Abstract
Introduction
Cytokine storm is one of the consequences of the severe forms of COVID-19 due to excessive immune response. In this study, we investigated the therapeutic effect of plasmapheresis and its role on the inflammatory cytokines levels in patients suffering from severe COVID-19.
Methods
In plasmapheresis group, 22 severe cases of COVID-19 receiving three cycles of plasmapheresis with time interval of 24–36 h and 22 COVID-19 patients as the control group were enrolled. Clinical history and laboratory parameters as well as IL-1, IL-6, IFN-γ and IL-17 cytokines serum levels in the time points of before and after plasmapheresis were studied.
Results
In severe COVID-19 patients, plasmapheresis significantly improved clinical and laboratory parameters such as cough, weakness, fever, blood oxygen saturation and CRP levels. Serum levels of IL-1, IL-6, IFN-γ and IL-17 in the group of patients receiving plasmapheresis, had a significant decrease following plasmapheresis courses. Although only IL-6 level in the control group had a significant decrease between the days 1–14 of disease. Also, at both time points of before and after plasmapheresis, serum levels of IL-1, IL-6, IFN-γ and IL-17 were inversely correlated to blood oxygen saturation.
Conclusion
Based on the obtained results, plasmapheresis therapy in severe forms of COVID-19 can effectively improve the clinical symptoms of the disease and reduce inflammatory markers. Therefore, it is suggested that plasmapheresis can be evaluated in standard treatment protocols for severe forms of COVID-19.
Keywords: COVID-19, Plasmapheresis, Cytokines, Inflammation
1. Introduction
Cytokine storm is one of the consequences of the COVID-19 involvement and subsequent severe immune response that causes severe systemic complications. In viral infections such as COVID-19, the innate immune system induces secretion of pro-inflammatory cytokines centered on IL-1, IL-6 and IFNs due to involvement of innate immunity receptors such as MDA-5 and RIG-1. The massive secretion of mentioned cytokines can cause severe immune system responses and infiltration of immune cells to affected organs such as lungs [[1], [2], [3]].
One of the main treatment strategies of COVID-19 patients is to modulate the immune response and suppress the excessive cytokines secretion. In the early stages cytokine storm in COVID-disease immunosuppressive drugs such as corticosteroids, as well as cytokine antagonist inhibitors are used. Other therapies such as intravenous immunoglobulins and Januskinase (JAKs) inhibitors have also been used to reduce inflammatory cytokine levels in COVID-19 patients which is directly related to reduced mortality [1,[4], [5], [6]].
One of the methods used in the clinic to reduce serum elements such as cytokine and coagulation factors is the physical elimination using plasmapheresis method [4]. Plasma Exchange (TPE) or plasmapheresis was first used in 1950s to control excessive viscosity in multiple myeloma patients. The basis of plasmapheresis is the physical removal of selected elements from the patient's plasma based on two methods of filtration or centrifugation. Studies on the effect of plasmapheresis on the several diseases such as COVID-19, influenza and sepsis showed role of plasmapheresis in controlling the symptoms of improving the general condition [[7], [8], [9], [10]].
Therefore, according to clinical observations, plasmapheresis has been able to improve the critical conditions of various diseases through dialysis and physical removal techniques and provide appropriate clinical outcome. So, in the present study, we aimed to investigate the therapeutic role of plasmapheresis in patients with severe form of COVID-19 and its effect on clinical symptoms, laboratory factors and inflammatory cytokines before and after plasmapheresis. Also, according to the obtained results, we investigated the correlation between the inflammatory cytokines serum levels with clinical and laboratory parameters in patients following plasmapheresis.
2. Material and methods
2.1. Patients and study design
This study was performed in the COVID-19 wards of Shahid Mohammadi hospital in Bandar Abbas, Iran. This study was approved by the ethics committee of Hormozgan University of Medical Sciences (HUMS.REC.1399.174) and Iran Clinical Trials Center (IRCT20200611047735N2). 22 patients who admitted to the COVID-19 wards of Shahid Mohammadi hospital in Bandar Abbas in the period between November and December 2020 were included in the study. All of the included patients were transferred to the ICU and did not improve their clinical symptoms after receiving standard severe COVID-19 treatments. The inclusion criteria were as follows: Patients with positive COVID-19 Real-time PCR result, dyspnea, tachypnea and respiratory rate more than 30 per minute, oxygen saturation lower than 88% and fraction of inspired oxygen/PaO2 ratio lower to equal 300 without oxygen therapy in room air, lung involvement more than 50% on CT scan, patient under mechanical ventilation, COVID-19 patient with multiple organ failure or presence of two highly positive inflammatory markers including: Ferritin greater than 1000 ng/mL, C-Reactive Protein (CRP) above 50 mg/dl, lactate dehydrogenase (LDH) greater than 1000 U/L, lack of proper response to antiviral and corticosteroid therapy.
