Abstract
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) is highly contagious, with a potential to cause large nosocomial outbreaks in the hospital setting. We report the advance deployment of comprehensive, multi-level infection control measures in a 3,700-bed large hospital to prevent nosocomial outbreaks of COVID-19 during the pandemic.
Methods
We implemented a series of dynamic infection control policies during the pandemic. A confirmed COVID-19 case was defined by positive real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay. All healthcare worker (HCW) having symptoms or close contact with the confirmed case received the RT-PCR test.
Results
A total of 5,722 patients were tested in our hospital from January to May 2020. Twenty-five patients were confirmed COVID-19, including two inpatients. A cluster of 4 HCWs with COVID-19 associated with the 2nd inpatient was identified in the early stage of epidemic. Our enhanced traffic control bundling, mask wearing, hand hygiene and environmental cleaning were reinforced after the outbreak. All other confirmed cases were identified at our outdoor quarantine station or epidemic clinic afterwards, and the results of testing for 146 symptomatic HCWs were all negative.
Conclusions
Integrated teamwork, advance deployment of infection control measures and efficient diagnostic testing and response protected HCW and facilities from large SARS-CoV-2 outbreaks and preserved the capacity and function of the health care system during the pandemic.
Keywords: SARS-CoV-2, COVID-19, Pandemic, Infection control, Hospital
At a glance commentary
Scientific background on the subject
COVID-19 has placed a large burden on hospitals and healthcare providers worldwide. Several nosocomial outbreaks of COVID-19 have been reported during the pandemic. Patients with existing diseases admitted in hospitals are at a higher risk of COVID-19 associated morbidity and mortality.
What this study adds to the field
The present study demonstrated a integrated team work, executing a comprehensive, dynamic infection control measures within a large hospital could efficiently mitigate COVID-19 nosocomial outbreaks and maintain the core function of the hospital.
Coronavirus disease 2019 (COVID-19) caused by severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China in late December 2019. On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic. SARS-CoV-2 can spread efficiently in humans, with a basic reproductive number of 2.2–2.5, as determined in Wuhan, suggesting high transmissibility [1,2]. Large outbreaks of SARS-CoV-2 infection in the community setting have been reported [3]. However, previous experience of an outbreak of MERS-CoV infection in Korea, where one single patient infected many others in an overcrowded hospital setting, raised the concerns about potential occurrence of large outbreaks in the hospital settings [4]. Furthermore, patients with existing diseases admitted in hospitals as well as health care workers are particularly vulnerable to SARS-CoV-2 infection and at a high risk for COVID-19-associated morbidity and mortality [5]. We herein report the preparedness of a large hospital in Taiwan to respond to the COVID-19 threat while maintaining core functions of health care during the pandemic.
Methods
Early stage preparedness
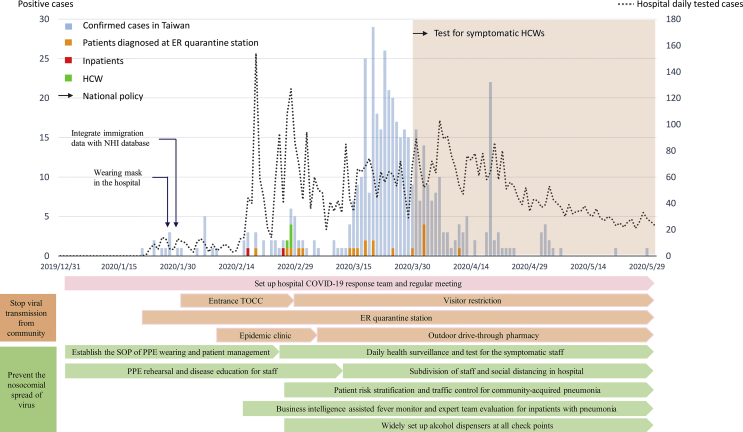
Chang Gung Memorial Hospital (CGMH) has, step-by-step, established a multi-disciplinary COVID-19 response team and started regular meetings every other day since January 3, 2020, soon after the pneumonia outbreak in Wuhan [Fig. 1]. We immediately proceeded to disseminate the epidemic and disease information to all staff and arrange personal protective equipment (PPE) rehearsal for the front-line medical personnel. We instituted the standards of PPE requirement, infection control measures and environmental cleansing for clinical care and transportation of confirmed or suspected COVID-19 patients in the hospital. The logistics department prepared a negative pressure room expansion plan and ventilator allocation for large-scale community outbreak, checked the storage and consumption of important medical supplies, including PPE and alcohol sanitizer.
