Abstract
Background
The use of cannabis for symptoms of endometriosis was investigated utilising retrospective archival data from Strainprint Technologies Ltd., a Canadian data technology company with a mobile phone application that tracks a range of data including dose, mode of administration, chemovar and their effects on various self-reported outcomes, including pelvic pain.
Methods
A retrospective, electronic record-based cohort study of StrainprintTM users with self-reported endometriosis was conducted. Self-rated cannabis efficacy, defined as a function of initial and final symptom ratings, was investigated across the included symptom clusters of cramps, pelvic pain, gastrointestinal pain, nausea, depression, and low libido. Cannabis dosage form, dose and cannabinoid ratio information was also recorded.
Results
A total number of 252 participants identifying as suffering endometriosis recorded 16193 sessions using cannabis between April 2017 and February 2020. The most common method of ingestion was inhalation (n = 10914, 67.4%), with pain as the most common reported symptom being treated by cannabis (n = 9281, 57.3%). Gastrointestinal symptoms, though a less common reason for cannabis usage (15.2%), had the greatest self-reported improvement after use. Inhaled forms had higher efficacy for pain, while oral forms were superior for mood and gastrointestinal symptoms. Dosage varied across ingestion methods, with a median dose of 9 inhalations (IQR 5 to 11) for inhaled dosage forms and 1 mg/mL (IQR 0.5 to 2) for other ingested dosage forms. The ratio of THC to CBD had a statistically significant, yet clinically small, differential effect on efficacy, depending on method of ingestion.
Conclusions
Cannabis appears to be effective for pelvic pain, gastrointestinal issues and mood, with effectiveness differing based on method of ingestion. The greater propensity for use of an inhaled dosage delivery may be due to the rapid onset of pain-relieving effects versus the slower onset of oral products. Oral forms appeared to be superior compared to inhaled forms in the less commonly reported mood or gastrointestinal categories. Clinical trials investigating the tolerability and effectiveness of cannabis for endometriosis pain and associated symptoms are urgently required.
Introduction
Endometriosis is a common, chronic inflammatory condition in women characterised by the presence of endometrial-like tissue found outside the uterus [1]. Prevalence rates of the disease have been estimated at between 5% [2] and 11% [3] of reproductive-aged women, impacting an estimated 176 million women worldwide. [4]. In addition, endometriosis can also affect post-menopausal women, ranging from between 2–5% of cases [5]. Endometriosis is associated with a variety of symptoms including chronic pelvic pain, fatigue, dysmenorrhoea (period pain), dyspareunia (painful sex), dyschezia (painful bowel movements) and dysuria (pain related to urination) [6,7]. In addition, co-morbid anxiety and/or depression in the endometriosis cohort is frequently reported [8,9], along with irritable bowel syndrome (IBS)-like gastrointestinal symptoms [10,11] and significant fatigue [6]. These symptoms contribute to substantial reductions in many aspects of quality-of-life including social, academic, work and sexual relationships [12,13]. In addition, endometriosis causes a noteworthy cost of illness burden to the economy, mostly due to lost productivity [14–16].
Recent studies have suggested that a dysfunction in the endocannabinoid system (ECS) is present in endometriosis patients [17,18], and that aspects of endometriosis-associated pain may be targeted by modulating the ECS [19].
Previous research on the use of illicit cannabis in women with endometriosis has shown promise in the treatment of endometriosis pain and co-morbid symptoms such as poor sleep, gastrointestinal upset and mood disorders [20–22]. However, these findings were all based on self-reported data and could not be reliably used to determine the relative effectiveness of different modes of cannabis administration, dose amount, cannabinoid ratios, or other determining factors (such as patient age), all of which may influence clinical outcomes.
This study sought to investigate the self-rated effectiveness, dosage forms, dosage amount and cannabinoid ratios of quality-assured legal cannabis products that women are using in the Canadian regulated market, via their use of the StrainprintTM smartphone application (“app”), which is used to track legal cannabis product usage for medicinal purposes.
Materials & methods
Procedure
Strainprint Technologies Ltd. is a Canadian-based data and analytics company that provides a free mobile phone app that allows individuals to prospectively track medicinal cannabis usage, including the varieties of cannabis, dosage form, dosage and changes in symptom severity of users over time. During the initial session, users enter basic demographic information such as age and gender, and then identify the medical conditions or symptoms they are experiencing as part of the onboarding process. Prior to using cannabis, users open the app and identify the symptom for which they will be using cannabis, which is then recorded as a “session”. A session was defined as the process by which participants select the symptom(s) they are currently experiencing (allowing categorization), self-rate the severity of the symptom(s) using an 11-point numerical rating scale (0 being lowest severity to 10 being highest) prior to cannabis use, select the product and dosage form they will use to address the aforementioned symptom(s), and record the dose taken. Product choices are pre-populated from a list of over 6500 laboratory-verified products in the StrainprintTM database offered by licenced medicinal Cannabis cultivators and distributors, including the records of the delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations and ratios, in addition to dominant terpene expressions within the database. Whilst the baseline severity of each symptom is obtained prior to taking the dose, the app prompts users after a set time period (20 minutes for inhaled forms and 90 minutes for oral forms) to revaluate the specific symptom severity scores.
