Supplemental digital content is available in the text.
KEY WORDS: Coagulopathy, PROMMTT, massive transfusion, shock, hemorrhagic, resuscitation
Abstract
BACKGROUND
Damage-control resuscitation (DCR) improves survival in severely bleeding patients. However, deviating from balanced transfusion ratios during a resuscitation may limit this benefit. We hypothesized that maintaining a balanced resuscitation during DCR is independently associated with improved survival.
METHODS
This was a secondary analysis of the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study. Patients receiving >3 U of packed red blood cells (PRBCs) during any 1-hour period over the first 6 hours and surviving beyond 30 minutes were included. Linear regression assessed the effect of percent time in a high-ratio range on 24-hour survival. We identified an optimal ratio and percent of time above the target ratio threshold by Youden’s index. We compared patients with a 6-hour ratio above the target and above the percent time threshold (on-target) with all others (off-target). Kaplan-Meier analysis assessed the combined effect of blood product ratio and percent time over the target ratio on 24-hour and 30-day survival. Multivariable logistic regression identified factors independently associated with 24-hour and 30-day survival.
RESULTS
Of 1,245 PROMMTT patients, 524 met the inclusion criteria. Optimal targets were plasma/PRBC and platelet/PRBC of 0.75 (3:4) and ≥40% time spent over this threshold. For plasma/PRBC, on-target (n = 213) versus off-target (n = 311) patients were younger (median, 31 years; interquartile range, [22–50] vs. 40 [25–54]; p = 0.002) with similar injury burdens and presenting physiology. Similar patterns were observed for platelet/PRBC on-target (n = 116) and off-target (n = 408) patients. After adjusting for differences, on-target plasma/PRBC patients had significantly improved 24-hour (odds ratio, 2.25; 95% confidence interval, 1.20–4.23) and 30-day (odds ratio, 1.97; 95% confidence interval, 1.14–3.41) survival, while on-target platelet/PRBC patients did not.
CONCLUSION
Maintaining a high ratio of plasma/PRBC during DCR is independently associated with improved survival. Performance improvement efforts and prospective studies should capture time spent in a high-ratio range.
LEVEL OF EVIDENCE
Epidemiologic/prognostic study, level II; Therapeutic, level IV.
Trauma deaths in the United States total an estimated 200,000 annually,1,2 and of these, approximately 50,000 are from acute hemorrhage.3 Recent analysis suggests that many of these deaths are potentially preventable;4–8 nearly 20,000 in-hospital trauma deaths per year could be eliminated by providing optimal clinical care to these critically injured bleeding patients.1 To reduce potentially preventable death from trauma, the most common cause of death, hemorrhage, requires improved therapeutic approaches.5–9
Recent advances in early hemorrhage control and resuscitation have directly addressed this vexing problem.3,10 The use of massive transfusion protocols (MTPs) supporting the practice of damage-control resuscitation (DCR) is associated with improved survival in severely injured trauma patients.11 The landmark Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study demonstrated improved 6-hour survival if ratios of plasma and platelet to packed red blood cell (PRBC) transfusion were closer to 1:1 as compared with less than 1:2.12 The subsequent Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) study did not demonstrate improved overall 24-hour mortality but did show that 1:1:1 plasma to platelets to PRBC transfusion decreased early death from exsanguination.13
However, global acceptance of and adherence to DCR principles remains surprisingly low. A survey of US trauma centers participating in the Trauma Quality Improvement Project indicated that up to 16% continue to use a resuscitation strategy that prioritizes PRBCs over plasma or platelets.14 The PROMMTT study also demonstrated significant practice variations among participating trauma centers with regard to blood product ratios during the course of a resuscitation. However, the effect of these practice variations resulting in wide swings in blood product ratios, often well below the hemostatic target, remains unknown. We hypothesized that maintaining a balanced resuscitation throughout DCR is independently associated with improved survival.
PATIENTS AND METHODS
This study was approved with a waiver of informed consent by the University of Pennsylvania Investigational Review Board. The investigators adhered to the policies regarding the protection of human subjects as prescribed by Code of Federal Regulations Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Patients and Data Collection
This was a secondary analysis of the PROMMTT study.12 The design of the PROMMTT study has been described in detail elsewhere.15 Briefly, this prospective observational study was conducted at 10 level I US trauma centers from July 2009 to October 2010. All 10 participating trauma centers had preexisting MTPs in place but demonstrated substantial variations in transfusion practice.15 Study subjects included patients who were at least 16 years of age, required the highest-level trauma activation, and received at least 1 U of PRBCs within the first 6 hours of admission. Patients were excluded if they were transferred from another facility, pronounced dead within 30 minutes of arrival, had more than 5 minutes of cardiopulmonary resuscitation before or within the first 30 minutes of admission, had burns of greater than 20% total body surface area, were diagnosed with inhalation injury, were prisoners, or were pregnant.
Resuscitation data were collected prospectively starting at 30 minutes following trauma center arrival (t30), at 15-minute intervals for two additional time points (t45 and t60), and then at 30-minute intervals thereafter by independent research assistants who were staffed 24/7. Resuscitation data collection continued until death, transfusion protocol discontinuation, or 2 hours after blood product transfusion for up to 6 hours. Study data audits assured complete and accurate records of each resuscitation.15
For this secondary analysis, we included massive transfusion (MT) patients who received more than 3 U of PRBCs within any 1-hour interval during the first 6 hours, which we termed critical administration threshold positive (CAT+).16 We also assessed alternative definitions of MT including the traditional 10 U or more of PRBC in 24 hours17 and resuscitation intensity.18 Our primary endpoint was 24-hour survival, and 30-day survival was a secondary endpoint.
