Abstract
Plant associated microbiomes are known to confer fitness advantages to the host. Understanding how plant factors including biochemical traits influence host associated microbiome assembly could facilitate the development of microbiome-mediated solutions for sustainable plant production. Here, we examined microbial community structures of a set of well-characterized Arabidopsis thaliana mutants disrupted in metabolic pathways for the production of glucosinolates, flavonoids, or a number of defense signalling molecules. A. thaliana lines were grown in a natural soil and maintained under greenhouse conditions for 4 weeks before collection of roots for bacterial and fungal community profiling. We found distinct relative abundances and diversities of bacterial and fungal communities assembled in the individual A. thaliana mutants compared to their parental lines. Bacterial and fungal genera were mostly enriched than depleted in secondary metabolite and defense signaling mutants, except for flavonoid mutations on fungi communities. Bacterial genera Azospirillum and Flavobacterium were significantly enriched in most of the glucosinolate, flavonoid and signalling mutants while the fungal taxa Sporobolomyces and Emericellopsis were enriched in several glucosinolates and signalling mutants. Whilst the present study revealed marked differences in microbiomes of Arabidopsis mutants and their parental lines, it is suggestive that unknown enzymatic and pleiotropic activities of the mutated genes could contribute to the identified host-associated microbiomes. Notwithstanding, this study revealed interesting gene-microbiota links, and thus represents valuable resource data for selecting candidate A. thaliana mutants for analyzing the links between host genetics and the associated microbiome.
Introduction
Plants interact with a vast diversity of microorganisms both above- and belowground, and the outcomes of those interactions may be either beneficial or detrimental to the plant. Essentially, the plant employs a range of strategies such as the action of constitutive and/or induced chemical compounds in combination with the plant innate immune system to assemble its associated microbiota [1]. The plant secondary metabolites glucosinolates (GLS) and flavonoids (FLVs) have been widely studied for several microbiota-mediating and plant protective functions [2]. For instance, GLS from the roots of Brassica species were found to inhibit microbial pathogens including Pseudomonas syringae, Alternaria brassicicola, Gaeumannomyces graminis, Botrytis cinerea, Fusarium oxysporum and Hyaloperonospora parasitica [3, 4]. The FLVs are well known for their chemoattractant and signalling function in legume-rhizobia interactions resulting in N-fixation and role in plant-mycorrhizal associations, but also as phytoanticipins [5, 6]. Phytohormones serve as signalling molecules in regulating the innate immune network, and salicylic acid (SA), jasmonic acid (JA), ethylene (ET) and abscisic acid (ABA) act as molecular switches in stimulating inducible defense against biotic and abiotic stresses [7, 8]. Owing to its robust and overarching activation of defense repertoires, the immune system is perceived to affect microbial community structures [1, 9, 10].
The biosynthetic pathways and genes involved in GLS [11, 12], FLV [13–15] and defense signalling [16–18] are well described, and research is directed towards exploiting these pathways to study the links between plant gene functions and microbiome assemblage. Several well-characterized mutants of the model plant, Arabidopsis thaliana (hereafter Arabidopsis) have become quintessential for studying those relationships. For example, Badri et al. [19] reported effects on microbial communities of a mutation in a plant ATP transporter involved in exudation of plant secondary metabolites, and further concluded that individual plant genes are actively involved in the interaction with microbial communities. By using GLS [20], FLV [19] and benzoxazinoid (BX) mutants [21], the influence of plant defensive secondary metabolites on the plant-associated microbiota has been demonstrated. For example, distinct microbiomes were observed in maize parental lines and their isogenic mutants (bx1, bx2 and bx6) carrying disruptions in genes encoding enzymes in different steps of the BX pathway [21]. This study further demonstrated a gatekeeper role of BXs in modulating plant-associated microbiomes associated with plant roots. In other studies, the coumarin-impaired mutants, myb72-2 and f6’h1 were used to demonstrate the impact of coumarins on microbial community structures [22, 23]. In addition, studies have used Arabidopsis mutants to examine the influence of phytohormones including fatty acid desaturases (FAD) on microbial community structures [24].
Mechanistic processes at the rhizoplane, including the gating role of plant secondary metabolites and defense signalling molecules (DSMs) could control the assembly of host specific microbiomes. We hypothesized that mutations in pathways for the synthesis of certain secondary metabolites and DSMs disrupt the ability of the plant to assemble an optimal microbiome. Findings from other studies using different experimental systems including either a single or a few mutants have reported contrasting effects of plant metabolites on the plant associated microbial community structures. Moreover, previous studies have relied on in-vitro systems where metabolites were exogenously applied and their effect on microorganisms examined. However, such studies do not always reveal the precise effects of these metabolites in a natural system. Our objective in the present study was to assess root microbiome assembly in natural soils in a range of plant mutants with gene disruption in different steps of defense-related biosynthetic or signalling pathways. For this, we selected a range of well-characterized Arabidopsis mutants disrupted in GLS, FLV and DSM synthesis and examined their effects on bacterial and fungal communities in a field soil. The analysis of these mutants using identical soils and growth conditions will provide comparable insights of the effects of these mutations on bacterial as well as fungal community structures, the latter has received little attention in previous studies.
Materials and methods
Plant material
We used 21 Arabidopsis mutants and their genetic background lines Col_0 and Ler_0 (Fig 1A–1C; S1 Table in S1 File). The parental line Col-0 is a natural accession that is maintained as a homozygous line, while Ler-0 carries X-ray induced mutations in the ERECTA gene [25], resulting in distinct chemical profiles [26], root morphology [27, 28], and resistance against F. oxysporum [29]. All GLS mutants are in a Col-0 background and similarly for the DSM mutants except for aba3_2, which was derived from Ler-0. The flavonoid tt mutants are in a Ler-0 background while pap1_D has Col-0 as its parental line. The GLS (cyp79B2 and cyp79B3), FLVs (tt3, tt5) and jasmonic acid (dde2) mutants were kindly provided by Profs. Judith Bender (Brown University), Wendy Peer (University of Maryland) and Paul Staswich (University of Nebraska), respectively. Other Arabidopsis lines were supplied by the Nottingham Arabidopsis Stock Centre (NASC), UK.
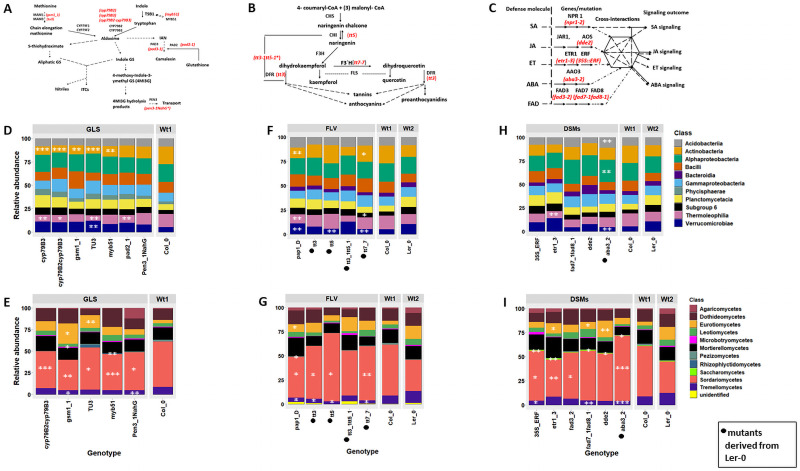
Fig 1. Plant biosynthetic and defense signalling pathways and microbial relative abundances.
