Abstract
The activity of the NF-κB family of transcription factors is regulated principally by phosphorylation and subsequent degradation of their inhibitory IκB subunits. Site-specific serine phosphorylation of IκBs by two IκB kinases (IKKα [also known as CHUK] and IKKβ) targets them for proteolysis. IKKα and -β have a unique structure, with an amino-terminal serine-threonine kinase catalytic domain and carboxy-proximal helix-loop-helix (HLH) and leucine zipper-like (LZip) amphipathic α-helical domains. Here, we describe the properties of two novel cellular isoforms of IKKα: IKKα-ΔH and IKKα-ΔLH. IKKα-ΔH and IKKα-ΔLH are differentially spliced isoforms of the IKKα mRNA lacking its HLH domain and both its LZip and HLH domains, respectively. IKKα is the major RNA species in most murine cells and tissues, except for activated T lymphocytes and the brain, where the alternatively spliced isoforms predominate. Remarkably, IKKα-ΔH and IKKα-ΔLH, like IKKα, respond to tumor necrosis factor alpha stimulation to potentiate NF-κB activation in HEK293 cells. A mutant, catalytically inactive form of IKKα blocked IKKα-, IKKα-ΔH-, and IKKα-ΔLH-mediated NF-κB activation. Akin to IKKα, its carboxy-terminally truncated isoforms associated with the upstream activator NIK (NF-κB-inducing kinase). In contrast to IKKα, IKKα-ΔLH failed to associate with either itself, IKKα, IKKβ, or NEMO-IKKγ-IKKAP1, while IKKα-ΔH complexed with IKKβ and IKKα but not with NEMO. Interestingly, each IKKα isoform rescued HEK293 cells from the inhibitory effects of a dominant-negative NEMO mutant, while IKKα could not. IKKα-ΔCm, a recombinant mutant of IKKα structurally akin to IKKα-ΔLH, was equally functional in these assays, but in sharp contrast, IKKβ-ΔCm, a structurally analogous mutant of IKKβ, was inactive. Our results demonstrate that the functional roles of seemingly analogous domains in IKKα and IKKβ need not be equivalent and can also exhibit different contextual dependencies. The existence of cytokine-inducible IKKα-ΔH and IKKα-ΔLH isoforms illustrates potential modes of NF-κB activation, which are not subject to the same in vivo regulatory constraints as either IKKα or IKKβ.
The NF-κB/Rel family of mammalian transcription factors represents a focal point for understanding how extracellular signals induce the expression of specific genes, which are involved in processes as diverse as cell division, inflammation, and apoptosis (programmed cell death) (3–5, 18, 31, 47, 49, 50). The Rel protein family can be classified into two structurally related groups. The first consists of p50 and p52, the products of the NF-κB1 and NF-κB2 genes, respectively (48). These proteins contain a 300-amino-acid sequence known as the Rel homology domain, which contains the information required for dimerization, nuclear translocation, and DNA binding (2, 42). The second group of Rel proteins includes RelA (p65), RelB, and c-Rel (the cellular homologue to the product of the v-Rel oncogene, isolated from the reticuloendotheliosis virus) (18). In addition to a Rel homology domain, these proteins have a transcriptional transactivation domain and form homo- and heterodimers with p50 and p52. The most common form of NF-κB is a heterodimer composed of p50 and RelA subunits.
NF-κB is anchored in the cytoplasm of most nonstimulated cells by a noncovalent interaction with an inhibitory protein, IκB (1). The principal IκB-like proteins are IκBα, -β, and -ɛ (3, 17). Additionally, the p105 and p100 products of the NF-κB1 and NF-κB2 genes can exert inhibitory effects on NF-κB (3, 48). Exposure of cells to proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) or interleukin-1 (IL-1), promotes the dissociation of IκB from NF-κB, unmasking the NF-κB nuclear localization signal, thereby allowing its nuclear translocation to upregulate specific gene expression (3, 48). The ability of TNF receptors to induce NF-κB activation requires the serine-threonine kinase RIP (receptor-interacting protein) (21) and adapter proteins belonging to the TRAF (TNF-receptor-associated factor) family (39), which lack enzymatic activity and share sequence homology at their C-terminal receptor-binding regions. In transfection studies, TRAF2 and RIP may mediate the activation of NF-κB in response to TNF-α, whereas TRAF6, MyD88, and IRAK are required for activation of NF-κB in response to IL-1 (7, 8, 13, 34, 51). However, studies using cells derived from RIP and TRAF2 knockout (KO) mice have shown that RIP is essential for NF-κB induction whereas TRAF2 is required for c-Jun N-terminal kinase (JNK) activation by TNF-α (21, 24, 54).
It has long been appreciated that the major regulatory step in NF-κB activation is the phosphorylation of IκB on two serine residues near the N terminus (serines 32 and 36 of IκBα) (6, 46). These two phosphorylation events target the IκB subunit for ubiquitination and subsequent degradation by the 26S proteosome, thus liberating NF-κB from its inhibitory constraint (9, 41). Initial attempts to identify the kinase(s) responsible demonstrated specific IκB kinase activity to be present in an ∼700-kDa cytoplasmic complex (10). Activation of IκB kinase activity within this complex can be mediated by MAP-ERK kinase kinase 1 (MEKK-1), although the precise mechanism of this pathway remains to be established (19, 22, 23, 35, 36). Attempts to identify the mechanism by which TRAF2 activates the NF-κB pathway led to the isolation of the NF-κB-inducing kinase (NIK) (30). NIK, like MEKK1 is a serine-threonine kinase of the MAPKK family. Phosphorylation of IκB in response to TNF-α requires NIK enzymatic activity (30, 43). However, NIK does not phosphorylate NF-κB directly but via two NIK-interacting kinases called IKKα and IKKβ (IκB kinases α and β) (38, 52). IKKα was independently cloned in a yeast two-hybrid screen with NIK as bait (38) and also by conventional biochemical purification of the major IκB kinase activity induced by TNF-α stimulation of HeLa cells (15, 33). IKKα had also been cloned previously in a search for myc-like genes and was termed CHUK (conserved helix-loop-helix [HLH] ubiquitous kinase) (12). The CHUK gene was shown to encode a 745-amino-acid polypeptide with an amino-terminal serine-threonine kinase catalytic domain, a carboxy-terminal HLH domain, and a leucine zipper-like (LZip) amphipathic α-helix juxtaposed in between the HLH and kinase domains (12). IKKβ, a structurally homologous kinase, was cloned by copurification with IKKα (33, 56) and by database-assisted searches for IKKα-related expressed sequence tags (52).
Two regulatory components of the 700-kDa cytoplasmic complex have also been identified: NEMO (NF-κB essential modulator) (53) (also termed IKKγ and IKKAP1) (32, 40) and IKAP (IKK complex-associated protein) (11). The former is a 50-kDa protein with a putative leucine zipper motif which can bind to IKKα and IKKβ complexes (perhaps via direct interactions with IKKβ) and appears to be essential for agonist-mediated stimulation of NF-κB. NEMO was isolated both by genetic complementation of an NF-κB activation-defective cell line (53) and by purification from the IκB kinase complex (32, 40). IKAP was isolated from affinity-purified IκB kinase complexes as a 150-kDa protein which binds to both NIK and the IKKs, presumably via one or more of its WD protein interaction domains (11). As both NEMO and IKAP have no discernible kinase or other enzyme activity, they appear to function as “scaffold,” or coordinating, proteins which may be required for correct formation of the IκB kinase complex and regulated interaction between the IKK complex and other upstream activators, like NIK and perhaps RIP.
IKKα and -β both possess all the hallmarks of IκB kinases, specifically phosphorylating serines 32 and 36 of IκBα, with both sites requiring phosphorylation in vivo to target IκBα for destruction. Initial studies demonstrated that activation of IKKα and -β occurred in response to NF-κB-activating agents and that mutant, catalytically inactive IKKα and -β blocked NF-κB stimulation by cytokines. Coexpression studies suggested that IKKα and -β can form both homo- and heterodimers via their LZip domains and that an IKKα-IKKβ heterodimer may be the functional IKK unit (33, 52, 56). Recombinant IKKα and IKKβ were shown to specifically phosphorylate IκB substrates in vitro, proving that they are indeed direct IκB kinases (23, 25, 55). Interestingly, site-directed mutation of the HLH domain in IKKα severely impaired its kinase activity without significantly reducing its interaction with IKKβ (56). Similarly, deletion of the IKKβ HLH domain failed to modify its interaction with either IKKα or NIK (52). Recent experiments indicate that the HLH domain of IKKβ functions as an essential positive effector of the kinase's amino-proximal catalytic domain (14). Furthermore, targeted inactivation of the IKKα and IKKβ genes in mice have revealed that only IKKβ is essential for mediating NF-κB activation by inflammatory-response cytokines (27, 28, 45). In contrast, IKKα was not required for activation of IKKβ or NF-κB by proinflammatory stimuli but was instead essential for keratinocyte differentiation (20, 26, 44). It remains to be determined if the essential role of IKKα in the differentiation of epidermal keratinocytes is in keeping with its role as an IκB kinase or if other, unknown IKKα substrates are involved in this developmental pathway. In addition, it remains unclear if other cellular kinases are complementing the loss of IKKα to activate NF-κB in response to proinflammatory signals and if the HLH domain of IKKα is essential for its functional activation, akin to IKKβ.
In this report, we describe the structure and properties of two novel cellular isoforms of IKKα which are produced by alternative mRNA maturation. The first, IKKα-ΔH, is strictly identical to IKKα from its N terminus until amino acid 576 and thereafter lacks the HLH-like domain present in IKKα and IKKβ. The second isoform, termed IKKα-ΔLH, contains an internal deletion removing the LZip domain and generating a premature stop codon in its stead, thereby removing the remainder of the carboxy terminus, including the HLH domain. Unlike IKKα, IKKα-ΔH and IKKα-ΔLH are differentially expressed in various cell lines and normal tissues and predominate over IKKα in activated T lymphocytes and the brain. Remarkably, both of these carboxy-terminally truncated forms of IKKα appropriately phosphorylate IκBα in response to TNF-α signaling with kinetics analogous to those of full-length IKKα, indicating that, unlike those of IKKβ, the HLH and LZip domains of IKKα are not essential for its functional activation.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney cells (HEK293) and HeLa cells were cultivated in Dulbecco's modified Eagle's medium (Gibco/BRL) containing 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin sulfate (50 μg/ml). Explanted BALB/c thymocytes were cultured in RPMI 1640 medium supplemented with penicillin, streptomycin, and 10% fetal bovine serum (Hyclone Inc.). In some experiments, T-cell cultures were stimulated with either 10 ng of the phorbol ester phorbol myristate acetate (PMA) (Sigma)/ml plus 100 ng of the calcium ionophore A23187 (Calbiochem)/ml or 100 ng of the T-cell mitogen concanavalin A (Amersham Pharmacia Biotech)/ml for 7 days prior to harvesting of total cellular RNAs.
cDNA library screening.
