Abstract
The ChAdOx1 nCoV-19 (ChA) (AstraZeneca) and Ad26.COV2.S (AD26) (Janssen) vaccines are virus-based coronavirus disease 2019 (COVID-19) vaccines used worldwide. In spring 2021, venous blood clots and thrombocytopenia were described in some vaccine recipients. We evaluated the frequency of severe adverse events (SAEs) documented in the EudraVigilance European database in young adult (18–64 years old) and older (≥65 years old) vaccine recipients up to 23 June 2021 and related them to coagulation disorders and arterial, cardiac, and nervous system events. Comparison between the frequency of SAEs and SAE-related deaths in ChA and AD26 vs. BNT162b2 COVID-19 (BNT) (Pfizer/BioNTech) vaccine recipients demonstrated: 1) ChA and AD26 recipients than BNT recipients had higher frequencies of not only SAEs caused by venous blood clots and hemorrhage, but also thromboembolic disease and arterial events, including myocardial infarction and stroke; 2) a corresponding higher frequency of SAE-related deaths. The frequency was higher in both young adults and older adults. Comparison between the frequency of SAEs and SAE-related deaths in AD26 vs. ChA recipients demonstrated in AD26 recipients: 1) lower frequency of thrombocytopenia; 2) lower frequency of SAEs in young adult recipients; 3) higher frequency of SAEs in older recipients. Interestingly, most of the venous thrombotic SAEs associated with ChA and AD26 vaccines were not associated with thrombocytopenia, suggesting that TTS (thrombosis with thrombocytopenia syndrome) is not the only type of thrombosis observed following virus-based vaccines. In conclusion, both virus-based COVID-19 vaccines show more SAEs than BNT, but the frequency of the SAE type in the different age groups differs, suggesting that the mechanisms responsible of SAEs overlap only partly.
Keywords: Virus-based COVID-19 vaccines, Severe adverse events, Venous thrombosis, Hemorrhage, Myocardial infarction
1. Introduction
Vaccines against severe acute respiratory syndrome coronavirus (SARS-CoV)-2 that cause an immune response against the SARS-CoV-2 spike protein are, at present, the primary method of fighting the coronavirus disease 2019 (COVID-19) pandemic. Real-world studies have described similar vaccine efficacy in preventing COVID-19 and severe COVID-19 [[1], [2], [3]]. To evaluate the benefit–risk profile of each vaccine, it is crucial to know the adverse events (AEs) each vaccine causes not only in the short-term but also in the mid/long-term, with particular reference to severe AEs (SAEs) and death. SAEs, consisting of events that occur extremely rarely in the healthy population, are promptly detected by pharmacovigilance, but SAEs consisting of events that happen relatively frequently in the population may be more challenging to discover because attributed to underlying disease and underreported [[4], [5], [6], [7]].
In March 2021, the ChAdOx1 nCoV-19 COVID-19 adenovirus-based vaccine (ChA) produced by AstraZeneca was associated with blood clots in unusual sites and bleeding events [8]. Subsequently, Greinacher and colleagues demonstrated thrombocyte aggregation in the presence of anti-platelet factor 4 antibodies (anti-PF4) [9] that were produced in response to SARS-CoV-2 spike protein, adenovirus proteins, and a co-factor present in ChA but not in the Ad26.COV2.S vaccine (AD26) (manufactured by Janssen), another adenovirus-based vaccine [10]. Kowarz and colleagues suggest in a version of a manuscript that has not completed journal peer review that a spliced spike soluble protein derived from the codon-optimized DNA present in the ChA and AD26 vaccines binds to ACE2 and promotes antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of endothelial cells when the recipients produce anti-spike antibodies [11].
In a further document, the European Medicine Agency (EMA) stated that ChA might cause cerebral venous sinus thrombosis (CVST), splanchnic venous thrombosis (SVT), and/or other rare thromboses in the presence of thrombocytopenia (thrombosis with thrombocytopenia syndrome, TTS) in about 10 ChA recipients out of one million doses (OMD) [12]. Presently, the EMA reports that thrombosis in combination with thrombocytopenia may occur in fewer than 1 in 10,000 people (the formal definition of the rarest AE) so that the actual frequency is not specified [13].
In a recent publication, we demonstrate that SAEs in ChA recipients are not only related to CVST and SVT, but also other blood clots and bleedings. In particular, we observed 151 and 33 SAEs/OMD in ChA recipients and recipients of BNT162b2 COVID-19 (BNT) vaccine (manufactured by Pfizer/BioNTech) based on nanoparticle mRNA-based technology [14], respectively. This observation means there are more blood clots and bleedings in ChA vs. BNT recipients of >1 SAE out of 10,000 doses [15].
In AD26 recipients as well, CVST was observed in about 1 recipient/OMD [16]. The EMA reports that thrombosis in combination with thrombocytopenia may occur in fewer than 1 in 10,000 people (the definition of the rarest AE) [17], but the frequency is not specified. Another EMA document reports that fatal outcomes have been reported, but again does not specify the frequency [18]. Therefore, it is unclear whether ChA and AD26 recipients have a similar frequency of blood clots and bleedings.
In the present manuscript, we compared the frequency of SAEs reported in the EudraVigilance European database [19] following the administration of the ChA, AD26, or BNT. In particular, we investigated not only the frequency of SAEs related to coagulation disorders (venous thrombotic and hemorrhagic events, including thrombocytopenia) but also to arterial, cardiac, and nervous system events. To discover whether SAE frequency differed from the baseline frequency of events in the unvaccinated population, we hypothesized that the BNT vaccine does not cause SAEs and that the SAEs observed in BNT recipients represent the baseline event frequency [15].
The specific objectives of the study were to verify: i) whether ChA and AD26 recipients have differing frequency of coagulation disorder-related SAEs and deaths; ii) whether the risk of virus-based vaccines is limited to thrombotic/hemorrhagic events or involves arterial events; iii) the frequency of SAEs and SAE-related deaths caused by cardiac and nervous system events in ChA and AD26 recipients.
2. Material and methods
2.1. Data source
Data on reported AEs after administration of the ChA [reported as “COVID-19 VACCINE ASTRAZENECA (CHADOX1 NCOV-19)”], AD26 [reported as “COVID-19 VACCINE JANSSEN (AD26.COV2.S)”] or BNT [reported as “COVID-19 MRNA VACCINE PFIZER-BIONTECH (TOZINAMERAN)”] vaccines were obtained from EudraVigilance [19] using the in-site tools Number of Individual Cases and Number of Individual Cases By Reaction Groups – By Seriousness. For in-depth analyses of SAEs, a list of single case reports was obtained using the Line Listing in-site tool using the parameters specified below. The data were downloaded from EudraVigilance on 23 June 2021.
Data on the total number of ChA, AD26, and BNT vaccine doses administered were downloaded from the European Centre for Disease Prevention and Control (ECDC) database COVID-19 Vaccine Tracker tool [20] on 21 June 2021, and were equal to 48,071,531 (ChA), 5,891,506 (AD26), and 213,021,122 (BNT) doses.
The vaccine administration data of the different age ranges were downloaded from the ECDC database and referred to “Vaccine rollout overview week 23rd, 2021” (www.ecdc.europa.eu/en/covid-19/vaccine-roll-out-overview).
2.2. Parameters for downloading SAE single case reports
A specific search was undertaken using the EudraVigilance Line Listing in-site tool using the following parameters:
-
•
Seriousness: Serious
-
•
Geographic Origin: All selected
-
•
Reporter Group: All selected
-
•
Sex: All selected
-
•
Age Group: All selected
-
•
Reaction Groups: All selected
-
•
Reported Suspected Reaction: Keywords specified below
-
•
Gateway Date: 2021
The keywords we used for the AE case report download are reported in the Supplementary material (Tables S1–S6).
The output of the Line Listing tool is an Excel™ table (Microsoft, Redmond, WA, USA) in which we reported AE single case reports, specifying: EU Local Number, Patient Age Group, Patient Sex, Reaction List PT (Duration – Outcome – Seriousness Criteria), Suspect/interacting Drug List (Drug Char – Indication PT – Action taken – [Duration – Dose – Route]), and the link for downloading the original single case report form.
2.3. Analyses of AE reaction groups
We used the EudraVigilance in-site tool Number of Individual Cases By Reaction Groups – By Seriousness to assess the absolute number of non-SAEs and SAEs for all vaccines. Data were organized by the tool into reaction groups. Eleven reaction groups were considered for further analyses.
2.4. Data normalization
2.4.1. Data normalization using the total number of administered doses
The total number of administered doses for all vaccines was downloaded on 21 June 2021, from the ECDC database using the COVID-19 Vaccine Tracker tool. Therefore, the number of events (recipients with AEs, SAEs, or death)/OMD was calculated using the following formula:
2.4.2. Normalization of AE data of administered doses by age range
For all vaccines, the number of administered doses in the different age ranges was obtained from the “COVID-19 Vaccine rollout overview week 23rd, 2021” data from the ECDC database for the available countries (25 out of 30) (Table S7). Not all European countries provided these data. Hence, we created six simulations to extend the available data to all of Europe. One of these simulations, “European mean”, considered the data of all 25 countries to calculate the percentage of administration in each age range. Each of the other five simulations considered the data of 5 of the 25 available countries chosen in a stochastic manner. For selecting the countries used in each of the five simulations, all 25 European countries were numbered using the “ = RAND()” formula provided by Excel. Therefore, we selected only the five countries with the highest numbers to calculate the percentage of administration in each age range. The countries selected in the five simulations were: Austria, Croatia, the Czech Republic, Luxembourg, and Portugal (Simulation 1); Belgium, the Czech Republic, Ireland, Italy, and Romania (Simulation 2); Bulgaria, Iceland, Italy, Malta, and Romania (Simulation 3); Greece, Ireland, Malta, Romania, and Sweden (Simulation 4); and Estonia, Latvia, Romania, Slovakia, and Sweden (Simulation 5). For all vaccines, the overall number of doses administered was distributed in the age ranges using the fraction of administered doses in each age range obtained from the six simulations with the following formula:
Consequently, the number of doses administered to each age range was obtained by multiplying the total number of administered vaccines according to the fraction of administered doses.
Data on the SAE distribution in each age range were obtained directly from the table generated by the Line Listing tool (the age ranges required for analyses were 18–64 years and ≥65 years).
Each datum on AE frequency normalized for the two age ranges was calculated six times using the percentage of the administered dose obtained in the six simulations and was reported as the mean (with SD or 95% confidence interval [CI]) of the obtained results.
2.5. Statistical analyses
Statistical analyses were conducted using Prism v.9.1.2 (GraphPad, San Diego, CA, USA). We evaluated the differences between the distribution of the SAEs and death events following ChA, AD26, and BNT vaccination in the two age ranges. The Kolmogorov–Smirnov normality test was used on data from each group before statistical evaluation. When three experimental groups were compared, if the Kolmogorov–Smirnov normality test was passed, significance was calculated using the Welch and Brown-Forsythe one-way analysis of variance (ANOVA) and Dunnett T3 multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values were reported. If the Kolmogorov–Smirnov normality test failed, significance was calculated using the Kruskal-Wallis test and Dunn's multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values were reported. When two experimental groups were compared, the unpaired t-test with Welch's correction was used if the Kolmogorov–Smirnov normality test passed, and the Mann–Whitney test was used if the Kolmogorov–Smirnov normality test failed.
