Abstract
Background
One possible reason for increased mortality due to SARS-CoV-2 in patients with diabetes is from the complication of diabetic ketoacidosis (DKA).
Objectives
To re-evaluate the association of SARS-CoV-2 and development of DKA and analyse the demographic and biochemical parameters and the clinical outcomes in COVID-19 patients with DKA.
Design
A systematic review and meta-analysis. Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was followed.
Methods
Electronic databases (Proquest, Medline, Embase, Pubmed, CINAHL, Wiley online library, Scopus and Nature) were searched from 1 December 2019 to 30 June 2021 in the English language using the following keywords alone or in combination: COVID-19 OR SARS-CoV-2 AND diabetic ketoacidosis OR DKA OR ketosis OR ketonemia OR hyperglycaemic emergency OR hyperglycaemic crisis. We included studies in adults and children of all ages in all healthcare settings. Binary logistic regression model was used to explore the effect of various demographic and biochemical parameters variables on patient’s final treatment outcome (survival or death).
Results
Of the 484 papers that were identified, 68 articles were included in the systematic review and meta-analysis (54 case report, 10 case series, and 4 cohort studies). Studies involving 639 DKA patients with confirmed SARS-CoV-2 [46 (7.2%) were children and 334 (52.3%) were adults] were analyzed. The median or mean patient age ranged from < 1 years to 66 years across studies. Most of the patients (n = 309, 48.3%) had pre-existing type 2 diabetes mellitus. The majority of the patients were male (n = 373, 58.4%) and belonged to Hispanic (n = 156, 24.4%) and black (n = 98, 15.3%) ethnicity. The median random blood glucose level, HbA1c, pH, bicarbonate, and anion gap in all included patients at presentation were 507 mg/dl [IQR 399–638 mg/dl], 11.4% [IQR 9.9–13.5%], 7.16 [IQR 7.00–7.22], 10 mmol/l [IQR 6.9–13 mmol/l], and 24.5 mEq/l [18–29.2 mEq/l]; respectively. Mortality rate was [63/243, 25.9%], with a majority of death in patients of Hispanic ethnicity (n = 17, 27%; p = 0.001). The odd ratios of death were significantly high in patients with pre-existing diabetes mellitus type 2 [OR 5.24, 95% CI 2.07–15.19; p = 0.001], old age (≥ 60 years) [OR 3.29, 95% CI 1.38–7.91; p = 0.007], and male gender [OR 2.61, 95% CI 1.37–5.17; p = 0.004] compared to those who survived.
Conclusion
DKA is not uncommon in SARS-CoV-2 patients with diabetes mellitus and results in a mortality rate of 25.9%. Mortality key determinants in DKA patients with SARS-CoV-2 infection are individuals with pre-existing diabetes mellitus type 2, older age [≥ 60 years old], male gender, BMI ≥ 30, blood glucose level > 1000 mg/dl, and anion gap ≥ 30 mEq/l.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-021-00740-6.
Keywords: SARS-Cov-2, Diabetes, COVID-19, Ketoacidosis, Systematic Review, Meta-Analysis
Background
Diabetes is a frequent comorbidity in patients with severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], with a reported prevalence ranging from 9 to 20% [1–4]. Diabetes is also associated with more than twofold higher risk of having severe or critical corona virus disease 2019 [COVID-19] illness and about threefold increased risk of in-hospital mortality compared to SARS-CoV-2 patients without diabetes [1–4]. A possible reason for increased mortality due to SARS-CoV-2 in patients with diabetes is from the complication of diabetic ketoacidosis (DKA), one of the most serious acute complications of diabetes. DKA is characterized by the presence of hyperglycaemia [usually < 800 mg/dl and generally between 350 to 500 mg/dl], arterial pH [≤ 7.30], anion gap [> 12 mEq/l], and serum bicarbonate [≤ 15 mmol/l] [5].
In light of newer case reports, case-series and cohort studies that were done to re-evaluate the association of SARS-CoV-2 and development of DKA, we aimed to analyse the demographic and biochemical parameters and the clinical outcomes in COVID-19 patients with DKA with larger and better-quality data.
Methods
Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) in conducting this systematic review and meta-analysis [6]. The following electronic databases were searched: PROQUEST, MEDLINE, EMBASE, PUBMED, CINAHL, WILEY ONLINE LIBRARY, SCOPUS and NATURE with Full Text. We used the following keywords: COVID-19 OR SARS-CoV-2 AND diabetic ketoacidosis OR DKA OR ketosis OR ketonemia OR hyperglycaemic emergency OR hyperglycaemic crisis OR euglycemia OR euglycemic. The search was limited to papers published in English between 1 December 2019 and 30 June 2021. Based on the title and abstract of each selected article, we selected those discussing and reporting occurrence of DKA in COVID-19 patients. We also utilized backward snowballing to increase the yield of potentially relevant articles (Additional file 1).
Inclusion–exclusion criteria
We included case reports, case series and cohort studies, but excluded editorials, commentaries, case and animal studies, discussion papers, preprints, news analyses, and reviews. We considered studies to be eligible regardless of experimental or observational design, and irrespective of their primary objective. However, we excluded studies that did not report data on DKA and SARS-CoV-2; studies that never reported details on SARS-CoV-2 identified cases with DKA; or studies that reported DKA in patients with negative PCR COIVD-19 tests. We evaluated studies that included all children and adults as our population of interest who experienced DKA and SARS-CoV-2 infection during the period from December 1, 2019 through June 30, 2021.
Data extraction
Four authors (S.A., A.A., A.R. and Z.A.) critically reviewed all of the studies retrieved and selected those judged to be the most relevant. The abstracts of all citations were examined thoroughly. Data were extracted from the relevant research studies using key headings, which are noted in Table 1, simplifying analysis, and review of the literature. Articles were categorized as case report, case series or cohort studies.
Table 1.
Summary of the characteristics of the included studies with evidence on diabetic ketoacidosis and SARS-CoV-2 (n = 68 studies), 2019–2021
| Author, year, study location | Study design, setting | Age (years)b | Male, n (%) | BMI (kg/m2)b | Ethnicitya | Type of diabetes | Use of SGLT2 inhibitors | Biochemical parameters at presentationb | NOS score; and Treatment outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood glucose (mg/dl) | HbA1c (%) | pH | Bicarbonate (mmol/l) | Anion gap (mEq/l) | |||||||||
| Albuali et al. 2021 [8], Saudi Arabia | Retrospective case report, single centre | 7 | 0 (0) | Not reported | 1 Arab | 1 Newly diagnosed | No | 555 | 10.3 | 7.10 | 10 | 23 |
(NOS, 6) 1 survived |
| Alfishawy et al. 2021 [9], Egypt | Retrospective case report, single centre | 17 | 1 (100) | Not reported | 1 Arab | 1 Newly diagnosed | No | 566 | 14.7 | 6.8 | Not reported | Not reported |
(NOS, 5) 1 survived |
| Ali et al. 2021 [10], Qatar | Retrospective case report, single centre | 53 | 1 (100) | Not reported | 1 Bengali | 1 Newly diagnosed | No | 295.2 | 6.9 | 6.831 | 5 | 35 |
(NOS, 6) 1 died |
| Alizadeh et al. 2021 [11], United States | Retrospective case report, single centre | 1.3 | 1 (100) | Not reported | Not reported | 1 Newly diagnosed | No | 805 | 9.5 | 7.0 | 4 | 40 |
(NOS, 6) 1 survived |
| Al-Naami et al. 2020 [12], Saudi Arabia | Retrospective case report, single centre | 46 | 1 [100] | 27 | 1 Arab | 1 Newly diagnosed | No | 657 | 13.5 | 7.4 | 29 | 26 |
(NOS, 5) 1 died |
| Alsadhan et al. 2020 [13], Saudi Arabia | Retrospective case series, single centre | 47 (42–62.5) | 3 (60) | 29.4 (26.8–29.4) | 5 Arab |
2 Pre-existing T2DM 1 Pre-existing T1DM 2 Newly diagnosed |
No | 491 (360–664) | 11.3 (10.4–14.8) | 7.14 (6.97–7.27) | 12.5 (8.5–14.1) | 25 (19.5–26) |
(NOS, 6) 4 survived 1 died |
| Añazco et al. 2021 [14], Peru | Retrospective case report, single centre | 41 | 0 (0) | > 30 | 1 Hispanic | 1 Pre-existing T2DM | No | 500 | Not reported | 7.29 | 20 | Not reported |
(NOS, 5) 1 died |
| Amesfoort et al. 2021 [71], The Netherlands | Retrospective case report, single centre | 21 | 0 (0) | Not reported | 1 White (Caucasian) | Not reported | No | 84.6 | Not reported | 7.34 | 8.7 | 23 |
(NOS, 6) 1 survived |
| Armeni et al. 2020 [15], United Kingdom | Retrospective case series, multicentre | 57 [48–64] | 7 (63.6) | 24·7 (21·3–28·5) |
5 Black 1 Asian 3 White (Caucasian) 2 Mixed |
2 Pre-existing T1DM 9 Pre-existing T2DM |
No | 486 (396–558) | 12.4 (10·7–14·2) | 7.2 (6.9–7.3) | 11.8 (7.8–15.4) | 14.8 (10.4–20.5) |
(NOS, 8) 10 survived 1 died |