Archival data in Excel format was provided by Strainprint Technologies Ltd. for the period April 2017 until February 2020. During this period, only pre-populated and lab-verified product selection was available, with no ability for manual entry by users. Demographic data and the breakdown of clinical indications and dosage forms from the data set are noted in Table 1. To facilitate multilevel analysis, symptoms were classified into clinically meaningful subgroups a priori, arranged as either “Pain” (including pelvic pain and cramps), “Gastrointestinal” (including gastrointestinal pain and nausea) and “Mood” (incorporating depression and low libido). Further characterisation was applied to the dosage form utilised by the users and included “Oral” (including edibles [mg], oils [mL], capsules [mg], tinctures [mL] or sprays [mL], “Topical” (including transdermal and topical) and “Inhaled” forms (“puffs” for smoked and vapourised).
Table 1. Demographic of StrainprintTM users with endometriosis.
| Number of StrainprintTM users | 252 |
| Total number of recorded cannabis sessions | 16187 |
| Mean (SD) | |
| Average age at first use of StrainprintTM app | 33.10 (7.8) |
| Average months using StrainprintTM app per user | 5.5 (7.4) |
| Average number of recorded cannabis sessions per user | 64.2 (170.8) |
| Average number of clinical indications per user | 2.6 (1.5) |
| Average number of dosage forms per user | 2.0 (1.4) |
| Dosage form breakdown (n = 16187 sessions) | N (%) |
| Inhaled products | |
| Vaporised | 6575 (40.6) |
| Smoked | 4191 (25.9) |
| Concentrate | 132 (0.84) |
| Dab bubbler/rig | 16 (0.10) |
| Orally ingested products | |
| Oil | 4041 (25.0) |
| Pill | 558 (3.5) |
| Edible | 389 (2.4) |
| Spray | 90 (0.56) |
| Tincture | 90 (0.56) |
| Oral | 52 (0.32) |
| Topical products | |
| Topical | 33 (0.20) |
| Transdermal | 20 (0.12) |
| Clinical indications breakdown (n = 16192 sessions) | N (%) |
| Pelvic Pain | 6864 (42.4) |
| Gastrointestinal Distress | 2461 (15.2) |
| Cramps | 2417 (14.9) |
| Nausea | 2254 (13.9) |
| Depression | 2138 (13.2) |
| Low Libido | 53 (0.33) |
Due to the nature of the research project utilising fully anonymised retrospective archival datasets, this project was exempt from ethical review by the Western Sydney University Human Ethics Committee as per the National Health & Medical Research Council (NHMRC) National Statement [23]. All individuals who register to use the StrainprintTM app signed a Consent to Collection and Use of Data form for research purposes
Data analysis
Self-rated cannabis efficacy, defined as a function of initial and final symptom ratings before and after cannabis use was utilised.
Where x = initial symptom rating (score out of 10) and y = final symptom rating (score out of 10).
Statistical analysis was carried out using multilevel linear regression to examine the effect of various independent factors on efficacy rating. Participants and sessions were classed as random effects to take into account dependence between observations in the same session from the same user, and all other factors were classed as fixed effects. Additionally, the ratio of THC to CBD was hypothesised to be an important determinant of efficacy, and due to the fact that some participants’ recorded cannabinoids with zero amounts of THC or CBD, trace levels (0.0001) of THC or CBD were assigned to these data points for the purpose of modelling, so that ratios could be calculated and missing data minimised.
Results
A total number of 252 participants were identified as having endometriosis, with a total of 16,193 sessions. Six sessions were excluded from this dataset due to ingestion method (suppository, n = 1), outlying THC (>500 mg/mL, n = 3), outlying CBD (>500 mg/mL, n = 1), and negative dosage (n = 1), yielding a total of 16,187 sessions included in the final analysis. Prevalence of cannabis use was rated highest for the pain subgroup (n = 9281/57.3%), followed by gastrointestinal (n = 4715/29.1%) and mood (n = 2191/13.5%).
The mean age at the beginning of each session of women identifying as having endometriosis within the StrainprintTM dataset was 35 years, with the majority using the app for approximately 5.5 months (See Table 1). Pulmonary administration was the favoured dosage form, with inhaled forms accounting for 67.4% of all dosage forms utilised by the cohort: Vapouriser (40.6%), Smoked (25.9%), dab bubbler or concentrate (0.9%). Orally ingested dosage forms were the next most utilised, accounting for 32.3% of the cohort: Oils (25.0%), Pills (3.5%), Edible products (2.4%), Tincture as spray or oral drops (1.4%). Topicals (0.2%) and transdermals (0.1%) had minimal reported usage in this dataset.
