Supplemental Digital Content is available in the text.
Keywords: cilostazol, clopidogrel, dual anti-platelet therapy, high-risk, noncardioembolic ischemic stroke, secondary prevention
Abstract
Background and Purpose:
Although dual antiplatelet therapy (DAPT) with aspirin and clopidogrel reduces the recurrence of ischemic stroke while significantly increasing the bleeding events compared with monotherapy, the CSPS.com trial (Cilostazol Stroke Prevention Study combination) showed that DAPT using cilostazol was more effective without the bleeding risk. In the CSPS.com trial, aspirin or clopidogrel was used as the underlying antiplatelet drug. The effectiveness and safety of each combination were examined and clarified.
Methods:
In the CSPS.com trial, a multicenter, open-label, randomized controlled study, patients with high-risk, noncardioembolic ischemic stroke 8 to 180 days after onset treated with aspirin or clopidogrel alone at the discretion of the physician in charge were recruited. Patients were randomly assigned to receive either monotherapy or DAPT using cilostazol and followed for 0.5 to 3.5 years. The primary efficacy outcome was first recurrence of ischemic stroke. The safety outcome was severe or life-threatening bleeding. The analysis was based on the underlying antiplatelet agents.
Results:
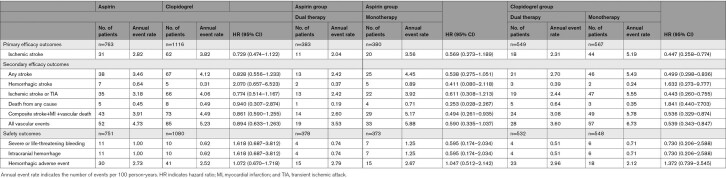
A total of 763 patients taking aspirin and 1116 taking clopidogrel were included in the intention-to-treat analysis. Although the clopidogrel group had more risk factors than the aspirin group, the primary efficacy outcome and safety outcome did not differ significantly between the 2 groups. In the aspirin group, the primary efficacy outcome and safety outcome did not differ significantly between the DAPT group and the aspirin-monotherapy group. In the clopidogrel group, the primary end point occurred at a rate of 2.31 per 100 patient-years in the DAPT group and 5.19 per 100 patient-years in the clopidogrel-monotherapy group (hazard ratio, 0.447 [95% CI, 0.258–0.774]). Safety outcome did not differ significantly between groups (0.51 per 100 patient-years versus 0.71 per 100 patient-years, respectively; hazard ratio, 0.730 [95% CI, 0.206–2.588]).
Conclusions:
The combination of cilostazol and clopidogrel significantly reduced the recurrence of ischemic stroke without increasing the bleeding risk in noncardioembolic, high-risk patients.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01995370. URL: https://www.umin.ac.jp/ctr/; Unique identifier: UMIN000012180.
Antiplatelet therapy is the most basic treatment for the prevention of a secondary stroke in patients with noncardioembolic ischemic stroke. The combination of antiplatelets with different mechanisms is expected to prevent the recurrence of ischemic stroke events more effectively than monotherapy. The combination of aspirin and clopidogrel inhibits platelet function more than either of these agents alone, and several clinical trials and meta-analyses have shown that the aspirin and clopidogrel combination reduces the recurrence of ischemic stroke slightly, while significantly increasing the frequency of bleeding events, compared with aspirin or clopidogrel alone.1–4 The relevant guidelines therefore recommend avoiding the use of dual antiplatelet therapies (eg, aspirin and clopidogrel) for preventing secondary stroke events in patients who are in the chronic phase of noncardioembolic ischemic stroke.5
Cilostazol selectively inhibits phosphodiesterase 3, and the results of the CSPS2 (Cilostazol Stroke Prevention Study 2) demonstrated that cilostazol treatment significantly reduces stroke recurrence with fewer bleeding events than aspirin.6 In light of the lower rate of bleeding events in patients treated with cilostazol, the addition of cilostazol to a regimen with another antiplatelet therapy has been expected to decrease the recurrence of stroke without increasing the bleeding risk. The CSPS.com trial (Cilostazol Stroke Prevention Study combination) showed that, compared with aspirin or clopidogrel alone, combination treatment with cilostazol reduced the recurrence of ischemic stroke in patients in the chronic stage without increasing the bleeding risk.7
In the CSPS.com trial, aspirin or clopidogrel was used as the underlying antiplatelet drug. A small number of trials has demonstrated some effectiveness and safety for the combination of aspirin and cilostazol.8,9 Since clopidogrel is reported to be more effective than aspirin,10 the combination of clopidogrel and cilostazol has been expected to be useful for secondary stroke prevention, with greater effectiveness. However, the clinical utility of this combination therapy has not been reported, to the best of our knowledge. Thus, the effectiveness and safety of the combination of cilostazol and clopidogrel were analyzed in patients enrolled in the CSPS.com trial.
Methods
Data Availability Statement
The deidentified individual participant data and the study protocol of the CSPS.com may be available upon request to Japan Cardiovascular Research Foundation. Researchers can request data disclosure until March 2022.
