p < 0.001.
In older adults, model building for RT of the dominant FDI resulted in three significant predictors (F(3,12) = 8.95, p = 0.002, Adj. R2 = 0.61); see Table 5. Specifically, this model indicated that longer RTs of the dominant FDI were related to higher SMC GABA+ levels in the dominant hemisphere (β = 0.09, p = 0.02), lower SMC GABA+ levels in the non-dominant hemisphere (β = −0.22, p < 0.001), and more disinhibitory M1ND−M1D l-IHi modulation during the preparatory period (β = 0.10, p = 0.02). The highest VIF score was 1.53. The model for the non-dominant FDI RT prediction also yielded three significant predictors (F(3,10) = 11.5, p = 0.001, Adj. R2 = 0.71); see Table 6. More specifically, during the preparatory period, longer RTs were related to more disinhibitory M1D−M1ND l-IHi modulation (β = 0.08, p = 0.01) and less disinhibitory PMdD−M1ND l-IHi modulation (β = −0.16, p < 0.001). During the premotor period, more CSEND facilitation predicted faster RTs of a non-dominant FDI response (β = −0.12, p < 0.001). The highest VIF score for this model was 2.05.
Table 5.
Linear regression model for older adults for predicting RT of the dominant FDI.
| Y = Dominant FDI RT | |||||
|---|---|---|---|---|---|
| Coefficients | Estimate (β) | Std. Error | t-value | p-value | Delta R 2 |
| (Intercept) | 0.593 | 0.089 | 6.693 | 0.000*** | |
| SMCND GABA+ | −0.224 | 0.044 | −5.139 | 0.000*** | 0.680 |
| SMCD GABA+ | 0.088 | 0.031 | 2.819 | 0.015* | 0.205 |
| M1ND–M1D l-IHiNORM(prep) | 0.102 | 0.039 | 2.619 | 0.022* | 0.177 |
| F(3,12)-statistic | 8.951 | ||||
| p-value | 0.002** | ||||
| Residual Std. Error | 0.038 | ||||
| Adj. R 2 | 0.614 | ||||
| Highest VIF score | 1.53 | ||||
p < 0.05.
p < 0.01.
p < 0.001.
Table 6.
Linear regression model for older adults for predicting RT of the non-dominant FDI.
| Y = Non-dominant FDI RT | |||||
|---|---|---|---|---|---|
| Coefficients | Estimate (β) | Std. Error | t-value | p-value | Delta R 2 |
| (Intercept) | 0.653 | 0.047 | 13.904 | 0.000*** | |
| PMDD–M1ND l-IHiNORM(prep) | −0.157 | 0.034 | −4.588 | 0.001*** | 0.473 |
| CSENORM(prem), ND | −0.115 | 0.025 | −4.676 | 0.001*** | 0.491 |
| M1D–M1ND l-IHiNORM(prep) | 0.084 | 0.024 | 3.485 | 0.006** | 0.273 |
| F(3,10)-statistic | 11.5 | ||||
| p-value | 0.001** | ||||
| Residual Std. Error | 0.036 | ||||
| Adj. R 2 | 0.708 | ||||
| Highest VIF score | 2.047 | ||||
p < 0.05;
p < 0.01;
p < 0.001.
4. Discussion
The present study yielded three major findings. First, aging effects were present for both TMS-derived measures for neurophysiological modulation, as well as for GABA+ levels in the primary motor areas. More specifically, older adults exhibited a reduced bilateral CSE suppression, and a reduced magnitude in bidirectional long latency interhemispheric (pre)motor-motor disinhibition during the preparatory period, as compared to young adults. Moreover, GABA+ levels in bilateral SMC were reduced in older as compared to young adults. Second, only in older adults, the exploratory correlational analyses indicated multiple associations between SMC GABA+ levels and task-related neurophysiological modulations. However, most associations did not survive the p-value correction for multiple testing. Third, according to the regression analyses, (slower) RTs within the older adults’ group could at least in part be explained by SMC GABA+ levels and task-related neurophysiology. Remarkably, in young adults, neither physiological (pre)motor-motor modulations, nor RTs were dependent on SMC GABA+ levels.
4.1. Age-related differences in neurophysiological modulations underlying increases in reaction time and the role of SMC GABA+ levels
4.1.1. Preparatory period
Overall, age-related differences in neurophysiology were most prominent during the preparatory period as compared to the premotor period (see also Cuypers et al., 2013; Cuypers et al., 2020). In accordance with findings of previous studies, the current results indicated a CSE suppression during the preparatory period (Hasbroucq et al., 1997; Touge et al., 1998; Sinclair and Hammond 2008; Duque and Ivry 2009; Kroeger et al., 2010; Hinder et al., 2012, 2018; Cuypers et al., 2013; Duque et al., 2014; Bestmann and Duque 2016; Klein et al., 2016). Importantly, the respective CSE suppression was reduced in older as compared to young adults, supporting our previous work (Cuypers et al., 2013).
Regarding the role of bilateral CSE suppression during the delayed period following a non-informative cue, two non-mutually exclusive hypotheses have been proposed (Bestmann and Duque 2016; Duque et al., 2017). First, CSE suppression might serve to reduce noise during motor preparation in order to assist the gain of excitatory inputs against a more quiescent background (Greenhouse et al., 2015; Quoilin and Derosiere 2015; Duque et al., 2017). In this view, an age-related decline in CSE suppression might reflect less effective background noise reduction. This could at least in part be reconciled with the hypothesis of age-related brain dedifferentiation (Cabeza 2002; Goh 2011; Grady 2012; King et al., 2017; Cassady et al., 2019). More specifically, brain dedifferentiation refers to a reduced distinctiveness and increased activation of brain regions across various cognitive and motor tasks in older adults. Interestingly, findings of Cassady et al. (2019) strongly suggest that age-related brain dedifferentiation might be a direct consequence of reduced SMC GABA levels. Results of the current study indeed indicated lower GABA+ levels in bilateral SMC in older as compared to young adults at the group level. Furthermore, in line with the dedifferentiation hypothesis, lower GABA+ levels (thus, possibly implying increased noise) in the non-dominant SMC predicted slower RTs of the dominant hand within the older adults group. The same association was observed between non-dominant SMC GABA+ levels and RTs of the non-dominant hand, but this result failed to reach significance (p = 0.08). Second, CSE suppression of a potentially selected effector could represent a mechanism for “impulse control ”, which entails a process that operates by temporarily inhibiting excitability at the spinal level (Touge et al., 1998; Hasbroucq et al., 1999; Duque et al., 2010; Kroeger et al., 2010). Impulse control thereby allows cortical excitatory preparatory processes to unfold without causing a premature release of the actual movement (Duque et al., 2017). Consistent with this hypothesis, our data showed that an overall disinhibition (rather than facilitation, see Figs. A.2 and A.3 in the appendix) of long latency M1-M1 and PMd-M1 IHi co-occurred with the CSE suppression in the target M1 in both age groups (see also Hinder et al., 2012, 2018). Remarkably, older adults exhibited reduced disinhibitory modulations of l-IHi (i.e., less release of inhibition) for both PMd-M1 and M1-M1 interactions, apparently due to a reduced baseline inhibitory tone (Figs. A.2 and A.3, and Table A2). Interestingly, an age-related reduction in resting-state intra-cortical inhibition is suggested to predict a reduced capacity to modulate inhibitory processes during a motor task, which relates to motor function declines (Heise et al., 2013). In our experiment, however, we did not probe baseline measures at complete rest, but at the start of each trial to control for the processes related to the task-driven increases in attention (Kastner et al., 1999; Labruna et al., 2011; Vassiliadis et al., 2020). An interesting hypothesis for future research would be to investigate to what extent our observed relation between motor performance and (dis)inhibitory fronto-motor modulations are driven by the inhibitory state measured at baseline. Overall, since l-IHi in rest appears not to be affected by aging (Talelli et al., 2008; Hermans et al., 2018), the present findings are indicative for an age-related difference in the task-related modulatory capacity of the GABAB receptor-mediated neuronal circuit. Accordingly, earlier studies reported age-related reductions in M1-M1 l-IHi modulation during voluntary contraction of the non-targeted homologue muscle (Talelli et al., 2008) and reduced modulations in contralateral silent period during a hand-foot coordination task (Fujiyama et al., 2009), denoting altered GABAB receptor activity under motor task conditions (Reis et al., 2008).
An intriguing result in older adults during the preparatory period was the discrepancy between modulation of bidirectional M1-M1 l-IHi and PMdD−M1ND l-IHi and their relation to RT performance. More specifically, in older adults, stronger long latency M1-M1 disinhibition was related to slower RTs, whereas stronger long latency PMdD−M1ND disinhibition was associated with faster RTs. The former result was rather unexpected, since young adults showed higher long latency M1-M1 disinhibition than older adults, but faster RTs. One could therefore speculate that M1-M1 disinhibition during the preparatory period in older adults reflects dysfunctional activation spreading. This was supported by an additional a-posteriori analysis, correlating preparatory M1-M1 l-IHi modulations with preparatory CSE modulations (see Fig. A6). Specifically, results indicated that only in young adults more long latency M1-M1 disinhibition was related to more CSE suppression, while no significant relation was observed in older adults.
Since the global preparatory CSE suppression likely contributes to a more quiescent background in the M1 area, a reduced CSE suppression combined with a reduced long latency PMd-M1 disinhibition suggests a lower signal-to-noise ratio in older adults in the target M1. This is supported by the finding that older adults with greater PMdD−M1ND disinhibition exhibited faster RTs. Since the respective PMdD−M1ND l-IHi modulation was only significantly related to RT in older, but not in young adults, the present findings are consistent with an overall increased reliance on premotor areas in older adults during the preparatory period (see also Berchicci et al., 2012; Hinder et al., 2012; Stewart et al., 2014).
4.1.2. Premotor period
Whereas CSE suppression during the preparatory period was reduced in older as compared to young adults, both age groups showed a comparable CSE facilitation towards EMG onset in the premotor period (see also Cuypers et al., 2013). More specifically, the bilateral CSE suppression during the preparatory period was followed by a CSE facilitation towards EMG onset in the premotor period during a (selected) unimanual or bimanual response (see also Fig. A1). This observation substantiates earlier reported CSE dynamics during both simple (Chen and Hallett 1999; Leocani et al., 2000; Levin et al., 2011; Hinder et al., 2012) and choice RT tasks (Duque et al., 2005, 2014; Duque and Ivry 2009; Kroeger et al., 2010; Cuypers et al., 2013; Bestmann and Duque 2016; Klein et al., 2016; Hinder et al., 2018). It has been argued that the discrepancy in CSE modulation between selected and non-selected response options reflects a competitive process that serves to assist action selection (Bestmann and Duque 2016; Duque et al., 2017). Notably, only in older adults, more CSE facilitation in the non-dominant hemisphere predicted faster non-dominant FDI RTs. Fujiyama et al. (2012) reported a similar association between CSE facilitation in the dominant hemisphere in the late premotor period and dominant FDI RT using a simple go/no-go task in older, but not in young adults.
