Abstract
Background:
In response to the COVID-19 pandemic, we scaled up telemedicine and rideshare services for clinic and laboratory visits for pediatric and adolescent patients with HIV.
Setting:
HIV subspecialty program for patients aged 0–24 years at Children's National Hospital, Washington, DC.
Methods:
Using the χ2 and Wilcoxon rank sum tests, we compared demographics, visit and laboratory data, and rideshare usage among patients who scheduled telemedicine at least once (telemedicine) versus those who never scheduled telemedicine (no-telemedicine) during the pandemic (April–September 2020). We compared the number and proportion of scheduled and completed clinic visits before the pandemic (April–September 2019) with those during the pandemic.
Results:
We analyzed 178 pediatric and adolescent patients with HIV (median age 17.9 years, 89.3% Black, 48.9% male patients, 78.7% perinatally infected), of whom 70.2% and 28.6% used telemedicine and rideshare, respectively. Telemedicine patients scheduled more visits (236 vs 179, P < 0.0001) and completed a similar proportion of visits (81.8% vs 86.0%, P = 0.3805) compared with no-telemedicine patients. Laboratory testing rates (81.3% versus 98.5%, P = 0.0005) were lower in telemedicine patients compared with no-telemedicine patients. Rideshare usage (12.4% versus 26.5%, P = 0.0068) was lower in telemedicine versus no-telemedicine patients. During the pandemic, most of the patients (81.0%) had HIV RNA <200 copies/mL. The total number of completed visits and the proportion of visits completed were similar before and during the pandemic.
Conclusion:
Most of the pediatric and adolescent patients with HIV used telemedicine and maintained HIV RNA <200 copies/mL during the pandemic. Despite rideshare usage, laboratory testing rates were lower with telemedicine compared with in-person visits.
Key Words: telemedicine, HIV, rideshare, COVID-19, children, youth
INTRODUCTION
Early data on the effects of the COVID-19 pandemic on people living with HIV in the United States indicated interruption of services and potential negative health outcomes.1,2 Specifically, youth living with HIV identified challenges, including loss of income and jobs, financial and food insecurity, and poor medication adherence.1 Since the pandemic, innovations in HIV service delivery were needed to ensure uninterrupted access and utilization of HIV treatment services for children and youth. Most (99%) of the surveyed HIV providers in the United States offered telemedicine during the pandemic, with approximately 47% of visits conducted this way.3 Subsequently, 57% of surveyed patients responded they were more likely to use telemedicine compared with in-person visits for their HIV care.4 Telemedicine reduced the need for in-person visits and transportation5,6; however, it required patients to have access to technology, private space, and the ability to complete laboratory testing. Youth living with HIV expressed privacy concerns when using telemedicine,7 highlighting that flexibility and tailored options must be considered for this population. In addition, the need for laboratory testing must be addressed with options such as supporting transportation and facilitating access to conveniently located testing sites to ensure timely laboratory testing. To date, there are limited data on access to HIV treatment services among children and youth during the COVID-19 pandemic in the United States.
Special Immunology Services (SIS) at Children's National Hospital (CNH) cares for approximately 200 children and youth living with HIV annually in the metropolitan District of Columbia (DC) area. Supported by federal Ryan White (RW) funds,8 SIS provides comprehensive HIV medical care, including case management, peer support, nutritional and mental health services, and access to resources such as food and transportation (including rideshare services through Uber Health). Patients are scheduled at 3- to 4-month intervals for routine HIV care and laboratory testing, as per pediatric and adolescent US Department of Health and Human Services (HHS) guidelines.9 At the beginning of the pandemic, SIS reduced the number of in-person medical visits and laboratory testing to the minimum needed. With the support of the CNH Telehealth Program,10 SIS launched and rapidly scaled up telemedicine services in April 2020. We developed a standard operating procedure including provider training and scheduling and visit workflows using the HIPAA-compliant Zoom platform. SIS also scaled up existing rideshare services with institutional and COVID-19 RW support. This study aimed to evaluate the effect of telemedicine on visit completion rates, rideshare usage, and related health outcomes among children and youth living with HIV receiving care at SIS during the pandemic.