| Batista et al. 2021 [16], Brazil | Retrospective case report, single centre | 56 | 1 (100) | 26.4 | 1 Hispanic | 1 Pre-existing T2DM | 1 Yes | 118 | 7.2 | 7.28 | 8.9 | 24.1 |
(NOS, 6) 1 survived |
| Cavalcanti et al. 2020 [17], United states | Retrospective case report, single centre | 23 | 1 (100) | Not reported | Not reported | 1 Newly diagnosed | No | 1384 | Not reported | 7.0 | Not reported | Not reported |
(NOS, 6) 1 died |
| Chamorro-Pareja et al. 2020 [18], United States | Retrospective cohort, single centre | 59 (42.3–70) | 32 (64) | 27.15 (23.2–33) |
15 Black 16 Hispanic 8 Other 3 White (Caucasian) 1 Asian 7 Unknown |
6 Pre-existing T1DM 44 Pre-existing T2DM 8 Newly diagnosed |
2 Yes | 506 (252–1485) |
HbA1c ≥ 8 (n = 30) HbA1c < 8 (n = 4) and HbA1c unknown (n = 16) |
Not reported | Not reported | 28.1 (14.3–41.2) |
(NOS, 6) 24 survived 25 died 1 hospitalized |
| Chan et al. 2020 [19], United States | Retrospective case reports, single centre | 50 (33.2–62) | 6 (100) | 24.7 (23.9–37.6) |
3 Black 3 Hispanic |
5 Pre-existing T2DM 1 Newly diagnosed |
No | 1014 (663–1116) | 12.7 (11.2–13.5) | 7.05 (6.83–7.21) | 7.3 (5.7–9.6) | 29 (27–32.2) |
(NOS, 6) 2 survived 4 died |
| Chee et al. 2020 [20], Singapore | Retrospective case report, single centre | 37 | 1 (100) | 22.6 | 1 Asian | 1 Newly diagnosed | No | 715 | 14.2 | 7.28 | 12 | 30 |
(NOS, 6) 1 survived |
| Croft et al. 2020 [21], United States | Retrospective case reports, single centre | 55 (41.5–60) | 2 (40) | 29.1 (20.6–33.6) |
1 Black 3 Hispanic |
5 Pre-existing T2DM | No | 399 (284–848) | 11.3 (9.6–13.4) | 7.1 (7.0–7.2) | Not reported | 21 (18–23) |
(NOS, 6) 3 survived 1 died 1 hospitalized |
| Daniel et al. 2020 [22], India | Retrospective case report, single centre | 15 | 0 (0) | 19 | 1 Indian | 1 Newly diagnosed | No | 414 | 13.5 | 6.9 | 2 | Not reported |
(NOS, 5) 1 survived |
| Dey et al. 2021 [23], Maldives | Retrospective case reports, single centre | 65.5 (53–65.5) | 2 (100) | Not reported | 2 Asian | 2 Pre-existing T2DM | No | 1084 (626–1084) | 9.8 (6.6–9.8) | Not reported | Not reported | Not reported |
(NOS, 5) 2 survived |
| Ebekozien et al. 2021 [24], United States | Retrospective cohort, multicentre |
≤ 19 = (n = 30) AND > 19 = (n = 25) |
23 (41.8) | > 30 (n = 9) |
30 Black 15 Hispanic 10 White (Caucasian) |
44 Pre-existing T1DM 11 Newly diagnosed |
No | Not reported | 11.1 (9–11.1) | Not reported | Not reported | Not reported |
(NOS, 8) 51 survived 4 died |
| Emara et al. 2020 [25], Saudi Arabia | Retrospective case report, single centre | 51 | 1 (100) | 21 | 1 Arab | 1 Pre-existing T2DM | No | 592 | 7.8 | 7 | Not reported | Not reported |
(NOS, 5) Not reported |
| Ghosh et al. 2021 [26], India | Retrospective case report, single centre | 60 | 1 (100) | Not reported | 1 Indian | 1 Newly diagnosed | No | 540 | 5.1 | 7.20 | 13 | 18 |
(NOS, 6) 1 survived |
| Goldman et al. 2020 [27], United Kingdom | Retrospective case reports, single centre | 50.5 (40.5–76.2) | Not reported | Not reported |
1 White (Caucasian) 2 Asian 1 Black |
3 Pre-existing T2DM 1 Newly diagnosed |
1 Yes | 378 (346–450) | 10.8 (9.5–10.8) | 7.17 (7.10–7.26) | 10 (7.5–14.8) | Not reported |
(NOS, 7) 1 survived 2 died 1 hospitalized |
| Gorthi et al. 2021 [28], United States | Retrospective case series, single centre | 65 (61.5–77) | 2 (40) | 28.6 (24.3–31.1) |
4 Black 1 White (Caucasian) |
3 Pre-existing T1DM 2 Pre-existing T2DM |
1 Yes | 587 (370.5–723) | 8.9 (8.1–10.4) | 7.31 (7.11–7.33) | 16 (7–18.5) | 26 (20–28.5) |
(NOS, 6) 4 survived 1 died |
| Haider et al. 2020 [29], United States | Retrospective case report, single centre | 46 | 0 (0) | Not reported | Not reported | 1 Pre-existing T1DM | No | 590 | Not reported | Not reported | Not reported | 18 |
(NOS, 6) 1 survived |
| Hawkes et al. 2021 [30], United States | Retrospective case reports, single centre | 6 [3–6] | 1 (50) | Not reported | Not reported | 2 Newly diagnosed | No | Not reported | Not reported | 7.17 (7.1–7.17) | 10.1 (10–10.1) | Not reported |
(NOS, 6) 2 survived |
| Heaney et al. 2020 [31], United States | Retrospective case report, single centre | 54 | 1 (100) | 42.56 | Not reported | 1 Newly diagnosed | No | 463 | Not reported | 7.193 | 9.9 | 31 |
(NOS, 6) 1 survived |
| Heidarpour et al. 2021 [32], Iran | Retrospective case report, single centre | 36 | 1 (100) | Not reported | 1 Persian | 1 Newly diagnosed | No | 500 | Not reported | 7 | 11 | Not reported |
(NOS, 6) 1 survived |
| Hollstein et al. 2020 [33], Germany | Retrospective case report, single centre | 19 | 1 (100) | Not reported | 1 White (Caucasian) | 1 Newly diagnosed | No | 552 | 16.8 | 7.1 | Not reported | Not reported |
(NOS, 6) 1 survived |
| Howard et al. 2021 [34], United States | Retrospective case reports, single centre | 14.5 (12–14.5) | 1 (50) | Not reported | Not reported | 2 Newly diagnosed | No | 518 (337–518) | 10.9 (10.8–10.9) | 6.91 (6.84–6.91) | 5 (3.7–5) | 27.5 (25–27.5) |
(NOS, 7) 2 survived |
| Ishii et al. 2021 [35], Japan | Retrospective case report, single centre | 33 | 0 (0) | Not reported | 1 Asian | 1 Newly diagnosed | No | 638 | 15.7 | 6.74 | 4.8 | 27.2 |
(NOS, 6) 1 survived |
| Kabashneh et al. 2020 [36], United States | Retrospective case report, single centre | 54 | 1 (100) | Not reported | Not reported | 1 Pre-existing T1DM | No | 1100 | Not reported | 6.79 | 4 | 46 |
(NOS, 6) 1 survived |
| Kaur et al. 2020 [37], United States | Retrospective case report, single centre | 43 | 1 (100) | Not reported | Not reported | 1 Pre-existing T2DM | No | 948 | Not reported | 6.96 | Not reported | 27 |
(NOS, 6) 1 died |
| Kim et al. 2020 [38], South Korea | Retrospective case reports, single centre | 65.5 (59–65.5) | 1 (50) | Not reported | 2 Asian | 2 Pre-existing T2DM | No | 672 (655–672) | 12 (11.4–12) | 7.381 | 18.1 | Not reported |
(NOS, 6) 1 died 1 hospitalized |
| Kuchay et al. 2020 [39], India | Retrospective case reports, single centre | 34 (30–34) | 3 (100) | 27.3 (26.2–27.3) | 3 Indian | 3 Newly diagnosed | No | 582 (555–582) | 12 (9.6–12) | 7.21 (7.07–7.21) | 13 (6.1–13) | 16.2 (11.9–16.2) |
(NOS, 6) 3 survived |
| Kulick-Soper et al. 2020 [40], United States | Retrospective case report, single centre | 52 | 0 (0) | Not reported | Not reported | 1 Newly diagnosed | No | 1114 | 17.4 | 7.25 | Not reported | 33 |
(NOS, 6) Not reported |
| Li et al. 2020 [41], China | Retrospective case reports, single centre | 44 (26–44) | 2 (66.7) | Not reported | 3 Asian | 3 Pre-existing T2DM | No | 382 (298–382) | Not reported | 7.22 (6.86–7.22) | Not reported | Not reported |
(NOS, 6) 1 survived 2 died |
| Marchon et al. 2020 [42], United Kingdom | Retrospective case reports, single centre | 28 | 0 (0) | Not reported | White (Caucasian) | 1 Pre-existing T1DM | No | 401.4 | 12.9 | 7.0 | 3.2 | Not reported |
(NOS, 6) 1 survived |
| Mondal et al. 2021 [43], India | Prospective case series, single centre | 54.8 ± (11.7) | Males were > females | 24.8 ± (1.92) | 26 Indian | 26 Pre-existing T2DM | No | Not reported | 10.1 ± (1.9) | Not reported | Not reported | Not reported |
(NOS, 6) 23 survived 3 died |
| Naguib et al. 2021 [44], United States | Retrospective case report, single centre | 8 | 0 (0) | > 35 | 1 Hispanic | 1 Newly diagnosed | No | 429 | 12 | 7.3 | 14 | 21 |
(NOS, 6) 1 survived |
| Nielsen-Saines et al. 2021 [45], United States | Retrospective case report, single centre | 7 | 1 (100) | 16.8 | 1 Hispanic | 1 Newly diagnosed | No | 470 | 14.8 | 7.01 | 3.5 | 32 |
(NOS, 6) 1 survived |
| Omotosho et al. 2021 [46], United States | Retrospective case report, single centre | 45 | 0 (0) | 25.39 | 1 Hispanic | 1 Pre-existing T2DM | No | 344 | 13.7 | 7.22 | 13 | 18 |
(NOS, 6) 1 survived |
| Oriot et al. 2020 [47], Belgium | Retrospective case report, single centre | 52 | 1 (100) | 29 | 1 White (Caucasian) | 1 Pre-existing T1DM | 1 Yes | 270 | 7.4 | 7.25 | 19 | 17 |
(NOS, 6) 1 hospitalized |
| Ozer et al. 2020 [48], Turkey | Retrospective case report, single centre | 42 | 0 (0) | Not reported | 1 White (Caucasian) | 1 Pre-existing T2DM | Yes | 196 | Not reported | 7.08 | 8.9 | 20 |
(NOS, 5) 1 survived |
| Palermo et al. 2020 [49], United States | Retrospective case reports, single centre | 49 (45–49) | 1 [50] | 30.5 (28–30.5) | Not reported |
1 Pre-existing T2DM 1 Newly diagnosed |
1 Yes | 395 (192–395) | 10 (7.5–10) | 7.21 (7.18–7.21) | 17.5 (15–17.5) | Not reported |
(NOS, 6) 2 survived |
| Panjawatanan et al. 2020 [50], United States | Retrospective case report, single centre | 59 | 1 [100] | 32 | Not reported | 1 Pre-existing T2DM | No | 387 | 11.3 | 7.25 | 19 | 13 |
(NOS, 6) 1 survived |
| Parwanto et al. 2020 [51], Indonesia | Retrospective case report, single centre | 51 | 1 (100) | Not reported | 1 Asian | 1 Pre-existing T2DM | No | 369 | Not reported | 7.22 | 9.3 | Not reported |
