Abstract
Background:
Studies of current opinion of our community members for the characteristics, mode, and location of use, use cases, and overall enthusiasm for point-of-care testing (POCT) diagnosis and management tools are needed.
Study Design and Methods:
Qualitative research methods were used to develop, refine, and evaluate hardcopy and electronic versions of a 45-item English language survey. The accuracy of the instrument was measured by recorded structured interview, and its precision was measured by comparison to its administration to a group of uncompensated volunteers.
Main Findings and Results:
Comparison of survey and structured interview data demonstrated high levels of accuracy. Highly concordant with significant levels of correlation and of direct association indicated favorable precision. Ninety-three percent of respondents believed that POCT could improve their care, and 56% identified having a POCT in their home as a top priority. Accuracy, insurance coverage, immediacy of results, and ease of use were identified as the most important characteristics of a POCT.
Conclusions:
Community members strongly support the development of accurate, in-home devices that produce immediate results that can be used to diagnose, manage, and encourage their adherence to treatments for their medical conditions.
Keywords: medical devices, point-of-care technologies, laboratory testing
Over the past decade, and especially during the COVID-19 pandemic, there has been a rapid increase of interest in and demand for point-of-care testing (POCT) solutions that enable health care delivery using telehealth tools. The advantages of expanding the role of the patient in the management of their own health and wellness through the use of devices and POCTs have long been recognized.1-3 Notably, POCT has shown to be extremely effective during disaster relief efforts, such as those during Hurricane Maria in Puerto Rico, and during public health crisis, such as the COVID-19 pandemic.1 Furthermore, it is important to note that an increasing number of Americans are providing care for aging community members in settings ideal for POCT solutions.4,5 Synchronous advances in point-of-care diagnostic tests, device development, clinical needs assessment, and market strategies have led to unprecedented opportunities for providing more efficient and patient-centered high-quality health care. Point-of-care products have the potential to both expedite the diagnosis and support patient-integrated management while also reducing transportation costs, delays, and caregiver inconvienience.6,7 Many publications have documented the advantages of POCT including its cost-effectiveness and high levels of patient satisfaction; however, less is known about POCT preferences in the COVID-19 pandemic era.6,8-15
The optimal use case scenarios for POCT are evolving, as increasing numbers of patients and clinicians are adopting devices such as home blood pressure cuffs, diabetes monitors, and activity monitors that connect with their electronic medical records. As the number and complexity of available POCTs for disease management have increased, potential uses have dramatically expanded. Concomitantly, patient preferences about use of POCT have become more diverse. For example, some patients prefer to receive the results of diagnostic testing in private, whereas others prefer to have diagnostic information be delivered by their clinician. Moreover, individual patients may have different preferences for how the results of different diagnostic tests are revealed. Many individuals prefer patient-integrated chronic disease management, some patients prefer to have a dominant role in their disease management, and others prefer that their care is largely clinician directed. Information on how these preferences are evolving and the extent to which the coronavirus pandemic has impacted them are urgently needed by developers, payers, and patients.6-8 We therefore developed and validated an instrument that allows community members to accurately and efficiently express their preferences for these important aspects of POCT.
METHODS
The need to understand preferences for medical POCTs and devices has led to the creation of survey instruments that gather developer, physician, and patient user requirements and use cases.1 Lists of the information that developers find useful to guide device design and function were generated through discussion of global POCT development objectives with key members of successful teams and from a well-established physician needs assessment tool. Each potential survey item was reviewed for clarity, accuracy, and completeness and modified by content area clinicians with published survey development expertise and by experts from the National Heart Lung Blood Institute of the National Institutes of Health. Individual items were modified to use the perspective and language of patients and home caregivers. Survey development was conducted with the prior approval of the University of Massachusetts Medical School Institutional Review Board (H-00018766).
The original draft survey was administered to a group of volunteers who had research and product development expertise with recording of the time to survey completion and subsequent group review of each survey item, with modification of items that were identified as imperfect. The modified instrument was presented to community volunteers. Responses to flyers by the UMass Memorial Medical Center Office of Volunteer Services resulted in 2 groups of 5 uncompensated community members who participated a 2-hour focus group on different days of January and early February 2020 before the COVID-19 pandemic was present in Massachusetts communities. Focus group sessions were recorded, coded, and conducted according to the study manual of operations.