The exclusion criteria of the patients were as also follows: Hemodynamic instability, coagulation disorders such as thrombocytopenia and dissatisfaction with participating in the study. Written consent was obtained from patients to enter the study. Clinical examinations as well as laboratory findings were recorded before starting and after the last cycle of plasmapheresis.
To compare the serum levels of patients treated with plasmapheresis with those who did not receive plasmapheresis (control group), 22 patients with COVID-19 who were hospitalized due to COVID-19 but did not have criteria of receiving plasmapheresis (according to the inclusion criteria) were selected. At the time of admission to the hospital and 14 days after hospital admission, blood samples were taken and clinical examinations and laboratory and cytokines measurements were done.
In order to perform plasmapheresis, the COVID-19 management scientific committee in Hormozgan University of Medical Sciences was developed the plasmapheresis protocol for the treatment of COVID-19 patients and the present study was performed according to the mentioned written protocol. After selecting patients for plasmapheresis, three sets of plasmapheresis with an interval of 24–36 h were performed based on daily evaluation. Plasmapheresis was performed by centrifugation with continuous flow method. The volume of plasma removed from each patient's blood for plasmapheresis depended on the patient's hematocrit and ranged from 30 cc/kg to 40 cc/kg and crystalloid solutions such as normal saline or 5% albumin solution and Fresh frozen plasma (FFP) was used as the replacement solution. Sodium citrate 4% also was used as an anticoagulant.
2.2. Data collection and inflammatory serum cytokine measurements
In order to evaluate the laboratory and serum levels of cytokines before and after plasmapheresis, blood samples were collected in two time points of before and after plasmapheresis. Then, serum was isolated from patients' blood samples and stored in a freezer at −70C until performing experiments. In order to evaluate the effect of plasmapheresis on the level of inflammatory cytokines, IL-6, IL-1β, IL-17, IFN-γ cytokines assays were done using commercial ELISA kits (Karmania Pars Gen, Iran), according to the manufacturer's instructions. Then, the association between serum levels of cytokines before and after plasmapheresis with clinical factors and laboratory findings were studied.
2.3. Statistical analysis
The statistical analysis was done using GraphPad PRISM software, version 8. Results have been reported as individual value, percentage or mean ± standard deviation. The obtained results were checked by Kolmogorov–Smirnov test for normality distribution. Independent sample t-test and Analysis of variance (ANOVA) tests were used to compare mean difference of variables between two and more than two groups, respectively. Pearson correlation test was to study the correlation between quantitative variables. Fisher's exact and Chi square tests were also used to compare proportions between the groups. P.value < 0.05 was considered as statistically significant.
3. Results
3.1. Demographic data and clinical outcome of plasmapheresis therapy in COVID-19 patients
As shown in Table 1 , 11 (50%) female and 11 (50%) men with the mean age of 62.8 ± 20.4 years and 58.5 ± 15.1 years, respectively were enrolled to the plasmapheresis treatment study. Based on Table 1, there was no significantly difference in terms of age and sex (P > 0.05). The underlying diseases in these patients are also shown in Table 1. In the other side, in the control group, 8 (36.4%) female and 14 (63.6%) male with the mean age of 48.79 ± 11.76 years and 48.13 ± 10.43 years, respectively were included in the study (P > 0.05). Also, the medications used in plasmapheresis and control treatment groups are shown in Table 2 .
Table 1.
Demographic variables of COVID-19 patients in Plasmapheresis and Control groups.
| Variables | Plasmapheresis | P. value | Control | P. value |
|---|---|---|---|---|
| Sex | Male: 11 (50%) Female: 11 (50%) |
>0.99 | Male: 14 (63.6%) Female: 8 (36.4%) |
0.13 |
| Age (Years) | Mean: 60.7 ± 17.3 Max: 88 Min:31 |
Mean:48.55 ± 11.04 Max:66 Min: 27 |
||
| Age in male participants (years) | 62.8 ± 20.4 | 0.58 | 48.79 ± 11.76 | 0.89 |
| Age in Female participants (years) | 58.5 ± 15.1 | 48.13 ± 10.43 | ||
| HIV positive | 1 (4.5%) | – | ||
| Hypertension | 7 (31.8%) | – | ||
| Cardiovascular diseases | 2 (9.1%) | – | ||
| Type II diabetes | 7 (31.8%) | – | ||
| Renal calculi | 2 (9.1%) | – | ||
| End-stage renal disease (ESRD) | 1 (4.5%) | – | ||
| Cerebrovascular accident (CVA) | 1 (4.5%) | – | ||
| Lung involvement (CT Scan) Mild (<30%) Moderate (30-<60%) Severe (>60%) |
Mild: 0 (0%) Moderate: 2 (9.1%) Severe: 20 (90.9%) |
Mild: 1 (4.5%) Moderate:11 (50%) Severe: 10 (45.5%) |
Quantitative results are shown as Mean ± SD.