Analytical Variables and Definitions
Blood product ratios for plasma to PRBC (plasma/PRBC) and platelets to PRBC (platelet/PRBC) were defined as the cumulative ratio at 6 hours or at the end of data collection, whichever came first. Optimal 6-hour ratios for plasma/PRBC and platelet/PRBC were then identified based on 24-hour survival using Youden’s index, max(sensitivity + specificity − 1). We also assessed optimal 6-hour ratios for 30-day survival.
We then evaluated the effect of time spent above and below these optimal blood product ratios. First, we performed linear regression analysis to assess the relationship between time over the optimal ratio and 24-hour survival. We then determined the percent time spent over the optimal ratio for both plasma/PRBC and platelet/PRBC for each patient. Percent time was calculated as the number of minutes spent above the optimal threshold divided by the total resuscitation time. Total resuscitation time was defined as 6 hours for survivors or the time of death for nonsurvivors (Supplemental Fig. 1, http://links.lww.com/TA/C3). We performed sensitivity analysis on alternative definitions for the percent time to include only time to hemostasis or only the first 2 hours of resuscitation since the majority of patients achieved hemostasis by 105 minutes.13
From the optimal ratio and the optimal percent time spent above this ratio, each patient’s DCR profile was then categorized as either on-target (6-hour ratio ≥ optimal target and percent time ≥ optimal target) and off-target (all others) for both plasma and platelets to PRBCs. Unadjusted Kaplan-Meier survival analysis was performed on the patient cohorts. Significant differences between patients who survived and those who died were identified, and multivariable logistic regression was performed to determine factors independently associated with 24-hour and 30-day survival.
Patient cohorts were assessed using descriptive statistics. Categorical variables were compared using the χ2 test, and continuous variables were evaluated using the Wilcoxon-Mann-Whitney test. Statistical significance was defined as p < 0.05. Power analysis indicated that 96 patients per cohort would be required to achieve 0.8 power for 24-hour survival. All analyses were performed using SAS Enterprise version 9.4 (Cary, NC). Article preparation was guided by the STROBE statement for the reporting of cohort studies in epidemiology (checklist provided as Supplemental Digital Content, http://links.lww.com/TA/C2).
RESULTS
Of 1,245 patients in the PROMMTT study, we identified 524 CAT+ patients who comprised our study cohort (Supplemental Fig. 2, http://links.lww.com/TA/C3; Supplemental Table 1, http://links.lww.com/TA/C3). Of the CAT+ patients, 78% were male, the median age was 37 years, and 37% had a penetrating injury (Table 1). Of note, there was no difference in outcome based on injury mechanism on unadjusted analysis. In addition, 408 (77.9%) survived 24 hours, and 355 (67.7%) survived 30 days (Table 2). Exsanguination was the most prevalent overall cause of death at 24 hours and 30 days. On-target plasma/PRBC patients had a lower rate of death from exsanguination compared with off-target patients at both 24 hours (10.3% vs. 20.6%, p = 0.003) and 30 days (11.7% vs. 20.6%, p = 0.011). Similarly, fewer on-target platelet/PRBC died from exsanguination at 24 hours (7.8% vs. 18.9%, p = 0.007) and 30 days (8.6% vs. 19.4%, p = 0.010) (Supplemental Table 2, http://links.lww.com/TA/C3). Youden’s index analysis identified the optimal 6-hour plasma/PRBC ratio of 0.77 (95% confidence interval [CI], 0.74–0.80) and the optimal platelet/PRBC ratio of 0.75 (95% CI, 0.70–0.80) (Supplemental Fig. 3, http://links.lww.com/TA/C3). This methodology was repeated for 30-day survival (Supplemental Tables 3 and 4, http://links.lww.com/TA/C3). From these results, a pragmatic optimal ratio of 0.75 (3:4) plasma/PRBC and platelet/PRBC was used for all subsequent analyses. Patients with a 6-hour plasma/PRBC ratio ≥ the optimal ratio had a 24-hour survival of 82.6% versus 73.4% (p = 0.012), while patients who had a 6-hour platelet/PRBC ratio ≥ the optimal ratio had a 24-hour survival of 86.2% versus 74.5% (p = 0.003).
TABLE 1.
