PURPOSE
Younger women are at risk for depression and related symptoms following breast cancer. The Pathways to Wellness study, a randomized, multi-institution, three-arm trial, tested the efficacy of two behavioral interventions for younger breast cancer survivors with elevated depressive symptoms: mindful awareness practices (MAPs) and survivorship education (SE) (Clincaltrials.gov identifier: NCT03025139).
METHODS
Women diagnosed with breast cancer at or before 50 years of age who had completed treatment and had elevated depressive symptoms were randomly assigned to 6 weeks of MAPs, SE, or wait-list control (WLC). Assessments were conducted preintervention and postintervention and at 3-month and 6-month postintervention follow-ups. Analyses compared each intervention to WLC using linear mixed models. The primary outcome was change in depressive symptoms from preintervention to postintervention on the Center for Epidemiologic Studies-Depression Scale; secondary outcomes included change in fatigue, insomnia, and vasomotor symptoms.
RESULTS
Two hundred forty-seven women (median age = 46 years) were randomly assigned to MAPs (n = 85), SE (n = 81), or WLC (n = 81). MAPs and SE led to significant decreases in depressive symptoms from preintervention to postintervention relative to WLC (mean change relative to WLC [95% CI]: MAPs, –4.7 [–7.5 to –1.9]; SE, –4.0 [–6.9 to –1.1]), which persisted at 6-month follow-up for MAPs (mean change relative to WLC [95% CI]: MAPs, –3.7 [–6.6 to –0.8]; SE, –2.8 [–5.9 to 0.2]). MAPs, but not SE, also had beneficial effects on fatigue, insomnia, and vasomotor symptoms that persisted at 6-month follow-up (P < .05).
CONCLUSION
Mindfulness meditation and SE reduced depressive symptoms in younger breast cancer survivors. These interventions can be widely disseminated over virtual platforms and have significant potential benefit for quality of life and overall survivorship in this vulnerable group.
INTRODUCTION
With advances in cancer detection and treatment, the number of women who survive breast cancer has increased significantly. As of January 1, 2019, there were more than 3.8 million women with a history of breast cancer living in the United States.1 Breast cancer is the most common cancer in younger women (< 50 years at time of diagnosis), who comprise approximately 19% of incident breast cancer cases.1 For many women, life after breast cancer includes adverse psychologic and physical sequelae, and this is particularly true for younger survivors.2,3 Younger women have greater psychologic morbidity after breast cancer than older women and age-matched women with no cancer history, including elevated levels of depression.2-5 Depression has been linked to treatment nonadherence6 and predicts shorter recurrence-free and overall survival in women with breast cancer,7 highlighting its clinical relevance. Younger breast cancer survivors (BCS) also report high levels of other behavioral symptoms (ie, fatigue, insomnia, and vasomotor symptoms) that cause significant impairment in quality of life.2,3,5,8,9 Behavioral disturbances have been documented up to 10 years after diagnosis, suggesting that effects may not improve without intervention.10
CONTEXT
Key Objective
Depression is common in younger breast cancer survivors, and many also report problems with fatigue, insomnia, and other symptoms. To our knowledge, there are no behavioral interventions designed to target depression in this vulnerable group.
Knowledge Generated
We found that two brief behavioral interventions—mindfulness meditation and survivor education—were both effective in reducing depressive symptoms relative to wait-list control. Mindfulness also reduced fatigue, insomnia, and vasomotor symptoms.
Relevance
These standardized intervention programs have the potential for wide dissemination, with significant potential benefit for quality of life and overall survivorship in this high-risk group.
Despite considerable need, there is a lack of interventions specifically designed for younger BCS beyond the acute phase of treatment.3 The current study was designed to test two interventions for this vulnerable group: mindfulness meditation and survivorship education (SE). With its focus on attention and emotion regulation, mindfulness has emerged as a promising intervention for reducing depression and other behavioral symptoms in patients with breast cancer and BCS,11,12 particularly those with elevated symptoms.13-15 In a phase II randomized controlled trial in younger BCS, we found beneficial effects of a brief mindfulness intervention on depressive symptoms, fatigue, sleep disturbance, and vasomotor symptoms.16 Education-based approaches have demonstrated efficacy in reducing depression and other behavioral symptoms in patients with cancer,17-19 with preliminary support for their benefit in younger BCS.20 Education-based approaches are used frequently in pretreatment settings (eg, chemotherapy teaching) without routine implementation in the survivorship period, and particularly not for younger BCS. These two interventions warrant further evaluation in larger samples and in multi-institutional settings as a means of translating cancer survivorship research into clinical care delivery.
METHODS
Overview of Trial Design
Pathways to Wellness is a randomized, three-arm, phase III trial designed to evaluate the efficacy of two distinct group interventions, mindful awareness practices (MAPs) and SE, for younger BCS with elevated depressive symptoms.21 Because both are credible interventions, the trial compared each program to wait-list control (WLC) in an efficient design. The primary outcome was change in depressive symptoms from preintervention to postintervention; assessments were also conducted over a 6-month follow-up to determine persistence of effects. Secondary outcomes included fatigue, insomnia, and vasomotor symptoms. The trial was conducted at three sites: UCLA Jonsson Comprehensive Cancer Center in Los Angeles, CA; Dana-Farber Cancer Institute in Boston, MA; and Johns Hopkins Kimmel Comprehensive Cancer Center in Baltimore, MD. The study was approved by the institutional review boards at each site and registered at ClincalTrials.gov (identifier: NCT03025139). All participants provided informed consent.