3. Results
3.1. The frequency of SAEs and death following vaccination is higher in ChA and AD26 than in BNT recipients
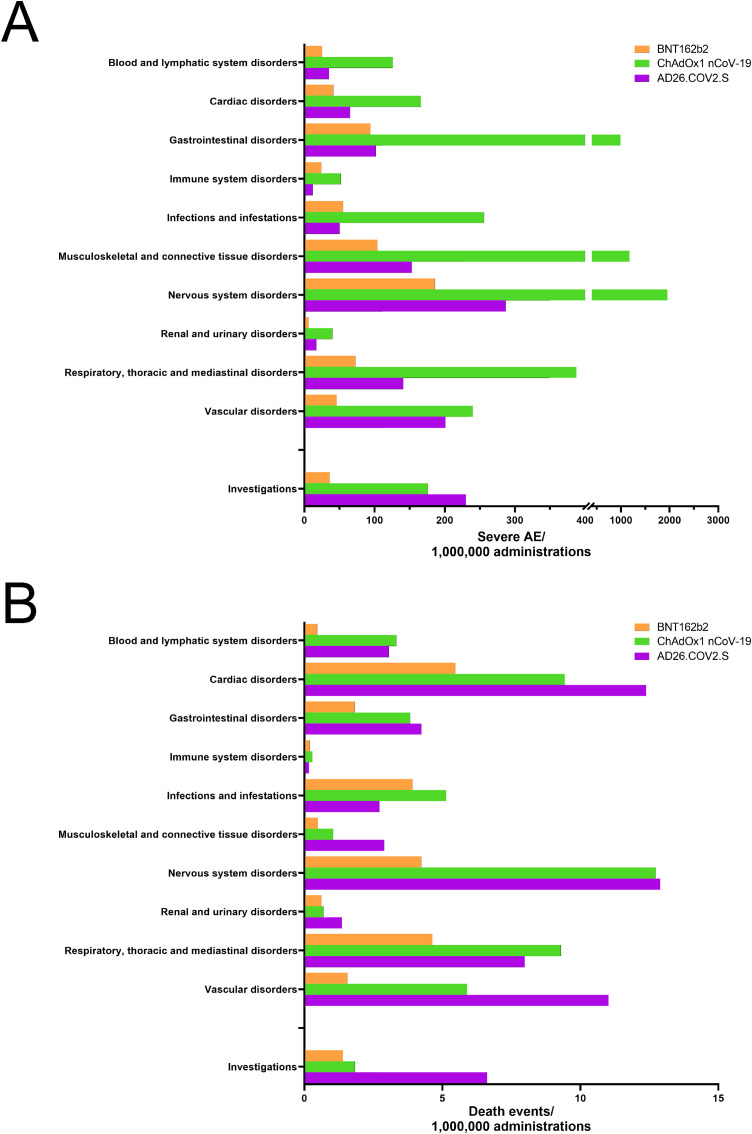
We first evaluated the number of SAEs reported by the European EudraVigilance databank and normalized it with the number of doses of each vaccine administered in European countries. We detected 9126, 1911, and 1104 SAEs/OMD in the ChA, AD26, and BNT recipients, respectively. To understand which body part showed higher SAE frequency in the ChA and AD26 recipients as compared to BNT recipients, we evaluated SAEs and deaths affecting different organs/tissues/systems (10 AE reaction groups), as reported by EudraVigilance following normalization with the number of doses of each vaccine administered in European countries (Fig. 1 A and B). We also considered the frequency of SAEs and death that required instrumental diagnostics (AE reaction group “investigations”).
Fig. 1.
Frequency of individual cases with AEs divided into reaction groups following BNT, AD26, or ChA vaccination. The frequency of individual cases of AEs organized by reaction groups, as reported in the EudraVigilance database, was calculated by normalizing the number of individual cases with the vaccine doses administered in Europe. SAEs (A) and SAE-related deaths (B) are reported. For focusing on life-threatening events, reaction groups with >2 deaths/OMD (the mean between ChA and AD26 recipients) are reported, except for “surgical and medical procedures” and “general disorders and administration site conditions”, which mainly refer to the vaccine inflammatory response. “Renal and urinary disorders” and “immune system disorder” are also reported despite the occurrence of <2 deaths/OMD (the mean between ChA and AD26 recipients).
Both SAEs and deaths were more frequent in ChA and AD26 recipients than in BNT recipients in all reaction groups except for “immune system disorders” and “infection and infestation”. Of note, vascular disorder-related deaths were 3.8- and 7.1-fold more frequent in ChA and AD26 recipients, respectively, compared to BNT recipients. In several reaction groups, AD26 recipients had lower SAE frequency than ChA recipients (Fig. 1A), but in the same reaction groups, AD26 and ChA recipients had similar frequencies of death (Fig. 1B). In particular, “cardiac disorders”, “nervous system disorders”, and “vascular disorders” caused a death frequency of >1/100,000 doses in AD26 recipients.
3.2. AD26 and ChA recipients had a higher frequency of SAEs and SAE-related death related to coagulation disorders and arterial, cardiac, and nervous system events than BNT recipients
Considering that most of the deaths following the virus-based vaccines were due to “cardiac disorders”, “nervous system disorders”, “respiratory, thoracic and mediastinal disorder”, and “vascular disorders” (Fig. 1A), and some recipients of the virus-based vaccines showed thrombocytopenia and venous blood clots possibly responsible for the abovementioned SAEs, we evaluated the SAEs and death events related to coagulation disorders (venous thrombotic, hemorrhagic events, thrombocytopenia) and arterial, cardiac, and nervous system events normalized with the number of doses administered (Figure S1) in vaccine recipients. AD26 and ChA recipients had much higher frequencies of SAEs and SAE-related death in each group of SAEs than BNT recipients. The hazard ratio (HR) of AD26 and ChA as compared to BNT ranged between 1.57 and 12.87 for SAEs and between 2.19 and 18.81 for death (Table 1 ).
Table 1.
HRs of specific SAEs and SAE-related deaths following ChA and AD26 vaccination, using BNT vaccination as the baseline reference.
| SAEs |
Death events |
|||
|---|---|---|---|---|
| ChA/BNT | AD26/BNT | ChA/BNT | AD26/BNT | |
| Venous thrombotic events | 7.03 | 8.34 | 7.63 | 5.96 |
| Hemorrhagic events | 5.74 | 3.01 | 4.01 | 2.85 |
| Thrombocytopenia | 12.87 | 7.61 | 18.81 | 12.31 |
| Arterial events | 5.11 | 8.28 | 3.71 | 5.44 |
| Cardiac events | 2.72 | 1.57 | 2.19 | 2.22 |
| Nervous system events | 4.13 | 4.22 | 4.05 | 3.73 |
To evaluate the data correctly, we needed to consider that the frequency of hemorrhagic, arterial, cardiac, and nervous system events in the general population (unvaccinated) increases with age. Such events are considered SAEs when occur following drug administration (in this case, vaccines). Therefore, even if the vaccine does not cause any AEs, we expect more SAEs in an older vaccinated population than in a young one. To evaluate whether the comparison among vaccine SAEs is correct, we evaluated the age of European people vaccinated with each vaccine. Not all European countries report to the ECDC the number of doses of a specific vaccine administered to people within an age range; therefore, the recipient age for less than half of the administered doses is known. Therefore, we inferred the number of people vaccinated in each age range with BNT, ChA, or AD26 by knowing the number of doses administered to the whole European population (with/without indication of the age range) and the percentage of vaccinated people in a particular age range, calculated based on the information from the abovementioned countries. Figure S2A shows that the BNT vaccine has been administered to people aged 18–59 years at a similar percentage to the ChA vaccine but smaller than that of the AD26 vaccine. Consequently, the AD26 vaccine has been administered to people aged ≥60 years at a lower percentage than the BNT and ChA vaccines. Moreover, the BNT vaccine has been administered much more often to people aged ≥70 years (Figure S2A) and ≥80 years (Figure S2C) than the ChA and AD26 vaccines. Therefore, in the hypothesis that vaccines do not cause SAEs, it is expected to find fewer SAEs in AD26 than ChA recipients and fewer SAEs in ChA than BNT recipients when the SAEs and deaths of the whole vaccinated population are evaluated. In the few cases in which events in the unvaccinated population are more frequent in young rather than older adults, the opposite would occur. This makes it difficult to compare the data.
To make the data comparable, we evaluated the SAEs and deaths in two age ranges: young adults (18–64 years old) and older adults (≥65 years old). The age distribution of vaccines in the young adults was similar among the three vaccines (0.3–11.2% difference among the same age range) (Figure S2B), minimizing the risk of bias due to the administration of different vaccines to people of different ages. The age distribution of vaccines in the older adults was similar between AD26 and ChA (no more than 5% differences among the same age range) (Figure S2C), rendering the SAEs and deaths following the administration of these vaccines comparable. On the contrary, among the older adults, about two-thirds of BNT doses had been administered to people aged ≥70 years, different from the AD26 and ChA vaccines, which have been administered mainly to people aged 60–69 years. Therefore, in the hypothesis that vaccines do not cause SAEs, it is expected to find more SAEs and deaths in BNT rather than AD26 and ChA older adult recipients. The expectation is enforced by the fact that in several countries, more BNT than ChA and AD26 doses were administered to people with co-morbidities [15].
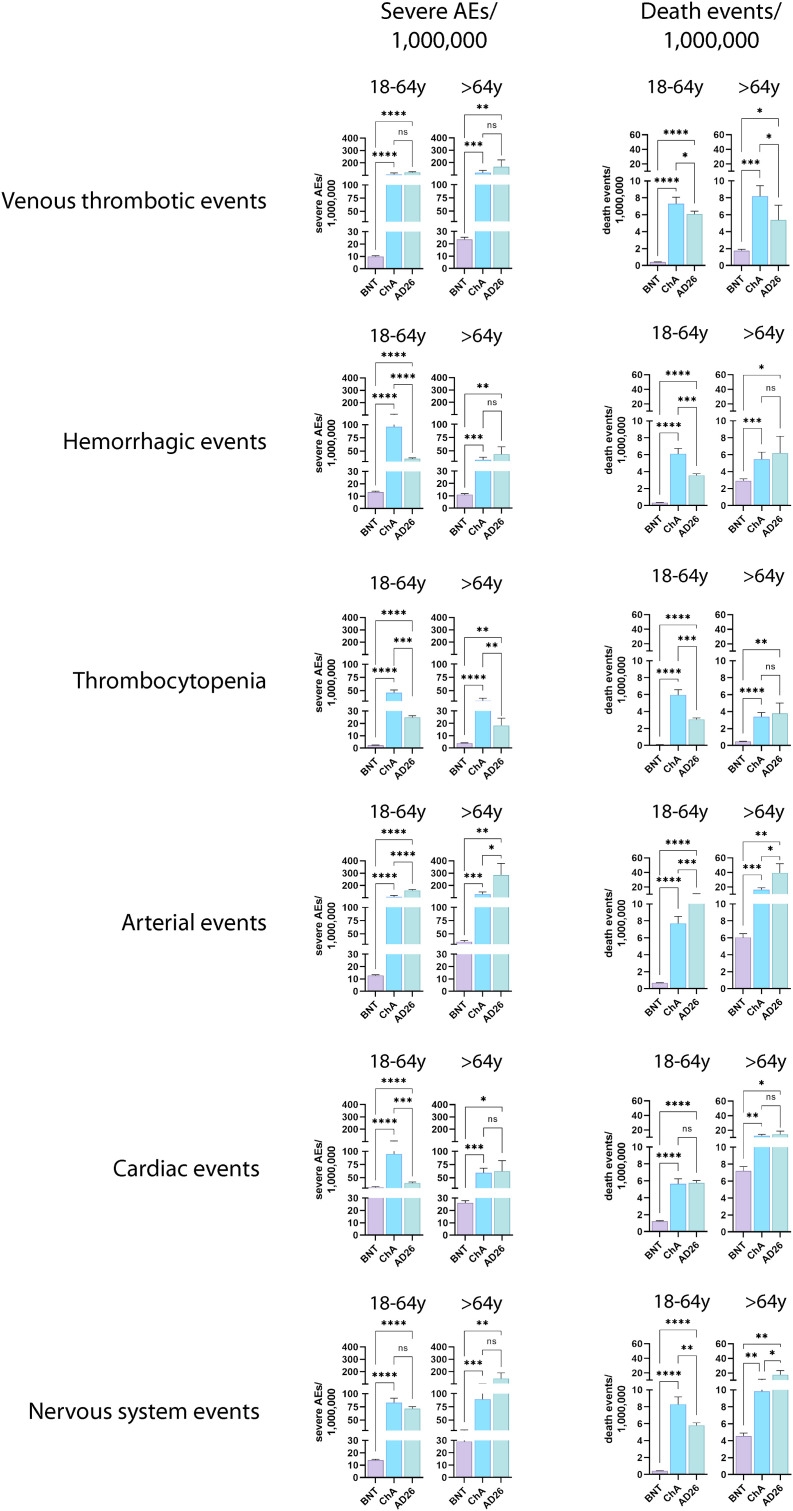
Fig. 2 and Table S8 and S9 show the normalized frequency of coagulation disorders, arterial, cardiac, and nervous system events, and SAE-related deaths in young adult and older adult BNT, ChA, and AD26 recipients. The frequency of events and SAE-related deaths in each event and age group was significantly higher in AD26 and ChA rather than BNT recipients, despite the bias concerning the more frequent administration of the BNT vaccine to older adults.