Pelvic Pain (42.4% of all participants) was the primary clinical indication for use of cannabis, with Gastrointestinal Distress (15.2%) and Cramps (14.9%) following in frequency. Nausea (13.9%) and Depression (13.2%) were also notable findings, with Low Libido (0.3%) being the least reported symptom.
Dosage varied according to ingestion method, but not for the symptom being treated, with the highest median dose being taken by those using inhalation (9 mg/mL or puff, IQR: 5, 11, n = 10,914). Lowest median dose was taken by those treating pain and mood via oral dosage forms, with a median dose of 1 mg/mL, capsule or piece (with an IQR of 0.5 to 2, n = 5220). The median dose for topical application was 2 mg/mL (IQR: 1.5 to 20, n = 53).
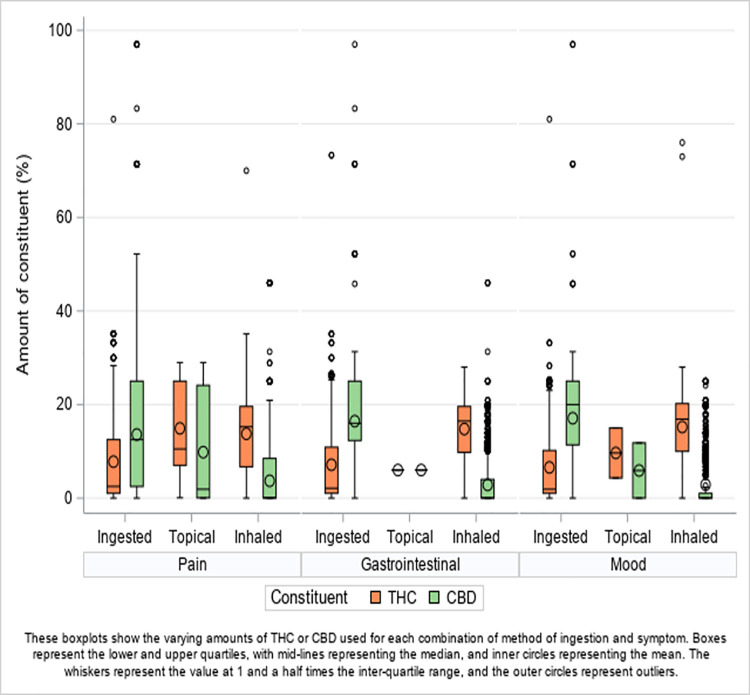
THC and CBD levels (and THC/CBD ratios) varied widely, depending on the ingestion method (Fig 1). Inhaled methods typically had a high THC to CBD ratio, with a median % or mg/mL THC of 16 (IQR 8 to 19.6), a median % or mg/mL CBD of 0.07 (IQR 0 to 8.01) and a median THC/CBD ratio of 90 (IQR 0.6 to 304). Ingested dosage forms exhibited the opposite tendency, having a high CBD to THC ratio, with a median for THC of 2.5 (IQR 1.0 to 12), a median for CBD of 13.5 (IQR 5 to 25) and a median THC/CBD ratio of 0.08 (IQR 0.04 to 1). The median amounts of THC and CBD for topical dosage forms was equal (median ratio of 1, IQR 0.4 to 13.2). The THC/CBD ratio remained similar as median ratios across the symptoms: Pain 0.95 (IQR 0.1 to 197) vs. Gastrointestinal Distress 0.88 (IQR 0.08 to 242) vs. Mood 0.89 (IQR 0.08 to 220), although approximately 8% less THC than CBD was used overall.
Fig 1. THC and CBD by symptom group and ingestion method.
Results of the multilevel model are reported in Table 2. Overall, cannabis was shown to have a positive effect, with a modelled mean baseline efficacy rating of 31.98 (95% CI 31.26 to 32.71, p<0.0001). The symptom with the largest effect was seen for gastrointestinal symptoms, with an estimated increase in efficacy of 9.02 (95% CI 8.15 to 9.90, p<0.0001) compared to pain. Mood symptoms had effects not significantly different from baseline of pain. Despite there being relatively few participants reporting use of topical administrations, this had the strongest effect of all the ingestion methods, with an estimated increase in efficacy of 13.64 (95% CI 6.11 to 21.17, p = 0.0004) in comparison to the baseline for inhalation. Orally ingested products had a slightly lower efficacy than the baseline observed for inhaled cannabis (-2.11 with 95% CI –3.23 to –1.00, p = 0.0002). Ingestion methods varied according to symptom. Examination of the interaction between symptom group and ingestion method revealed an increase in efficacy when taking oral cannabis for gastrointestinal symptoms, with an estimate of 7.82 (95% CI 6.26 to 9.37, p<0.0001) adding to the effect of oral and gastrointestinal seen alone. There was also an increase in efficacy for those treating mood with orally ingested forms (5.16 with 95% CI 2.96 to 7.36, p<0.0001). Effects of topical administration on gastrointestinal and mood symptoms were intriguing, but should be treated with caution as these subgroups had minimal usage in this dataset.