As for CSE modulations, M1-M1 IHi modulations were not affected by aging during the premotor period, which is in accordance with the results of Hinder et al. (2012) who used a simple pre-cued RT task. Strikingly, in young adults, greater short latency M1ND−M1D disinhibition was predictive for slower RTs of the dominant FDI. In an additional a-posteriori analysis, results indicated that greater short latency M1ND−M1D disinhibition during the premotor period was related to less facilitatory modulation of CSE of the dominant M1 in young adults (see Fig. A7). Together, these findings suggest that the conditioning M1 is not facilitating subsequent movement initiation directly through target M1 CSE facilitation. Along these lines, a recent study reported that young adults with more resting-state M1-M1 short and long latency interhemispheric inhibition exhibited higher resting-state CSE in the target M1 by means of MEP recruitment curves, suggesting that the ability to recruit a larger number of corticospinal pathways within the target M1 is related to the ability to activate local inhibitory circuits (Hermans et al., 2018). Interestingly, some studies suggest M1-M1 IHi modulations to be engaged in maintaining a continuous intrinsic state of inhibition towards movement onset (Kroeger et al., 2010; Liuzzi et al., 2010). In this view, the observed relationship between M1-M1 IHi modulations and RT or CSE could be interpreted as a role of the conditioning M1 in controlling the background noise in the target M1 to an optimal level, which assists the gain of excitatory processes, expressed by the CSE modulation towards facilitation (Duque et al., 2017).
Regarding PMd-M1 IHi modulations in older adults, the present study yielded two notable results. First, higher GABA+ levels in the dominant SMC were significantly related to lower PMdND−M1D l-IHiNORM during the premotor period, when the target (dominant) FDI was required not to respond (i.e., during a unimanual response condition of the non-targeted, non-dominant FDI). More specifically, the data suggest that higher GABA+ levels in the dominant SMC were associated with PMdND−M1D l-IHi modulation towards more inhibition, while lower GABA+ levels were related to modulation towards disinhibition and even facilitation. A plausible hypothesis to be tested in future studies is that more inhibitory PMd-M1 l-IHi modulation contributes to performance in terms of low error rate, i.e., by suppressing the intrinsic tendency for mirror movements (e.g., Swinnen 2002; Swinnen and Wenderoth 2004; Boisgontier et al., 2014) when the effector is not selected for movement.
A second prominent finding was that PMdD−M1ND s-IHi modulation was disinhibitory/facilitatory in older, but not in young adults (see also Fig. A5 in the appendix). In line with this result, Hinder et al. (2012) reported PMdD−M1ND disinhibitory modulations between IS onset and response initiation to be present in older adults only. The most plausible explanation for the age-related increase in disinhibitory/facilitatory PMdD−M1ND s-IHi modulation during the premotor period could be that it reflects an overall increased recruitment of premotor areas in older adults (Berchicci et al., 2012; Hinder et al., 2012; Stewart et al., 2014). However, whether this increased PMd recruitment is either compensatory or dysfunctional is hard to determine since no relation with RT was evident in the current data. Notably, findings of Hinder et al. (2012) suggested that in older adults more PMd-M1 disinhibition early in the preparatory period was related to faster RTs, while more PMd-M1 disinhibition in the late premotor period was related to slower RTs. This suggests that early PMd-M1 disinhibition is beneficial, whereas on the contrary, late PMd-M1 disinhibition is detrimental for fast motor responses. In accordance, results of an additional a-posteriori analysis showed that in both age groups more PMdD−M1ND s-IHi disinhibition was related to less CSEND facilitation during the premotor period (see Fig. A8 in the appendix). Since in older adults, less CSEND facilitation was predictive for slower RTs, this additional analysis supports indirectly the hypothesis that age-related increases in PMd-M1 disinhibition in anticipation of a motor response are detrimental, rather than beneficial for fast RTs.
4.1.3. Age-related differences in the role of SMC GABA+ levels
In contrast to young adults, SMC GABA+ levels in older adults explained at least in part both task-related physiological modulations, as well as RT. One could therefore speculate that when GABA levels fall below a critical limit, as it might be the case in older adults, the consequent lower signal-to-noise ratio becomes a critical factor in the effectiveness of the (pre)motor-motor modulatory capacity and motor behavioural output. On the other hand, if GABA levels reach above that critical limit, a further increase in GABA levels would not affect physiological dynamics and motor behavior. Likewise, this would explain why SMC GABA levels did neither predict RT (Greenhouse et al., 2017), nor task-related neurophysiological dynamics (Cuypers et al., 2020) in young adults. However, caution is needed to not oversimplify the interaction between GABA level and its functionality. For example, regarding RT of the dominant FDI in older adults, the current findings indicated that faster RTs were related to more SMCND GABA+, but less SMCD GABA+. It is difficult to interpret this discrepancy without considering other properties of the GABAergic system, for example receptor availability (Cuypers et al., 2020), and future multimodal studies are needed to further elucidate this complexity. Taken together, these data underscore that task-related neurophysiological modulations might just reflect the tip of the iceberg: when considering the (non-)existence of relationships between neurophysiological dynamics and motor behavior, this should best be interpreted with respect to trait-based interindividual differences in GABA levels and GABA receptor availability. Indeed, similar to the neurochemical GABA+ measure, in young adults neurophysiological (pre)motor-motor modulations during motor preparation were overall not predictive for RT performance. This suggests that the observed significant modulations during motor preparation within this group may rather be an epiphenomenal byproduct of brain physiology, than causal to RT motor behavior.
4.2. Hemispheric (a)symmetry of M1-M1 and PMd-M1 interactions
An important novel aspect in this study was that both IHi directions, originating in either PMd or M1, as well as CSE in both the dominant and non-dominant hemisphere, were probed in one single experimental design. This offered a unique opportunity to directly compare interhemispheric (im)balances of the (pre)motor-motor pathways during a delayed choice RT task. Importantly, irrespective of age, only PMd-M1 IHi modulations, but not M1-M1 IHi modulations, were dependent on the measured direction. This suggests a more lateralized PMd function during motor planning, as compared to M1 (but see Duque et al., 2007). Overall, irrespective of which hand was selected, these findings indicate a more dominant PMdD−M1ND interaction as compared to the PMdND−M1D interaction (see also Fujiyama et al., 2016a, Fujiyama et al. 2016b) in right-handed subjects, while interactions between homologue M1s are more balanced.
4.3. Limitations and future directions
The current study is subject to some limitations. Most important are the methodological limitations of the normalization/correction procedure when comparing MRS-derived GABA+ levels directly between age groups. Due to the lack of a golden standard, different correction methods have been used in literature so far (e.g., Gao et al., 2013; Mooney et al., 2017; Porges et al., 2017a; Chalavi et al., 2018; Hermans et al., 2018; Ferland et al., 2019; Cuypers et al., 2020), which might explain the inconsistent conclusions regarding the aging effect on GABA+ levels (Porges et al., 2017b; Maes et al., 2018). In line with previous studies (e.g., Chalavi et al., 2018; Cuypers et al., 2020; Maes et al., 2021) and in order to obtain an accurate GABA+ estimation for each age group, we normalised the GABA+ measurements to a voxel that is representative for each investigated population. An important limitation of this procedure is that the observed age differences in GABA+ levels may also be in part driven by age differences in tissue composition, despite the alpha correction (see Table S.1 and Figure S.1 in the Supplementary Material for an a posteriori re-analysis of the aging effect on GABA+ levels, using a different normalization procedure). Nevertheless, our results showed lower overall GABA+ levels in older adults in the SMC voxels, which may have functional implications. Future research is warranted to establish the most appropriate metric for GABA+ (i.e., overall or tissue-specific) for the study of brain–behavior associations. A second methodological limitation in the GABA+ processing, specifically for older adults, is the external validity of the internal standard. More specifically, even though the water signal was corrected for tissue-specific relaxation times and visible water content (Harris et al., 2015), the assumed parameters in these corrections were derived from studies with participants younger than 51 years (Wansapura et al., 1999; Lu et al., 2005; Piechnik et al., 2009). It is currently unknown whether (and/or to what extent) these parameters would further change with older age (> 60 years). Thus, it cannot be ruled out that particular age-related biophysical changes in the water signal could have an effect on the results.
Third, as interhemispheric interactions assessed with TMS likely involve glutamatergic projections that cross the corpus callosum before synapsing with GABAergic interneurons (Carr and Sesack 1998; Daskalakis et al., 2002; Chen 2004; Lee et al., 2007; Reis et al., 2008; Palmer et al., 2012), the resulting MEP amplitudes following dual-site TMS do not exclusively reflect GABAergic neurotransmission. Nevertheless, given that modulations in contralateral silent period, which also reflects GABAB receptor-mediated neurotransmission, are also reduced in older adults during specific motor tasks (Fujiyama et al., 2009), and baclofen (a GABAB agonist) strengthens resting-state interhemispheric inhibition to a great extent (Irlbacher et al., 2007), we propose that a change in GABAergic neurotransmission is very likely contributing to our observed aging effects on the TMS metrics, although an additional contribution of changes in glutamatergic neurotransmission cannot be precluded.
A fourth limitation encompasses the limited amount of time points for TMS assessment during a trial due to the comprehensive TMS design of the current study, i.e., including bidirectional PMd-M1 and M1-M1 s-IHi and l-IHi assessment. Therefore, modulations during time intervals where early selection processes are expected (i.e., immediately after IS onset; Koch et al., 2006; O’Shea et al., 2007) were not assessed, although it would have been informative to collect data in this phase as well.
A fifth limitation is the exploratory nature of the correlation analyses between SMC GABA+ availability and task-related neurophysiology. This resulted in a high number of statistical tests, which in turn resulted in rather conservative corrected p-values (e.g., Lee and Lee 2018). Consequently, almost none of the observed correlations in older adults remained significant after correction. Although one reason could be that both metrics differ significantly in spatial resolution [i.e., for MRS, a relatively large voxel size is necessary to offset the inherent low signal to noise ratio for GABA spectra (Mullins et al., 2014)], it would be interesting to test the observed trends for associations in older adults in future studies in a hypothesis-driven manner to either confirm or reject these associations.
In contrast to young adults, older adults did not show significant interhemispheric inhibition at the time of WS onset (see Figs. A.2.–A.5 in the appendix), irrespective of IHi type (s-IHi versus l-IHi), target hemisphere and conditioned region. This raises the question whether the used TMS stimulation parameters were effective in older adults to evoke interhemispheric inhibition. Therefore, another limitation of our study is that we did not record resting-state TMS data to validate this. However, previous studies of our group, using identical stimulation parameters, indicated that in rest interhemispheric inhibition can be evoked in both young and older age groups, irrespective of IHi type, target hemisphere and conditioned region (Fujiyama et al., 2016b; Hermans et al., 2018).
Lastly, it should be noted that the interpretations concerning aging effects are inferred from statistical contrasts between a young adult group (< 33 years) and an older adult group (> 60 years) in a cross-sectional study design, which is why caution is needed to interpret group differences in MRS and TMS measures as changes of these measures over time. On a similar note, some caution is warranted to interpret the observed age-related alterations in neurochemical/-physiological characteristics as being the actual “cause ” of age-related RT slowing, since the used analyses were merely correlational in nature.