METHODS
This was a retrospective cohort study of patients aged 0–24 years who received care at SIS. From patients seen during April–September 2020 (described as “during the pandemic”), data were collected from electronic medical records: demographics (age at the end of study period, sex, and race), HIV acquisition mode, time since HIV diagnosis at the end of study period, scheduled and completed medical visit information (telemedicine, in-person), rideshare usage (to attend in-person visits or to complete laboratory testing at CNH or local laboratory sites for those who attended telemedicine visits), laboratory testing completion for each medical visit, time between laboratory testing and medical visit, and laboratory test results if obtained for each visit (HIV RNA and CD4 count). During the pandemic, patients were advised to alternate in-person and telemedicine visits when feasible, and antiretroviral therapy refills were not contingent on medical visits. The type of visit scheduled was based on patient preference and not on clinical or laboratory criteria. Demographics, visit information, rideshare usage, and laboratory outcomes were compared between patients who scheduled telemedicine at least once during the study period (classified as “telemedicine”) versus those who never scheduled telemedicine (classified as “no-telemedicine”). The χ2 and Wilcoxon rank sum tests were used to compare categorical and continuous variables, respectively.
To examine the relationship of the pandemic with visit completion, we compared medical visit information of patients seen during the pandemic versus patients seen during the same period in 2019 (April−September 2019, described as “prepandemic”). We used paired t tests to compare the number and proportion of visits completed during the 2 periods. SAS Studio was used for analysis (SAS Institute, Cary, North Carolina). This study was approved by the CNH Institutional Review Board, with informed consent waived.
RESULTS
We analyzed data for 178 individual patients (median age 17.9 years, 89.3% Black, 48.9% male patients, 78.7% perinatally infected) who scheduled visits during the pandemic. Of the 125 (70.2%) telemedicine patients, 72 (57.6%) scheduled only telemedicine visits (i.e. no in-person visits), whereas the remaining scheduled a combination of telemedicine and in-person visits (data not shown). Telemedicine patients were of a similar age (median 18.0 years; IQR 15.5, 20.4) compared with no-telemedicine patients (median 17.1 years; IQR 13.2, 20.7; P = 0.4910). There was a nonsignificant higher proportion of perinatally infected children and youth in the telemedicine group compared with the no-telemedicine group (82.4% versus 69.8%, P = 0.0609) (Table 1).
TABLE 1.
Patient Characteristics and Outcomes by Telemedicine Use During the Pandemic Study Period (April 2020 to September 2020)
| All (n = 178) | Telemedicine (n = 125) | No-telemedicine (n = 53) | P | |
| Patient characteristics* | ||||
| Median age at the end of study period, years (IQR) | 17.9 (15.0, 20.5) | 18.0 (15.5, 20.4) | 17.1 (13.2, 20.7) | 0.4910 |
| Race/ethnicity, n (%): | ||||
| Black | 159 (89.3) | 114 (91.2) | 45 (84.9) | |
| White | 4 (2.3) | 3 (2.4) | 1 (1.9) | |
| Hispanic | 9 (5.1) | 3 (2.4) | 6 (11.3) | 0.1381 |
| Asian | 4 (2.3) | 3 (2.4) | 1 (1.9) | |
| Other/unknown | 2 (1.1) | 2 (1.6) | 0 (0.0) | |
| Current sex, n (%): | ||||
| Male† | 87 (48.9) | 62 (49.6) | 25 (47.2) | 0.7668 |
| Female | 91 (51.1) | 63 (50.4) | 28 (52.8) | |
| HIV acquisition mode, n (%): | ||||
| Perinatal | 140 (78.7) | 103 (82.4) | 37 (69.8) | 0.0609 |
| Nonperinatal | 38 (21.3) | 22 (17.6) | 16 (30.2) | |
| Median years since HIV diagnosis at the end of study period (IQR) | 11.9 (4.2, 16.2) | 13.0 (5.1, 16.2) | 7.1 (3.0, 15.4) | 0.0461 |