Table 2. Final model results.
| Factor | Factor level | Estimate (95% CI) | P-value |
|---|---|---|---|
| Intercept | 31.98 (31.26, 32.71) | <0.0001 | |
| Centred age (age at session–mean age) | 0.26 (0.20, 0.31) | <0.0001 | |
| Log10(THC/CBD) | 1.72 (1.22, 2.23) | <0.0001 | |
| Symptom (class) | Pain | Reference category | |
|
|
Gastrointestinal | 9.02 (8.15, 9.90) | <0.0001 |
| Mood | 0.66 (-0.58, 1.90) | 0.2952 | |
| Ingestion method (class) | Inhaled | Reference category | |
| Ingested | -2.11 (-3.23, -1.00) | 0.0002 | |
| Topical | 13.64 (6.11, 21.17) | 0.0004 | |
| Interaction: Log10(THC/CBD) and ingestion method (class) | Inhaled | ||
| Ingested | -4.75 (-5.62, -3.88) | <0.0001 | |
| Topical | -2.15 (-8.51, 4.22) | 0.5084 | |
| Interaction: Centred age (age at session–mean age) and dosage | -0.007 (-0.009, -0.004) | <0.0001 | |
| Interaction: Log10(THC/CBD) and dosage | 0.03 (0.008, 0.04) | 0.0039 | |
| Interaction: Symptom (class) and Ingestion method (class) | Gastrointestinal * Inhaled | Reference category | |
| Mood * Inhaled | Reference category | ||
| Pain * Ingested | Reference category | ||
| Pain * Topical | Reference category | ||
| Pain * Inhaled | Reference category | ||
| Gastrointestinal * Ingested | 7.82 (6.26, 9.37) | <0.0001 | |
| Gastrointestinal * Topical | -3.04 (-40.64, 34.57) | 0.8743 | |
| Mood * Ingested | 5.16 (2.96, 7.36) | <0.0001 | |
| Mood * Topical | 33.90 (1.34, 66.46) | 0.0413 | |
The effect of THC/CBD ratio gave an increase in efficacy of 1.72 (95% CI 1.22 to 2.23, p<0.0001) for every ten-fold increase in THC. Age also had a statistically significant effect, with an increase in efficacy of 0.26 (95% CI 0.20 to 0.31, p<0.0001) for each year increase in age, with older women having better overall efficacy.
As seen in Fig 1, the relationship between THC and CBD levels differed between ingestion methods, and the model showed that efficacy also differed subsequently. There was no evidence of a difference in efficacy according to the ratio of THC to CBD between topical and inhaled treatments. However, there was a difference between inhaled and orally ingested cannabis in combination with the ratio of THC to CBD, where for ingested methods, there was a reduction in efficacy of 4.75 (95% CI 5.62 to 3.88) for every 10-fold increase in THC compared to CBD (an increase in efficacy for every 10-fold increase in CBD compared to THC).
Discussion
Our findings demonstrate that women self-reporting endometriosis used cannabis to manage a variety of their symptoms, including pelvic pain and gastrointestinal tract (GIT) symptoms. Inhaled forms of cannabis were the most commonly used, followed by oral ingestion, where both were similarly efficacious in terms of alleviating symptoms. In particular, there was no difference between these two routes of administration with respect to pelvic pain. This may be due to the different time points that effectiveness scores were taken in the StrainprintTM app: 20 minutes from intake for inhaled vs. 90 minutes for oral. Therefore, the effectiveness scores are unlikely to capture this important difference in speed of onset for pain relief. Orally ingested forms appeared to be superior compared to inhaled forms for the less commonly reported mood or gastrointestinal issues.
Age-associated increases in effectiveness was also observed within the dataset, with better subject-perceived effectiveness of cannabis preparations existing for a variety of symptoms, whether using inhaled or orally ingested dosage forms, seeming to increase with greater age. Why perception of effectiveness increased with age is unknown, but could plausibly be due to age-related change to organ function causing increased sensitivity to drugs/medications [24]. changing hormone levels [25] or the possible decline in endocannabinoid system functioning associated with ageing [26,27]. It may also be due to the changes in pain perception that occur over time in people with endometriosis [28,29].