A) Biosynthetic pathway of aliphatic and indolic GLS in Arabidopsis. Adapted from Frerigmann et al, (2014). IAN (Indole-3-acetonitrile), TSB1 (tryptophan synthase beta subunit 1). B) The flavonoid biosynthetic pathway. CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol reductase; F3H, flavonol 3-hydroxylase; FLS, flavonol synthase. *(double mutant). Adapted from Buer et al., (2009, 2010). C) The defense signalling pathways. The phytohormones salicylic acid (SA), jasmonic acid (JA), ethylene (ET), abscisic acid (ABA) mediate defense signalling in plants. Fatty acid desaturase (FAD) is also involved in defense including the regulation of JA and SA pathways. Defense signal interactions to fine-tune defense signalling outcomes is also shown. NPR1 (Non-expressor of PR genes1), AAO3 (Abscisic acid synthase), AOS (Allene oxide synthase). Both disrupted genes from which mutants were derived and mutants (indicated in red) are shown. Class-level relative abundances (RAs) of microbial communities observed in Arabidopsis genotypes. D) Bacterial and E) fungal RAs at class level in GLS mutants and the parental line, Col_0. F) Bacterial and G) fungal RA at class level in FLV mutants and respective parental lines, Col_0 and Ler-0. H) Bacterial and I) fungal RAs at class level in DSMs and their respective parental lines, Col_0 and Ler-0. Test of statistical significance was performed by comparing each mutant to their respective background parental lines, and the significantly affected taxa are indicated as *** (P<0.001), ** (P<0.01) and * (P<0.05). ANOVA tests were followed by the Tukey–Kramer post hoc test using the Benjamini and Hochberg (BH) FDR for multiple comparisons. Only the most abundant 10 taxa were used in this analysis. Microbial taxa with small effect sizes were removed by filtering (effect size = 0.8). The analysis was performed using the STAMP software (v2.1.3).
Experimental design
Arabidopsis seeds were sown in pots (8cm x 8cm x 6cm) with moistened field soil (fine sand 32.2%, coarse sand 52.8%, humus 4.7%, clay 3%, silt 7.3%) [30], pH 5.95, collected from a fallow field at the Jyndevad Research station (N 46° 28’ 20.716’’/E 9° 28’ 45.347’’) in Denmark. Microbiome analysis of the soil revealed high abundance of bacterial phyla Acidobacteria and Proteobacteria and the fungal classes Sordariomycetes and Mortierellomycetes [21]. Each pot represented a biological replicate of individual genotypes and was replicated 5 times for all genotypes. Seeds were stratified and pots completely randomized and maintained in a greenhouse under 2017 summer conditions (S1 Fig in S1 File). Seedlings (five plants per pot) were maintained by capillary watering (100 ml) and weed removal every week. Sampling was done after 4 weeks of plant growth, by uprooting each plant and gently shaking roots to remove loosely adhering soils. Roots (with remaining attached fine soils) of the 5 plants in each pot was pooled and placed into 2 ml collection tubes, to represent one replicate. The samples were frozen in liquid nitrogen and stored at -20°C. Subsequently, the samples were lyophilized and ground with 3 sterile metal balls (size 2.88mm) using a Geno/Grinder 2010 at a rate of 1500 rpm for 2 minutes before DNA extraction.
Sample processing, sequence analysis and statistics
Sample DNA extraction and library preparation were essentially performed as previously described [21]. Briefly, we extracted DNA using the PowerLyzer™ Power Soil® DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA). The bacterial primers S-D-Bact-0341-b-S-17, 5′-CCTACGGGNGGCWGCAG-3′ and S-D-Bact-0785-a-A-21, 5′-GACTACHVGGGTATCTAATCC-3′ [31] and the fungal primers fITS7, 5′-GTGARTCATCGAATCTTTG-3′ and ITS4, 5′-TCCTCCGCTTATTGATATGC-3′ [32] were used to amplify the V3/V4 region of the bacterial 16S rRNA and the fungal internal transcribed spacer 2 (ITS2) region, respectively. A dual indexing strategy was used, and PCR conditions were as described [21]. For detailed library and PCR conditions see Additional file 1 in S1 File. Samples were sequenced using the Illumina MiSeq platform at Eurofins MWG (Ebersberg, Germany). All sequence files were deposited in the NCBI Sequence Read Archive (SRA) under the accession number PRJNA579829.
Sequence analysis including demultiplexing, operational taxonomic units (OTUs) clustering at 97% similarity cutoff value, chimera detection and removal, and OTU table creation were performed using VSEARCH version 2.6 [33], as described in [21]. Taxonomy assignments were carried out in QIIME version 1.9 [34], respectively using SILVA 132 [35] and UNITE version 7.2 [36] reference databases for bacterial and fungal OTUs. Downstream data exploration and visualization was performed in the R statistical package [37] using the ggplot2 (v3.3.2) package. Microbial community diversity estimations including alpha and beta diversities, species richness and dissimilarity were performed using the ‘vegan’ package [38] and ‘phyloseq’ [39]. We applied a minimum cutoff of 700 and 500 reads respectively, for bacteria and fungi and the reads and OTU distribution were visualized (S2 Fig in S1 File). Following this, samples with less than 3 replicates were removed and OTUs represented in less than 3 samples in the total dataset were also excluded prior to downstream analysis.
To determine statistically significant differences in taxonomic profiles, we performed a multiple group analysis by comparing sequences assigned to different class level taxa (top 10) in mutants and their respective parental lines (Col_0 or Ler-0) using the STAMP software v2.1.3 [40, 41]. Genotypes were compared by ANOVA, followed by the Tukey–Kramer post hoc test (p < 0.05) using the Benjamini and Hochberg (BH) FDR for multiple comparisons. Class taxa with small effect sizes were removed by filtering (effect size = 0.8).
Bacterial and fungal alpha diversities (observed and Shannon) were estimated by rarifying the OTU table 100 times at a depth of 700 reads for bacteria and 500 reads for fungi and the mean of the diversity estimates of 100 trials was used. To identify the effect of host genotype on alpha diversity, we partitioned the data for each mutant and parental line and performed Kruskal-Wallis test, followed by pairwise Wilcoxon rank sum tests with Benjamini-Hochberg correction. The OTU tables were transformed to relative abundances (RAs) for beta diversity analysis for each genotype groups (GLS, FLV and DSM mutants), and further visualized using unconstrained principal coordinates analysis (PCoA). Permutational multivariate analysis of variance (PERMANOVA) based on a Bray-Curtis dissimilarity matrix was carried out by using 1000 permutations to detect significant differences in the overall bacterial and fungal community composition, using the “adonis” test from the “vegan” package [38]. We further confirmed significant genotypic variations on microbial community structures using generalized linear models (GLMs), where genotype was fitted to each OTU using the mvabund package (function manyglm with a negative binomial distribution) [42]. The likelihood-ratio test was used, and p-values were adjusted for multiple testing. To further analyse specific genotype effects, we split the data for each mutant and parental line and performed PERMANOVA analysis. We performed differential analysis using DESeq2 (version 1.22.2) [43] to determine bacterial and fungal taxa that were significantly different in mutants and parental lines.
Results
We studied microbiome composition in Arabidopsis plants carrying mutations in specific steps of the glucosinolates (GLS), flavonoids (FLV) and defense signaling molecules (DSM) pathways (Fig 1A–1C). The bacterial community profiling yielded a total of 717,379 sequence reads clustering into 6,471 OTUs while the fungal profiling yielded 703,675 sequences which clustered into 344 OTUs after quality filtering. The sequence read statistics are provided (S2 Table in S1 File).
Microbial abundance is affected in most mutants
Bacterial and fungal class relative abundances were distinct in the GLS, FLV and DSM mutants compared to parental lines (Fig 1). We found the most highly significant differences (P<0.001) in the mean abundance of reads belonging to the bacterial classes Actinobacteria, Thermoleophilia and Verrucomicrobiae in the GLS upstream mutants cyp79B3, cyp79B2cyp79B3 and tu3 (Fig 1D). Both cyp79B3 and cyp79B2cyp79B3 have upstream mutations that lacks the ability to form aldoxime from tryptophan and, thus, are deficient in indolic GLS [44, 45], while tu3 lacks C6, C7, and C8 aliphatic glucosinolates [12]. Similarly, the fungal class Eurotiomycetes was highly enriched in gsm1_1 and had the least abundance in Col_0 (Fig 1E). Dothidiomycetes was strongly enriched in myb51(impaired in IGS and camalexin synthesis) (Fig 1E).