Baseline Demographics Comparison for CAT+ Cohort
| Plasma/PRBC | Platelet/PRBC | ||||||
|---|---|---|---|---|---|---|---|
| CAT+ | Off-target | On-target | p | Off-target | On-target | p | |
| (n = 524) | (n = 311) | (n = 213) | (n = 408) | (n = 116) | |||
| Age, y | 37 (24–52) | 40 (25–54) | 31 (22–50) | 0.002 | 36 (23–52) | 43 (24–55) | 0.064 |
| Men | 408 (77.9) | 245 (78.8) | 163 (76.5) | 0.615 | 323 (79.2) | 85 (73.3) | 0.222 |
| Penetrating | 191 (36.5) | 106 (34.1) | 85 (39.9) | 0.205 | 148 (36.3) | 43 (37.1) | 1.000 |
| Blunt | 326 (62.2) | 199 (62.0) | 127 (59.6) | 0.358 | 255 (62.5) | 71 (61.2) | 0.885 |
| Other | 7 (1.3) | 6 (1.9) | 1 (0.5) | 0.297 | 5 (1.2) | 2 (1.7) | 1.000 |
| ISS | 29 (17–41) | 27 (17–41) | 29 (19–38) | 0.320 | 29 (17–38) | 29 (18–42) | 0.420 |
| AIS head | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0.464 | 0 (0–3) | 0 (0–4) | 0.013 |
| AIS chest | 3 (0–4) | 3 (0–4) | 3 (0–4) | 0.436 | 3 (0–4) | 3 (0–3) | 0.020 |
| AIS abdomen | 2 (0–4) | 2 (0–4) | 3 (0–4) | 0.508 | 2 (0–4) | 2 (0–4) | 0.820 |
| Systolic BP, mm Hg | 98 (80–122) | 99 (78–123) | 98 (80–120) | 0.925 | 99 (80–122) | 98 (81–123) | 0.507 |
| HR, beats per minute | 111 (89–132) | 110 (89–132) | 112 (89–131) | 0.644 | 113 (96–98) | 105 (86–123) | 0.097 |
| Temperature, °C | 35.0 (35.6–36.6) | 36.1 (35.4–36.5) | 36.1 (35.6–36.7) | 0.353 | 36.1 (35.6–36.6) | 35.9 (35.6–36.3) | 0.126 |
| GCS | 5 (3–15) | 6 (3–15) | 3 (3–15) | 0.661 | 7 (3–15) | 3 (3–14) | 0.022 |
| pH | 7.2 (7.1–7.3) | 7.2 (7.1–7.3) | 7.2 (7.1–7.3) | 0.637 | 7.2 (7.1–7.3) | 7.3 (7.1–7.3) | 0.008 |
| Base deficit | 1.0 (0.5–3.8) | 0.6 (0.3–5.0) | 1.0 (1.0–2.6) | 0.855 | 1.0 (0.2–2.9) | 2.5 (0.9–4.9) | 0.452 |
| Lactate, mg/dL | 5.2 (3.3–8.8) | 5.3 (3.5–8.1) | 5.1 (3.3–9.2) | 0.864 | 5.3 (3.2–8.3) | 5.1 (3.7–8.9) | 0.983 |
| Hemoglobin, g/dL | 11.1 (9.4–12.7) | 11.1 (9.6–12.7) | 11.2 (9.3–12.8) | 0.940 | 11.4 (9.9–12.8) | 10.1 (8.8–12.0) | 0.001 |
| PTT, s | 30.4 (25.2–38.8) | 28.0 (24.8–35.6) | 32.5 (26.5–41.8) | <0.001 | 30.1 (25.1–36.7) | 33.0 (26.4–41.0) | 0.072 |
| PT, s | 15.9 (14.1–18.7) | 15.0 (12.8–17.7) | 17.3 (15.1–19.9) | <0.001 | 15.6 (14.0–18.4) | 17.4 (14.7–21.2) | 0.004 |
| INR | 1.3 (1.2–1.6) | 1.2 (1.1–1.5) | 1.4 (1.2–1.7) | <0.001 | 1.3 (1.2–1.6) | 1.4 (1.2–1.7) | 0.032 |
| OR intervention | 424 (80.9) | 254 (81.7) | 170 (79.8) | 0.675 | 331 (81.1) | 93 (80.2) | 0.923 |
All values are presented as n (%) or median (IQR).
CAT+, patients requiring more than 3 U of PRBCs within any hour during the first 6 hours.
On-target, patients with a 6-hour ratio >0.75 and who spent ≥40% of their time above this threshold.
Off-target, all other patients not meeting on-target criteria.
BP, blood pressure; GCS, Glasgow Coma Scale; HR, heart rate; INR, international normalized ratio; IQR, interquartile range; ISS, Injury Severity Score; PT, prothrombin time; PTT, partial thromboplastin time.
TABLE 2.