An MPC-11 mouse myeloma cDNA library was prepared in λ-ZapII(XR) (Stratagene Inc.) and screened with IKKα-specific probes along with a BALB/c lung λ-Zap II library (Stratagene Inc.) and a BXSB mouse spleen λ-gt-10 library (kindly provided by Konrad Huppi) as previously described (12).
Plasmids.
Murine IKKα was amplified by PCR from pBluescript KS(+) (Stratagene) and cloned into pcDNA3.1 (Invitrogen, Carlsbad, Calif.) in frame with a C-terminal hemagglutinin (HA) epitope tag to generate pcDNA-IKKα-HA, Myc-NIK, IKKα-T7, NF-κB-dependent luciferase, and Rous sarcoma virus long terminal repeat-driven β-galactosidase (RSV–β-Gal) reporter plasmids were all as previously described (16). The kinase-deficient IKKα-(K44A)-HA mutant was generated by PCR (16). pcDNA3.1 FLAG-IKKβ and FLAG-NIK expression vectors were kind gifts of Randy Noelle. IKKα-ΔLH and IKKα-ΔH were cloned by PCR from pBluescript KS(+) in frame with a carboxy-terminal V5 epitope tag in pcDNA3.1/V5/His-TOPO as described by the manufacturer (Invitrogen Inc.). IKKα-ΔCm (amino acids 1 to 451 of IKKα), a recombinant derivative of IKKα-ΔLH lacking its unique 20-amino-acid C-terminal tail, was also cloned by PCR in frame with the C-terminal V5 epitope of pcDNA3.1/V5/His-TOPO. IKKβ-ΔCm (amino acids 1 to 454; structurally analogous to IKKα-ΔCm) was amplified from a human IKKβ construct with the primer pair 5′-TAGAGAACCGCACTGCTTACTGGCT-3′ and 5′-GGCGGCTCGCTGTCCCTGCT-3′ into pcDNA3.1/V5/His-TOPO. IKKα-KΔm (amino acids 1 to 345; specifying the kinase catalytic domain) was amplified from a human IKKα expression vector (a kind gift of Steven Pullen) with the primer pair 5′-CCGATGGACTACAAAGACGA-3′ and 5′-TCAAGTTTCACGCTCAATACGAG-3′ into pcDNA3.1/V5/His-TOPO. A complete NEMO coding sequence (53) was cloned by reverse transcriptase (RT)-PCR with the primer pair 5′-ACACTGTCCTGTTGGATGAA-3′ and 5′-CTCTATGCATCCATGACAT-3′ from the EL4 murine T-cell line. Two independent 1.3-kb full-length clones yielded a sequence identical to that previously published (53) except for one base change (C38T) converting amino acid 13 from threonine to methionine. The NEMO cDNA was subcloned into pcDNA3.1(+) in frame with a carboxy-terminal Myc epitope tag coding sequence. Δ-NEMO (an N-terminal truncation, leaving amino acids 235 to 419) was amplified from a full-length cDNA clone with the primer pair 5′-CCAACTCTTAGACTACGACAG-3′ and 5′-CTCTATGACCTCCATGACAT-3′, initially cloned into the TA cloning vector pCR2.1 (Invitrogen) and subsequently released by EcoRI digestion and recloned in frame with an N-terminal M45 epitope tag into the CMX mammalian expression vector (37).
RT-PCRs of IKKα isoforms.
As indicated in Results, expression of IKKα, IKKα-ΔH, and IKKα-ΔLHa and -b transcripts were distinguished by RT-PCR assays. Total cellular RNAs (5 μg) were extracted from various cell lines and tissues with triazol reagent (Roche Molecular Biochemicals) and reverse transcribed into cDNAs in a 20-μl RT reaction. The RNAs were preincubated with 10 pmol of an anchored oligo(dT) primer, 5′-AGCTCCGGAATTCGGTTTTTTTTTTTTVN-3′, in up to 12 μl of sterile, distilled H2O at 70°C for 10 min and quick chilled on ice. After a brief centrifugation, the RT reactions were performed with a SUPERSCRIPT II RT kit (BRL Life Technologies) as recommended by the manufacturer. Briefly, the reaction was initially supplemented with 4 μl of 5× first-strand buffer (BRL Life Technologies), 2 μl of 0.1 M dithiothreitol and 1 μl of a 10 mM mixture of all four dinucleoside triphosphates. After a second preincubation at 42°C for 2 min, 1 μl (200 U) of SUPERSCRIPT II, a mutant form of Moloney murine leukemia virus RT lacking RNase activity, was added, and the reaction was allowed to proceed at 42°C for 50 min followed by inactivation at 70°C for 15 min. The resultant cDNAs were used directly in 40-μl PCRs containing 20 pmol of each primer, 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.01% Triton X-100, 1.5 mM MgCl2, and 2 U of Taq polymerase (Promega Inc.). All RT-PCRs were performed with a 5′ amplimer present in all forms of IKKα (α, 5′-ACCATTTGCATCCAGAAGTTTATC-3′; bp 1241 to 1264) and one of four 3′ primers: (i) β, 5′-CAGGAGGTCTGTGCTTTAGCTG-3′ (1,761 to 1,782 bp in all forms of IKKα), (ii) δ, 5′-TGCTCAGGTGACCAAACAGCT-3′ [1,861 to 1,881 bp of IKKα and CHUK(ΔLHa)], (iii) γ, 5′-GCAAAAAGAATACCAAAACAGGAT-3′ (1,879 to 1,902 bp of IKKα-ΔH and IKKα-ΔLHb), and (iv) ɛ, 5′-GATAACCAATGACACCAACCTC-3′ (1,620 to 1,641 bp in all forms of IKKα). In some PCRs (see Fig. 1 and 3), 20 pmol of 5′ α was mixed with 10 pmol each of δ and γ. PCRs were submitted to a hot-start protocol (AmpliWax Gems; Perkin-Elmer Inc.) followed by a 4-min preincubation at 94°C and 26 cycles (30 s at 94°C, 1 min at 62°C, and 1 min at 72°C). The reaction products were resolved by 6% polyacrylamide gel electrophoresis (PAGE).
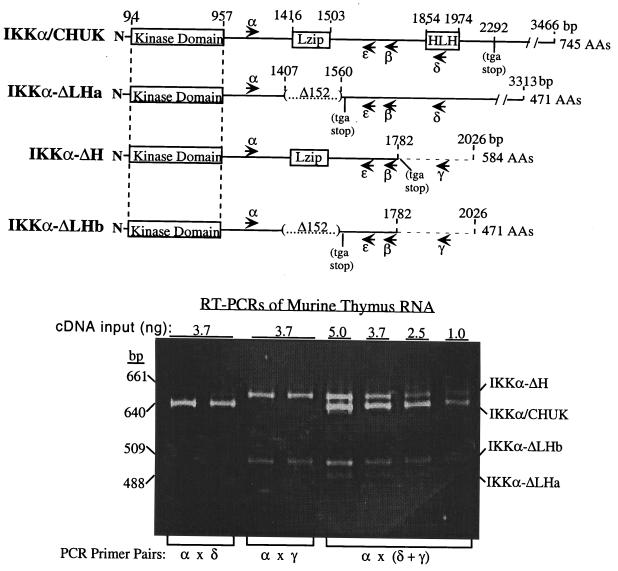
FIG. 1.
Structural comparisons of four IKKα mRNAs and an RT-PCR assay for their relative detection. Comparative diagrams of four different IKKα cDNA clones, each obtained as multiple isolates from three independent cDNA libraries (murine BXSB spleen, BALB/c lung, and MPC-11 plasma cell tumor libraries). A 152-nucleotide internal deletion in the IKKα-ΔLHa and -b isoforms, removing nucleotides 1408 to 1559, deletes the LZip domain and inserts a translation stop codon after a unique 20-amino-acid carboxy-terminal tail downstream of IKKα alanine 451. A novel 3′ NCS in the IKKα-ΔH isoform is shown to replace all IKKα coding sequences downstream of nucleotide 1782, terminating the translation reading frame at a new stop codon eight unique amino acids after IKKα proline 576. IKKα-ΔLHb contains the same 3′ NCS as IKKα-ΔH. The locations of PCR primer pairs used in RT-PCRs discussed throughout the text are shown in each cDNA. The PCR primers α, β, and ɛ anneal to sequences in all four mRNAs, as opposed to primers δ and γ, which only anneal to sequences in the IKKα HLH domain or the novel 3′ NCS in isoforms IKKα-ΔH and IKKα-ΔLHb, respectively. At the bottom, a representative RT-PCR assay of BALB/c thymus total RNA reveals that all four IKKα mRNAs can be reliably detected and quantitated by employing a mixture of primer pairs α, δ, and γ. Varying the input of total cDNA template reveals that the relative intensities of bands in each lane of the ethidium bromide-stained gel are comparable to the relative abundance of their specific mRNA species. The identity of each band was confirmed by restriction enzyme mapping and DNA sequencing. The ratios of expression of each mRNA species in individual lanes were derived by densitometric scanning of individual lanes with the NIH Image program followed by the appropriate length corrections.
