Vaccine-induced immunity is crucial to combat the COVID-19 pandemic, but titres of antibodies against the SARS-CoV-2 spike protein 1 (S1) can decrease over time.1 The efficacy of a third vaccine dose was recently reported in people aged older than 60 years who had received two doses of BNT162b2 (Pfizer-BioNTech) at least 5 months earlier.2 A significant proportion of patients under immunosuppression (solid organ transplant recipients and patients with autoimmune diseases) with a previously inadequate anti-S1 response after two vaccine doses seroconverted after an additional vaccine dose.3, 4 Lower seroconversion rates than in healthy controls have been reported in patients with rheumatic diseases receiving immunomodulatory therapies.5, 6
In line with recommendations from Swiss health authorities and after obtaining approval by the Ethical Committee of St Gallen, Switzerland, a third vaccine dose was offered to all patients with rheumatoid arthritis who participated in the RECOVER trial and who had not developed an anti-S1 response within 12 weeks after the standard vaccination regimen. Of note, disease-modifying antirheumatic drugs (DMARDs) had not been paused during the previous vaccination period.
Written consent was obtained from all patients. Serum samples were collected before and 2 weeks after the third vaccination. Quantitative antibody testing was performed using the Roche Elecsys Anti-SARS-CoV-2 spike subunit 1 assay (West Sussex, UK), which measures antibodies against SARS-CoV-2 S1 (range 0·4–2500 U/mL) and against SARS-CoV-2 nucleoprotein to identify patients with asymptomatic SARS-CoV-2 infection. The results of this assay have been demonstrated to correlate with in-vitro neutralisation of SARS-CoV-2 with a suggested cutoff level of 133 U/mL.7
17 patients with rheumatoid arthritis who showed no or minimal serological response to two doses of an mRNA-based anti-SARS-CoV-2 vaccine were eligible to receive a third dose between July 14 and August 25, 2021. Baseline characteristics are shown in the appendix. Vaccine from the same manufacturer was used for all three doses. Most patients were being treated with a combination of a conventional synthetic DMARD and a biologic (five [29%] patients) or a Janus kinase (JAK) inhibitor (five [29%] patients). The other patients were being treated with monotherapy (conventional synthetic DMARD, n=1; biological DMARD, n=3; or JAK inhibitor, n=3). 16 of 17 patients agreed to temporarily discontinue DMARD therapy: methotrexate and JAK inhibitors were paused 1 week before and restarted 2 weeks after the third vaccine dose, and biological DMARDs were paused 2 weeks before and restarted 2 weeks after the third vaccine dose. One patient stayed on leflunomide and a tumour necrosis factor inhibitor because of a previous relapse of concomitant Crohn's disease.
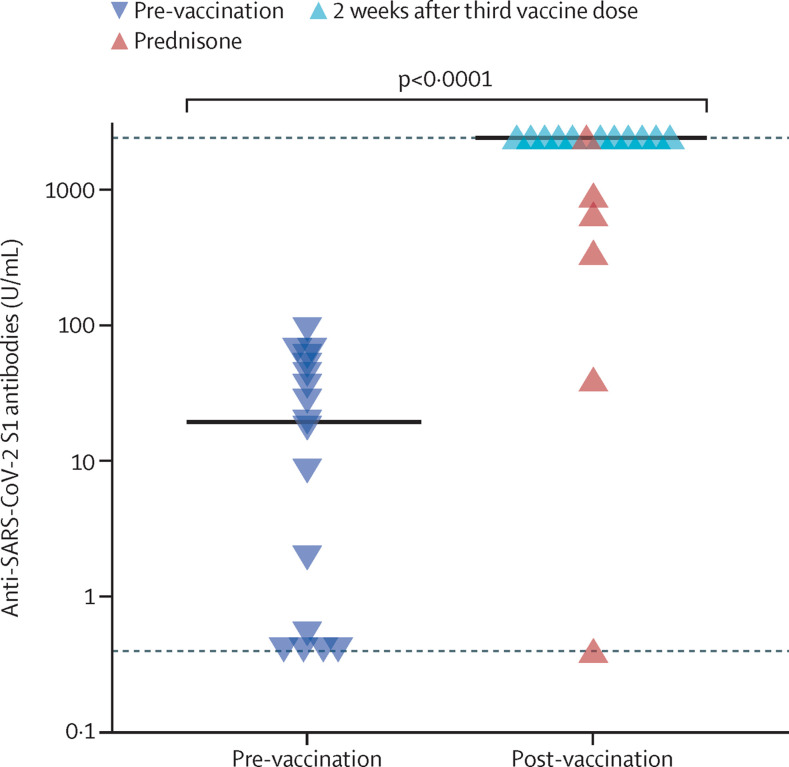
Low or absent anti-S1 antibodies were confirmed immediately before the third vaccine dose (median 19·5 U/mL [IQR 0·45–48]). 2 weeks after the third vaccine dose, a significant increase in anti-S1 antibodies was observed (median 2500 U/mL [IQR 798–2500]; p<0·0001). 12 (71%) patients had maximal anti-S1 titres (assay ceiling at 2500 U/mL), and three patients had moderate anti-S1 titres (figure ). Two patients who continued on 5 mg prednisone daily, did not have titres above the threshold of 133 U/mL after the third vaccine dose despite pausing DMARD medication; one of these patients had received a combination of methotrexate and baricitinib, and the other was on leflunomide, hydroxychloroquine, and anakinra.