Comparison of Transfusion Requirements and Mortality for CAT+ Cohort
| Plasma/PRBC | Platelet/PRBC | ||||||
|---|---|---|---|---|---|---|---|
| CAT+ | Off-target | On-target | p | Off-target | On-target | p | |
| (n = 524) | (n = 311) | (n = 213) | (n = 408) | (n = 116) | |||
| 6-h Plasma/PRBC | 0.72 (0.47–0.96) | 0.50 (0.33–0.67) | 1.00 (0.86–1.04) | <0.001 | 0.67 (0.40–0.90) | 0.87 (0.67–1.00) | <0.001 |
| 6-h Platelet/PRBC | 0.29 (0.00–0.80) | 0.00 (0.00–0.73) | 0.54 (0.00–0.86) | <0.001 | 0.00 (0.00–0.50) | 1.03 (0.86–1.33) | <0.001 |
| 6-h Plasma | 6 (3–11) | 4 (2–8) | 8 (6–14) | <0.001 | 5 (3–10) | 8 (4–13) | 0.001 |
| 6-h Platelet | 6 (0–12) | 0 (0–6) | 6 (0–12) | 0.002 | 0 (0–6) | 12 (6–18) | <0.001 |
| 6-h PRBC | 8 (5–15) | 8 (5–16) | 8 (5–14) | 0.668 | 8 (5–15) | 8 (6–14) | 0.697 |
| 24-h Plasma/PRBC | 0.75 (0.50–1.00) | 0.52 (0.33–0.70) | 1.00 (0.86–1.22) | <0.001 | 0.69 (0.44–0.94) | 0.83 (0.67–1.10) | <0.001 |
| 24-h Platelet/PRBC | 0.40 (0.00–0.75) | 0.23 (0.00–0.73) | 0.55 (0.00–0.86) | <0.001 | 0.00 (0.00–0.55) | 1.00 (0.75–1.24) | <0.001 |
| 24-h Plasma | 7 (4–14) | 5 (2–11) | 10 (6–16) | 0.504 | 7 (4–14) | 9 (5–16) | 0.013 |
| 24-h Platelet | 6 (0–12) | 3 (0–12) | 6 (0–12) | <0.001 | 0 (0–11) | 12 (6–18) | <0.001 |
| 24-h PRBC | 10 (6–18) | 9 (6–19) | 10 (6–16) | <0.001 | 10 (6–19) | 9 (6–16) | 0.851 |
| Died in 3 h | 57 (10.9) | 49 (15.8) | 8 (3.8) | <0.001 | 54 (13.2) | 3 (2.6) | 0.002 |
| Died in 6 h | 87 (16.6) | 68 (21.9) | 19 (8.9) | <0.001 | 79 (19.4) | 8 (6.9) | 0.002 |
| Died in 24 h | 116 (22.1) | 83 (26.7) | 33 (15.5) | 0.003 | 103 (25.2) | 13 (11.2) | 0.002 |
| Died in 30 d | 169 (32.3) | 113 (36.3) | 56 (26.3) | 0.020 | 137 (33.6) | 32 (27.6) | 0.269 |
All values are n (%) or median (IQR).
CAT+, patients requiring more than 3 U of PRBCs within any hour during the first 6 hours.
On-target, patients with a 6-hour ratio >0.75 and who spent ≥40% of their time above this threshold.
Off-target, all other patients not meeting on-target criteria.
IQR, interquartile range.
Linear regression assessing the effect of percent time (minutes) over the optimal ratio of 0.75 on predicted survival demonstrated improved 24-hour survival with increased time over this ratio for both plasma/PRBC (p = 0.010) and platelet/PRBC (p = 0.011) (Fig. 1). Youden’s index was then used to determine an optimal percent of time over the target ratio. This demonstrated an optimal threshold of 42% resuscitation time spent over the optimal target ratio for both plasma/PRBC and platelet/PRBC (Supplemental Tables 3 and 4, http://links.lww.com/TA/C3). A pragmatic 40% time threshold was selected for both plasma/PRBC and platelet/PRBC for subsequent analyses. On sensitivity analysis, using alternative time exposure intervals for calculating the optimal percent time above a target ratio of 0.75 did not improve model performance; however, using the time exposure interval of “time to hemostasis” effectively differentiated 24-hour survivors from nonsurvivors (Supplemental Table 5, http://links.lww.com/TA/C3).
Figure 1.
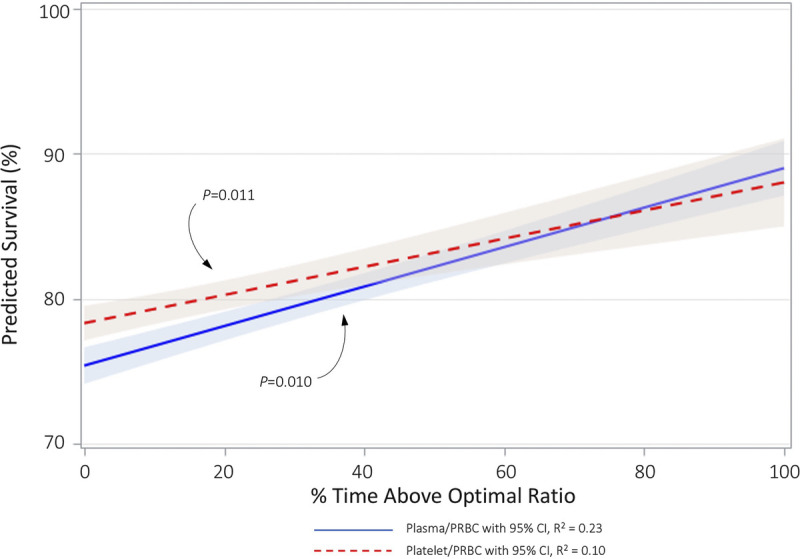
Linear regression showing predicted survival (%) as a function of percent time spent at or above the optimal ratio of 0.75 (3:4) for both plasma/PRBC and platelet/PRBC.
Based on cumulative 6-hour plasma/PRBC ratio and percent time over this optimal ratio, 213 (40.6%) were classified as on-target, while 311 (59.4%) were classified as off-target. On-target plasma/PRBC patients had a 24-hour survival of 83.3% (177 of 213 patients) as compared with 73.3% (228 of 311 patients) for off-target patients (p = 0.003) (Table 2, Fig. 2A). Similarly, for platelet/PRBC, 116 (22.1%) were classified as on-target, while 408 (77.8%) were classified as off-target. On-target platelet/PRBC patients had a 24-hour survival of 88.8% (103 of 116 patients) compared with 74.8% (305 of 408 patients) for off-target patients (p = 0.002) (Table 2, Fig. 2B). Unadjusted survival analysis demonstrated early separation of the survival curves for these patients with significantly greater survival in the on-target group for both plasma/PRBC (p = 0.001 by log rank) and platelet/PRBC (p = 0.001 by log rank) (Fig. 3).