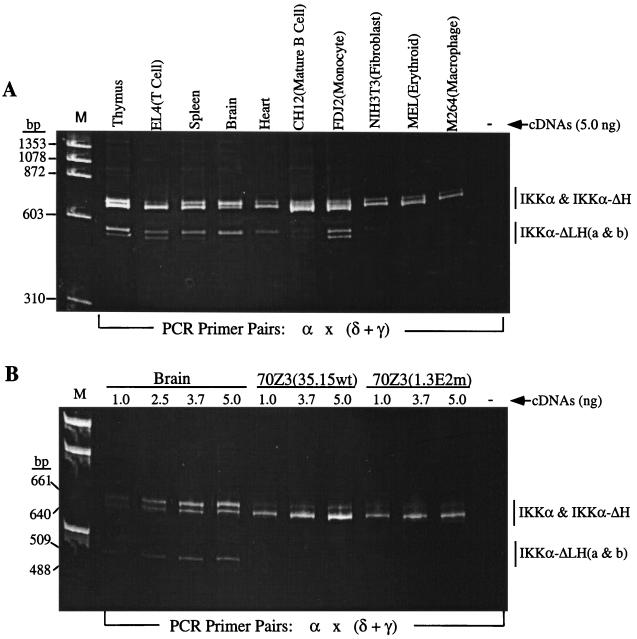
FIG. 3.
RT-PCR analysis of murine tissues and cell lines reveals four IKKα isoforms. (A) Following reverse transcription with an anchored oligo(dT) primer (as described in Materials and Methods), PCRs were performed on 5 ng of total cDNAs with a mixture of primer pairs α, δ, and γ. (B) Comparisons of IKKα RT-PCR products in a cDNA dose-response analysis of brain, 70Z3(35.15wt) and 70Z3(1.3E2 mutant) cells. The PCR products were separated on a 6% polyacrylamide gel and stained with ethidium bromide. The locations and sequences of PCR primers are provided in Fig. 1 and in Materials and Methods, respectively.
Luciferase reporter assays.
HEK293 cells were transfected by the calcium phosphate method essentially as previously described (16) with 4 μg of expression plasmid, together with 0.5 and 0.25 μg of NF-κB luciferase reporter and RSV–β-Gal reference vectors, respectively. The total DNA concentrations in each transfection were kept constant by the inclusion of appropriate empty vector. Twenty-four hours posttransfection, the cells were stimulated where appropriate with TNF-α (10 ng/ml) for 6 h prior to cell lysis. Luciferase and β-Gal assays were performed as detailed in the Promega assay kit. Luciferase activity was found to vary over a threefold range, although activities for individual plasmid preparations were qualitatively identical.
Antibodies and recombinant proteins.
Anti-T7 and Anti-V5 antibodies were obtained from Novagen and Invitrogen, respectively, and recombinant TNF-α was from GIBCO-BRL. GST-IκBα(1-62) was produced as previously described (16) and purified by standard procedures.
Immune complex kinase assays.
HEK293 cells (2.5 × 106 in 10-cm-diameter plates) were transfected with 10 μg of kinase expression plasmid by the calcium phosphate method and stimulated 24 h later in Dulbecco's modified Eagle's medium with the appropriate agonist at 37°C for the times indicated. The cells were washed with ice-cold phosphate-buffered saline and lysed with Triton X-100 lysis buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 40 mM β-glycerophosphate, 200 μM sodium orthovanadate, 10−4 M phenylmethylsulfonyl fluoride, 1 mg of leupeptin/ml, 1 μM pepstatin A, 1% Triton X-100). Proteins from the lysates (500 μg) were incubated with specific anti-HA (12CA5) or V5 epitope (Invitrogen Inc.) antibodies preadsorbed to protein A-Sepharose-coated beads for 2 h at 4°C. The immune complexes were washed three times with Triton X-100 lysis buffer and twice with kinase assay buffer (20 mM HEPES, pH 7.4, 20 mM MgCl2, 1 mM dithiothreitol, 10 mM p-nitrophenylphosphate). IKKα activity was assayed by resuspending the final pellet in 40 μl of kinase buffer containing 50 μM [α-32P]ATP (5,000 cpm/pmol) (Amersham) and 0.25 mg of glutathione-S-transferase GST-IKKα(1-62)/ml. The reaction was incubated for 10 min at 30°C and stopped with Laemmli sample buffer. Samples were resolved by sodium dodecyl sulfate (SDS)-PAGE (10% acrylamide), and phosphorylation was determined by exposure in a PhosphorImager (Molecular Dynamics). Kinase activity was found to vary over a twofold range, although activities for individual plasmid preparations were qualitatively identical.
Immunoblotting.
Cell lysates were prepared in Triton X-100 lysis buffer as described above for the kinase assays. The proteins in cellular lysates were separated by SDS–7% PAGE and electroblotted onto Hybond-C Extra membranes (Amersham). The protein blots were exposed to specific primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies, which were subsequently detected by enhanced-chemiluminescence immunodetection (Amersham) by standard procedures.
In vitro translation.
Constructs in pcDNA3.1 were translated in a Promega rabbit reticulocyte in vitro translation kit either with [35S]methionine (Amersham) or with unlabeled methionine according to the manufacturer's instructions.
RESULTS
Structural comparisons of murine IKKα, IKKα-ΔH, and IKKα-ΔLHa and -b cDNA clones.
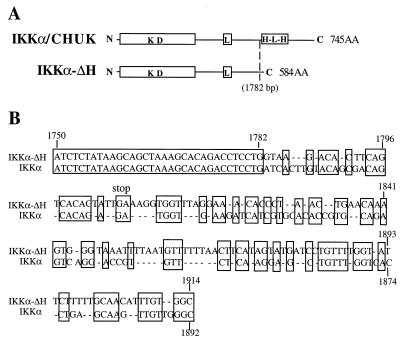
An RT-PCR strategy, devised to clone novel proteins sharing domains with the c-MYC protein, resulted in the isolation of a cDNA clone encoding a new member of the HLH and leucine zipper gene families (12). This novel gene was originally named the CHUK gene, for conserved HLH ubiquitous kinase (12), and was subsequently shown to be the IKKα component of the IκB kinase complex (15, 33, 38). Consequently, we refer to the full-length murine form of the protein as IKKα throughout this report. Subsequent screening of several murine cDNA libraries (BALB/c lung, BXSB spleen, and MPC-11 mouse myeloma libraries) with IKKα-specific probes produced multiple isolates of three other IKKα cDNAs with overlapping and different structural features. Thus, alternative IKKα transcripts are expressed by different cell types. As shown in Fig. 1 and 2, IKKα-ΔH is a unique isoform which is identical to IKKα until residue 576 (nucleotide 1782), where the former cDNA has a novel 3′ noncoding sequence (NCS). The presence of a translation stop, after eight additional codons in IKKα-ΔH, truncates the polypeptide chain 24 amino acids upstream of the HLH domain, replacing the remainder of the protein with a short, 8-amino-acid carboxy-terminal extension (Fig. 2A). In addition, the alternative 3′ NCS in IKKα-ΔH exhibited significant homology with the sequence of the HLH domain, indicating that this 3′ NCS is likely specified by an alternative splice to a duplicated exon which has undergone extensive sequence divergence (Fig. 2B). IKKα-ΔLHa and IKKα-ΔLHb are two other isoforms of the full-length IKKα transcript, both bearing the same 152-bp deletion of nucleotides 1408 to 1559. This deletion excises the LZip domain downstream of residue 451 and then switches the reading frame to generate a translation stop codon after adding a short 20-amino-acid carboxy-terminal tail (Fig. 1). The remainder of the IKKα-ΔLHa mRNA is structurally identical to full-length IKKα mRNA, while the related IKKα-ΔLHb mRNA isoform possesses the same 3′ NCS as IKKα-ΔH, again at nucleotide 1782 (Fig. 1).
FIG. 2.
Nucleotide sequence comparisons of IKKα and IKKα-ΔH. The site of insertion (A) and primary structure (B) of a novel 3′ NCS in IKKα-ΔH are shown. Nucleotides conserved between IKKα and IKKα-ΔH are boxed. The novel 3′ NCS in IKKα-ΔH is also present at the same location in the IKKα-ΔLHb isoform (see maps in Fig. 1).
Unlike IKKα, IKKα-ΔH and IKKα-ΔLHa and -b are differentially expressed.
We employed RT-PCR to investigate the expression patterns of the four IKKα transcripts in a variety of cell types and normal murine tissues. An RT-PCR strategy was designed to coamplify all four isoforms and to distinguish their PCR products on a 6% polyacrylamide gel. We paired a 5′ Pan IKKα amplimer, which is conserved in all four sequences (nucleotides 1241 to 1264; see the location of α primer in Fig. 1), with four different 3′ primers: (i) 3′ Pan IKKα/CHUK 1761-1782, which is between the LZip and HLH domains and present in all four sequences, (β in Fig. 1); (ii) 3′ IKKα 1861-1881 in the HLH domain (δ in Fig. 1); (iii) 3′ IKKα-ΔH 1879-1902 in the 3′ NCS of IKKα-ΔH and IKKα-ΔLHb (γ in Fig. 1); and (iv) 3′ IKKα 1620-1641, which, like primer β, is between the LZip and HLH domains and is present in all four sequences (ɛ in Fig. 1). PCR amplification of anchored oligo(dT)-primed cDNAs with α versus δ produced IKKα- and IKKα-ΔLHa-specific bands of 640 and 488 bp (Fig. 1 and 3). RT-PCR performed with α versus γ yielded IKKα-ΔH and IKKα-ΔLHb bands of 661 and 509 bp (Fig. 1 and 3). Amplifications with a mixture of all three primers produced all four bands with similar relative intensities (Fig. 1 and 3B). The identities of the four bands were confirmed by restriction digestion and DNA sequencing (data not shown). The IKKα-ΔH and IKKα-ΔLHa bands were 21 bp larger than the IKKα and IKKα-ΔLHb species, since the distances between α and δ versus α and γ differed by 21 bp (see the sequence comparisons of IKKα and IKKα-ΔH [Fig. 2]). PCRs performed with increasing doses of cDNA templates indicated that the relative intensities of the individual bands in each amplification were close approximations of the relative quantities of their mRNAs (see the cDNA dose response analyses of thymus, brain, and 70Z3 lines [Fig. 1 and 3B]). To independently determine the relative amounts of the IKKα-ΔLHa and -b isoforms in comparison to IKKα and IKKα-ΔH, RT-PCRs were performed with primer pairs conserved in all four isoforms (IKKα 5′ and 3′ Pan primers) which flanked the site of the 152-bp (LZip) deletion in the IKKα-ΔLHa and -b isoforms (see the locations of primers α, β, and ɛ in Fig. 1 and 4). As shown in Fig. 4, these results are in good agreement with those of the RT-PCRs shown in Fig. 1 and 3.