A multiple test analysis revealed significant enrichment of a number of bacterial taxa in FLV mutants pap1_D (FLV overexpressed mutant) and tt7_7 compared to their parental lines. Verrucomicrobiae was strongly enriched in tt5 and tt7_7 (deficient in flavonoid 3’-hydroxylase activity and lacking orthodihydroxy flavonoids such as quercetin and kaempferol) compared to Ler_0 (Fig 1F). Similarly, we found significant differences in the relative abundances of Sordariomycetes and Tremellomycetes among the mutants (Fig 1G). In the DSM mutant aba3_2, Acidobacteria and Alphaproteobacteria were enriched while Verrucomicrobiae was depleted compared to the parental line Ler_0 (Fig 1H). Thermoleophilia was strongly depleted in etr1_3. Sordariomycetes was enriched, while Tremellomycetes was depleted in aba3_2 (Fig 1I). The relative abundance of other fungal taxa including Eurotiomycetes and Leotiomycetes were also significantly affected.
GLSs, FLVs and DSMs all affect root microbial diversity
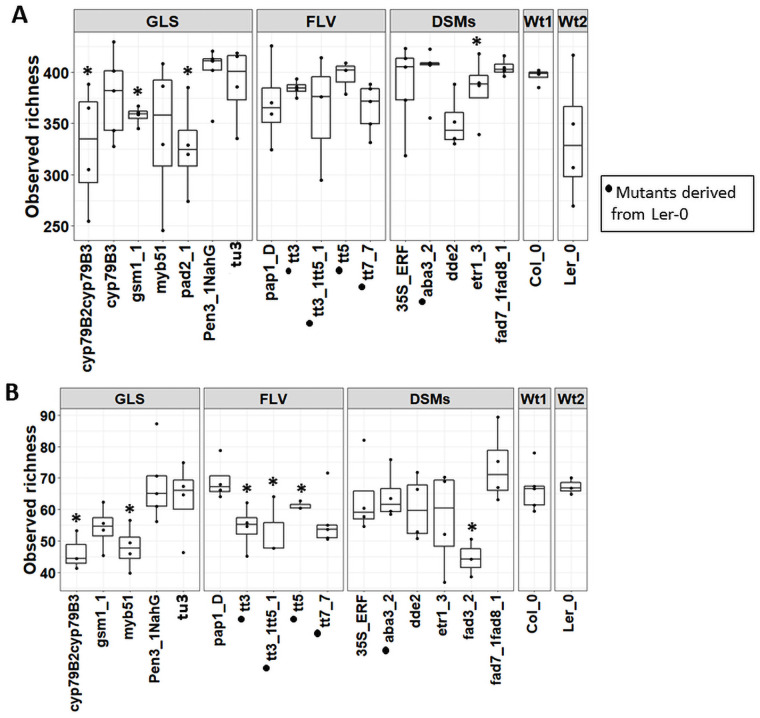
We found significant differences in bacterial observed richness (P<0.05) in the GLS mutants cyp79B2cyp79B3, gsm1_1 and pad2_1 and in the DSM mutant dde2 (Fig 2, S3 Table in S1 File). Shannon diversity was also significantly different (P<0.05) among the pad2_1 and dde2 mutants (S3A Fig in S1 File, S3 Table in S1 File). Bacterial alpha diversity was not significantly different in any of the FLV lines; however, the observed richness was higher in these mutants (the tt lines) compared to Ler_0. For fungi, the observed richness was significantly lower (P< 0.05) in the GLS cyp79B2cyp79B3, myb51, the FLVs tt3, tt3_1tt5_1, tt5 and the DSM mutant fad3_2 compared to Ler_0 (Fig 2, S3 Table in S1 File). In addition, Shannon diversity was significantly different (P<0.05) in the DSM mutant aba3_2 (S3B Fig in S1 File, S3 Table in S1 File).
Fig 2. Alpha diversity (observed) of bacterial (A) and fungal (B) communities in roots of Arabidopsis secondary metabolite and signalling mutants and their parental lines (Col_0 and Ler-0).
Significant differences in alpha diversity in secondary metabolite and signalling mutants and their parental lines are indicated as *, **, and *** for P <0.05, P<0.01 and P<0.001 respectively. The analysis was performed using the Kruskal-Wallis test.
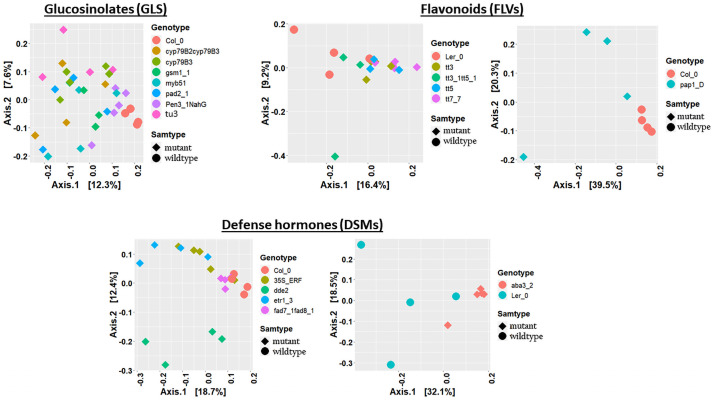
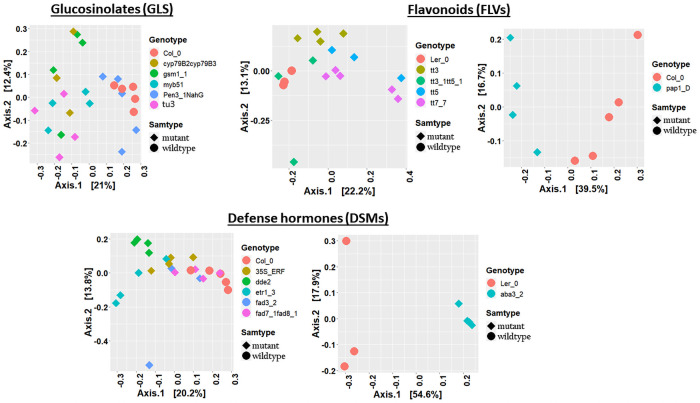
Bacterial beta diversity analysis and visualization using PCoA ordination plots showed a clear separation of GLS mutants from Col_0 (Fig 3A). PERMANOVA revealed significant differences on the bacterial communities (Adonis, bacteria: R2 = 0.31, P< 0.001; Table 1; S4 Table in S1 File,). We likewise observed clustering in the fungal PCoA plots, except that Col_0 and pen3_1_NahG (impaired in both IGS and SA) clustered together (Fig 4A), and the GLS mutation also significantly affected fungal communities (Adonis, R2 = 0.37, P< 0.001, Table 1; S5 Table in S1 File). The tu3 mutant explained the highest proportion of the variation on both bacterial (Adonis, R2 = 0.34, P<0.01, Table 1) and fungal (Adonis, R2 = 0.41, P<0.01, Table 1) communities. The pen3_1_NahG mutant, which is disrupted in both metabolism and transport of tryptophan-derived secondary metabolites and SA synthesis, expectedly had a strong effect on bacterial communities. However, pen3_1_NahG line also carries mutation in PEN3 gene known for cell-wall defense enhancement [46], thus, its effect on the microbiome should be interpreted cautiously. Similarly, PCoA ordination plots showed clustering in the FLV mutants and parental lines in both bacterial and fungal datasets (Figs 3B and 4B) and significant differences were confirmed by PERMANOVA for bacterial (Adonis, R2 = 0.36, P< 0.001, Table 1; S4 Table in S1 File)) and fungal (Adonis, R2 = 0.38, P<0.01, Table 1; S5 Table in S1 File) community structures. Further PERMANOVA analysis using datasets consisting of individual mutants and their parental lines revealed the highest effects of tt3_1_tt5_1 and tt7_7 mutants on bacterial (Adonis; R2 = 0.28, P<0.05, Table 1) and fungal (Adonis; R2 = 0.33, P<0.05, Table 1) communities, respectively. A minor but significant effect of tt3 (Adonis; R2 = 0.31, P<0.05, Table 1) and pap1_D (Adonis; R2 = 0.33, P<0.05, Table 1) was detected on the fungal communities.
Fig 3. Principal coordinate analysis (PCoA) plots of bacterial communities in Arabidopsis secondary metabolite and signalling mutants, and their parental lines.