| Visits* | ||||
| Total scheduled visits, n | 315 | 236 | 79 | <0.0001 |
| Scheduled telemedicine visits, n (%) | 171 (54.3) | 171 (72.5) | NA | NA |
| Proportion of completed visits, n (%): | ||||
| All visits | 261 (82.9) | 193 (81.8) | 68 (86.0) | 0.3805 |
| Telemedicine only | 142 (83.0) | 142 (83.0) | NA | NA |
| In-person only | 119 (82.6) | 51 (78.5) | 68 (86.1) | 0.2300 |
| Completed laboratory testing for completed visit, n (%) | 224 (85.8) | 157 (81.4) | 67 (98.5) | 0.0005 |
| Median days between laboratory testing and visit (IQR) | 0 (0, 11) | 2 (0, 19) | 0 (0, 0) | <0.0001 |
| Number of rideshare users, n (%)‡ | 34 (19.1) | 21 (16.8) | 13 (24.5) | 0.2304 |
| Visits completed where rideshare was used, n (%) | 42 (13.3) | 24 (12.4) | 18 (26.5) | 0.0068 |
| Laboratory outcomes | ||||
| HIV RNA, n (%):§ | ||||
| <20 copies/mL | 125 (57.9) | 93 (61.6) | 32 (49.2) | 0.2405 |
| 20–200 copies/mL | 50 (23.1) | 32 (21.2) | 18 (27.7) | |
| >200 copies/mL | 41 (19.0) | 26 (17.2) | 15 (23.1) | |
| CD4 cell count, n (%):§ | ||||
| <200 cells/mm3 | 11 (5.0) | 9 (5.8) | 2 (3.0) | 0.2468 |
| 200–499 cells/mm3 | 34 (15.3) | 27 (17.4) | 7 (10.4) | |
| ≥500 cells/mm3 | 177 (79.7) | 119 (76.8) | 58 (86.6) |
Bold values are considered statistically significant with a P-value <0.05.
Proportions were calculated based on the relevant denominator for the variable (e.g.: denominator used for “telemedicine visits completed” was “scheduled telemedicine visits” for that column; denominator used for “number of patients who used rideshare” was “number of unique patients” for that column).
Includes one transgender male patient
Includes in-person visits where rideshare was used and telemedicine visits where rideshare was used to complete laboratory testing.
Laboratory testing was completed at 224 visits by patients living with HIV. There were missing data for 8 HIV RNA and 2 CD4 counts.
A total of 315 visits were scheduled by all patients, half of which were telemedicine (n = 171, 54.3%). Visit completion rates were high for all visits (82.9%), telemedicine visits only (83.0%), and in-person visits only (83.0%). Telemedicine patients scheduled more visits (n = 236; 1.9 visits per patient) compared with no-telemedicine patients (n = 79; 1.5 visits per patient; P < 0.0001), with most (72.5%) of their scheduled visits being telemedicine visits. Telemedicine and no-telemedicine patients had similar proportions of completed visits (all visits 81.8% versus 86.0%, P = 0.3805; in-person visits only 78.5% versus 86.1%, P = 0.2300) (Table 1).
Most patients completed laboratory testing (85.8%) around the time of their visit (median 0 days; IQR 0, 11). Laboratory testing was completed by fewer telemedicine patients (81.4%) compared with no-telemedicine patients (98.5%, P = 0.0005), and there was a longer time between laboratory tests and the visit in telemedicine patients (median 2 days; IQR 0, 19) compared with no-telemedicine patients (median 0 days; IQR 0, 0; P < 0.0001). Thirty-four patients (19.1%) used rideshare to attend in-person visits or to complete laboratory testing at CNH or local laboratory sites. Patients used rideshare services for 13.3% of all completed visits (including rideshare for in-person visits and laboratory testing for telemedicine visits). Rideshare usage to complete visits and/or laboratory testing (12.4% vs 26.5%, P = 0.0068) was lower in telemedicine versus no-telemedicine patients. During the pandemic, most of the children and youth living with HIV had HIV RNA <200 copies/mL (81.0%) and CD4 count ≥500 cells/mm3 (79.7%). The HIV RNA and CD4 count were similar between telemedicine and no-telemedicine children and youth living with HIV (Table 1).