Participants
Women were eligible if they met the following criteria: (1) breast cancer diagnosis (stage 0, I, II, or III) at or before 50 years of age; (2) within 5 years of diagnosis; (3) completion of surgery, radiation, and/or chemotherapy at least 6 months previously; (4) ability to complete questionnaires in English; (5) ability to participate in the intervention; and (6) presence of at least mild depressive symptoms as indicated by score ≥ 5 on the Patient Health Questionnaire—8 (PHQ-8), a standardized depression screening measure.22 This PHQ-8 score was used through May 2019 and then liberalized to a score of ≥ 3 to enhance recruitment. Exclusion criteria were (1) recurrent or metastatic breast cancer; (2) another interval cancer diagnosis following breast cancer diagnosis (excluding nonmelanoma skin cancer); (3) current mindfulness practice; (4) pregnancy; and (5) a serious chronic medical or psychiatric condition that could detract from intervention participation or measurement of outcomes.
Procedure
Recruitment was conducted from February 2017 to September 2019 using institutional and community resources, including regional cancer registries. Recruitment procedures included mailed invitation letters, electronic mailings, flyers and social media announcements, and direct invitation by clinicians.
Participants were screened by phone to determine interest and eligibility. If a woman met eligibility criteria, was willing to be randomly assigned, and available for the next scheduled class series, an in-person enrollment visit was conducted at which time the informed consent was signed and preintervention assessments were completed, including questionnaires and blood draw for immune evaluation. Participants were then randomly assigned to MAPs, SE, or WLC (1:1:1 ratio), after stratifying on study site and PHQ-8 score (≤ 7, ≥ 8), using permuted blocks (sizes 3 and 6). Postintervention assessments were conducted within 2 weeks after intervention completion, and follow-up assessments were conducted 3 and 6 months after intervention completion. The postintervention and 6-month assessments were conducted in person and included questionnaires and blood draw (with the exception of 6-month follow-up for the final cohort, which was questionnaire-only because of COVID-19). The 3-month assessment was questionnaire-only and did not require an in-person visit. The questionnaire battery was administered electronically using REDCap and included measures assessing demographic and medical history as well as validated scales to assess primary and secondary outcomes. Of note, the PHQ-9 was included in the questionnaire battery, and the protocol required assessment of suicide risk if suicidal ideation was endorsed. Participants randomly assigned to WLC were given the option to participate in MAPs or SE after their 6-month follow-up; 31 (38%) elected to participate in MAPs and received the intervention either in person or via Zoom.
Interventions
MAPs and SE interventions were conducted in groups, which met in person for 2-hour sessions conducted weekly for 6 weeks.
Mindful awareness practices.
MAPs for BCS is a standardized intervention that includes presentation of theoretical materials on mindfulness, relaxation, and the mind-body connection; experiential practice of meditation; and a psychoeducational component for young BCS. Lecture, discussion, and group process focus on solving problems concerning impediments to effective practice, working with difficult thoughts and emotions, managing pain, and cultivation of loving kindness. Written materials were provided with a summary of information covered each week. Participants were instructed to practice mindfulness exercises at home on a daily basis (5-20 minutes/d) and advised in the informal use of mindfulness in daily life. Two 1-hour in-person group booster sessions were provided approximately 4 and 8 weeks after the 6-week program, which included guided meditations and discussion of how to maintain a mindfulness practice. Classes were led by experienced mindfulness instructors who received specialized training in the program (Appendix 1, online only, for instructor selection criteria).
Survivor education.
SE is a standardized educational program designed for younger BCS that used a written curriculum and annotated slides that were developed by the study investigators and covered major topic areas: quality of life after breast cancer; medical management and quality of care after treatment; relationships and work-life balance; body image, sexuality and fertility; energy balance, nutrition, and physical activity; and cancer in the family, genetics, and related issues. Written materials and community resources were provided to participants in a notebook. Two newsletters were provided approximately 4 and 8 weeks after the 6-week program with tailored health information for younger survivors; these were designed to match the booster sessions for MAPs. Classes were led by trained nurses or physicians with expertise in breast cancer survivorship.
Intervention adherence and fidelity.
Adherence was monitored by taking attendance at each session and completing homework logs (MAPs only). To evaluate fidelity, instructors completed weekly checklists to assess coverage of key program elements. In addition, sessions were recorded and reviewed by Diana Winston, Director of Mindfulness Training at Mindful Awareness Research Center (for MAPs), and study oncologists (for SE) at each site. Feedback was provided as necessary to ensure consistency across instructors and sites.
Study Outcomes
The primary outcome variable was depressive symptoms assessed using the Center for Epidemiologic Studies-Depression scale (CES-D). The CES-D is a reliable, validated, and widely used measure that includes affective, cognitive, and somatic symptoms of depression.23 A CES-D score of 16 or greater is indicative of clinically significant depressive symptoms.
Secondary outcome variables included symptoms that are common in young BCS: fatigue (Fatigue Symptom Inventory24), insomnia (Insomnia Severity Index25), and vasomotor symptoms (hot flashes and night sweats; Breast Cancer Prevention Trail Symptom Checklist26).
Sample Size
The study was designed to provide 80% power to detect medium effect sizes of d = 0.50 for change in CES-D from preintervention to postintervention for MAPs and SE compared with WLC,16 using an α of .027 for each test to control familywise error rate at 0.05 (Dunnett's procedure).27,28 Assuming correlation between baseline (BL) and change scores of –0.50, analysis of covariance power methods indicated that 58 evaluable participants per arm were required. Adjusting for 10%-15% attrition, the target sample size was 70 per arm. The study was not powered to test differences between interventions.
Statistical Methods
BL differences across study arms were assessed using chi-square, Kruskal-Wallis, and analysis of variance tests. Outcome analyses were conducted under the intent-to-treat principle, including all participants in their assigned condition, using linear mixed models fitted to all available data for each outcome variable, including data of participants with incomplete follow-up. Models included fixed effects for time and condition and random effects for individuals, and controlled for study site and variables with BL imbalance or that differed between participants retained and lost to follow-up.