PCoA of bacterial communities in GLS (A), FLV (B) and DSM (C) genotypes. Individual genotypes and sample groups are shown in different colours and shapes.
Table 1. Permutation analysis of variance (PERMANOVA) for individual mutants and background parental lines.
Adonis tests were based on Bray-Curtis dissimilarity matrices for both bacterial fungal communities using 1000 permutations.
| Dataset/ Factor | Genotype description | Bacteria (R2) | Fungi (R2) |
|---|---|---|---|
| GLS (Mut1) | GLS mutants and parental lines | 0.31*** | 0.37*** |
| FLV (Mut2) data | FLV mutants and parental lines | 0.36*** | 0.38** |
| DSM (Mut3) | Defense signalling mutants and parental lines | 0.39*** | 0.40*** |
| GLS | |||
| Col_0_cyp79B3 | IGS partial disruption) | 0.26** | -- |
| Col_0_cyp79B2cyp79B3 | Lacks IGS and camalexin (Blocked in the production of I3AOx) | 0.28* | 0.33* |
| Col_0_myb51 | IGS synthesis disruption | 0.26* | 0.30* |
| Col_0_gsm1_1 | Reduced amounts of many aliphatic glucosinolates | 0.26* | 0.34* |
| Col_0_tu3 | Deficient in aliphatic GLS with heptyl and octyl core groups | 0.34* | 0.41* |
| Col_0_ pad2-1 | Partially blocks camalexin | 0.28* | -- |
| Col_0_ pen3_1_NahG | Disruption in both IGS synthesis and SA signalling pathways. | 0.20** | 0.16* |
| FLV | |||
| Ler-0_tt7_7 | Deficient in flavonoid 3’-hydroxylase activity and lacks orthodihydroxy flavonoids such as quercetin and kaempferol | 0.23* | 0.33* |
| Ler-0_tt3 | Excess quercetin, kaempferol | -- | 0.31* |
| Ler-0_tt5 | Low-level quercetin production | n.s | n.s |
| Ler-0_ tt3_1tt5_1 | Double mutation, disruption of the synthesis of brown pigment | 0.28* | n.s |
| Col_0_pap1_D | Overexpressed (anthocyanin) mutant | n.s | 0.35* |
| DSMs | |||
| Col_0_dde2 | JA deficient | 0.33* | 0. 41* |
| Ler-0_ aba3_2 | ABA deficient | 0.24* | 0.54* |
| Col_0_ etr1_3 | Ethylene responsive | 0.33* | 0.32* |
| Col_0_35S::ERF | Ethylene (overexpressed) | 0.19* | 0.24* |
| Col_0_ fad3_2 | Fatty acid desaturase (FAD) deficient | -- | 0.25* |
| Col_0_ fad7_1fad8_1 | FAD double mutation | 0.31* | -- |
Significance of test indicated as
*** for p<0.001,
** p <0.01,
*p<0.05 and R2 for the proportion of variation explained. I3AOx (Indole-3-aldoxime) IGS (indole glucosinolate).
-- not computed due to few sample (<3) replicates and n.s (not significant).
Fig 4. Principal coordinate analysis (PCoA) plots of fungal communities in Arabidopsis secondary metabolite and signalling mutants, and their parental lines.
PCoA of fungal communities in GLS (A), FLV (B) and DSM (C) genotypes. Individual genotypes and sample groups are shown in different colours and shapes.
The DSM mutants and their parental lines likewise showed clear clustering of bacterial and fungal communities (Figs 3C and 4C) and were further confirmed to be significantly different (bacteria: Adonis; R2 = 0.39, P<0.001, Table 1; S4 Table in S1 File, and fungi: Adonis; R2 = 0.40, P<0.001, Table 1; S5 Table in S1 File). PERMANOVA performed on the individual mutants and their parental lines showed that the highest proportion of variation was contributed by the dde2 and etr1_3 mutations on the bacterial communities (dde2: Adonis; R2 = 0.33, P<0.001; etr1_3 Adonis; R2 = 0.33, P<0.001, Table 1), and the aba3_2 mutation on the fungal communities (Adonis; R2 = 0.54, P<0.001, Table 1).
GLS, FLV and DSM mutations specifically enrich or deplete microbial taxa
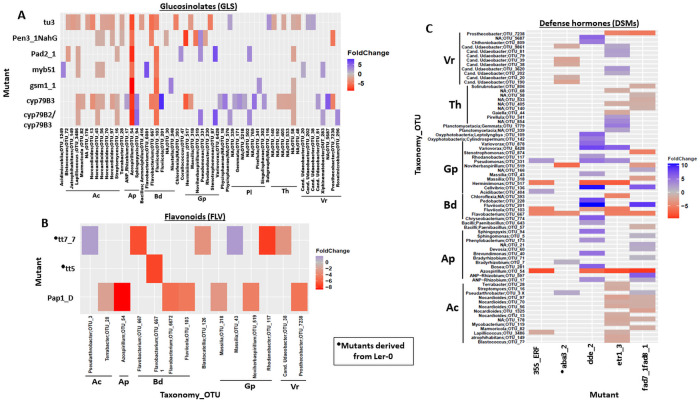
Next, we performed differential analysis to determine bacterial and fungal OTUs (bOTUs and fOTUs, respectively) that had significantly different relative abundances between mutants and parental lines. Most of the differentially affected genera belong to dominant microbial taxa and are mostly enriched than depleted when secondary metabolite and signaling pathways are altered, except for flavonoid mutations on fungi communities (S6 Table in S1 File). For GLS, the highest numbers of differentially abundant bOTUs were observed in upstream mutated lines tu3 and cyp79B3 (Fig 5A; S7 Table in S1 File). Bacterial phyla Actinobacteria, Alphaproteobacteria and Bacteroidetes were among the most highly enriched taxa in many of the GLS mutants. bOTUs assigned to the genera Nocardioides and Azospirillum were the most highly enriched in several GLS mutants. Other significantly enriched genera included Streptomyces and Fluviicola in mutants such as tu3, myb51 and cyp79B3 (Fig 5A; S7 Table in S1 File). Planctomycetes genera were mostly depleted in cyp79B3. Massilia and Flavobacterium were also significantly enriched in cyp79B3 and tu3. These results indicate that both indolic and aliphatic GLSs regulate microbial members.
Fig 5. Heat map of differentially abundant bacterial OTUs identified by comparing secondary metabolite and signalling mutants and their parental lines.
A) Heat map of differentially abundant bacterial OTUs identified by comparing GLS mutants and parental lines. B) Heat map of differentially abundant bacterial OTUs identified by comparing FLVs mutants and parental lines. C) Heat map of differentially abundant bacterial OTUs identified by comparing DSMs mutants and parental lines. Foldchanges are indicated in blue and red respectively for parental lines and mutants. ANP-Rhizobium (Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium), Candidatus Udaeobacter (Cand. Udaeobacter), Candidatus Xiphinematobacter (Cand. Xiphinematobacter), Ac (Actinobacteria), Ap (Alphaproteobacteria), Bd (Bacteroidetes), Gp (Gammaproteobacteria), Pl (Planctomycetes), Th (Thermoleophilia), Vr (Verrucomicrobiae). Analysis was performed using DESeq2 package.
We found the lowest number of significantly affected bOTUs in the FLV mutants, with most of these taxa enriched in tt7_7 and pap1-D (Fig 5B; S8 Table in S1 File). bOTUs belonging to Flavobacterium were enriched in the FLV mutants, and Rhodanobacter and Azospirillum were significantly enriched in tt7_7 and pap1-D, respectively. The DSM mutant dde2 was mostly depleted in Proteobacteria and Bacteroidetes genera while Actinobacteria genera were enriched in etr1_3 and fad7_1fad8_1. Other mutants such as 35S::ERF, aba3_2 and dde_2 were enriched in Azospirillum, Flavobacterium and Fluviicola (Fig 5C; S9 Table in S1 File).