Fig. 1.
The number of tested cases and number of confirmed COVID-19 cases in CGMH, and key national and hospital infection control measures deployed along the different stages of the epidemic in Taiwan. Abbreviations used: ER: emergency room; HCW: health care worker; TOCC: travel, occupation, contacts, cluster; SOP: standard operation procedure; PPE: personal protective equipment.
Measures to stop viral transmission from community to hospital
We started a detailed triage at the emergency room (ER) and all the hospital entrances. The triage included infrared thermal camera scanning for fever, respiratory symptom querying and collecting personal histories of travel, occupation, contacts, and clusters (TOCC). Patients, families, visitors, and healthcare workers (HCW) were asked to wear surgical mask and complete hand disinfection before entering the hospital. A relevant travel history was crucial in the beginning of this epidemic, and about 80% of Taiwan's confirmed COVID-19 cases were imported. Taiwan government integrated the citizens' immigration database with the National Health Insurance (NHI) database since January 27, 2020, and all visitors' travel histories can be accessed by reading their NHI cards.
Visitors with travel history to the endemic areas within 14 days would not be allowed to enter the hospital. Any patients presenting with fever or respiratory symptoms as well as having a relevant travel history would be routed to our epidemic clinic or outdoor quarantine station at the emergency room based on the severity of their symptoms. The epidemic clinic was located at the corner of one hospital building. It is separated from other outpatient clinics and had an independent entrance and air-conditioning. In response to the increasing cases and local clusters in community, we applied a strict visitor restriction for hospitalized patients and opened our outdoor drive-through pharmacy on February 29, 2020.
Measures to prevent nosocomial spread of COVID-19
We adopted the concept of enhanced traffic control bundling (eTCB), and distributed patients with community-acquired pneumonia requiring hospitalization to different zones of the hospital by their risk stratification [6]. Patients with typical symptoms fulfilling the diagnostic definition of notifiable disease COVID-19 by Taiwan Centers for Disease Control (Taiwan CDC) would be admitted to negative pressure room in the isolation ward (red zone). The quarantine ward (yellow zone) housed suspected patients with atypical manifestations. There were independent routes and designated elevators for patient transportation from emergency room to the red/yellow zones. HCWs wore standard PPE (N95 respirator, glove, water resistant gown and facial shield) prior to entering red/yellow zones. Patients could be transferred to general ward (green zone) if COVID-19 were excluded by negative RT-PCR and after an assessment by infectious disease (ID) specialists.
The Department of Information Technology developed an active hospital-wide inpatient fever surveillance system. With the assistance from the real-time fever dashboard, our ID specialists can timely identify the febrile patients in his responsible ward and evaluate the possibility of COVID-19. All physicians can also contact the responsible ID specialist for inpatients with pneumonia un-responding to initial treatment. If COVID-19 is suspected by our expert team, comprising pulmonologists and ID specialists, patients will be transferred to the quarantine ward (yellow zone) where a diagnostic RT-PCR will be performed.
To prevent a massive staff knockout by SARS-CoV-2 during the nosocomial outbreak, we subdivided our staff into different groups in every clinical unit. Every group worked at different area or station and used different offices to prevent prolonged contact or interaction. We postponed or canceled non-urgent surgeries and procedures, reduced inpatient services to keep a sufficient manpower. We also requested all staff members to report their body temperature and respiratory symptoms, if any, daily through the hospital information system or staff health surveillance app. Staff would be requested to stop working immediately as soon as they developed fever or any respiratory or systemic symptoms. Hospital staff with symptoms would be asked to take two real-time reverse transcriptase–polymerase chain reaction (RT-PCR) tests, at least 24 h apart, before they were allowed to go back to work after improvement of the symptoms. We continuously trained and supervised sanitors to enhance adequate environmental cleansing and widely set up alcohol dispensers at every checkpoint to mitigate the possibility of fomite transmission.