Figure.
Anti-SARS-CoV-2 S1 serum antibodies from patients with rheumatoid arthritis
Patients with rheumatoid arthritis eligible for an additional vaccine dose (n=17) before and 2 weeks after their third vaccination. Symbols show individual values, horizontal bars show medians, horizontal dotted lines show lower (0·4 U/mL) and upper (2500 U/mL) detection limits. Red triangles show anti-S1 titres for patients on prednisone. Statistical analysis was performed using the Mann-Whitney U test for non-parametric data. S1=spike protein 1.
Six (35%) patients used concomitant prednisone at a median dose of 5 mg (IQR 5–5) daily. Anti-S1 titres were significantly higher in patients not taking prednisone than in patients on prednisone (median 2500 U/L [IQR 2500–2500] vs 515 U/mL [119–859]; p=0·001).
Local pain was reported in six (35%) patients and systemic vaccine associated side-effects were reported in nine (53%) patients following the third vaccine dose. All patients had stable disease before DMARDs were paused. Before the third vaccine dose (with methotrexate, JAK inhibitors, and biological DMARDs already discontinued), nine (53%) patients remained in remission or had low disease activity, whereas eight patients developed at least moderate disease activity according to the Clinical Disease Activity Index. Two patients with low disease activity before the third vaccine dose flared and an increase in disease activity to moderate–high was noted. In contrast to organ transplant recipients who require continuous immunosuppression to prevent graft rejection, temporary discontinuation of DMARD therapy in patients with rheumatoid arthritis could increase vaccine-induced immune responses. A randomised controlled trial demonstrated an increase in vaccine immunogenicity in response to an influenza vaccine in patients with rheumatoid arthritis who paused methotrexate for 2 weeks after vaccination without increases in disease activity.8 Of note, mRNA vaccines are unique with regard to intracellular processing, antigen presentation, and immunogenicity. Due to the uncertainty about whether and when patients with an inadequate response to three doses of an mRNA-based SARS-CoV-2 vaccine would be eligible for subsequent doses and the lack of approved vector-based or protein-based vaccines in Switzerland, we recommended this treatment discontinuation interval to our patients receiving third doses of vaccine to optimise the vaccine-induced anti-S1 response.
The importance of an adequate vaccine-induced humoral immune response against SARS-CoV-2 is supported by the observation that breakthrough infections in fully vaccinated health-care workers correlated to lower titres of neutralising antibodies.9 It is currently unknown whether immunocompromised patients would get more benefit from an additional dose of the same or a different vaccine.10 Our data suggest that temporary discontinuation of DMARD therapy represents an option to increase vaccine immunogenicity, but the risk for worsening of rheumatoid arthritis disease activity needs to be considered. Of note, continuous use of low-dose prednisone (median dose 5mg daily [IQR 5–5]) resulted in lower anti-S1 titres.
Limitations of this report are the small cohort size, a short follow-up, and the use of a numerical anti-S1 response cutoff as possible correlates of vaccine-induced protection.
In summary, our data demonstrate that a homologous additional vaccine dose and temporary discontinuation of DMARD therapy results in a significant anti-S1 response in the majority of patients with rheumatoid arthritis who did not have an anti-S1 response to a previous standard two-dose vaccination regimen. Increase in disease activity during DMARD discontinuation was observed in almost half of the patients. Studies with larger patient cohorts will allow analysis of the effect of different DMARD regimens on the kinetics of anti-S1 titres and whether and how long treatment discontinuation is necessary to optimise vaccine-induced anti-S1 responses.
KS contributed to project administration, investigation, formal analysis, visualisation, and review and editing of the report. NV and SP were involved in investigation, formal analysis, and development of methodology. BL contributed to conceptualisation, investigation, and formal analysis. WCA was involved in investigation and development of methodology. JvK contributed to conceptualisation, investigation, and review and editing of the report. AR-R was the project lead, and was involved in conceptualisation, writing the original draft of the report, investigation, and formal analysis. KS reports support for travel or meeting attendance from AbbVie Germany. WCA reports grants from the Gottfried & Julia Bangerter-Rhyner Stiftung, FUNGINOS, and the Swiss National Science Foundation; an internal grant from Kantonsspital St Gallen; honoraria from Pfizer and Medscape; and compensation for participation on a data safety monitoring board from Merck Sharp & Dohme and Sanofi. JvK reports honoraria from AbbVie, Bristol Myers Squibb (BMS), Boehringer-lngelheim, GlaxoSmithKline, Menarini, Novartis, Pfizer, and Sanofi. AR-R reports consulting fees from AbbVie, Gilead, Lilly, BMS, and Sanofi; honoraria from AbbVie, Pfizer, Sanofi, UCB, BMS, Lilly, Gilead, and Roche; payment for expert testimony from AbbVie and Gilead; support for travel or meeting attendance from Sanofi, Roche, and AbbVie; and compensation for participation on a data safety monitoring board from R-Pharm. All other authors declare no competing interests.
Supplementary Material
References
- 1.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly CM, Teles M, Frey S, et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221206. published online Sept 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubbert-Roth A, Vuilleumier N, Ludewig B, et al. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021;3:e470–e472. doi: 10.1016/S2665-9913(21)00186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 7.Resman Rus K, Korva M, Knap N, et al. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.