Figure 2.
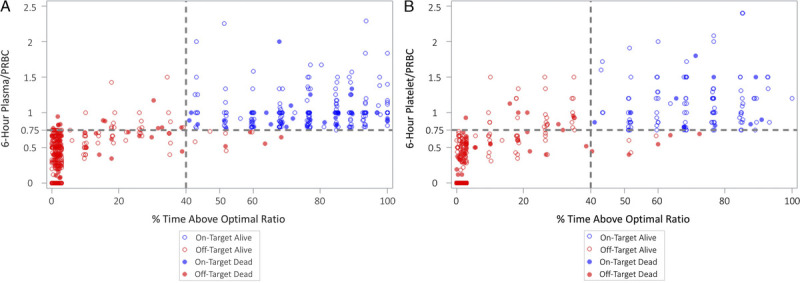
Scatter plot of 6-hour blood product ratio and percent time spent at or above the optimal ratio of 0.75 (3:4) divided into On-Target (blue) and Off-Target (red) cohorts. Overplotting was avoided by a 2% noise reduction through jittering the graph. (A), Plasma/PRBC. (B), Platelet/PRBC.
Figure 3.
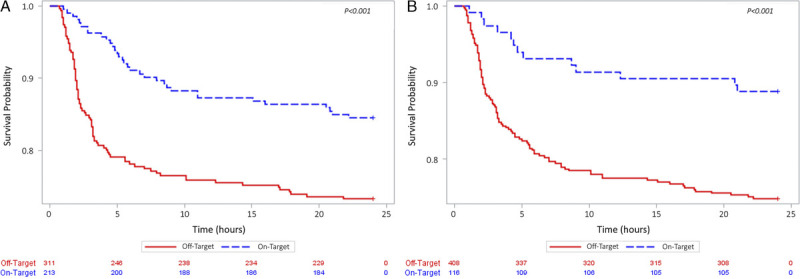
Kaplan-Meier 24-hour survival analysis on the On-Target (blue) and Off-Target (red) cohorts. (A), Plasma/PRBC. (B), Platelet/PRBC.
In comparing patients who survived to those who died, we included Injury Severity Score, Abbreviated Injury Scale (AIS) chest, heart rate, prothrombin time, on-target plasma/PRBC, and on-target platelet/PRBC as covariates in our final model for 24-hour survival. For 30-day survival, we included age, AIS head, admission hemoglobin, prothrombin time, on-target plasma/PRBC and on-target platelet/PRBC. This demonstrated an independent survival benefit for on-target plasma/PRBC at 24 hours (odds ratio [OR], 2.25; 95% CI, 1.20–4.23; p = 0.012) and 30 days (OR, 1.97; 95% CI, 1.14–3.41; p = 0.015) (Fig. 4). However, no such independent association was identified for on-target platelet/PRBC. Increased time spent above the optimal plasma/PRBC ratio by 30-minute intervals (OR, 1.16; 95% CI, 1.07–1.25; p < 0.001), 1-hour intervals (OR, 1.34; 95% CI, 1.14–1.57; p < 0.001), and 3-hour intervals (OR, 2.38; 95% CI, 1.48–3.82; p < 0.001) was also independently associated with improved 24-hour survival. However, a similar independent association was not demonstrated for increased 30-minute intervals (OR, 1.10; 95% CI, 0.98–1.23; p < 0.108), 1-hour intervals (OR, 1.20; 95% CI, 0.96–1.51; p < 0.108), or 3-hour intervals (OR, 1.75; 95% CI, 0.89–3.44; p < 0.108) above a high platelet/PRBC ratio.
Figure 4.
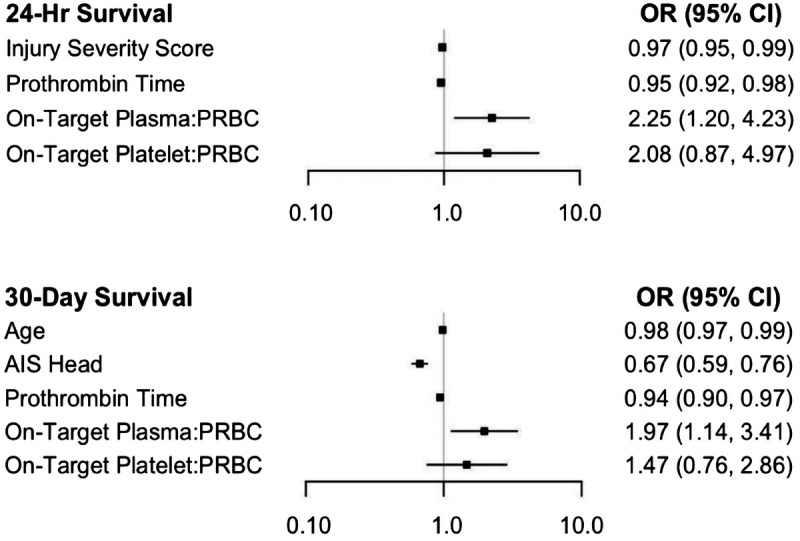
Multivariable logistic regression model assessing survival at 24 hours and 30 days.