(NOS, 5) 1 died |
| Pasquel et al. 2021 [52], United States | Retrospective cohort, multicentre | 56 ± (17) | 120 (57.1) | 31 ± (9) | Not reported | Not reported | Not reported | 523 ± (228) | 11.3 ± (2.7) | Not reported | 12.2 ± (4.5) | 27 ± (8) |
(NOS, 8) 146 survived 64 died |
| Pikovsky et al. 2021 [53], United Kingdom | Retrospective case reports, single centre | 34 (34–34) | 0 (0) | 26.5 (25–26.5) |
1 Asian 1 White (Caucasian) |
1 Pre-existing T2DM 1 Newly diagnosed |
No | 77.4 (75.6–77.4) | 11.5 | 7.0 (6.9–7.0) | 6.6 (6.2–6.6) | 21 |
(NOS, 6) 2 survived |
| Plasencia-Dueñas et al. 2021 [54], Peru | Retrospective case reports, single centre | 64 (42.5–71.2) | 3 (75) | Not reported | 4 Hispanic | 4 Newly diagnosed | No | 740 (489–1108) | Not reported | 7.17 (6.86–7.3) | 11.6 (4–17.6) | Not reported |
(NOS, 5) Not reported |
| Potier et al. 2021 [55], France | Retrospective case report, single centre | 31 | 1 (100) | Not reported | 1 White (Caucasian) | 1 Newly diagnosed | No | 427 | Not reported | 7.25 | 8 | Not reported |
(NOS, 6) 1 survived |
| Rabizadeh et al. 2020 [56], Iran | Retrospective case report, single centre | 16 | 1 [100] | 17.7 | 1 Persian | 1 Newly diagnosed | No | 512 | 12.9 | 6.95 | 8 | Not reported |
(NOS, 5) 1 survived |
| Ramos-Yataco et al. 2021 [57], Peru | Retrospective case reports, single centre | 49 (33–49) | 3 (100) | Not reported | 3 Hispanic | 3 Newly diagnosed | No | 679 (625–679) | 4.5 | 7.1 (6.6–7.1) | 8 (4–8) | 10 |
(NOS, 5) 3 survived |
| Ramos-Yataco et al. 2021 [58], Peru | Retrospective case series, single centre | 66 (42.5–72.5) | 3 (60) | Not reported | 5 Hispanic | 5 Pre-existing T2DM | No | 538 (465.5–617.5) | 5.9 (5.6–6.7) | 7.2 (6.8–7.2) | 7.7 (4.2–10.7) | 15 (14.5–17) |
(NOS, 5) 5 survived |
| Rao et al. 2021 (59), United States | Retrospective case series, single centre | 39 (20–54) | 3 (42.8) | 28.6 (26.8–34) |
4 White (Caucasian) 3 Hispanic |
6 Pre-existing T2DM 1 Newly diagnosed |
No | 311 (282–596) | 12.8 (10.1–13.9) | 7.25 (7.18–7.37) | 13 (9–19) | 21 (19–33) |
(NOS, 6) 6 survived 1 died |
| Reddy et al. 2020 (60), India | Retrospective case reports, single centre | 45 (30–45) | 2 (100) | Not reported | 2 Indian |
1 Pre-existing T2DM 1 Newly diagnosed |
No | 568 (555–568) | 11.1 (9.6–11.1) | 7.18 (7.07–7.18) | 9.5 (6.1–9.5) | 14 (11.9–14) |
(NOS, 6) 2 survived |
| Shankar et al. 2021 [61], India | Retrospective case reports, single centre | 13 (11–15) | 3 (60) | Not reported | 5 Indian |
2 Pre-existing T1DM 3 Newly diagnosed |
No | 425 (343–513) | 13.5 (11.9–15.5) | Not reported | 10 (3.7–13.7) | Not reported |
(NOS, 5) 5 survived |
| Singh et al. 2021 [63], United States | Retrospective case series, single centre | 42.5 (32.2–60.2) | 7 (87.5) | 27.3 (24.5–39.9) |
1 Black 6 Hispanic 1 Bengali |
1 Pre-existing T1DM 5 Pre-existing T2DM 2 Newly diagnosed |
1 Yes | 454 (375–543) | 11.4 (10.7–14.4) | 7.15 (7.1–7.3) | 12.5 (7.7–15.5) | 26.5 (22.5–28) |
(NOS, 6) 5 survived 3 died |
| Singh et al. 2021 [62], United States | Retrospective case series, single centre | 47 (35–79) | 7 (63.6) | 25.7 (23.4–29.3) |
6 Hispanic 2 Black 2 White (Caucasian) 1 Arab |
8 Pre-existing T2DM 2 Newly diagnosed 1 Pre-existing T1DM |
1 Yes | 974 (610–1284) | 13.8 (11.8–15.5) | 7.01 (6.9–7.2) | 5 (4–10) | 34 (30–37) |
(NOS, 7) 4 survived 7 died |
| Singh et al. 2021 [64], United States | Retrospective case report, single centre | 24 | 1 (100) | 32.1 | Not reported | 1 Pre-existing T1DM | No | 507 | 15.8 | 7.16 | 2 | 30.6 |
(NOS, 6) 1 died |
| Smati et al. 2020 [65], France | Retrospective case report, single centre | 36 | 0 (0) | 35.2 | 1 Black | 1 Gestational diabetes | No | 111 | 6.1 | 7.22 | 5.8 | Not reported |
(NOS, 6) 1 survived |
| Soliman et al. 2020 [66], Qatar | Retrospective case report, single centre | 0.7 | Not reported | - | 1 Arab | 1 Newly diagnosed | No | 571 | 8.5 | 7.08 | 7 | 18 |
(NOS, 6) 1 survived |
| Stack et al. 2020 [67], United States | Retrospective case report, single centre | 40 | 1 (100) | Not reported | Not reported | 1 Pre-existing T1DM | No | 328 | 11.5 | Not reported | 18 | 20 |
(NOS, 6) 1 survived |
| Stevens et al. 2021 [68], United States | Retrospective cohort, multicentre | 63.6 ± (14.2) | 108 (68.8) | < 18.5 (4.5%); 18.5 < 25.0 (29.3%); 25.0 < 30.0 (30.6%); > 30.0 (29.3%) |
84 Hispanic 35 Black |
156 Pre-existing T2DM 1 Pre-existing T1DM |
Not reported | > 250 (n = 124) | 10.7 ± (2.8) | Not reported | Not reported | Not reported |
(NOS, 6) 99 survived 58 died |
| Suwanwongse et al. 2021 [69], United States | Retrospective case reports, single centre | 51 (18–51) | 2 (66.7) | 33 (32–33) | Not reported | 3 Newly diagnosed | No | 496 (353–496) | 11.4 (10.4–11.4) | 7.1 (7.12–7.3) | 17 (15–17) | 25 (19–25) |
(NOS, 6) 3 survived |
| Thorne et al. 2021 [70], United Kingdom | Retrospective case series, single centre | 31 (25.5–39.5) | 0 (0) | 32.5 (29.7–39) | Not reported | 4 Newly diagnosed | No | Not reported | Not reported | 7.4 (7.22–7.45) | 14.5 (8.1–16.2) | Not reported |
(NOS, 6) 4 survived |
| Vasconez et al. 2020 [72], United States | Retrospective case report, single centre | 16 | 0 (0) | Not reported | Not reported | 1 Pre-existing T1DM | No | 687 | 13.5 | 6.77 | 3 | 21 |
(NOS, 6) 1 survived |
| Wallett et al. 2021 [73], United Kingdom | Retrospective case series, single centre | Not reported | Not reported | Not reported | White (Caucasian) |
5 Pre-existing T1DM 15 Pre-existing T2DM |
Not reported | 465.3 | Not reported | 7.15 | 11.4 | Not reported |
(NOS, 5) Not reported |
| Xu and Zia 2020 [74], United States | Retrospective case report, single centre | 55 | 1 (100) | Not reported | Not reported | 1 Pre-existing T2DM | 1 Yes | 525 | Not reported | 7.11 | 8 | 31 |
(NOS, 6) 1 survived |
| Zavaleta et al. 2020 [75], Peru | Retrospective case reports, single centre | 64 (42.5–71.2) | 3 (75) | Not reported | 4 Hispanic |
1 Newly diagnosed 3 Unknown diabetes type |
No | 740 (641–1108) | 14.3 (12–14.3) | 7.17 (6.86–7.3) | 11.6 (4–17.6) | Not reported |
(NOS, 6) 2 survived 2 died |
DKA Diabetic ketoacidosis, SGLT2 Sodium-glucose Cotransporter-2, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2; NOS Newcastle Ottawa Scale, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
aPatients with black ethnicity include African-American, Black African, African and Afro-Caribbean patients
bData are presented as median (25th-75th percentiles), or mean ± (SD)
The following data were extracted from selected studies: authors; publication year; study location; study design and setting; age; proportion of male patients; patient body mass index [BMI] and ethnicity; type of diabetes [newly diagnosed or pre-existing]; use of sodium-glucose transport protein 2 [SGLT2] inhibitors; patient biochemical parameters at hospital presentation [blood glucose level, HbA1c, pH, bicarbonate, and anion gap]; assessment of study risk of bias; and treatment outcome [survived or died].
Quality assessment
The quality assessment of the studies was undertaken based on the Newcastle–Ottawa Scale (NOS) to assess the quality of the selected studies [7]. This assessment scale has two different tools for evaluating case–control and cohort studies. Each tool measures quality in the three parameters of selection, comparability, and exposure/ outcome, and allocates a maximum of 4, 2, and 3 points, respectively. High-quality studies are scored greater than 7 on this scale, and moderate-quality studies, between 5 and 7 [7]. Quality assessment was performed by five authors (A.S.A., M.A.A., S.A.A., M.H.A., and H.A.) independently, with any disagreement to be resolved by consensus.
Data analysis
Descriptive statistics were used to describe the data. For continuous variables, mean and standard deviation were used to summarize the data; and for categorical variables, frequencies and percentages were reported. Differences between the COVID-19 and DKA survival group and COVID-19 and DKA death group were analyzed using the Chi-square (χ2) tests (or Fisher's exact tests for expected cell count < 5 in more than 20% of the cells).
To explore the effect of various demographic and biochemical parameters variables on patient’s final treatment outcome [survival or death] in COVID-19 cases who presented with DKA and included in our review, binary logistic regression model with the univariate and multivariate logistic regression of the complete model; and their odd ratios [ORs], confidence intervals (CIs) and p-values were produced; and forest plots were generated for visualization purposes. All p-values were based on two-sided tests and significance was set at a p-value less than 0.05. R version 4.1.0 with the packages finalfit and forestplot was used for all statistical analyses.