The primary hypothesis was that participants in each intervention would show a greater reduction in CES-D from BL to postintervention than WLC participants. Differences between conditions in change over time were tested using condition-by-time interaction terms. We also tested for differences in change over time in CES-D from BL to 3-month follow-up and from BL to 6-month follow-up for each intervention versus WLC. Similar analyses were conducted for secondary outcomes. Standardized effect sizes were obtained by fitting the models using outcome variables standardized using the standard deviation of the full sample at BL. Post hoc, we compared difference in change over time in the proportion of participants scoring at or above the CES-D clinical threshold of 16 using a generalized linear mixed model with a logit link. Dunnett's procedure was used to control the familywise error rate at 0.05 for each set of two intervention-to-control comparisons; this entailed using an α of .027 for each test.27,28 All tests were two-sided. Analyses were conducted using Stata SE 15.1.
RESULTS
Characteristics of Study Participants
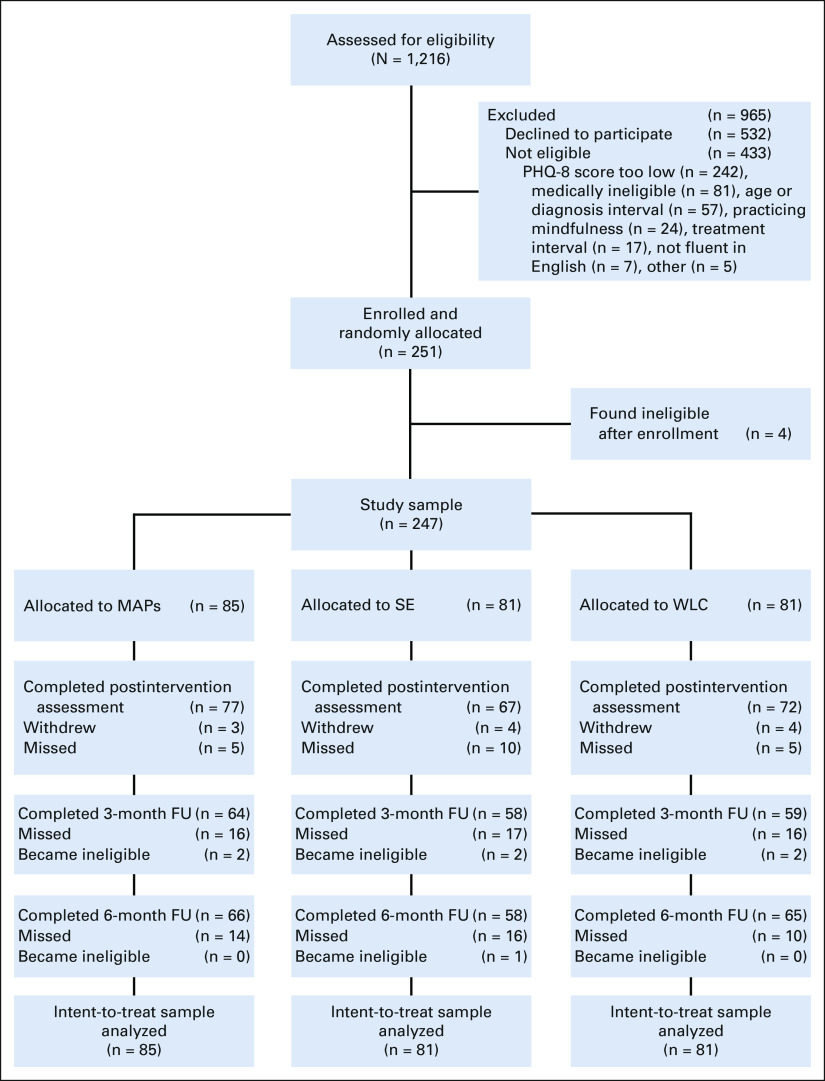
Figure 1 shows participant flow and reasons for ineligibility. Over 2.5 years of recruitment, 1,525 women expressed interest in the study, 1,216 were screened for eligibility, and 247 were determined to be eligible and able to participate. Among those screened, the primary reason for ineligibility was a low score on the depression screener. Of note, only 16 of 247 (6.5%) evaluable participants had PHQ-8 scores < 5 on screening. Of the 247 women enrolled and randomly assigned, 87.4% completed the postintervention assessment. Seven women had a cancer recurrence during follow-up and became ineligible for study completion. Comparison of completers versus noncompleters revealed significant differences across sites, P = .03; site was included as a covariate in all analyses.
FIG 1.
CONSORT diagram showing number of women screened, enrolled, and randomly assigned to the three study groups, as well as completion of study assessments. FU, follow-up; MAPs, mindful awareness practices; PHQ-8, Patient Health Questionnaire—8; SE, survivorship education; WLC, wait-list control.