The time to survey completion characteristic was recorded for each participant, and sessions were securely recorded and coded. All focus group members simultaneously identified each item as correctly capturing their opinion, as unclear, or as not correctly capturing their intended response. All items with one or more nonpositive responses were discussed and modified, and revisions were iteratively revoted. Each discussion session was coded as follows: item revision consensus achieved with minimal iterative modification, consensus on revision achieved after iterative modifications, group consensus regarding revision was not achieved, item identified as not appropriate, or other.
Concurrent coding of each item was compared with subsequent coding of the recording by 2 members of the research team who were unaware of the concurrent code. Discrepant responses were discussed by all 3 auditors, and a consensus code was recorded. Final coding was reconciled against the original and postrevision versions of the item language. Interrater correlation coefficients were calculated using the Fleiss κ test. κ Values of 0.75 or greater were considered excellent agreement, and a value greater than 0.4 was considered fair to good. Descriptive statistics were calculated for demographic survey items.
Hardcopy and electronic versions of the resulting survey were created. Every focus group member completed a hardcopy survey. Ten participants completed the focus group version of the survey, and 267 individuals completed the electronic survey. The survey was administered between May 13 and August 12, 2020, after the peak of the COVID-19 pandemic, by single invitation, to a group of uncompensated individuals who had given permission to be contacted for medical research. Surveys were distributed through researchmatch.org and research volunteer registries maintained by the University of Massachusetts. Respondent preferences were recorded on a 5-point Likert scale, and mean responses were calculated and tabulated. Correlation of the responses of the focus group was compared with the electronic survey by Pearson product-moment correlation, and the nature of the correlation was explored using a linear regression analysis. A P value <0.05 was considered significant.
SURVEY DEVELOPMENT AND RESULTS
All of the 5 female and 5 male focus group members completed the survey and session. The ages of the participants ranged from 22 to 91 years, 2 identified as Black, and 1 identified as Hispanic. Survey completion times ranged from 8 to 17 minutes, with an average (SD) of 12.5 (2.8) minutes. The survey item completion rate was 98%. The 18 questions of the instrument included 45 items. The focus group voted to approve 38 items with minimal iterative discussion and 7 after modification, and none were identified for removal. The concurrent coding of every discussion was concordant with the assessment of the independent reviewers, with a Fleiss κ characteristic of 1.0. In comparison to the focus group, more of the 267 survey respondents identified as female, White, older-age category, having higher levels of education, and as using a medication(s).
The survey item with the strongest degree of agreement was a belief the POCT could improve their care. Ninety-four percent of respondents agreed or strongly agreed. The site of use for POCTs was identified by 93% of respondents as being in their home rather than in a medical office. Sixty-five percent of respondents reported using a POCT, and 56% identified having POCT in their home as a top priority. The best reason for immediate availability of POCT results was identified by 35% of respondents as so that their doctor could help them sooner, by 28% as obviating the need to travel, by 27% as enabling self-management, and by 5% as allowing for private diagnosis. Seventy-three percent responded that they would only use a POCT that was recommended by their doctor.
Ninety-three percent of patients expected to use POCTs in their own home rather than in a medical office. The type of device that would get the most use in the community was identified by 44% of respondents as one that enabled self-care, by 25% of respondents as one that provided access to medical care, by 17% as one that increased their independence, and by 11% as a device that makes them safe. When asked to identify up to 3 most important characteristics of a POCT, 79% identified test accuracy, 51% identified insurance coverage for its cost, 50% identified immediacy of results, 43% identified ease of use, 33% identified affordability, 15% identified that the target body fluid is easy to collect, 14% identified that the device fits into their lifestyle, and 3% identified that the size of the device not excessive Figure 1. Fifty-three percent of respondents favored established technologies, 40% preferred the newest tests, and only 7% demanded exhaustive testing. The responses of the focus group were similar in degree and distribution to those of the survey respondents.
FIGURE 1.