Results
Study characteristics and quality
A total of 557 publications were identified (Fig. 1). After scanning titles and abstracts, we discarded 162 duplicate articles. Another 119 irrelevant articles were excluded based on the titles and abstracts. The full texts of the 201 remaining articles were reviewed, and 133 irrelevant articles were excluded. As a result, we identified 68 studies that met our inclusion criteria [8–75]. The detailed characteristics of the included studies are shown in Table 1. Among the included studies, 11 reported DKA and COVID-19 in children [8, 11, 22, 30, 34, 44, 45, 56, 61, 66, 72], 56 reported DKA and COVID-19 in adults [9, 10, 12–21, 23, 25–29, 31–33, 35–43, 46–55, 57–60, 62–65, 67–71, 73–75], and only 1 study reported DKA and COVID-19 in both children and adults [24]. There were 54 case report [8–12, 14, 16, 17, 19–23, 25–27, 29–42, 44–51, 53–58, 60, 61, 64, 66, 67, 69, 71, 72, 74, 75], 10 case series [13, 15, 28, 43, 58, 59, 62, 63, 70, 73] and 4 cohort [18, 24, 52, 68] studies. These studies were conducted in United States (n = 29), United Kingdom (n = 6), India (n = 6), Peru (n = 5), Saudi Arabia (n = 4), France (n = 2), Qatar (n = 2), Iran (n = 2), The Netherlands (n = 1), Turkey (n = 1), Brazil (n = 1), Belgium (n = 1), South Korea (n = 1), Japan (n = 1), Germany (n = 1), Singapore (n = 1), Indonesia (n = 1), Maldives (n = 1), China (n = 1), and Egypt (n = 1). Only 4 studies were performed within a multi-centre settings [15, 24, 52, 68]. The median NOS score for these studies was 6 (range, 5–8). Among the 68 included studies, 65 studies were moderate-quality studies (i.e., NOS scores were between 5 and 7) and 3 studies demonstrated a relatively high quality (i.e., NOS scores > 7); Table 1 (Additional file 2).
Fig. 1.
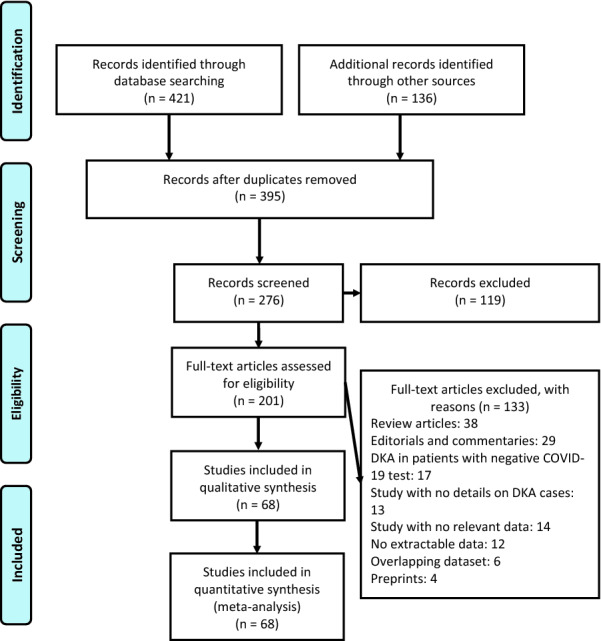
Flow diagram of literature search and data extraction from of studies included in the systematic review and meta-analysis
Demographic and clinical characteristics of DKA patients with SARS-CoV-2 infection
The included studies had a total of 639 DKA patients with confirmed SARS-CoV-2 infection as detailed in Table 1. Amongst these 639 patients, 46 (7.2%) were children and 334 (52.3%) were adults. The median or mean patient age ranged from < 1 years to 66 years across studies. There was an increased male predominance in DKA patients diagnosed with SARS-CoV-2 in most of the studies [n = 373, 58.4%] [9–13, 15–17, 19, 20, 23, 25, 26, 31–33, 36, 37, 39, 45, 47, 50–52, 55–58, 60–64, 67–69, 74, 75] and majority of the patients belonged to Hispanic (n = 156, 24.4%) and black (n = 98, 15.3%) ethnicity [14–16, 18, 19, 21, 24, 27, 28, 44–46, 54, 57, 58, 62, 63, 65, 68, 75]. The median BMI for all included patients was 27.3 kg/m2 [interquartile range (IQR) 24.8–30.6 kg/m2]. Most of the patients (n = 309, 48.3%) had pre-existing type 2 diabetes mellitus, however, some of the cases were pre-existing type 1 diabetes mellitus (n = 73, 11.4%) and about (n = 75, 11.7%) of the patients were newly diagnosed diabetes mellitus with SARS-CoV-2. Only 11 (1.7%) of all cases were taking SGLT2 inhibitors.
Biochemical parameters at presentation
The median random blood glucose level, HbA1c, pH, bicarbonate, and anion gap in all included patients at presentation were 507 mg/dl [IQR 399–638 mg/dl], 11.4% [IQR 9.9–13.5%], 7.16 [IQR 7.00–7.22], 10 mmol/l [IQR 6.9–13 mmol/l], and 24.5 mEq/l [18–29.2 mEq/l]; respectively. Five patients had blood glucose < 250 mg/dl at presentation (euglycemic DKA) [16, 53, 65, 71]; one was on SGLT2 inhibitor medication [16] while seven patients had gestational diabetes mellitus [53, 65, 70].
Patient clinical outcome and predictors of mortality
Patients were stratified based on treatment outcome (if survived or died). A summary of the demographic, biochemical and clinical predictors with regards to final treatment outcome in 243 patients who had either survived (n = 180) or died (n = 63) is shown in Table 2. Most patients had an age of < 60 years old (n = 95, 39.1%)]. Majority of the patients were male (n = 134, 55.1%); and most of the cases belonged to Hispanic (n = 53, 21.8%) and black ethnicity (n = 45, 18.5%). There was a high obesity rate [BMI ≥ 30: n = 27, 11.1%]. Diabetes types among those patients were approximately identical [newly diagnosed (n = 61, 25.1%); pre-existing diabetes mellitus type 1 (n = 60, 24.7%); and pre-existing diabetes mellitus type 2 (n = 60, 24.7%)]. Most patients presented with a random blood glucose level in the range of 500 mg/dl and 1000 mg/dl [n = 61, 25.1%]. About 69 (28.4%) of the patients had an HbA1c higher than ≥ 10%. As expected with the acute DKA complication, most patients had abnormal arterial pH [pH between 7–7.34: n = 78, 32.1%; and pH < 7.00: n = 29, 11.9%]. Also, most patients had low bicarbonate [≤ 11 mmol/l: n = 69, 28.4%] and high anion gap [between 21–30 mEq/l: n = 39, 16%; and between 31–50 mEq/l: n = 20, 8.2%]; Table 2.
Table 2.
Demographic data of the SARS-CoV-2 patients with diabetic ketoacidosis, stratified by treatment outcome (n = 68 studies), 2019–2021
| Variable | Findingsb | |||
|---|---|---|---|---|
| All (n = 243) | Survived (n = 180) | Died (n = 63) | p-valuec | |
| Age (years) | ||||
| < 60 | 95 (39.1) | 80 (44.4) | 15 (23.8) | 0.021* |
| ≥ 60 | 35 (14.4) | 17 (9.4) | 18 (28.6) | |
| Gender | ||||
| Female | 95 (39.1) | 80 (44.4) | 15 (23.8) | 0.015* |
| Male | 134 (55.1) | 90 (50) | 44 (69.8) | |
| BMI (kg/m2) | ||||
| < 30 | 44 (18.1) | 32 (17.8) | 12 (19) | 0.338 |
| ≥ 30 | 27 (11.1) | 17 (9.4) | 10 (15.9) | |
| Ethnicity | ||||
| Arab | 10 (4.1) | 7 (3.9) | 3 (4.8) | 0.001* |
| Asian | 13 (5.3) | 8 (4.4) | 5 (7.9) | |
| Blacka | 45 (18.5) | 42 (23.3) | 3 (4.8) | |
| Hispanic | 53 (21.8) | 36 (20) | 17 (27) | |
| Indian | 14 (5.8) | 13 (7.2) | 1 (1.6) | |
| Bengali | 2 (0.8) | 1 (0.5) | 1 (1.6) | |
| Persian | 2 (0.8) | 2 (1) | 0 | |
| White (Caucasian) | 27 (11.1) | 24 (13.3) | 3 (4.8) | |
| Diabetes type | ||||
| Newly diagnosed | 61 (25.1) | 55 (30.5) | 6 (9.5) | 0.000* |
| Pre-existing type 1 diabetes mellitus | 60 (24.7) | 55 (30.5) | 5 (7.9) | |
| Pre-existing type 2 diabetes mellitus | 60 (24.7) | 42 (23.3) | 24 (38.1) | |
| Use of SGLT2 inhibitors | ||||
| Yes | 8 (3.3) | 6 (3.3) | 2 (3.2) | 0.000* |
| No | 185 (76.1) | 149 (82.8) | 36 (57.1) | |
| Blood glucose | ||||
| < 500 mg/dl | 45 (18.5) | 38 (21.1) | 7 (11.1) | 0.048* |
| Between 500–1000 mg/dl | 61 (25.1) | 44 (24.4) | 17 (27) | |
| > 1000 mg/dl | 13 (5.3) | 6 (3.3) | 7 (11.1) | |
| HbA1c (%) | ||||
| < 10 | 24 (9.9) | 17 (9.4) | 6 (9.5) | 0.096 |
| ≥ 10 | 69 (28.4) | 57 (31.7) | 12 (19) | |
| pH | ||||
| > 7.35 | 8 (3.3) | 7 (3.9) | 1 (1.6) | 0.047* |
| Between 7–7.34 | 78 (32.1) | 58 (32.2) | 20 (31.7) | |
| < 7 | 29 (11.9) | 18 (10) | 11 (17.5) | |
| Bicarbonate (mmol/l) | ||||
| Above 20 | 3 (1.2) | 2 (1.1) | 1 (1.6) | 0.818 |
| Between 12–20 | 40 (16.5) | 29 (16.1) | 11 (17.5) | |
| Between 2–11 | 69 (28.4) | 54 (30) | 15 (23.8) | |
| Anion gap (mEq/l) | ||||
| Between 12–20 | 26 (10.7) | 22 (12.2) | 4 (6.3) | 0.327 |
| Between 21–30 | 39 (16) | 29 (16.1) | 10 (15.9) | |
| Between 31–50 | 20 (8.2) | 12 (6.7) | 8 (12.7) | |
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, SGLT2 Sodium-glucose Cotransporter-2, BMI body mass index
aPatients with black ethnicity include African-American, Black African, African and Afro-Caribbean patients
bData are presented as number (%)
cChi-square (χ2) test was used to compare between survival and death groups
Those patients who died were more likely to have been older in age [≥ 60 years old: 28.6% vs 23.8%; p = 0.021]; and more likely to be men [male gender: 69.8% vs 23.8%; p = 0.015]. Majority of patients who died had a Hispanic ethnicity (n = 17, 27%; p = 0.001). Patients with a pre-existing type 2 diabetes mellitus type had the highest mortality rate compared to other diabetes types [n = 24, 38.1%; p = 0.000]. In addition, patients who died had higher random blood glucose level at admission [(blood glucose between 500–1000 mg/dl: 27% vs 24.4%) and (blood glucose > 1000 mg/dl: 11.1% vs 3.3%); p = 0.048]; and experienced more severely low pH than those who survived [pH < 7: 17.5% vs 10%; p = 0.047]. Moreover, more patients had high anion gap in the mortality group [anion gap between 31–50 mEq/l: 12.7% vs 6.7%, p = 0.327]. However, a higher proportion of patients had low bicarbonate [bicarbonate between 2–11 mmol/l: 23.8% vs 30%; p = 0.818] and glycated haemoglobin was raised more in the survival group [HbA1c ≥ 10%: 19% vs 31.7%; p = 0.096].