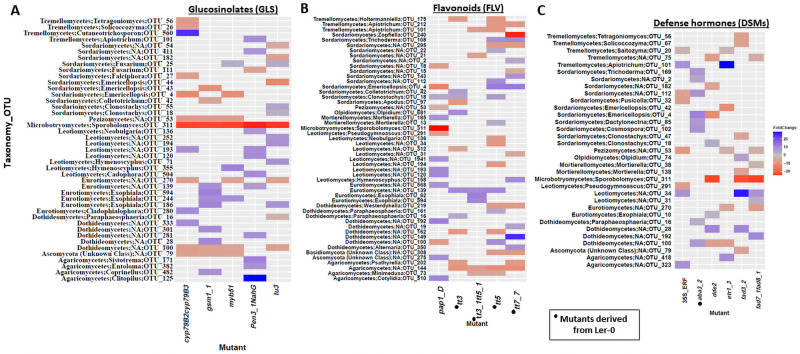
fOTUs assigned to Sporobolomyces and Emericellopsis were the most highly enriched genera in the cyp79B2cyp79B3 and tu3 mutants (Fig 6A; S10 Table in S1 File). In addition, the fungal family Ceratobasidiaceae was strongly enriched in tt3, tt5, tt7_7 and pap1_D. A blast analysis of reads assigned this taxon to Waitea circinata/Rhizoctonia spp. These strong enrichment of the family Ceratobasidiaceae in tt3, tt5, tt7_7 and pap1_D compared with parental lines could suggest an antagonistic effect of FLVs on fungal members of this taxa. We also observed differential abundances of notable antagonistic fungi such as Clonostachys rosea in tt3, tt5 and pap1_D, and the genus Trichoderma in tt5 and tt7_7 (Fig 6B; S11 Table in S1 File). Similarly, in the DSM mutants, Sporobolomyces was significantly enriched in dde2, fad3_2 and fad7_1fad8_1, while Emericellopsis was highly enriched in dde2 and etr1_3. Conversely, aba3_2 was strongly depleted in Emericellopsis and Trichoderma while the ethylene mutants etr1_3 and 35S::ERF had increased abundances of Saitozyma (Fig 6C; S12 Table in S1 File).
Fig 6. Heat map of differentially abundant fungal OTUs identified by comparing secondary metabolite and signalling mutants and their parental lines.
A) Heat map of differentially abundant fungal OTUs identified by comparing GLS mutants and parental line. B) Heat map of differentially abundant fungal OTUs identified by comparing FLVs mutants and parental line. C) Heat map of differentially abundant fungal OTUs identified by comparing DSMs mutants and parental line. Foldchanges are indicated in blue and red respectively in parental lines and mutants. ANP-Rhizobium (Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium), Candidatus Udaeobacter (Cand. Udaeobacter), Candidatus Xiphinematobacter (Cand. Xiphinematobacter). The analysis was performed using DESeq2 package.
Discussion
GLSs have notable effects on the host root-associated microbiome
Our results revealed notable effects of glucosinolates (GLS) mutants on relative abundances, and alpha- and beta-diversities of microbial communities, suggesting that specific GLS metabolites affect the composition of the root microbiome. The separation of GLS mutants from the parental line in the PCoA plots suggests differential effects of the GLS mutations on the Arabidopsis microbiome, thus supporting previous studies [47]. Specifically, tu3 that carries a gene disruption upstream of the aliphatic GLS pathway, had the strongest effect on both bacterial and fungal communities. Aliphatic GLS and their hydrolysis products have been reported to have higher effects on microorganisms compared to indole glucosinolates (iGLS) [48]. The toxicity of aliphatic GLS towards microorganisms is attributed to the complex degradation products isothiocyanates, thiocyanates, oxazolidinethiones and nitriles that are produced from the enzymatic cleavage of glucosinolates by myrosinase [49].
The strong effect of tu3 also confirmed that gene disruptions at the initial steps of a biosynthetic pathway generally have more pronounced effects on the host-associated microbiota [21]. However, other mutants including cyp79B3 and the double mutant cyp79B2cyp79B3 in the same indole GLS pathways or the gsm1_1 in the aliphatic GLS pathways, with upstream gene disruptions only had minor, but significant, effects on the microbiome. These results suggest a differential regulatory role of metabolic genes and their effects on host associated microbiota. Comparatively, the variations in glucosinolates profiles could cause the differential effects of mutants tu3 and gsm1-1 on microbial communities. The tu3 produce glucosinolates that are deficient in gsm1-1 (that is, aliphatic glucosinolates with butyl, pentyl, or hexyl core groups) but lacks aliphatic glucosinolates with heptyl and octyl core groups [3, 12]. Brader et al. [50] found differences in the induction of the CYP79B2 and CYP79B3 genes upon treatment with culture filtrates of the bacterium Erwinia carotovora. Hence, it is possible that unknown enzymatic and pleiotropic activities of the mutated genes could contribute to the observed differences of microbial communities. Furthermore, Ludwig-Müller et al. [51] reported that several TU mutants, having different contents of GLS intermediate products, developed varying degrees of clubroot disease symptoms caused by Plasmodiophora brassicae. Together, these suggest distinct gene functions in GLS pathways which possibly underline mechanistic processes in microbiome assembly.
GLSs had distinct effects on specific microbial groups as confirmed by the identification of a range of bacterial and fungal taxa responding to the different GLS mutations. The increased abundance of individual bacterial genera such as Azospirillum and Fluviicola as well as the fungal genera Sporobolomyces and Emericellopsis in several of the GLS mutants further confirmed the selective effects of metabolites on individual microbial taxa and thus corroborates previous studies [21, 23]. For instance, soils amended with isothiocyanates (allyl, butyl, phenyl, and benzyl ITC) were reported to affect fungal communities more dramatically than bacterial communities [52]. The authors observed changes in community composition including increased Humicola abundance in allyl ITC and Mortierella abundance in butyl ITC amended soils, while the bacterial phylum Firmicutes temporally increased in response to amendment with allyl ITC [52]. Plant metabolic compounds with antimicrobial properties including GLS are known to be part of the boundary layers of the root rhizoplane that modulate root microbiome assemblage [53]. Other genera such as Nocardioides and the plant beneficial taxa Streptomyces and Flavobacterium were also enriched in most of the GLS mutants. Azospirillum contains several beneficial species, widely known for their plant growth promoting traits including nitrogen fixation and synthesis of phytohormones and other compounds required for both biotic and abiotic stress tolerance [54]. The yeast Sporobolomyces, that was enriched in the GLS lines and the other mutants, is an abundant member of the plant mycobiome [55, 56] and is antagonistic against pathogens [57]. Also, the strongly enriched genus Emericellopsis in both the indole GLS mutants cyp79B2cyp79B3, myb51 and aliphatic GLS lines gsm1_1 and tu3 is known to possess biocontrol traits via the antimicrobial compound emericellipsin A, and have been shown to suppress the pathogen Aspergillus niger [58]. In addition, the strong enrichment of Fusarium in pen3_1_NahG could suggest that both physical and chemical barriers (GLS and SA) affect this genus [59, 60].
Flavonoids have a higher effect on root-associated fungal communities
Flavonoids (FLVs) are some of the most studied phytochemicals due to their profound role in plant-microbe interactions. Analysis of microbiota from FLV mutants impaired in different steps of the FLV pathway revealed differential effects on bacterial and fungal communities. While the FLV mutations did not affect bacterial alpha diversities significantly, weak but significant effects of the individual FLV mutations were observed on microbial community composition. The FLV mutant tt7_7 which lacks orthodihydroxy flavonoids (for example quercetin, naringenin, genistein, luteolin, daidzein, and morin), and accumulates pelargonidin rather than the cyanidin found in wild-type plants [61], had significant effects on both bacterial and fungal communities. Differential responses of pelargonidin and cyanidin to both fungi and bacteria have been reported earlier [62]. The orthodihydroxy flavonoids have been shown to mediate plant-microbe interactions, especially in nodule formation and in enhancing arbuscular mycorrhizal colonization, and by inhibiting bacterial and fungal pathogens [63, 64]. Hence, the disruption of the synthesis of these FLVs would likely affect microbial communities and the strong enrichment of the bacterial genera Flavobacterium and Rhodanobacter in tt7_7 could suggest a modulating role of orthodihydroxy flavonoids on bacterial communities. Similarly, the pap1_D which accumulates anthocyanin pigments, (mainly cyanins) and other secondary metabolites [65, 66] significantly affected only the fungal communities.