Fig. 2.
Frequency of individual cases with specific SAEs and SAE-related deaths among BNT, ChA, and AD26 recipients divided according to age group. The frequency of individual cases with specific AEs divided according to age range (18–64 years and >64 years) was obtained by normalizing the number of individual cases with the doses apparently administered to each age range in Europe at week 23 (Table S7). We needed to evaluate the doses apparently administered to each age range in Europe. Hence, we considered the doses administered by the European countries providing data on the administration of each vaccine to these age ranges and established a method for evaluating the variance of the doses (see the Method section for details). The figure shows the frequency (mean ± SD) of individual cases with SAEs and SAE-related deaths consisting of coagulation disorders (venous thrombotic and hemorrhagic events, thrombocytopenia) and arterial, cardiac and nervous system events. Significance was calculated using the Welch and Brown-Forsythe one-way ANOVA; the difference between ChA and BNT, AD26 and BNT, and ChA and AD26 was assessed with Dunnett T3 multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values are reported. ns (not significant) = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Among the most relevant SAEs in the ChA and AD26 recipients, venous thrombotic SAEs were detected at a similar frequency (about 1–2 SAEs out of 10,000 doses) in young adults and older adults. Surprisingly, an even higher frequency of arterial events was observed in both ChA and AD26 recipients, but the frequency of these SAEs in AD26 recipients, with particular reference to older adults, was higher than in ChA recipients. Indeed, in AD26 recipients, arterial-related events were responsible for about four deaths out of 100,000 doses. A high frequency of cardiac event-related death was observed in older adult ChA and AD26 recipients, showing about one death out of 100,000 doses. Interestingly, young adult ChA recipients had more cardiac-related SAEs than young adult BNT and AD26 recipients, with AD26 recipients showing similar SAE frequency to BNT recipients. However, the frequency of cardiac event-related death in young adult recipients was similar for ChA and AD26 recipients and higher than for BNT recipients. A high frequency of nervous system events was observed in ChA and AD26 recipients. In particular, older adult AD26 recipients showed high SAE frequency, leading to about two deaths out of 100,000 doses. Notably, the ChA recipients showed more SAEs than AD26 recipients for hemorrhagic events (young adults only) and thrombocytopenia, with a two times higher frequency.
3.3. The frequency of SAEs and SAE-related deaths derived from thrombosis and coagulation disorders is much higher than the TTS frequency in AD26 and ChA recipients
The frequency of vaccine recipients with SAEs or death by venous thrombosis, hemorrhage, and/or thrombocytopenia was equal to 195 SAEs/OMD and 12.1 deaths/OMD in ChA recipients, as expected [15], and was slightly lower in AD26 recipients (173 SAEs/OMD and 9.5 deaths/OMD) (Table S10). In both the ChA and AD26 recipients, thrombocytopenia frequency was much lower than that for venous thrombosis, suggesting that not all people with venous thrombotic events had thrombocytopenia. Moreover, not all recipients with thrombocytopenia had thrombosis (Figure S3). Therefore, the frequency of vaccine recipients with TTS must be lower than that of recipients with thrombosis.
To evaluate the frequency of TTS in ChA and AD26 recipients, we evaluated the frequency of recipients with thrombosis and thrombocytopenia. The analysis suggested that TTS frequency was equal to 19.4 SAEs/OMD in ChA recipients, causing 3.6 deaths/OMD, and was lower in AD26 recipients (11.2 SAEs/OMD, causing 2.0 deaths/OMD). As expected, in both ChA and AD26 recipients, TTS was more frequent in young adults than in older adults (Table S10). Therefore, in ChA recipients, TTS was responsible for about 20% of the venous thrombotic SAEs and 10% of the SAEs caused by venous thrombosis, hemorrhage, and/or thrombocytopenia (Table S10). In AD26 recipients, the percentages were even lower (Table S10).
3.4. Thromboembolic disease is the most frequent venous thrombotic SAE in ChA and AD26 recipients
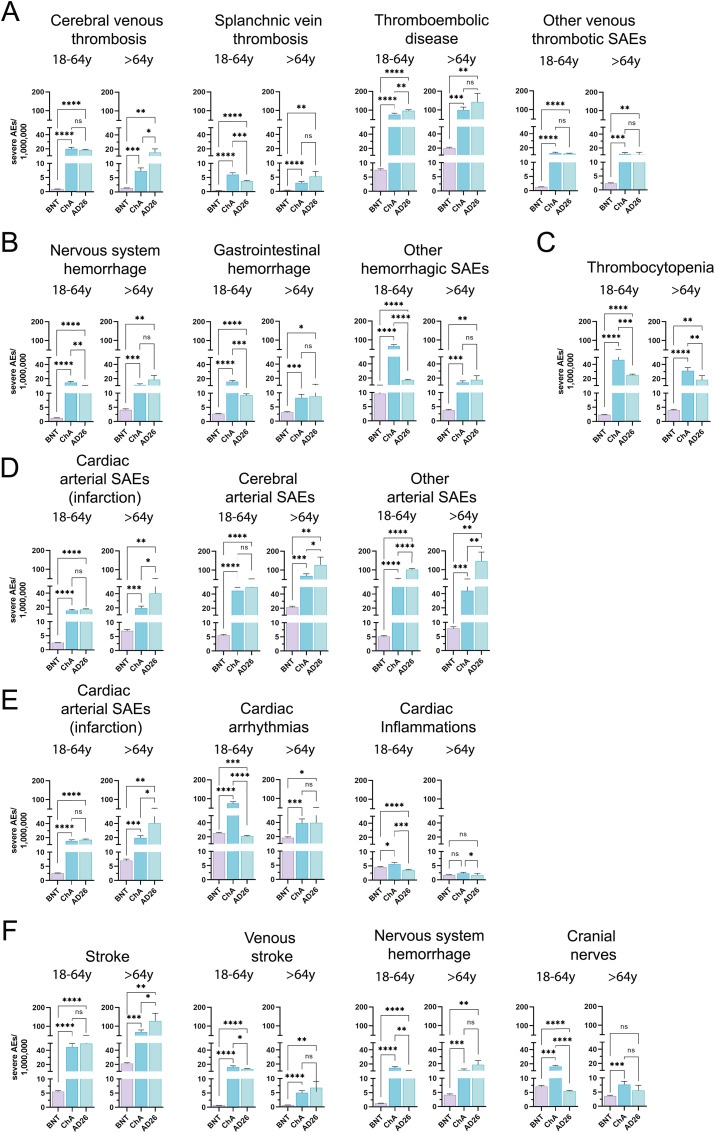
We evaluated the venous thrombotic SAEs of vaccines in all (Table S12), young adult (18–64 years old), and older adult (≥65 years old) recipients (Fig. 3 A). Cerebral venous thrombosis (CVT) was virtually absent in BNT recipients and was observed at a similar frequency in young adult ChA and AD26 recipients (about 20 SAEs/OMD). As expected, CVT was more frequent in young adults than in older adult ChA recipients [15] but showed a similar frequency in young adult and older adult AD26 recipients. Among the ChA and AD26 recipients, there were higher numbers of CVT-related deaths in young adults (2–4 deaths/OMD) rather than older adult recipients (about 1 death/OMD) (Fig. 4 A). If the CVT frequency in BNT recipients was considered equal to the baseline event frequency in unvaccinated people, the HR in the young adult ChA and AD26 recipients was about 22 and 20, respectively (Table 2 ).
Fig. 3.
Frequency of individual cases with specific SAEs among BNT, ChA, and AD26 recipients divided according to age group. The frequency of individual cases with specific AEs divided according to age range (18–64 years and >64 years) was obtained by normalizing the number of individual cases with the doses supposedly administered to each age range in Europe at week 23 (Table S7). To do this, we considered the doses administered by the European countries providing data on the administration of each vaccine to these age ranges and established a method for evaluating the variance of the doses (see the Method section for details). The figure shows the frequency (mean ± SD) of individual cases with SAEs consisting of (A) venous thrombotic SAEs (CVT, SVT, thromboembolic disease, other venous thrombotic events); (B) hemorrhagic SAEs (nervous system hemorrhage, gastrointestinal hemorrhage, other hemorrhagic events); (C) thrombocytopenia; (D) arterial SAEs (cardiac arterial events – myocardial infarction, cerebral arterial events, other arterial events); (E) cardiac SAEs (cardiac arterial events – myocardial infarction, arrhythmias, cardiac inflammation); myocardial infarction SAEs are also reported in (D); (F) nervous system SAEs (stroke, venous stroke, nervous system hemorrhage, cranial nerves); nervous system hemorrhage is also reported in (B). Significance was calculated using the Welch and Brown-Forsythe one-way ANOVA test; the difference between ChA and BNT, AD26 and BNT, and ChA and AD26 was assessed using Dunnett T3 multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values are reported. ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
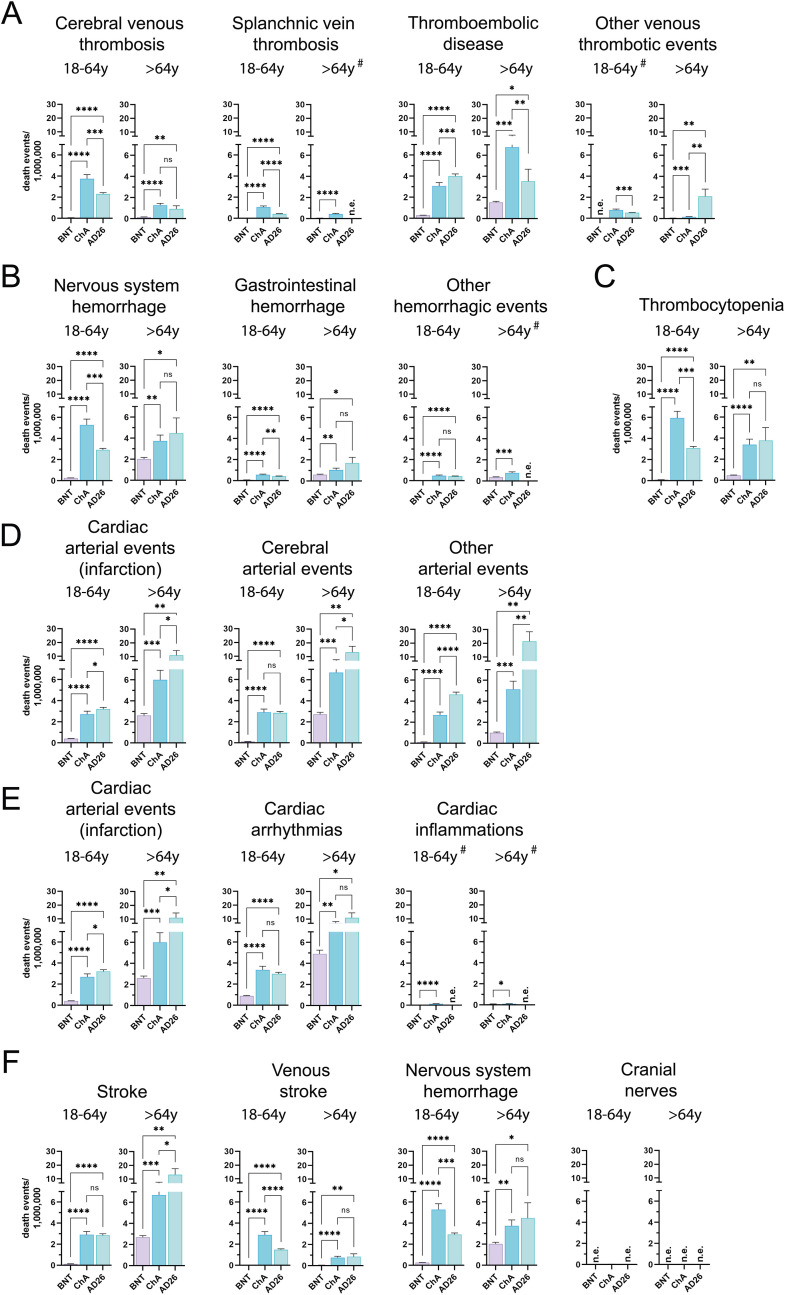
Fig. 4.