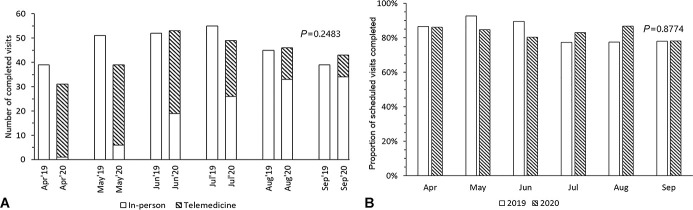
There were more patients seen during the pandemic compared with prepandemic (178 versus 163), and we did not observe a trend in declined visits during the months preceding the pandemic (data not shown). There was no significant difference in the number of completed visits (261 versus 281; P = 0.2483) and in the proportion of completed visits during the pandemic compared with prepandemic (82.9% versus 83.4%, P = 0.8774) (Fig. 1).
FIGURE 1.
A, Number of completed visits and (B) proportion of scheduled visits completed at Special Immunology Services in Children's National Hospital during April–September 2019 and April–September 2020.
DISCUSSION
Our study reports sustained number of visits and retention in care during the COVID-19 pandemic among predominantly Black children and youth living with HIV in metropolitan DC. The COVID-19 pandemic posed a significant health burden to the lives of Black people, who are also disproportionately affected by the HIV epidemic in the United States.5,11,12 Providing telemedicine with rideshare helped to address access to care, transportation barriers, and infection control requirements associated with the pandemic.
Although some US-based clinics caring for people living with HIV reported an increase in delayed or postponed visits during the pandemic,2,13 we report similar volumes of visits during the pandemic study period. Telemedicine patients had a nonsignificant lower visit completion rate for their in-person visits compared with no-telemedicine patients. This is likely multifactorial, including telemedicine patient's experience of easy replacement of a missed in-person visit with a telemedicine visit and telemedicine patients potentially experiencing more transportation and logistical barriers at baseline that lead to more missed in-person visits. Despite the high proportion of completed telemedicine visits (83%) during the pandemic, we did not observe the low no-show rates for telemedicine (4%–6%), as reported among adult infectious diseases, adult HIV, and general adolescent clinics in the United States.14,15 This may be attributed to differences in logistical and technological barriers associated with the use of telemedicine,5,16,17 patient privacy concerns,7,18 and differences in baseline patterns of engagement in care.
More than half of scheduled visits during the pandemic in our study were telemedicine visits, likely due to the ease of scheduling and attending those visits compared with in-person visits.4,18 Telemedicine also allowed for interventions such as video directly observed therapy and enhanced adherence counseling in home settings. Interestingly, many patients chose to schedule only in-person visits or only telemedicine visits during the pandemic, reflecting the importance of acknowledging patient preference when scheduling in-person or telemedicine visits.
Many of our patients and their families reside in DC neighborhoods that have been affected by high rates of COVID-19,19 further demarcating existing and COVID-19–related health, social, and economic disparities. As with other areas in the United States, public transportation in DC was heavily scaled back from March 2020; stay-at-home orders came into effect from April 2020 through June 2020.20 During this period, in addition to facilitating access to services for patients who attended in-person visits, rideshare became an important facilitator for telemedicine patients who required transportation support to complete laboratory testing.
Despite rideshare usage, we identified drawbacks of using telemedicine, including lower rates of laboratory testing completion and longer time between laboratory testing and visits. Other clinics observed similar challenges in obtaining laboratory testing and opted to extend the HHS recommendation of 3- to 4-month intervals for routine HIV testing9 to longer intervals for stable virologically suppressed patients.18 However, this is less desirable for immunocompromised patients living with HIV and people who have significant adherence issues or comorbidities. Reassuringly, the 2-day delay in laboratory testing completion among telemedicine patients is unlikely to negatively affect their clinical management. It is unclear whether a further scale-up of rideshare would be adequate to improve laboratory testing completion rates for patients using telemedicine. In conjunction with rideshare, we plan to evaluate the use of mobile health interventions for timely laboratory testing completion; the utilization of mobile health interventions has proven successful in increasing engagement of care for people with HIV and is an effective communication modality for youth.21–23
Most importantly, during the pandemic, most of the children and youth living with HIV (telemedicine and no-telemedicine patients) in our study maintained HIV RNA <200 copies/mL. In contrast to adult studies that have shown that a transition to telemedicine resulted in either improved24,25 or worsened26 virologic suppression rates, our study showed similar rates of HIV RNA <200 copies/mL in telemedicine and no-telemedicine patients. Recently updated HHS pediatric HIV guidelines highlight the role of telemedicine in caring for the pediatric HIV population.9 We have now incorporated telemedicine and rideshare within our package of routine HIV prevention and care services.
