Supplemental Digital Content is available in the text.
Keywords: biomarker, COVID-19, inflammation, ischemic stroke, mortality
Abstract
Background and Purpose:
We sought to determine if biomarkers of inflammation and coagulation can help define coronavirus disease 2019 (COVID-19)–associated ischemic stroke as a novel acute ischemic stroke (AIS) subtype.
Methods:
We performed a machine learning cluster analysis of common biomarkers in patients admitted with severe acute respiratory syndrome coronavirus 2 to determine if any were associated with AIS. Findings were validated using aggregate data from 3 large healthcare systems.
Results:
Clustering grouped 2908 unique patient encounters into 4 unique biomarker phenotypes based on levels of c-reactive protein, D-dimer, lactate dehydrogenase, white blood cell count, and partial thromboplastin time. The most severe cluster phenotype had the highest prevalence of AIS (3.6%, P<0.001), in-hospital AIS (53%, P<0.002), severe AIS (31%, P=0.004), and cryptogenic AIS (73%, P<0.001). D-dimer was the only biomarker independently associated with prevalent AIS with quartile 4 having an 8-fold higher risk of AIS compared to quartile 1 (P=0.005), a finding that was further corroborated in a separate cohort of 157 patients hospitalized with COVID-19 and AIS.
Conclusions:
COVID-19–associated ischemic stroke may be related to COVID-19 illness severity and associated coagulopathy as defined by increasing D-dimer burden.
Acute ischemic stroke (AIS) is a major contributor of morbidity and mortality in patients with coronavirus disease 2019 (COVID-19).1,2 In one retrospective series, AIS was reported in ≈1% and associated with mortality in 64% of hospitalized patients.3 Although elevated serum D-dimer has emerged as a marker of COVID-19-associated coagulopathy, it remains to be determined if markers of coagulation or inflammation can be used to define COVID-19–associated ischemic stroke.4,5
We sought to determine if clusters of biomarkers were associated with incident AIS in patients hospitalized with COVID-19 using unsupervised machine learning cluster analysis and hypothesized that biomarkers reflecting increased disease severity and coagulopathy would be associated with AIS. We further sought to validate findings related to AIS in an aggregate cohort of patients with COVID-19 and AIS admitted to 3 healthcare systems in New York City.
Methods
Study Population and Outcomes
The data that support the findings of this study are available from the corresponding author upon reasonable request. Cluster analysis was performed in a retrospective cohort of 5652 patients with COVID-19 admitted to the Montefiore Health System, between March 1, 2020 and May 8, 2020. Results related to AIS were additionally validated using aggregate categorical data from Montefiore Health System, Columbia University Medical Center, and New York University Langone Health comprising a total of 7 hospitals. COVID-19 was defined as a positive severe acute respiratory syndrome coronavirus 2 viral real-time reverse transcription polymerase chain reaction. D-dimer in 2 centers was assayed using fibrinogen equivalent units with a reference range <0.5 µg/mL and an upper measurement limit of 20 µg/mL and as D-dimer units with a reference range <243 ng/mL and a measurement limit of 10 000 ng/mL in the third. Cluster outcomes including AIS and composite thrombotic events (myocardial infarction, pulmonary embolism, deep vein thrombosis, and acute limb thrombosis) were screened using the International Classification of Diseases codes and confirmed by manual chart review. Myocardial infarction was defined clinically and included type 1 and type 2. All of the other composite thrombotic events required confirmatory diagnostic imaging. AIS was adjudicated using the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification scheme.6 Of the cryptogenic subgroup in the validation cohort, 72% (n=51) underwent complete evaluation as defined by relevant vessel imaging, cardiac echocardiogram, and cardiac rhythm analysis.
Statistical Analysis
Common readily available serological biomarkers reported in association with illness severity including CRP (C-reactive protein), D-dimer, LDH (lactate dehydrogenase), white blood cell count, partial thromboplastin time, sedimentation rate, interleukin-6, fibrinogen, and ferritin were included in the cluster analysis.7 Encounters with missing cluster biomarkers and biomarkers with >30% missing values were excluded. Only the first available biomarker value was included and encounters with hospitalizations greater than 30 days were excluded. Agglomerative hierarchical clustering was used to detect patterns in the Montefiore Health System cohort based on the distribution of biomarker quartiles in an unsupervised fashion using R 4.0.3 (R core team, 2020). Patients were iteratively grouped based on similarities in biomarker levels with a stopping point of 4, and outcomes were stratified by cluster (Figure I and Table I in the Data Supplement). Relative risk of all outcomes conferred by individual biomarkers was calculated using inverse-probability weighted logistic regression adjusting for baseline demographics, comorbidities, and clinical variables (Table IV in the Data Supplement) Machine learning model details and a checklist of the minimum information about clinical artificial intelligence modeling guidelines are available in the Data Supplement (Table VI in the Data Supplement).8 This study was approved by the Institutional Review Boards of each institution. Written informed consent was not required and was, therefore, waived for this study per center-specific guidelines and recommendations.