Design and Patients
Details regarding the CSPS.com trial rationale, design, and methods have been described elsewhere.11 The protocol for the CSPS.com trial was approved by the ethics committee at each participating site, and all patients provided written, informed consent before randomization. In that multicenter, randomized, open-label, parallel-group trial, patients at 292 sites in Japan underwent assignment following randomization from December 2013 through March 2017. The steering committee extended the period of enrollment for 1 year to increase the number of anticipated patients. Any event related to the primary and secondary outcomes was reviewed by the event review committee, which was blinded to the patients’ antiplatelet medications.
The trial’s eligible patients were subjects between 20 and 85 years old who had experienced a noncardioembolic ischemic stroke, as identified on magnetic resonance imaging, between 8 and 180 days before the start of the protocol treatment. These patients were administered either aspirin or clopidogrel alone as antiplatelet therapy after providing informed consent. The choice of whether to use aspirin or clopidogrel before randomization depended on the physician in charge. The patients were also required to meet one or more of the following 3 criteria indicating a high risk of stroke recurrence: (1) ≥50% stenosis of a major intracranial artery (to the level of A2, M2, or P2); (2) ≥50% stenosis of an extracranial artery (common carotid artery, internal carotid artery, vertebral artery, brachiocephalic artery, or subclavian artery); and (3) 2 or more of the following risk factors: age ≥65 years, diabetes, hypertension, peripheral arterial disease, chronic kidney disease, history of ischemic stroke other than the qualifying stroke for the trial, history of ischemic heart disease, and current smoking.11
In the CSPS.com trial, the patients were randomly assigned, in a 1:1 ratio using a block-randomization scheme, to receive either monotherapy with aspirin (81 or 100 mg) or clopidogrel (50 or 75 mg), administered once daily; or dual therapy using cilostazol (100 mg, twice daily; the recommended dose for stroke prevention in Japan) in combination with either aspirin (81 or 100 mg) or clopidogrel (50 or 75 mg), administered once daily. In Japan, clopidogrel at 50 mg is approved for older (eg, ≥75 years old) and low-weight patients (≤50 kg body weight). For the prevention of adverse drug reactions such as headache and tachycardia, treating physicians provided the option of initiating cilostazol treatment at 100 mg/day and increasing to 200 mg/day within 15 days. Changes in the choice of these 3 antiplatelet medications were not permitted after informed consent was obtained. The data of the CSPS.com trial were analyzed based on the underlying antiplatelet agents.
Outcomes
The primary efficacy outcome was the first recurrence of ischemic stroke. The secondary efficacy outcomes were (1) any stroke (ischemic or hemorrhagic); (2) hemorrhagic stroke (intracerebral or subarachnoid hemorrhage); (3) ischemic stroke or transient ischemic attack; (4) death from any cause; (5) a composite of stroke, myocardial infarction, and vascular death; and (6) all vascular events, including stroke, myocardial infarction, and other vascular events.