Table 2.
Medications intake in two groups of Plasmapheresis and Control.
| Medications | Plasmapheresis (n = 22) | Control (n = 22) | Dosage |
|---|---|---|---|
| Kaletra | 18 (81.8%) | 13 (59%) | P.O, Two tabs, twice a day (BD) for 14 days. |
| Methylprednisolone | 18 (81.8%) | 0 | I.V, 50–250 mg, daily. |
| Interferon (IFN) beta-1a 12000000 | 17 (77.3%) | 0 | S.C, Every other day for 5 doses. |
| Atazanavir | 2 (9.1%) | 0 | P.O, 300 mg daily for 14 days |
| Dexamethasone | 1 (4.5%) | 19 (86.4%) | I.V, 8 mg every 8 h, daily according to patient's status. |
| Aspirin (ASA) | 4 (18.2%) | 0 | P.O, 80 mg daily. |
| Vancomycin | 1 (4.5%) | 0 | I.V, 1 gr, twice a Day (BD) for 10 days. |
| Meropenem | 1 (4.5%) | 0 | I.V, Meropenem 1gr every 8 h for 5 days. |
According to Table 3 , there was a significant reduction in the clinical symptoms of fever, muscular pain, weakness, cough and shortness of breath in patients after treating with plasmapheresis. The biggest difference was related to the reduction of shortness of breath in patients after completing the plasmapheresis treatment (P: 0.0004).
Table 3.
Clinical data of COVID-19 patients in Plasmapheresis and control groups.
| Plasmapheresis |
Control |
|||||
|---|---|---|---|---|---|---|
| Clinical signs and symptoms | Before (n = 22) | After (n = 22) | P.value | Before (n = 22) | After (n = 22) | P.value |
| Fever °C) | 36.8 ± 0.4 | 36.7 ± 0.3 | 0.73 | 36.65 ± 0.35 | 26.59 ± 0.22 | 0.45 |
| Chills | 2 (9.1%) | 0 (0%) | 0.48 | 4 (18.8%) | 0 (0%) | 0.1 |
| headache | 4 (18.2%) | 0 (0%) | 0.1 | 9 (40.9%) | 0 (0%) | <0.0001 |
| Sore throat | 2 (9.1%) | 0 (0%) | 0.48 | 9 (40.9%) | 0 (0%) | <0.0001 |
| diarrhea | 3 (13.6%) | 0 (0%) | 0.23 | 2 (9.1%) | 0 (0%) | 0.48 |
| Muscular pain | 7 (31.8%) | 1 (4.5%) | 0.04 | 12 (54.5%) | 0 (0%) | <0.0001 |
| weakness | 13 (59%) | 3 (13.6%) | 0.0040 | 12 (54.5%) | 0 (0%) | <0.0001 |
| Cough | 12 (54.5%) | 2 (9.1%) | 0.0028 | 11 (50%) | 0 (0%) | 0.0002 |
| Shortness of breath | 14 (63.6%) | 2 (9.1%) | 0.0004 | 12 (54.5%) | 0 (0%) | <0.0001 |
Quantitative results are shown as Mean ± SD.
Clinical and laboratory findings in patients receiving plasmapheresis, as shown in Table 4 , heart rate, blood oxygen saturation and CRP levels showed significant difference between two time points of before and after plasmapheresis (P < 0.05). The notable point was the significant changes in blood oxygen saturation from 89.5 ± 4.4% before plasmapheresis to 94.3. 5.2% after plasmapheresis (P: 0.013). Also, serum 3+ positive CRP level dropped from 68.2% of patients before plasmapheresis to 22.7% of patients after plasmapheresis (P: 0.01). According to the final follow-up results of patients in the plasmapheresis group, 6 (27.3%) of patients were connected to mechanical ventilation and 5 (22.7%) of them died. 17 (77.3%) others discharged from the ICU.
Table 4.