Potential determining variables associated in survival and death groups were analyzed through binary logistic regression analysis and shown in Fig. 2, Fig. 3 and Fig. 4. As expected, old age [≥ 60 years] (OR 3.29, 95% CI 1.38–7.91; p = 0.007), male gender (OR 2.61, 95% CI 1.37–5.17; p = 0.004), and BMI ≥ 30 kg/m2 (OR 1.57, 95% CI 0.56–4.4; p = 0.389) are associated with increased odd ratio for death; Fig. 2. Among the diabetes types, patients who presented with pre-existing diabetes mellitus type 2 had a very high OR of dying (OR 5.24, 95% CI 2.07–15.19; p = 0.001). In opposite, patients with pre-existing diabetes mellitus type 1 had a much lower OR of 0.83 for mortality (95% CI 0.23–2.92); Fig. 3. Other predictors for increased risk of succumbing included blood glucose level ≥ 1000 mg/dl (OR 3.02, 95% CI 0.88–10.67), low pH of < 7 (OR 4.28, 95% CI 0.64–24.3), and high anion gap [between 31 and 50 mEq/l] (OR 3.38, 95% CI 0.89–14.83); Fig. 3 and Fig. 4.
Fig. 2.
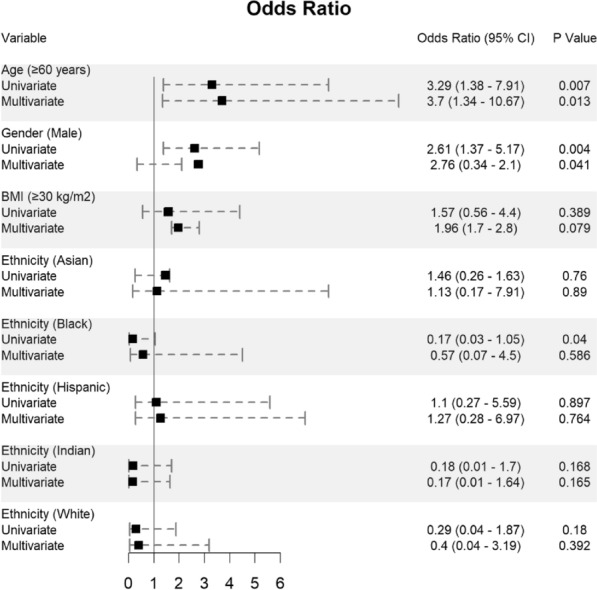
Predictors of mortality in patients hospitalized for DKA and SARS-CoV-2 (n = 243)
Fig. 3.
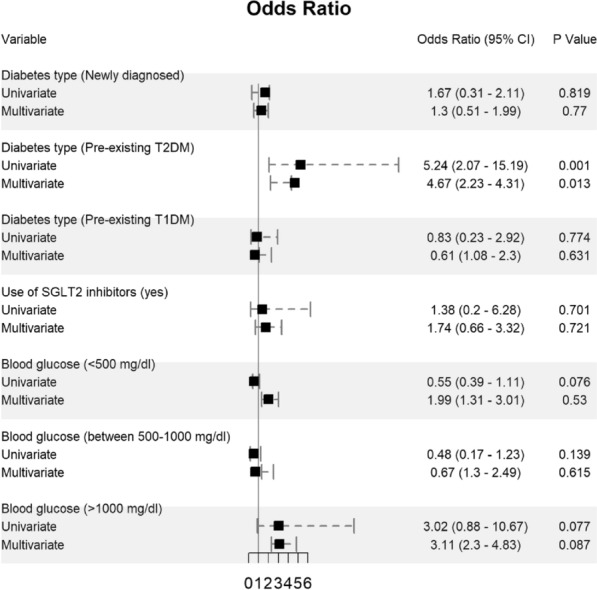
Predictors of mortality in patients hospitalized for DKA and SARS-CoV-2 (n = 243)
Fig. 4.
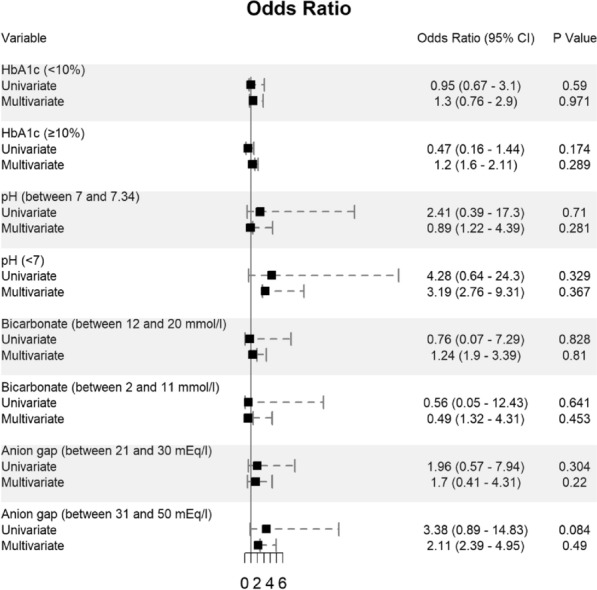
Predictors of mortality in patients hospitalized for DKA and SARS-CoV-2 (n = 243)
These variables were considered needing further evaluation and, thus, were included in multivariate regression analysis. Nevertheless, multivariate analysis confirmed old age [≥ 60 years], male gender, and a pre-existing diabetes mellitus type 2 were significantly associated with increased death. Although univariate analysis showed black ethnicity was significantly associated with increased mortality (p = 0.04), however, this finding was not reciprocated by multivariate analysis; Fig. 2.
Discussion
This is the largest meta-analysis on the development of DKA in patients with SARS-CoV-2. This study involving 639 patients from 68 observational studies found majority of the DKA patients diagnosed with SARS-CoV-2 were adults (52.3%), men (58.4%) and had pre-existing type 2 diabetes mellitus (48.3%).
DKA is one of the most common and serious hyperglycaemic emergency; and is considered a precipitating event that frequently occurs due to infection [often pneumonia or urinary tract infection], and discontinuation of or inadequate insulin therapy [76, 77]. Adults of any age may develop severe SARS-CoV-2 and experience adverse outcomes, especially those with comorbidities [78, 79]. Most children with SARS-CoV-2 have mild symptoms or have no symptoms at all [80], however, adults are at higher risk to experience more severe COVID-19 infection than children [81]. Factors proposed to explain the difference in severity of COVID-19 in children and adults include: 1- age-related increase in endothelial damage and changes in clotting function; 2- higher density, increased affinity and different distribution of angiotensin converting enzyme 2 receptors and transmembrane serine protease 2; 3- pre-existing coronavirus antibodies (including antibody-dependent enhancement) and T cells; 4- immunosenescence and inflammaging, including the effects of chronic cytomegalovirus infection; 5- a higher prevalence of comorbidities associated with severe COVID-19 and 6- lower levels of vitamin D [82]. Hence, lower rate of children SARS-CoV-2 patients with DKA in our review can be justified by the fact that the high severity of COVID-19 tends to be much lower in children compared to adults.
DKA is thought to happen most often in patients with diabetes mellitus type 1 [49, 83], however, this conceptualization is not true and we report fourfold higher rate of DKA in the diabetes mellitus type 2 patients compared to diabetes mellitus type 1. Type 2 diabetes mellitus patients have high susceptibility to DKA under stressful conditions such as trauma, surgery or infections [83]; and majority of the DKA cases worldwide occur in patients with type 2 diabetes due to its higher prevalence [84, 85]. DKA occurs more commonly in adult COVID-19 patients with type 2 diabetes mellitus mainly because the worldwide prevalence of diabetes mellitus type 2 is estimated at 9.3 percent in adults, equivalent to 463 million people [86]. Type 2 diabetes accounts for over 90 percent of patients with diabetes [86, 87].
In our review, males gender predominated development of DKA in SARS-CoV-2 patients, a finding suggested in most of the reports [11–13, 15–17, 19, 20, 23, 25, 26, 31, 33, 36, 37, 39, 47, 50, 52, 55–58, 60–64, 69, 74, 75] and in contradiction with data from other reports suggesting an equal proportion of DKA cases in COVID-19 patients for both genders [30, 34, 38, 49]. Lifestyle, body fat distribution, hormonal factors, susceptibility to glucotoxicity and lipotoxicity, and changes in insulin sensitivity have been described as potential factors of DKA and possible mechanisms of male predominance [88]. However, male excess in DKA in our review might be attributed mainly to the differences in the inclusion criteria and the population age groups included in the studies; or can be explained by social factors as women are often the primary caregivers for their families, assuming the responsibility of family members’ disease management, at the expense of their own treatment [89].
A comparison of the current results with findings from previous studies can offer some validation of the findings of this present meta-analysis and identify methodological differences in their approaches. Regarding the mortality rate in patients who developed DKA during SARS-CoV-2 infection, we report an overall similar and slightly lower death rate [25.9%] compared to the previous two systematic meta-analyses [28.9 and 29%, respectively] [90, 91]. The current meta-analysis is more comprehensive and included a total of 68 studies [8–75] including a total of 639 patients; whose details on final treatment outcome were available; in comparison to smaller sample size in previous meta-analyses [sample size: n = 45 and n = 21, respectively] [90, 91]. The inclusion of 48 recently published studies [8–14, 16, 21, 23–26, 28, 30, 32–35, 40, 42–46, 48, 50, 51, 53, 54, 56–59, 61–64, 66–75] contributed to the refinement on evidence of the demographic, biochemical, and clinical characteristics; in addition to final therapy outcome in COVID-19 patients with DKA.
