In their response to our manuscript, Hasmann et al. [1] describe important aspects of the quantitative antibody response after SARS-CoV-2 immunization. Slight differences in the response rates in different studies may also relate to different SARS-CoV-2 related test systems being used. Hereby, we are pleased to report the antibody and T-cellular response levels to SARS-CoV-2 vaccination in the largest cohort of dialysis patients reported to date [2]. De novo reactivity of anti-spike S1 IgG antibodies - Figure 1a (QuantiVac from Euroimmun[3]), anti-SpikeS1 receptor binding domain (RBD) IgG antibodies - Figure 1b (Euroimmun [4]), and de novo T-cellular SARS-CoV-2 immune response measurements as assessed by an specific interferon-γ release assay (IGRA, Euroimmun [2,5,6]) at the T2 time point are included in this letter. The T2 time point includes blood samples from study participants eight weeks after the first vaccination and five (BNT162b2 mRNA) / four weeks (mRNA-1273) after booster vaccination.
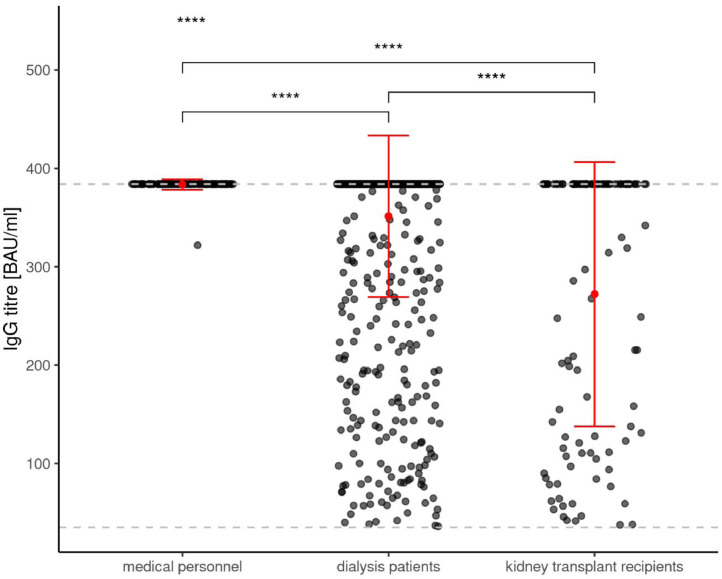
Figure 1a.
shows the de novo prevalence of anti-Spike S1 immunoglobulin G antibody levels in humoral responsive participants to SARS-CoV-2 mRNA vaccination at the time point T2 in medical personnel, dialysis patients and kidney transplant recipients.
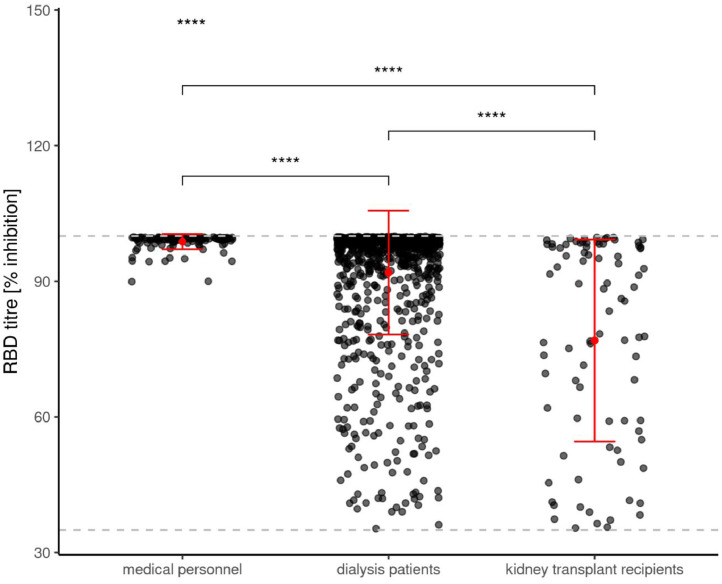
Figure 1b.
shows the de novo prevalence of anti-Spike S1 immunoglobulin G antibody against receptor binding domain (RBD) levels in humoral responsive participants to SARS-CoV-2 mRNA vaccination at T2 in medical personnel, dialysis patients and kidney transplant recipients.