The safety outcomes were severe or life-threatening bleeding as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries classification, which includes intracranial hemorrhage and bleeding resulting in substantial hemodynamic compromise requiring treatment.
Statistical Analysis
Efficacy analyses were conducted in the intention-to-treat population, focused only on time to first event. Safety analyses were conducted with patients who had received at least one dose of a trial regimen. The treatment groups were compared using the log-rank test. Cox proportional hazard models were used to calculate the hazard ratios (HRs) and 95% CIs for the comparison of the dual therapy group with the monotherapy group. Annual recurrence rates were estimated using the person-year method. Subgroup analyses were performed following stratification by age, sex, type of ischemic stroke (atherothrombotic or lacunar), stenosis of extracranial arteries, stenosis of intracranial arteries, modified Rankin Scale score, medical history and complications, current smoking status, obesity, and time to randomization. Tests for interactions between the treatment arms and subgroups were performed using the Cox proportional hazards model. Two-sided P <0.05 were considered significant. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Aspirin Group Versus Clopidogrel Group
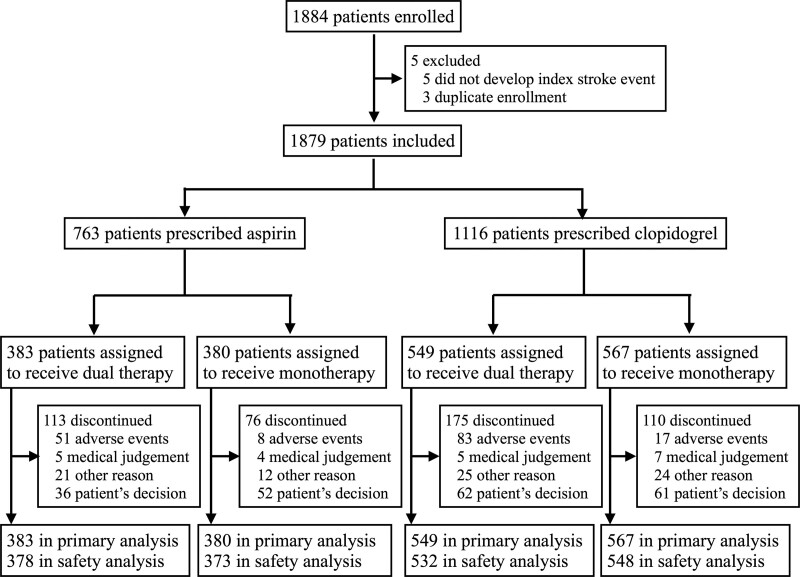
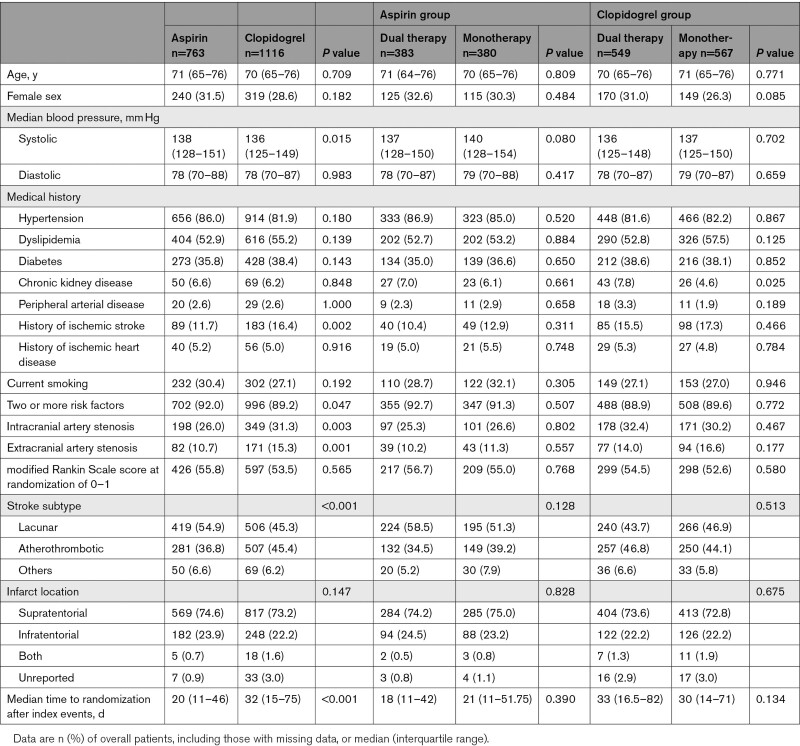
At randomization and before enrollment in the study, clopidogrel was being taken by 1116 (59%) of the 1879 patients, and aspirin was being taken by the remaining 763 (41%) patients (Figure 1). Some of the background clinical features of the aspirin and clopidogrel groups differed. The clopidogrel group had a significantly higher prevalence of a history of ischemic stroke compared with the aspirin group (183 [16.4%] patients versus 89 [11.7%] patients, respectively; P=0.0021), intracranial artery stenosis (349 [31.3%] versus 198 [26.0%] patients, P=0.0028), extracranial artery stenosis (171 [15.3%] versus 82 [10.7%] patients, P=0.0014), and atherothrombotic stroke subtype (507 [45.4%] patients versus 281 [36.8%] patients, P<0.0001). The systolic blood pressure was significantly lower in the clopidogrel group (median 136 versus 138 mm Hg, P=0.0148). The median time to randomization was significantly longer in the patients treated with clopidogrel than in those treated with aspirin (32 [15–75] days versus 20 [11–46] days, respectively; P<0.0001; Table 1).
Figure 1.
Study flow chart.
Table 1.
Baseline Characteristics
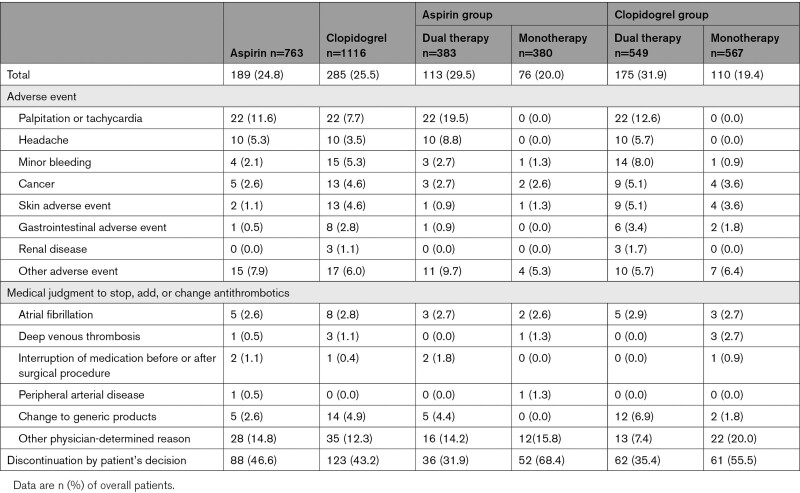
Despite the slightly higher prevalence of risk factors among the patients in the clopidogrel group, the clopidogrel group and the aspirin group did not differ significantly in the primary ischemic stroke recurrence outcome (62 patients [3.82 per 100 patient-years] versus 31 patients [2.82 per 100 patient-years], respectively; HR, 0.729 [95% CI, 0.474–1.122]), in any of the secondary efficacy outcomes, or in the safety outcome (10 patients [0.62 per 100 patient-years] versus 11 patients [1.00 per 100 patient-years], respectively; HR, 1.618 [95% CI, 0.687–3.812]) (Table 2). The rate of discontinuing follow-up for reasons other than the development of a major event did not differ significantly between the clopidogrel and aspirin groups (285 patients [25.5%] versus 189 patients [24.8%], respectively; P=0.625; Table 3).