The preferred dosage form in previous research investigating illicit cannabis usage in endometriosis cohorts in Australia and New Zealand was via the inhaled route (including smoked and vapourised), with 61.9% of Australian [20] and 67.8% of New Zealand [21] respondents favouring this method of administration. Whilst it is plausible that inhaled forms are favoured within illicit markets due to a scarcity of other dosage forms, such a similarity within our legal cannabis dataset suggests other factors may be involved in the choice to use inhaled forms.
A key factor in the use of inhaled forms for endometriosis may be that there is a faster onset of pharmacological action, and therefore symptom reduction, by cannabinoids (and terpenes) administered via the inhaled route, usually within 5–10 minutes, in contrast to the 45–180 minutes for oral dosage forms [30]. The absorption and bioavailability of THC and other cannabinoids via smoking has been typically reported between 2% and 56% due to variables such as the cannabis incineration temperature, the inhalation number and duration, and inhalation volume/hold-time, etc. However, it is generally higher than the slow rate of absorption and poor bioavailability (4% to 20%) of oral dosage forms [31]. The difference in this speed of onset means that both routes of administration may play a vital role as women with endometriosis have both chronic pain and more acute pain, often described as endometriosis “flares” [32,33], which are characterised as episodes of acute and intense breakthrough pain. Such sudden-onset pain events require a fast analgesic onset for immediate symptom relief and may explain the prevalence of the inhaled route for dose delivery. This may also be relevant for patients with Deep Infiltrating Endometriosis (DIE) who suffer from pelvic floor muscle hypertonic dysfunction [34], whereby the muscle relaxant activity associated with cannabis [35] may also play an important role. Finally, another factor favouring the inhaled route is that women have previously reported that they have chosen inhaled forms due its ease of dose control, in order to titrate a balance between pain relief and impairment [36]. Lastly, women may be favouring inhaled dosage forms due to familiarity from previous recreational use [20,21], with smoked cannabis being the most common method of administration historically [37].
The effect of changing the THC:CBD ratio appeared to have only a minor impact on pelvic pain, suggesting that a precise titration of THC is important for symptom management, but that higher levels may overly impair or increase known side-effects, potentially causing issues with participants being able to perform Activities of Daily Living (ADL). This may also suggest that women who use illicit cannabis for therapeutic purposes may not always receive the ideal cannabinoid ratio for pelvic pain as an oral dosage form due to unknown cannabinoid levels and lack of standardisation. Average illicit cannabis THC potency has increased in strength over the last 50 years due to the decentralised development of underground selective-breeding programs and widespread adoption of the “sinsemilla” (i.e., without seeds) growing technique, combined with a consequent market erosion for lower potency seeded cannabis. Although connoisseur high-potency varieties were available historically, cannabis in the 1960s more commonly had a typical THC concentration of between 1–5%. This compares to chemovars today commonly ranging in content between 15–30% THC [37,38], usually at the expense of CBD content. This higher ratio of THC in illicit cannabis may have contributed to the pain reduction found in previous research [20,21] featuring an endometriosis cohort using illicit cannabis. However, the implied high absolute levels of THC may not be needed to gain optimal symptom control, as lower doses are readily implemented via inhaled dose titration. Nevertheless, future research is needed to explore this aspect of dosing in more detail.
Plausible mechanisms exist for improvements in the GIT and mental health symptoms reported. Although THC plays a role in gastrointestinal symptoms, including nausea and vomiting, CBD has documented anti-inflammatory and antioxidant activity [39], with a noted fatty acid amide hydrolase (FAAH) inhibition demonstrated to induce anti-inflammatory effects in the GIT [40,41]. CBD has documented anti-emetic and anti-nausea activity [42], and emergent evidence of CBD being of benefit in gastrointestinal conditions has been reported [43,44]. Further, a clinical endocannabinoid deficiency [45,46] has been posited for conditions such as irritable bowel syndrome, a common co-morbid diagnosis in people with endometriosis [47,48]. However, CBD is not a ligand for the CB1 and CB2 receptor, so other mechanisms must be extant. For example, CBD exhibits known anxiolytic and antidepressant activity via modulation of the serotonin (5HT1A) receptor [49,50].
Absorption of CBD and other cannabinoids may be increased with oral dosage forms by utilising an oil (e.g., olive oil, medium-chain triglycerides, etc) as the carrier/excipient, or if taken with meals that contain fats, resulting in a longer duration of effect (6–8 hours) [30]. Oral administration also provides patients with more stable plasma levels and, therefore, a more sustained therapeutic benefit. This route also provides higher concentrations of CBD compared to inhaled products, which may explain why there was a greater reported effectiveness in mood and GIT symptoms in the present data set.
Whilst topical dosage forms demonstrated a notable effect on pain compared to inhalation, the actual number of participants reporting use of this dosage form was very small, therefore caution should be applied in interpreting or extrapolating from this data. Topical applications offer a novel method of dose delivery and may be useful for localised symptoms and effects, but do have noted variability in both onset and duration of systemic effect [30]. With a dearth of evidence for this dosage form, both generally and in the endometriosis cohort particularly, further research is required to learn about cannabinoid tolerability, ratios and extent of therapeutic effect.