We reported an only marginally lower seroconversion rate of 95.3% in dialysis patients compared with an immunocompetent medical personnel group. Focusing on antibody levels in positively responding study participants, clear cut differences in dialysis patients can be appreciated when compared to the medical personnel group (see figure 1). The quantitative anti-Spike S1-IgG levels in responsive participants are lowest in kidney transplant recipients, intermediate in dialysis patients and highest in medical personnel (Figure 1a). Hereby, anti-Spike S1 IgG antibody levels are beyond the upper limit of 384 BAU/ml in all but one of the medical personnel (99.2%), in the majority (85.7%) of dialysis patients, and to a lesser extent (44.6%) in responding transplant recipients. A very similar pattern can be observed regarding anti-Spike S1-RBD antibody levels in all subgroups (Figure 1b) as being considered a surrogate parameter for SARS-Cov-2 neutralizing capacity [4].
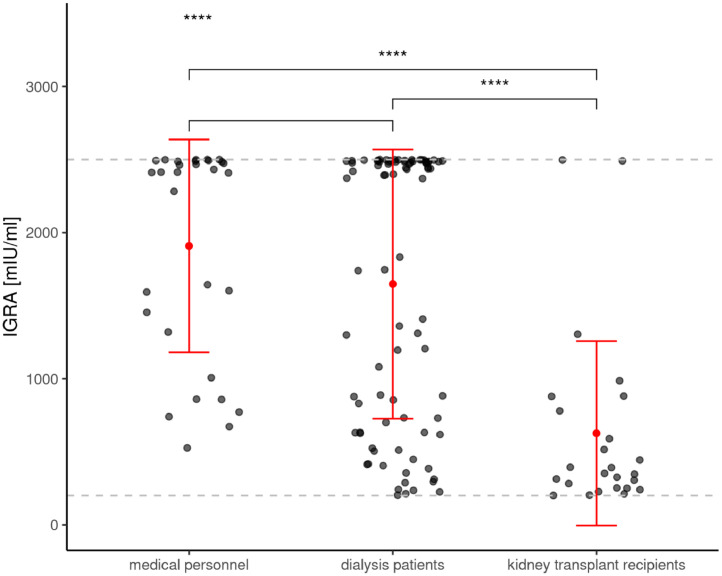
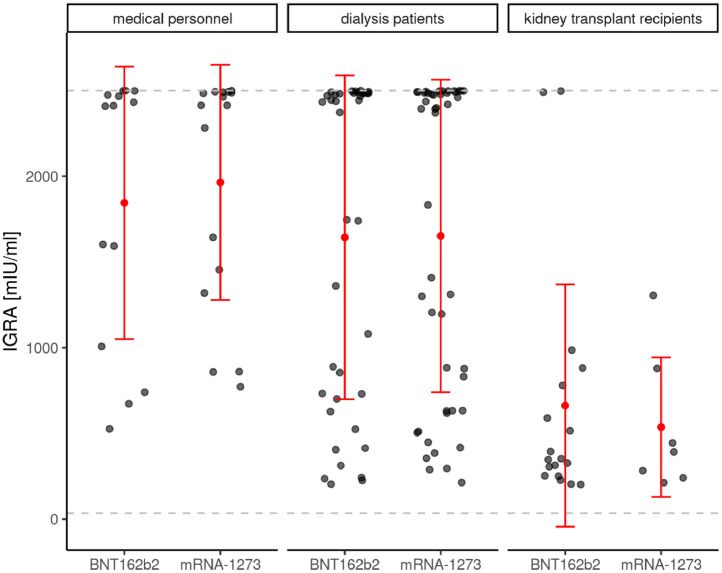
Similar to the seroconversion rate, the de novo T-cellular conversion rate of 78.2% via IGRA measurements in dialysis patients was almost comparable to medical personnel (85.7%) [2]. In contrast to antibody levels, IGRA levels in vaccination responding dialysis patients were not different from medical personnel, while markedly decreased levels were noted in responding transplant recipients (Figure 1c).
Figure 1c.
shows de novo prevalence of T-cellular response levels as assessed by interferon-γ release assay (IGRA) in T-cellular responsive participants (positive means ≥100 mIU/ml) at T2 in medical personnel, dialysis patients and kidney transplant recipients.