The current results indicate that the relation between bulk GABA+ levels and GABAergic neurotransmission is complex. Therefore, future studies using multimodal approaches, for example by mapping GABA receptor availability as well, are recommended and are expected to deliver additional understanding about how different surrogates of the GABAergic system interact and shape human motor behavior. Finally, this study presents a robust paradigm to investigate the (pre)motor-motor inter-hemispheric interplay throughout motor preparation in healthy adults of distinct ages. Similar future approaches would be useful to unravel interhemispheric (pre)motor-motor interactions in pathological conditions, as well as their impact on motor control. Such studies justify research on healthy aging processes to constitute a reference for elucidating abnormal or pathological neurophysiological and neurochemical processes with a typically higher incidence in the aged population, such as in stroke or dementia (e.g., Rossini et al., 2007).
5. Conclusions
To the best of our knowledge, this is the first study that presents such a comprehensive perspective on bilateral CSE and inter-hemispheric (pre)motor-motor dynamics at both short and long latencies, during a pre-cued bimanual choice RT task in a single study design. Moreover, associations between these neurophysiological dynamics and static MRS GABA+ levels in SMC were explored, as well as their contribution to RT in healthy young and older adults. Results yielded that older adults exhibited a reduced bilateral CSE suppression as well as a reduced magnitude of bidirectional long latency interhemispheric (pre)motor-motor disinhibition during the preparatory period, suggesting deficiencies in GABAB receptor-mediated neurotransmission. Our findings also indicated that task-related (pre)motor-motor modulations and slower RTs in older adults might at least in part be explained by lower GABA+ levels in bilateral sensorimotor cortices. In contrast, neither physiological (pre)motor-motor modulations nor RTs were dependent on SMC GABA+ levels in young adults.
Supplementary Material
Acknowledgments
The authors wish to thank Ir. P. Meugens and R. Clerckx for their crucial assistance in the development of the setup and the automatization of data processing, respectively. We additionally thank Dr. L. Hermans, S. Vanuytven, K. Quadflieg and S. Denissen for their assistance with data collection.
Funding
This work was supported by the Research Fund KU Leuven (C16/15/070), Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (G089818N, G039821N, I005018N, AUHL/11/01 R-3987) and by the FWO-FNRS (EOS 30446199, MEMODYN). Celine Maes and Melina Hehl are funded by a fellowship grant from FWO. Shanti Van Malderen is funded by a fellowship grant from the Special Research Fund (BOF) of Hasselt University (BOF21DOC12). This work applies tools developed under NIH grants R01 EB016089, R01 EB023963 and P41 EB015909 (awarded to R. Edden and coworkers). Mark Mikkelsen is supported by NIH grant K99 EB028828. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviation:
- CS
conditioning stimulus
- CSE
corticospinal excitability
- CSENORM(prep)
modulation of corticospinal excitability during the preparatory period
- CSENORM(prem)
modulation of corticospinal excitability during the premotor period
- D
dominant
- dsTMS
dual-site transcranial magnetic stimulation
- EMG
electromyography
- FDI
first dorsal interosseus muscle
- GABA
gamma-aminobutyric acid
- GABA+
gamma-aminobutyric acid with the contribution of co-edited macro-molecules
- IHi
interhemispheric interaction
- IHiNORM(prep)
modulation of interhemispheric interaction during the preparatory period
- IHiNORM(prem)
modulation of interhemispheric interaction during the premotor period
- IS
imperative signal
- ISI
inter-stimulus interval
- l-IHi
long latency interhemispheric interaction
- LED
light-emitting diode
- M1
primary motor cortex
- MEP
motor evoked potential
- MRS
magnetic resonance spectroscopy
- ND
non-dominant
- PMd
dorsal premotor cortex
- rMT
resting motor threshold
- RT
reaction time
- s-IHi
short latency interhemispheric interaction
- SMC
sensorimotor cortex
- TMS
transcranial magnetic stimulation
- TS
test stimulus
- VIF
variance inflation factor
- WS
warning signal
- 75%EMG
75% of the time between IS onset and EMG onset
APPENDIX
Table A1.
Group comparisons of CSE, expressed in mV, at WS and IS. If the assumption of equal variances was met, a parametric unpaired t-test was used. Otherwise, a Welch test was used (indicated by superscript “W”). A false discovery rate (FDR) correction method (Benjamini and Hochberg 1995) was applied to correct for multiple testing. No significant results were observed.
| Time point | Target hemisphere | Young (mean ± SD) | Older (mean ± SD) | t-value (DF) | p-value |
|---|---|---|---|---|---|
| WS | D | 2.11 ± 0.94 | 1.92 ± 1.25 | 1.22 (184)W | 0.23 |
| ND | 2.16 ± 1.26 | 2.09 ± 1.42 | 0.38 (198) | 0.70 | |
| IS | D | 1.58 ± 0.71 | 1.63 ± 1.17 | −0.32 (163)W | 0.73 |
| ND | 1.53 ± 0.96 | 1.73 ± 1.13 | −1.38 (198) | 0.18 |
Abbreviations: CSE, corticospinal excitability; WS, warning signal; IS, imperative signal; D, dominant hemisphere; ND, non-dominant hemisphere.
Table A2.
Group comparisons of s-IHi and l-IHi metrics at WS and IS. If the assumption of equal variances was met, a parametric unpaired t-test was used. Otherwise, a Welch test was used (indicated by superscript “W ”). A false discovery rate (FDR) correction method (Benjamini and Hochberg 1995) was applied to correct for multiple testing. Values in bold survived FDR correction.
| Time point | IHi type | Target hemisphere | Young (mean ± SD) | Older (mean ± SD) | t-value (DF) | p-value |
|---|---|---|---|---|---|---|
| WS | M1-M1 s-IHi | D | 0.80 ± 0.24 | 0.93 ± 0.31 | −3.52 (189)W | <0.001 *** |
| ND | 0.82 ± 0.23 | 1.05 ± 0.43 | −4.58 (151)W | <0.001 *** | ||
| M1-M1 l-IHi | D | 0.75 ± 0.19 | 0.94 ± 0.25 | −5.96 (186)W | <0.001 *** | |
| ND | 0.82 ± 0.23 | 0.91 ± 0.54 | −1.50 (133)W | 0.14 | ||
| PMd-M1 s-IHi | D | 1.04 ± 0.21 | 0.97 ± 0.26 | 1.89 (198) | 0.06 | |
| ND | 0.99 ± 0.23 | 1.02 ± 0.26 | −0.80 (198) | 0.43 | ||
| PMd-M1 l-IHi | D | 0.93 ± 0.22 | 0.98 ± 0.19 | −1.51 (198) | 0.13 | |
| ND | 0.82 ± 0.21 | 1.01 ± 0.32 | −4.99 (172)W | <0.001 *** | ||
| IS | M1-M1 s-IHi | D | 0.85 ± 0.25 | 0.92 ± 0.27 | −1.90 (198) | 0.06 |
| ND | 0.86 ± 0.25 | 0.87 ± 0.29 | −0.38 (198) | 0.70 | ||
| M1-M1 l-IHi | D | 1.05 ± 0.46 | 1.01 ± 0.27 | 0.73 (159) | 0.47 | |
| ND | 1.09 ± 0.41 | 1.01 ± 0.35 | 1.46 (198) | 0.14 | ||
| PMd-M1 s-IHi | D | 1.06 ± 0.31 | 1.03 ± 0.24 | 0.76 (187)W | 0.45 | |
| ND | 0.99 ± 0.21 | 0.88 ± 0.30 | 3.02 (180)W | 0.003 ** | ||
| PMd-M1 l-IHi | D | 1.10 ± 0.28 | 1.04 + 0.29 | 1.48 (198) | 0.14 | |
| ND | 0.99 ± 0.28 | 1.00 ± 0.22 | −0.21 (189)W | 0.84 |
Abbreviations: s-IHi, short latency interhemispheric interaction; l-IHi, long latency interhemispheric interaction; WS, warning signal; IS, imperative signal; D, dominant hemisphere; ND, non-dominant hemisphere; M1, primary motor cortex; PMd, dorsal premotor cortex.
p (uncorrected) < 0.01
p (uncorrected) < 0.001
Table A3.
Pearson’s r or Spearman’s ρ correlations (indicated by superscript “ρ”) between SMC GABA+ levels and corticospinal excitability (CSE) modulation: r(p-value) or ρ(p-value). No result survived FDR correction.
| YOUNG | OLDER | ||||
|---|---|---|---|---|---|
| Dominant | Non-dominant | Dominant | Non-dominant | ||
| Preparatory Period | 0.33(0.12) ρ | 0.15(0.49) | −0.14(0.52) ρ | −0.16(0.50) ρ | |
| Premotor Period | Selected | −0.08(0.70) ρ | −0.03(0.90) ρ | 0.29(0.18) ρ | 0.34(0.14) ρ |
| Non-selected | −0.06(0.79) ρ | −0.14(0.52) | 0.09(0.67) ρ | 0.02(0.93) ρ | |
| Bimanual | 0.02(0.92) ρ | −0.02(0.92) | 0.07(0.76) ρ | 0.47(0.04) ρ, * | |
| No response | 0.20(0.35) ρ | −0.02(0.94) ρ | −0.38(0.07) | 0.16(0.49) | |
p (uncorrected) < 0.05.
Table A4.
Pearson’s r or Spearman’s ρ correlations (indicated by superscript “ρ”) correlations between SMC GABA+ levels and modulation of the M1-M1 short latency interhemispheric interaction (s-IHiNORM): r(p-value) or ρ(p-value). No result survived FDR correction.
| YOUNG | OLDER | ||||
|---|---|---|---|---|---|
| Dominant | Non-dominant | Dominant | Non-dominant | ||
| Preparatory Period | 0.27(0.21) ρ | 0.06(0.80) | 0.09(0.67) | 0.13(0.59) ρ | |
| Premotor Period | Selected | −0.25(0.27) ρ | −0.05(0.84) ρ | 0.03(0.88) | 0.05(0.85) ρ |
| Non-selected | −0.02(0.93) ρ | −0.04(0.84) | 0.29(0.19) | 0.34(0.14) | |
| Bimanual | −0.22(0.33) ρ | −0.16(0.46) | −0.21(0.34) | 0.09(0.72) | |
| No response | −0.04(0.84) ρ | −0.26(0.22) | 0.10(0.65) ρ | −0.00(0.99) | |
Table A5.
Pearson’s r or Spearman’s ρ correlations (indicated by superscript “ρ”) between SMC GABA+ levels and modulation of the PMd-M1 short latency interhemispheric interaction (s-IHiNORM): r(p-value) or ρ(p-value). No result survived FDR correction.
| YOUNG | OLDER | ||||
|---|---|---|---|---|---|
| Dominant | Non-dominant | Dominant | Non-dominant | ||
| Preparatory Period | −0.08(0.73) ρ | −0.27(0.20) | −0.10(0.66) | 0.04(0.86) | |
| Premotor Period | Selected | 0.35(0.10) ρ | 0.24(0.28) | 0.13(0.56) ρ | −0.20(0.41) |
| Non-selected | 0.41(0.05) ρ | −0.16(0.46) ρ | −0.24(0.29) ρ | 0.39(0.10) | |
| Bimanual | 0.13(0.55) ρ | 0.09(0.70) | 0.13(0.57) | −0.06(0.81) | |
| No response | −0.12(0.59) ρ | −0.11(0.60) | −0.09(0.70) | −0.14(0.58) ρ | |
Table A6.