Table 2.
Efficacy and Safety Outcomes
Table 3.
Reasons for the Discontinuation of Trial Drugs
Efficacy and Safety Outcomes in the Aspirin Group
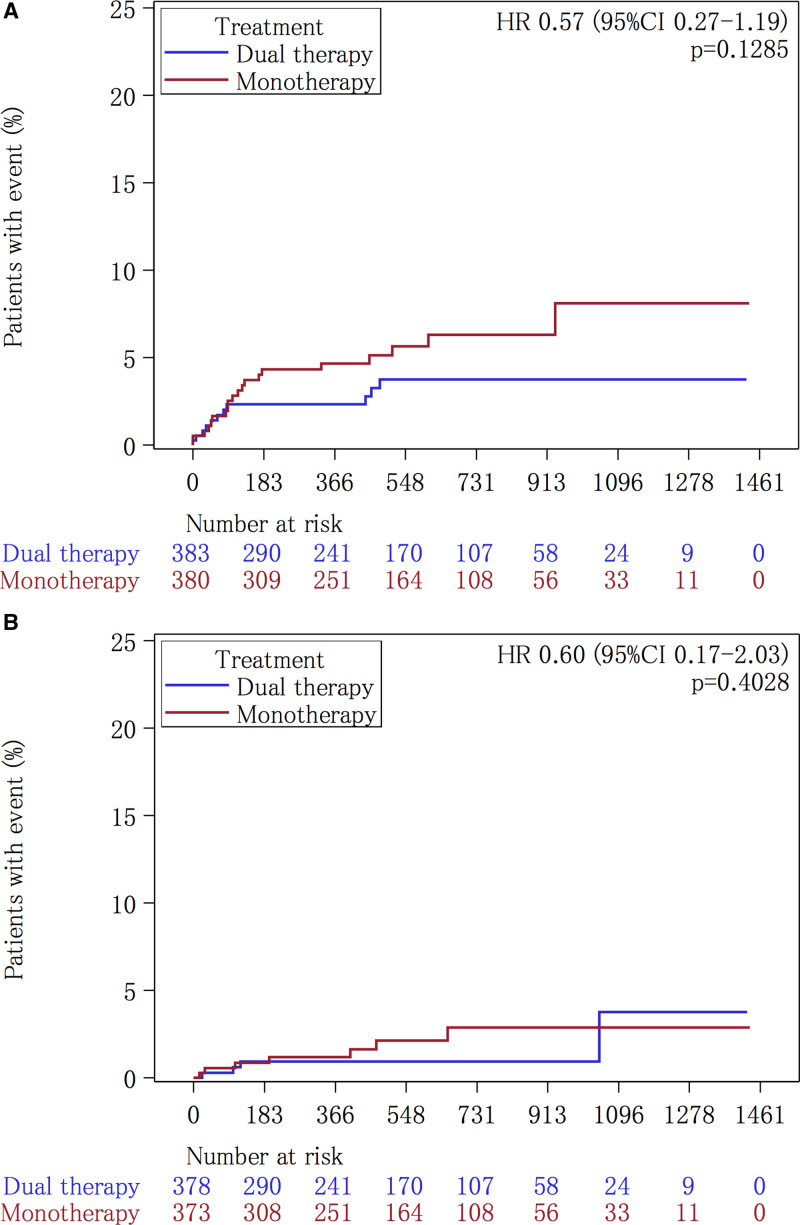
The clinical background characteristics did not differ significantly between the dual therapy patients who received the added cilostazol and the aspirin-monotherapy patients (Table 1). The primary end point of ischemic stroke occurred in 11 (2.04 per 100 patient-years) of the 383 patients during follow-up in the dual therapy group and in 20 (3.56 per 100 patient-years) of the 380 patients in the monotherapy group (HR, 0.569 [95% CI, 0.273–1.189]) (Table 2, Figure 2A). None of the secondary efficacy outcomes was significantly different (Table 2). The rate of the safety outcome of severe or life-threatening hemorrhage did not differ significantly between these 2 groups (4 patients [0.74 per 100 patient-years] versus 7 patients [1.25 per 100 patient-years], respectively; HR, 0.595 [95% CI, 0.174–2.034]) (Table 2, Figure 2B). The rate of discontinuation for reasons other than the development of a major event was significantly higher in the dual therapy patients compared with the aspirin-monotherapy patients (113 patients [29.5%] versus 76 patients [20.0%], respectively; P=0.001). Palpitations or tachycardia and headache were common reasons for discontinuation in the dual therapy group (Table 3).