Case definition
Patients were confirmed SARS-CoV-2 infection by positive result of RT-PCR assay from a nasopharyngeal or oropharyngeal sample. RNA was extracted from nasopharyngeal or oropharyngeal swabs of patients suspected of having SARS-CoV-2 infection, and then RT-PCR assay was conducted to amplify and test three target genes, including RNA-dependent RNA polymerase gene (RdRp), envelope protein gene and nucleocapsid protein gene [7].
Healthcare associated infection
The case definitions of healthcare associated infection (HAI) and healthcare associated bloodstream infection (HABSI) are according to the healthcare associated infection surveillance definition by Taiwan CDC [8]. The incidence density of HAI/HABSI was determined as overall HAI/HABSI cases per 1000 patient-days.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was applied to analyze the difference of HAI/HABSI incidence between the study period (Jan, 2020 ∼ Dec, 2020) and one year before (Jan, 2019 ∼ Dec, 2019). A P value < 0.05 was considered statistically significant. All statistical calculations were performed by standard programs of the Statistical Package for the Social Sciences for Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Deployment of infection control measures and numbers of confirmed cases
CGMH at Linkou is a 3700-bed tertiary medical center in northern Taiwan. We started our ER outdoor quarantine station since the identification of the first case of COVID-19 on January 21, 2020 in Taiwan and this strategy was applied to all hospital entrances on January 29. Our HCWs and patient also followed our national policy, wearing mask in hospital since late January. A total of 5722 patients were tested for SARS-CoV-2 in our hospital during the study period between December 31, 2019 and May 31, 2020. Twenty-five patients were confirmed COVID-19, including two inpatients at the time of diagnosis [Fig. 1]. Our first inpatient was a case of community-acquired pneumonia, and COVID-19 was diagnosed on Feb 17, 2020, two weeks after her hospitalization. On hundred fifty-four HCWs having close contact with the patient were tested, and all showed negative results in RT-PCR. However, four of our HCWs (three nurses and one sanitor) were infected in a nosocomial outbreak associated with the second inpatient diagnosed on Feb 26, 2020. This patient, who had no abnormal TOCC, was not diagnosed until she developed a pneumonia 10 days after admission. We did a thorough investigation for the outbreak and its association with the first inpatient. The first and second inpatients were hospitalized with five days apart, and they were admitted into two different departments in two different buildings respectively. During the hospitalization, the two patients did not receive examination or treatment in the same space at the same time. The medical staff who take care of them are also in different groups. The four infected HCWs didn't having contact with the first inpatient. Based on the investigation result, we believed the second inpatient is a case of community acquired COVID-19, and she was asymptomatic upon admission [9]. Responding to the outbreak, we implemented a more rigorous visitor restriction and opened an outdoor pharmacy to reduce the people flow in hospital. We universally requested our staff and patients to wear surgical masks and enhance hand hygiene, the outbreak ward was closed after a complete environmental cleaning. All clerk, intern, post-graduate year residents were all prohibited from taking care of patient in the isolation/quarantine ward. Our eTCB was reinforced by widely testing SARS-CoV-2 and isolating or quarantining all patients with respiratory or systemic symptoms or pulmonary infiltrates found on chest films. After global pandemic was declared in March 2020, all the confirmed cases were identified at our outdoor quarantine station or epidemic clinic, and the results of PCR testing for 146 symptomatic HCWs in CGMH were all negative.
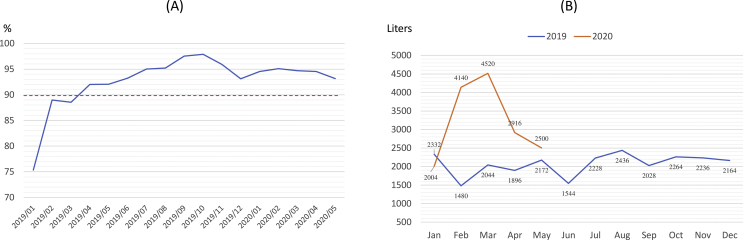
The percentage of staff daily respiratory symptom and body temperature report was 75.3% at the beginning of implementation and continued to exceed 90% since March 2020 [Fig. 2A]. Our hospital 75% alcohol consumption during study period was remarkable higher than the same period in 2019, with a peak level in global pandemic March 2020. The average of monthly alcohol consumption during study period is 3208 L, which was 155% of our average 2069 L in 2019 [Fig. 2B].