Results
Cluster Analysis Results
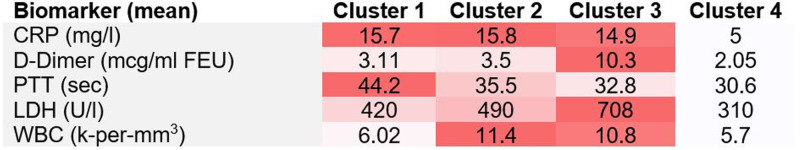
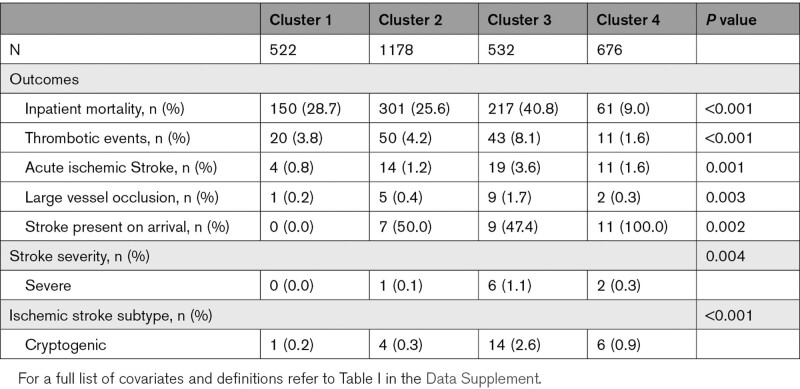
Clustering identified CRP, D-dimer, LDH, white blood cell, and partial thromboplastin time as fitting inclusion criteria and grouped 2908 patients into 4 clusters. Cluster 3 (n=532) had the highest mean D-dimer (10.31 µg/mL fibrinogen equivalent units, P<0.001) and LDH (707.97 U/L, P<0.001), relatively high CRP (14.91 mg/L, P<0.001) and white blood cell count (10.83 k-per-mm3, P<0.001), and low partial thromboplastin time (32.79 s, P<0.001), whereas cluster 4 (n=676) had the lowest mean values for all 5 biomarkers (Figure 1). Cluster 3 had the highest prevalence of AIS (3.6%, P<0.001), in-hospital AIS (53%, P<0.002), large vessel occlusion (53%, P<0.003), severe AIS (31%, P=0.004), and cryptogenic AIS (73%, P<0.001). Cluster 3 also had the highest prevalence of composite thrombotic events (8.1%, P<0.001) and COVID-19–associated disease severity as judged by mortality (41%, P<0.001). Cluster 4 on the other hand had no cases of in-hospital AIS (P<0.001), the lowest prevalence of composite thrombotic events (1.6%, P<0.001) and was the least severe as judged by the lowest associated mortality (9%, P<0.001; Table).
Figure 1.
Biomarker composition of each cluster. CRP indicates C-reactive protein; LDH, lactate dehydrogenase; PTT, partial thromboplastin time; and WBC, white blood cells.
Table.