The tt3 mutant which accumulates high concentrations of both kaempferol and quercetin [13] had a more profound effect on fungal communities compared to the bacterial communities. Both kaempferol and quercetin are highly secreted in Arabidopsis [67], and their accumulation were likely to affect microbial communities. Quercetin enhances mycorrhizal-plant symbiosis by stimulating host penetration and hyphal growth [5], while kaempferol inhibits germination of pathogenic fungal spores [68]. However, Vikram et al. [69] reported that kaempferol and quercetin disrupts quorum sensing and biofilm formation in bacterial communities. Schultz et al. [70] found higher relative abundances of Proteobacteria in a quercetin-treated soil compared with non-treated soil. Moreover, alpha diversity indices were observed to significant decline after quercetin treatment [70]. Yu et al., [71] showed that flavones lead to enrichment of the plant beneficial Oxalobacteraceae in the rhizosphere of maize. Guenoune et al. [72] reported antifungal effects of the FLV maackiain against the fungal pathogen Rhizoctonia solani. Moreover, the enrichment of some members of the order Pleosporales (genus Paraphaeosphaeria) in tt3 and tt5, Westerdykella in tt5 and tt7_7 or depletion of Alternaria in tt7_7, suggests differential effects of FLVs on members of this order. The species Holtermanniella takashimae, which was enriched in tt3 and tt5, was reported to be negatively co-occurring with Fusarium species that were pathogenic in wheat [73].
Although tt5 (impaired in naringenin chalcone) did not affect microbial community composition significantly, naringenin chalcone inhibits spore germination of plant pathogens [68]. In addition, Vandeputte et al. [68, 74] demonstrated that plant produced naringenin and catechin is important in reducing the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. The higher number of differentially abundant fungal taxa compared to bacterial taxa, also point to a higher effect of FLVs on fungi, thus supporting the profound role of FLVs on fungi [75].
Defense signalling mutations have complex effects on microbial taxa
Defense signaling molecules (DSMs) including JA, ABA, SA, FAD and the gaseous molecule ET are well known for their role in mediating plant-microbe interactions. We found that DSM mutants distinctively affected microbial relative abundances and alpha- and beta diversity, thus confirming previous studies [24, 76, 77]. Both etr1_3 (ET insensitive) and 35S::ERF (high ET synthesis) displayed noticeable differences in bacterial and fungal relative abundances and diversity, and thus supports previous studies [78, 79]. The distinct effect of etr1_3 and 35S::ERF could be caused by their differential activation of ethylene. The etr1_3 has reduced ethylene binding activity while 35S::ERF encodes a transcription factor that regulates plant-microbe interactions, as well as integration of signaling pathways to activate ethylene and jasmonate-dependent responses to pathogens [80, 81]. Using a sterile system with artificially constructed bacterial community, Bodenhausen et al. [78] showed that the ethylene-insensitive mutant ein2 assemble distinct bacterial community compared with the parental line, with a noticeable enrichment of the genus Variovorax. Comparably, our study revealed differential enrichment of the genus Variovorax, suggesting a selective effect of etr1_3 on this genus. ABA is an essential molecule in modulating abiotic stress (e.g drought stress and salinity stress), as well as overall plant associated microbial communities [82]. We found that the ABA deficient mutant aba3_2 affected both bacterial and fungal communities, but surprisingly, only a few bacterial taxa at the genus level, including Bradyrhizhobium and Pseudarthrobacter were slightly enriched, whereas several fungal genera were enriched. These results indicate a higher antagonistic effect of ABA on fungal communities. In another study, exogenous application of ABA was found to change community composition as well as enrich the genera Massilia, Cellvibrio, Limnobacter [83]. Also, the JA mutant dde2 (impaired in JA biosynthesis) significantly affected bacterial fungal community composition, with a strong enrichment of bacterial taxa corroborating previous studies [77, 78]. In addition, FAD is pivotal in the phytohormone signalling network by modulating both the SA [84] and JA pathways [85], and its role in mediating plant-microbe interactions has been reported [18, 24]. Likewise, our study revealed distinct effects of FAD mutants on microbial community structures, as both Azospirillum and Sporobolomyces were strongly enriched in fad3_2 and fad7_1fad8_1, as was also observed in a previous study in which the Arabidopsis triple mutant fad3fad7fad8 was enriched in several species within Alpha- and Gammaproteobacteria [24]. Differential effects of FAD genes on microbial taxa have been reported, for instance, while the transcription of the FAD3 gene was shown to be unresponsive upon inoculation of the bacterial pathogen Xanthomonas campestris [84], the FAD7 gene was induced by fungal effectors [86]. The distinct enrichment of a number of bacterial genera in the different DSM mutants is indicative of the selective effects of DSMs in shaping the Arabidopsis root microbiome. However, the resident microbial community can interfere with the plant-hormonal pathways [87]. For example, Finkel et al. [88] demonstrated that the bacterial genus Variovorax utilizes an auxin-degradation operon to alter plant-hormone balances, enabling it to reverse the severe inhibition of root growth that was induced by a wide diversity of bacterial strains. Thus, the analysis of the effect of plant DSMs on microbiomes should be done with caution.
The increasing interest in studying plant metabolites will enable us to better quantify plant host effects on associated microbial communities. However, it is currently challenging to quantify specific effects and mechanisms of important metabolites on plant microbiomes due to methodological limitations. For example, when using mutants, pleiotropic effects arising from gene disruptions in both metabolic and hormonal pathways, makes it impossible to account for individual effects of targeted compounds on microbial community structures. Moreover, because immune signaling activation is complex due to hormonal crosstalk mechanisms it is difficult to quantify the effects of individual hormones on microbial communities. We therefore suggest that, in future studies, detailed analyses should include mutants having complete abolishment of interactive pathways and be complemented with other omics analysis techniques. Furthermore, the host-associated microbiota can alter metabolite synthesis and are also capable of producing several phytohormones [89] and it is therefore important to adopt experimental approaches that will be able to strictly account for plant-derived compounds and their impact on the plant microbiome.
Conclusion
Arabidopsis mutants carrying gene disruptions in pathways of the plant secondary metabolites GLS and FLV, or the signalling molecules SA, ABA, ET, or FAD, assembled distinct microbiomes compared to their parental lines. Most earlier studies on the effects of disruption of metabolic pathway have only considered bacterial communities. In this study, we demonstrated dramatic effects of such mutations also on fungal communities. We found distinct relative abundances and diversities of bacterial and fungal taxa in the mutants. Differential analysis at OTU level revealed significantly affected taxa between the mutants and parental lines. Also, the bacterial and fungal genera were mostly enriched than depleted in mutants, except for flavonoid mutations on fungi communities. These results strongly support the perception that many synthesized plant secondary metabolites and DSMs regulate the assembly of the plant root microbiome. However, the interconnectedness in metabolic and signalling pathways presents a high complexity, and thus, mutant lines with several mutations with the possible elimination of overlapping defense-signalling functions are suggested for further studies. The present screening study revealed significant gene-microbiota links, and thus serve as an important resource for in-depth plant-omics analysis in the future.
Supporting information
(PDF)
Acknowledgments
We thank Profs. Judith Bender (Brown University, USA), Wendy Peer (University of Maryland, USA) and Paul E. Staswich (University of Nebraska, USA) for kindly providing the Arabidopsis mutants. We thank Simone Ena Rasmussen and Mathilde Schiøtt Dige for their excellent laboratory and technical assistance.
Data Availability
The MiSeq paired end reads for bacterial 16 s rRNA gene (V3-V4) and fungal ITS2 regions have been deposited in the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA579829.
Funding Statement
Aarhus University (project number 22550) Independent Research Fund Denmark (DFF), grant number 6111-00065B.