Fig. 2.
Autonomous health management by healthcare workers and consumption of alcohol per month during the pandemic. (A) The percentage of staff daily body temperature and health status report. (B) The monthly hospital alcohol consumption (liters), January 2019 to May 2020.
Healthcare associated infection
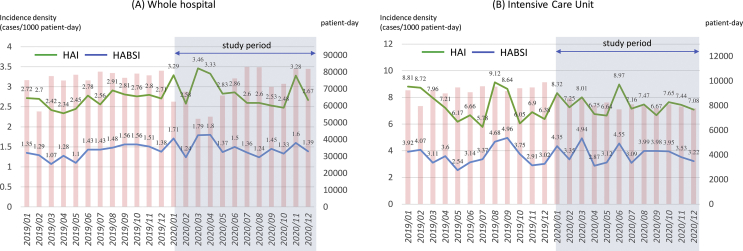
The whole hospital HAI incidence density had a monthly average 2.66 ± 0.18 per 1000 patient-days in 2019 and 2.88 ± 0.36 per 1000 patient-days in 2020 (p = 0.151). And HABSI incidence density was 1.37 ± 0.16 in 2019 and 1.48 ± 0.2 in 2020 (p = 0.223) [Fig. 3A]. Although there was no statistical significance noted between the two study periods, a small HAI peak (Mar, 2020 to May, 2020) was observed after our hospital COVID-19 cluster in Feb, 2020. The increasing peak probably was related to our reduction of inpatient services and cancelation of non-urgent medical services. Our hospital inpatient services had approximately 25% reduction in this 3-month period (monthly average patient-days: 76,013 in 2019 and 57,310 during Mar 2020 ∼ May, 2020).
Fig. 3.
The monthly incidence density (cases per 1000 patient-days) of healthcare associated infection, healthcare associated blood stream infection and patient-days during Jan, 2019 to Dec, 2020. (A) Whole hospital (B) Intensive Care Unit. Abbreviations used: HAI: healthcare associated infection; HBSI: healthcare associated bloodstream infection.
The HAI and HABSI in the intensive care unit (ICU) were also had no difference between the two periods (HAI: 7.37 ± 1.22 in 2019, 7.45 ± 0.7 in 2020, p = 0.833; HABSI: 3.59 ± 0.73 in 2019, 3.75 ± 0.65 in 2020, p = 0.554) [Fig. 3B].
Discussion
The high transmissibility before symptom onset and efficiency of human to human transmission of SARS-CoV-2 suggest that it is more difficult to contain the virus in the hospital setting than other respiratory viruses. There was no difference in the infection rates of HCW that can be related to the exposure risk of working areas in a study in Madrid, Spain [10]. The non-first-line HCWs had a higher infection rate than first-line HCWs in a large hospital in Wuhan, China [11]. A single unsuspected case of SARS-CoV-2 led to 6 major clusters involving 5 hospital wards and an outside nursing home and dialysis unit, with infection ultimately confirmed among 80 staff members and 39 patients, 15 of whom died [12].
Insufficient protective measures and lack of alert to the disease could have put non-first-line HCWs at a higher risk for the infection. The nosocomial outbreak of our four HCWs associated with the second inpatient developed in the general medicine ward. These findings highlighted the importance of advance deployment of infection control measures, and strict adherence to PPE protocol for all hospital staff. Wearing surgical masks and hand hygiene may decrease in transmission between patients and HCWs and among HCWs. Decreased hand hygiene compliance was more frequent on the outbreak ward in a quasi-experimental cluster-control outbreak evaluation in New York city hospital [13]. Universal masking of HCWs and patients in hospital was associated with a significantly lower rate of SARS-CoV-2 positivity among HCWs [14]. Our early universal masking policy coupled with hand hygiene might play an important role in mitigating the nosocomial outbreak. Although the first inpatient was diagnosed after two weeks hospitalization, there was no nosocomial transmission of virus. And the outbreak associated with our second inpatient was soon contained within the first generation of transmission. The transmission and its associated impact were mitigated, compared to the spread of nosocomial Middle East respiratory syndrome and severe acute respiratory syndrome (SARS) that usually involved four to five generation of transmission in the healthcare setting in previous reports [15].