Outcomes Stratified by Cluster
Individual Biomarkers and Validation
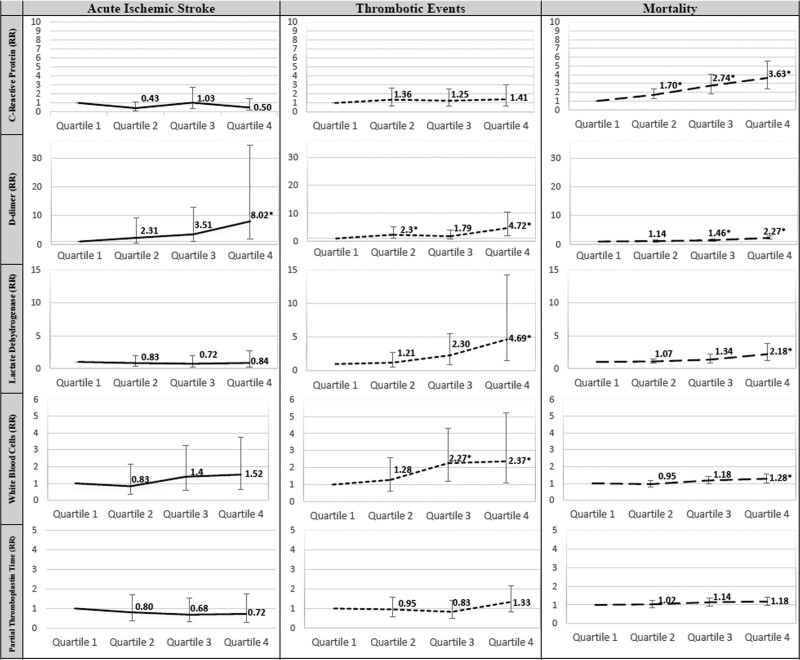
When testing the effect of isolated biomarkers in the fully adjusted model, only D-dimer was associated with prevalent AIS with quartile 4 having an 8-fold higher relative risk of AIS compared to quartile 1 (relative risk, 8.02 [95% CI, 1.86–34.61], P=0.005; Figure 2). In a separate multicenter validation cohort (n=158), patients with cryptogenic stroke (n=71) were significantly more likely to have elevated D-dimer defined as >10mcg/mL fibrinogen equivalent units (or >10 000 ng/mL D-dimer units) compared with the cardioembolic (n=61) and atherosclerotic/lacunar stroke subtypes (n=26; 50.8% versus 32.7% versus 4.5%, P=0.0064; Table III in the Data Supplement)
Figure 2.
Adjusted risk of stroke, composite thrombotic events, and mortality by each biomarker. Error bars signify 95% CI. RR (relative risk; y axis). Adjusted for all variables in Table I in the Data Supplement. Quartile 1 is reference. *P<0.05.
Discussion
We used unsupervised clustering to study biomarker profiles of patients with COVID-19 and AIS. Similarly, another study used cluster analysis to identify patterns of increased stroke-related mortality in patients with COVID-19.9 We chose this method because stroke subtyping schemes like TOAST by nature cannot classify novel stroke mechanisms beyond the cryptogenic category. The model identified 4 unique clusters and the cluster with the highest COVID-19–associated illness severity, as defined by mortality, had the largest AIS prevalence, whereas the least severe cluster had no episodes of in-hospital AIS. Additionally, the most severe cluster was least likely to have a discovered stroke cause (cryptogenic) other than perhaps COVID-19. When testing the effect of lone biomarkers, only D-dimer was associated with AIS and was further elevated in patients with cryptogenic AIS compared to those with other stroke subtypes in a separate multicenter cohort.
Although exploratory and requiring further study, we conclude that excess prevalence of AIS in patients with COVID-19 is associated with higher COVID-19 disease severity and associated coagulopathy. We, therefore, suggest that COVID-19–associated ischemic stroke may be defined as AIS with an otherwise cryptogenic cause after full diagnostic evaluation in the setting of (1) severe systemic COVID-19 illness or (2) COVID-19–associated coagulopathy as defined by a high D-dimer burden. We acknowledge that there may be significant overlap between the two criteria and that both are subjective—currently lacking consensus or standardized definitions.
A major limitation to this study is a likely underestimate of AIS and thrombotic events as we only include imaging-confirmed cases. Nonetheless, outcomes reported in this study are similar to those in other similar cohorts.10 Furthermore, because our inclusion of biomarkers was limited by their availability in the medical record and to the first available level, we could not study the effect of some biomarkers on AIS (sedimentation rate, interleukin-6, fibrinogen, ferritin) and acknowledge that biomarker associations related to in-hospital AIS are likely less accurate than those related to AIS present on arrival. Finally, although D-dimer was independently associated with AIS, we acknowledge that the wide confidence interval conferred by the low number of events requires confirmatory study.
Despite these limitations, the findings presented herein support other reports that link COVID-19-associated coagulopathy and cryptogenic stroke and suggest that COVID-19–associated ischemic stroke may be mediated by COVID-19 illness severity and associated coagulopathy.3–5
Acknowledgments
We dedicate this work to the memory of our colleagues who have lost their lives to coronavirus disease 2019 (COVID-19).
Sources of Funding
None.