DISCUSSION
In this secondary analysis of PROMMTT, we sought to determine whether maintaining a balanced resuscitation throughout DCR is associated with improved survival in severely injured patients at risk for death from hemorrhage. This study demonstrates that early empiric resuscitation should target a high ratio of both plasma and platelets to PRBC (≥0.75) and that this high resuscitation ratio needs to be maintained throughout the resuscitation to optimize survival. In particular, maintaining this high ratio of plasma to PRBC for at least 40% of the patient’s initial 6 hours of resuscitation was independently associated with improved 24-hour and 30-day survival and a lower frequency of cause of death from exsanguination at 24 hours and 30 days. Furthermore, every 30-minute increment in maintaining this high ratio was independently associated with improved survival. Although unadjusted analysis indicated an association between maintaining a high ratio of platelet to PRBC, this association did not persist on adjusted analysis.
The concept of DCR has transformed trauma resuscitation practice over the past three decades by emphasizing a number of concepts including minimizing crystalloid-based resuscitation, optimizing transfusion ratios, and administering hemostatic adjuncts.19–23 Despite this evolution, hemorrhage remains the leading cause of preventable death from trauma.9,24,25 This prompted us to consider the potential role of dynamic component ratios on survival and whether belatedly “catching up” to a balanced ratio could compromise some of the benefit seen with DCR.
To study this effect, we sought to identify a subpopulation within the PROMMTT study truly at risk for death from exsanguination. Traditionally, the MT population meets these criteria; however, the historic metric of receiving ≥10 U of PRBC within 24 hours introduces survivor bias. Recently, Savage et al. defined MT by rate and volume termed CAT+: any patient who received at least 3 U of PRBC in any 1 hour over the course of 24 hours.16,26 This definition mitigates the survival bias that occurs when early death precludes the patient from receiving 10 U of PRBCs. In our analysis, we used a modified form of the CAT definition of more than 3 U during the index 6 hours as opposed to the 24 hours used in the traditional CAT definition since the average time to hemostasis in the PROMMTT study was 105 minutes.13
After selecting these CAT+ patients who were actively exsanguinating, like others, we sought to derive an optimal blood product ratio.11,13,17,27–31 In our analysis, the optimal plasma/PRBC ratio of 0.75 is consistent with previous studies.17,27,32–34 In addition, our 0.75 optimal platelet/PRBC ratio was consistent with a high ratio of 0.66 previously analyzed by Brown et al.32 and Holcomb et al.33 It is possible, however, that the optimal platelet/PRBC ratio may indeed be higher than 0.75.29,35,36
Although there has been much attention on cumulative blood product ratios, very little work to date has focused on how blood product ratios over time impacts survival.11 Recent studies have indicated an important element of timeliness in DCR, though.37 Meyer et al.38 used the PROPPR data set to demonstrate the association between every minute delay in the activation and arrival of the first blood cooler and mortality. Regardless of the ratio, there was a 5% increase in the odds of mortality for every minute transfusion was delayed. In our study, we evaluated the effect of both the cumulative ratio (≥0.75 or <0.75) and the duration of maintaining that ratio (≥40% or <40% resuscitation time) on survival. Ultimately, a 6-hour plasma/PRBC of at least 0.75 for at least 40% of the resuscitation time was independently associated with improved 24-hour and 30-day survival. Furthermore, for every 30 minutes, 1 hour, and 3 hours over this ratio, 24-hour survival was also improved.
The strength of this study is that these results were obtained from the prospective multicenter PROMMTT study conducted at 10 level 1 trauma centers that met rigorous selection criteria for study participation. Nonetheless, our secondary analysis of this study contains several important limitations. First, confounding variables unmeasured in this study included resuscitation adjuncts such as calcium,39 tranexamic acid,40 recombinant factor VIIa,41 fibrinogen,42 prothrombin complex concentrate,43 and vasopressin.44 Crystalloid was considered; however, it did not reach statistical significance. Second, relatively few patients received platelets, and the various MTPs used by participating PROMMTT sites provided platelets later in the resuscitation.12 In the original study, only 343 (38%) of study patients received platelets, and the first platelet transfusion was given at a median of 121 minutes (interquartile range, 80–187 minutes). As a result, our adjusted analysis on time-in-target for platelets/PRBCs may have been underpowered to detect a clinically meaningful survival difference with optimal platelet transfusion. Nonetheless, we were able to calculate an optimal platelet/PRBC ratio for 24-hour mortality adjusted for age, sex, systolic blood pressure, heart rate, Glasgow Coma Scale score, international normalized ratio, AIS head, AIS chest. Finally, Youden’s index may not be the ideal approach to determining an optimal blood product ratio since it weighs sensitivity and specificity equally. In evaluating MTPs and DCR, it may be better to optimize sensitivity to maximize survival. In addition, some literature has suggested that there may be a U-shape curve surrounding the optimal ratio.45,46 Youden’s index further limits our ability to assess for such relationship.