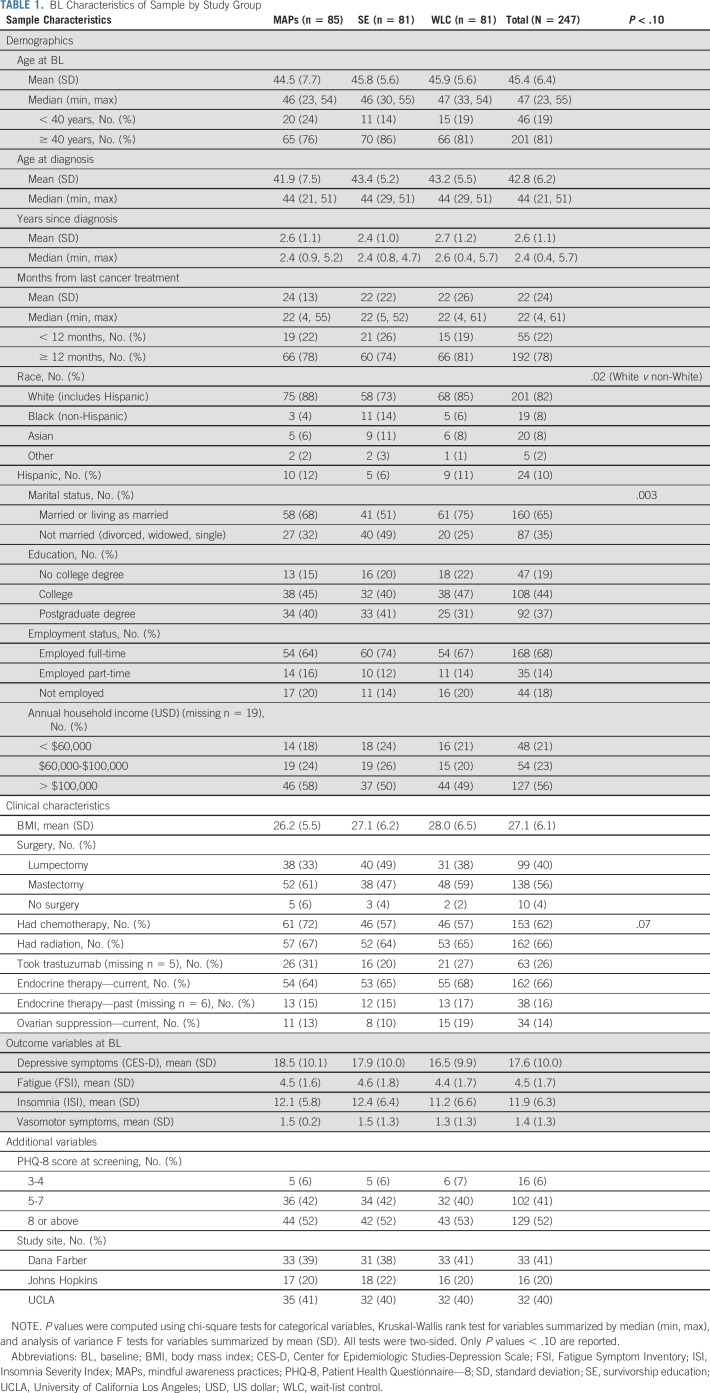
Table 1 provides demographic and clinical characteristics of the sample as well as means for the primary and secondary outcome variables at BL. On average, women were 45 years old, primarily White, and married or living as married, and had been diagnosed 2.6 years earlier. The majority had been treated with radiation (66%) and/or chemotherapy (62%), and most were receiving endocrine therapy (66%). Race and marital status were significantly different across study arms and were included as covariates in all analyses. Mean CES-D scores were in the clinically significant range (≥ 16), with no significant differences between groups. Mean scores on the Fatigue Symptom Inventory were also in the clinically elevated range (≥ 3)29 at BL and mean scores on the Insomnia Severity Index indicated subclinical insomnia (≥ 8),30 with no differences across study arms.
TABLE 1.
BL Characteristics of Sample by Study Group
Intervention Characteristics and Attendance
MAPs and SE groups comprised 3-14 women, with group size dependent on the number of eligible women available at the scheduled class time, and how the random assignment distributed participants. Both MAPs and SE were reasonably well attended; the mean number of classes attended was 4.5 for MAPs (SD = 1.9, range 0-6) and 3.8 for SE (SD = 2.1, range 0-6).
Primary Outcome: Depressive Symptoms
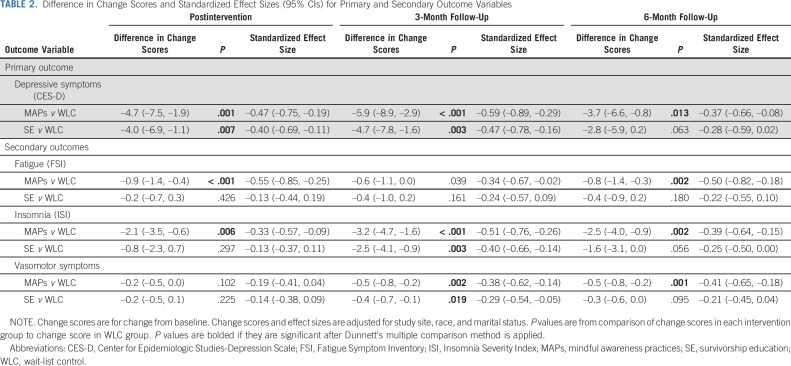
Linear mixed models were fit to compare each intervention group to WLC on change in depressive symptoms, controlling for study site, race, and marital status. Table 2 reports differences in change scores for MAPs versus WLC and SE versus WLC, as well as standardized effect sizes and P values for these differences. Mean scores at each assessment are reported in Appendix Tables A1 and A2 (online only). Trajectories in each condition are depicted in Figure 2.
TABLE 2.
Difference in Change Scores and Standardized Effect Sizes (95% CIs) for Primary and Secondary Outcome Variables
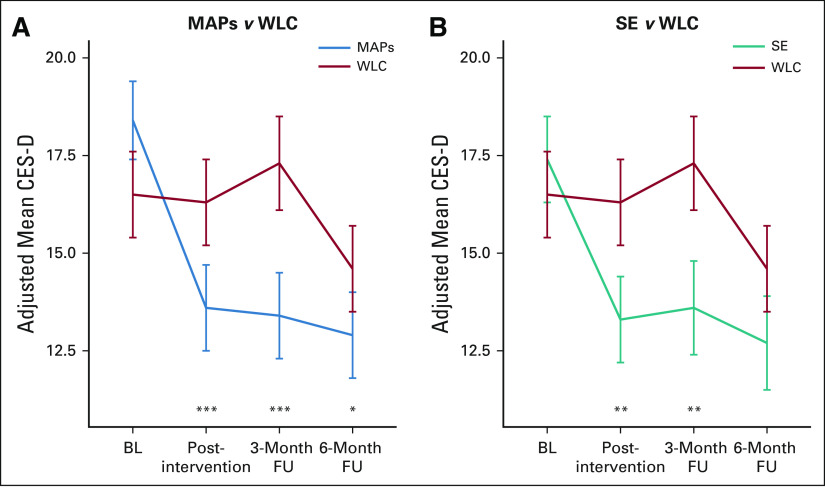
FIG 2.