Frequency of individual cases with specific SAE-related death among BNT, ChA, and AD26 recipients divided according to age group. The frequency of individual cases with specific AEs divided according to age range (18–64 years and >64 years) was obtained by normalizing the number of individual cases with the doses supposedly administered to each age range in Europe at week 23 (Table S7). To do this, we considered the doses administered by the European countries providing data on the administration of each vaccine to these age ranges and established a method for evaluating the variance of the doses (see the Method section for details). The figure shows the frequency (mean ± SD) of individual cases with deaths related to SAEs consisting of (A) venous thrombotic SAEs (CVT, SVT, thromboembolic disease, other venous thrombotic events); (B) hemorrhagic SAEs (nervous system hemorrhage, gastrointestinal hemorrhage, other hemorrhagic events); (C) thrombocytopenia; (D) arterial SAEs (cardiac arterial events – myocardial infarction, cerebral arterial events, other arterial events); (E) cardiac SAEs (cardiac arterial events – myocardial infarction, arrhythmias, cardiac inflammation); myocardial infarction SAEs are also reported in (D); (F) nervous system SAEs (stroke, venous stroke, nervous system hemorrhage, cranial nerves); nervous system hemorrhage is also reported in (B). When deaths were observed for all three vaccines, significance was calculated using the Welch and Brown-Forsythe one-way ANOVA test; the difference between ChA and BNT, AD26 and BNT, and ChA and AD26 was assessed with Dunnett T3 multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values are reported. When deaths were observed for only two vaccines (#), the unpaired t-test with Welch's correction was used. ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Table 2.
HRs of specific SAEs and SAE-related deaths in the age groups following ChA and AD26 vaccination, using BNT vaccination as the baseline reference.
| HR of SAEs mean ± SD (95% CI) |
HR of death events mean ± SD (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 18-64 y |
>65 y |
18-64 y |
>65 y |
|||||
| ChA/BNT | AD26/BNT | ChA/BNT | AD26/BNT | ChA/BNT | AD26/BNT | ChA/BNT | AD26/BNT | |
| Cerebral venous thrombosis | 21.59 ± 2.05 (19.44–23.74) |
19.78 ± 1.20 (18.52–21.04) |
5.45 ± 0.70 (4.72–6.18) |
11.34 ± 3.70 (7.46–15.22) |
57.00 ± 5.42 (51.32–62.69) |
35.33 ± 2.14 (33.08–37.58) |
8.17 ± 1.04 (7.08–9.27) |
5.99 ± 1.95 (3.94–8.04) |
| Splanchnic vein thrombosis | 14.58 ± 1.38 (13.13–16.03) |
9.09 ± 0.55 (8.51–9.67) |
7.05 ± 0.90 (6.11–7.99) |
12.52 ± 4.08 (8.24–16.80) |
18.49 ± 1.76 (16.65–20.34) |
7.40 ± 0.45 (6.93–7.87) |
7.99 ± 1.02 (6.92–9.06) |
N.A.a |
| Thromboembolic disease | 10.09 ± 0.96 (9.08–11.10) |
12.85 ± 0.78 (12.03–13.67) |
5.03 ± 0.64 (4.36–5.70) |
7.19 ± 2.34 (4.73–9.65) |
10.58 ± 1.01 (9.53–11.64) |
13.85 ± 0.84 (12.97–14.73) |
4.39 ± 0.56 (3.81–4.98) |
2.32 ± 0.76 (1.52–3.11) |
| Other venous thrombotic events | 9.53 ± 0.91 (8.58–10.48) |
9.17 ± 0.56 (8.58–9.75) |
4.83 ± 0.62 (4.18–5.47) |
4.38 ± 1.43 (2.88–5.88) |
N.A.b | N.A.b | 3.03 ± 0.39 (2.63–3.44) |
38.42 ± 12.52 (25.28–51.56) |
| Nervous system hemorrhage | 11.42 ± 1.09 (10.28–12.56) |
7.98 ± 0.48 (7.47–8.49) |
2.63 ± 0.34 (2.28–2.98) |
4.49 ± 1.46 (2.95–6.02) |
20.62 ± 1.96 (18.56–22.67) |
11.41 ± 0.69 (10.68–12.14) |
1.84 ± 0.23 (1.60–2.09) |
2.22 ± 0.72 (1.46–2.98) |
| Gastrointestinal hemorrhage | 5.63 ± 0.53 (5.07–6.19) |
3.33 ± 0.20 (3.12–3.55) |
2.63 ± 0.33 (2.28–2.98) |
2.81 ± 0.92 (1.85–3.77) |
7.57 ± 0.72 (6.82–8.33) |
5.67 ± 0.34 (5.31–6.03) |
1.73 ± 0.22 (1.50–1.97) |
2.80 ± 0.91 (1.85–3.76) |
| Other hemorrhagic events | 7.06 ± 0.67 (6.36–7.76) |
1.80 ± 0.11 (1.69–1.92) |
3.67 ± 0.47 (3.18–4.16) |
4.67 ± 1.53 (3.07–6.27) |
26.98 ± 2.57 (24.29–29.67) |
23.75 ± 1.45 (22.23–25.27) |
2.02 ± 0.26 (1.75–2.28) |
N.A.a |
| Thrombocytopenia | 19.47 ± 1.85 (17.52–21.41) |
10.48 ± 0.64 (9.81–11.15) |
7.72 ± 0.98 (6.69–8.74) |
4.60 ± 1.50 (3.03–6.18) |
63.37 ± 6.02 (57.05–69.69) |
32.98 ± 2.00 (30.88–35.08) |
7.20 ± 0.92 (6.23–8.16) |
8.07 ± 2.63 (5.31–10.83) |
| Cardiac arterial events (infarction) | 6.05 ± 0.57 (5.45–6.65) |
6.74 ± 0.41 (6.31–7.17) |
2.79 ± 0.35 (2.41–3.16) |
5.81 ± 1.89 (3.82–7.80) |
6.64 ± 0.63 (5.98–7.30) |
7.83 ± 0.47 (7.33–8.32) |
2.30 ± 0.29 (1.99–2.61) |
4.23 ± 1.38 (2.78–5.67) |
| Cerebral arterial events | 8.05 ± 0.76 (7.24–8.85) |
8.91 ± 0.54 (8.34–9.48) |
3.26 ± 0.42 (2.83–3.70) |
6.03 ± 1.96 (3.96–8.09) |
21.07 ± 2.00 (18.97–23.17) |
20.68 ± 1.26 (19.36–22.0) |
2.46 ± 0.31 (2.13–2.79) |
4.89 ± 1.59 (3.22–6.56) |
| Other arterial events | 9.79 ± 0.93 (8.82–10.77) |
19.73 ± 1.20 (18.47–20.98) |
5.60 ± 0.71 (4.85–6.34) |
18.66 ± 6.08 (12.28–25.04) |
20.20 ± 1.92 (18.19–22.22) |
34.81 ± 2.12 (32.59–37.03) |
5.10 ± 0.65 (4.41–5.78) |
21.34 ± 6.95 (14.05–28.64) |
| Cardiac arterial events (infarction) | 6.05 ± 0.57 (5.45–6.65) |
6.74 ± 0.41 (6.31–7.17) |
2.79 ± 0.35 (2.41–3.16) |
5.81 ± 1.89 (3.82–7.80) |
6.55 ± 0.62 (5.89–7.20) |
7.83 ± 0.47 (7.33–8.32) |
2.30 ± 0.29 (2.00–2.61) |
4.23 ± 1.38 (2.78–5.67) |
| Cardiac arrhythmias | 2.99 ± 0.28 (2.70–3.29) |
0.82 ± 0.05 (0.77–0.87) |
2.14 ± 0.27 (1.85–2.42) |
2.17 ± 0.71 (1.43–2.91) |
3.79 ± 0.36 (3.41–4.17) |
3.37 ± 0.20 (3.16–3.59) |
1.46 ± 0.19 (1.26–1.65) |
2.26 ± 0.74 (1.49–3.04) |
| Cardiac inflammations | 1.25 ± 0.12 (1.12–1.37) |
0.80 ± 0.05 (0.75–0.84) |
1.32 ± 0.17 (1.15–1.50) |
0.97 ± 0.32 (0.64–1.31) |
3.37 ± 0.32 (3.03–3.70) |
N.A.a | 1.22 ± 0.16 (1.05–1.38) |
N.A.a |
| Stroke | 8.11 ± 0.75 (7.32–8.90) |
8.99 ± 0.67 (8.29–9.70) |
3.29 ± 0.44 (2.83–3.75) |
6.06 ± 1.92 (4.04–8.08) |
21.23 ± 1.97 (19.17–23.30) |
20.86 ± 1.54 (19.24–22.48) |
2.48 ± 0.33 (2.13–2.83) |
4.92 ± 1.56 (3.28–6.56) |
| Venous stroke | 24.83 ± 2.36 (22.35–27.31) |
20.21 ± 1.23 (18.92–21.50) |
7.59 ± 0.97 (6.57–8.60) |
10.36 ± 3.38 (6.82–13.90) |
58.66 ± 5.58 (52.80–64.51) |
30.23 ± 1.84 (28.30–32.15) |
13.90 ± 1.77 (12.05–15.76) |
15.37 ± 5.01 (10.11–20.62) |
| Nervous system hemorrhage | 11.42 ± 1.09 (10.28–12.56) |
7.98 ± 0.48 (7.47–8.49) |
2.63 ± 0.34 (2.28–2.98) |
4.49 ± 1.46 (2.95–6.02) |
20.62 ± 1.96 (18.56–22.67) |
11.41 ± 0.69 (10.68–12.14) |
1.84 ± 0.23 (1.60–2.09) |
2.22 ± 0.72 (1.46–2.98) |
| Cranial nerves | 2.26 ± 0.22 (2.03–2.48) |
0.76 ± 0.05 (0.71–0.80) |
2.11 ± 0.27 (1.83–2.39) |
1.55 ± 0.51 (1.02–2.08) |
N.A.b | N.A.c | N.A.c | N.A.c |
No death events reported for AD26 vaccine.
No death events reported for BNT vaccine.
No death events reported for both vaccines.
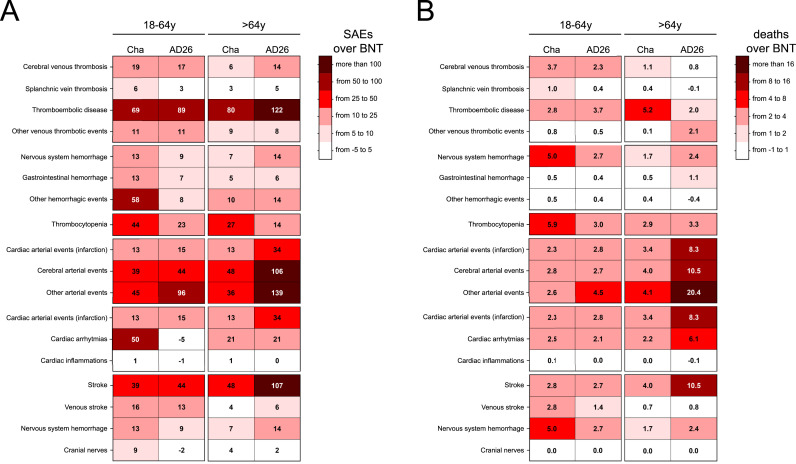
The most frequent venous thrombosis event in ChA and AD26 recipients was the thromboembolic disease, showing a similar frequency in the ChA and AD26 recipients (about 1 SAEs/10,000 doses). The frequency of SAE-related death was similar to that of CVT in young adults (3–4 deaths/OMD) and was higher in older adult recipients (3–7 deaths/OMD). Considering that thromboembolic disease was observed even in BNT recipients (likely representing its baseline frequency), the frequency of SAEs and death caused by the virus-based vaccines must be evaluated by subtracting the SAEs observed following BNT from the SAEs observed following virus-based vaccines. After subtracting the baseline frequency, Fig. 5 shows that in ChA and AD26 recipients, a frequency of thromboembolic disease higher than expected is observed. The excess affects 69 and 89 young adult recipients/OMD and 80 and 122 older adult recipients/OMD following ChA and AD26, respectively, whereby the HR of death in young adult ChA and AD26 recipients was about 11 and 14, respectively (Table 2).