Prospective Observational Multicenter Major Trauma Transfusion initially showed a protective association with mortality and higher transfusions during the index 24 hours. Expanding on the initial 24 hours, our secondary analysis indicated a protective association when considering both the optimal ratio and time above that ratio at both 24 hours and at 30 days. Regarding the generalizability of these results, some may note that this study included a large proportion of patients with penetrating injuries and that all participating level I trauma centers had preexisting MTPs. Both observations are true; however, we do not believe that they limit the generalizability of our findings. The pragmatic nature of the PROMMTT study actually captured important practice variations in resuscitation that enhanced the richness of this data set.15 This feature of the data allowed us to identify a clearly superior resuscitation strategy that should prove effective for any patient with hemorrhagic shock and trauma-induced coagulopathy regardless of the original traumatic mechanism.
While PROPPR and PROMMTT were prospective trials analyzing transfusion ratios, there remains a paucity of prospective data regarding the impact of maintaining the optimal transfusion ratios.12,13 We believe that future DCR studies and performance improvement efforts should include prospectively collected resuscitation signatures. This signature would assess not only how quickly the team recognizes and responds to an exsanguinating patient but also how well they adhere to DCR principles throughout the resuscitation. Through the assessment of how well the team stays on-target, future damage-control metrics can be created and used as benchmarks in performance improvement efforts such as the American College of Surgery Trauma Quality Improvement Guidelines.
CONCLUSION
In this secondary analysis of the prospective observational PROMMTT study, we found that maintaining a high ratio of plasma/PRBC during DCR is independently associated with improved survival at 24 hours and 30 days. Performance improvement reviews and future prospective studies should capture time spent in a high-ratio range.
Supplementary Material
AUTHORSHIP
A.M.H., Z.G., C.L.M., B.S.A., S.H., P.Z.C., C.E.W., and J.W.C. designed the study. A.M.H., Z.G., D.S., D.R.S., and J.W.C. searched the literature. Z.G., E.E.F., C.E.W., and J.W.C. collected the data. A.M.H., Z.G., E.E.F., C.L.M., D.N.H., A.J.Y., C.E.W., and J.W.C. analyzed the data. A.M.H., Z.G., D.S., E.F., C.L.M., D.R.S., D.N.H., B.S.A., A.J.Y., S.H., P.Z.C., C.E.W., and J.W.C. participated in data interpretation. A.M.H., Z.G., D.R.S., and J.W.C. drafted the article, which all authors critically revised and approved.
ACKNOWLEDGMENT
This work is supported by the US Army Medical Research and Materiel Command under contract number W81XWH-18-C-0163.
DISCLOSURE
C.L.M. is an employee of Arcos Medical, Inc. and owns stock in Arcos Medical, Inc. The rest of the authors do not have any other personal or institutional interest with regard to the authorship and/or publications of this article.
The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
Footnotes
Published online: April 24, 2021.
This study was presented at the 50th Annual Meeting of Western Trauma Association, February 28 to March 5, 2021, in Big Sky, Montana (virtual).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Allyson M. Hynes, Email: allyson.hynes@pennmedicine.upenn.edu.
Zhi Geng, Email: zgeng@pennmedicine.upenn.edu.
Daniela Schmulevich, Email: daniela.schmulevich@pennmedicine.upenn.edu.
Erin E. Fox, Email: Erin.E.Fox@uth.tmc.edu.
Christopher L. Meador, Email: chris.meador@arcosmedical.com.
Dane R. Scantling, Email: Dane.Scantling@Pennmedicine.upenn.edu.
Daniel N. Holena, Email: Daniel.Holena@pennmedicine.upenn.edu.
Benjamin S. Abella, Email: Benjamin.Abella@pennmedicine.upenn.edu.
Andrew J. Young, Email: andrew.young@osumc.edu.
Sara Holland, Email: Sara.Holland@pennmedicine.upenn.edu.
Pamela Z. Cacchione, Email: pamelaca@nursing.upenn.edu.
Charles E. Wade, Email: Charles.E.Wade@uth.tmc.edu.