Adjusted mean scores for depressive symptoms at each assessment in (A) MAPs versus WLC and (B) SE versus WLC. Means are from linear mixed models and are adjusted for study site, race, and marital status. Difference in change since BL; *P < .027 (Dunnett multiplicity threshold); **P < .01; ***P < .001. BL, baseline; CES-D, Center for Epidemiologic Studies-Depression scale; FU, follow-up; MAPs, mindful awareness practices; SE, survivorship education; WLC, wait-list control.
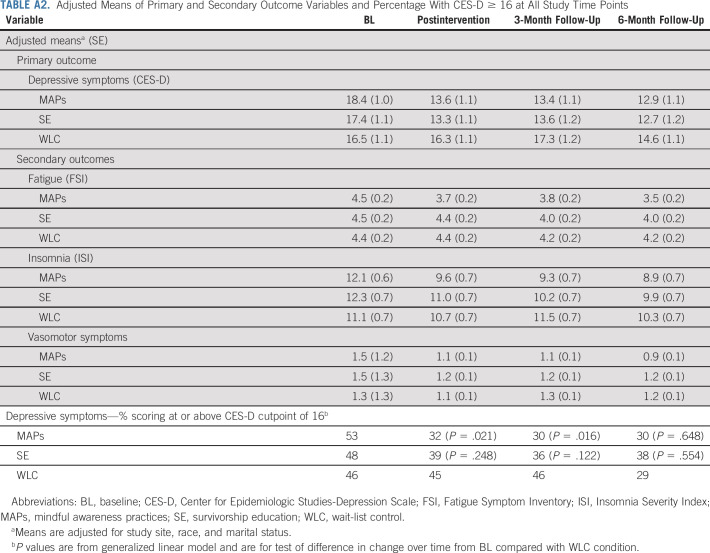
MAPs led to a statistically significant reduction in CES-D from preintervention to postintervention relative to WLC. This effect persisted at 3-month and 6-month follow-ups, with mean CES-D scores falling below the clinical cutpoint at each follow-up assessment. In post hoc analysis, there was a statistically significant decrease in the percentage of participants who scored in the clinical range of the CES-D in the MAPs condition relative to WLC at postintervention (change from 53% to 32% in MAPs v 46% to 45% in WLC, P = .021) and 3-month follow-up (change from 53% to 30% in MAPs v 46% to 46% in WLC, P = .016); this difference was no longer significant at 6-month follow-up (Appendix Table A2).
SE led to a statistically significant reduction in CES-D from preintervention to postintervention relative to WLC, which persisted at 3-month follow-up and became marginal at 6-month follow-up. Although the percentage with elevated CES-D scores in the SE condition decreased, this was not significantly different from WLC at any timepoint (P > .10; Appendix Table A2). Additional adjustment for chemotherapy did not change results for MAPs or SE. There was no evidence of any bias in missingness at the 3-month follow-up that might influence conclusions about intervention benefit (Appendix 1).
Secondary Outcomes: Fatigue, Insomnia, and Vasomotor Symptoms
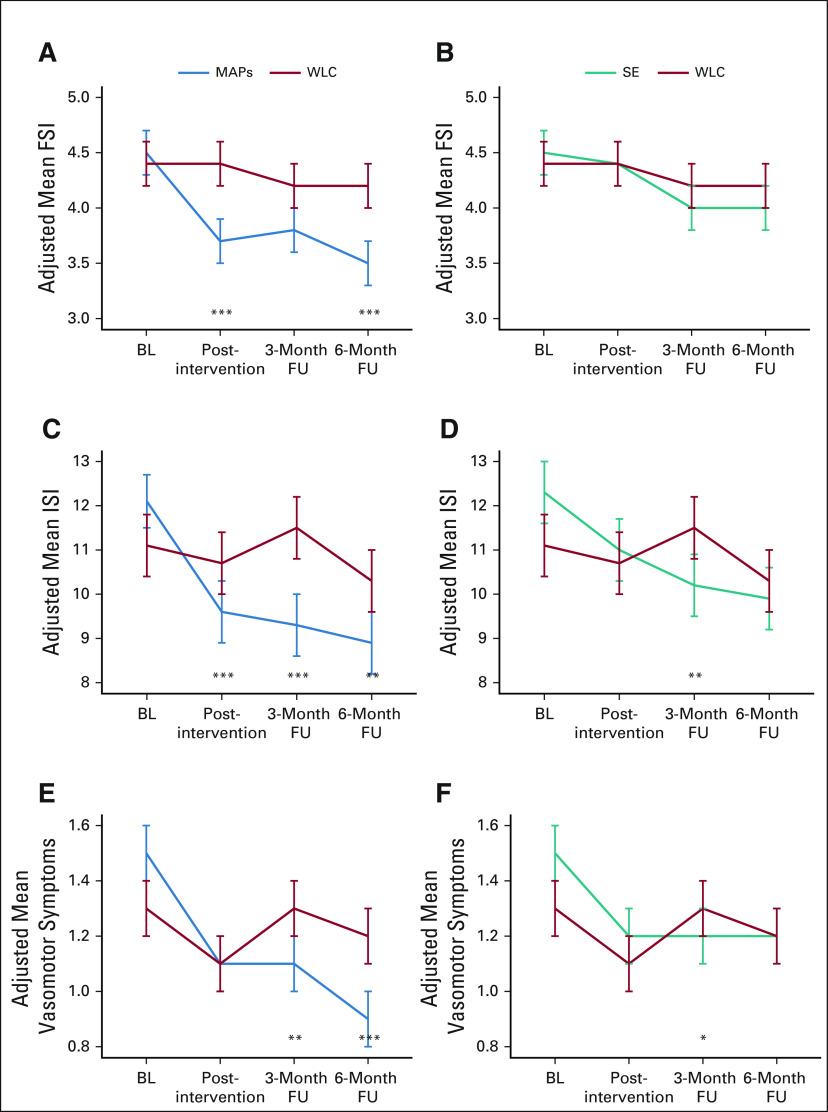
As reported in Table 2 and depicted in Figure 3, MAPs led to significant decreases in fatigue and insomnia from preintervention to postintervention relative to WLC. These effects generally persisted over follow-up, although mean levels remained in the clinical range for fatigue and in the subthreshold range for insomnia. MAPs also showed reductions in vasomotor symptoms that were marginal at post-treatment and significant at 3-month and 6-month follow-ups.