Disclosures
Dr Altschul reports grants and personal fees from Microvention, grants from Medtronic, and personal fees from Stryker outside the submitted work. Dr Frontera reports grants from National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) and grants from NIH/National Institute on Aging (NIA) outside the submitted work. Dr Liberman reports grants from NIH/NINDS outside the submitted work. The other authors report no conflicts.
Supplemental Materials
Online Figure I
Online Tables I–VI
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- LDH
- lactate dehydrogenase
- TOAST
- Trial of ORG 10172 in Acute Stroke Treatment
This manuscript was sent to Jean-Claude Baron, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.035045.
For Sources of Funding and Disclosures, see page e709.
The podcast and transcript are available at https://www.ahajournals.org/str/podcast.
Contributor Information
Natalie T. Cheng, Email: ncheng@montefiore.org.
Jorge Luna, Email: jl2708@columbia.edu.
Joshua Willey, Email: joshua.benton@einsteinmed.org.
Amelia K. Boehme, Email: akb2188@cumc.columbia.edu.
Kathryn Kirchoff-Torres, Email: kkirchof@montefiore.org.
Daniel Labovitz, Email: dlabovit@montefiore.org.
Ava L. Liberman, Email: avaliberman@gmail.com.
Peter Mabie, Email: Peter.mabie@einsteinmed.org.
Khadean Moncrieffe, Email: kmoncrie@montefiore.org.
Ainie Soetanto, Email: asoetant@montefiore.org.
Andrea Lendaris, Email: alendari@montefiore.org.
Johanna Seiden, Email: jseiden@montefiore.org.
Inessa Goldman, Email: INGOLDMA@montefiore.org.
David Altschul, Email: david.flomenbaum@einsteinmed.org.
Ryan Holland, Email: ryhollan@montefiore.org.
Joshua Benton, Email: joshua.benton@einsteinmed.org.
Joseph Dardick, Email: joseph.dardick@einsteinmed.org.
Jenelys Fernandez-Torres, Email: fernandez.jenelys@gmail.com.
David Flomenbaum, Email: david.flomenbaum@einsteinmed.org.
Jenny Lu, Email: jenny.lu@einsteinmed.org.
Avinash Malaviya, Email: avinash.malaviya@einsteinmed.org.
Nikunj Patel, Email: nikunjpatel332@gmail.com.
Aureliana Toma, Email: toma.aureliana@gmail.com.
Aaron Lord, Email: Aaron.Lord@nyulangone.org.
Koto Ishida, Email: koto.ishida@nyulangone.org.
Jose Torres, Email: Jose.Torres2@nyulangone.org.
Thomas Snyder, Email: Thomas.Snyder@nyulangone.org.
Jennifer Frontera, Email: jenfrontera@hotmail.com.
Shadi Yaghi, Email: shadiyaghi@yahoo.com.
References
- 1.Altschul DJ, Esenwa C, Haranhalli N, Unda SR, de La Garza Ramos R, Dardick J, Fernandez-Torres J, Toma A, Labovitz D, Cheng N, et al. Predictors of mortality for patients with COVID-19 and large vessel occlusion. Interv Neuroradiol. 2020;26:623–628. doi: 10.1177/1591019920954603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskandar EN, Altschul DJ, de la Garza Ramos R, Cezayirli P, Unda SR, Benton J, Dardick J, Toma A, Patel N, Malaviya A, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96:e1527–e1538. doi: 10.1212/WNL.0000000000011356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, et al. Prevalence and outcomes of D-Dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 7.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 8.Norgeot B, Quer G, Beaulieu-Jones BK, Torkamani A, Dias R, Gianfrancesco M, Arnaout R, Kohane IS, Saria S, Topol E, et al. Minimum information about clinical artificial intelligence modeling: the MI-CLAIM checklist. Nat Med. 2020;26:1320–1324. doi: 10.1038/s41591-020-1041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridman S, Bres Bullrich M, Jimenez-Ruiz A, Costantini P, Shah P, Just C, Vela-Duarte D, Linfante I, Sharifi-Razavi A, Karimi N, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. doi: 10.1212/WNL.0000000000010851 [DOI] [PubMed] [Google Scholar]
- 10.Elbadawi A, Elgendy IY, Sahai A, Bhandari R, McCarthy M, Gomes M, Bishop GJ, Bartholomew JR, Kapadia S, Cameron SJ. Incidence and outcomes of thrombotic events in symptomatic patients with COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:545–547. doi: 10.1161/ATVBAHA.120.315304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.