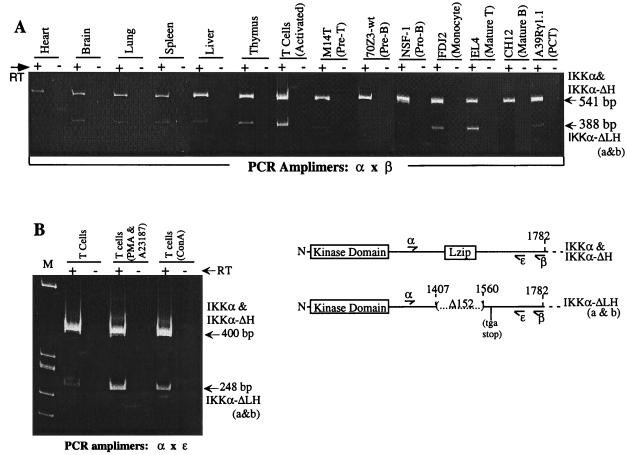
FIG. 4.
RT-PCR analysis to compare the expression of the IKKα-ΔLH isoforms to those of IKKα-ΔH and IKKα. (A) PCRs were performed with 5 ng of the indicated total cDNAs using the primer pair α and β, which produce a band of 541 bp for IKKα and IKKα-ΔH and a band of 388 bp for IKKα-ΔLHa and -b. (B) PCRs were performed with 5 ng of cDNA from unstimulated, in vitro-cultured T cells or from T cells after 7 days of tissue culture with the indicated mitogens (as described in Materials and Methods). Primer pairs α and ɛ produce a band of 400 bp for IKKα-ΔH and IKKα and a band of 288 bp for IKKα-ΔLHa and -b. The PCR products were analyzed on a 6% polyacrylamide gel and revealed by ethidium bromide staining as described in the legend to Fig. 3, but the gel was run 1 h longer to achieve greater fragment resolution. The locations of PCR primer pairs are provided in adjacent diagrams of the amplified portions of the IKKα isoforms, and their sequences are given in Materials and Methods. +, present; −, absent.
IKKα is the major mRNA species in most cell types and tissues, while the three new mRNA isoforms are differentially expressed. Numerous experiments with a variety of murine tissue samples reveal that the relative expression of IKKα-ΔH in comparison to that of full-length IKKα follows a rank order pattern of brain > thymus > spleen > lung = liver > heart, where IKKα-ΔH predominates over IKKα in the brain but is only ∼5% of IKKα in the heart (Fig. 1 and 3A and B and data not shown). In a larger survey of a variety of established cell lines, IKKα-ΔH varied from being almost undetectable to about 20% of IKKα (Fig. 3A and data not shown). In contrast, the IKKα-ΔLH isoforms were more apparent in the thymus (∼30% of IKKα) than in all other tissues (10 to 20% of IKKα) except for the brain, where IKKα and IKKα-ΔLHa were comparably expressed (Fig. 3A, 3B, and 4A and data not shown). In established cell lines, the IKKα-ΔLH isoforms were more strongly expressed in a mature T-cell lymphoma (EL4) (at least 50% of all forms of IKKα) and a monocytic leukemia (FDJ2) (∼25% of IKKα) than in other cell types (including immature B and T lymphocytes, macrophages, fibroblasts, and erythroid and epithelial cells), where they were weakly expressed (Fig. 3A and 4A). Interestingly, the IKKα-ΔLH isoforms were differentially enhanced relative to IKKα and IKKα-ΔH upon mitogenic costimulation of normal T cells with a phorbol ester and a calcium ionophore (PMA and A23187) (Fig. 4A and B) or concanavalin A, a T-cell specific lectin (Fig. 4B). Remarkably, the level of the IKKα-ΔLH isoform in PMA-plus-A23187-stimulated T cells became similar to the combined expression of IKKα and IKKα-ΔH (Fig. 4B). The IKKα-ΔLHb isoform tends to predominate over the ΔLHa species except in the more strongly expressing EL4 and FDJ2 lines, where they accumulate to similar levels. Interestingly, the IKKα-ΔLH isoforms were absent and IKKα-ΔH was barely detectable in the parental 70Z3 pre-B line and in its 1.3E2 (Δ-NEMO) mutant (Fig. 3B), which has been shown to require NEMO complementation to achieve NF-κB activation (53). Stimulation of either parental 70Z3 cells or the 1.3E2 mutant with NF-κB-inducing stimuli like lipopolysaccharide or PMA also failed to induce the appearance of the smaller IKKα isoforms (data not shown).
Attempts to definitively identify either of the endogenous polypeptides corresponding to the two novel, truncated IKKα isoforms have been problematic. This is in part due to the generally low abundances of the full-length IKKα kinase in some cell types, particularly T lymphocytes, where the truncated isoforms were found to predominate over the full-length form by quantitative RT-PCRs. We also lack specific antisera, which would detect only either the IKKα-ΔH or IKKα-ΔLH polypeptides by virtue of their short, unique carboxy termini. All commercially available antibodies that identify IKKα in whole-cell lysates or cytoplasmic extracts also tend to reveal multiple smaller protein species in blotting experiments in addition to the expected 85- to 90-kB full-length IKKα. Therefore, until antibodies directed against either the unique 8 or 20 carboxy-terminal amino acids of IKKα-ΔH or IKKα-ΔLH respectively are available to us (work in progress), it is not possible to prove that smaller protein species bearing the apparent molecular weights of the smaller isoforms are not degradation products of full-length IKKα.
Polypeptides encoded by IKKα-ΔH and IKKα-ΔLH upregulate NF-κB.
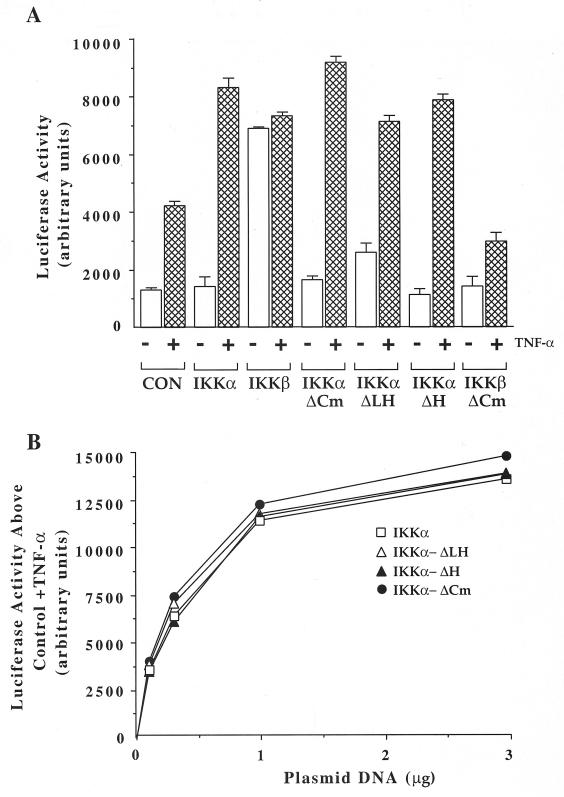
Activation of NF-κB can be readily detected in transient-transfection assays using an NF-κB-dependent reporter gene construct. We next investigated whether the IKKα-ΔH and IKKα-ΔLH proteins, like IKKα and IKKβ, would activate NF-κB and also potentiate its induction by TNF-α. Cotransfection of IKKα leads to a twofold increase in TNF-α-stimulated luciferase activity, with little difference in basal NF-κB-driven luciferase activity. As shown in Fig. 5A and B, IKKα-ΔH and IKKα-ΔLH also increase the ability of TNF-α to stimulate NF-κB-dependent luciferase activity. As the amount of plasmid encoding each IKKα isoform was increased, the TNF-α-induced luciferase activity increased correspondingly in a fashion similar to that of IKKα (Fig. 5B). Western blot experiments conducted on HEK293 cells transfected with each of the IKKα isoforms revealed similar levels of protein expression throughout the dose-response analysis (data not shown). Given that each expression vector is limiting at its lowest DNA input but relative activities remain comparable throughout, these observations are not due to differences attributable to overexpression. Hence, in comparison to IKKα and IKKβ, the two new smaller IKKα isoforms are comparably efficient at potentiating NF-κB activation in response to TNF-α. To verify that the short carboxy-terminal extensions of IKKα-ΔH and IKKα-ΔLH had no unanticipated effects on their activities, we removed the 20-amino-acid tail of the smaller IKKα-ΔLH protein. As shown in Fig. 5 and other figures below, IKKα-ΔCm, a recombinant form of IKKα-ΔLH lacking its 20-amino-acid tail, was equally capable of enhancing TNF-α stimulation of the NF-κB luciferase reporter. However, further deletion of the remaining 106 amino acids of IKKα separating the amino-proximal kinase and LZip domains inactivated the protein kinase assays (Fig. 7A, and data not shown). IKKβ was constitutively active and could enhance the activity of the NF-κB-driven luciferase reporter independently of cytokine stimulation, in agreement with another report (33). In sharp contrast to IKKα-ΔCm, IKKβ-ΔCm (amino acids 1 to 454), a structurally analogous recombinant form of IKKβ (amino acids 1 to 451), was inactive in the NF-κB reporter assay (Fig. 5A). Thus, IKKα does not appear to have the same activation constraints as IKKβ.
FIG. 5.
Truncated IKKα isoforms and full-length IKKα activate NF-κB with similar potencies. (A) HEK293 cells were transiently transfected with plasmids encoding IKKα, IKKα-ΔCm, IKKα-ΔLH, IKKα-ΔH, IKKβ, or IKKβ-ΔCm as indicated, together with reporter plasmid (NF-κB–luciferase). Control cells (CON) received only reporter plasmid. Twenty-four hours posttransfection, the cells were stimulated or not for 6 h with TNF-α (10 ng/ml) prior to cell lysis and luciferase activity measurement as described in Materials and Methods. The error bars indicate standard deviations. +, present; −, absent. (B) HEK293 cells were transiently transfected with increasing concentrations of vectors encoding HA epitope-tagged IKKα and IKKα-ΔCm or V5 epitope-tagged IKKα-ΔLH and IKKα-ΔH, along with reporter (NF-κB–luciferase) and reference control (RSV–β-Gal) plasmids. Twenty-four hours posttransfection, the cells were stimulated for 6 h with TNF-α (10 ng/ml) prior to lysis and luciferase measurements. The data represent the increase in activity above that obtained with TNF-α alone and are the means of two experiments (n = 2). Error bars are not shown for clarity, but the error was less than 4% of the mean in all cases. Variations in transfection efficiencies were corrected against a cotransfected RSV–β-Gal control vector, which is not subject to regulation by NF-κB (16).