In this context, it is likely that these quantitative differences regarding vaccination related humoral and T-cellular responses between all three study groups (considering only responding study participants) may be important for effective protection against SARS-CoV-2 infection[7], especially regarding novel more infectious variants. Future studies, investigating antibody and/or cellular response fading over longer time periods, will be needed to explore threshold values with clinical consequences for loss of vaccination related protection and/or need for booster vaccinations [8,9]. Nevertheless, it also needs to be considered that immunocompromised (ie dialysis patients) or immunosuppressed (ie transplant recipients) populations may have delayed immune responses compared to an immunocompetent population. Delayed humoral responses, as we have already illustrated in the time course of T0 (before 1st vaccination), T1 (before 2nd vaccination) and T2 (8 weeks after 1st vaccination) [2], could theoretically lead to a further increase in the humoral response at later time points [10], [11], [12].
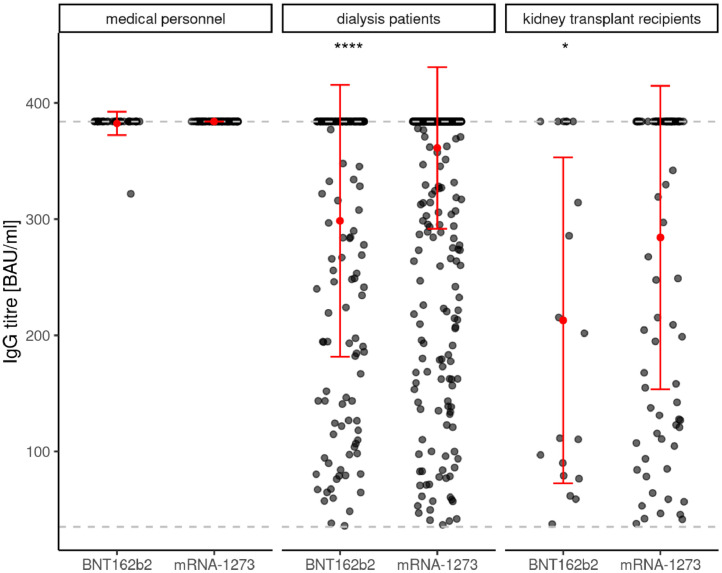
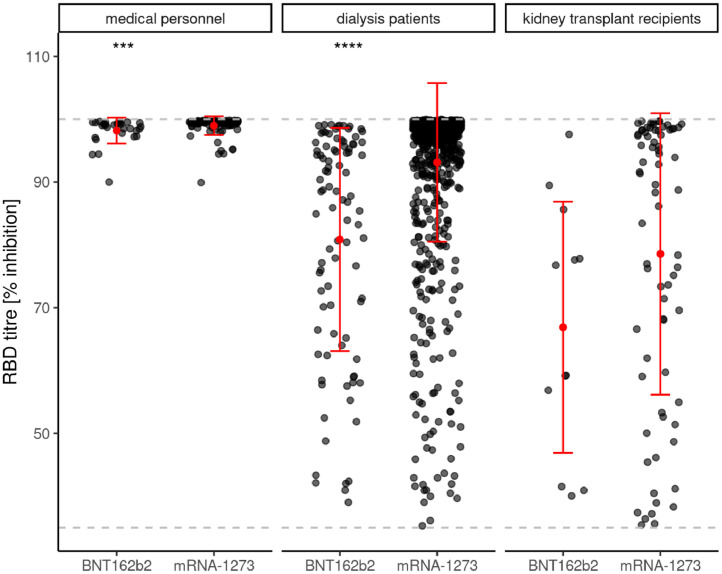
A second major and novel finding of our original study was that the seroconversion success rate in dialysis patients was dependent on the mRNA vaccine type with a clear advantage for mRNA-12732. Comparing vaccination related antibody titres at T2 of positively responding study participants, certain differences can be seen dependent on the use of BNT162b2 mRNA or mRNA-1273 in particular in dialysis patients but also in all three study subgroups (Figure 1d). While levels of anti-Spike S1 IgG antibody titres were independent of vaccine type in medical personnel, these titres were significantly higher in dialysis patients as well as in transplant recipients using mRNA-1273 vaccine compared to BNT162b2 mRNA. Despite a difference between the two vaccine types, anti-Spike S1 RBD antibody titres were all above 90% in medical personnel using either mRNA vaccines. In dialysis patients, this difference in anti-Spike S1-RBD antibody formation was pronounced, with higher levels for mRNA-1273, whereas in transplant recipients there was only a trend in favor of mRNA-1273 (Figure 1e). In contrast, no vaccine dependent differences were observed regarding de novo T-cell immunity according to IGRA levels in any subgroup of responding study participants (Figure 1f).