Figure 2.
Kaplan-Meier analysis of outcomes in the aspirin group. The Kaplan-Meier curves for time to the first event of the primary efficacy outcome, defined as ischemic stroke (A), and to the safety outcome of severe or life-threatening bleeding (B), are shown. HR indicates hazard ratio.
Efficacy and Safety Outcomes in the Clopidogrel Group
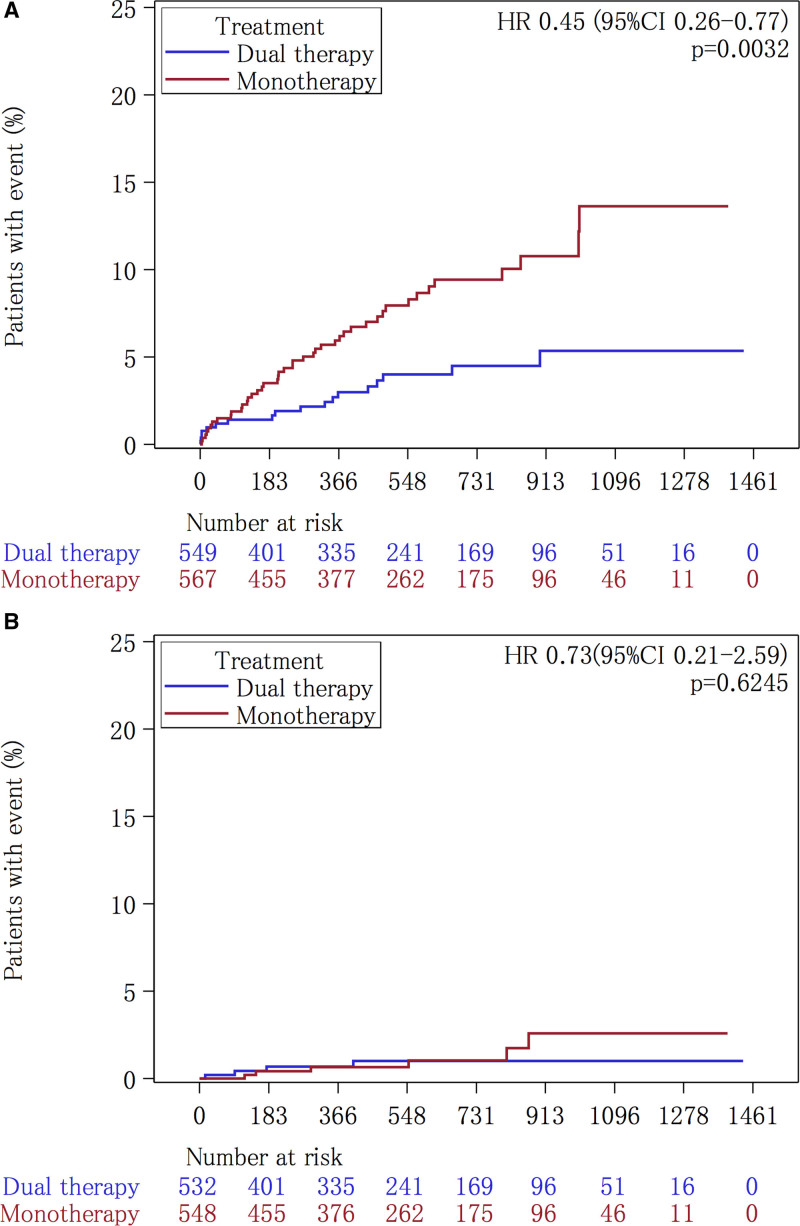
Regarding the patients’ clinical background characteristics, the prevalence of chronic kidney disease was significantly higher in the cilostazol and clopidogrel dual therapy group than in the clopidogrel-monotherapy group (43 [7.8%] patients versus 26 [4.6%] patients, respectively; P=0.0253) (Table 1). The primary end point of ischemic stroke occurred in 18 (2.31 per 100 patient-years) of the 549 patients during follow-up in the dual therapy group and in 44 (5.19 per 100 patient-years) of the 567 patients in the monotherapy group (HR, 0.447 [95% CI, 0.258–0.774]) (Table 2, Figure 3A). Any stroke, ischemic stroke or transient ischemic attack, composite vascular events and all vascular events were also significantly lower in the dual therapy group (Table 2). The rate of the safety outcome of severe or life-threatening hemorrhage did not differ significantly between the 2 groups (4 patients [0.51 per 100 patient-years] versus 6 patients [0.71 per 100 patient-years], respectively; HR, 0.730 [95% CI, 0.206–2.588]) (Table 2, Figure 3B). The rate of discontinuation for reasons other than the development of a major event was significantly higher in the dual therapy patients than in the clopidogrel-monotherapy patients (175 [31.9%] patients versus 110 [19.4%] patients, respectively; P<0.001). As for the aspirin group, palpitations or tachycardia and headache were common reasons for discontinuation in the dual therapy group. Minor bleeding and skin adverse events were common in the clopidogrel group, especially in the patients treated with cilostazol (dual therapy; Table 3).