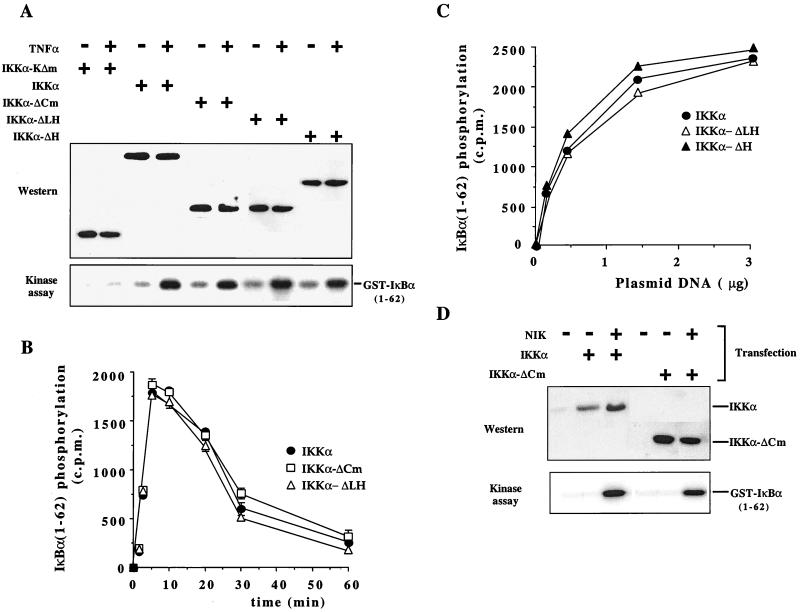
FIG. 7.
Truncated IKKα isoforms are inducible IκBα kinases. (A) HEK293 cells were transiently transfected with plasmids encoding (+) either HA epitope-tagged IKKα or IKKα-ΔCm or V5 epitope-tagged IKKα-ΔLH, IKKα-ΔH, or IKKα-KΔm, as indicated. Thirty hours posttransfection, the cells were stimulated for 5 min with TNF-α (10 ng/ml) prior to lysis and subsequent HA-IKK or V5-IKK immunoprecipitation. The immunoprecipitates were then analyzed for IκBα kinase activity with GST-IκBα(1-62) as a substrate (bottom). Whole-cell lysates underwent immunoblotting to determine the level of expression of the various IKKα constructs (top). (B) In a separate experiment, HEK293 cells were transiently transfected with HA-IKKα, HA-IKKα-ΔCm, or IKKα-ΔLH, and 24 h posttransfection, the cells were stimulated or not for the times indicated with TNF-α (10 ng/ml) prior to cell lysis, HA-IKK immunoprecipitation, and determination of IKK activity. The data are expressed as the increase in 32P incorporation into the GST-IκBα(1-62) substrate relative to the basal activity and are means of single determinations pooled from three experiments (n = 3; the error bars represent ranges). (C) HEK293 cells were transiently transfected with increasing concentrations of plasmid encoding either HA-IKKα, V5 epitope-tagged IKKα-ΔLH, or IKKα-ΔH. Twenty-four hours posttransfection, the cells were stimulated or not for 5 min with TNF-α (10 ng/ml) prior to cell lysis, immunoprecipitation of exogenously expressed IKK, and determination of IKK activity. The data are expressed as the increase in 32P incorporation into the GST-IκBα(1-62) substrate relative to the basal activity and are means of single determinations pooled from two experiments (the error was less than 4%). (D) HEK293 cells were transiently transfected with plasmid encoding epitope-tagged IKKα, IKKα-ΔCm, or NIK as indicated (+), and 30 h posttransfection, the cells were lysed and IKK activity was determined as for panel A. Construct expression levels (top) were also determined.
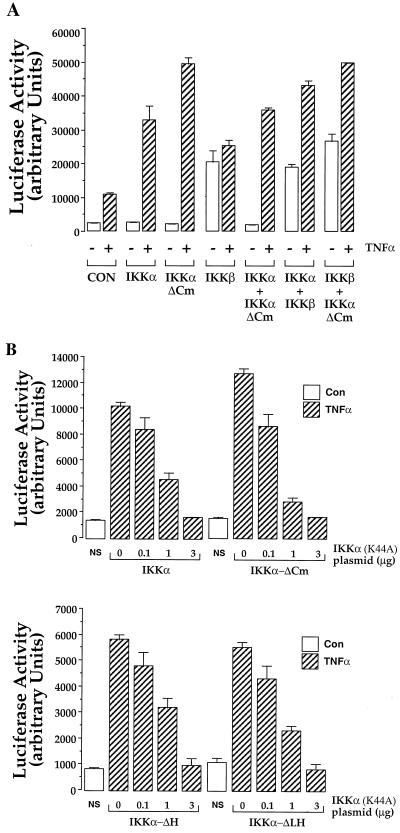
It was previously reported that IKKα and IKKβ coimmunoprecipitate and may function as a heterodimer (33, 52, 55, 56) (see below). We compared the effects of each kinase alone and in pairwise combinations on the TNF-α-dependent induction of NF-κB activity in HEK293 cells. Again, IKKβ was found to be dominant in that it always induced NF-κB-dependent luciferase activity in the absence of TNF-α stimulation (Fig. 6A and data not shown). However, the presence of either IKKα or IKKα-ΔCm and IKKβ or IKKα plus IKKα-ΔCm always led to an increase in TNF-α-stimulated luciferase activity, the magnitude of which was similar to that elicited when either IKKα or IKKα-ΔCm was expressed alone (Fig. 6A). As expected, IKKα-ΔLH and IKKα-ΔH performed comparably in this mixing experiment (data not shown). Since the effects of the carboxy-terminally truncated IKKα isoforms were not additive with either IKKα or IKKβ, we conclude that their NF-κB induction pathways are not completely independent but are at least partially overlapping, perhaps in their dependence on a common, limiting upstream activator like NIK.
FIG. 6.
IKKα and IKK-ΔCm activate NF-κB by overlapping pathways. (A) HEK293 cells were transiently transfected with plasmids encoding IKKα, IKKα-ΔCm, or IKKβ as indicated, either separately (4 μg) or in combination (2 μg each), together with reporter (NF-κB–luciferase) and reference control (RSV–β-Gal) plasmids as in Fig. 5. Control cells (CON) received only reporter plasmid. Twenty-four hours posttransfection, the cells were stimulated or not for 6 h with TNF-α (10 ng/ml) prior to cell lysis and luciferase activity measurement as described in Materials and Methods. +, present; −, absent. (B) HEK293 cells were transiently transfected with plasmid encoding IKKα or IKKα-ΔCm (1 μg per well) (top) or, in an independent experiment, IKKα-ΔLH and IKKα-ΔH (bottom) together with reporter plasmid (NF-κB–luciferase; 0.5 μg per well) and increasing concentrations of plasmid encoding catalytically inactive IKKα (IKKα-K44A). Control cells (NS) received only reporter plasmid. Twenty-four hours after transfection, the cells were stimulated or not (NS) for 6 h with TNF-α (10 ng/ml) prior to cell lysis and luciferase activity measurement. Luciferase activity is expressed as arbitrary units normalized to β-Gal activity. The data are means ± range of duplicates from a single experiment representative of three performed.
The cascade of cytoplasmic proteins leading from TNF-α receptors to phosphorylation of IκB includes NIK and the IKKs (30). Thus, catalytically inactive mutant forms of these proteins are able to attenuate TNF-α-mediated activation of NF-κB (30, 38, 52). If the alternative isoforms of IKKα share a common activator (as suggested by the results in Fig. 6A), then we might expect a catalytically inactive mutant of IKKα to titrate out the ability of an upstream activator to stimulate them. This hypothesis was tested by transiently transfecting HEK293 cells with the NF-κB-dependent reporter construct, either IKKα, IKKα-ΔCm, IKKα-ΔH, or IKKα-ΔLH, and increasing concentrations of plasmid encoding a catalytically inactive form of IKKα, IKKα(K44A) (Fig. 6B). Increasing concentrations of IKKα(K44A) elicit a dose-dependent reduction in the ability of IKKα, IKKα-ΔCm, IKKα-ΔH, and IKKα-ΔLH to potentiate the TNF-α-mediated activation of the NF-κB reporter construct. Hence, some of the upstream components in the activation pathways may be shared between IKKα and its carboxy-terminally truncated isoforms.
IKKα-ΔH and IKKα-ΔLH are TNF-α-inducible IκBα kinases.