Pearson’s r or Spearman’s ρ correlations (indicated by superscript “ρ”) between target SMC GABA+ levels and modulation of the M1-M1 long latency interhemispheric interaction (l-IHiNORM): r(p-value) or ρ(p-value). No result survived FDR correction.
| YOUNG | OLDER | ||||
|---|---|---|---|---|---|
| Dominant | Non-dominant | Dominant | Non-dominant | ||
| Preparatory Period | −0.18(0.40) ρ | −0.08(0.73) | −0.13(0.55) | 0.57(0.01) ** | |
| Premotor Period | Selected | −0.34(0.12) ρ | −0.40(0.06) | 0.07(0.75) | 0.15(0.55) ρ |
| Non-selected | 0.02(0.93) ρ | 0.20(0.36) | −0.18(0.43) ρ | −0.03(0.91) | |
| Bimanual | 0.13(0.56) ρ | −0.11(0.61) | 0.22(0.33) | −0.25(0.31) | |
| No response | −0.29(0.18) ρ | −0.16(0.46) | −0.07(0.74) | −0.17(0.48) ρ | |
p (uncorrected) < 0.01.
Table A7.
Pearson’s r or Spearman’s ρ correlations (indicated by superscript “ρ”) between SMC GABA+ levels and modulation of the PMd-M1 long latency interhemispheric interaction (l-IHiNORM): r(p-value) or ρ(p-value). Values in bold survived FDR correction.
| YOUNG | OLDER | ||||
|---|---|---|---|---|---|
| Dominant | Non-dominant | Dominant | Non-dominant | ||
| Preparatory Period | −0.09(0.70) ρ | −0.24(0.26) ρ | 0.53(0.01) ** | −0.07(0.77) | |
| Premotor Period | Selected | 0.09(0.69) ρ | 0.19(0.38) ρ | −0.34(0.13) | 0.08(0.74) |
| Non-selected | 0.20(0.37) ρ | 0.08(0.71) ρ | −0.62(0.00) ** | 0.18(0.44) | |
| Bimanual | −0.18(0.43) ρ | −0.07(0.77) | −0.12(0.60) | −0.03(0.89) | |
| No response | −0.26(0.26) ρ | 0.10(0.65) | −0.46(0.03) * | 0.08(0.72) | |
p (uncorrected) < 0.05.
p (uncorrected) < 0.01.
Fig. A1.
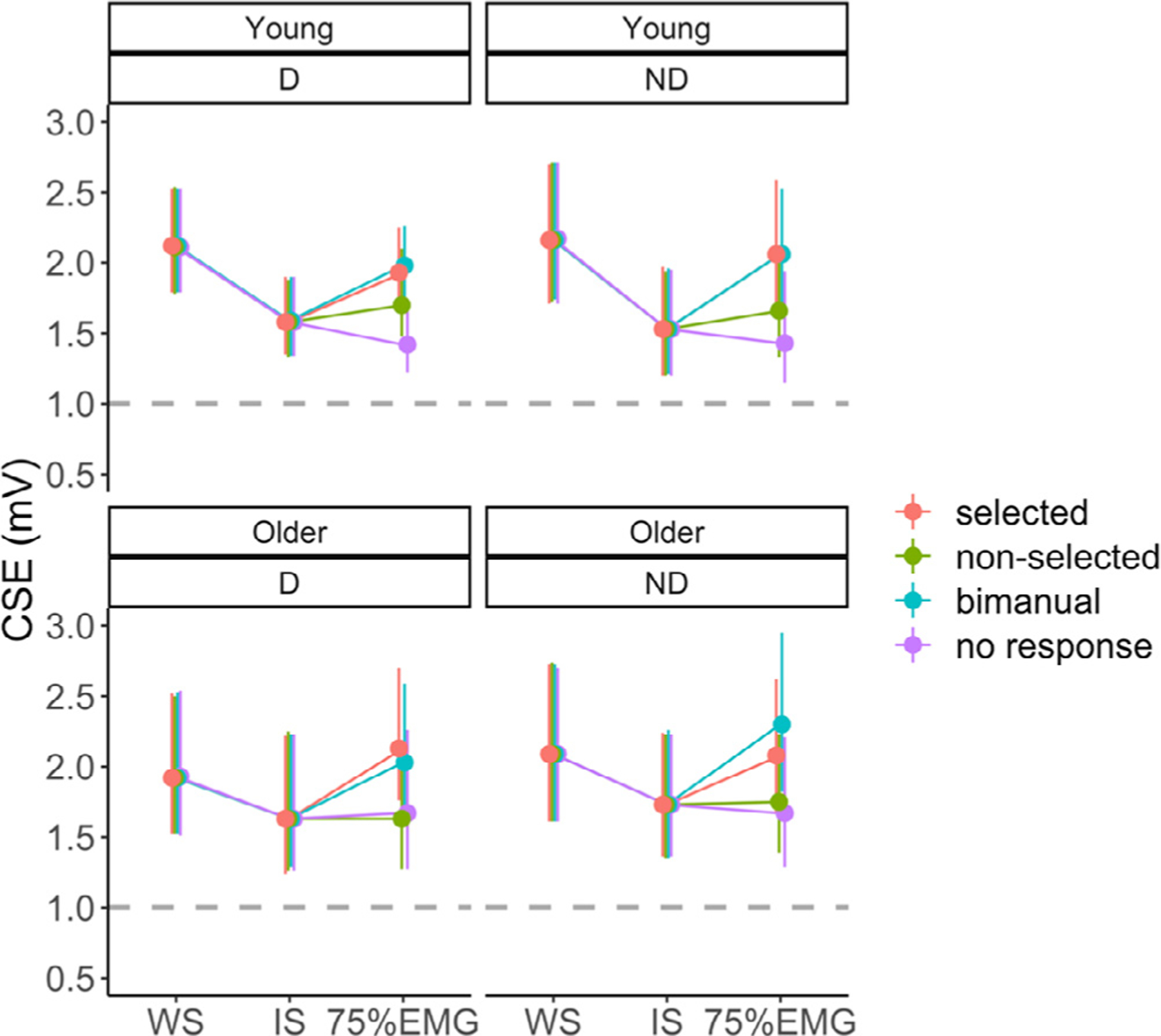
Corticospinal excitability (CSE) at each time point during a trial, for each age group (Young vs. Older) and target hemisphere (D vs. ND). Line plots in different colours represent the different response conditions (selected vs. non-selected vs. bimanual vs. no response). The dashed line indicates CSE at rest. Error bars indicate 95%CIs. Abbreviations: D, dominant; ND, non-dominant; WS, warning signal; IS, imperative signal; 75%EMG, 75% of the time between IS onset and EMG onset.
Fig. A2.
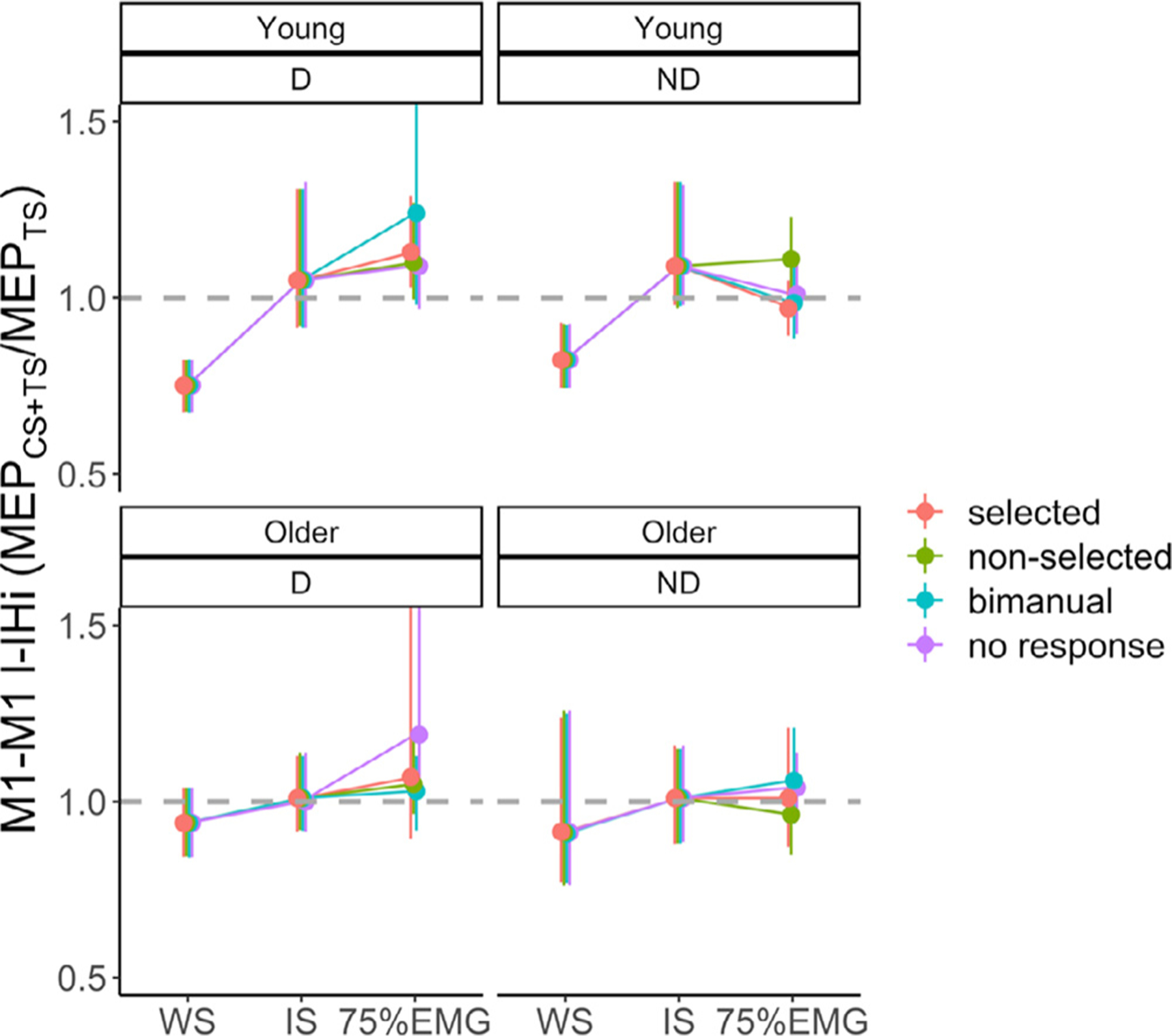
Long latency interhemispheric M1-M1 interactions (M1-M1 l-IHi) at each time point during a trial, for each age group (Young vs. Older) and target hemisphere (D vs. ND). Line plots in different colours represent the different response conditions (selected vs. non-selected vs. bimanual vs. no response). Values below the dashed line indicate an inhibitory M1-M1 l-IHi, whereas values above the dashed line indicate a facilitatory M1-M1 l-IHi. Error bars indicate 95%CIs. Abbreviations: D, dominant; ND, non-dominant; WS, warning signal; IS, imperative signal; 75%EMG, 75% of the time between IS onset and EMG onset; M1, primary motor cortex; CS, conditioning stimulus; TS, test stimulus.
Fig. A3.