Fig. 5.
Frequency of SAEs and SAE-related deaths due to specific AEs in ChA and AD26 recipients over that in BNT recipients. (A) The frequency of SAEs in ChA and AD26 recipients over those in BNT recipients, divided according to age group. In the hypothesis that the BNT vaccine does not cause SAEs (i.e., the SAE frequency in BNT recipients reflects the frequency of severe events in the unvaccinated population), the number in each rectangle represents the frequency of SAEs/OMD caused (not observed) by ChA and AD26. Darker colors indicate a higher frequency of events caused by the virus-based vaccines. White represents SAEs with a similar frequency between the virus-based vaccines and BNT. (B) The frequency of SAE-related deaths in ChA and AD26 recipients over those in BNT recipients, divided according to age group. In the hypothesis that BNT does not cause death (i.e., the frequency of deaths in BNT recipients reflects the frequency of deaths in the unvaccinated population), the number in each rectangle represents the frequency of deaths/OMD caused (not observed) by the ChA and AD26 vaccines. Darker colors indicate a higher frequency of deaths caused by the virus-based vaccines. White indicates SAE-related deaths with a similar frequency between the virus-based vaccines and BNT.
Fig. 3, Fig. 4, Fig. 5 show that SVT and venous thrombotic events different from those mentioned above were detected in ChA and AD26 recipients at a higher frequency than in BNT recipients but were less relevant from the quantitative point of view, even if they were responsible for some deaths.
3.5. TTS is more frequent in CVT than in the other thrombotic SAEs in ChA and AD26 recipients
In Section 3.3 and Table S10, we show that only 17.5% and 8.5% of venous thrombotic events consisted of TTS in ChA and AD26 recipients, respectively. We evaluated if TTS frequency was low even in vaccine recipients with CVT. Table S11 shows that 59% of young adult ChA recipients with CVT had thrombocytopenia, and 83% of the recipients who died due to CVT had thrombocytopenia. If possible gaps in the preparation of the AE reports by physicians were considered, the latter percentage might mean that virtually all life-threatening CVT in young adult ChA recipients are associated with thrombocytopenia. Older adult ChA recipients with CVT had lower percentages of thrombocytopenia and related death (Table S11).
Young adult AD26 recipients with CVT had a similar percentage of TTS to ChA recipients (Table S11). In older adult AD26 recipients, CVT, despite being more frequent than in ChA recipients (15.3 vs 7.4), was virtually not associated with thrombocytopenia.
3.6. Nervous system hemorrhage is the most life-threatening hemorrhagic SAE in ChA and AD26 recipients
We evaluated the hemorrhagic SAEs of vaccines in all (Table S12), young adult (18–64 years old), and older adult (≥65 years old) recipients (Fig. 3B). Nervous system and gastrointestinal hemorrhage were relatively frequent in young adult and older adult ChA and AD26 recipients (8–19 SAEs/OMD) and less frequent in BNT recipients so that 5–15 more SAEs/OMD were observed in the ChA and AD26 recipients. Interestingly, a high frequency of non-cerebral, non-gastrointestinal hemorrhagic SAEs was observed in young adult ChA recipients (exceeding BNT by 58 SAEs/OMD) (Fig. 3, Fig. 5), but not in older adult ChA or young adult and older adult AD26 recipients. Nervous system hemorrhage was the only life-threatening hemorrhage observed in the ChA and AD26 recipients (Fig. 4B), with 5.0 and 2.7 more deaths than BNT recipients in young adult recipients and an HR of about 21 and 11, respectively (Fig. 5 and Table 2).
Thrombocytopenia was much more frequent in ChA and AD26 than in BNT recipients (Fig. 3C). However, it was more frequent in young adult ChA rather than AD26 recipients, with 44 and 23 more SAEs than BNT recipients, respectively, causing a relevant frequency of death in young adult ChA recipients (5.9 more deaths/OMD than for BNT) (Fig. 4, Fig. 5).
3.7. Arterial SAEs are the most life-threatening in older adult AD26 recipients
We evaluated the cardiac (myocardial infarction), cerebral (stroke), and non-cardiac, non-cerebral arterial SAEs in all (Table S12), young adult (18–64 years), and older adult (≥65 years) recipients (Fig. 3D). In young adult BNT recipients, the frequency of arterial SAEs was no more than 5 SAEs/OMD, and in young adult ChA and AD26 recipients, the frequency of cardiac, cerebral, and non-cardiac, non-cerebral arterial SAEs was about 16 (both), 47 (both), and 50 (ChA), or 100 (AD26) SAEs/OMD, respectively. The frequency of stroke and non-cardiac, non-cerebral arterial SAEs was even more striking in older adult AD26 recipients, exceeding that in BNT recipients by 34–139 SAEs/OMD (Fig. 5). Moreover, in older adult AD26 recipients, 10–20 deaths/OMD were correlated to arterial SAEs (Fig. 4D), exceeding that in BNT recipients by 8–20 deaths/OMD (Fig. 5) and with HR = 4–21 (Table 2). The greater SAEs and deaths compared to the BNT vaccine are surely more relevant than that reported, given the bias concerning the age distribution of older adult vaccine recipients (Figure S2C), with BNT recipients being older than ChA and AD26 recipients. In fact, arterial events are much more frequent in older rather than young people in the vaccine untreated population.
3.8. Cardiac arrhythmias are more frequent in ChA and AD26 recipients
To determine whether there were cardiac SAEs different from myocardial infarction in ChA and AD26 recipients, we evaluated the frequency of arrhythmias and cardiac inflammations in the vaccine recipients (Table S12) (Fig. 3E). The frequency of cardiac arrhythmia-related SAEs was similar (about 23 SAEs/OMD) in young adult BNT and AD26 recipients and was much higher in ChA recipients, exceeding that in BNT recipients by 50 SAEs/OMD (Fig. 5). In older adult ChA and AD26 recipients, the frequency of cardiac arrhythmia-related SEAs was twice that observed in BNT recipients. Interestingly, there were more cardiac arrhythmia-related deaths not only in older adults but even in young adult ChA and AD26 recipients (2.1–6.1) than in BNT recipients (Fig. 5).
In young adult vaccine recipients, there were more cardiac inflammation-related SAEs than in older adult recipients (Fig. 3E), and the differences among the BNT, ChA, and AD26 vaccines were not relevant (Fig. 5).
3.9. Venous strokes are more frequent in ChA and AD26 recipients
To determine whether there were SAEs affecting the central nervous system and different from stroke and hemorrhage in ChA and AD26 recipients, we evaluated the frequency of venous stroke and SAEs affecting the cranial nerves (Fig. 3F) (Table S12).
Venous stroke was very rare in BNT recipients and was observed at a frequency of about 15/OMD in young adult ChA and AD26 recipients, with some deaths (Fig. 3, Fig. 4F). There were 13–16 more SAEs/OMD than that in BNT recipients (Fig. 5), with HR = 25–20 (Table 2). Cranial nerve-related SAEs were observed in BNT recipients, particularly the young adults (about 7 SAEs/OMD). In the same age range, compared to BNT recipients, cranial nerve SAEs were more than twice as frequent in ChA recipients, while AD26 recipients had a lower frequency thereof.
3.10. The frequency of SAEs in older adult AD26 recipients and arterial SAEs in young adult and older adult AD26 recipients was higher than in ChA recipients
There were many more SAEs and SAE-related deaths for the ChA and AD26 vaccines than for the BNT vaccine concerning coagulation disorders and arterial, cardiac, and nervous system events. The greater number of SAEs and deaths in the ChA and AD26 recipients is relevant in most of the AE types we considered and were present in both young adults and older adults (Fig. 5). However, several differences were observed in the frequency of specific SAEs in the ChA and AD26 recipients.
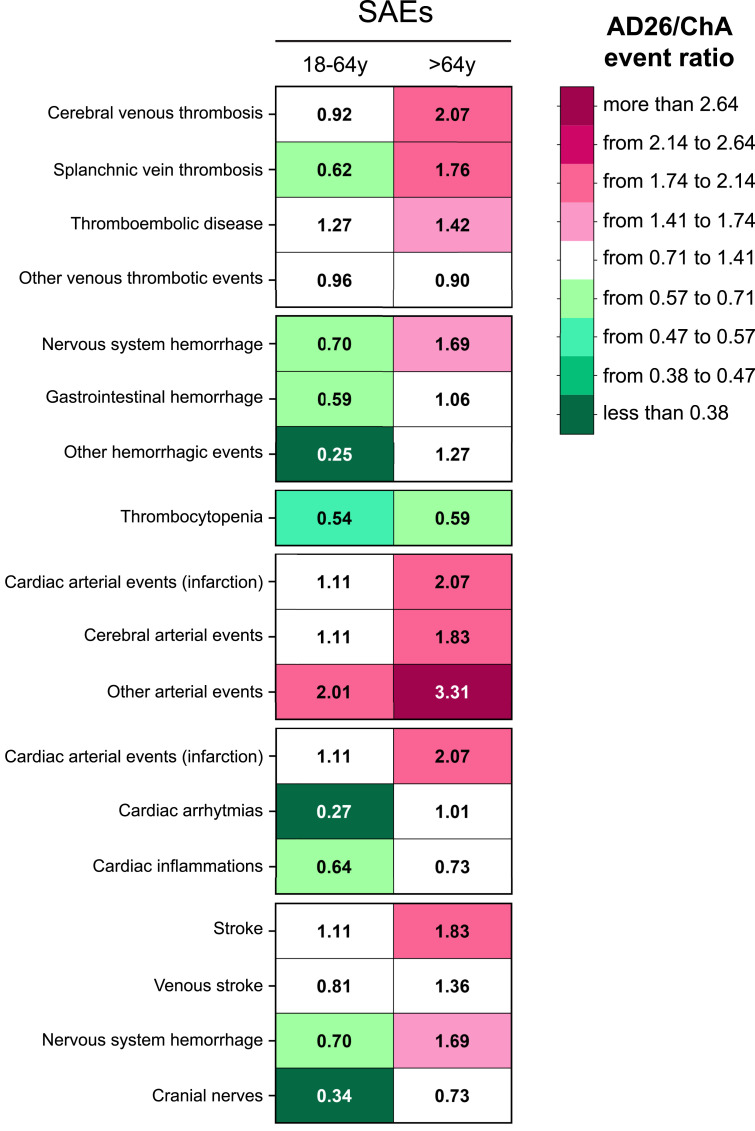
To determine whether there were systematic differences between SAE frequency in the ChA and AD26 recipients, we evaluated the SAE frequency ratio in young adult and older adult ChA and AD26 recipients. Fig. 6 shows that the SAE frequency in the ChA and AD26 recipients was 3–4-fold in favor of either the former or the latter. There was a similar SAE frequency for only 15 out of 36 SAEs in the ChA and AD26 recipients (white background). The differences suggest that: 1) with the relevant exception of arterial events, the ChA vaccine is associated with more SAEs than the AD26 vaccine in young adults; 2) the AD26 vaccine is associated with more SAEs than the ChA vaccine in older adults; 3) AD26 vaccination is followed by more arterial events than ChA vaccination, specifically concerning myocardial infarction and stroke in older adults and non-cardiac, non-cerebral arterial events in both young adults and older adults; 4) despite thrombocytopenia being caused even by the AD26 vaccine, it was more frequent in ChA than in AD26 recipients. Indeed, TTS was more frequent in ChA rather than AD26 recipients (Section 3.3).
Fig. 6.
The ratio between SAE frequency in ChA and AD26 recipients. The ratio between the SAE frequency (SAEs/OMD) observed in AD26 vs. ChA recipients (numbers within the rectangles), divided according to age group. Darker pink colors indicate a higher frequency of the events in AD26 recipients as compared to ChA recipients. Darker green colors indicate a higher frequency of the events in ChA recipients as compared to AD26 recipients (the darkest green indicates that an SAE frequency in ChA recipients is 2.64-fold greater than that in AD26 recipients). White indicates SAEs with a similar frequency.