Para clinical characteristics of COVID-19 patients in Plasmapheresis and Control groups.
| Plasmapheresis |
Control |
|||||
|---|---|---|---|---|---|---|
| Para clinical variables | Before (n = 22) | After (n = 22) | P.value | Before (n = 22) | After (n = 22) | P.value |
| Systolic pressure | 117.2 ± 17.5 | 115.2 ± 22.7 | 0.69 | 121.2 ± 7.7 | 117.7 ± 7.5 | 0.15 |
| Diastolic pressure | 67 ± 16.9 | 73.3 ± 11 | 0.19 | 83 ± 8.3 | 77.1 ± 8 | 0.06 |
| Respiratory rate (Per minute) | 20 ± 4 | 18 ± 2 | 0.23 | 21 ± 2 | 20 ± 3 | 0.08 |
| Heart rate (Per minute) | 90 ± 7 | 81 ± 10 | 0.006 | 86 ± 8 | 88 ± 9 | 0.41 |
| Oxygen Saturation level (SO2) % | 89.5% ± 4.4 | 94.3% ± 5.2 | 0.013 | 91.7% ± 5.4 | 96.9% ± 1.2 | <0.0001 |
| PaO2/FiO2 (PF) ratio | 65.36 ± 13.83 | 89.66 ± 21.11 | <0.0001 | 182 ± 56.69 | 252.8 ± 52.56 | <0.0001 |
| WBC count × 103/microliter | 6.83 ± 3.15 | 8.71 ± 4.04 | 0.46 | 5.71 ± 2.1 | 7.1 ± 1.7 | 0.0042 |
| Lymphocyte percent | 17.4 ± 9.7 | 13.8 ± 9.9 | 0.119 | 24.1 ± 12.2 | 3.04 ± 11.5 | 0.02 |
| Neutrophil percent | 76.9 ± 10.2 | 80.2 ± 12.5 | 0.19 | 67.9 ± 14.9 | 61.3 ± 13.1 | 0.03 |
| Platelet count × 103/microliter | 195.1 ± 65.3 | 170.3 ± 85.7 | 0.169 | 185.5 ± 43.4 | 190.2 ± 46.8 | 0.73 |
| C-Reactive Protein (CRP) | Neg: 1 (4.5%) 1+: 3 (13.6%) 2+: 3 (13.6%) 3+: 15 (68.2%) |
Neg: 8 (36.4%) 1+:6 (27.3%) 2+: 3 (13.6%) 3+: 5 (22.7%) |
0.01 | Neg: 2 (9.1%) 1+: 3 (13.7%) 2+: 5 (22.7%) 3+: 12 (54.5%) |
Neg: 18 (81.8%) 1+:0 (0%) 2+: 3 (13.6%) 3+: 1 (4.6%) |
<0.0001 |
| Potassium (mg/dl) | 4.36 ± 1.19 | 4.11 ± 0.53 | 0.366 | – | – | – |
| Sodium | 138 ± 4.2 | 137.4 ± 3.8 | 0.593 | – | – | – |
| PTT (second) | 36 ± 8.3 | 38.4 ± 15.6 | 0.495 | 33.7 ± 5.72 | 34.57 ± 8 | 0.69 |
| PT (second) | 14.1 ± 1.7 | 13.1 ± 2.4 | 0.073 | 13.8 ± 1.7 | 14 ± 1.6 | 0.78 |
| International Normalized Ratio (INR) | 1.16 ± 0.18 | 1.12 ± 0.1 | 0.252 | 1.15 ± 0.13 | 1.15 ± 0.2 | 0.42 |
| ALT (IU/L) | 50.3 ± 37.5 | 59.3 ± 46.7 | 0.235 | – | – | – |
| AST (IU/L) | 38.5 ± 15.7 | 43.3 ± 19.19.4 | 0.337 | – | – | – |
| LDH (IU/L) | 668.9 ± 267.4 | 637.3 ± 210 | 0.616 | 607.5 ± 256.2 | 443 ± 119.8 | 0.009 |
| Ferritin (pg/ml) | 878.9 ± 833.4 | 813.9 ± 558.9 | 0.769 | – | – | – |
| Creatinine (mg/dl) | 1.34 ± 0.88 | 1.35 ± 1.34 | 0.984 | – | – | – |
Quantitative results are shown as Mean ± SD.