References
- 1.Colaianni NR, Parys K, Lee HS, Conway JM, Kim NH, Edelbacher N, et al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe 2021;29(4):635–649.e9. doi: 10.1016/j.chom.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020;184(1):39–52. doi: 10.1104/pp.20.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tierens KFMJ, Thomma BPHJ, Brouwer M, Schmidt J, Kistner K, Porzel A, et al. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 2001;125(4):1688–99. doi: 10.1104/pp.125.4.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus JF, Gardner PA, Kirkegaard JA, Desmarchelier JM. Biofumigation: Isothiocyanates released from brassica roots inhibit growth of the take-all fungus. Plant Soil. 1994;162(1):107–12. [Google Scholar]
- 5.Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol Fertil Soils. 2012;48(2):123–49. [Google Scholar]
- 6.Buer CS, Imin N, Djordjevic MA. Flavonoids: New roles for old molecules. J Integr Plant Biol. 2010;52(1):98–111. doi: 10.1111/j.1744-7909.2010.00905.x [DOI] [PubMed] [Google Scholar]
- 7.Pieterse CMJ, Leon-Reyes A, Van Der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5(5):308–16. doi: 10.1038/nchembio.164 [DOI] [PubMed] [Google Scholar]
- 8.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal Modulation of Plant Immunity. Annu Rev Cell Dev Biol 2012;28(1):489–521. doi: 10.1146/annurev-cellbio-092910-154055 [DOI] [PubMed] [Google Scholar]
- 9.Teixeira PJPL, Colaianni NR, Law TF, Conway JM, Gilbert S, Li H, et al. Specific modulation of the root immune system by a community of commensal bacteria. Proc Natl Acad Sci. 2021;118(16). doi: 10.1073/pnas.2100678118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringlis IA, Pieterse CMJ. Evolutionary “hide and seek” between bacterial flagellin and the plant immune system. Cell Host Microbe. 2021;29(4):548–50. doi: 10.1016/j.chom.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 2006;57:303–33. doi: 10.1146/annurev.arplant.57.032905.105228 [DOI] [PubMed] [Google Scholar]
- 12.Haughn GW, Davin L, Giblin M, Underhill EW. Biochemical genetics of plant secondary metabolites in Arabidopsis thaliana. Plant Physiol 1991;217–26. doi: 10.1104/pp.97.1.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol 2001;126(2):536–48. doi: 10.1104/pp.126.2.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito K, Yonekura-Sakakibara K, Nakabayashi R, Higashi Y, Yamazaki M, Tohge T, et al. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol Biochem. 2013;72:21–34. doi: 10.1016/j.plaphy.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Weston LA, Mathesius U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol. 2013;39(2):283–97. doi: 10.1007/s10886-013-0248-5 [DOI] [PubMed] [Google Scholar]
- 16.Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15(6):747–54. doi: 10.1046/j.1365-313x.1998.00265.x [DOI] [PubMed] [Google Scholar]
- 17.Soliman S, El-Keblawy A, Mosa K, Helmy M, Wani S. Understanding the phytohormones biosynthetic pathways for developing engineered environmental stress-tolerant crops. In: Biotechnologies of Crop Improvement, Volume 2: Transgenic Approaches. 2018. p. 417–50. [Google Scholar]
- 18.Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci. 2002;98(16):9448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badri D V., Quintana N, El Kassis EG, Kim HK, Choi YH, Sugiyama A, et al. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 2009;151(4):2006–17. doi: 10.1104/pp.109.147462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressan M, Roncato M-A, Bellvert F, Comte G, el Haichar F Z, Achouak W, et al. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J. 2009;3(11):1243–57. doi: 10.1038/ismej.2009.68 [DOI] [PubMed] [Google Scholar]
- 21.Kudjordjie EN, Sapkota R, Steffensen SK, Fomsgaard IS, Nicolaisen M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome. 2019;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci. 2018;115(22):E5213–22. doi: 10.1073/pnas.1722335115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voges MJEEE, Bai Y, Schulze-Lefert P, Sattely ES. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc Natl Acad Sci. 2019;116(25):12558–12565. doi: 10.1073/pnas.1820691116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kniskern JM, Traw MB, Bergelson J. Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. MPMI. 2007;20(12):1512–22. doi: 10.1094/MPMI-20-12-1512 [DOI] [PubMed] [Google Scholar]
- 25.Zapata L, Ding J, Willing EM, Hartwig B, Bezdan D, Jiao WB, et al. Chromosome-level assembly of Arabidopsis thaliana Ler reveals the extent of translocation and inversion polymorphisms. Proc Natl Acad Sci. 2016;113(28):E4052–60. doi: 10.1073/pnas.1607532113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mönchgesang S, Strehmel N, Schmidt S, Westphal L, Taruttis F, Müller E, et al. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Sci Rep 2016;6:29033. doi: 10.1038/srep29033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cajero Sánchez W, García-Ponce B, Sánchez M de la P, Álvarez-Buylla ER, Garay-Arroyo A. Identifying the transition to the maturation zone in three ecotypes of Arabidopsis thaliana roots. Commun Integr Biol. 2018;11(1). doi: 10.1080/19420889.2017.1395993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price CG, Knee EM, Miller JA, Shin Di, Mann J, Crist DK, et al. Following Phenotypes: An Exploration of mendelian genetics using Arabidopsis plants. Am Biol Teach. 2018;80(4):291–300. [Google Scholar]
- 29.Diener AC, Ausubel FM. RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics. 2005;171(1):305–21. doi: 10.1534/genetics.105.042218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiss A, Fomsgaard IS, Mathiassen SK, Kudsk P. Silicone tube microextraction for repeated sampling of benzoxazinoids in the root zone and analysis by HPLC /MS-MS. J Allelochem Interact. 2018;2018:27–37. [Google Scholar]
- 31.Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, et al. New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol. 2012;82(3):666–77. doi: 10.1111/j.1574-6941.2012.01437.x [DOI] [PubMed] [Google Scholar]
- 32.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013. Jan;41(1):e1. doi: 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high- throughput community sequencing data. Nature Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinger L, Bonin A, Alsos IG, Bálint M, Bik H, Boyer F, et al. DNA metabarcoding—Need for robust experimental designs to draw sound ecological conclusions. Mol Ecol. 2019;28(8):1857–62. doi: 10.1111/mec.15060 [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. R: A language and environment for statistical computing. 2017.
- 38.Oksanen AJ, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, et al. Package ‘ vegan’ 2019.