Figure 1d.
shows the de novo prevalence of anti-Spike S1 immunoglobulin G antibody levels in humoral responsive participants to different SARS-CoV-2 mRNA vaccinations at T2 in medical personnel, dialysis patients and kidney transplant recipients.
Figure 1e.
shows the de novo prevalence of anti-Spike S1 RBD antibody levels in humoral responsive participants to different SARS-CoV-2 mRNA vaccinations at T2 in medical personnel, dialysis patients and kidney transplant recipients.
Figure 1f.
shows the de novo prevalence of T-cellular response levels as assessed by interferon-γ release assay (IGRA) in T-cellular responsive participants (positive means ≥100 mIU/ml) to different SARS-CoV-2 mRNA vaccinations at T2 in medical personnel, dialysis patients and kidney transplant recipients. T2 = 8 weeks after 1st SARS-CoV-2 vaccination and five (BNT162b2 mRNA – Tozinamaran, BioNTech-Pfizer) or four weeks (mRNA-1273 – Elasomeran, Moderna) after 2nd vaccination, respectively. The lower dashed gray lines indicate the threshold for a positive humoral or cellular immune response in each test. The upper dashed gray lines indicate the upper limit of quantitation of the tests. Red dot indicate mean values, red lines show the standard deviation. For this evaluation, all participants with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination were excluded. Humoral vaccination responses were assessed as positive, when de novo production of the IgG antibody to the Spike S1 protein was above lower test limit. A positive IGRA response required de novo positivity above a threshold value of 100 mIU/ml, as being recommended by the manufactures. *Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T1 or T2) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive. **Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
In conclusion, these results demonstrate that even in vaccination responders eight weeks after vaccination start, the level of anti-Spike S1 antibody formation is impaired in immunocompromised dialysis patients. This difference increases further in immunosuppressed kidney transplant recipients, where also the degree of a positive T-cell response is decreased compared to both other groups. In this non-randomised observational study, the use of mRNA-1273 led to higher seroconversion rates and higher antibody but not IGRA titres than BNT162b2 mRNA among dialysis patients and kidney transplant recipients who mounted a response. Longitudinal follow up investigations are under way to explore the clinical consequences of these subgroup and vaccine type specific humoral and T-cellular immunization differences.
Author Contributions
Julian Stumpf and Christian Hugo contributed to study design, data collection, data interpretation, and drafting of the manuscript. All authors accepted the final version of the manuscript.
Ethic declaration
According to the professional code of conduct for doctors (§15) the clinical trial was submitted to the ethical institutional review boards at Technische Universität Dresden (TU Dresden) responsible for the coordinating investigator (BO-EK-45012021), as well as at the University of Leipzig (046/21-lk) and Saxon Medical Association (Sächsische Landesärztekammer – EK-BR-10/21-1) responsible for further participating trial sites.
Financial Disclosure statement
EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany provided antibody ELISAs for this study.
This study was funded by the Else Kröner Fresenius Stiftung, Bad Homburg v. d. H., grant number Fördervertrag EKFS 2021_EKSE.27
The authors declare no further funding was received for this study.
Declaration regarding data sharing
After publication of the primary objective, the data might be provided to interested scientists on request (e.g. for meta-analyses, health related registers or other scientific questions) in an anonymized way within five years, if the members of the DIA-Vacc group agree.
ClinicalTrials.gov Identifier
Table 1.
g) Adapted from Hausmann et al. Comparison of SARS-CoV-2 S Antibody response rate and median antibody titre after vaccination between hemodialysis patients and healthy controls/medical personnel using the median and the interquartile range.