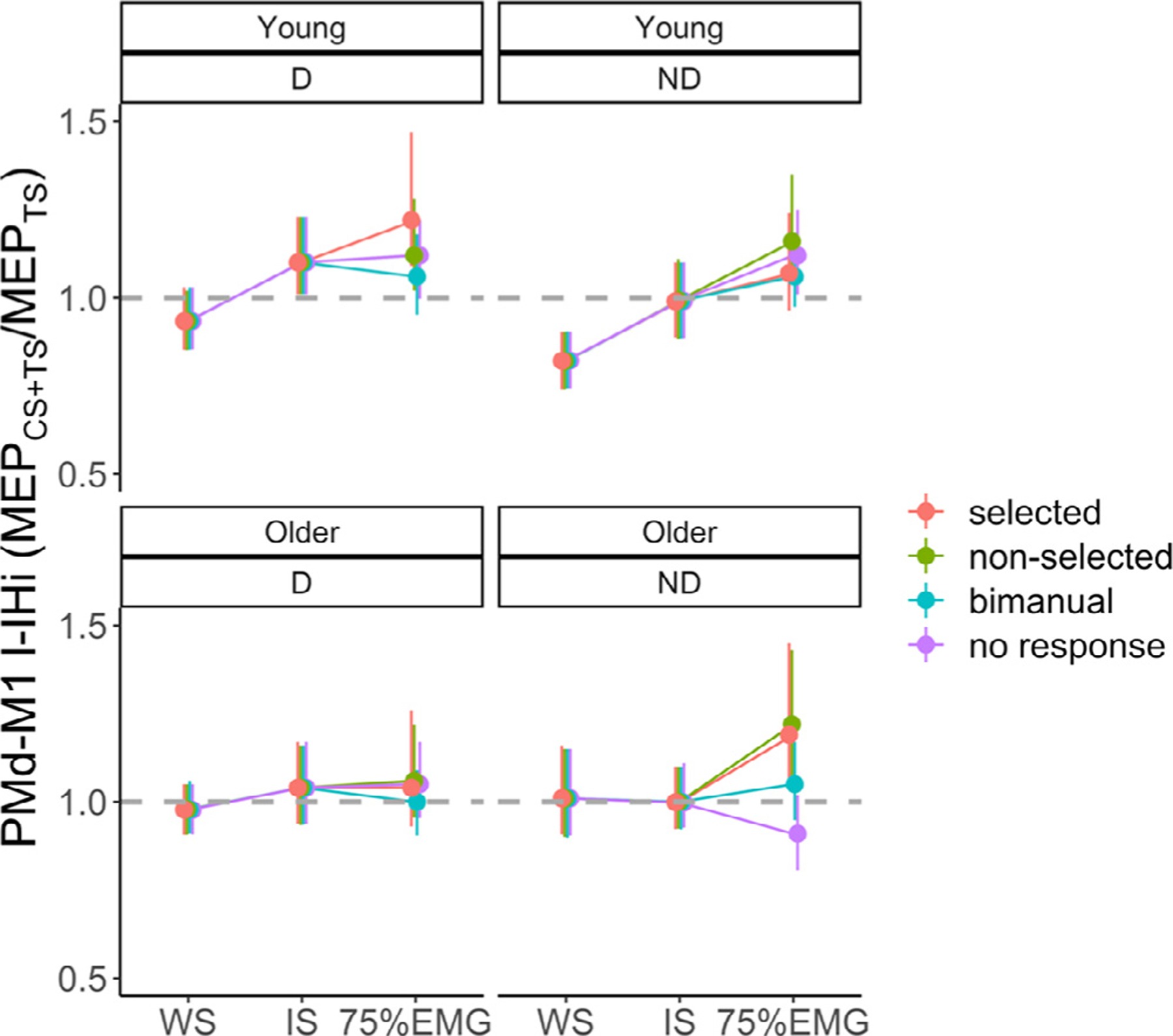
Long latency interhemispheric PMd-M1 interactions (PMd-M1 l-IHi) at each time point during a trial, for each age group (Young vs. Older) and target hemisphere (D vs. ND). Line plots in different colours represent the different response conditions (selected vs. non-selected vs. bimanual vs. no response). Values below the dashed line indicate an inhibitory PMd-M1 l-IHi, whereas values above the dashed line indicate a facilitatory PMd-M1 l-IHi. Error bars indicate 95%CIs. Abbreviations: D, dominant; ND, non-dominant; WS, warning signal; IS, imperative signal; 75%EMG, 75% of the time between IS onset and EMG onset; PMd, dorsal premotor cortex; M1, primary motor cortex; CS, conditioning stimulus; TS, test stimulus.
Fig. A4.
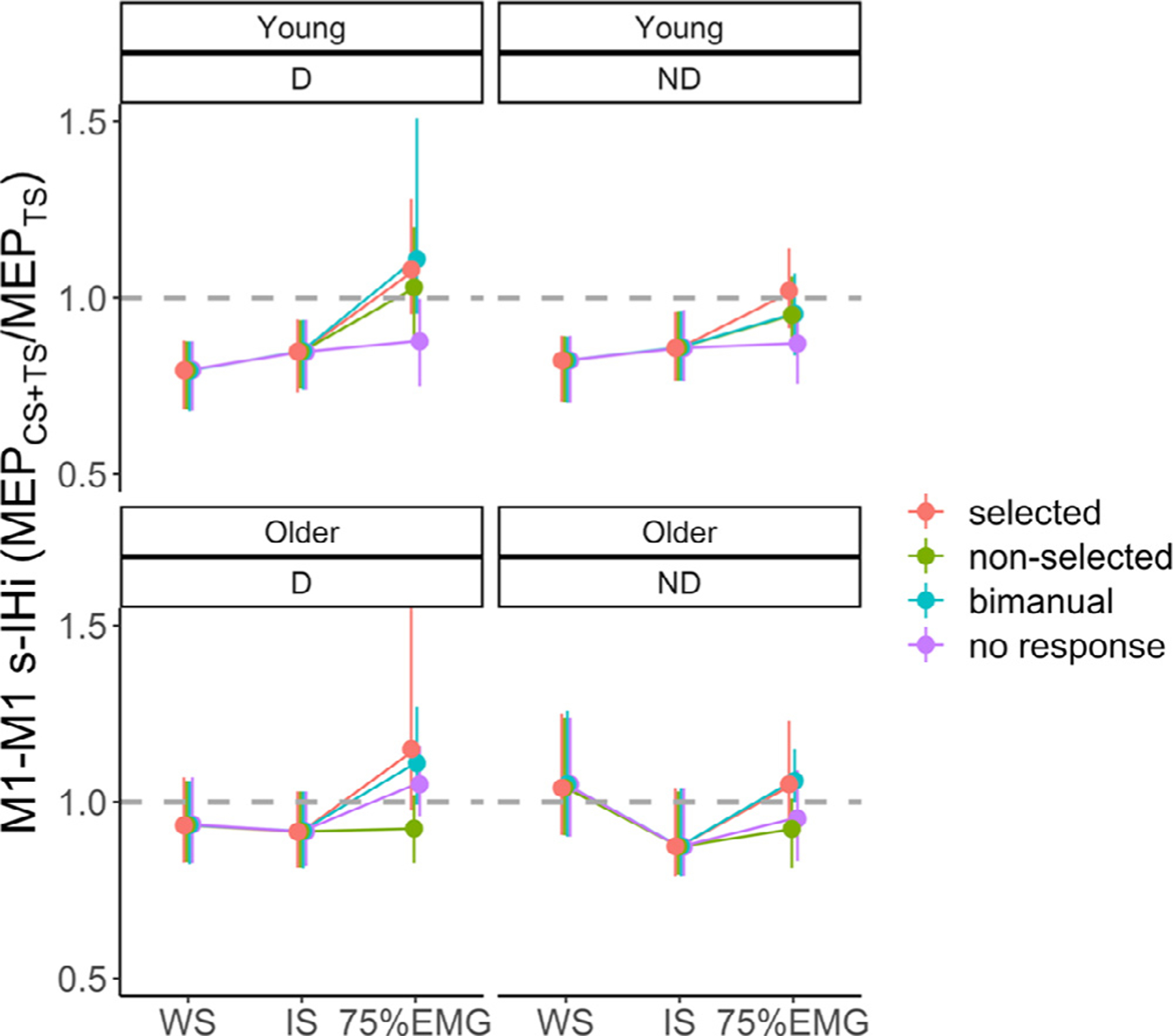
Short latency interhemispheric M1-M1 interactions (M1-M1 s-IHi) at each time point during a trial, for each age group (Young vs. Older) and target hemisphere (D vs. ND). Line plots in different colours represent the different response conditions (selected vs. non-selected vs. bimanual vs. no response). Values below the dashed line indicate an inhibitory M1-M1 s-IHi, whereas values above the dashed line indicate a facilitatory M1-M1 s-IHi. Error bars indicate 95%CIs. Abbreviations: D, dominant; ND, non-dominant; WS, warning signal; IS, imperative signal; 75%EMG, 75% of the time between IS onset and EMG onset; M1, primary motor cortex; CS, conditioning stimulus; TS, test stimulus.
Fig. A5.