3.11. ChA and AD26 show similar levels of SAEs with regards to the considered events
To determine the burden of SAEs and SAE-related deaths from vaccines, it would be incorrect to sum the above-described SAEs and deaths. In fact, a recipient may have developed and died from >1 SAE. Therefore, we evaluated the number of recipients affected by SAEs and who died from SAEs following BNT, ChA, and AD26 vaccination. The data did not involve all recipients with SAEs and SAE-related death following vaccination, but exclusively involved recipients experiencing coagulation disorders and arterial, cardiac, and nervous system events.
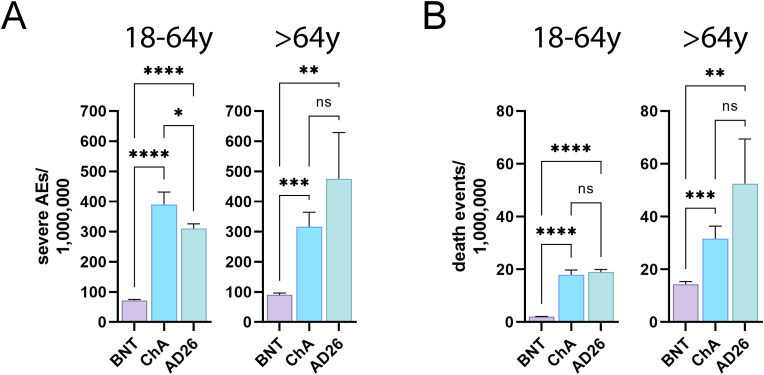
Fig. 7 shows that a much higher frequency of ChA and AD26 recipients than BNT recipients were affected by SAEs. In particular, there were 200–400 more SAEs/OMD for the ChA and AD26 vaccines than for the BNT vaccine (i.e., 2–4 SAEs related to coagulation disorders and arterial, cardiac, and nervous system events for every 10,000 doses). There were slightly more SAEs for the ChA vaccine than for the AD26 vaccine in the young adult recipients and a similar SAE frequency to the AD26 vaccine in older adults, even if there was a tendency for more SAEs for the AD26 vaccine in this subpopulation.
Fig. 7.
The frequency of BNT, ChA, and AD26 recipients with ≥1 SAEs relative to coagulation disorders and arterial, cardiac and nervous system events and deaths caused by the SAEs. (A) The frequency (mean ± SD)/OMD of BNT, ChA, and AD26 recipients with ≥1 SAEs relative to coagulation disorders (venous thrombotic, hemorrhagic events, thrombocytopenia) and arterial, cardiac, and nervous system events divided according to age range (18–64 years and >64 years). (B) The frequency (mean ± SD)/OMD of BNT, ChA, and AD26 recipients who died due to ≥1 SAEs relative to coagulation disorders (venous thrombotic, hemorrhagic events, thrombocytopenia), arterial, cardiac, and nervous system events, divided according to age range (18–64 years and >64 years). Significance was calculated using the Welch and Brown-Forsythe one-way ANOVA test; the difference between ChA and BNT, AD26 and BNT, and ChA and AD26 was assessed with Dunnett T3 multiple-comparison post-hoc analysis. Multiplicity-adjusted p-values are reported. ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 7 also shows a much higher frequency of SAE-related death in ChA and AD26 recipients than in BNT recipients. The greater frequency compared to the BNT vaccine was: 1) about 16 deaths/OMD in young adults, meaning 1.5 more deaths for the ChA and AD26 vaccines every 100,000 doses; 2) 17–38 deaths/OMD in older adults, meaning 1.5–4 more deaths for the ChA and AD26 vaccines every 100,000 doses. In ChA and AD26 young adult and recipients the frequency of death associated with the vaccines was similar, even if in old adult AD26 recipients a tendency for more SAE-associated deaths is observed.
4. Discussion
SARS-CoV-2 promotes humoral immunity dysregulation and autoantibody production, possibly contributing to the complication of COVID-19 [[21], [22], [23], [24], [25], [26], [27]]. Among the SARS-CoV-2 proteins, the spike protein, whose production is favored by each vaccine, carries a relevant number of amino acid sequences similar to those of human self-tissues (molecular mimicry), resulting in a higher possibility of the production of antibodies cross-reactive with human proteins [28]. Despite that, venous thrombosis and thrombocytopenia are much more frequent in ChA recipients than in BNT recipients [15,29,30], and we demonstrate here that coagulation disorders and arterial events were much more frequent in ChA and AD26 recipients rather than BNT recipients, with a high SAE and SAE-related deaths frequency in both young adult and older adult recipients. Therefore, the data suggest that the viral spike protein produced following vaccination with the abovementioned vaccines is not the main party responsible for the SAEs of ChA and AD26 vaccination. However, virus involvement in autoimmune diseases has long been known [31]. Indeed, several viral proteins show molecular mimicry [32,33]. Therefore, the virus-based ChA and AD26 vaccines may be associated with a higher frequency of SAEs because of adenoviral proteins or the production of aberrant spike proteins, as discussed below.
4.1. The higher frequency of SAEs related to coagulation disorders and arterial, cardiac, and nervous system events in ChA and AD26 recipients than BNT suggest that ChA and AD26 cause or participate in causing such events
To assess the safety profile of a drug, the temporal association of an undesirable event with drug administration is not sufficient to demonstrate the cause-effect relationship between the drug and the event despite the event being formally defined AE or side effect (usually related to a drug). In fact, undesirable events are present in the untreated population regardless of drug administration and it must be considered that they occur with the same frequency (reference frequency) after drug administration. If the frequency is higher, the drug is known to cause the AE.
To assess whether ChA and AD26 cause SAEs, we used BNT recipients as a reference here and in a previous study [15]. Considering that BNT is not a long-term treatment, it is unlikely to protect people from unwanted events. Consequently, the frequency of SAEs in BNT can be equal to that of the untreated population or higher, if BNT also causes coagulation disorders and/or arterial, cardiac, and nervous system SAEs.
Fig. 5 shows the frequency of SAE and SAE-related deaths in ChA and AD26 recipients over that in BNT recipients, suggesting that ChA and AD26 cause arterial, cardiac and nervous system SAE and coagulation disorders and SAE-related deaths and/or favor them in subjects with co-morbidities. Data suggest that ChA and AD26 cause 2–4 SAEs related to the abovementioned events per 10,000 vaccinations and 1.5–3.5 SAE-related deaths per 100,000 vaccinations. We believe our study suggests a causal relationship between virus-based vaccines and such events, but further studies are needed to confirm our observation, rule out possible confounding factors, and establish the actual frequency of such SAEs.
Our study does not investigate the mechanism(s) underlying these unwanted events, but some studies suggest that they are due to the production of anti-PF4 antibodies, platelet activation, and thrombocytopenia or other mechanisms [9,11,[34], [35], [36]]. In the next few sections, we will discuss the hypotheses on this topic in more depth.
4.2. Most thromboses observed in ChA and AD26 recipients are not due to TTS
TTS (also termed vaccine-induced immune thrombocytopenia and thrombosis, VITT) is a syndrome first described in ChA recipients in March 2021 [8]. TTS is caused by the production of anti-PF4 antibodies, causing platelet activation. The last related statement from EMA, Medicines and Healthcare products Regulatory Agency (MHRA), and Health Canada reported that TTS frequency in ChA recipients is 9.3, 15.1 and 21.9 per OMD, respectively [[37], [38], [39]]. The EudraVigilance data showed similar results considering recipients with venous thrombosis and thrombocytopenia (Table S10), with about 19 TTS/OMD and about four TTS-dependent deaths/OMD. However, the analysis also demonstrates that ChA recipients have a much higher frequency of thrombosis-related SAEs (about 100 SAEs/OMD, Figure S2), so that about 80 thromboses/OMD appear to be unassociated with thrombocytopenia. If considering all coagulation disorders in ChA recipients, they would cause about 200 SAEs/OMD and 12 deaths/OMD. In conclusion, ChA-dependent TTS appears to cause only 10% and 30% of coagulation disorder-related SAEs and deaths, respectively. There may be some reasons, including underreporting of thrombocytopenia in the databank, but it is reasonable to suppose that the majority of thromboses observed in the ChA recipients were observed in the absence of thrombocytopenia.
The idea that virus-based vaccines cause coagulation disorders with mechanisms different from that favoring massive platelet aggregation and thrombocytopenia was confirmed by the analysis of data from the AD26 recipients. Interestingly, there was a normalized number of venous thrombotic events similar to that of ChA recipients for AD26 recipients, but less thrombocytopenia. Indeed, there were only 11 TTS/OMD and about two TTS-related deaths/OMD in AD26 recipients (Table S10). If we hypothesize that thrombocytopenia was absent from the records of the patients with SAEs merely because of incomplete filling of the reports by physicians, the AD26 vaccine would have equal or even higher frequency of TTS than ChA vaccine considering that, in Europe, AD26 entered clinical use after ChA and when TTS for ChA and AD26 vaccinations (the latter in the USA) became known. Therefore, it seems likely that the AD26 vaccine determines thrombocytopenia in fewer recipients than the ChA vaccine, but nevertheless causes a large number of thrombotic events (131 SAEs/OMD), slightly more than the ChA vaccine.
The frequency of TTS depends on the recipient's age and the type of thrombosis. The analysis shown in Table S10 suggests that, as expected, TTS in the ChA and AD26 recipients was more frequent in young adults than in older adults [15]. Moreover, different from other thromboses, TTS frequency was higher in CVT and CVT-related death (Table S11), suggesting that CVT is mainly caused by anti-PF4 antibody production and massive platelet activation/aggregation, as demonstrated by the outstanding study by Greinacher and colleagues [9]. In conclusion, thrombosis in several virus-based vaccine recipients is not associated with thrombocytopenia, but the lack of thrombocytopenia cannot lead to the conclusion that the vaccine is not responsible for thrombosis. Indeed, the frequency of thrombosis without thrombocytopenia is much higher in ChA and AD26 recipients than in BNT recipients, suggesting a pathogenetic relationship. This is important from the clinical point of view.
4.3. What mechanism(s) is responsible for arterial events and venous thrombosis without thrombocytopenia?
Several mechanisms have been proposed to explain venous thrombosis caused by virus-based vaccines [36,40]. Most of them hypothesize the mechanisms determining the presence of anti-PF4 antibodies in virus-based vaccine recipients that activate platelets and favor their aggregation. It is assumed that massive platelet aggregation causes: 1) coagulation in atypical venous sites and/or disseminated intravascular coagulation; 2) thrombocytopenia. Here, we confirm that some types of severe thrombosis (e.g., CVT) following ChA or AD26 vaccination are frequently associated with thrombocytopenia, but most of the thromboses (even severe) caused by virus-based vaccines are not associated with thrombocytopenia. Therefore, there are two possible explanations: 1) thrombosis is caused by anti-PF4 antibodies that activate platelets in specific sites only and does not cause thrombocytopenia; 2) thrombosis is caused by mechanism(s) different from anti-PF4 antibodies in the absence of thrombocytopenia.
The first hypothesis implies that platelets activated by low levels of anti-PF4 antibodies aggregate mainly in sites where there are predisposing factors, in either veins or arteries, without causing massive consumption of platelets and thrombocytopenia. The second mechanism may synergize with the first mechanism, and the hypothesis states that virus-based vaccines cause a pro-inflammatory response. Indeed, it has been hypothesized that aberrantly spliced spike soluble proteins derived from the codon-optimized DNA present in the ChA and AD26 vaccines are produced by the cells in which the adenovirus enters [11]. The aberrant proteins bind to ACE2 expressed by vascular endothelial cells and promote endothelial cell ADCC and CDC. Therefore, ADCC and CDC might activate a local inflammatory response, favoring platelet aggregation in sites where predisposing factors are present due to underlying diseases. Furthermore, other mechanisms, including the interaction of non-replicating adenoviral vectors with mast cells and endothelial cells and the production of interleukin-6, may explain the activation of a local inflammatory response by virus-based vaccines independent of anti-PF4 antibodies [36].
The abovementioned idea may also explain the arterial events observed frequently in recipients of virus-based vaccines (Fig. 3D). They are more frequent in older adult rather than young adult recipients, as expected if the autoimmune response hypothesis is excluded and if the inflammatory response present in the endothelium with plaques is considered [41,42]. Moreover, they are more frequent in AD26 rather than ChA recipients, different from TTS that depends on anti-PF4 antibody production and is more frequent in ChA recipients [43].