- 39.McMurdie PJ, Holmes S, Kindt R, Legendre P, O’Hara R. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. Watson M, editor. PLoS One. 2013. Apr;8(4):e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–4. doi: 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks DH, Beiko RG. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26(6):715–21. doi: 10.1093/bioinformatics/btq041 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Naumann U, Wright ST, Warton DI. Mvabund- an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol. 2012;3(3):471–4. [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):1–21. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sønderby IE, Geu-Flores F, Halkier BA. Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci. 2010;15(5):283–90. doi: 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 45.Frerigmann H, Gigolashvili T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant. 2014;7(5):814–28. doi: 10.1093/mp/ssu004 [DOI] [PubMed] [Google Scholar]
- 46.Stein M, Dittgen J, Sanchez-Rodrıguez C, Hou B-H, Molina A, Schulze-Lefert P, et al. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micallef S, Colón-Carmona A. Genetic and developmental control of rhizosphere bacterial communities. Mol Microb Ecol Rhizosph. 2013;1:257–63. [Google Scholar]
- 48.Kirkegaard JA, Sarwar M. Biofumigation potential of brassicas I. Variation in glucosinolate profiles of diverse field-grown brassicas. Plant Soil. 1998;71–89. [Google Scholar]
- 49.Burel C, Boujard T, Kaushik SJ, Boeuf G, Mol KA, Van Der Geyten S, et al. Effects of rapeseed meal-glucosinolates on thyroid metabolism and feed utilization in rainbow trout. Gen Comp Endocrinol. 2001;124(3):343–58. doi: 10.1006/gcen.2001.7723 [DOI] [PubMed] [Google Scholar]
- 50.Brader G, Tas E, Palva ET. Jasmonate-dependent induction of indole glucosinolates in arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol. 2001;126(2):849–60. doi: 10.1104/pp.126.2.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig-Müller J, Pieper K, Ruppel M, Cohen JD, Epstein E, Kiddle G, et al. Indole glucosinolate and auxin biosynthesis in Arabidopsis thaliana (L.) Heynh. glucosinolate mutants and the development of clubroot disease. Planta. 1999;208(3):409–19. doi: 10.1007/s004250050576 [DOI] [PubMed] [Google Scholar]
- 52.Hu P, Hollister EB, Somenahally AC, Hons FM, Gentry TJ. Soil bacterial and fungal communities respond differently to various isothiocyanates added for biofumigation. Front Microbiol 2014; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Heijden MGA, Schlaeppi K. Root surface as a frontier for plant microbiome research: Proc Natl Acad Sci. 2015;112(8):2299–300. doi: 10.1073/pnas.1500709112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukami J, Cerezini P, Hungria M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express. 2018;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sapkota R, Knorr K, Jørgensen LN, O Hanlon K a, Nicolaisen M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015;207(4):1134–44. doi: 10.1111/nph.13418 [DOI] [PubMed] [Google Scholar]
- 56.Sapkota R, Jørgensen LN, Nicolaisen M. Spatiotemporal variation and networks in the mycobiome of the wheat canopy. Front Plant Sci. 2017;8:1357. doi: 10.3389/fpls.2017.01357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wachowska U, Borowska J. Antagonistic yeasts competes for iron with winter wheat stem base pathogensAntagonistische Hefen konkurrieren mit Winterweizenhalmbasis-Pathogenen um Eisen. Gesunde Pflanz. 2014;66(4):141–8. [Google Scholar]
- 58.Rogozhin EA, Sadykova VS, Baranova AA, Vasilchenko AS, Lushpa VA, Mineev KS, et al. A novel lipopeptaibol emericellipsin a with antimicrobial and antitumor activity produced by the extremophilic fungus emericellopsis alkalina. Molecules. 2018;23(11). doi: 10.3390/molecules23112785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edgar CI, McGrath KC, Dombrecht B, Manners JM, Maclean DC, Schenk PM, et al. Salicylic acid mediates resistance to the vascular wilt pathogen Fusarium oxysporum in the model host Arabidopsis thaliana. Australas Plant Pathol. 2006;35(6):581–91. [Google Scholar]
- 60.Berrocal-Lobo M, Molina A. Arabidopsis defense response against Fusarium oxysporum. Trends Plant Sci. 2008;13(3):145–50. doi: 10.1016/j.tplants.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 61.Koornneef M, Luiten W, de Vlaming P, Schram AW. A gene controlling flavonoid 3’ hydroxylation in Arabidopsis. Arab Inf Serv. 1982;19(1980):113–5. [Google Scholar]
- 62.Lingua G, Bona E, Manassero P, Marsano F, Todeschini V, Cantamessa S, et al. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int J Mol Sci. 2013;14(8):16207–25. doi: 10.3390/ijms140816207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah A, Smith DL. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy. 2020;10(8). [Google Scholar]
- 64.Sugiyama A, Yazaki K. Flavonoids in plant rhizospheres: Secretion, fate and their effects on biological communication. Plant Biotechnol. 2014;31(5):431–43. [Google Scholar]
- 65.Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved myb regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12(12):2383. doi: 10.1105/tpc.12.12.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi MZ, Xie DY. Features of anthocyanin biosynthesis in pap1-D and wild-type Arabidopsis thaliana plants grown in different light intensity and culture media conditions. Planta. 2010;231(6):1385–400. doi: 10.1007/s00425-010-1142-9 [DOI] [PubMed] [Google Scholar]
- 67.Buer CS, Djordjevic MA. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot. 2009;60(3):751–63. doi: 10.1093/jxb/ern323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, Jaziri M El, et al. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAQ1. Appl Environ Microbiol. 2010;76(1):243–53. doi: 10.1128/AEM.01059-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol. 2010;109(2):515–27. doi: 10.1111/j.1365-2672.2010.04677.x [DOI] [PubMed] [Google Scholar]
- 70.Schütz V, Frindte K, Cui J, Zhang P, Hacquard S, Schulze-Lefert P, et al. Differential Impact of Plant Secondary Metabolites on the Soil Microbiota. Front Microbiol 2021; 12: 1–17. doi: 10.3389/fmicb.2021.666010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu P, He X, Baer M, Beirinckx S, Tian T, Moya YAT, et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat Plants 2021; 7: 481–499. doi: 10.1038/s41477-021-00897-y [DOI] [PubMed] [Google Scholar]
- 72.Guenoune D, Galili S, Phillips DA, Volpin H, Chet I, Okon Y, et al. The defense response elicited by the pathogen Rhizoctonia solani is suppressed by colonization of the AM-fungus Glomus intraradices. Plant Sci. 2001;160(5):925–32. doi: 10.1016/s0168-9452(01)00329-6 [DOI] [PubMed] [Google Scholar]
- 73.Rojas EC, Sapkota R, Jensen B, Jørgensen HJL, Henriksson T, Jørgensen LN, et al. Fusarium head blight modifies fungal endophytic communities during infection of wheat spikes. Microb Ecol. 2020;79(2):397–408. doi: 10.1007/s00248-019-01426-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, Rajaonson S, et al. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology. 2011;157(7):2120–32. doi: 10.1099/mic.0.049338-0 [DOI] [PubMed] [Google Scholar]
- 75.Scervino JM, Ponce MA, Erra-Bassells R, Vierheilig H, Ocampo JA, Godeas A. Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol Res. 2005;109(7):789–94. doi: 10.1017/s0953756205002881 [DOI] [PubMed] [Google Scholar]
- 76.Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349(6250):860–4. doi: 10.1126/science.aaa8764 [DOI] [PubMed] [Google Scholar]
- 77.Carvalhais LC, Dennis PG, Badri D V, Kidd BN, Vivanco JM, Schenk PM. Linking jasmonic acid signaling, root exudates, and rhizosphere microbiomes. Mol Plant Microbe Interact 2015;28(9):1049–58. doi: 10.1094/MPMI-01-15-0016-R [DOI] [PubMed] [Google Scholar]
- 78.Bodenhausen N, Bortfeld-Miller M, Ackermann M, Vorholt JA. A Synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 2017;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doornbos RF, Geraats BPJ, Kuramae EE, Van Loon LC, Bakker P a HM. Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. Mol Plant Microbe Interact. 2011;24(4):395–407. doi: 10.1094/MPMI-05-10-0115 [DOI] [PubMed] [Google Scholar]
- 80.Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008;55(2):175–87. doi: 10.1111/j.1365-313X.2008.03495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12(23):3703–14. doi: 10.1101/gad.12.23.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones P, Garcia BJ, Furches A, Tuskan GA, Jacobson D. Plant host-associated mechanisms for microbial selection. Front Plant Sci. 2019;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalhais LC, Dennis PG, Schenk PM. Plant defence inducers rapidly influence the diversity of bacterial communities in a potting mix. Appl Soil Ecol. 2014;84:1–5. [Google Scholar]
- 84.Buell CR, Somerville SC. Expression of defense-related and putative signaling genes during tolerant and susceptible interactions of Arabidopsis with Xanthomonas campestris pv. campestris. MPMI. 1995. p. 435–43. [Google Scholar]
- 85.Kirsch C, Takamiya-Wik M, Reinold S, Hahlbrock K, Somssich IE. Rapid, transient, and highly localized induction of plastidial ω-3 fatty acid desaturase mRNA at fungal infection sites in Petroselinum crispum. Proc Natl Acad Sci. 1997;94(5):2079–84. doi: 10.1073/pnas.94.5.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avila CA, Arévalo-Soliz LM, Jia L, Navarre DA, Chen Z, Howe GA, et al. Loss of function of FATTY ACID DESATURASE7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012;158(4):2028–41. doi: 10.1104/pp.111.191262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Compant S, Samad A, Faist H, Sessitsch A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J Adv Res. 2019;(March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finkel OM, Salas-González I, Castrillo G, Conway JM, Law TF, Teixeira PJPL, et al. A single bacterial genus maintains root growth in a complex microbiome. Nature. 2020;587(7832):103–8. doi: 10.1038/s41586-020-2778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;1–15. [DOI] [PubMed] [Google Scholar]