Release of NF-κB from its IκBα inhibitor requires the latter's phosphorylation at serines 32 and 36 (6, 46). To assess the relative abilities of IKKα, IKKα-ΔH, and IKKα-ΔLH to phosphorylate IκBα in response to TNF-α stimulation, in-vitro kinase assays were performed with GST-IκBα(1-62) as a substrate in either anti-HA or anti-V5 immunoprecipitates of HEK293 cells transfected with HA epitope-tagged IKKα and IKKα-ΔCm or V5 epitope-tagged IKKα-ΔH and IKKα-ΔLH (Fig. 7A). HEK293 cells transiently transfected with each of the IKKα isoforms expressed similar amounts of immunodetectable proteins with the expected molecular masses (Fig. 7A, top). TNF-α stimulation of HEK293 cells transfected with each IKKα isoform resulted in an increase in immunoprecipitable kinase activity towards GST-IκBα(1-62) (Fig. 7A, bottom). However, further truncation of IKKα-ΔCm by removing its carboxy-terminal 106 amino acids to leave an intact amino-terminal kinase domain (IKKα-KΔm [Fig. 7A]) inactivated its TNF-α-inducible IκBα kinase activity, implying that a block of amino acids residing between the kinase and LZip domains of IKKα are part of a cytokine response domain. As anticipated from the NF-κB reporter assay results, all experiments performed with IKKα-ΔH, IKKα-ΔLH, or the recombinant IKKα-ΔCm produced comparable results, indicating that neither the LZip domain of IKKα-ΔH nor the short carboxy-terminal extensions of either short isoform had significant effects on IκBα phosphorylation in this assay. Indeed, time course experiments revealed that the activation profiles of IKKα, IKKα-ΔCm, and IKKα-ΔLH enzymatic activities in response to TNF-α stimulation were superimposable (Fig. 7B). To determine whether IKKα and its shorter isoforms function equivalently when exogenously expressed at different levels, dose-response kinase assays were performed (Fig. 7C). These experiments revealed that IKKα and its shorter isoforms IKKα-ΔH and IKKα-ΔLH perform in similar TNF-α-activatable manners at different levels of expression. We next explored whether the IKKα upstream activating kinase, NIK, would also activate IKKα-ΔCm. Transfection of HEK293 cells with IKKα-ΔCm, IKKα-ΔH, or IKKα-ΔLH and the upstream activating kinase, NIK, resulted in a similar increase in IκBα kinase activity (Fig. 7C and data not shown), further suggesting that IKKα and its smaller isoforms have common upstream activators. In a separate set of experiments, HEK293 cells were transfected with IKKα or IKKα-ΔLH together with MEKK-1. MEKK-1 failed to appreciably activate IKKα and IKKα-ΔLH but activated JNK (data not shown). Control immune-complex kinase assays performed with a mutated GST-IκBα(1-62) (serines 32 and 36 mutated to alanines) failed to support phosphorylation (data not shown). Hence, similar to full-length IKKα, its shorter cellular isoforms are IκBα kinases, which can be activated by both TNF-α and NIK.
Associations of the IKKα isoforms with IKKβ and NIK.
IKKα possesses functional domains (Fig. 1) known to play roles in protein-protein association, while they are both absent in the IKKα-ΔLH isoform. Cotransfection assays demonstrate that IKKα and IKKβ coimmunoprecipitate (33, 52, 56) and may also form homodimers (55). Even though the absence of both LZip and HLH domains in the IKKα-ΔLH isoform appears to have no effect on its TNF-α activation, it may prevent its interactions with regulatory components of the IKK signalosome complex.
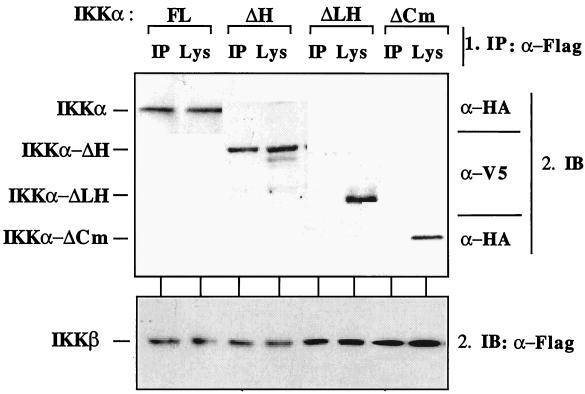
In an in vivo approach to analyzing the association of IKKs, HEK293 cells were cotransfected with IKKβ, IKKα, IKKα-ΔH, IKKα-ΔLH, or IKKα-ΔCm followed by selective immunoprecipitation of either IKKα or IKKβ (Fig. 8). IKKβ coprecipitated with IKKα and its IKKα-ΔH isoform, supporting earlier work showing that the HLH domains of IKKβ and IKKα were not essential for their interaction (33, 52, 55, 56). However, neither IKKα-ΔLH nor IKKα-ΔCm coprecipitated with either IKKβ (Fig. 8) or IKKα (data not shown). Control immunoblots revealed that all three kinases were expressed in each cell lysate (Fig. 8). These results show that interactions between IKKα-ΔLH or IKKα-ΔCm and IKKβ are not essential for their TNF-α-inducible kinase activities.
FIG. 8.
Association of IKKα isoforms and IKKβ in vivo. HEK293 cells were transiently transfected with plasmids encoding epitope-tagged versions of IKKα, IKKα-ΔCm, IKKα-ΔLH, or IKKα-ΔH together with FLAG-IKKβ as indicated. FL, full-length IKKα. Thirty hours posttransfection, the cells were lysed and FLAG-IKKβ was immunoprecipitated. Samples were divided in two, and the presence of IKKα (top) and IKKβ (bottom) in both the IKKβ immunoprecipitates (IP) and the total cell lysates (Lys) were revealed by immunoblotting (IB) with appropriate antibodies. α-Flag, anti-FLAG antibody.
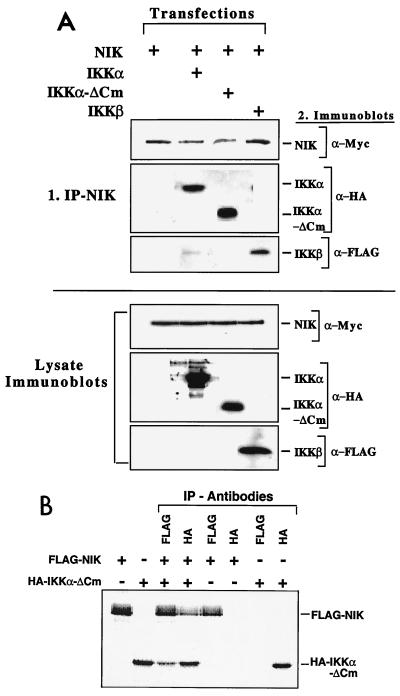
IKKα was identified as being a NIK-binding protein in a yeast two-hybrid screen (38). Deletion of the entire C-terminal tail (up to the leucine zipper) of IKKβ did not prevent its association with the putative upstream activator NIK (52). In transient-expression studies, NIK activated both IKKα and IKKα-ΔCm (Fig. 7C). Hence, the smaller IKKα isoforms are likely to interact with NIK. To directly assay for the ability of NIK to bind to isoforms of IKKα lacking both LZip and HLH protein-protein interaction domains, coimmunoprecipitation experiments were performed with HEK293 cells transiently transfected with IKKβ, IKKα-ΔCm, or IKKα and NIK. As shown in Fig. 9A (top), NIK coimmunoprecipitated with each polypeptide, and as expected, similar results were obtained with IKKα-ΔH and IKKα-ΔLH (data not shown). Immunoblots of cellular lysates revealed that comparable amounts of all transfected proteins were expressed, indicating similar efficiencies of interaction between the different isoforms of IKKα and NIK (Fig. 9A, bottom). An independent in vitro approach was also undertaken to confirm the association of IKKα-ΔCm and NIK (Fig. 9B). HA epitope-tagged IKKα-ΔCm and FLAG epitope-tagged NIK were produced by in vitro translation in the presence of [35S]methionine. These proteins were preincubated for 15 min at 30°C prior to selective immunoprecipitation with either anti-HA or anti-FLAG antibodies. IKKα-ΔCm associates with NIK in this assay, irrespective of whether NIK or IKKα-ΔCm was immunoprecipitated first. Quantitative analyses revealed that the proportions of IKKα-ΔCm and NIK that were coprecipitated represented ∼80% of the proteins (Fig. 9B). Hence, the IKKα-ΔCm isoform of IKKα lacking the LZip and HLH domains efficiently coimmunoprecipitates with NIK in vitro and in vivo.
FIG. 9.
IKKα-ΔCm associates with NIK in vivo and in vitro. (A) (Top) HEK293 cells were transiently transfected with plasmids encoding HA-IKKα, HA-IKKα-ΔCm, FLAG-IKKβ, Myc-NIK, or combinations thereof. Thirty hours posttransfection, NIK was immunoprecipitated and samples were resolved on SDS–9% PAGE gels, which were immunoblotted with appropriate primary antibodies. HA-p44MAPK was used in cotransfections as a negative control and did not associate with NIK (not shown). (Bottom) Comparable expression of each transfected construct was verified by immunoblotting of transfected cell lysates (50 μg of protein) with appropriate antibody. The data are from one experiment done twice. +, present; −, absent. (B) HA-IKKα-ΔCm and FLAG-NIK were translated in vitro with [35S]methionine and preincubated for 15 min at 30°C prior to immunoprecipitation with either anti-HA (IKKα-ΔCm) or anti-FLAG (NIK) antibodies as indicated. Proteins from immunoprecipitates were resolved on SDS-PAGE (8% acrylamide gels), dried, and exposed in a phosphorimager.
IKKα-ΔH, IKKα-ΔLH, and IKKα-ΔCm polypeptides fail to associate with NEMO.
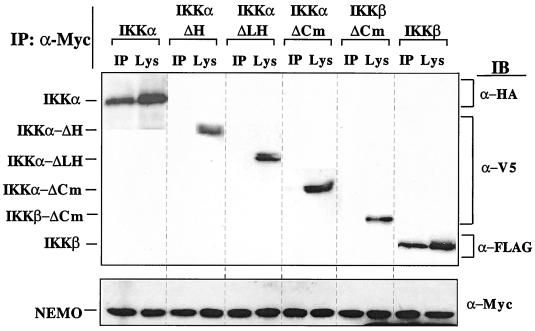
Complementation rescue of two cell types which were unresponsive to NF-κB-activating agonists, along with purification of the IκB kinase complex, resulted in the identification and cloning of NEMO (32, 40, 53). NEMO appears to be a prerequisite for activation of NF-κB. It does not exhibit enzymatic activity but possesses a putative LZip domain and several coiled-coil motifs which may mediate interaction with other elements of the NF-κB signaling cascade. In vitro-translated NEMO coimmunoprecipitates with IKKβ and to a lesser extent with IKKα (40, 53). Cotransfection studies were performed to determine whether each IKKα isoform interacted with NEMO. Transient transfection of HEK293 cells with IKKβ, IKKα-ΔH, IKKα-ΔLH, IKKα-ΔCm, IKKα, or NEMO followed by NEMO immunoprecipitation revealed that NEMO associated with both IKKα and IKKβ in vivo but failed to associate with the smaller IKKα isoforms (Fig. 10), indicating that the HLH domain of IKKα was essential for interaction with NEMO.
FIG. 10.