3.2. Effect of plasmapheresis therapy on the serum cytokine levels in COVID-19 patients
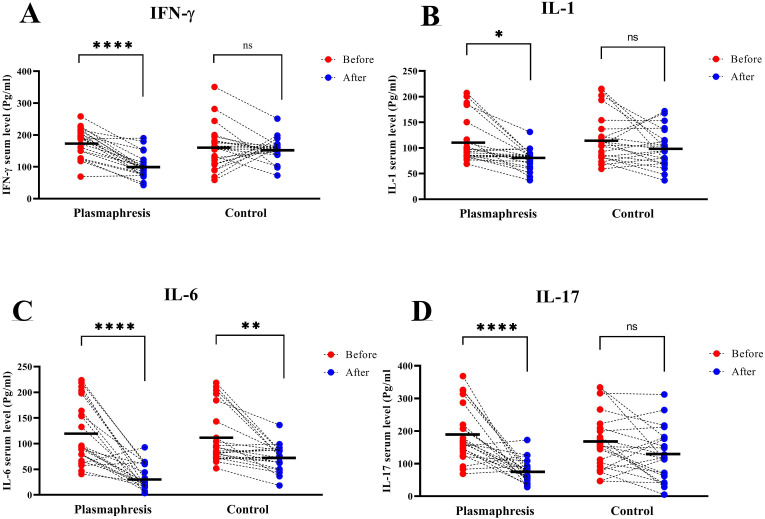
In the study of serum levels of inflammatory cytokines in COVID-19 patients receiving plasmapheresis compared with patients who did not receive plasmapheresis, as shown in Fig. 1 , the serum level of IFN-γ in the group of patients receiving plasmapheresis, before and after plasmapheresis showed a significant reduction from 177.9 ± 45.2 pg/ml and 101 ± 38.2 pg/ml (P < 0.0001), while there was no statistically significant change in the control group (P > 0.05). (Fig. 1A). Serum IL-1 level were also showed a significant reduction from 111.4 ± 43.7 pg/ml and 78.9 ± 20 pg/ml, respectively (P < 0.05), without significant difference between serum IL-1 level on the first and the fourteenth days of disease in control group. (P > 0.05). (Fig. 1B). Also, as shown in Fig. 1C, in the plasmapheresis group, serum levels of IL-6 before and after plasmapheresis were 119.7 ± 60.4 pg/ml and 33.8 ± 23.3 pg/ml, respectively (P < 0.0001). Moreover, in the control group, IL-6 was observed with a significant reduction from 112.2 ± 53.7 pg/ml to 73 ± 24.6 pg/ml in the two time points of the first and the fourteenth days of disease (P < 0.01). Serum levels of IL-17 cytokine, as shown in Fig. 1D, treatment of plasmapheresis significantly reduced serum IL-17 from 189.2 ± 84.4 pg/ml to 76.9 ± 34.3 pg/ml (P < 0.0001). However, the serum level of IL-17 in patients in the control group did not show a significant decrease (P > 0.05).
Fig. 1.
Evaluation of serum levels of cytokines before and after plasmapheresis in patients with Covid-19 compared with the control group. A: Evaluation of IFN-γ serum level in Plasmapheresis group in compare with the control group. B: Evaluation of IL-1 serum level in Plasmapheresis group in compare with the control group. C: Evaluation of IL-6 serum level in Plasmapheresis group in compare with the control group. D: Evaluation of IL-17 serum level in Plasmapheresis group in compare with the control group.
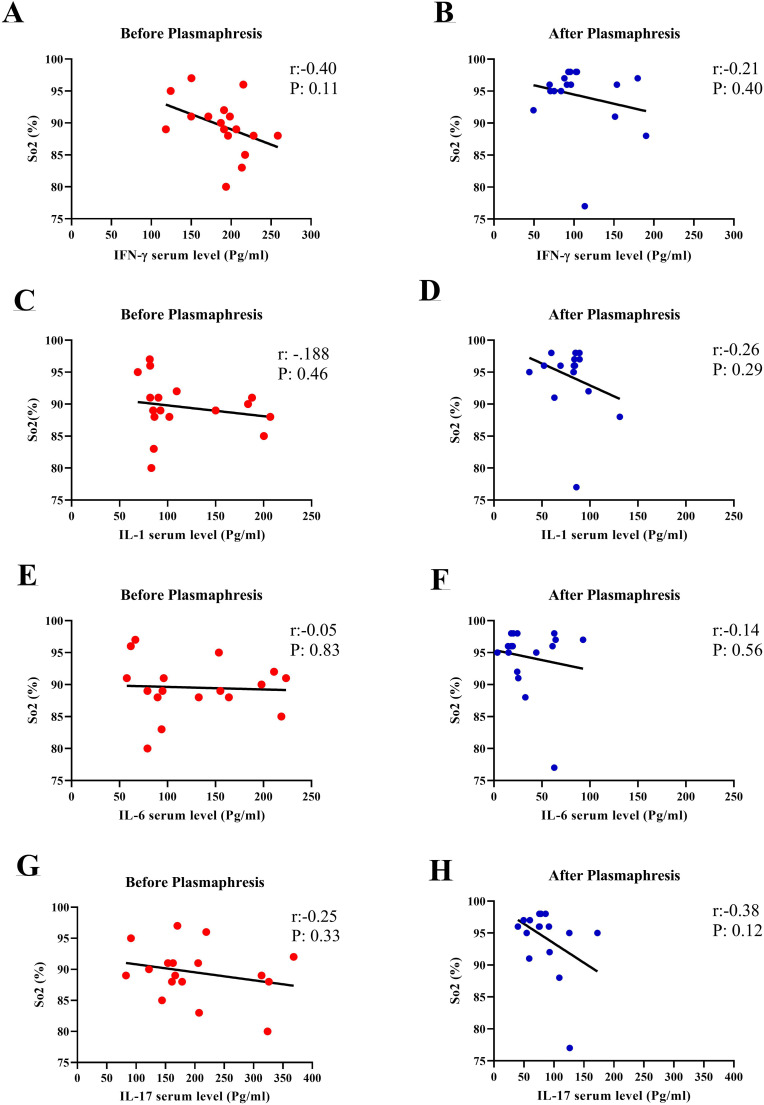
3.3. Correlation between serum levels of cytokines and blood oxygen saturation levels
As seen earlier in Table 4, a significant increase in oxygen saturation levels was observed after plasmapheresis treatment in patients with COVID-19. Fig. 2 also shows the correlation between serum levels of cytokines and blood oxygen saturation levels. In all cases, the serum cytokines were inversely correlated to the blood oxygen saturation levels, so that with increasing levels of cytokines, the amount of blood oxygen saturation levels decreased. This inverse correlation was more highlighted after plasmapheresis, compared to pre-plasmapheresis point. (Fig. 2A–H).