We demonstrate that thrombosis without thrombocytopenia is the most frequent thrombosis observed in recipients of virus-based vaccines and that arterial events are as frequent as venous thrombotic events, and this finding has to be kept in mind in clinical practice. More investigations are needed to discover if thrombosis without thrombocytopenia and arterial events are still dependent on anti-PF4 antibodies or not.
4.4. Why does the frequency of some types of coagulation disorders and arterial events differ in ChA and AD26 recipients?
Both ChA and AD26 are virus-based vaccines, and we may expect similar types of vaccine-dependent SAEs. In comparing the SAEs of those vaccines with BNT SAEs, the similarities between the SAE type and frequency of virus based-vaccines greatly exceed the differences. Indeed, the ChA and AD26 vaccines share several properties, including the use of a replicating defective adenovirus and the use of DNA to carry the information for spike protein synthesis.
However, there were several differences in the ChA and AD26 SAE type and frequency. These involved the frequency of not only some types of SAEs (e.g., hemorrhage in young adult recipients and arterial events in older adult recipients, Fig. 2), but also the frequency of SAE-related deaths, particularly in older adult recipients (e.g., thromboembolic disease, cardiac infarction, stroke, other arterial events, Fig. 4). Moreover, as discussed earlier, there were more cases of TTS and hemorrhage in young adult ChA recipients than in AD26 recipients. Finally, a few types of SAEs in young adults (e.g., “other hemorrhagic SAEs” and “cardiac arrhythmias”) were observed almost exclusively in the ChA recipients. In our opinion, the differences are not due to reporting bias and their relevance from the quantitative point of view makes it unlikely that the differences observed were by chance. Therefore, they must be dependent on the difference between the two vaccines.
As far as we know, the differences between the vaccines are as follows: 1) The type of adenovirus is different (human Ad26 and chimpanzee adenovirus in the AD26 and ChA vaccines, respectively); 2) the human cell lines by which adenovirus are produced are different: T-REx-293 cells for ChA and PER.C6 TetR cells for AD26 [44]; 3) ChA contains a large number of host cell proteins differently from AD26 [10,44,45]; 4) chimpanzee adenovirus has a stronger negative charge than human AD26 [40]. Molecular simulations suggest that the chimpanzee adenovirus charge, combined with aspects of the virus shape, could allow it to bind to the positively charged PF4 protein [46]; 5) The number of viral particle in the AD26 vaccine is 3.3-fold higher than that in the ChA vaccine [47,48], possibly implying a higher spike protein and adenoviral protein burden. 6) EDTA is present in the ChA vaccine but not in the AD26 vaccine [47,48], and it has been hypothesized that it favors the development of inflammation at the vaccine inoculation site.
The more relevant difference in SAE type and frequency should be investigated and may have important follow-up for improving vaccine safety.
4.5. Why, in older adult recipients, is the frequency of SAEs higher in AD26 than ChA recipients?
Among the differences between the virus-based vaccines, the different frequency of SAEs in young adult and older adult vaccine recipients is relevant. After subtracting the baseline frequency of the SAEs, the frequency of most of the studied SAEs is higher in young adult than older adult ChA recipients (Fig. 5). On the contrary, the frequency of most of the studied SAEs is higher in older adult than young adult AD26 recipients. The phenomenon is even more relevant when analyzing the frequency of SAE-related deaths in AD26 recipients (Fig. 5). Furthermore, ChA causes more SAEs than AD26 in young adult recipients and AD26 causes more SAEs than ChA in older adult recipients (Fig. 6). Only 2 exceptions were observed: thrombocytopenia (more frequent in young and older adult ChA recipients) and “other arterial events” (more frequent in young and older adult AD26 recipients). In our opinion, it is not possible that the differences were dependent on the age distribution of ChA and AD26 vaccine recipients, as the age of recipients in young adult and older adult groups had very similar distributions (Figure S2B and S2C).
The reason for such a different distribution of SAEs is not clear. One of those may be the well-known higher frequency of thrombocytopenia due to anti-PF4 antibodies in young adult compared to older adult ChA recipients, resulting in TTS [8,9,15]. Hence, the lower frequency of TTS in AD26 recipients may partly explain our observation.
Another reason could be the presence of anti-adenovirus antibodies in vaccine recipients. Some people have antibodies to Ad26 (the human virus used to make the AD26 vaccine) [49]. In ChA, the use of chimpanzee adenovirus, which does not infect humans, can avoid the problem of pre-existing antibodies. The statement is basically true only for people living in Western countries. Indeed, neutralizing antibodies against some chimpanzee adenovirus serotypes have been detected in humans from sub-Saharan Africa, Brazil, and China [50,51]. We can hypothesize that pre-existing anti-Ad26 antibodies are more frequent in older adults than young adults and that their presence modifies the frequency of SAEs related to coagulative disorder and arterial, cardiac, and nervous system events. However, Dan Barouch and colleagues studying the effect of an adenovirus-based HIV vaccine suggest they do not [52]. Further studies are needed to understand the different frequencies of SAEs in young adults and older adult recipients of virus-based vaccines.
4.6. Is the assumption that the BNT vaccine does not cause any SAE verified?
In a recent publication, we showed that BNT recipients sometimes had a similar frequency of events to that in the unvaccinated population, but it is more often lower by a factor of 2–4-fold, suggesting that the reporting of events in vaccine recipients is lower than that in the unvaccinated population [15]. In our opinion, one from among the several reasons for this phenomenon appears the most relevant. When physicians observe an event (e.g., cardiac infarction leading to patient death) in a vaccine recipient with a predisposing disease (e.g., coronary ischemia), they do not report the event as an SAE-related death because they think it is more likely that the recipient died because of the predisposing disease and not because of the vaccine. This behavior is reasonable but is wrong for two reasons: 1) even in the case the recipient died because of the predisposing disease and not because of the vaccine, the decision of physicians changes the rate of events in the vaccinated population; 2) frequently, an adverse event caused by a drug is more frequent in the population with a predisposing disease than in the healthy population; nonetheless, the drug may be responsible for the event and the death. The comparison between vaccinated and unvaccinated populations will make clear whether the drug is responsible for the event.
On this basis, it is very difficult to say whether comparing the frequency of the event following the vaccination with that in the unvaccinated population can determine whether a vaccine causes an event. A very recent study comparing the incidence of potential AEs among BNT recipients with that among matched unvaccinated people aids this task [53], even if the authors do not consider the population of persons with a previous diagnosis of that event (reasonably introducing a bias). Among the events considered by the authors and that overlap with our categories (cerebrovascular accident, intracranial hemorrhage, myocardial infarction, myocarditis, deep vein thrombosis, pulmonary embolism, thrombocytopenia), all categories showed a risk ratio of <1, except myocardial infarction (1.07, 95% CI 0.74–1.60) and myocarditis (3.24, 95% CI 1.55–12.44). Therefore, we may conclude that, among them, the only SAE in which the frequency of events in BNT recipients cannot be considered the frequency at baseline is the cardiac inflammation.
The other SAEs observed in the BNT recipients at a high frequency are arrhythmias. However, arrhythmias were not considered in the abovementioned study [53]. It is known that the incidence of new arrhythmias in the general population is very high (4720 per million per year), corresponding to 197–393/OMD new arrhythmias within 15 or 30 days following vaccination [54], but it is difficult to evaluate the frequency of arrhythmias requiring hospitalization or causing death, so it is difficult to state if the frequency of about 20 SAEs/OMD in BNT recipients (Fig. 3E) is similar to or higher than that observed in the unvaccinated population.
4.7. Does the actual frequency of SAEs in ChA and AD26 recipients match the frequency we describe here?
It is well known that SAE frequency can be over- or under-evaluated due to overreporting and underreporting, respectively.
The reasoning we reported in Section 4.6 suggests that vaccine SAEs are underreported. For these reasons, we compared the SAE frequency in ChA and AD26 recipients with that in BNT recipients. In this manner, we eliminated: 1) bias in vaccine recipient selection; 2) people's different lifestyles during the SARS-CoV-2 infection period, possibly decreasing the frequency of some events; and mostly 3) AE underreporting due to: i) no reporting of events in recipients with predisposing factors (see section above); ii) the distance between the event and vaccination (particularly when the events manifests weeks after the vaccination); iii) physicians considering SAE reporting a task that is not useful; and, in some cases, iv) the idea that deems it appropriate to not highlight SAEs for fear of discouraging citizens' vaccination.
Regarding thrombosis, ChA- and AD26-related overreporting in the weeks following agencies’ declaration of SAEs due to thrombotic events such as CVST, SVT, and disseminated intravenous coagulation cannot be ruled out. However, other SAEs we analyzed (e.g., arterial events, including stroke and myocardial infarction) were never considered to be caused by virus-based vaccines. Therefore, overreporting of such events should be excluded.
To evaluate overreporting of thrombosis-related SAEs, we considered the percentage of deaths following SAEs in BNT, ChA, and AD26 recipients across age groups (Table S13). If overreporting had been present in ChA and AD26 recipients (e.g., hospitalization for mild blood loss), the death rate would have been lower. In contrast, ChA and AD26 recipients had similar or even higher death rates than BNT recipients, so overreporting does not appear to be present.
4.8. Limitations of the study
Our study has four main limitations. First, when analyzing an AE database, underreporting of some events and overreporting of other events can be anticipated. The robust institutional efforts used to organize the pharmacovigilance of COVID-19 vaccines may limit these biases, but such biases cannot be excluded with certainty. To reduce underreporting/overreporting biases, we used as the control the AEs reported for another vaccine assuming that the reporting bias was comparable, as discussed in Section 4.7.
Second, we reported the AE rate for only two age groups (18–64 and ≥ 65 years) with a big range. However, the EudraVigilance AE records no longer report the vaccine recipient's age. Therefore, a more complete analysis of the data was not possible. Moreover, the SAE frequency in the two age groups may be approximate due to incomplete information on the stratification of vaccinated people, as the number of vaccinated people in each age range was inferred from data from some European countries.
Third, we know the sex of people who developed AEs but not the number of females and males vaccinated with a specific vaccine in each age range. Therefore, it was not possible to stratify SAEs and death by sex.
Fourth, we did not know the health status of the vaccine recipients; however, many more people aged ≥70 years have been vaccinated with the BNT rather than ChA and AD26 vaccines (Figure S2), and these people have a much higher incidence of co-morbidities, and reasonably, of events unrelated to vaccination reported as SAEs and deaths [15]. Therefore, a higher frequency of SAEs and deaths in BNT recipients as compared with ChA and AD26 recipients might be expected. On the contrary, we found the opposite.
5. Conclusions
We have demonstrated a higher rate of coagulative disorder and arterial, cardiac, and nervous system events in ChA and AD26 rather than BNT recipients, with recipients developing ≥1 of these SAEs with a frequency of 3–5 SAEs out of 10,000 doses and 3–5 deaths out of 100,000 doses. As far as concerns several SAEs, ChA causes more SAEs than AD26 in young adults, and AD26 causes more SAEs than ChA in older adults. We observed a frequency of thrombosis caused by virus-based vaccines much higher than reported by regulatory agencies worldwide. The discrepancy is because regulatory agencies consider only thrombosis associated with thrombocytopenia (TTS) being caused by virus-based vaccines.
Our data may aid the evaluation of the risk–benefit ratio of the ChA and AD26 vaccines, taking into account the depth of the pandemic in a particular country. The vaccines’ risk–benefit ratio should be evaluated more carefully in countries that can buy and distribute mRNA-based vaccines, with low infection rates and almost reaching herd immunity.
Author statement
Luigi Cari: Conceptualization, Data download, Formal analysis, Visualization Mahdieh Naghavi Alhosseini: Data download, Formal analysis, Visualization Paolo Fiore: Data download, Formal analysis Sabata Pierno: Formal analysis Sabrina Pacor: Formal analysis Alberta Bergamo: Formal analysis Gianni Sava: Conceptualization, Writing—original draft Giuseppe Nocentini: Conceptualization, Project administration, Funding acquisition, Writing—original draft
Funding
This research was funded by Ministero dell’Università e della Ricerca (MUR) (grant number FISR2020IP_03103, granted to G.N.).
Declaration of competing interest
The authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. All this includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, and royalties.