Truncated IKKα isoforms do not associate with NEMO. HEK293 cells were transiently transfected with plasmids encoding epitope-tagged versions of either IKKα, IKKα-ΔCm, IKKα-ΔLH, IKKα-ΔH, IKKβ-ΔCm, or IKKβ, together with NEMO-Myc. Thirty hours posttransfection, NEMO was immunoprecipitated and samples were resolved on SDS-PAGE (9% acrylamide gel) followed by immunoblotting (IB) with the appropriate primary antibody. (Top) Expression of all forms of IKKα and IKKβ was verified in immunoprecipitates (IP) and whole-cell lysates (Lys). (Bottom) Protein expression of NEMO was verified in both immunoprecipitates and whole-cell lysates. The data are from a single experiment performed twice with similar results.
IKKα-ΔCm, IKKα-ΔH, and IKKα-ΔLH rescue NF-κB activity from the inhibitory effects of a dominant-negative NEMO mutant.
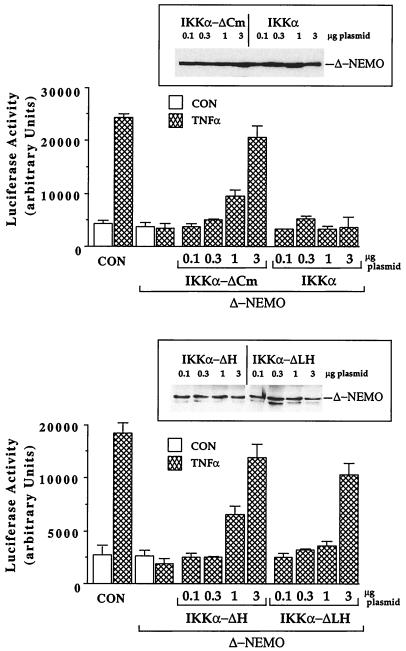
To further elaborate functional distinctions between the activation pathways of IKKα and those of its truncated isoforms, we assessed the effects of a dominant-negative mutant of NEMO on the abilities of IKKα, IKKα-ΔCm, IKKα-ΔH, and IKKα-ΔLH to activate NF-κB in response to TNF-α. In agreement with a previous report (32), an amino-terminally truncated mutant of NEMO inhibited the ability of IKKα to stimulate TNF-α-inducible NF-κB activation at all dosages (Fig. 11). In contrast, IKKα-ΔH, which lacks the HLH domain; IKKα-ΔLH; and IKKα-ΔCm, which lack the IKKα LZip and HLH domains and failed to interact with NEMO in vivo (Fig. 10), efficiently rescue NF-κB induction from the inhibitory effects of the same NEMO mutant (Fig. 11). These and other results presented here support the view that the carboxy-terminally truncated IKKα isoforms activate NF-κB by an IKKβ- and NEMO-independent pathway.
FIG. 11.
Truncated IKKα isoforms rescue NF-κB activation from the inhibitory effects of a dominant-negative NEMO mutant, but IKKα does not. HEK293 cells were transiently transfected with increasing concentrations of plasmid encoding either HA epitope-tagged IKKα or IKKα-ΔCm (top) or, in an independent experiment, V5 epitope-tagged IKKα-ΔLH or IKKα-ΔH (bottom) together with 1.5 μg of a Δ-NEMO expression plasmid (an amino-terminally truncated, dominant-inhibitory form of NEMO) (32), 0.5 μg of NF-κB–luciferase reporter, and 0.25 μg of RSV–β-Gal reference control plasmids. Control cells (CON) received only the reporter and reference control plasmids. Twenty-four hours posttransfection, the cells were stimulated for 6 h with TNF-α (10 ng/ml) prior to lysis and luciferase activity measurement. Luciferase activity is expressed as arbitrary units normalized to β-Gal activity, and the values are means ± range of duplicate determinations from an experiment performed three times with comparable results. The inset shows an immunoblot of Δ-NEMO protein expression in lysates (100 μg of protein) from the IKKα- and IKKα-ΔCm-transfected cells.
DISCUSSION
The study of the cascade of events triggered by the binding of cytokines such as TNF-α and IL-1 to their receptors, leading to the activation of the cytoplasmically anchored transcription factor NF-κB, has recently seen considerable advances. A major breakthrough was the identification of IKKα and subsequently IKKβ as cytoplasmic kinases which phosphorylate IκB family members at physiologically relevant sites and thereby target them for proteasome-mediated degradation (15, 33, 38, 52, 55, 56). IKKα and IKKβ proteins are 52% similar at the primary-sequence level and share two carboxy-proximal structural domains resembling LZip and HLH motifs (12). Since LZip and HLH domains are thought to play roles in protein-protein interactions, the IKKs may employ these domains to recruit proteins involved in their regulation or to facilitate binding to specific substrates. Recent experiments on the regulation of IKKβ activation suggest that the probable interaction of the carboxy-proximal HLH and amino-proximal catalytic domains are required for its cytokine-induced activation (14).
Identification of novel, differentially expressed IKKα isoforms.
In this report, we describe the cloning and patterns of expression of three unique IKKα mRNAs (IKKα-ΔH and IKKα-ΔLHa and -b) and the functional properties of their two encoded proteins. IKKα-ΔH is identical to IKKα up to residue 576 (nucleotide 1782), where a novel 3′ NCS replaces the rest of the full-length IKKα mRNA. After adding 8 unique amino acids, a translation termination codon truncates the polypeptide encoded by IKKα-ΔH 24 amino acids upstream of the HLH domain (Fig. 1 and 2). Interestingly, the unique 3′ NCS in IKKα-ΔH exhibits significant homology with the sequence of the HLH domain, perhaps indicating that the 3′ NCS is specified by an alternative splice to a duplicated exon with extensive sequence divergence (Fig. 2A). In addition to IKKα-ΔH, IKKα-ΔLHa and IKKα-ΔLHb are isoforms of the full-length IKKα transcript possessing the identical 152-bp deletion of IKKα (nucleotides 1408 to 1559 [numbered according to reference 12]). This internal 152-bp deletion removes the LZip domain downstream of residue 451. The same deletion changes the remainder of the translation reading frame to encounter a termination codon after a short stretch of 20 unique amino acids (Fig. 1). The IKKα-ΔLHa transcript is otherwise identical to the full-length IKKα mRNA, while its related IKKα-ΔLHb isoform also possesses the same 3′ NCS as IKKα-ΔH, again inserted after IKKα nucleotide 1782 (Fig. 1). Consequently, IKKα-ΔLHa and IKKα-ΔLHb encode the same 471-amino-acid carboxy-terminally truncated IKKα polypeptide which lacks precisely the LZip and HLH domains. In surprising contrast to the full-length IKKα transcript, RT-PCR analysis reveals that its three smaller isoforms are differentially expressed in normal murine tissues and established cell lines (Fig. 3 and 4). IKKα is the predominant mRNA in most instances, except for the thymus and brain. In normal T lymphocytes, transcripts encoding the smaller IKKα polypeptides predominate over full-length IKKα, and the relative steady-state amounts of the IKKα-ΔLH isoforms are also preferentially increased by T-cell-mitogenic stimuli. Preferential expression of the IKKα-ΔLH isoforms are also observed for the EL4 mature T-cell lymphoma, while they are very weakly expressed in immature T cells, immature and mature B cells, and most other cell types (Fig. 3A and B and 4A). In the brain, the IKKα-ΔH isoform is the predominant IKKα mRNA (Fig. 3B).
Like IKKα, the IKKα-ΔH and IKKα-ΔLH polypeptides are TNF-α-inducible, NF-κB-activating IκBα kinases.
By a combination of NF-κB-driven luciferase gene reporter assays, immune complex kinase assays, and coimmunoprecipitations with other known components of the ∼700- to 900-kDa IKK complex, we find that the IKKα-ΔH- and IKKα-ΔLH-encoded proteins behave in a fashion similar to that of full-length IKKα by several but not all criteria. First, plasmid dose-response curves reveal that all forms of IKKα activate comparable levels of NF-κB luciferase activity, even at limiting dosages (Fig. 5B). Second, each form of IKKα correctly phosphorylates IκBα (on serines 32 and 36) in response to either TNF-α signaling or overexpression of the upstream activator NIK (Fig. 7A and D). Third, IKKα-ΔCm, a recombinant form of IKKα-ΔLH lacking its unique 20-amino-acid C-terminal tail, activates NF-κB and phosphorylates IκBα like IKKα-ΔLH and IKKα-ΔH with an enzymatic time course superimposable on that of full-length IKKα (Fig. 5 and 7). Fourth, the abilities of IKKα-ΔH, IKKα-ΔLH, and IKKα-ΔCm to activate NF-κB are not appreciably enhanced by coexpression with either IKKα or IKKβ, while all of them are inhibited by a kinase-inactive, ATP-binding domain mutant of IKKα (Fig. 6). We conclude that the smaller isoforms of IKKα retain a number of functions of the complete polypeptide without its LZip and HLH domains. In addition, the unique, carboxy-terminal tail extremities of IKKα-ΔH and IKKα-ΔLH do not contribute to their functional activities in these assays.