Limitations of this study include that the data was cross-sectional and that it captured regular users rather than de novo use longitudinally. Additionally, during the timeline of this captured dataset (April 2017—February 2020), the predominant dosage form reported by the StrainprintTM app for the Canadian regulated medicinal cannabis market was heavily weighted towards dried cannabis flos (flower). This suggests that the higher percentage of cannabis-inhaling users presented in the data may have reflected this predominance and is not necessarily due only to perceived effectiveness.
Conversely, given the average use of the StrainprintTM app was approximately 6 months, coupled with an average of 64.2 recorded medication sessions per user, a greater proportion of those who experienced improvement may have continued to log data, due to the perceived effectiveness of the app and the cannabis dosage form being utilised.
Conclusion
With emerging evidence internationally demonstrating that women are utilising illicit cannabis as a self-management strategy for the pain and the associated symptoms of endometriosis, this paper demonstrates that Canadian women are also utilising legally obtained and quality-assured products to manage endometriosis symptoms across domains such as pelvic pain, gastrointestinal symptoms and mood. Cannabis appears to be effective across all reported symptoms, with a noted propensity for inhaled delivery due to the potential increased speed of onset of effects versus the slower onset of oral products, particularly for pelvic pain. Conversely, oral forms appeared to be superior for the less reported mood and gastrointestinal categories, possibly due to higher CBD concentrations in the products utilised. Whilst topical products demonstrated a good effect on pain, due to a very small data set, caution should be exercised in interpreting or extrapolating from this data.
The importance of quality-assured and standardised (i.e., cannabinoid potency and ratios) cannabis products to obtain reproduceable clinical results, and to mitigate possible adverse effects due to potential adulteration or contamination of products, is an important clinical consideration for medicinal cannabis moving forward, particularly when considering the urgent need for clinical trials investigating the safety, tolerability and effectiveness of cannabis for endometriosis pain and associated symptoms.
Acknowledgments
The authors wish to thank Strainprint Technologies Ltd. for their support and data access.
Data Availability
The data underlying this manuscript was based on data collected by Strainprint Technologies Ltd mHealth app between April 2017 until February 2020. Permission to use this data was granted by David Berg, President of Strainprint Technologies Ltd. To request access to this data please contact David Berg at dave@strainprint.ca. The authors confirm they did not have any special access privileges that others would not have.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24. Epub 2016/12/07. doi: 10.1093/humrep/dew293 . [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. Epub 2015/06/13. doi: 10.1016/S0140-6736(15)60692-4 ; PubMed Central PMCID: PMC4561509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowlands IJ, Abbott JA, Montgomery GW, Hockey R, Rogers P, Mishra GD. Prevalence and incidence of endometriosis in Australian women: a data linkage cohort study. BJOG. 2021;128(4):657–65. Epub 2020/08/07. doi: 10.1111/1471-0528.16447 . [DOI] [PubMed] [Google Scholar]
- 4.Adamson GD, Kennedy S., Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. Journal of Endometriosis. 2010;2(1):3–6. [Google Scholar]
- 5.Ianieri MM, Buca DIP, Panaccio P, Cieri M, Francomano F, Liberati M. Retroperitoneal endometriosis in postmenopausal woman causing deep vein thrombosis: case report and review of the literature. Clin Exp Obstet Gynecol. 2017;44(1):148–50. Epub 2017/01/01. . [PubMed] [Google Scholar]
- 6.Ramin-Wright A, Schwartz ASK, Geraedts K, Rauchfuss M, Wölfler MM, Haeberlin F, et al. Fatigue–a symptom in endometriosis. Human Reproduction. 2018;33(8):1459–65. doi: 10.1093/humrep/dey115 [DOI] [PubMed] [Google Scholar]