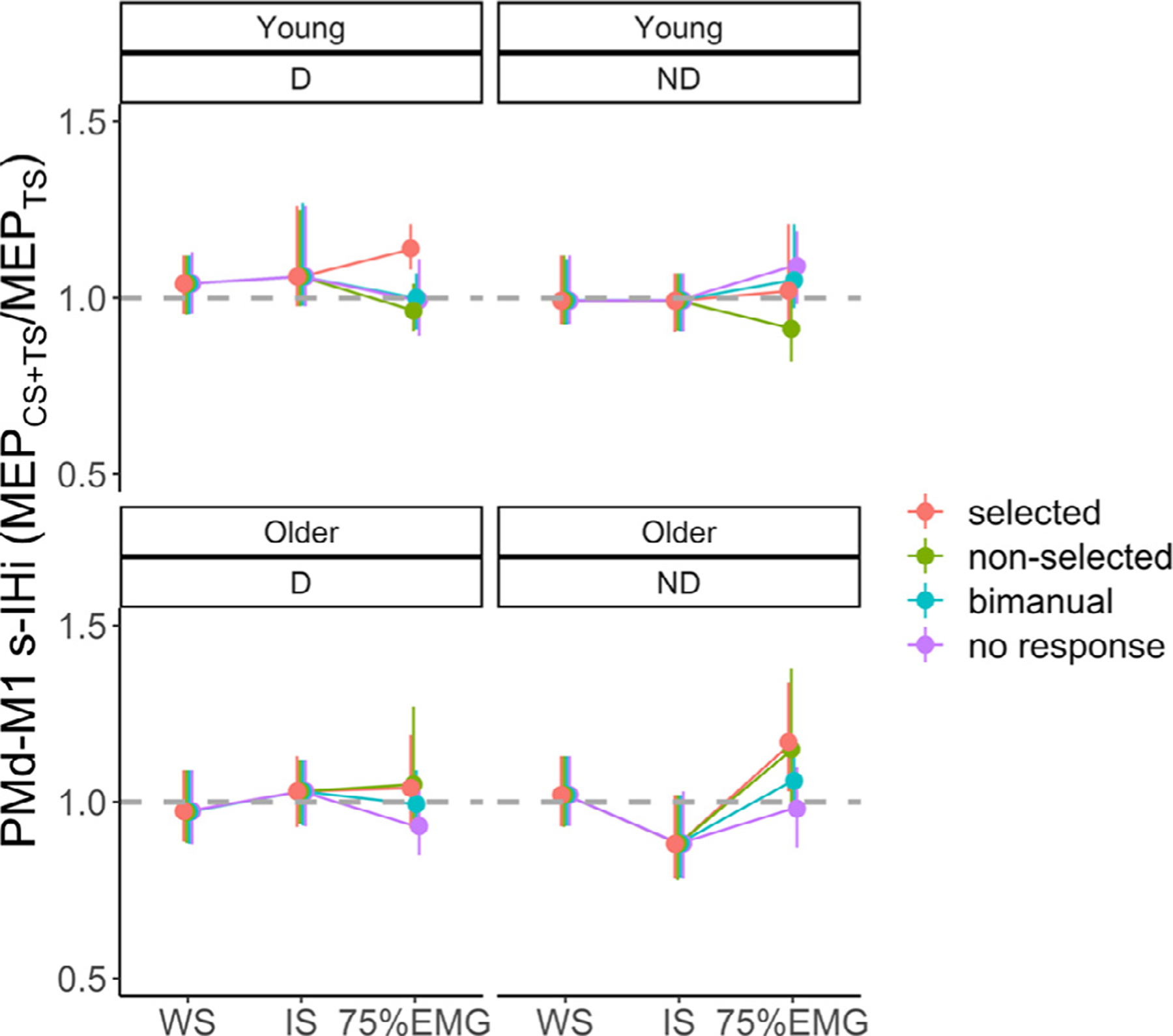
Short latency interhemispheric PMd-M1 interactions (PMd-M1 s-IHi) at each time point during a trial, for each age group (Young vs. Older) and target hemisphere (D vs. ND). Line plots in different colours represent the different response conditions (selected vs. non-selected vs. bimanual vs. no response). Values below the dashed line indicate an inhibitory PMd-M1 s-IHi, whereas values above the dashed line indicate a facilitatory PMd-M1 s-IHi. Error bars indicate 95%CIs. Abbreviations: D, dominant; ND, non-dominant; WS, warning signal; IS, imperative signal; 75%EMG, 75% of the time between IS onset and EMG onset; PMd, dorsal premotor cortex; M1, primary motor cortex; CS, conditioning stimulus; TS, test stimulus.
Fig. A6.
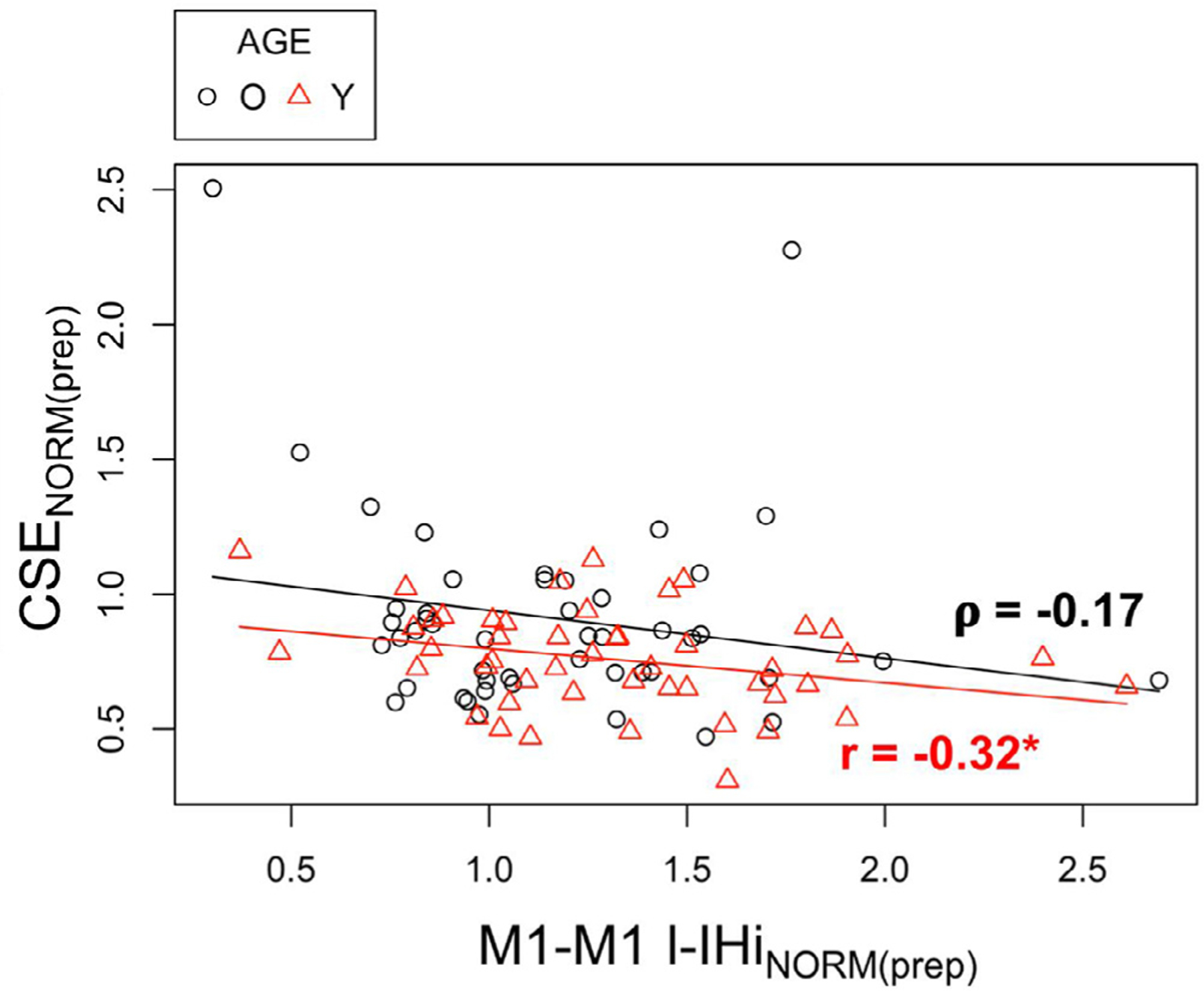
Relationship between (1) CSENORM(prep) of the target M1 and (2) M1-M1 l-IHiNORM(prep) for young (red triangles) and older adults (black circles). Results are collapsed for both directions (l-IHiNORM(prep)) and bilateral M1 (CSENORM(prep)). Abbreviations: O, Older adults; Y, Young adults; r, Pearson’s r correlation coefficient; ρ, Spearman’s ρ correlation coefficient; CSENORM(prep), modulation of corticospinal excitability during the preparatory period; M1, primary motor cortex; l-IHiNORM(prep), modulation of long latency interhemispheric interaction during the premotor period; *, p < 0.05.
Fig. A7.
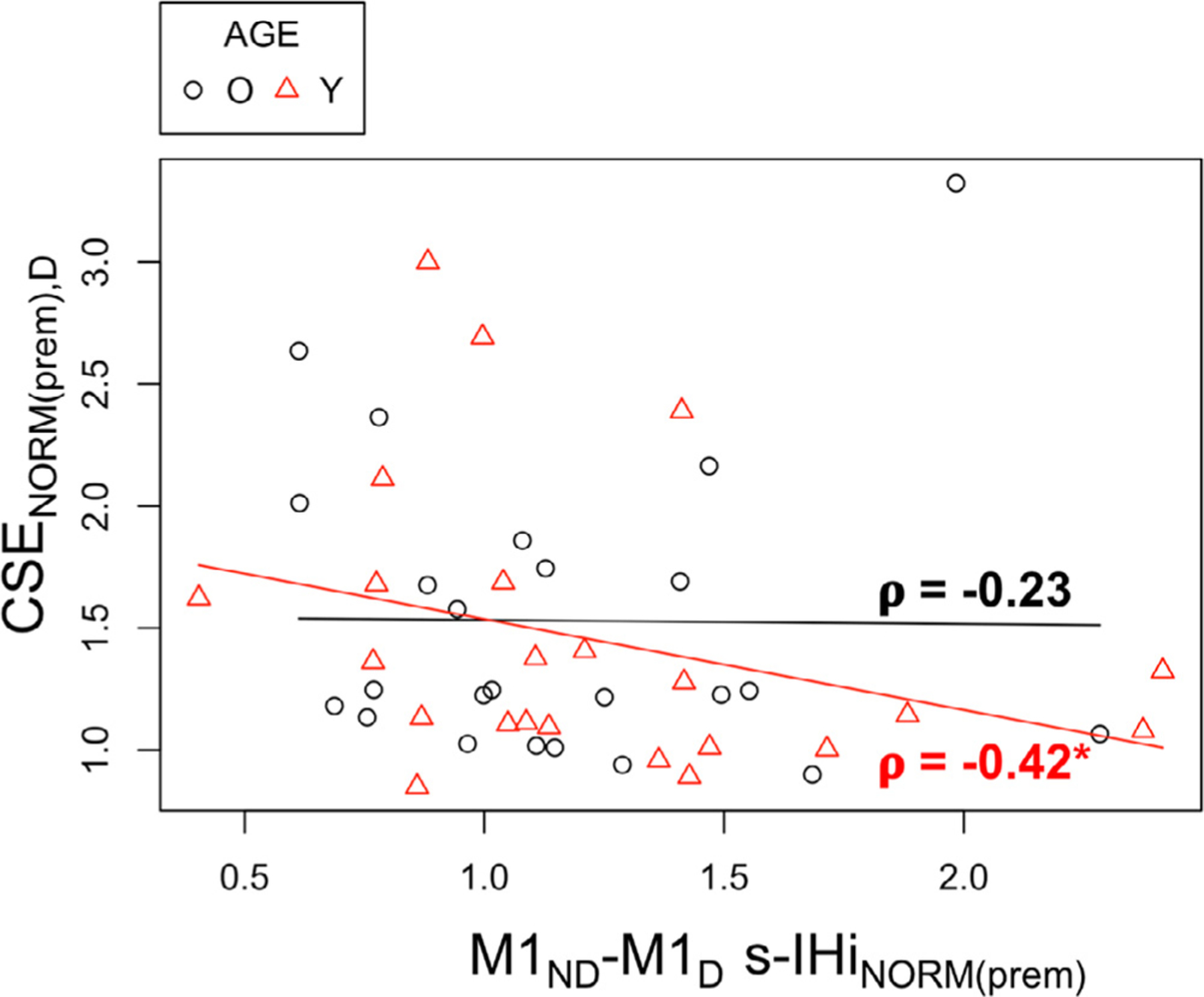
Relationship between (1) CSENORM(prem) of the dominant M1 and (2) M1ND−M1D s-IHiNORM(prem) when the target (dominant) FDI is selected for a unimanual response, for young (red triangles) and older adults (black circles). Abbreviations: O, Older adults; Y, Young adults; ρ, Spearman’s ρ correlation coefficient; CSENORM(prem), modulation of corticospinal excitability during the premotor period; M1, primary motor cortex; ND, non-dominant; D, dominant; s-IHiNORM(prem), modulation of short latency interhemispheric interaction during the premotor period; FDI, first dorsal interosseus; *, p < 0.05.
Fig. A8.