The existence of functional, differentially expressed isoforms of IKKα can conceivably provide cells with alternative modes of NF-κB activation under some physiological and tissue-specific circumstances. The targeted KOs of the IKKα and IKKβ genes in mice were recently reported by several groups to result in strikingly diverse developmental defects, with the former blocking keratinocyte differentiation (20, 26, 44) and the latter causing severe liver degeneration and apoptosis, resulting in embryonic lethality (27, 28, 45). Furthermore, the loss of IKKβ resulted in an almost-complete block of the ability of TNF-α and IL-1 to activate NF-κB and phosphorylate IκB in mouse embryonic fibroblasts (MEFs), indicating that IKKα at best only weakly compensates for the loss of IKKβ (27, 28, 45). In contrast, loss of the related IKKα protein had little if any effect on the ability of inflammatory-response cytokines to induce NF-κB in MEFs and fetal liver cells (20, 26, 44) or in thymocytes derived from RAG-2-deficient mice reconstituted with IKKα KO fetal liver cells (44). However, one of these reports also showed a reduction in NF-κB binding activity and diminished expression of NF-κB target genes in MEFs of IKKα−/− mice, suggesting that IKKα is a pleotropic contributor to NF-κB activity, even though a substantial amount of NF-κB activity remains in its absence. In addition, it remains a formal possibility that IKKα loss is compensated for by other kinases in addition to IKKβ. Alternatively, IKKα loss may be sufficiently compensated for in these mutant mice by the presence of functionally adequate (albeit not wild-type) signalsomes harboring less-active IKKβ homodimers (32). It is also important to note that, given the mortality of the IKKα KO mice, it was not possible to score for other physiological defects in adult animals. Nevertheless, the dissimilar embryonic phenotypes of the IKKα and IKKβ KO mice indicate that some dominant in vivo functions of the two IκB kinases are different, which remains puzzling in light of (i) the strong structural similarities of IKKα and IKKβ, (ii) their coisolation from a ∼700- to 900-kDa cytoplasmic complex from HeLa cells (15, 33), (iii) their abilities to activate NF-κB by IκB phosphorylation in response to cytokine signaling in transfected HeLa and HEK293 cells (15, 33, 38, 52, 56), (iv) their abilities as homogeneous recombinant proteins to correctly phosphorylate IκBα (25, 55), and (v) the inhibitory effects of both IKKα dominant-negative mutants (15, 33, 38) and IKKα antisense expression (15) on TNF-α-induced NF-κB activation and IκB phosphorylation. Taken together, these observations argue that the individual roles of IKKα and IKKβ in NF-κB activation are complex and may differ in various cellular backgrounds and physiological situations. Even though IKKα and IKKβ appear to be present in similar amounts in the ∼700- to 900-kDa IKK complex (15, 33), recent evidence suggests that IKKβ may be the dominant IκBα kinase activated in response to inflammatory mediators like TNF-α and IL-1 (14), in contrast to two earlier reports (29, 33), making this new data somewhat controversial. Nevertheless, IKKβ remains insufficient to activate NF-κB in all cellular backgrounds (such as developing keratinocytes), and it remains to be demonstrated that IKKβ is sufficient in vivo to physiologically activate NF-κB in response to all its diverse activating stimuli and in adult animals. Two additional NF-κB-stimulating agonists, phorbol esters (PMA) and oxidative stress (H2O2), produced similar degrees of IKKα and IKKα-ΔCm activation in transfected HEK293 cells, indicating that they can be general activators of NF-κB, at least in some cellular backgrounds (data not shown). The elevated expression of alternative isoforms of IKKα in activated T cells and in the brain demonstrated in this report provides the interesting possibility that novel cytoplasmic signaling complexes containing alternative forms of IKKα may contribute to NF-κB activation. Alternatively, the smaller IKKα isoforms may preferentially target different IκBs, retaining different members of the NF-κB family, or even target novel IKKα substrates not recognized by the NEMO-dependent IKK complex.
Modes of IKKα-ΔH and IKKα-ΔLH activation are not subject to the same constraints as full-length IKKα and IKKβ: multiple signaling pathways for NF-κB activation.
Even though a number of functional attributes are shared among the IKKα, IKKα-ΔH, and IKKα-ΔLH polypeptides, there are clear dissimilarities between the mechanisms and apparent requirements for activation of the smaller IKKα isoforms and those for IKKα and IKKβ. For instance, IKKα-ΔH associates with IKKα and IKKβ, but IKKα-ΔLH and IKKα-ΔCm do not (Fig. 8). Therefore, and in agreement with earlier reports (52, 56), we find that the LZip domain is required for IKKα-IKKβ interaction but the HLH domain is not. However, interactions of the smaller isoforms of IKKα (IKKα-ΔLH and IKKα-ΔCm) with either IKKα or IKKβ are not required for NF-κB activation, given the comparable activities of IKKα-ΔH, IKKα-ΔLH, and IKKα-ΔCm. In addition, unlike IKKα and IKKβ, the smaller forms of IKKα do not associate with NEMO (Fig. 10), which has been shown to be a common component of the ∼700- to 900-kDa IKK complex and to be essential for IKKα and IKKβ activation (32, 40, 53). We also show that IKKα-ΔCm, IKKα-ΔH, and IKKα-ΔLH can each rescue the cytokine-inducible NF-κB responsiveness of cells from the inhibitory effects of an amino-terminal deletion mutant of NEMO, while full-length IKKα cannot (Fig. 11). Thus, the smaller forms of IKKα neither dock with nor require NEMO for their functional activation, in contrast to IKKα and IKKβ. Furthermore, the unique properties of the IKKα-ΔH isoform demonstrate that IKKα and IKKβ association per se via their LZip domains does not ensure the functional docking of the kinase heterodimer to NEMO, unless each kinase molecule also contains an H-L-H domain (Fig. 8, 10, and 11). By analogy these observations also indicate that the HLH motifs of IKKα and IKKβ appear to function like dominant effector domains, funneling them into NEMO-dependent complexes.
It is important to note in this context that complementation of NEMO loss in an NF-κB-nonresponsive mutant of the 70Z3 murine pre-B-cell line and in a rat fibroblast line, which failed to activate NF-κB in response to the human T-cell leukemia virus type 1 Tax gene product, restored their abilities to activate NF-κB in response to TNF-α, IL-1, and Tax (53). Their NEMO requirement for NF-κB activation implies that the endogenous smaller cellular isoforms of IKKα in these cellular backgrounds are insufficient by themselves for mediating inducible NF-κB activation. We find that expression of the smaller IKKα isoforms is considerably weaker in fibroblastic cells and virtually absent in parental 70Z3 and 70Z3(1.3E2) NEMO mutant cells even after exposure to NF-κB-activating stimuli (Fig. 3A and B and data not shown).
An important functional distinction among IKKα-ΔH, IKKα-ΔLH, and IKKα-ΔCm is the ability of the two smaller IKKα isoforms (ΔLH and ΔCm) to respond to TNF-α and NIK signaling without their carboxy-proximal HLH domains. Delhase and colleagues reported that IKKβ requires its HLH domain to interact with the protein's amino-terminal kinase-catalytic domain, which can even occur if the individual domains are expressed as separate proteins, presumably by virtue of their abilities to productively coassociate in NEMO-dependent IKK complexes in vivo (14). In agreement with Delhase et al., we find that IKKβ-ΔCm, a recombinant carboxy-terminal-truncation mutant of IKKβ (amino acids 1 to 454) that is structurally analogous to the functional IKKα-ΔCm protein (amino acids 1 to 451), is incapable of activating NF-κB. However, our findings appear to be at odds with two other reports, which showed that a recombinant mutant of IKKα, bearing site-directed replacements of two amino acids in the HLH motif, was functionally inactive (36, 56). It is conceivable that the IKKα(HLH)− mutant functions like a dominant-negative form of IKKα in vivo by retaining its ability to associate with NEMO and IKKβ but disrupting the conformation of the ∼700- to 900-kDa IKK complex, thereby inhibiting its ability to transduce cytokine signals to phosphorylate IκB. Alternatively, this site-directed mutation may have caused misfolding of IKKα. The abilities of IKKα-ΔH, IKKα-ΔLH, and IKKα-ΔCm, but not IKKα, to rescue cells from a dominant-negative NEMO mutant would be in keeping with this argument. Finally, the absence of activity of IKKβ-ΔCm, a recombinant mutant of IKKβ that is structurally analogous to functionally active IKKα-ΔCm, strongly suggests that the amino-terminal catalytic domains of IKKα and IKKβ are not functionally analogous and are likely to be activated in vivo by distinct mechanisms.
ACKNOWLEDGMENTS
We thank Randy Noelle for the FLAG-IKKβ and FLAG-NIK expression constructs, Steven Pullen for a human IKKα baculovirus expression vector, Carol Sibley for providing the wild-type 70Z/3-35.15 pre-B line and its 1.3E2 variant subclone, and Konrad Huppi for the BXSB spleen λ-gt-10 cDNA library. We also gratefully acknowledge the expert assistance of Gail Habicht (Department of Pathology, SUNY at Stony Brook) in the preparation of primary murine thymocyte cultures.
This work was supported in part by NIH grants CA36246 and GM26939 and a research contract from Small Molecule Therapeutics Inc. (for the functional analysis of IKKα-ΔH) awarded to K.B.M.
REFERENCES
- 1.Baeuerle P, Baltimore D. IkappaB: a specific inhibitor of the NF-kappaB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-kappaB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A., Jr The NF-kappa B and Ikappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Beg A, Baltimore A. The IkappaB proteins: multifunctional regulators of Rel.NF-κB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 5.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IkappaB-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 8.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IkappaB alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 11.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IkappaB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 12.Connelly M A, Marcu K B. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase catalytic domain. Cell Mol Biol Res. 1995;41:537–549. [PubMed] [Google Scholar]
- 13.Croston G E, Cao Z, Goeddel D V. NF-kappaB activation by interleukin-1 (IL-1) requires an IL-1 receptor-associated protein kinase activity. J Biol Chem. 1995;270:16514–16517. doi: 10.1074/jbc.270.28.16514. [DOI] [PubMed] [Google Scholar]
- 14.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 15.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 16.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E J, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S, May M J, Kopp E B. NF-kappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore T D, Koedood M, Piffat K A, White D W. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 19.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. MEK kinase is involved in tumor necrosis factor alpha-induced NF-kappaB activation and degradation of IkappaB-alpha. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 21.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkappaB kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Peet G W, Pullen S S, Schembri-King J, Warren T C, Marcu K B, Kehry M R, Barton R, Jakes S. Recombinant IkappaB kinases alpha and beta are direct kinases of IkappaB-alpha. J Biol Chem. 1998;273:30736–30741. doi: 10.1074/jbc.273.46.30736. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte J C, Verma I M. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 28.Li Z W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling L, Cao Z, Goeddel D V. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malinin N L, Boldwin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 31.Mayo M W, Wang C Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A., Jr Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 32.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IkappaB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 34.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 35.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemoto S, DiDonato J A, Lin A. Coordinate regulation of IkappaB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-kappaB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obert S, O'Connor R J, Schmid S, Hearing P. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 39.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 40.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 41.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of IkappaB alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;12:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 43.Song H Y, Regnier C, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-B and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Fuentes M E, Yamaguchi K, Durnin M H, Dalrymple S A, Hardy K L, Goeddel D V. Embryonic lethality, liver degeneration, and impaired NF-kappaB activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 46.Traenckner E, Pahl H, Henkel T, Schmidt K, Wilk S, Baeuerle P. Phosphorylation of human IkappaB-alpha on serines 32 and 36 controls IkappaB-alpha proteolysis and NF-kappaB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 48.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 49.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 50.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 51.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 52.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 53.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 54.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel D V, Mak T W. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 55.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 56.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]