Consistent with previous meta-analysis, we found development of DKA in SARS-CoV-2 patients was highest in the Hispanics and blacks (24.4% and 15.3%, respectively) [91]. Moreover, we found mortality rate in DKA patients infected with COVID-19 was significantly very high in patients with Hispanic ethnicity [27%, p = 0.001] in whom risk of acquiring SARS-CoV-2 and clinical prognosis of this viral infection was previously described as high and poor [92, 93]. Because most of the studies included in our review that reported the ethnicity of DKA cases infected with COVID-19 were either from the United States of America, India or United Kingdom; representation of other ethnicities at risk to develop DKA during COVID-19 can be misleading. For instance, we report a low prevalence of DKA in Asian population, yet, a systematic review and meta-analysis reported the highest DKA incidence rates in Chinese people [94].
In line with our findings, severely low pH (i.e. pH of < 7) has been identified as an important predictor of mortality in patients with DKA and COVID-19 compared to those who survived (p = 0.047) [90, 91]. Very high uncontrolled random blood glucose level (> 1000 mg/dl) was the other biochemical parameter at presentation that differed significantly between the survival and death groups in DKA patients infected with SARS-CoV-2 (p = 0.048); a finding suggested in previous studies [91, 95, 96] and in contradiction with data from case reports demonstrating death in DKA cases during COVID-19 infection when their blood glucose levels were kept at < 500 mg/dl [27, 59, 63]. Moreover, increasing age in combination with male gender and BMI ≥ 30 might denote seriously sick patients who can potentially have more morbidity and propensity to die. The majority of patients hospitalized with SARS-CoV-2 are older and seemed to have underlying medical conditions [97, 98], with increased age being associated with clinical severity, including case fatality [97, 99]. Fortunately, however, mortality from DKA in elderly people have also declined dramatically during the past 10 years [100]. Therefore, these patients should be identified at the earliest and treated preferably in a special care set up to avoid morbidity and mortality. It is worth mentioning increasing age in patients may result in increased hospital stay and might put SARS-CoV-2 patients at risk to develop medical complications like coagulopathy, pneumonia, acute respiratory distress syndrome, organ failure and nosocomial coinfections [97, 101]. The presence of these factors in severely ill patients may have necessitated the use of advanced therapies like renal replacement therapy or ventilator support which would have delayed hospital discharge [102]. Although COVID-19 has a higher survival rate than other chronic diseases, the incidence of complications in the geriatric population are considerably high, with more systemic complications [103]. Of the patients admitted to hospital for management of COVID-19, 49.7% (36,367 of 73,197) had at least one complication [104]. Overall, complications and worse functional outcomes in patients admitted to hospital with SARS-CoV-2 are high in old people, and even in young, previously healthy individuals; and COVID-19 complications could strain health system for years.
In our review, the odd ratio of mortality was the highest in DKA patients with the pre-existing the diabetes type 2 variable [OR 5.24, 95% CI 2.07–15.19; p = 0.001]; and DKA patients with pre-existing type 1 diabetes had very low OR of death [OR 0.83, 95% CI 0.23–2.92; p = 0.774]. In diabetes mellitus type 2 diabetes, underlying severe illness is almost always the direct cause of both the DKA and ensuing death; while in diabetes mellitus type 1 diabetes, DKA is most often caused by missed insulin doses but death is rare with prompt treatment [49].
There is growing evidence to suggest that SARS-CoV-2 might cause diabetes in some people [105, 106]. In our study, out of the 639 DKA patients infected with SARS-CoV-2, there was (n = 75, 11.7%) newly diagnosed diabetes mellitus cases at admission. Of these 75 cases, 22 had HbA1c > 9.0% (ranging from 9.5% to 17.4%) [8, 9, 11, 12, 20, 22, 33–35, 39, 40, 44, 45, 53, 56, 67, 69] and three of which had a BMI > 30 [44, 69], suggesting these patients had undiagnosed diabetes mellitus and improbable was caused by SARS-CoV-2 infection. DKA in COVID-19 patients was the least to occur in newly diagnosed diabetes cases probably as a result of increased diabetes screening and early recognition, DKA now occurs more frequently in persons with established diabetes rather than at the time of the initial diagnosis [100]. COVID-19 likely unmasked existing diabetes mellitus by aggravating its metabolic complications rather than causing the new-onset diabetes in these patients.
Out of the 11 (1.7%) DKA cases infected with COVID-19 who were taking SGLT2 inhibitors, two patient [18.2%] were diagnosed with SGLT2-associated euglycemic DKA [blood glucose < 250 mg/dl at presentation] [16, 48]; in addition to seven patients who had gestational diabetes mellitus [53, 65, 70]. Euglycemic DKA is a rare life-threatening complication associated with the use of SGLT2 inhibitors in patients with type 2 diabetes that may be unnoticed, particularly in COVID-19 pandemic, due to the absence of significant hyperglycaemia, delaying its treatment [16]. Given their undisputed cardiovascular and renal benefits, these medications are common in patients with type 2 diabetes [107]. There are recommendations that patients using SGLT2 inhibitors should be monitored for ketosis using available home testing kits in case of infections and should discontinue the medication in case of SARS-CoV-2 while the administration of insulin is considered the safest pharmacotherapy choice [108].
Limitations
First, while most of the evidence discussed were based on few cohorts, some case series and many case reports, many of these are small and not necessarily generalizable to the current COVID-19 clinical environment. Second, to asses factors associated with mortality, larger cohort of patients is needed. Third, almost all studies included in this review were retrospective in design which could have introduced potential reporting bias due to reliance on clinical case records. Fourth, study was not registered in Prospero, an international prospective register of systematic reviews, as this might have added extra work and the merit was mostly limited to the avoidance of duplication. Last, the study population included paediatric patients and hence its results cannot be generalized to adult patients.
Conclusion
Patients with diabetes are at increased risk of severe complications from SARS-CoV-2 which may include DKA. Acute diabetes-related DKA in SARS-CoV-2 patients lead to increased mortality; key determinants are individuals with pre-existing diabetes mellitus type 2, older age [≥ 60 years old], male gender, BMI ≥ 30, blood glucose level > 1000 mg/dl, and anion gap ≥ 30 mEq/l.
Supplementary Information
Additional file 2. Search outcomes of all studies found via electronic search databases.
Acknowledgements
We would like to thank authors and their colleagues who contributed to the availability of evidence needed to compile this article. We would also like to thank the reviewers for very helpful and valuable comments and suggestions for improving the paper.
Abbreviations
- COVID-19
Coronavirus disease 2019
- DKA
Diabetic ketoacidosis
- NOS
Newcastle–Ottawa scale
- PRISMA
Preferred Reporting Items for systematic reviews and meta-Analyses
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SGLT2
Sodium-glucose Cotransporter-2 inhibitors
Authors' contributions
SA, AA, ZA, AR and AAO contributed equally to the systematic review. SA, AA, ZA and AR were the core team leading the systematic review. SA, AA, AR and ZA identified and selected the studies. ASA, MAA, SAA, MHA, and HA did the quality assessment of the studies. SA, MAK, FMA and HM collected the data. SA, AMA, KD and AAO analyzed the data. SA, AA, AR, ZA, JA and AAO drafted the manuscript. All authors approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data are available upon request, please contact author for data requests.
Declarations
Ethics approval and consent to participate
This review is exempt from ethics approval because we collected and synthesized data from previous clinical studies in which informed consent has already been obtained by the investigators.
Consent for publication
All authors agreed to this publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saad Alhumaid, Email: saalhumaid@moh.gov.sa.
Abbas Al Mutair, Email: abbas4080@hotmail.com.
Zainab Al Alawi, Email: zalalwi@kfu.edu.sa.
Ali A. Rabaan, Email: arabaan@gmail.com
Mohammed A. Alomari, Email: amoualomari@gmail.com
Sadiq A. Al Salman, Email: sadiqa@moh.gov.sa
Mohammed H. Al Hassan, Email: mohealhassan@moh.gov.sa
Hesham Alhamad, Email: halhamad@moh.gov.s.
Mustafa A. Al-kamees, Email: mualkhamis@moh.gov.sa
Fawzi M. Almousa, Email: fmalmosa@moh.gov.sa
Hani N. Mufti, Email: fmuftihn@ngha.med.sa
Ali M. Alwesabai, Email: aalwesabai@moh.gov.sa
Kuldeep Dhama, Email: akdhama@rediffmail.com.
Jaffar A. Al-Tawfiq, Email: jaffar.tawfiq@jhah.com
Awad Al-Omari, Email: awad.omari@drsulaimanalhabib.com.