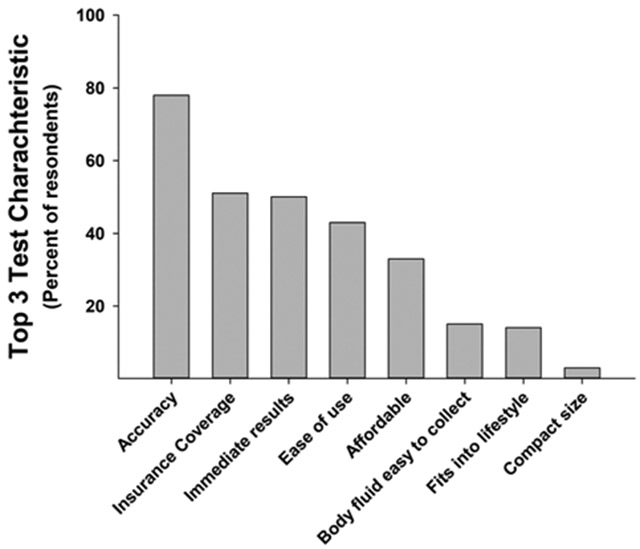
Patient preferences for the most important characteristics of a POCT among 267 respondents who were able to select up to 3 characteristics.
Comparison of the responses of the focus group and the survey respondents regarding the benefits of POCTs is presented in Table 1, and that for their POCT concerns is presented in Table 2. The responses to individual items of the focus group were significantly correlated with the responses from the survey with a Pearson product-moment correlation of 0.87 (P < 0.001). Tests for normality and constant variance justified the linear regression analysis that revealed a significant direct association with a slope of 1.1 and an adjusted R2 value of 0.75 (P < 0.001; Fig. 2).
TABLE 1.
Comparison of Responses Regarding the Benefits to Patients of POCT
| Survey Item | Focus Group* | Survey* |
|---|---|---|
| I believe that POCT could improve my care. | 1.6 | 1.6 |
| POCTs will save time by reducing the number of visits or calls to my doctor. | 1.7 | 1.7 |
| POCTs will reduce my need to go to the hospital or a specialty clinic. | 2.0 | 1.8 |
| The use of POCTs will increase my adherence to my treatment plan. | 2.0 | 2.1 |
| POCTs will increase diagnostic certainty. | 2.1 | 2.3 |
| POCTs improve my doctor's confidence in my diagnosis. | 2.2 | 2.2 |
| POCTs could improve the management of my medical condition. | 2.2 | 1.8 |
| POCTs improve my satisfaction with my medical care. | 2.2 | 2.0 |
| POCTs enable more effective targeted treatment of my medical condition. | 2.3 | 2.0 |
| POCTs may reduce medical errors. | 2.2 | 2.5 |
| Using POCTs will enhance my ability to communicate with my doctor. | 2.3 | 2.0 |
| Using POCTs could enhance my relationship with my doctor. | 2.4 | 2.4 |
| POCTs will increase my doctor's job satisfaction. | 2.4 | 2.7 |
| POCTs might decrease the need for taking an antibiotic. | 3.0 | 2.2 |
Response categories: 1, strongly agree; 2, agree; 3, neutral; 4, disagree; 5, strongly disagree.
Group mean response. Italicized items had mean responses in the neutral or disagree range. The focus groups were conducted before and the survey was administered after the penetration of COVID-19 into respondent communities.
TABLE 2.
Comparison of Responses Regarding Patient Concerns About POCT
| Survey Item | Focus Group* | Survey* |
|---|---|---|
| I am concerned that my insurance might not cover the costs of POCTs. | 2.1 | 2.1 |
| I am concerned that POCTs might not provide definitive results. | 2.5 | 2.8 |
| The results of the test might be difficult to handle if it delivered bad news. | 2.6 | 3.1 |
| The use of POCT could cause overreliance on tests rather than on my doctor's evaluation. |
2.9 | 2.9 |
| Teaching me how to use the tests may be too hard. | 2.9 | 4.1 |
| I might not know enough about how to manage the condition to use the results of the test. | 2.9 | 3.0 |
| The costs of POCTs are too high. | 3.0 | 2.9 |
| My doctor(s) might rely too much on POCTs. | 3.2 | 3.0 |
| I might have difficulty interpreting the results of a POCT. | 3.2 | 3.3 |
| POCT leads to too much testing | 3.3 | 3.5 |
| The accuracy of POCT is not good enough. | 3.5 | 3.0 |
| The results of POCTs are not available quickly enough. | 3.5 | 3.8 |
| It would take me too long to use a POCT. | 3.5 | 4.2 |
| POCTs are too difficult for me to use. | 3.8 | 4.2 |
Response categories: 1, strongly agree; 2, agree; 3, neutral; 4, disagree; 5, strongly disagree.
Group mean response. Italicized items had mean responses in the neutral or disagree range.
FIGURE 2.