Fig. 2.
Evaluation of the correlation between serum cytokine levels and Saturated oxygen level percent (SO2) in COVID-19 patients, before and after plasmapheresis. A and B: The correlation between IFN-γ serum level and SO2%, Before and after plasmapheresis. C and D: The correlation between IL-1 serum level and SO2%, Before and after plasmapheresis. E and F: The correlation between IL-6 serum level and SO2%, Before and after plasmapheresis. G and H: The correlation between IL-17 serum level and SO2%, Before and after plasmapheresis.
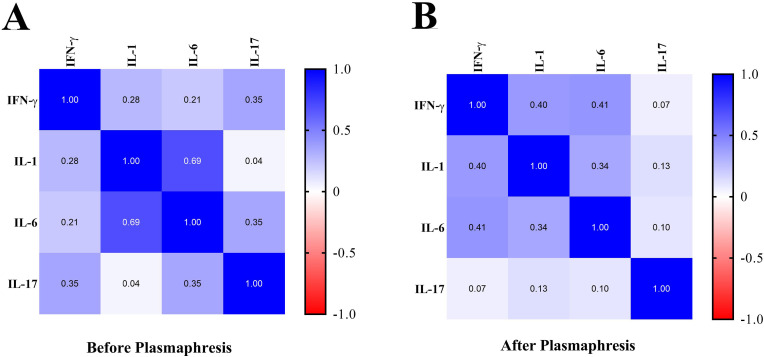
3.4. Correlation between the assessed serum cytokines before and after plasmapheresis treatment
Another finding was the correlation between the assessed serum cytokines before and after plasmapheresis treatment in COVID-19 patients. As shown in Fig. 3 A, before plasmapheresis, there was a direct correlation between the studied cytokines serum levels. The correlation was the most directly among IL-6 and IL-1 cytokines (Pearson correlation: 0.69). Also, the lowest correlation was observed between IL-17 and IL-1 with Pearson correlation equal to 0.04. Also, the correlation between serum levels of cytokines after plasmapheresis is shown in Fig. 3B. In the post-plasmapheresis time point, all cytokines were directly correlated together, with the highest direct correlation between IL-1 and IL-6 (Pearson correlation: 0.34) and the lowest correlation between IL-17 and IFN-γ (Pearson correlation: 0.07).
Fig. 3.
Evaluation of the correlation between serum levels of cytokines, before and after plasmapheresis in COVID-19 patients. A: The Pearson correlation table between serum levels of cytokines, before plasmapheresis. B: The Pearson correlation table between serum levels of cytokines, after plasmapheresis.
4. Discussion
To date, the treatments to control cytokine storm are corticosteroids, intravenous immunoglobulins, Januskinase (JAKs) inhibitors and cytokine antagonists such as IL-6 inhibitors [1,4,5]. However, one study noted the difficulty of deciding to suppress the immune system due to secondary infections and complicated treatment processes [6]. In the present study, plasmapheresis therapy was done to reduce the serum levels of inflammatory cytokines including IL-1, IL-6, IL-17 and IFN-γ. As seen in the results, the use of plasmapheresis significantly reduced the inflammatory cytokines serum levels in COVID-19 patients. In general, rapid control of inflammatory cytokine levels in COVID-19 patients is one of the main strategies in COVID-19 severe conditions.