Figure 3.
Kaplan-Meier analysis of outcomes in the clopidogrel group. The Kaplan-Meier curves for the time to the first event of the primary efficacy outcome, defined as ischemic stroke (A), and to the safety outcome of severe or life-threatening bleeding (B), are shown. HR indicates hazard ratio.
The results of the subgroup analysis demonstrated that the dual therapy was more effective than the monotherapy in male patients (9/379 [2.37%] patients versus 36/418 [8.61%] patients, respectively; HR, 0.285 [95% CI, 0.137–0.593]; Figure in the Data Supplement).
In the whole group analysis, there was no significant interaction of subgroup (aspirin or clopidogrel)-by-treatment (cilostazol or not), which was tested using the multivariate Cox proportional hazards model with the main effect of subgroup and treatment; the adjusted HR was 0.79 [95% CI, 0.31–1.97] for the efficacy analysis and 1.23 [95% CI, 0.21–7.17] for the safety analysis.
Discussion
To the best of our knowledge, this is the first study to show that the combination of clopidogrel and cilostazol reduces the rate of secondary ischemic stroke without increasing the bleeding risk. There have been several previous reports on the combined use of aspirin and cilostazol. These trials of the combination of aspirin and cilostazol described a tendency toward more effective reductions of the recurrent ischemic stroke rate and the progression of intracranial artery stenosis compared with those seen with aspirin alone. The TOSS (Trial of Cilostazol in Symptomatic Intracranial Arterial Stenosis) demonstrated that the progression of intracranial artery stenosis was significantly lower in the cilostazol and aspirin group than in the aspirin group (6.7% versus 28.8%, respectively; P=0.008) in follow-up of only 6 months.8 The CATHARSIS trial (Cilostazol-Aspirin Therapy Against Recurrent Stroke With Intracranial Artery Stenosis) showed that progression of intracranial stenosis was observed in 9.6% of the cilostazol and aspirin dual therapy group and in 5.6% of the aspirin-monotherapy group, with no significant intergroup difference (P=0.53). CATHARSIS also demonstrated that the mean annual recurrence ratio of ischemic stroke was 2.5% in the cilostazol and aspirin dual therapy group and 4.5% in the aspirin-monotherapy group (adjusted HR, 0.47 [95% CI, 0.13–1.73]).9 The number of cases included in the CATHARSIS trial was too small to allow conclusions about the clinical outcome differences, but treatment with the combination of cilostazol and aspirin might be expected to reduce the risk of recurrent ischemic stroke. A clinical trial in the acute phase, the ADS study (Acute Aspirin Plus Cilostazol Dual Therapy for Noncardiogenic Stroke Patients Within 48 Hours of Symptom Onset) showed that the combination of cilostazol and aspirin, while safe, did not reduce the rate of short-term neurological worsening after 14 days in noncardioembolic stroke patients.12 In the present analysis of a subset of the CSPS.com data, the combination of cilostazol and aspirin did not result in significant differences in the clinical outcome compared with aspirin alone in the chronic phase. This lack of significance may reflect the fact that the sample size was small and the analysis was underpowered, or that the incidence rate of vascular events was lower in the aspirin group.
In the analysis described here, patients in the clopidogrel group had a higher prevalence of risk factors than those in the aspirin group. Based on the results of a randomized, blinded trial of CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events), which showed that clopidogrel was safe and more effective than aspirin in atherothrombotic stroke patients,10 treating physicians currently tend to select clopidogrel for their higher-risk patients, such as those with large-artery stenosis and a previous history of stroke before the trial. Thus, in the present study, a higher rate of primary events occurred in the clopidogrel group compared with the aspirin group. Notably, in these higher-risk patients (especially those with an atherothrombotic infarction), adding cilostazol to a clopidogrel regimen significantly reduced the rate of recurrent ischemic stroke compared with clopidogrel alone. Another reason explaining the usefulness of the combination of cilostazol and clopidogrel may be the higher prevalence of poor metabolizers with a polymorphism of CYP2C19 in Asian patients, including Japanese patients.13 Poor metabolizers might not achieve full clinical antiplatelet inhibition with clopidogrel alone. A meta-analysis demonstrated that, in patients with acute ischemic stroke or transient ischemic attack treated with clopidogrel, carriers of CYP2C19 loss-of-function alleles are at greater risk of both stroke and composite vascular events with similar bleeding rates compared with noncarriers.14 However, another meta-analysis that included patients with mostly coronary artery disease demonstrated that there was no significant association between the patient genotype and cardiovascular events.15 Compared with the trials conducted with patients in the acute phase, the clinical trials in Japanese stroke patients showed that the poor metabolizers were not at greater risk of cerebrovascular events.16 The addition of cilostazol is expected to further promote the antiplatelet effect of clopidogrel. The combination of aspirin and cilostazol, which have different mechanisms of action, may result in an additive increase in antiplatelet activity. On the other hand, the antiplatelet effect pathway of clopidogrel includes the elevation of intracellular cyclic adenosine monophosphate,17,18 which indicates that cilostazol is able to enhance clopidogrel’s effect synergistically and to enhance other pleotropic effects such as those related to vasodilatory properties.19 Other reported predictors of clopidogrel resistance are the concomitant use of other drugs or the presence of vascular risk factors, in particular smoking and diabetes.20 However, no other significant characteristic clinical features were identified in the subanalysis presented here.