| dialysis patients | p-value | medical personnel (healthy controls) | kidney transplant recipients | p-value | test method | cut-off / unit (upper limit of test) | |
|---|---|---|---|---|---|---|---|
| Danthu et al., CJASN 2021 | RR 81% (N=78) Ab titre 278 (83-526) | < 0.001 | RR 100% (N= 7) Ab titre 1082 (735-1662) | LIAISON SARS-CoV-2 TrimericS IgG (DiaSorin) | > 13 AU/ml | ||
| Espi et al., Kidney Int 2021 | RR 82% (N=106) Ab titre 176 | < 0.001 | RR 100% (N=30) Significant higher titre, but no median reported | Maglumi® SARS-CoV-2 S-RBD IgG test(Snibe Diagnostic) | > 1 AU/ml | ||
| Grupper et al., CJASN 2021 | RR 96% (N=56) Ab titre 2900 (1128-5651) | < 0.001 | RR 100% (N=95) Ab titre 7401 (3687-15471) | SARS-CoV-2 IgG II (Abbott) | > 50 AU/ml | ||
| Jahn et al., vaccines 2021 | RR 93% (N=72) Ab titer titre.5 (89.6-606) | < 0.001 | 100% (N=16) Ab titre 800 (520-800) | LIAISON® SARS-CoV-2 TrimericS IgG (Diasorin) | AU/ml | ||
| Simon et al., NDT 2021 | RR 91% (N=81) Ab titre 171 | < 0.001 | RR 100% (N=80) Ab titre 2500 | Elecsys Anti-SARS-CoV-2 S (Roche) | > 0,4 U/ml | ||
| Paal et al., CKJ 2021 | RR 96.6% (N=179) Ab titre 253.5 (64.2 - 679) | < 0.001 | RR 97.1%(N=70) ##Ab titre 1756 (971.5- 2436.5 | Elecsys Anti-SARS-CoV-2 S (Roche) | > 0,8 U/ml | ||
| Yanay et al., Kidney Int 2021 | RR 90% (N=160) Ab titre 116.5 (66-160) | < 0.001 | RR 100% (N=132) Ab titre 176.5 (142-235) | LIAISON SARS-CoV-2 TrimericS IgG (DiaSorin) | AU/ml | ||
| Stumpf et al., Lancet Regional Health 2021 | |||||||
| IgG S1 SARS-CoV-2-Ab | RR 94.5 % (N = 1074 of 1136) Ab titre 384 (384 - 384) | < 0.001 | RR 98.5 % (N = 132 of 134) Ab titre 384 (384 - 384) | RR 33.6 % (N = 112 of 333) Ab titre 8.0 (3.2 - 127.1) | < 0.001 | SARS-CoV-2-QuantiVac (Euroimmun) | ≥ 35.2 BAU/ml(> 384 BAU/ml) |
Figure 1g) shows a table, adapted from Hasmann et al. For further comparison, SARS-CoV-2 S1 antibody response rate and median antibody titres after vaccination in transplant recipients are also included. Within these groups comparison of all vaccination related study participants (not just the positive responding as shown above in figures 1a-d) are being considered. * response rate (RR), defined as antibody titre above the cut-off of the assay antibody (Ab). # p-value comparing the median Ab titre, if reported ## cohort of non dialysis patients.
Data analysis and graphics were performed using the statistical software R[13] and the ggplot2 package[14]. The following tests were used: Kruskal–Wallis test for comparisons among groups; Wilcoxon test for paired groups. For significance levels in the figures 1a-f, p<0.05 = *, p<0.01 = **, p<0.001 = *** and p<0.0001 = ****.
Declaration of Interests
The authors declare no conflicts of interest.
Acknowledgement
DIA-Vacc- Investigators
References
- 1.Hasmann S, Paal M, Füeßl L, Fischereder M, Schönermarck U. Humoral immunity to SARS-CoV-2 vaccination in hemodialysis patients. Lancet Reg Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stumpf J, Siepmann T, Lindner T. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer B, Torriani G, Yerly S. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin Microbiol Infect. 2020;26(10):1386–1394. doi: 10.1016/j.cmi.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EUROIMMUN Medizinische Labordiagnostika AG aPc. SARS-CoV-2 NeutraLISA. 31.03.2021 2021. https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_F.pdf.
- 5.Brand I, Gilberg L, Bruger J. Broad T Cell Targeting of Structural Proteins After SARS-CoV-2 Infection: High Throughput Assessment of T Cell Reactivity Using an Automated Interferon Gamma Release Assay. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.688436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifacius A, Tischer-Zimmermann S, Dragon AC. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54(2):340-54 e6. doi: 10.1016/j.immuni.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DS, Cromer D, Reynaldi A. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Carr EJ, Kronbichler A, Graham-Brown M. Systematic Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients with Chronic Kidney Disease. Kidney Int Rep. 2021 doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrotri M, Navaratnam AMD, Nguyen V. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rincon-Arevalo H, Choi M, Stefanski AL. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60) doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 11.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I, Gautier G, Perrin P. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA. 2021 doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. 4.0.1 ed. [Google Scholar]
- 14.Wickham H. Springer International Publishing; Cham, Switzerland: 2016. Ggplot2: Elegant graphics for data analysis. 2 ed. [Google Scholar]