References
- 1.Mantovani A, Byrne CD, Zheng M-H, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Al-Rifai RH, Saha S. Diabetes prevalence and mortality in COVID-19 patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021 doi: 10.1007/s40200-021-00779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus: a meta-analysis. Diab Res Clin Pract. 2020;164:108200. doi: 10.1016/j.diabres.2020.108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, Sperling MA, Codner E. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diab. 2018;19:155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. pp. 1–12. [Google Scholar]
- 8.Albuali WH, AlGhamdi NA. Diabetic ketoacidosis precipitated by atypical coronavirus disease in a newly diagnosed diabetic girl. J Taibah Univ Sci. 2021 doi: 10.1016/j.jtumed.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfishawy M, Nassar M, Mohamed M, Fatthy M, Elmessiery RM. New-onset type 1 diabetes mellitus with diabetic ketoacidosis and pancreatitis in a patient with COVID-19. Sci Afr. 2021;13:e00915. doi: 10.1016/j.sciaf.2021.e00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali E, Badawi M, Ahmed A, Abdelmahmuod E, Ibrahim W. Severe SARS-CoV-2 infection presenting with acute kidney injury and diabetic ketoacidosis complicated by pancreatitis in a 53-year man with hypertension. Clinical Case Reports. 2021;9(3):1202–1206. doi: 10.1002/ccr3.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alizadeh F, O’Halloran A, Alghamdi A, Chen C, Trissal M, Traum A, DeCourcey D. Toddler with new onset diabetes and atypical hemolytic-uremic syndrome in the setting of COVID-19. Pediatrics. 2021 doi: 10.1542/peds.2020-016774. [DOI] [PubMed] [Google Scholar]
- 12.Al-Naami AQ, Khan LA, Zaidan FI, Al-Neami IA, Awad AS, Hobani AI, Sheikh AH, Ahmadini MA. Hyperglycemic hyperosmolar state (HHS) with new-onset diabetes mellitus in a patient with SARS CoV-2 infection. Authorea Preprints. 2020 doi: 10.22541/au.160611667.79425739/v1. [DOI] [Google Scholar]
- 13.Alsadhan I, Alruwashid S, Alhamad M, Alajmi S, Alshehri S, Alfadhli E, Ekhzaimy A. Diabetic ketoacidosis precipitated by Coronavirus disease 2019 infection: case series. Curr Therap Res. 2020;93:100609. doi: 10.1016/j.curtheres.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Añazco PH, Balta FM, Córdova-Cueva L. Bilateral renal infarction in a patient with severe COVID-19 infection. Braz J Nephrol. 2021 doi: 10.1590/2175-8239-jbn-2020-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armeni E, Aziz U, Qamar S, Nasir S, Nethaji C, Negus R, Murch N, Beynon HC, Bouloux P, Rosenthal M. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8(8):660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batista DV, de Almeida Vieira CAF, Costa TA, Lima EG. COVID-19-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes on SGLT2 inhibitor: a case report. Diabetol Int. 2021;12(3):313–316. doi: 10.1007/s13340-020-00473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamorro-Pareja N, Parthasarathy S, Annam J, Hoffman J, Coyle C, Kishore P. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KH, Thimmareddygari D, Ramahi A, Atallah L, Baranetsky NG, Slim J. Clinical characteristics and outcome in patients with combined diabetic ketoacidosis and hyperosmolar hyperglycemic state associated with COVID-19: a retrospective, hospital-based observational case series. Diab Res Clin Pract. 2020;166:108279. doi: 10.1016/j.diabres.2020.108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diab Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft A, Bucca A, Jansen JH, Motzkus C, Herbert A, Wang A, Hunter BR. First-time diabetic ketoacidosis in Type 2 diabetics with Covid-19 infection: a novel case series. J Emerg Med. 2020;59(5):e193–e197. doi: 10.1016/j.jemermed.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel S, Gadhiya B, Parikh A, Joshi P. COVID-19 in a child With diabetic ketoacidosis: an Instigator, a deviator or a spectator. Indian Pediatr. 2020;57(10):969. doi: 10.1007/s13312-020-2008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey RK, Hilmy AI, Imad HA, Yoosuf AA, Latheef AA. COVID-19 and emergencies in patients with diabetes: two case reports. J Med Case Rep. 2021;15(1):1–5. doi: 10.1186/s13256-020-02659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebekozien O, Agarwal S, Noor N, Albanese-O’Neill A, Wong JC, Seeherunvong T, Sanchez J, DeSalvo D, Lyons SK, Majidi S. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metabol. 2021;106(4):e1755–e1762. doi: 10.1210/clinem/dgaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emara MH, Mazid U, Atta MA, Elshahat S, Mahros AM. Ketonuria with or without ketoacidosis as the presenting manifestation of SARS-CoV-2 (COVID-19) among uncontrolled type 2 diabetic patients. Med Hypothes. 2020;144:110226. doi: 10.1016/j.mehy.2020.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh R, Dubey S, Roy D, Ray A, Pandit A, Ray BK, Benito-León J. Choreo-ballistic movements heralding COVID-19 induced diabetic ketoacidosis. Diab Metabol Synd. 2021;15(3):913. doi: 10.1016/j.dsx.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman N, Fink D, Cai J, Lee Y-N, Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diab Res Clin Pract. 2020;166:108291. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorthi RS, Kamel G, Dhindsa S, Nayak RP. COVID-19 presenting with diabetic ketoacidosis: a case series. AACE Clin Case Rep. 2021;7(1):6–9. doi: 10.1016/j.aace.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haider MB, Abbas F, Hafeez W. A 46-year-old woman who presented with diabetic ketoacidosis and COVID-19 pneumonia with multiple pulmonary thromboemboli: a case report. Am J Case Rep. 2020;21:e925794–e925801. doi: 10.12659/AJCR.925794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkes CP, Willi SM. A trend towards an early increase in ketoacidosis at presentation of paediatric type 1 diabetes during the coronavirus-2019 pandemic. Diab Med. 2021 doi: 10.1111/dme.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaney AI, Griffin GD, Simon EL. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020;38(11):2491. doi: 10.1016/j.ajem.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidarpour M, Vakhshoori M, Haghighatpanah MA, Ashrafi L, Khorvash F, Iraj B. Rhabdomyolysis plus hypocalcemia and diabetic ketoacidosis as concurrent rare COVID-19 manifestations. Case Rep Med. 2021 doi: 10.1155/2021/6625086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollstein T, Schulte DM, Schulz J, Glück A, Ziegler AG, Bonifacio E, Wendorff M, Franke A, Schreiber S, Bornstein SR. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020;2(10):1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 34.Howard MB, Basu S, Sherwin E, Cohen JS. Triple threat: New presentation with diabetic ketoacidosis, COVID-19, and cardiac arrhythmias. Am J Emerg Med. 2021 doi: 10.1016/j.ajem.2021.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii K, Suwanai H, Saito T, Motohashi N, Hirayama M, Kondo A, Sano K, Shikuma J, Ito R, Miwa T. A case of diabetic ketoacidosis in a patient with COVID-19 and newly diagnosed type 1 diabetes. Clin Case Rep. 2021 doi: 10.1002/ccr3.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabashneh S, Ali H, Alkassis S. Multi-organ failure in a patient with diabetes due to COVID-19 with clear lungs. Cureus. 2020 doi: 10.7759/cureus.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur P, Posimreddy S, Singh B, Qaqa F, Habib HA, Maroules M, Shamoon F. COVID-19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020 doi: 10.12890/2020_001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim N-Y, Ha E, Moon JS, Lee Y-H, Choi EY. Acute hyperglycemic crises with coronavirus disease-19. Diab Metabol J. 2020;44(2):349–353. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr. 2020;14(6):2039–2041. doi: 10.1016/j.dsx.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulick-Soper CV, McKee JL, Wolf RL, Mohan S, Stein JM, Masur JH, Lazor JW, Dunlap DG, McGinniss JE, David MZ. Pearls & Oy-sters: Bilateral globus pallidus lesions in a patient with COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchon KA, Nunn MO, Chakera AJ. Images of the month: An incidental finding of spontaneous pneumomediastinum (Hamman's syndrome) secondary to diabetic ketoacidosis during the coronavirus pandemic. Clin Med. 2020;20(6):e275–e277. doi: 10.7861/clinmed.2020-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondal S, DasGupta R, Lodh M, Gorai R, Choudhury B, Hazra AK, Ganguly A. Predictors of new-onset diabetic ketoacidosis in patients with moderate to severe COVID-19 receiving parenteral glucocorticoids: a prospective single-centre study among Indian type 2 diabetes patients. Diabetes Metab Syndr. 2021;15(3):795–801. doi: 10.1016/j.dsx.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naguib MN, Raymond JK, Vidmar AP. New onset diabetes with diabetic ketoacidosis in a child with multisystem inflammatory syndrome due to COVID-19. J Pediatr Endocrinol Metab. 2021;34(1):147–150. doi: 10.1515/jpem-2020-0426. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen-Saines K, Li E, Olivera AM, Martin-Blais R, Bulut Y. Case report: insulin-dependent diabetes mellitus and diabetic keto-acidosis in a child with COVID-19. Front Pediatr. 2021;9:33. doi: 10.3389/fped.2021.628810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omotosho YB, Ying GW, Stolar M, Mallari AJP. COVID-19-induced diabetic ketoacidosis in an adult with latent autoimmune diabetes. Cureus. 2021 doi: 10.7759/cureus.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oriot P, Hermans MP. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg. 2020 doi: 10.1080/17843286.2020.1780390. [DOI] [PubMed] [Google Scholar]
- 48.Ozer O, Yorulmaz G. Euglycemic diabetic ketoacidosis associated with empagliflozin use in the course of the SARS-Cov-2 pandemic. J Coll Physicians Surg Pak. 2020 doi: 10.29271/jcpsp.2020.Supp2.110. [DOI] [PubMed] [Google Scholar]
- 49.Palermo NE, Sadhu AR, McDonnell ME. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105(8):2819–2829. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panjawatanan P, Jha S, Hughes J, Riesenfeld E. A case of concomitant COVID-19 infection-induced acute respiratory distress syndrome and diabetic ketoacidosis: another challenge in fluid management. Cureus. 2020 doi: 10.7759/cureus.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parwanto MLE, Digambiro RA, Nusantara DU, Rarasati T. Coronavirus disease 2019 (COVID-19): A case report in a patient with diabetic ketoacidosis and hypertension. Bali Med J. 2020;9(3):624–629. doi: 10.15562/bmj.v9i3.1939. [DOI] [Google Scholar]
- 52.Pasquel FJ, Messler J, Booth R, Kubacka B, Mumpower A, Umpierrez G, Aloi J. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4(3):e211091. doi: 10.1001/jamanetworkopen.2021.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pikovsky M, Tan MY, Ahmed A, Sykes L, Agha-Jaffar R, Christina K. Euglycaemic ketoacidosis in pregnant women with COVID-19: two case reports. BMC Pregnancy Childbirth. 2021;21(1):1–7. doi: 10.1186/s12884-021-03928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plasencia-Dueñas EA, Concepción-Zavaleta MJ, Gonzáles-Yovera JG. Pancreatic enzyme elevation patterns in patients with diabetic ketoacidosis: does severe acute respiratory syndrome coronavirus 2 play a role? Pancreas. 2021;50(2):e19. doi: 10.1097/MPA.0000000000001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potier L, Julla J, Roussel R, Boudou P, Gauthier D, Ketfi C, Gautier J. COVID-19 symptoms masking inaugural ketoacidosis of type 1 diabetes. Diab Metabol. 2021;47(1):101162. doi: 10.1016/j.diabet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabizadeh S, Hajmiri M, Rajab A, Kouchak HE, Nakhjavani M. Severe diabetic ketoacidosis and coronavirus disease 2019 (COVID-19) infection in a teenage patient with newly diagnosed diabetes. J Pediatr Endocrinol Metab. 2020;33(9):1241–1243. doi: 10.1515/jpem-2020-0296. [DOI] [PubMed] [Google Scholar]