Limitations
Some limitations of the present analysis need to be acknowledged. First, this result was a subanalysis of the CSPS.com data. The CSPS.com trial was designed to assess the effectiveness and safety of adding cilostazol to an aspirin or clopidogrel regimen, and the trial could not include the case numbers expected in the protocol. The power was not sufficient to permit a direct comparison of the 2 dual-therapy groups, that is, the utility of separating the dual-therapy patients into distinct clopidogrel and aspirin groups. Thus, some outcomes could not achieve statistical significance, especially in the aspirin group. A larger clinical trial might be able to assess the precise clinical effectiveness of the combination of cilostazol and aspirin.
Second, the patients in the present analyses were all of Japanese heritage. Most of the large clinical trials of cilostazol have been conducted with East Asian patients. It is not yet clear whether the results of these trials (including the CSPS.com trial) can be generalized to other populations.
Conclusions
In a subanalysis of the CSPS.com data, the present study demonstrated that, compared with clopidogrel alone, the combination of cilostazol and clopidogrel reduced the recurrence of ischemic stroke in patients at the chronic stage without increasing the bleeding risk. To the best of our knowledge, the combination of cilostazol and clopidogrel has not been well studied. Since the present findings were obtained in a study population consisting solely of Japanese patients, further clinical trials will be needed to investigate the efficacy and safety of combined treatment with cilostazol and clopidogrel in other high-risk, noncardioembolic ischemic stroke populations.
Sources of Funding
CSPS.com (PI: Dr Yamaguchi) was conducted under a trial contract between the consignee, Japan Cardiovascular Research Foundation, and the consignor, Otsuka Pharmaceutical Co, Ltd. Japan Cardiovascular Research Foundation received funding for trial implementation and management from Otsuka Pharmaceutical Co, Ltd. Otsuka Pharmaceutical Co, Ltd, did not directly contribute to trial design, data management, or statistical analysis and did not contribute to any issues on this subanalysis study. Statistical analysis of this article was performed in the Department of Data Science, National Cerebral and Cardiovascular Center (Omae, Ishida). This study was funded mainly by the Japan Agency for Medical Research and Development to Toyoda K (AMED, 20lk0201094h0002, and 20lk0201109h0001).
Disclosures
Dr Hoshino reports personal fees from Daiichi-Sankyo and Pfizer outside the submitted work. Dr Toyoda reports personal fees from Daiichi-Sankyo, Bayer, Bristol-Myers-Squibb, Boehringer-Ingelheim, and Takeda, outside the submitted work. Dr Uchiyama reports receiving grant support, honoraria, advisory board fees, and lecture fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Takeda, Healios, Otsuka, and Sanofi. Dr Kimura received lecture fees from Bristol-Myers Squibb, Boehringer Ingelheim, Bayer, and Daiichi Sankyo and research funding from Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Medtronic, and Teijin Pharma. Dr Sakai reports receiving grant support and lecture fees from Asahi-Intec, Daiichi Sankyo, Medtronic, NeuroVasc, Stryker, and Terumo. Dr Houkin reports receiving honoraria as principle investigator of a clinical trial from Healios. Dr Yamaguchi reports receiving lecture fee from Daiichi Sankyo. Dr Minematsu reports receiving honoraria from Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi Sankyo, EPS Corporation, Fuji Film Pharma, Healios, Mitsubishi Tanabe, Nippon Chemiphar, Otsuka, Pfizer, Sanofi, and Stryker. Dr Terayama reports receiving grant support, consulting fees, and honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo. Dr Yasuda reports receiving grant support, lecture fees, and clinical trial fees from Abbott, Bristol-Myers Squibb, Daiichi Sankyo, and Takeda. Dr Yamaguchi reported personal fees from Daiichi-Sankyo and Pfizer outside the submitted work. The other authors report no conflicts.
Supplemental Materials
Online Figure
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CAPRIE
- Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events
- CATHARSIS
- Cilostazol-Aspirin Therapy Against Recurrent Stroke With Intracranial Artery Stenosis
- CSPS2
- Cilostazol Stroke Prevention Study 2
- CSPS.com
- Cilostazol Stroke Prevention Study combination
- DAPT
- dual antiplatelet therapy
- HR
- hazard ratio
- TOSS
- Trial of Cilostazol in Symptomatic Intracranial Arterial Stenosis
This manuscript was sent to Harold P. Adams, Jr, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.034378.
For Sources of Funding and Disclosures, see page 3439.
Contributor Information
Kazunori Toyoda, Email: toyoda@ncvc.go.jp.
Katsuhiro Omae, Email: k.omae@ncvc.go.jp.
Noriyuki Ishida, Email: Ishida.noriyuki.r89@kyoto-u.jp.