Approximately one-fifth of individuals with mild COVID-19 were asymptomatic at admission [16], and potential viral transmission from asymptomatic patients and HCWs may also play a role in nosocomial outbreak [11]. Maintaining a low threshold for suspicion of infection and early detection for symptomatic staff and patient during hospitalization can help to early identify source of the infection. Our daily staff's health and temperature report and inpatient real-time fever surveillance system timely identify the symptomatic individuals such that rapid response can be initiated. A universal testing of patients upon admission has been implemented in a high CVOID-19 prevalence area to screen asymptomatic patients, determine hospital infection control practices, and also protect the whole health care teams [17]. The benefit of universal PCR test to screen asymptomatic patients on admission should be evaluated according to the community prevalence, testing resources and the vulnerability of the patients or residents.
The complete vaccination with authorized SARS-CoV-2 mRNA vaccines is highly effective at preventing symptomatic COVID-19 among HCWs in United States [18]. Coupling with consistent infection control practices, a prompt reduction of COVID-19 infection in HCWs is observed even prior to the completion of the second vaccine dose [19]. In addition to adherence to recommended infection control and prevention practices, a critical component of controlling the COVID-19 pandemic and protecting HCP is ensuring high coverage with safe and effective COVID-19 vaccines.
Two clusters of infections in HCWs occurred in the neurosurgery ward of a Wuhan hospital in the early stage of the epidemic in China [20]. Similar to our situation, the two outbreaks were associated with two un-identified index inpatients. Such observation again highlighted the necessity of early deployment of patient traffic control according to their risk stratification. This traffic control bundle policy combined with hand disinfection at checkpoints has been shown to effectively minimize nosocomial SARS infection in HCWs according to a report from Taiwan before [21]. In order to maintain a more sufficient workforce, we postponed unurgent surgeries and procedures, reduced 20% inpatient services during this period. The burnout of clinical staff may result in inadequate hand hygiene practices and lapses in other infection control procedures. In a previous study, reducing the workload of nursing staff is a promising strategy to help control HAI in acute care facilities [22].
Hand hygiene with alcohol-based hand rub is widely used around the world as one of the most effective, simple and low-cost procedures against COVID-19 cross-transmission [23]. Good hand hygiene programs are also dependent on systemic components of the healthcare facility, including access to supplies, how nurses are organized and trained [24]. The hand hygiene education and audit of HCWs are our regular activities every year. Our alcohol consumption had a remarkable 155% increase during the pandemic, indicating a higher awareness of hand washing in staff and patients. It is also well known that improving hand hygiene compliance among HCWs reduces HAIs in hospital settings [25]. There was no obvious change in our major infection control and bundle care policies for the prevention of HAI during this period. The impact of alcohol consumption and hand hygiene on nosocomial infection was not observed in out hospital, where the hand hygiene adherence and compliance rate all reaching 95% in recent years. A small increasing of whole hospital HAI was observed during Mar, 2020 to May, 2020 due to 25% reduction in hospital patient-days and inpatients’ medical conditions during the same period were generally more severe that requiring urgent medical care.
In conclusion, the study demonstrated that practically executing a comprehensive, dynamic infection control policy to stop viral transmission from the community and further spread within a large hospital could efficiently prevent or mitigate COVID-19 nosocomial outbreak, while maintain the capacity and core function of the hospital.
Funding
The study was supported by the Ministry of Science and Technology (MOST 109-2327-B-182-002), Taiwan.