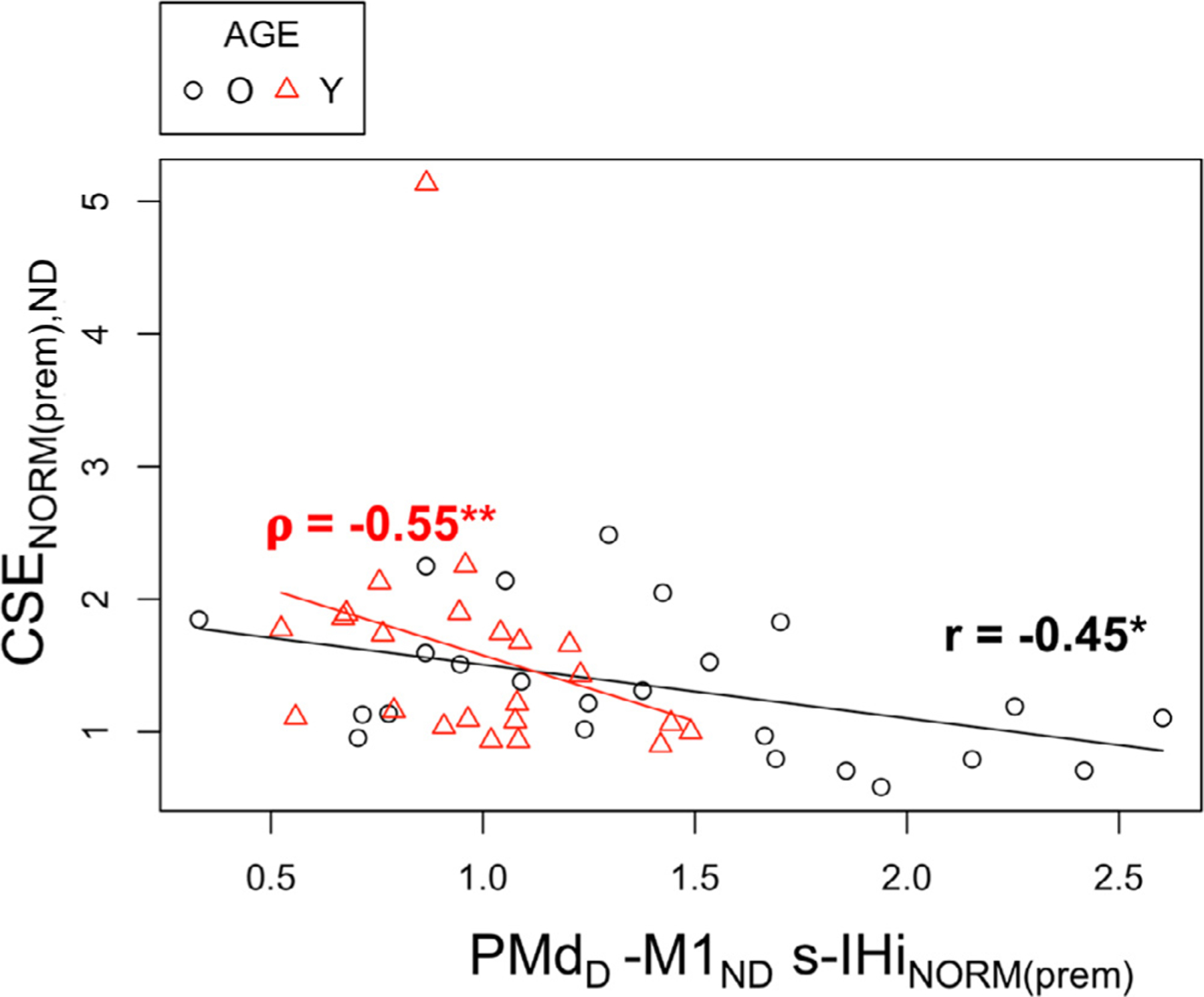
Relationship between (1) CSENORM(prem) of the non-dominant M1 and (2) PMdD−M1ND s-IHiNORM(prem) when the target (non-dominant) FDI is selected for a unimanual response, for young (red triangles) and older adults (black circles). Abbreviations: O, Older adults; Y, Young adults; r, Pearson’s r correlation coefficient; ρ, Spearman’s ρ correlation coefficient; CSENORM(prem), modulation of corticospinal excitability during the premotor period; M1, primary motor cortex; PMd, dorsal premotor cortex; ND, non-dominant; D, dominant; s-IHiNORM(prem), modulation of short latency inter-hemispheric interaction during the premotor period; FDI, first dorsal interosseus; *, p < 0.05; **, p < 0.01.
Footnotes
Disclosure statement
The authors disclose no conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118500.
Data and code availability statement
Ethical approval granted by the local ethics committee does not permit the publication of data online. If readers would like to access these data, additional ethical approval (on an individual user and purpose basis) will be required. Such requests can be made directly to the local medical ethical committee. The authors are also happy to support such ethical approval applications. Requests for custom MATLAB codes can be addressed to the corresponding author.
References
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Bäumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, Münchau A, 2006. Magnetic stimulation of human premotor or motor cortex produces inter hemispheric facilitation through distinct pathways. J. Physiol 572, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc 57, 289–300. [Google Scholar]
- Berchicci M, Lucci G, Pesce C, Spinelli D, Di Russo F, 2012. Prefrontal hyperactivity in older people during motor planning. Neuroimage 62, 1750–1760. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Duque J, 2016. Transcranial magnetic stimulation : decomposing the processes underlying action preparation. Neurosci. 22, 392–405. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, van Ruitenbeek P, Leunissen I, Chalavi S, Sunaert S, Levin O, Swinnen SP, 2016. Nucleus accumbens and caudate atrophy predicts longer action selection times in young and old adults. Hum. Brain Mapp 4639, 4629–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Wittenberg GF, Fujiyama H, Levin O, Swinnen SP, 2014. Complexity of central processing in simple and choice multi limb reaction-time tasks. PLoS ONE 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A, 1996. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 144, 160–170. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P, 2010. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 20, 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17, 85–100. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR, 1998. Callosal terminals in the rat prefrontal cortex: synaptic targets and association with GABA-immuno reactive structures. Synapse 29, 193–205. [DOI] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, Seidler RD, Polk TA, 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalavi S, Pauwels L, Heise KF, Zivariadab H, Maes C, Puts NAJ, Edden RAE, Swinnen SP, 2018. The neurochemical basis of the contextual interference effect. Neurobiol. Aging 66, 85–96. [DOI] [PubMed] [Google Scholar]
- Chen R, 2004. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp. Brain Res 154, 1–10. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M, 1999. The time course of changes in motor cortex excitability associated with voluntary movement. Can J. Neurol. Sci 26, 163–169. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J-Y, 2003. Organization of Ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol 89, 1256–1264. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF, 2005. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45, 801–814. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Hehl M, van Aalst J, Chalavi S, Mikkelsen M, Van Laere K, Dupont P, Mantini D, Swinnen SP, 2020a. Age-related GABAergic differences in the primary sensorimotor cortex: a multimodal approach combining PET. MRS and TMS. Neuroimage 226, 117536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Maes C, Swinnen SP, 2018. Aging and GABA. Aging (Albany NY) 10, 1186–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Marsman A, 2021. Transcranial magnetic stimulation and magnetic resonance spectroscopy: opportunities for a bimodal approach in human neuroscience. Neuroimage 224, 117394. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Thijs H, Duque J, Swinnen SP, Levin O, Meesen RLJ, 2013. Age-related differences in corticospinal excitability during a choice reaction time task. Age (Omaha) 35, 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Verstraelen S, Maes C, Hermans L, Hehl M, Heise K-F, Chalavi S, Mikkelsen M, Edden R, Levin O, Sunaert S, Meesen R, Mantini D, Swinnen SP, 2020b. Task-related measures of short-interval intra cortical inhibition and GABA levels in healthy young and older adults: a multimodal TMS-MRS study. Neuroimage 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R, 2002. The mechanisms of inter-hemispheric inhibition in the human motor cortex. J. Physiol 543, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf RA 2019. In Vivo NMR spectroscopy: principles and techniques. Third. ed. [Google Scholar]
- Depestele S, Ross V, Verstraelen S, Brijs K, Brijs T, Dun K, Van, Meesen R, 2020. The impact of cognitive functioning on driving performance of older persons in comparison to younger age groups : a systematic review. Transp. Res. Part F Psychol. Behav 73, 433–452. [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC, 1998. Magnetic transcranial stimulation at intensities below active motor threshold activates intra-cortical inhibitory circuits. Exp. Brain Res 119, 265–268. [DOI] [PubMed] [Google Scholar]
- Duque J, Greenhouse I, Labruna L, Ivry RB, 2017. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 40, 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB, 2009. Role of corticospinal suppression during motor preparation. Cereb. Cortex 19, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Cazares C, Ivry RB, 2014. Dissociating the influence of response selection and task anticipation on cortico-spinal suppression during response preparation 65, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Richard B 2010. Evidence for two concurrent inhibitory mechanisms during response preparation. 30:3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG, 2005. Kinematically specific inter-hemispheric inhibition operating in the process of generation of a voluntary movement. Cereb. Cortex 15, 588–593. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG, 2007. Inter-manual differences in movement-related inter-hemispheric inhibition. J. Cogn. Neurosci 19, 204–213. [DOI] [PubMed] [Google Scholar]
- Dyke K, Pépés SE, Chen C, Kim S, Sigurdsson HP, Draper A, Husain M, Nachev P, Gowland PA, Morris PG, Jackson SR, 2017. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage 152, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: a batch-processing tool for the quantitative analysis of GABA-edited MRS spectra. J. Magn. Reson. Imag 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Yordanova J, Kolev V 2006. Effects of aging on slowing of motor-response generation. 59:22–29. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD, 1992. Inter-hemispheric Inhibition of the Human Motor Cortex. J. Physiol 453, 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland MC, Therrien-Blanchet JM, Lefebvre G, Klees-Themens G, Proulx S, Théoret H, 2019. Longitudinal assessment of 1H-MRS (GABA and GLX) and TMS measures of cortical inhibition and facilitation in the sensorimotor cortex. Exp. Brain Res 237, 3461–3474. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, Summers JJ, 2009. Age-related differences in inhibitory processes during inter-limb coordination. Brain Res. 1262, 38–47. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Tandonnet C, Garry MI, Summers JJ, 2012. Age-related differences in corticomotor excitability and inhibitory processes during a Visuomotor RT Task. J Cogn Neurosci 24, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Van Soom J, Rens G, Cuypers K, Heise K-F, Levin O, Swinnen SP, 2016a. Performing two different actions simultaneously: the critical role of inter-hemispheric interactions during the preparation of bimanual movement. CORTEX 77, 141–154. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, Swinnen SP, 2016b. Age-related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J. Neurosci 36, 1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in. Brain GABA Levels. Neuroimage. 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U, 2009. Modulation of inter-hemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J. Physiol 587, 5393–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JOS, 2011. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2, 30–48. [PMC free article] [PubMed] [Google Scholar]
- Golan H, Talpalar AE, Schleifstein-Attias D, Grossman Y, 1996. GABA metabolism controls inhibition efficacy in the mammalian CNS. Neurosci. Lett 217, 25–28. [DOI] [PubMed] [Google Scholar]
- Grady C, 2012. Trends in neurocognitive aging. Nat. Rev. Neurosci 13, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, King M, Noah S, Maddock RJ, Ivry RB, 2017. Individual differences in resting cortico spinal excitability are correlated with reaction time and GABA content in motor cortex. J. Neurosci 37, 2686–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Saks D, Hoang T, Ivry RB 2015. Inhibition during response preparation is sensitive to response complexity. 2792–2800. [DOI] [PMC free article] [PubMed]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I, 2001. Inter-hemispheric facilitation of the hand motor area in humans. J. Physiol 531, 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Edden RAE, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation and tissue relaxations. J. Magn. Reson. Imaging 42, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M 1999. The time-course of preparatory spinal and cortico-spinal inhibition : an H-reflex and transcranial magnetic stimulation study in man. 33–41. [DOI] [PubMed]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï C-A, 1997. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Cogn. Brain Res 5, 185–192. [DOI] [PubMed] [Google Scholar]
- Heise K-F, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC, 2013. The aging motor system as a model for plastic changes of GABA-mediated intra-cortical inhibition and their behavioral relevance. J. Neurosci 33, 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, Leunissen I, Pauwels L, Cuypers K, Peeters R, Puts NAJ, Edden RAE, Swinnen SP, 2018a. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci 38, 7844–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, Levin O, Maes C, van Ruitenbeek P, Heise KF, Edden RAE, Puts NAJ, Peeters R, King BR, Meesen RLJ, Leunissen I, Swinnen SP, Cuypers K, 2018b. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Hinder MR, Fujiyama H, Summers JJ, 2012. Premotor-motor inter-hemispheric inhibition is released during movement initiation in older but not young adults. PLoS ONE 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Puri R, Kemp S, Waitzer S, Reissig P, Stöckel T, Fujiyama H, 2018. Distinct modulation of interhemispheric inhibitory mechanisms during movement preparation reveals the influence of cognition on action control. Cortex 99, 13–29. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J, 2007. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol 17, 234–242. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow Jv., Brandt SA, 2007. Effects of GABA A and GABA B agonists on interhemispheric inhibition in man. Clin. Neurophysiol 118, 308–316. [DOI] [PubMed] [Google Scholar]