Shinichiro Uchiyama, Email: suchiyama@iuhw.ac.jp.
Kazumi Kimura, Email: k-kimura@nms.ac.jp.
Nobuyuki Sakai, Email: n.sakai@siren.ocn.ne.jp.
Yasushi Okada, Email: okada.yasushi.yh@mail.hosp.go.jp.
Kortaro Tanaka, Email: kortaro@sonata.plala.or.jp.
Hideki Origasa, Email: horigasa@med.u-toyama.ac.jp.
Hiroaki Naritomi, Email: naritomi@kyowakai.com.
Kiyohiro Houkin, Email: houkin@med.hokudai.ac.jp.
Keiji Yamaguchi, Email: takeyama41119@ybb.ne.jp.
Masanori Isobe, Email: m-isobe@rio.odn.ne.jp.
Kazuo Minematsu, Email: kminemat@ncvc.go.jp.
Masayasu Matsumoto, Email: matsumoto-m@sakai-hospital.jp.
Teiji Tominaga, Email: tomi@nsg.med.tohoku.ac.jp.
Hidekazu Tomimoto, Email: tomimoto@clin.medic.mie-u.ac.jp.
Yasuo Terayama, Email: teray032154@gmail.com.
Satoshi Yasuda, Email: syasuda@cardio.med.tohoku.ac.jp.
Takenori Yamaguchi, Email: takeyama41119@ybb.ne.jp.
References
- 1.Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Risk-benefit profile of long-term dual- versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann Intern Med. 2013;159:463–470. doi: 10.7326/0003-4819-159-7-201310010-00006 [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 3.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al. ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 5.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 6.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, et al. ; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- 7.Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, Sakai N, Okada Y, Tanaka K, Origasa H, et al. ; CSPS.com Trial Investigators. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2019;18:539–548. doi: 10.1016/S1474-4422(19)30148-6 [DOI] [PubMed] [Google Scholar]
- 8.Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7 [DOI] [PubMed] [Google Scholar]
- 9.Uchiyama S, Sakai N, Toi S, Ezura M, Okada Y, Takagi M, Nagai Y, Matsubara Y, Minematsu K, Suzuki N, et al. ; CATHARSIS Study Group. Final results of Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis (CATHARSIS). Cerebrovasc Dis Extra. 2015;5:1–13. doi: 10.1159/000369610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CAPRIE steering committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 11.Toyoda K, Uchiyama S, Hoshino H, Kimura K, Origasa H, Naritomi H, Minematsu K, Yamaguchi T; CSPS.com Study Investigators. Protocol for Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com): a randomized, open-label, parallel-group trial. Int J Stroke. 2015;10:253–258. doi: 10.1111/ijs.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki J, Iguchi Y, Urabe T, Yamagami H, Todo K, Fujimoto S, Idomari K, Kaneko N, Iwanaga T, Terasaki T, et al. ; ADS Investigators. Acute aspirin plus cilostazol dual therapy for noncardioembolic Stroke Patients Within 48 Hours of Symptom Onset. J Am Heart Assoc. 2019;8:e012652. doi: 10.1161/JAHA.119.012652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, Noh GJ, Njau R, Close S, Wise S, et al. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929–940. doi: 10.1177/0091270009355161 [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Chen W, Xu Y, Yi X, Han Y, Yang Q, Li X, Huang L, Johnston SC, Zhao X, et al. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. 2017;135:21–33. doi: 10.1161/CIRCULATIONAHA.116.024913 [DOI] [PubMed] [Google Scholar]
- 15.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Yamagami H, Ihara M, Miyata T, Miyata S, Hamasaki T, Amano S, Fukuma K, Yamamoto H, Nakagawara J, et al. Association of CYP2C19 polymorphisms with clopidogrel reactivity and clinical outcomes in chronic ischemic stroke. Circ J. 2019;83:1385–1393. doi: 10.1253/circj.CJ-18-1386 [DOI] [PubMed] [Google Scholar]
- 17.Geiger J, Brich J, Hönig-Liedl P, Eigenthaler M, Schanzenbächer P, Herbert JM, Walter U. Specific impairment of human platelet P2Y(AC) ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arterioscler Thromb Vasc Biol. 1999;19:2007–2011. doi: 10.1161/01.atv.19.8.2007 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Colman RW. Thrombin regulates intracellular cyclic AMP concentration in human platelets through phosphorylation/activation of phosphodiesterase 3A. Blood. 2007;110:1475–1482. doi: 10.1182/blood-2006-10-052522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi T, Hara H. Protective effects of cilostazol against hemorrhagic stroke: current and future perspectives. J Pharmacol Sci. 2016;131:155–161. doi: 10.1016/j.jphs.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 20.Wiśniewski A, Filipska K. The phenomenon of clopidogrel high on-treatment platelet reactivity in ischemic stroke subjects: a comprehensive review. Int J Mol Sci. 2020;21:E6408. doi: 10.3390/ijms21176408 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified individual participant data and the study protocol of the CSPS.com may be available upon request to Japan Cardiovascular Research Foundation. Researchers can request data disclosure until March 2022.