- 7.Markham R, Luscombe G.M., Manconi F., Fraser I.S. A detailed profile of pain in severe endometriosis. Journal of endometriosis and pelvic pain disorders. 2019;11:85–94. [Google Scholar]
- 8.Cavaggioni G, Lia C, Resta S, Antonielli T, Benedetti Panici P, Megiorni F, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res Int. 2014;2014:786830. doi: 10.1155/2014/786830 ; PubMed Central PMCID: PMC4090426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagana AS, La Rosa VL, Rapisarda AMC, Valenti G, Sapia F, Chiofalo B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–30. Epub 2017/05/30. doi: 10.2147/IJWH.S119729 ; PubMed Central PMCID: PMC5440042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ek M, Roth B, Ekstrom P, Valentin L, Bengtsson M, Ohlsson B. Gastrointestinal symptoms among endometriosis patients—A case-cohort study. BMC Womens Health. 2015;15:59. Epub 2015/08/15. doi: 10.1186/s12905-015-0213-2 ; PubMed Central PMCID: PMC4535676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saidi K, Sharma S, Ohlsson B. A systematic review and meta-analysis of the associations between endometriosis and irritable bowel syndrome. Eur J Obstet Gynecol Reprod Biol. 2020;246:99–105. Epub 2020/02/01. doi: 10.1016/j.ejogrb.2020.01.031 . [DOI] [PubMed] [Google Scholar]
- 12.De Graaff AA, D’Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, Consortium WE, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–85. doi: 10.1093/humrep/det284 . [DOI] [PubMed] [Google Scholar]
- 13.Armour M, Sinclair J, Ng CHM, Hyman MS, Lawson K, Smith CA, et al. Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey. Sci Rep. 2020;10(1):16253. Epub 2020/10/03. doi: 10.1038/s41598-020-73389-2 ; PubMed Central PMCID: PMC7529759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–9. Epub 2012/03/17. doi: 10.1093/humrep/des073 . [DOI] [PubMed] [Google Scholar]
- 15.Armour M, Lawson K, Wood A, Smith CA, Abbott J. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. PLoS One. 2019;14(10):e0223316. Epub 2019/10/11. doi: 10.1371/journal.pone.0223316 ; PubMed Central PMCID: PMC6786587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–73 e8. Epub 2011/07/02. doi: 10.1016/j.fertnstert.2011.05.090 ; PubMed Central PMCID: PMC3679489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilgic E, Guzel E, Kose S, Aydin MC, Karaismailoglu E, Akar I, et al. Endocannabinoids modulate apoptosis in endometriosis and adenomyosis. Acta Histochem. 2017;119(5):523–32. Epub 2017/05/28. doi: 10.1016/j.acthis.2017.05.005 . [DOI] [PubMed] [Google Scholar]
- 18.Sanchez AM, Cioffi R, Vigano P, Candiani M, Verde R, Piscitelli F, et al. Elevated Systemic Levels of Endocannabinoids and Related Mediators Across the Menstrual Cycle in Women With Endometriosis. Reprod Sci. 2016;23(8):1071–9. Epub 2016/02/19. doi: 10.1177/1933719116630414 . [DOI] [PubMed] [Google Scholar]
- 19.Bouaziz J, Bar On A, Seidman DS, Soriano D. The Clinical Significance of Endocannabinoids in Endometriosis Pain Management. Cannabis Cannabinoid Res. 2017;2(1):72–80. Epub 2017/09/02. doi: 10.1089/can.2016.0035 ; PubMed Central PMCID: PMC5436335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinclair J, Smith CA, Abbott J, Chalmers KJ, Pate DW, Armour M. Cannabis Use, a Self-Management Strategy Among Australian Women With Endometriosis: Results From a National Online Survey. J Obstet Gynaecol Can. 2019. Epub 2019/11/15. doi: 10.1016/j.jogc.2019.08.033 . [DOI] [PubMed] [Google Scholar]
- 21.Armour M, Sinclair J, Noller G, Girling J, Larcombe M, Al-Dabbas MA, et al. Illicit Cannabis Usage as a Management Strategy in New Zealand Women with Endometriosis: An Online Survey. Journal of women’s health (2002). 2020. Epub 2020/12/05. doi: 10.1089/jwh.2020.8668 . [DOI] [PubMed] [Google Scholar]
- 22.Armour M, Sinclair J, Chalmers KJ, Smith CA. Self-management strategies amongst Australian women with endometriosis: a national online survey. BMC Complement Altern Med. 2019;19(1):17. Epub 2019/01/17. doi: 10.1186/s12906-019-2431-x ; PubMed Central PMCID: PMC6332532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Statement on Ethical Conduct in Human Research Canberra, ACT: The National Health and Medical Research Council; 2018. Available from: www.nhmrc.gov.au/guidelines/publications/e72.