REFERENCES
- 1.Hashmi ZG, Haut ER, Efron DT, Salim A, Cornwell EE, 3rd, Haider AH. A target to achieve zero preventable trauma deaths through quality improvement. JAMA Surg. 2018;153(7):686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. [DOI] [PubMed] [Google Scholar]
- 3.Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370–379. [DOI] [PubMed] [Google Scholar]
- 4.Berwick D, Downey A, Cornett E. A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths after Injury. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 5.Eastridge BJ Hardin M Cantrell J, et al. Died of wounds on the battlefield: Causation and implications for improving combat casualty care. J Trauma. 2011;71(Suppl 1):S4–S8. [DOI] [PubMed] [Google Scholar]
- 6.Eastridge BJ Mabry RL Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S437. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, Browder T, Noguchi TT, Demetriades D. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63(6):1338–1346. [DOI] [PubMed] [Google Scholar]
- 8.Davis JS, Satahoo SS, Butler FK, Dermer H, Naranjo D, Julien K, Van Haren RM, Namias N, Blackbourne LH, Schulman CI. An analysis of prehospital deaths: who can we save? J Trauma Acute Care Surg. 2014;77(2):213–218. [DOI] [PubMed] [Google Scholar]
- 9.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(Suppl 6):S3–S11. [DOI] [PubMed] [Google Scholar]
- 10.King DR. Initial care of the severely injured patient. N Engl J Med. 2019;380(8):763–770. [DOI] [PubMed] [Google Scholar]
- 11.Cannon JW Khan MA Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:605–617. [DOI] [PubMed] [Google Scholar]
- 12.Holcomb JB Del Junco DJ Fox EE, et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holcomb JB Tilley BC Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camazine MN, Hemmila MR, Leonard JC, Jacobs RA, Horst JA, Kozar RA, Bochicchio GV, Nathens AB, Cryer HM, Spinella PC. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S48–S53. Lippincott Williams and Wilkins. [DOI] [PubMed] [Google Scholar]
- 15.Rahbar MH Fox EE Del Junco DJ, et al. Coordination and management of multicenter clinical studies in trauma: experience from the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study. Resuscitation. 2012;83(4):459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Time matters in 1: 1 resuscitations: concurrent administration of blood: plasma and risk of death. J Trauma Acute Care Surg. 2014;77(6):833–837. [DOI] [PubMed] [Google Scholar]
- 17.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. [DOI] [PubMed] [Google Scholar]
- 18.Rahbar E Fox EE Del Junco DJ, et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S16–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris T, Davenport R, Mak M, Brohi K. The evolving science of trauma resuscitation. Emerg Med Clin North Am. 2018;36(1):85–106. [DOI] [PubMed] [Google Scholar]
- 20.Cotton BA, Reddy N, Hatch QM, Lefebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bickell WH, Wall MJ, Jr., Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–1109. [DOI] [PubMed] [Google Scholar]
- 22.Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, Liscum KR, Wall MJ, Jr., Mattox KL. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652–663. [DOI] [PubMed] [Google Scholar]
- 23.Cotton BA, Guy JS, Morris JA, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115–121. [DOI] [PubMed] [Google Scholar]
- 24.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC, Moore E, Medicine P. Early predictors of postinjury multiple organ failure from the departments of surgery. Arch Surg. 1994;129(1):39–45. [DOI] [PubMed] [Google Scholar]
- 25.Holcomb JB. Transport time and preoperating room hemostatic interventions are important: improving outcomes after severe truncal injury. Crit Care Med. 2018;46(3):447–453. [DOI] [PubMed] [Google Scholar]
- 26.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–402. [DOI] [PubMed] [Google Scholar]
- 27.Sperry JL Ochoa JB Gunn SR, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–993. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–119. [DOI] [PubMed] [Google Scholar]
- 29.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197(5):565–570. [DOI] [PubMed] [Google Scholar]
- 30.Ho AM, Dion PW, Yeung JH, Holcomb JB, Critchley LA, Ng CS, Karmakar MK, Cheung CW, Rainer TH. Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116(3):716–728. [DOI] [PubMed] [Google Scholar]
- 31.Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–584. [DOI] [PubMed] [Google Scholar]
- 32.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cushieri J, Sperry JL. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73(2):358–364. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb JB Wade CE Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. [DOI] [PubMed] [Google Scholar]
- 34.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50(6):1370–1383. [DOI] [PubMed] [Google Scholar]
- 35.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328. [DOI] [PubMed] [Google Scholar]
- 36.Inaba K, Lustenberger T, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, Nelson J, Talving P, Demetriades D. The impact of platelet transfusion in massively transfused trauma patients. J Am Coll Surg. 2010;211(5):573–579. [DOI] [PubMed] [Google Scholar]
- 37.Meyer DE, Vincent LE, Fox EE, O’Keeffe T, Inaba K, Bulger E, Holcomb JB, Cotton BA. Every minute counts: time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg. 2017;83(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer DE, Cotton BA, Fox EE, Stein D, Holcomb JB, Cohen M, Inaba K, Rahbar E. A comparison of resuscitation intensity and critical administration threshold in predicting early mortality among bleeding patients: a multicenter validation in 680 major transfusion patients. J Trauma Acute Care Surg. 2018;85:691–696. Lippincott Williams and Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackay EJ, Stubna MD, Holena DN, Reilly PM, Seamon MJ, Smith BP, Kaplan LJ, Cannon JW. Abnormal calcium levels during trauma resuscitation are associated with increased mortality, increased blood product use, and greater hospital resource consumption: a pilot investigation. Anesth Analg. 2017;125(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113–119. [DOI] [PubMed] [Google Scholar]
- 41.Hauser CJ Boffard K Dutton R, et al. Results of the control trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. [DOI] [PubMed] [Google Scholar]
- 42.Wafaisade A, Lefering R, Maegele M, Brockamp T, Mutschler M, Lendemans S, Banerjee M, Bouillon B, Probst C. Administration of fibrinogen concentrate in exsanguinating trauma patients is associated with improved survival at 6 hours but not at discharge. J Trauma Acute Care Surg. 2013;74(2):387–395. [DOI] [PubMed] [Google Scholar]
- 43.McSwain N, Jr., Barbeau J. Potential use of prothrombin complex concentrate in trauma resuscitation. J Trauma. 2011;70(Suppl 5):S53–S56. [DOI] [PubMed] [Google Scholar]
- 44.Sims CA Holena D Kim P, et al. Effect of low-dose supplementation of arginine vasopressin on need for blood product transfusions in patients with trauma and hemorrhagic shock: a randomized clinical trial. JAMA Surg. 2019;154(11):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra B, Mori A, Cameron PA, Fitzgerald M, Paul E, Street A. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury. 2010;41(1):35–39. [DOI] [PubMed] [Google Scholar]
- 46.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