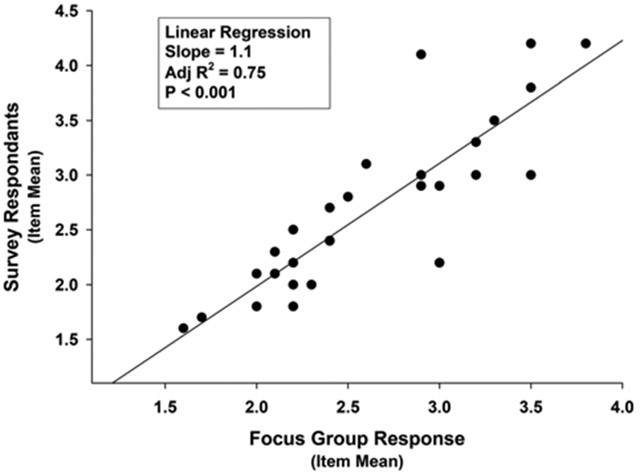
Linear relation of survey respondent with focus group participant responses to individual POCT survey items.
DISCUSSION
The main findings of this validated end-user POCT survey for patients, which was developed using established qualitative research methods, were responses that indicated overwhelming support for the development of POCTs. “I believe that POCT could improve my care” was the item with the strongest level of agreement, and 56% of respondents identified having a POCT in their home as a top priority. Reducing the number of visits or calls to their doctor was also a top priority. The top concern identified was that their insurance company would not cover the cost of the POCT. The highest levels of disagreement were with items stating that POCTs were too difficult or time-consuming for patients to use. Patients expect accurate tests that deliver immediate results in the privacy of their own homes.
Even postpandemic respondents to this survey identified chronic disease management twice as often as they identified diagnosis as the dominant use case. Respondents agreed that POCT would encourage adherence to their treatment regimen and expressed a variety of models for co-managing their diseases with their doctor(s), including management models with high degrees of independence.
We used qualitative research methods that included uncompensated community volunteers to create, develop, refine, and validate an instrument that measures key aspects of the POCT preferences of community members. The instrument was refined by a focus group of uncompensated adult volunteer English-speaking subjects who had sex, race, ethnicity, and age characteristics that were reflective of the 2010 US census.16 The group also included individuals for whom English was not their first language. Favorable ease of use can be inferred from its 12.5-minute time to survey completion and its 98% rate of completion of all 45 items.
Survey accuracy was established by demonstrating that the survey responses to individual items were concordant with those intended by the respondents during structured interviews. Survey precision was measured by correlation of the prepandemic focus group responses to items with the postpandemic responses to the survey. The statistically significant correlation coefficient of 0.87 indicated high levels of correlation. Linear regression analysis was used to explore whether the relation was direct or indirect (inverse) and revealed a significant direct relationship with a slope of 1.1. It also revealed that 25% of the variance of responses was not explained by concordant preferences and is due to other factors. Some of the unexplained variance may be due to changes in attitudes due to the pandemic, differences in the demographics of the respondents, differences of the electronic and hardcopy versions of the survey, or other factors.
External validity is also supported by the correlation of the focus group responses to survey items with those of the survey respondents and by their concordance with the results of prior surveys. The strong patient preference for convenience of POCT and concerns about insurance coverage have been identified in the Deloitte 2018 Healthcare Consumer Survey17 and are part of the World Health Organization criteria for POCT.18
The open comment section of the survey also captured remarkably favorable preferences for developing POCTs and input that indicated a belief that community members should have a pathway for expressing their preferences regarding which medical technologies should be developed. A strong desire for POCT development can also be inferred from the agree and disagree characteristics for the benefits and concerns sections of the survey. All of the POCT benefits items were scored agree or neutral, whereas more of the concern items scored in the disagree than in the agree range.
Although the early experience with our patient-facing survey instrument has been favorable, there are important limitations to its validation. The relatively small number of focus group volunteers makes it possible that the views of some segments of our community may not have been represented. The fact that they were regionally selected and not chosen at random makes it possible that groups from other regions might generate different results. In addition, this study does not provide insight into how foreign language translations of the survey might perform.
This survey is an advance because it provides a mechanism for adult English-speaking members of our communities to accurately and efficiently express their preferences regarding point-of-care medical tests. The survey found that even before the COVID-19 epidemic had a palpable impact in the communities of the focus group members, there was strong support among our volunteers for the development of in-home medical testing technologies. Patients want accurate, immediately available, at-home diagnostics and therapeutics that enable their preferred disease management strategies.