- 24.Cherry KE, Morton MR. Drug sensitivity in older adults: the role of physiologic and pharmacokinetic factors. Int J Aging Hum Dev. 1989;28(3):159–74. Epub 1989/01/01. doi: 10.2190/00X7-HVXQ-D3BG-MK76 . [DOI] [PubMed] [Google Scholar]
- 25.van den Beld AW, Kaufman JM, Zillikens MC, Lamberts SWJ, Egan JM, van der Lely AJ. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018;6(8):647–58. Epub 2018/07/19. doi: 10.1016/S2213-8587(18)30026-3 ; PubMed Central PMCID: PMC6089223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3326–41. Epub 2012/10/31. doi: 10.1098/rstb.2011.0388 ; PubMed Central PMCID: PMC3481530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, et al. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech Ageing Dev. 2015;150:55–64. Epub 2015/08/19. doi: 10.1016/j.mad.2015.08.005 . [DOI] [PubMed] [Google Scholar]
- 28.Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update. 2014;20(5):737–47. doi: 10.1093/humupd/dmu025 ; PubMed Central PMCID: PMC4501205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, et al. Functional Connectivity is Associated With Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J Pain. 2016;17(1):1–13. doi: 10.1016/j.jpain.2015.09.008 ; PubMed Central PMCID: PMC4698023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–9. Epub 2018/01/09. doi: 10.1016/j.ejim.2018.01.004 . [DOI] [PubMed] [Google Scholar]
- 31.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–804. Epub 2007/08/23. doi: 10.1002/cbdv.200790152 ; PubMed Central PMCID: PMC2689518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madrigal J. Four effective ways to deal with endometriosis flare-ups 2018. Available from: https://endometriosis.net/living/flare-ups/2021. [Google Scholar]
- 33.Hand S. The panic a flare-up can create 2019. Available from: https://endometriosis.net/living/flare-panic/2021. [Google Scholar]
- 34.Mabrouk M, Raimondo D, Del Forno S, Baruffini F, Arena A, Benfenati A, et al. Pelvic floor muscle assessment on three- and four-dimensional transperineal ultrasound in women with ovarian endometriosis with or without retroperitoneal infiltration: a step towards complete functional assessment. Ultrasound Obstet Gynecol. 2018;52(2):265–8. Epub 2017/10/13. doi: 10.1002/uog.18924 . [DOI] [PubMed] [Google Scholar]
- 35.Pertwee RG. Neuropharmacology and therapeutic potential of cannabinoids. Addict Biol. 2000;5(1):37–46. Epub 2000/01/01. doi: 10.1080/13556210071252 . [DOI] [PubMed] [Google Scholar]
- 36.Sinclair J, Armour S., Akowuah J., Proudfoot A., Armour M. Should I inhale?”—Perceptions, barriers, and drivers for medicinal cannabis use amongst Australian women with primary dysmenorrhoea: A qualitative study. Preprint Research Square. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wills S. Cannabis use and abuse by man: an historical perspective. In: Brown DT, editor. The Genus Cannabis. The Netherlands: Harwood Academic Publishers; 1998. p. 1–28. [Google Scholar]
- 38.Peters J, Chien J. Contemporary Routes of Cannabis Consumption: A Primer for Clinicians. J Am Osteopath Assoc. 2018;118(2):67–70. Epub 2018/01/31. doi: 10.7556/jaoa.2018.020 . [DOI] [PubMed] [Google Scholar]
- 39.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol—recent advances. Chem Biodivers. 2007;4(8):1678–92. Epub 2007/08/23. doi: 10.1002/cbdv.200790147 . [DOI] [PubMed] [Google Scholar]
- 40.D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20(3):568–70. doi: 10.1096/fj.05-4943fje . [DOI] [PubMed] [Google Scholar]
- 41.Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87(11):1111–21. Epub 2009/08/20. doi: 10.1007/s00109-009-0512-x . [DOI] [PubMed] [Google Scholar]
- 42.Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology (Berl). 2011;215(3):505–12. Epub 2011/01/19. doi: 10.1007/s00213-010-2157-4 . [DOI] [PubMed] [Google Scholar]
- 43.De Filippis D, Esposito G, Cirillo C, Cipriano M, De Winter BY, Scuderi C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011;6(12):e28159. Epub 2011/12/14. doi: 10.1371/journal.pone.0028159 ; PubMed Central PMCID: PMC3232190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154(5):1001–8. Epub 2008/05/13. doi: 10.1038/bjp.2008.177 ; PubMed Central PMCID: PMC2451037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett. 2008;29(2):192–200. Epub 2008/04/12. . [PubMed] [Google Scholar]
- 46.Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016;1(1):154–65. Epub 2016/07/01. doi: 10.1089/can.2016.0009 ; PubMed Central PMCID: PMC5576607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lea R, Whorwell PJ. Irritable bowel syndrome or endometriosis, or both? Eur J Gastroenterol Hepatol. 2003;15(10):1131–3. Epub 2003/09/23. doi: 10.1097/00042737-200310000-00012 . [DOI] [PubMed] [Google Scholar]
- 48.DiVasta AD, Zimmerman LA, Vitonis AF, Fadayomi AB, Missmer SA. Overlap Between Irritable Bowel Syndrome Diagnosis and Endometriosis in Adolescents. Clin Gastroenterol Hepatol. 2020. Epub 2020/03/19. doi: 10.1016/j.cgh.2020.03.014 . [DOI] [PubMed] [Google Scholar]
- 49.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–43. doi: 10.1007/s11064-005-6978-1 . [DOI] [PubMed] [Google Scholar]
- 50.Campos AC, Guimaraes FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl). 2008;199(2):223–30. Epub 2008/05/01. doi: 10.1007/s00213-008-1168-x . [DOI] [PubMed] [Google Scholar]