FIG 3.
Adjusted mean scores for (A, B) fatigue, (C, D) insomnia, and (E, F) vasomotor symptoms at each assessment in (A, C, and E) MAPs versus WLC and (B, D, and F) SE versus WLC. Means are from linear mixed models and are adjusted for study site, race, and marital status. Difference in change since BL; *P < .027 (Dunnett multiplicity threshold); **P < .01; ***P < .001. BL, baseline; FSI, Fatigue Symptom Inventory; FU, follow-up; ISI, Insomnia Severity Index; MAPs, mindful awareness practices; SE, survivorship education; WLC, wait-list control.
For SE, there was no significant effect on fatigue, and significant decreases in insomnia relative to WLC were observed only at 3-month follow-up. SE also led to significant reductions in vasomotor symptoms at 3-month follow-up only.
Adverse Events
No adverse events were reported directly resulting from the interventions; however, one participant randomly assigned to SE, who did not attend any classes or participate in any follow-up assessments, attempted suicide and was hospitalized 6 months after enrollment. Across the study, there were 22 women who endorsed suicidal ideation at enrollment on the PHQ-9 (with no intention or plan), reflecting the severity of depressive symptoms in the sample.
DISCUSSION
This phase III, multi-institution trial examined the efficacy of two 6-week, group-based behavioral interventions for younger BCS with elevated depressive symptoms. Both interventions had good adherence, with more than 70% of participants attending at least half of the classes. Both were also effective in reducing depressive symptoms, the primary trial outcome, supporting their utility in this vulnerable population.
The MAPs intervention led to significant declines in depressive symptoms at postintervention that were maintained over the follow-up, with rates of clinically significant depressive symptoms falling from 52% at BL to 30% at 3 and 6 months postintervention, respectively. The MAPs program also had beneficial effects on fatigue, insomnia, and vasomotor symptoms that persisted over the follow-up, with small to medium effect sizes. Although earlier studies have documented beneficial effects of mindfulness in BCS,11-15 this has not been demonstrated in younger BCS in a well-powered trial. In addition, the durability of these effects based on previous reports is unclear, either because longer-term (> 2 month) follow-ups were not conducted12,15 or because improvements dissipated over time.16 The persistence of effects observed in the current trial may be related to the booster sessions, which were designed specifically to support women in maintaining their practice, and to the ability of these young women to implement the techniques they learned. Of note, these techniques included approaches for dealing with negative thoughts as well as physical sensations, which may account for the scope of symptoms influenced by MAPs. MAPs has also been shown to influence biologic processes that are known to play a role in behavioral symptoms in cancer populations (eg, inflammation).31
SE also led to significant reductions in depressive symptoms at postintervention that persisted at 3 months and became marginally significant at 6 months. However, there was minimal evidence for SE effects on fatigue, insomnia, or vasomotor symptoms. Education interventions have shown beneficial effects on depressive symptoms and other behavioral symptoms (including fatigue) in patients with cancer and survivors in previous trials,18,19,32 although only a few of these studies enrolled patients with elevated symptoms. The results suggest that supportive psychoeducation may be useful for general depressive symptoms but that more targeted approaches may be required to improve specific symptoms, particularly if they have persisted beyond the acute phase of diagnosis and treatment.33,34 Indeed, participants in the SE groups indicated that the information might have been more useful to them if presented earlier in the cancer trajectory, during or in the immediate aftermath of treatment.
Limitations of this trial include the unblinded nature of the random assignment allocation, which is not feasible with a behavioral intervention. In addition, the conduct at comprehensive cancer centers rather than community centers, and associated lack of substantial diversity in study participants, limits the generalizability of the results.
Women diagnosed with breast cancer before 50 years of age are at increased risk for psychologic and behavioral problems that may not be adequately addressed even in highly resourced cancer centers. Here, we show that two interventions specifically designed for younger survivors are effective in reducing depressive symptoms and, in the case of mindfulness, also improve related symptoms that pose serious threats to women's health and well-being after cancer. These standardized programs have the potential for wide dissemination over virtual platforms, with significant potential benefit for quality of life and overall survivorship in this high-risk group.
ACKNOWLEDGMENT
The authors thank the mindfulness instructors and nurse educators for their role in delivering the interventions in this study, Diana Winston for overseeing the mindfulness intervention, as well as the research staff and study participants.
APPENDIX 1
Criteria for Selection of Mindfulness Instructors:
Completion of teacher training related to mindfulness (eg, mindfulness-based stress reduction [MBSR] teacher training).
Mindfulness teaching experience over a reasonable period (5 years or more), such as teaching MBSR courses or equivalent.