Our study had a small sample size and was limited to a single center that received supplemental COVID-19 RW funding and may not be generalizable to the general population. Furthermore, technological and transportation barriers and patient use of mental health services were not included in this study. Despite these limitations, our study is the first to show the effectiveness of telemedicine and rideshare for maintaining access to HIV services among children and youth living with HIV in the United States. In addition to our ongoing studies on the relationship between telemedicine and rideshare to the adherence and virologic outcomes, we are conducting a study on the acceptability of the telemedicine for mental health services among our patients.
CONCLUSIONS
Digital health equity is essential to achieving overall health equity,16,27 and telemedicine has the potential to bridge pediatric health care gaps.28 Telemedicine combined with rideshare support ensured uninterrupted access to HIV care among pediatric and adolescent patients with HIV at SIS. Most of the patients remained in care, used telemedicine, and maintained HIV RNA <200 copies/mL during pandemic. Despite rideshare usage, laboratory testing rates were lower with telemedicine visits compared with in-person visits. Future research is essential to optimize HIV prevention and care through telemedicine and to explore innovative service delivery models for children and youth living with HIV.
ACKNOWLEDGMENTS
The authors thank children, youth, and families who participated in this study. The authors also thank the Telehealth Program staff at Children's National Hospital for their support during this project. Special Immunology Services at Children's National Hospital received the following Ryan White HIV/AIDS grants support: DC DOH HASTA_REGEIS04.26.19, 20D050 Part A Program for Regional Early Intervention Services and CARES Act COVID Relief; 20D032 Suburban MD Part A, Part A Minority AIDS Initiative (MAI); 20D405 & 20D013 DC Part A, Part A MAI and Part B, RWA&B.11.10.16; and HRSA-17-039/MedStar Health Research Institute Part D Coordinated HIV Services and Access to Research for Women, Infants, Children and Youth (WICY), and CARES Act COVID Relief. The funding provided by RWHAP has allowed us to care for children and youth at risk of and living with HIV in the metropolitan DC area. The views represented in this article are our own and do not represent those of the RWHAP or HRSA.
Footnotes
A.M.N. is supported by K08 HD094638. WLAK is supported by a Mid-Atlantic Center for AIDS Research (CFAR) Consortium Scholars Program awarded from the District of Columbia CFAR, an NIH-funded program (P30AI117970), which is supported by the following NIH cofunding and participating institutes and centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, National Institute on Aging, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Minority Health and Health Disparities, National Institute of Dental and Craniofacial Research, National Institute of Nursing Research, Fogarty International Center, and Office of AIDS Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Armbruster M, Fields EL, Campbell N, et al. Addressing health inequities exacerbated by COVID-19 among youth with HIV: expanding our toolkit. J Adolesc Health. 2020;67:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao S, Li Z, Weissman S, et al. Disparity in HIV service interruption in the outbreak of COVID-19 in South Carolina. AIDS Behav. 2021;25:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson L, Kates J. Delivering HIV care and prevention in the COVID era: a National survey of Ryan White providers. Available at: https://kff.org/report-section/delivering-hiv-care-prevention-in-the-covid-era-a-national-survey-of-ryan-white-providers-issue-brief/. Accessed February 14, 2021.
- 4.Dandachi D, Dang BN, Lucari B, et al. Exploring the attitude of patients with HIV about using telehealth for HIV care. AIDS Patient Care STDS. 2020;34:166–172. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal D, Fowler EJ, Abrams M, et al. COVID-19 - implications for the health care system. N. Engl. J. Med. 2020;383:1483–1488. [DOI] [PubMed] [Google Scholar]
- 6.Manglani M, Gabhale Y, Lala MM, et al. Reaching the unreached: providing quality care to HIV-infected children through telemedicine-an innovative pilot initiative from Maharashtra, India. Int J Pediatr. 2020;2020:6432476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman CA, Kacanek D, Nichamin M, et al. Using social media and technology to communicate in pediatric HIV research: qualitative study with young adults living with or exposed to perinatal HIV. JMIR Pediatr. Parent. 2020;3:e20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Resources and Services Administration Ryan White and Global HIV/AIDS Program. About the ryan white HIV/AIDS program. Available at: https://hab.hrsa.gov/about-ryan-white-hivaids-program. Accessed February 15, 2021.