- 57.Ramos-Yataco A, Capcha KJM, Harbuz-Miller I. The peruvian experience: new-onset diabetes presenting with diabetic ketoacidosis in patients with COVID19 infections. JES. 2021;5(Supplement 1):A403–A404. [Google Scholar]
- 58.Ramos-Yataco A, Meza K, Farfán-García RC, Ortega-Rojas S, Salinas-Mamani I, Ontaneda ISA, Correa R. DKA in patients with pre-existing type 2 diabetes mellitus related to COVID-19: a case series. Endocrinol Diab Metabol Case Rep. 2021 doi: 10.1530/EDM-20-0148. [DOI] [Google Scholar]
- 59.Rao S, Ali K, Rivas M, Nugent K. Diabetic ketoacidosis in patients with COVID-19. Am J Med Sci. 2021 doi: 10.1016/j.amjms.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shankar GH, Sharma V, Sarangi B, Walimbe A, Markal KP, Reddy VS. Surge in diabetic ketoacidosis in children with Type 1 diabetes during COVID-19 pandemic—a report from a tertiary care center in Pune, India. J Pediatr Crit Care. 2021;8(2):110. doi: 10.4103/JPCC.JPCC_162_20. [DOI] [Google Scholar]
- 62.Singh B, Kaur P, Majachani N, Patel P, Reid R-JR, Maroules M. COVID-19 and combined diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic coma: report of 11 cases. J Investig Med High Impact Case Rep. 2021 doi: 10.1177/23247096211021231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh B, Patel P, Kaur P, Majachani N, Maroules M. COVID-19 and diabetic ketoacidosis: report of eight cases. Cureus. 2021 doi: 10.7759/cureus.14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh S, Foster A, Khan Z, Siddiqui A, Atere M, Nfonoyim JM. COVID-19-induced diabetic ketoacidosis and acute respiratory distress syndrome in an obese 24-year-old type I diabetic. Am J Case Rep. 2020;21:e925586–e925591. doi: 10.12659/AJCR.925586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smati S, Mahot P, Bourdiol A, Ploteau S, Hadjadj S, Cariou B. Euglycaemic ketoacidosis during gestational diabetes with concomitant COVID-19 infection. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soliman A, Al-Amri M, Ellithy K, Alaaraj N, Hamed N, De Sanctis V. Newly-onset type 1 diabetes mellitus precipitated by COVID-19 in an 8-month-old infant. Acta Bio Medica Atenei Parmensis. 2020;91(3):e2020046. doi: 10.23750/abm.v91i3.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stack A, Terpak L, Masri G. Diabetic ketoacidosis in a patient with COVID-19. Consultant. 2020;60(9):3–4. [Google Scholar]
- 68.Stevens J, Bogun M, McMahon D, Zucker J, Kurlansky P, Mohan S, Yin M, Nickolas T, Pajvani U. Diabetic ketoacidosis and mortality in COVID-19 infection. SSRN J. 2021 doi: 10.2139/ssrn.3768538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93(2):1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorne I, Steele S, Martineau M, Girling J. Case series of COVID-19 infection in pregnancy complicated by ketoacidosis and symptomatic breathlessness. Obstet Med. 2021 doi: 10.1177/1753495X211024511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Amesfoort JE, Werter DE, Painter RC, Hermans FJ. Severe metabolic ketoacidosis as a primary manifestation of SARS-CoV-2 infection in non-diabetic pregnancy. BMJ Case Rep. 2021;14(4):e241745. doi: 10.1136/bcr-2021-241745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasconez WA, Escobar CLB, Agarwal N, Solano JP, Sanchez JE. Severe diabetic ketoacidosis in a child with type-1 diabetes, asthma, and COVID-19. J Pediatr Intensive Care. 2020 doi: 10.1055/s-0040-1713164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallett L, Kempegowda P, Melson E, Juszczak A, Thomas L, Zhou D, Holmes C, Karamat MA, Ghosh S, Hanif W. Differences in presentation, severity and management of DKA in type 1 and type 2 diabetes during the COVID-19 pandemic. Clin Med. 2021;21(Suppl 2):1–2. doi: 10.7861/clinmed.21-2-s1. [DOI] [PubMed] [Google Scholar]
- 74.Xu C, Zia U. Recovery from acute kidney injury with diabetic ketoacidosis following SARS-CoV-2 infection: a case report and literature review. Cureus. 2020 doi: 10.7759/cureus.11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zavaleta MJC, Flórez CDA, Dueñas EAP, Arroyo JCC. Diabetic ketoacidosis during COVID-19 pandemic in a developing country. Diab Res Clin Pract. 2020;168:108391. doi: 10.1016/j.diabres.2020.108391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. JCDR. 2015 doi: 10.7860/JCDR/2015/8586.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Randall L, Begovic J, Hudson M, Smiley D, Peng L, Pitre N, Umpierrez D, Umpierrez G. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care. 2011;34(9):1891–1896. doi: 10.2337/dc11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al Mutair A, Alhumaid S, Alhuqbani WN, Zaidi ARZ, Alkoraisi S, Al-Subaie MF, AlHindi AM, Abogosh AK, Alrasheed AK, Alsharafi AA. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: an observational cohort study. Eur J Med Res. 2020;25(1):1–8. doi: 10.1186/s40001-020-00462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Omari A, Alhuqbani WN, Zaidi ARZ, Al-Subaie MF, AlHindi AM, Abogosh AK, Alrasheed AK, Alsharafi AA, Alhuqbani MN, Salih S. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2020;13(11):1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 81.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2021;106(5):429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 83.Barski L, Nevzorov R, Jotkowitz A, Rabaev E, Zektser M, Zeller L, Shleyfer E, Harman-Boehm I, Almog Y. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am J Med Sci. 2013;345(4):326–330. doi: 10.1097/MAJ.0b013e31827424ab. [DOI] [PubMed] [Google Scholar]
- 84.Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20(3):620–628. doi: 10.1111/dom.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680–1686. doi: 10.2337/dc15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.International Diabetes Federation. IDF Diabetes Atlas, 9th ed, 2019, 2021. https://www.diabetesatlas.org. Accessed 13 Oct 2021.
- 87.Association AD. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Tan H. Male predominance in ketosis-prone diabetes mellitus. Biomed Rep. 2015;3(4):439–442. doi: 10.3892/br.2015.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barski L, Harman-Boehm I, Nevzorov R, Rabaev E, Zektser M, Jotkowitz AB, Zeller L, Shleyfer E, Almog Y. Gender-related differences in clinical characteristics and outcomes in patients with diabetic ketoacidosis. Gend Med. 2011;8(6):372–377. doi: 10.1016/j.genm.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 90.Papadopoulos VP, Koutroulos M-V, Zikoudi D-G, Bakola S-A, Avramidou P, Touzlatzi N, Filippou DK. Diabetes-related acute metabolic emergencies in COVID-19 patients: a systematic review and meta-analysis. Diabetol Int. 2021 doi: 10.1007/s13340-021-00502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020;14(6):1563–1569. doi: 10.1016/j.dsx.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macias Gil R, Marcelin JR, Zuniga-Blanco B, Marquez C, Mathew T, Piggott DA. COVID-19 pandemic: disparate health impact on the Hispanic/Latinx population in the United States. J Infect Dis. 2020;222(10):1592–1595. doi: 10.1093/infdis/jiaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez-Diaz CE, Guilamo-Ramos V, Mena L, Hall E, Honermann B, Crowley JS, Baral S, Prado GJ, Marzan-Rodriguez M, Beyrer C. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol. 2020;52:46–53.e2. doi: 10.1016/j.annepidem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farsani SF, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. doi: 10.1136/bmjopen-2017-016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al Mutair A, Al Mutairi A, Alhumaid S, Maaz Abdullah S, Zia Zaidi AR, Rabaan AA, Al-Omari A. Examining and investigating the impact of demographic characteristics and chronic diseases on mortality of COVID-19: Retrospective study. PLoS ONE. 2021;16(9):e0257131. doi: 10.1371/journal.pone.0257131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al Mutair A, Al Mutairi A, Zaidi ARZ, Salih S, Alhumaid S, Rabaan AA, Al-Omari A. Clinical Predictors of COVID-19 Mortality Among Patients in Intensive Care Units: A Retrospective Study. International journal of general medicine. 2021;14:3719. doi: 10.2147/IJGM.S313757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alhumaid S, Al Mutair A, Al Alawi Z, Al Salman K, Al Dossary N, Omar A, Alismail M, Al Ghazal AM, Jubarah MB, Al SH. Clinical features and prognostic factors of intensive and non-intensive 1014 COVID-19 patients: an experience cohort from Alahsa, Saudi Arabia. Eur J Med Res. 2021;26(1):1–13. doi: 10.1186/s40001-021-00517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, Alosco ML, Solomon TM, Nowinski CJ, McHale L. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360–370. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 100.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. Morb Mortal Wkly Rep. 2018;67(12):362. doi: 10.15585/mmwr.mm6712a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alhumaid S, Al Mutair A, Al Alawi Z, Alshawi AM, Alomran SA, Almuhanna MS, Almuslim AA, Bu Shafia AH, Alotaibi AM, Ahmed GY. Coinfections with bacteria, fungi, and respiratory viruses in patients with SARS-CoV-2: a systematic review and meta-analysis. Pathogens. 2021;10(7):809. doi: 10.3390/pathogens10070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rabaan AA, Al-Ahmed SH, Garout MA, Al-Qaaneh AM, Sule AA, Tirupathi R, Mutair AA, Alhumaid S, Hasan A, Dhawan M. Diverse immunological factors influencing pathogenesis in patients with COVID-19: a review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens. 2021;10(5):565. doi: 10.3390/pathogens10050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA, Tirupathi R, Mutair AA, Alhumaid S, Al-Omari A, Dhawan M. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436. doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas M Drake AMR, Cameron J Fairfield, Conor Egan, Stephen R Knight, Riinu Pius, Hayley E Hardwick, Lisa Norman, Catherine A Shaw, Kenneth A McLean, A A Roger Thompson, Antonia Ho, Olivia V Swann, Michael Sullivan, Felipe Soares, Karl A Holden, Laura Merson, Daniel Plotkin, Louise Sigfrid, Thushan I de Silva, Michelle Girvan, Clare Jackson, Clark D Russell, Jake Dunning, Tom Solomon, Gail Carson, Piero Olliaro, Jonathan S Nguyen-Van-Tam, Lance Turtle, Annemarie B Docherty, Peter JM Openshaw, J Kenneth Baillie, Ewen M Harrison, Malcolm G Semple. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet. 2021;398(10296):223–37. [DOI] [PMC free article] [PubMed]
- 105.Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3(2):123–125. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diab Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dass B, Beck A, Holmes C, Morton G. Euglycemic DKA (euDKA) as a presentation of COVID-19. Clin Case Rep. 2021;9(1):395–398. doi: 10.1002/ccr3.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vitale RJ, Valtis YK, McDonnell ME, Palermo NE, Fisher ND. Euglycemic diabetic ketoacidosis with COVID-19 infection in patients with type 2 diabetes taking SGLT2 inhibitors. AACE Clin Case Rep. 2021;7(1):10–13. doi: 10.1016/j.aace.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Search outcomes of all studies found via electronic search databases.
Data Availability Statement
Data are available upon request, please contact author for data requests.