Plasmapheresis therapy is performed with two methods of discontinuous and continuous flow. In the discontinuous flow method, 125–375 ml of blood is taken from the patient and after mixing with anticoagulants using a high-speed centrifuge, the plasma or other desired plasma components are separated and the rest is returned to the patient blood circulation. In the continuous flow method, the volume of blood that is out of the body at any one time is about 80 ml and performs in a shorter time. As a result, it causes less hemodynamic disturbances which is suitable for patients suffering from severe anemia and younger ages. Plasmapheresis usually takes about 4 h in the discontinuous method and about 1.5 h in the continuous flow method. However, the continuous flow method requires the involvement of two peripheral vessels. The collected plasma is eventually discarded and replaced by alternative components such as Fresh Frozen Plasma (FFP), albumin or crystalloid fluids [11]. The cellular part of the blood is then returned to the patient. Also, anticoagulants such as Citrate, Sodium citrate, Trisodium citrate, ACDA and ACDB are usually used to prevent blood clotting. However, complications of plasmapheresis include hypotension, arrhythmia, thrombocytopenia and electrolyte disorders [4,11].
One of the main obtained results of the present study was the positive therapeutic effect of plasmapheresis on severe COVID-19 and also significant reduction of inflammatory cytokines serum levels in COVID-19 patients. In addition, plasmapheresis was directly related to the improvement of clinical symptoms such as increased blood oxygen saturation level, fever, shortness of breath, as well as laboratory findings such as decreased serum CRP. In addition to the present study, several other studies have demonstrated the role of plasmapheresis in clinical improvement. In a study by Adeli et al., they showed that using plasma exchange, the clinical symptoms as well as cytokine storm were controlled in Eight severe COVID-19 patients with antiviral and corticosteroid therapies failure [8]. In another study, in six COVID-19-associated meningoencephalitis patients, plasmapheresis reduced serum ferritin and improved disease-related clinical signs [9]. Morath et al. also conducted a study on plasma exchange of five COVID-19 patients suffering from respiratory failure, vasopressor-dependent shock, refractory fever, and increased cytokine IL-6. The results showed that plasma exchange decreased serum levels of CRP, IL-6, ferritin, LDH, D-dimer [12].
The above mentioned reports and other studies on the role of plasmapheresis in the treatment of COVID-19 patients confirm the obtained results of our study [9,[13], [14], [15]]. However, the strength of our study is the higher number of samples than previous studies and the measurement of a wide range of clinical and laboratory parameters along with measuring the serum level of inflammatory cytokines. In addition to the studies on COVID-19, in 2004 Kawashima et al. used plasma exchange in influenza infection treatment and they reported reduced IL-6 cytokine levels and the improving condition in the influenza infected patients [16]. Also, in a systematic review study, the results showed that the treatment of patients with sepsis using plasma exchange was associated with a reduction in patient mortality; Which confirms the important role of this type of treatment in infectious diseases [17]. In one of the studies performed on hepatitis C virus (HCV) patients, it was found that treatment with therapeutic apheresis, in addition to improving the symptoms of the disease, also reduces the viral load in the blood of patients [18]. Therefore, this result can be generalized to COVID-19 disease and can be considered as another dimension of this type of treatment in viral diseases.
All of the above reports indicate the effectiveness of plasmapheresis treatment in inflammatory conditions. However, in this method, we observed a decrease in coagulation factors such as a platelets count and induced PT and PTT time, which is one of the side effects of plasmapheresis which requires coagulation considerations before and after plasmapheresis in patients. However, reduction of coagulation factors in COVID-19 patients due to plasmapheresis can also have positive aspects and prevent coagulopathies. Other findings of the present study were related to opposite correlations between serum levels of inflammatory cytokines and blood oxygen saturation levels in COVI-19 patients. As seen in the results, blood oxygen saturation level showed reverse correlation in IL-1, IL-6, IL-17 and IFN-γ cytokines, which confirms the negative role of inflammatory cytokines in the deterioration of COVID-19 patients.
5. Conclusion
In conclusion, based on the results of the present study, plasmapheresis treatment in patients with severe forms of COVID-19 can effectively improve the clinical symptoms of the disease and reduce inflammatory factors in patients. Therefore, it is suggested that this treatment method can be evaluated in standard treatment protocols related to severe forms of COVID-19 in order to prevention of cytokine storm complications in COVID-19 or similar inflammatory conditions.
Authors' contributions
Patient selection, treatments and follow-ups after treatment carried out by MH, MSV, HRS, AG. MH and ARN designed the study SB, HA and ARN performed the immunological examinations. ARN, SB, HA and MH had major contributors in writing the manuscript. All authors also read critically and approved the final manuscript.
CRediT authorship contribution statement
Mehdi Hassaniazad: Conceptualization, Methodology, Resources, Funding acquisition, Writing – original draft. Mohammad Sadegh Vahedi: Investigation, Resources, Writing – original draft. Hamid Reza Samimagham: Methodology, Validation, Investigation, Resources, Writing – original draft. Abdollah Gharibzadeh: Methodology, Validation, Investigation, Resources, Writing – original draft. Shirin Beyranvand: Investigation, Software, Formal analysis, Writing – original draft. Hossein Abbasi: Investigation, Software, Formal analysis, Writing – original draft. Amin Reza Nikpoor: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Project administration.