ACKNOWLEDGMENTS
The authors recognize the thoughtful comments of Erin Iturriaga, PhD, and Jue Chen, PhD, of the National Heart, Lung, and Blood. Institute, National Institutes of Health that contributed to the quality of the survey. They also acknowledge the tireless administrative support of Mary Dubuque and Anne Davenport without which this project could not have been completed.
This work was supported by National Institutes of Health Grant U54-HL 143541.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Kost GJ, Hale KN, Brock TK, et al. Point-of-care testing for disasters: needs assessment, strategic planning, and future design. Clin Lab Med. 2009;29(3):583–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tideman PA, Tirimacco R, Senior DP, et al. Impact of a regionalised clinical cardiac support network on mortality among rural patients with myocardial infarction. Med J Aust. 2014;200(3):157–160. [DOI] [PubMed] [Google Scholar]
- 3.Service NH. Whole Systems Demonstrators: an overview of telecare and telehealth [Electronic]. 2009. Available at: https://joinup.ec.europa.eu/collection/ehealth/document/uk-whole-systems-demonstrators-overview-telecare-and-telehealth. Accessed June 23, 2020.
- 4.Dang S, Dimmick S, Kelkar G. Evaluating the evidence base for the use of home telehealth remote monitoring in elderly with heart failure. Telemed J E Health. 2009;15(8):783–796. [DOI] [PubMed] [Google Scholar]
- 5.Ding EY, Ensom E, Hafer N, et al. Point-of-care technologies in heart, lung, blood and sleep disorders from the Center for Advancing Point-of-Care Technologies. Curr Opin Biomed Eng. 2019;11:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvin CA, Lo SF, Deuser SM, et al. Impact of point-of-care testing on patients' length of stay in a large emergency department. Clin Chem. 1996;42(5):711–717. [PubMed] [Google Scholar]
- 7.Tsai WW, Nash DB, Seamonds B, et al. Point-of-care versus central laboratory testing: an economic analysis in an academic medical center. Clin Ther. 1994;16(5):898–910; discussion 854. [PubMed] [Google Scholar]
- 8.Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. BMJ. 1998;316(7137):1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteve M, Rosinach M, Llordes M, et al. Case-finding in primary care for coeliac disease: accuracy and cost-effectiveness of a rapid point-of-care test. United European Gastroenterol J. 2018;6(6):855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendriksen JM, Geersing GJ, van Voorthuizen SC, et al. The cost-effectiveness of point-of-care D-dimer tests compared with a laboratory test to rule out deep venous thrombosis in primary care. Expert Rev Mol Diagn. 2015;15(1):125–136. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic SM, Kostic M. Cost-effectiveness of introducing point-of-care test for detection of level of glycogen phosphorylase in early diagnostic algorithm of acute coronary syndrome. Value Health Reg Issues. 2016;10:79–84. [DOI] [PubMed] [Google Scholar]
- 12.Nshimyumukiza L, Douville X, Fournier D, et al. Cost-effectiveness analysis of antiviral treatment in the management of seasonal influenza A: point-of-care rapid test versus clinical judgment. Influenza Other Respi Viruses. 2016;10(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajasingham R, Pollock NR, Linas BP. The cost-effectiveness of a point-of-care paper transaminase test for monitoring treatment of HIV/TB co-infected persons. Open Forum Infect Dis. 2017;4(4):ofx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owusu-Edusei K Jr.., Gift TL, Ballard RC. Cost-effectiveness of a dual non-treponemal/treponemal syphilis point-of-care test to prevent adverse pregnancy outcomes in sub-Saharan Africa. Sex Transm Dis. 2011;38(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 15.Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. Am J Med Sci. 2008;336(3):254–264. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau. Public Information Office. United States Census 2010. Washington, DC: U.S. Census Bureau Public Information Office. Available at: http://www.census.gov/2010census/. Accessed August 10, 2020. [Google Scholar]
- 17.Deloitte. Deloitte 2018 Healthcare Consumer Survey. Deloitte Insights. 2018; 25. Available at: https://www2.deloitte.com/us/en/insights/industry/health-care/patient-engagement-health-care-consumer-survey.html. Accessed June 23, 2020. [Google Scholar]
- 18.Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95(9):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]