Personal experience with multiday silent retreats.
Analyses to Evaluate Potential Bias in Missingness at the 3-Month Follow-up:
The 3-month follow-up was missed by 16, 17, and 16 patients on mindful awareness practices, survivorship education, and wait-list control (WLC), respectively. We conducted additional analyses to determine whether women who completed the postintervention assessment but missed the 3-month follow-up differed from those who completed both the postintervention assessment and 3-month follow-up. The results showed no significant differences in postintervention Center for Epidemiologic Studies-Depression Scale (CES-D) scores between participants who did versus who did not complete the 3-month follow-up in mindful awareness practices or survivorship education (both P > .5). There was a marginally significant difference in postintervention CES-D scores between participants who did versus who did not complete the 3-month follow-up in WLC (mean CES-D = 14.9 for those who did complete the 3-month follow-up; mean CES-D = 21.1 for those who did not complete the 3-month follow-up; difference = 6.2, P = .07). This suggests that in WLC, there was more dropout at 3-month follow-up among women with higher CES-D scores at postintervention. This could have biased the change scores for WLC, which would have made the interventions appear less effective.
Analyses Omitting Participants With Patient Health Questionnaire—8 Screening Scores of 3 or 4:
As described in Methods, there was a change in participant entry criteria from Patient Health Questionnaire—8 score of at least 5 to at least 3. In total, there are only 16 participants (6.5%) with scores of 3 or 4. All 16 were in the fifth (final) cohort, which started at approximately the same time for all sites. By site, there were nine at DF (9% of their participants), two at JH (4% of their participants), and five at LA (5% of their participants); thus, there was no substantial differences by site. As a sensitivity analysis, we conducted the primary analysis omitting the 16 individuals with Patient Health Questionnaire—8 screening scores of 3 or 4. There were only minor changes to estimates and the results were similar to the main analysis.
TABLE A1.
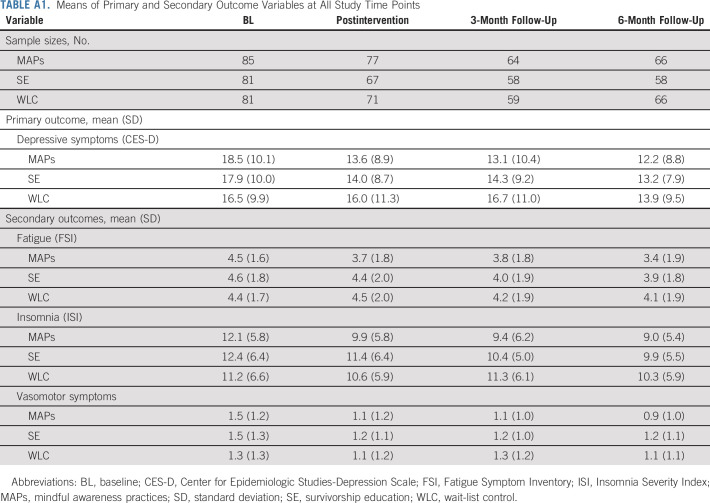
Means of Primary and Secondary Outcome Variables at All Study Time Points
TABLE A2.
Adjusted Means of Primary and Secondary Outcome Variables and Percentage With CES-D ≥ 16 at All Study Time Points
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Travel, Accommodations, Expenses: Novartis
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Hadine Joffe
Employment: Merck
Leadership: Merck
Stock and Other Ownership Interests: Merck, ArsenalBio, Tango Therapeutics
Consulting or Advisory Role: Kandy Therapeutics, Sojournix, Eisai, Jazz Pharmaceuticals
Research Funding: Pfizer, Merck, QUE Oncology, Kandy Therapeutics
Patricia A. Ganz
Leadership: Intrinsic LifeSciences
Stock and Other Ownership Interests: Xenon Pharma, Intrinsic LifeSciences, Silarus Therapeutics, Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma, Ambys Medicines, Global Blood Therapeutics, GlaxoSmithKline, Ionis Pharmaceuticals, Akebia Therapeutics, Protagonist Therapeutics, Regeneron, Sierra Oncology, Rockwell Medical Technologies Inc, Astellas Pharma, Gossamer Bio, American Regent, Disc Medicine, Blue Note Therapeutics
Research Funding: Sierra Oncology
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, UpToDate royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 Virtual San Antonio Breast Cancer Symposium, December 8-12, 2020.
SUPPORT
Supported by NCI R01 CA200977 as well as NCI P30 CA016042 (UCLA), P30 CA006973 (JHU), and NIH National Center for Advancing Translational Science UL1TR001881 (UCLA). Additional support was provided by the Breast Cancer Research Foundation (BCRF) to J.E.B., A.H.P., and P.A.G., who discloses that she is a member of the BCRF Scientific Advisory Board, and by the Komen Foundation SAC 170001 to A.C.W. and SAC 100008 to A.H.P.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00279.