Acknowledgments
We thank the EMA for making public the data relating to vaccine AEs, and the European countries for making public the number of people from different age groups to whom a specific vaccine had been administered.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2021.102742.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vasileiou E., Simpson C.R., Robertson C., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., de Lusignan S., Docherty A., Ford D., Hobbs R., Joy M., Katikireddi S.V., Marple J., McCowan C., McGagh D., McMenamin J., Moore E., Murray J.-L., Pan J., Ritchie L., Shah S.A., Stock S., Torabi F., Tsang R.S.M., Wood R., Woolhouse M., Sheikh A. SSRN Electron. J.; 2021. Effectiveness of First Dose of COVID-19 Vaccines against Hospital Admissions in Scotland: National Prospective Cohort Study of 5.4 Million People. [DOI] [Google Scholar]
- 2.Bernal J.L., Andrews N., Gower C., Stowe J., Robertson C., Tessier E., Simmons R., Cottrell S., Roberts R., O'Doherty M., Brown K., Cameron C., Stockton D., McMenamin J., Ramsay M. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. MedRxiv. 2021 doi: 10.1101/2021.03.01.21252652. 2021.03.01.21252652. [DOI] [Google Scholar]
- 3.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazell L., Shakir S.A.W. Under-reporting of adverse drug reactions. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Varallo F.R., Guimarães S. de O.P., Abjaude S.A.R., Mastroianni P. de C. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev. Esc. Enferm. USP. 2014;48:739–747. doi: 10.1590/S0080-623420140000400023. [DOI] [PubMed] [Google Scholar]
- 6.Moore T., Bennett C. Underreporting of hemorrhagic and thrombotic complications of pharmaceuticals to the U.S. Food and drug administration: empirical findings for warfarin, clopidogrel, ticlopidine, and thalidomide from the southern network on adverse reactions (SONAR) Semin. Thromb. Hemost. 2012;38:905–907. doi: 10.1055/s-0032-1328890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Rubio F., Calderón-Larrañaga A., Poblador-Plou B., Navarro-Pemán C., López-Cabañas A., Prados-Torres A. Underreporting of recognized adverse drug reactions by primary care physicians: an exploratory study, Pharmacoepidemiol. Drug Saf. 2011;20:1287–1294. doi: 10.1002/pds.2172. [DOI] [PubMed] [Google Scholar]
- 8.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): 29 March 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-29-march-2021_en.pdf [Google Scholar]
- 9.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greinacher A., Selleng K., Wesche J., Handtke S., Palankar R., Aurich K., Lalk M., Methling K., Völker U., Hentschker C., Michalik S., Steil L., Schönborn L., Beer M., Franzke K., Rangaswamy C., Mailer R.K., Thiele T., Kochanek S., Krutzke L., Siegerist F., Endlich N., Warkentin T.E., Renné T. Towards understanding ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) Res. Sq. 2021 doi: 10.21203/rs.3.rs-440461/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowarz E., Krutzke L., Reis J., Bracharz S., Kochanek S., Marschalek R. “Vaccine-Induced Covid-19 Mimicry” Syndrome: splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Res. Sq. 2021 doi: 10.21203/rs.3.rs-558954/v1. [DOI] [Google Scholar]
- 12.EMA - European Medicines Agency . 2021. AstraZeneca's COVID-19 Vaccine: Benefits and Risks in Context.https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context [Google Scholar]
- 13.EMA - European Medicines Agency, Vaxzevria (previously COVID-19 Vaccine AstraZeneca), (n.d.). https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca.
- 14.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Şahin U., Jansen K.U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 15.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J. Autoimmun. 2021;122:102685. doi: 10.1016/j.jaut.2021.102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for COVID-19 Vaccine Janssen: 22 April 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-covid-19-vaccine-janssen-22-april-2021_en.pdf [Google Scholar]
- 17.EMA - European Medicines Agency, COVID-19 Vaccine Janssen, (n.d.). https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen.
- 18.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Janssen: Link between the Vaccine and the Occurrence of Thrombosis in Combination with Thrombocytopenia.https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-covid-19-vaccine-janssen-link-between-vaccine_en.pdf [Google Scholar]
- 19.EMA - European Medicines Agency, EudraVigilance – european database of suspected adverse drug reaction reports, (n.d.). http://www.adrreports.eu/en/index.html.
- 20.ECDC - European Centre for Disease Prevention and Control, ECDC's COVID-19 Vaccine Tracker, (n.d.). https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab.
- 21.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;80–:370. doi: 10.1126/science.abd4585. eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodruff M.C., Ramonell R.P., Lee F.E.-H., Sanz I. Broadly-targeted autoreactivity is common in severe SARS-CoV-2 Infection. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.10.21.20216192. [DOI] [Google Scholar]
- 24.Zhou Y., Han T., Chen J., Hou C., Hua L., He S., Guo Y., Zhang S., Wang Y., Yuan J., Zhao C., Zhang J., Jia Q., Zuo X., Li J., Wang L., Cao Q., Jia E. Clinical and autoimmune characteristics of severe and critical cases of COVID‐19. Clin. Transl. Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R., Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021;20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., Coppi A., Lucas C., Silva J., Oh J.E., Song E., Perotti E.S., Zheng N.S., Fischer S., Campbell M., Fournier J.B., Wyllie A.L., Vogels C.B.F., Ott I.M., Kalinich C.C., Petrone M.E., Watkins A.E., Dela Cruz C., Farhadian S.F., Schulz W.L., Ma S., Grubaugh N.D., Ko A.I., Iwasaki A., Ring A.M. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 28.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., McCowan C., Agrawal U., Shah S.A., Ritchie L.D., Murray J., Pan J., Bradley D.T., Stock S.J., Wood R., Chuter A., Beggs J., Stagg H.R., Joy M., Tsang R.S.M., de Lusignan S., Hobbs R., Lyons R.A., Torabi F., Bedston S., O'Leary M., Akbari A., McMenamin J., Robertson C., Sheikh A. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., Lidegaard Ø., Tapia G., Gulseth H.L., Ruiz P.L.-D., Watle S.V., Mikkelsen A.P., Pedersen L., Sørensen H.T., Thomsen R.W., Hviid A. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina V., Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38:235–245. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 33.Saeki Y., Ishihara K. Infection–immunity liaison: pathogen-driven autoimmune-mimicry (PDAIM) Autoimmun. Rev. 2014;13:1064–1069. doi: 10.1016/j.autrev.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Thiele T., Ulm L., Holtfreter S., Schönborn L., Kuhn S.O., Scheer C., Warkentin T.E., Bröker B.M., Becker K., Aurich K., Selleng K., Hübner N.-O., Greinacher A. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138:299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sørvoll I.H., Horvei K.D., Ernstsen S.L., Lægreid I.J., Lund S., Grønli R.H., Olsen M.K., Jacobsen H.K., Eriksson A., Halstensen A.M., Tjønnfjord E., Ghanima W., Ahlen M.T. An observational study to identify the prevalence of thrombocytopenia and anti‐PF4/polyanion antibodies in Norwegian health care workers after COVID‐19 vaccination. J. Thromb. Haemostasis. 2021;19:1813–1818. doi: 10.1111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzarone B., Veneziani I., Moretta L., Maggi E. Pathogenic mechanisms of vaccine-induced immune thrombotic thrombocytopenia in people receiving anti-COVID-19 adenoviral-based vaccines: a proposal. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.728513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): 14 July 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-14-july-2021_en.pdf [Google Scholar]
- 38.Medicines and Healthcare products Regulatory Agency . 2021. Coronavirus Vaccine - Weekly Summary of Yellow Card Reporting - Updated 30 September 2021.https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#annex-1-vaccine-analysis-print MHRA. [Google Scholar]
- 39.Health Canada . 2021. Reported Side Effects Following COVID-19 Vaccination in Canada - September 24, 2021.https://health-infobase.canada.ca/covid-19/vaccine-safety/ [Google Scholar]
- 40.Ledford H. COVID vaccines and blood clots: what researchers know so far. Nature. 2021;596:479–481. doi: 10.1038/d41586-021-02291-2. [DOI] [PubMed] [Google Scholar]
- 41.Dhawan U.K., Singhal A., Subramanian M. Dead cell and debris clearance in the atherosclerotic plaque: mechanisms and therapeutic opportunities to promote inflammation resolution. Pharmacol. Res. 2021;170:105699. doi: 10.1016/j.phrs.2021.105699. [DOI] [PubMed] [Google Scholar]
- 42.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul-Ehrlich-Institut . 2021. Reports on Suspected Cases of Adverse Effects and Vaccination Complications Following a Vaccination for the Protection against COVID-19 (Reporting Period 27 December 2020 to 31 July 2021)https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/safety-reports/safety-report-27-december-31-july-2021.pdf?__blob=publicationFile&v=5 (German only) [Google Scholar]
- 44.Michalik S., Siegerist F., Palankar R., Franzke K., Schindler M., Reder A., Seifert U., Cammann C., Wesche J., Steil L., Hentschker C., Gesell-Salazar M., Reisinger E., Beer M., Endlich N., Greinacher A., Völker U. Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines. Res. Sq. 2021 doi: 10.21203/rs.3.rs-736157/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krutzke L., Roesler R., Wiese S., Kochanek S. Process-related impurities in the ChAdOx1 nCov-19 vaccine. Res. Sq. 2021 doi: 10.21203/rs.3.rs-477964/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker A.T., Boyd R.J., Sarkar D., Vant J., Crespo A.T., Waraich K., Truong C.D., Bates E., Wilson E., Chan C.K., Lipka-Lloyd M., Fromme P., Nagalo M.B., Heurich M., Williams D., Chiu P.-L., Rizkallah P.J., Parker A.L., Singharoy A., Borad M.J. The structure of ChAdOx1/AZD-1222 reveals interactions with CAR and PF4 with implications for vaccine-induced immune thrombotic thrombocytopenia. BioRxiv. 2021:2021. doi: 10.1101/2021.05.19.444882. 05.19.444882. [DOI] [Google Scholar]
- 47.EMA - European Medicines Agency . 2021. Vaxzevria (Previously COVID-19 Vaccine AstraZeneca) : EPAR - Product Information.https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf [Google Scholar]
- 48.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Janssen : EPAR - Product Information.https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf [Google Scholar]
- 49.Barouch D.H., V Kik S., Weverling G.J., Dilan R., King S.L., Maxfield L.F., Clark S., Ng’ang’a D., Brandariz K.L., Abbink P., Sinangil F., de Bruyn G., Gray G.E., Roux S., Bekker L.-G., Dilraj A., Kibuuka H., Robb M.L., Michael N.L., Anzala O., Amornkul P.N., Gilmour J., Hural J., Buchbinder S.P., Seaman M.S., Dolin R., Baden L.R., Carville A., Mansfield K.G., Pau M.G., Goudsmit J. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dicks M.D.J., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., Hill A.V.S., Cottingham M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris S.J., Sebastian S., Spencer A.J., Gilbert S.C. Simian adenoviruses as vaccine vectors. Future Virol. 2016;11:649–659. doi: 10.2217/fvl-2016-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barouch D.H., Tomaka F.L., Wegmann F., Stieh D.J., Alter G., Robb M.L., Michael N.L., Peter L., Nkolola J.P., Borducchi E.N., Chandrashekar A., Jetton D., Stephenson K.E., Li W., Korber B., Tomaras G.D., Montefiori D.C., Gray G., Frahm N., McElrath M.J., Baden L., Johnson J., Hutter J., Swann E., Karita E., Kibuuka H., Mpendo J., Garrett N., Mngadi K., Chinyenze K., Priddy F., Lazarus E., Laher F., Nitayapan S., Pitisuttithum P., Bart S., Campbell T., Feldman R., Lucksinger G., Borremans C., Callewaert K., Roten R., Sadoff J., Scheppler L., Weijtens M., Feddes-de Boer K., van Manen D., Vreugdenhil J., Zahn R., Lavreys L., Nijs S., Tolboom J., Hendriks J., Euler Z., Pau M.G., Schuitemaker H. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M.A., Lipsitch M., Kohane I., Netzer D., Reis B.Y., Balicer R.D. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2110475. NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khurshid S., Choi S.H., Weng L.-C., Wang E.Y., Trinquart L., Benjamin E.J., Ellinor P.T., Lubitz S.A. Frequency of cardiac rhythm abnormalities in a half million adults. Circ. Arrhythmia Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.