Ethics approval and consent to participate
This research was approved by the Chang Gung Medical Foundation Institutional. Review Board (202001179B0).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Acknowledgments
The authors acknowledge the infection control nurses, clinical microbiologists, and physicians in the Infection Control Working Group of Chang Gung Memorial Hospital: Li-Yueh Huang, Yueh-Pi Chiu, Chun-Sui Lin, Ting-Ying Chung, Kuei-Chu Hou, Mei-Lien Chen, Yu-Chuan Huang, Li-Mei Tsai, Yu-Hua Su, Hsiu-Ping Wu, Shu-Ling Liu, Hsiao-Ni Wang, Li-Fang Chang, Shu-Hui Shen, Yun-Chi Hung, En-Chi Liu, Yi-Chuan Chen, Chiu-Lan Yeh, Hsiao-Chi Chang, Yu-Ching Chen, Ya-Ting Wu, Ching-Yu Wang, Yi-Rong Lu, Mao-Cheng Ge, Jeng-How Yang, Yen-Mu Wu.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Q., Chen Y.C., Chen C.L., Chiu C.H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I., Clapham H.E., et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho S.Y., Kang J.M., Ha Y.E., Park G.E., Lee J.Y., Ko J.H., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz J., King C.C., Yen M.Y. Protecting healthcare workers during the coronavirus disease 2019 (COVID-19) outbreak: lessons from Taiwan's SARS response. Clin Infect Dis. 2020;71:858–860. doi: 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taiwan Center of Disease Control . 2020. Definition of healthcare associated infection.https://www.cdc.gov.tw/En/ [Google Scholar]
- 9.Huang P.Y., Wu T.S., Cheng C.W., Chen C.J., Huang C.G., Tsao K.C., et al. A hospital cluster of COVID-19 associated with a SARS-CoV-2 superspreading event. J Microbiol Immunol Infect. 2021 Jul doi: 10.1016/j.jmii.2021.07.006. S1684-1182(21)00145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folgueira M.D., Munoz-Ruiperez C., Alonso-Lopez M.A., Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedrXiV. 2020 https://www.medrxiv.org/content/10.1101/2020.04.07.20055723v2 2020.04.07.20055723v2 [Preprint]. 2020 [cited April 27, 2020] [Google Scholar]
- 11.Lai X., Wang M., Qin C., Tan L., Ran L., Chen D., et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lessells R., Moosa Y., de Oliveira T. 2020. Report into a nosocomial outbreak of coronavirus disease 2019 (COVID-19) at Netcare St. Augustine's Hospital.https://www.groundup.org.za/media/uploads/documents/staugustineshospitaloutbreakinvestigation_finalreport_15may2020.pdf/ [Google Scholar]
- 13.Lesho E.P., Walsh E.E., Gutowski J., Reno L., Newhart D., Yu S., et al. A cluster-control approach to a coronavirus disease 2019 (COVID-19) outbreak on a stroke ward with infection control considerations for dementia and vascular units. Infect Control Hosp Epidemiol. 2021;42:1333–1339. doi: 10.1017/ice.2020.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703–704. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowell G., Abdirizak F., Lee S., Lee J., Jung E., Nishiura H., et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim G.U., Kim M.J., Ra S.H., Lee J., Bae S., Jung J., et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26:948.e1–948.e3. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton D., Fuchs K., D'Alton M., Gofman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilishvili T., Fleming-Dutra K.E., Farrar J.L., Gierke R., Mohr N.M., Talan D.A., et al. Interim estimates of vaccine effectiveness of pfizer-BioNTech and moderna COVID-19 vaccines among health care personnel - 33 U.S. Sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunbar E., Godbout E., Pryor R., Rozycki H.J., Bearman G. Impact of coronavirus disease 2019 (COVID-19) vaccination program on healthcare worker infections in an academic hospital. Infect Control Hosp Epidemiol. 2021;10:1–2. doi: 10.1017/ice.2021.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Zhou Q., He Y., Liu L., Ma X., Wei X., et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55:2000544. doi: 10.1183/13993003.00544-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen M.Y., Lin Y.E., Lee C.H., Ho M.S., Huang F.Y., Chang S.C., et al. Taiwan's traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2011;77:332–337. doi: 10.1016/j.jhin.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimiotti J.P., Aiken L.H., Sloane D.M., Wu E.S. Nurse staffing, burnout, and health care–associated infection. Am J Infect Control. 2012;40:486–490. doi: 10.1016/j.ajic.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . 2020. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected.https://www.who.int/publications/i/item/10665-331495/ [Google Scholar]
- 24.Peters A., Lotfinejad N., Simniceanu A., Pittet D. The economics of infection prevention: why it is crucial to invest in hand hygiene and nurses during the novel coronavirus pandemic. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet D., Hugonnet S., Harbarth S., Mourouga P., Sauvan V., Touveneau S., et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]