Declaration of competing interest
All authors declare no conflicts of interest in relation to the subject matter. We have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Acknowledgment
This work was supported by the Hormozgan University of Medical Sciences, Iran. (Grant number: 990342).
References
- 1.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swol J., Lorusso R. Wiley Online Library; 2020. Additive Treatment Considerations in COVID‐19—The Clinician's Perspective on Extracorporeal Adjunctive Purification Techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balagholi S., Dabbaghi R., Eshghi P., Mousavi S.A., Heshmati F., Mohammadi S. Potential of therapeutic plasmapheresis in treatment of COVID-19 patients: immunopathogenesis and coagulopathy. Transfus. Apher. Sci. 2020:102993. doi: 10.1016/j.transci.2020.102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqi F., Dhawan N., Morgan R., Dinh J., Nedd K., Yatzkan G. Treatment of severe COVID-19 with tocilizumab mitigates cytokine storm and averts mechanical ventilation during acute respiratory distress: a case report and literature review. Trop. Med. Infect. Dis. 2020;5(3):112. doi: 10.3390/tropicalmed5030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Zhang Y., Xu X., Du W., Su K., Zhu C., Chen Y., Lei S., Zheng S., Jiang J. Evaluation of plasma exchange and continuous veno‐venous hemofiltration for the treatment of severe avian influenza a (H7N9): a cohort study. Ther. Apher. Dial. 2015;19(2):178–184. doi: 10.1111/1744-9987.12240. [DOI] [PubMed] [Google Scholar]
- 8.Adeli S.H., Asghari A., Tabarraii R., Shajari R., Afshari S., Kalhor N., Vafaeimanesh J. Using therapeutic plasma exchange as a rescue therapy in CoVID-19 patients: a case series. Pol. Arch. Intern. Med. 2020;135:455–458. doi: 10.20452/pamw.15340. [DOI] [PubMed] [Google Scholar]
- 9.Dogan L., Kaya D., Sarikaya T., Zengin R., Dincer A., Akinci I.O., Afsar N. Plasmapheresis treatment in COVID-19–related autoimmune meningoencephalitis: case series. Brain Behav. Immun. 2020;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patidar G.K., Land K.J., Vrielink H., Rahimi‐Levene N., Dann E.J., Al‐Humaidan H., Spitalnik S.L., Dhiman Y., So‐Osman C., Hindawi S.I. Vox Sanguinis; 2021. Understanding the Role of Therapeutic Plasma Exchange in COVID‐19: Preliminary Guidance and Practices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarfraz A., Singh-Makkar S., Sarfraz Z., Hathaway P.T., III Therapeutic plasma exchange and COVID-19: a rapid review. J. Clin. Immunol. Immunother. 2020;6 041. [Google Scholar]
- 12.Morath C., Weigand M.A., Zeier M., Speer C., Tiwari-Heckler S., Merle U. Plasma exchange in critically ill COVID-19 patients. Crit. Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemian S.M., Shafigh N., Afzal G., Jamaati H., Tabarsi P., Marjani M., Malekmohammad M., Mortazavi S.M., Khoundabi B., Mansouri D. Plasmapheresis Reduces Cytokine and Immune Cell Levels in COVID-19 Patients with Acute Respiratory Distress Syndrome (ARDS) Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J., Li T., Yan X., Chen L., Zhang S. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108408. 108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluck W.L., Callahan S.P., Brevetta R.A., Stenbit A.E., Smith W.M., Martin J.C., Blenda A.V., Arce S., Edenfield W.J. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir. Med. 2020;175:106188. doi: 10.1016/j.rmed.2020.106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima H., Togashi T., Yamanaka G., Nakajima M., Nagai M., Aritaki K., Kashiwagi Y., Takekuma K., Hoshika A. Efficacy of plasma exchange and methylprednisolone pulse therapy on influenza-associated encephalopathy. J. Infect. 2005;51(2):E53–E56. doi: 10.1016/j.jinf.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer E., Houston B.L., Kumar A., Abou-Setta A.M., Friesen C., Marshall J.C., Rock G., Turgeon A.F., Cook D.J., Houston D.S. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit. Care. 2014;18(6):1–8. doi: 10.1186/s13054-014-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szczepiorkowski Z.M., Winters J.L., Bandarenko N., Kim H.C., Linenberger M.L., Marques M.B., Sarode R., Schwartz J., Weinstein R., Shaz B.H. Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the Apheresis Applications Committee of the American Society for Apheresis. J. Clin. Apher. 2010;25(3):83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]