AUTHOR CONTRIBUTIONS
Conception and design: Julienne E. Bower, Ann H. Partridge, Antonio C. Wolff, Elissa D. Thorner, Michael R. Irwin, Hadine Joffe, Catherine M. Crespi, Patricia A. Ganz
Financial support: Julienne E. Bower, Ann H. Partridge, Antonio C. Wolff, Michael R. Irwin, Catherine M. Crespi, Patricia A. Ganz
Administrative support: Ann H. Partridge, Antonio C. Wolff, Michael R. Irwin, Patricia A. Ganz
Provision of study materials or patients: Ann H. Partridge, Antonio C. Wolff, Michael R. Irwin, Patricia A. Ganz
Collection and assembly of data: Julienne E. Bower, Ann H. Partridge, Antonio C. Wolff, Elissa D. Thorner, Laura Petersen, Patricia A. Ganz
Data analysis and interpretation: Julienne E. Bower, Ann H. Partridge, Antonio C. Wolff, Michael R. Irwin, Hadine Joffe, Laura Petersen, Catherine M. Crespi, Patricia A. Ganz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Targeting Depressive Symptoms in Younger Breast Cancer Survivors: The Pathways to Wellness Randomized Controlled Trial of Mindfulness Meditation and Survivorship Education
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Travel, Accommodations, Expenses: Novartis
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Hadine Joffe
Employment: Merck
Leadership: Merck
Stock and Other Ownership Interests: Merck, ArsenalBio, Tango Therapeutics
Consulting or Advisory Role: Kandy Therapeutics, Sojournix, Eisai, Jazz Pharmaceuticals
Research Funding: Pfizer, Merck, QUE Oncology, Kandy Therapeutics
Patricia A. Ganz
Leadership: Intrinsic LifeSciences
Stock and Other Ownership Interests: Xenon Pharma, Intrinsic LifeSciences, Silarus Therapeutics, Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma, Ambys Medicines, Global Blood Therapeutics, GlaxoSmithKline, Ionis Pharmaceuticals, Akebia Therapeutics, Protagonist Therapeutics, Regeneron, Sierra Oncology, Rockwell Medical Technologies Inc, Astellas Pharma, Gossamer Bio, American Regent, Disc Medicine, Blue Note Therapeutics
Research Funding: Sierra Oncology
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, UpToDate royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.DeSantis CE, Ma J, Gaudet MM, et al. : Breast cancer statistics, 2019. CA Cancer J Clin 69:438-451, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Carreira H, Williams R, Muller M, et al. : Associations between breast cancer survivorship and adverse mental health outcomes: A systematic review. J Natl Cancer Inst 110:1311-1327, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard-Anderson J, Ganz PA, Bower JE, et al. : Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst 104:386-405, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Suppli NP, Johansen C, Christensen J, et al. : Increased risk for depression after breast cancer: A nationwide population-based cohort study of associated factors in Denmark, 1998-2011. J Clin Oncol 32:3831-3839, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Champion VL, Wagner LI, Monahan PO, et al. : Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer 120:2237-2246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusufov M, Nathan M, Wiley A, et al. : Predictors of increased risk for early treatment non-adherence to oral anti-estrogen therapies in early-stage breast cancer patients. Breast Cancer Res Treat 185:53-62, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Groenvold M, Petersen MA, Idler E, et al. : Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 105:209-219, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Brand JS, Fang F, et al. : Time-dependent risk of depression, anxiety, and stress-related disorders in patients with invasive and in situ breast cancer. Int J Cancer 140:841-852, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Avis NE, Crawford S, Manuel J: Quality of life among younger women with breast cancer. J Clin Oncol 23:3322-3330, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Maass S, Boerman LM, Verhaak PFM, et al. : Long-term psychological distress in breast cancer survivors and their matched controls: A cross-sectional study. Maturitas 130:6-12, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Schell LK, Monsef I, Wockel A, et al. : Mindfulness-based stress reduction for women diagnosed with breast cancer. Cochrane Database Syst Rev 3:CD011518, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman CJ, Ersser SJ, Hopkinson JB, et al. : Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: A randomized, controlled trial. J Clin Oncol 30:1335-1342, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Wurtzen H, Dalton SO, Elsass P, et al. : Mindfulness significantly reduces self-reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer 49:1365-1373, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Andersen SR, Wurtzen H, Steding-Jessen M, et al. : Effect of mindfulness-based stress reduction on sleep quality: Results of a randomized trial among Danish breast cancer patients. Acta Oncol 52:336-344, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Lengacher CA, Reich RR, Paterson CL, et al. : Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. J Clin Oncol 34:2827-2834, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bower JE, Crosswell AD, Stanton AL, et al. : Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer 121:1231-1240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda A, Yamaoka K, Tango T, et al. : Effectiveness of psychoeducational support on quality of life in early-stage breast cancer patients: A systematic review and meta-analysis of randomized controlled trials. Qual Life Res 23:21-30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faller H, Schuler M, Richard M, et al. : Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. J Clin Oncol 31:782-793, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Mustian KM, Alfano CM, Heckler C, et al. : Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue A meta-analysis. JAMA Oncol 3:961-968, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheier MF, Helgeson VS, Schulz R, et al. : Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J Clin Oncol 23:4298-4311, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Ganz PA, Bower JE, Partridge AH, et al. : Screening for depression in younger breast cancer survivors: Outcomes from use of the 9-item Patient Health Questionnaire. JNCI Cancer Spectr 5:pkab017, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16:606-613, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS: The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measur 1:385-401, 1977 [Google Scholar]
- 24.Hann DM, Jacobsen PB, Azzarello LM, et al. : Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual Life Res 7:301-310, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Savard MH, Savard J, Simard S, et al. : Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology 14:429-441, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Stanton AL, Bernaards CA, Ganz PA: The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst 97:448-456, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Dunnett CW: A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096-1121, 1955 [Google Scholar]
- 28.Hasselblad V: Power considerations for trials of two experimental arms versus a standard active control or placebo. Stat Meth Med Res 25:1824-1835, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Donovan KA, Jacobsen PB, Small BJ, et al. : Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage 36:480-487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin CM, Belleville G, Belanger L, et al. : The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34:601-608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower JE, Irwin MR: Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun 51:1-11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bower JE, Bak K, Berger AM, et al. : Screening, assessment and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology Clinical Practice Guideline adaptation. J Clin Oncol 32:1840-1850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun YH, Lee KS, Kim YW, et al. : Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: A randomized controlled trial. J Clin Oncol 30:1296-1303, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Ganz PA, Greendale GA, Petersen L, et al. : Managing menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Natl Cancer Inst 92:1054-1064, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00279.