- 9.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/PedARV_GL.pdf. Accessed April 12, 2021.
- 10.Siwicki B. Children's National jumps short-term telehealth hurdles while focused on long-term transformation. HealthcareIT News 3/17/21. Available at: https://healthcareitnews.com/news/childrens-national-jumps-short-term-telehealth-hurdles-while-focused-long-term-transformation. Accessed July 4, 2021.
- 11.Goyal MK, Simpson JN, Boyle MD, et al. Racial and/or ethnic and socioeconomic disparities of SARS-CoV-2 infection among children. Pediatrics. 2020;146:e2020009951. [DOI] [PubMed] [Google Scholar]
- 12.Rossen LM, Branum AM, Ahmad FB, et al. Excess deaths associated with COVID-19, by age and race and ethnicity - United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridgway JP, Schmitt J, Friedman E, et al. HIV care continuum and COVID-19 outcomes among people living with HIV during the COVID-19 pandemic, Chicago, IL. AIDS Behav. 2020;24:2770–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood SM, White K, Peebles R, et al. Outcomes of a rapid adolescent telehealth scale-up during the COVID-19 pandemic. J. Adolesc. Health. 2020;67:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvajal KL, Pillai P, Winbush A, et al. 127. Walking to the virtual era. Analysis of the telehealth experience in the infectious diseases clinic during the COVID pandemic. Open Forum Infect. Dis. 2020;7:S193. [Google Scholar]
- 16.Crawford A, Serhal E. Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health. J. Med. Internet Res. 2020;22:e19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwadz M, Campos S, Freeman R, et al. Black and Latino persons living with HIV evidence risk and resilience in the context of COVID-19: a mixed-methods study of the early phase of the pandemic. AIDS Behav. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers BG, Coats CS, Adams E, et al. Development of telemedicine infrastructure at an LGBTQ+ clinic to support HIV prevention and care in response to COVID-19, providence, RI. AIDS Behav. 2020;24:2743–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Government of the District of Columbia. COVID-19 surveillance. Available at: https://coronavirus.dc.gov/data. Accessed January 3, 2021.
- 20.Washington Metropolitan Area Transit Authority. Metro and COVID-19: steps we've taken. Available at: https://mata.com/service/covid19/COVID-19.cfm. Accessed February 22, 2021.
- 21.Evans YN, Golub S, Sequeira GM, et al. Using telemedicine to reach adolescents during the COVID-19 pandemic. J. Adolesc. Health. 2020;67:469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canan CE, Waselewski ME, Waldman ALD, et al. Long term impact of PositiveLinks: clinic-deployed mobile technology to improve engagement with HIV care. PLoS One. 2020;15:e0226870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zurlo J, Du P, Haynos A, et al. OPT-in for life: a mobile technology-based intervention to improve HIV care continuum for young adults living with HIV. Health Promot. Pract. 2020;21:727–737. [DOI] [PubMed] [Google Scholar]
- 24.Saifu HN, Asch SM, Goetz MB, et al. Evaluation of human immunodeficiency virus and hepatitis C telemedicine clinics. Am J Manag Care. 2012;18:207–212. [PubMed] [Google Scholar]
- 25.Young JD, Patel M, Badowski M, et al. Improved virologic suppression with HIV subspecialty care in a large prison system using telemedicine: an observational study with historical controls. Clin Infect Dis. 2014;59:123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinelli MA, Hickey MD, Glidden DV, et al. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS. 2020;34:2328–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood BR, Young JD, Abdel-Massih RC, et al. Advancing digital health equity: a policy paper of the infectious diseases society of America and the HIV medicine association. Clin Infect Dis. 2021;72:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon DU, Belcher HME. COVID-19 pandemic health Disparities and pediatric health care-the promise of telehealth. JAMA Pediatr. 2021;175:345–346. [DOI] [PubMed] [Google Scholar]