- Jordan TC, Rabbit PMA, 1977. Response times to stimuli of increasing complexity as a function of ageing. Br. J. Psychol 68, 189–201. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, Weerd P De, Desimone R, Ungerleider LG, 1999. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22, 751–761. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, 1992. Receptor subtypes involved in callosally-induced postsynaptic potentials in rat frontal agranular cortex in vitro. Exp. Brain Res 88, 33–40. [DOI] [PubMed] [Google Scholar]
- King BR, van Ruitenbeek P, Leunissen I, Cuypers K, Heise K-F, Santos Monteiro T, Hermans L, Levin O, Albouy G, Mantini D, Swinnen SP, 2017. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cereb. Cortex 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P-A, Duque J, Labruna, Ivry RB, 2016. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. Neuroimage 125, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC, 2006. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J. Neurosci 26, 7452–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev V, Falkenstein M, Yordanova J 2006. Motor-response generation as a source of aging-related behavioural slowing in choice-reaction tasks. 27:1719–1730. [DOI] [PubMed] [Google Scholar]
- Kroeger J, Bäumer T, Jonas M, Rothwell JC, Siebner HR, Münchau A, 2010. Charting the excitability of premotor to motor connections while withholding or initiating a selected movement. Eur. J. Neurosci 32, 1771–1779. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD 1993. Corti-cocortical inhibition in human motor cortex. 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R, 2005. Interactions between long latency afferent inhibition and inter-hemispheric inhibitions in the human motor cortex. J. Physiol 563, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Fernández-Del-Olmo M, Ivry RB, 2011. Comparison of different baseline conditions in evaluating factors that influence motor cortex excitability. Brain Stimul. 4, 152–155. [DOI] [PubMed] [Google Scholar]
- Lebon F, Greenhouse I, Labruna L, Vanderschelden B, Papaxanthis C, Ivry RB, 2016. Influence of delay period duration on inhibitory processes for response preparation. Cereb. Cortex 26, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R, 2007. The effects of inhibitory and facilitatory intra-cortical circuits on inter-hemispheric inhibition in the human motor cortex. J. Physiol 580, 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee DK, 2018. What is the proper way to apply the multiple comparison test? Kor. J. Anesthesiol 71, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R 2018. Emmeans: estimated Marginal Means, aka Least-Squares Means.
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M, 2000. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123, 1161–1173. [DOI] [PubMed] [Google Scholar]
- Levin O, Cuypers K, Netz Y, Thijs H, Nuttin B, Helsen WF, Meesen RLJ, 2011. Age-related differences in human corticospinal excitability during simple reaction time. Neurosci. Lett 487, 53–57. [DOI] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ, 2014. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci. Biobehav. Rev 43, 100–117. [DOI] [PubMed] [Google Scholar]
- Liuzzi G, Hörniß V, Hoppe J, Heise K, Zimerman M, Gerloff C, Hummel FC, 2010. Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb. Cortex 20, 1323–1331. [DOI] [PubMed] [Google Scholar]
- Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, Van Zijl PCM, 2005. Routine clinical brain MRI sequences for use at 3.0 tesla. J. Magn. Reson. Imaging 22, 13–22. [DOI] [PubMed] [Google Scholar]
- Maes C, Cuypers K, Heise K, Edden RAE, Gooijers J, Swinnen SP 2021. GABA levels are differentially associated with bimanual motor performance in older as compared to young adults. 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Gooijers J, Orban de Xivry J-J, Swinnen SP, Boisgontier MP, 2017. Two hands, one brain, and aging. Neurosci. Biobehav. Rev 75, 234–256. [DOI] [PubMed] [Google Scholar]
- Maes C, Hermans L, Pauwels L, Chalavi S, Leunissen I, Levin O, Cuypers K, Peeters R, Sunaert S, Mantini D, Puts NAJ, Edden RAE, Swinnen SP, 2018. Age-related differences in GABA levels are driven by bulk tissue changes. Hum. Brain Mapp 39, 3652–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR, 2002. Neurophysiological correlates of age-related changes in human motor function. Neurology. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Singh KD, Brealy JA, Linden DEJ, Evans CJ, 2016. Quantification of γ-aminobutyric acid (GABA) in 1H MRS volumes composed heterogeneously of grey and white matter. NMR Biomed. 29, 1644–1655. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang Y-Z, Rothwell JC, 2004. Inter-hemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J. Physiol 561, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Cirillo J, Byblow WD, 2017. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J. Neurophysiol 118, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Edden RAE, 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh I-J, Castillo G, Hoque T, Chen R, 2009. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb. Cortex 19, 1654–1665. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MFS, 2007. Functional specificity of human premotor-motor cortical interactions during action selection. Eur. J. Neurosci 26, 2085–2095. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The Assessment and Analysis of Handedness: the Edinburgh Inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME, 2012. The Cellular Basis of GABAB-mediated inter-hemispheric inhibition. Science (80-) 335, 989–993. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-solé J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M, 1992. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain 115, 1045–1059. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, Pascual-Leone A, Rosenow F, Rothwell JC, Ziemann U, 2008. State of the art : pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 1, 151–163. [DOI] [PubMed] [Google Scholar]
- Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P, 2009. Functional changes in CSF volume estimated using measurement of water T2 relaxation. Magn. Reson. Med 61, 579–586. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R. Core Team. 2017. Linear and Nonlinear Mixed Effects Models.
- Porges EC, Woods AJ, Edden RAE, Puts NAJ, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA, 2017a. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, Williamson JB, Cohen RA, Edden RAE, Harris AD, 2017b. Impact of tissue correction strategy on GABA-Edited MRS findings. Neuroimage 162, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, 2012. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc 60, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Derosiere G, 2015. Global and specific motor inhibitory mechanisms during action preparation. J. Neurosci 35, 16297–16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Core Team. 2017. R: a Language and environment for statistical computing.
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG, 2008. Contribution of trans-cranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol 586, 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggeveen AB, Prime DJ, Ward LM, 2007. Lateralized readiness potentials reveal motor slowing in the aging brain. J. Gerontol 62B, 78–84. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C, 1994. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin. Neurophysiol 91, 79–92. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J 2007. Clinical neurophysiology of aging brain : from normal aging to neuro degeneration. 83:375–400. [DOI] [PubMed] [Google Scholar]
- RStudio Team. 2020. RStudio: integrated development environment for R.
- Rushworth MFS, Johansen-Berg H, Göbel SM, Devlin JT, 2003. The left parietal and premotor cortices: motor attention and selection. Neuroimage 20. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 2000. Aging and measures of processing speed 54, 35–54. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MFS, Passingham RE, Mills KR, 1998. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. a study using transcranial magnetic stimulation. Brain 121, 785–799. [DOI] [PubMed] [Google Scholar]
- Serbruyns L, Gooijers J, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP, 2015. Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum sub-regions. Brain Struct Funct 220, 273–290. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR, 2008. Reduced intra-cortical inhibition during the fore period of a warned reaction time task. Exp Brain Res 186, 385–392. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011a. The role of GABA in human motor learning. Curr Biol 21, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC, 2011b. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 589, 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Tran X, Cramer SC, 2014. Age-related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults 19, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD, 2010. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci 13, 825–827. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, 2002. Inter-manual coordination : from behavioral principles to neural-network interactions. Nat. Rev. Neurosci 3, 350–361. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N, 2004. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn. Sci 8, 18–25. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS, 2008. The effect of age on task-related modulation of inter-hemispheric balance. Exp. Brain Res 186, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC, 1998. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr. Clin. Neurophysiol. - Electromyogr. Mot. Control 109, 489–495. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, de Beaumont L, Marjańska M, Doyon J, Pascual-Leone A, Lassonde M, Théoret H, 2013. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate + glutamine. J. Neurophysiol 109, 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadis P, Derosiere G, Grandjean J, Duque J, 2020. Motor training strengthens corticospinal suppression during movement preparation. J. Neurophysiol. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U, 2007. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J. Neurosci 27, 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS, 1999. NMR relaxation times in the human brain at 3.0 Tesla. J. Magn. Reson. Imaging 9, 531–538. [DOI] [PubMed] [Google Scholar]
- Woods DL, Wyma JM, William Yund E, Herron TJ, Reed B, 2015. Age-related slowing of response selection and production in a visual choice reaction time task. Front. Hum. Neurosci 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association, 2013. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Med. Assoc 310, 2191–2194. [DOI] [PubMed] [Google Scholar]
- Yasen AL, Smith J, Christie AD, 2017. Reliability of glutamate and GABA quantification using proton magnetic resonance spectroscopy. Neurosci. Lett 643, 121–124. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Hohnsbein J, Falkenstein M 2004. Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes : evidence from high-resolution event-related potentials. 127:351–362. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P, 1997. Localization of the motor hand area to a knob on the pre-central gyrus. a new landmark. Brain 120, 141–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ethical approval granted by the local ethics committee does not permit the publication of data online. If readers would like to access these data, additional ethical approval (on an individual user and purpose basis) will be required. Such requests can be made directly to the local medical ethical committee. The authors are also happy to support such ethical approval applications. Requests for custom MATLAB codes can be addressed to the corresponding author.


