PURPOSE
Among Bruton's tyrosine kinase inhibitors, acalabrutinib has greater selectivity than ibrutinib, which we hypothesized would improve continuous therapy tolerability. We conducted an open-label, randomized, noninferiority, phase III trial comparing acalabrutinib and ibrutinib in patients with chronic lymphocytic leukemia (CLL).
METHODS
Patients with previously treated CLL with centrally confirmed del(17)(p13.1) or del(11)(q22.3) were randomly assigned to oral acalabrutinib 100 mg twice daily or ibrutinib 420 mg once daily until progression or unacceptable toxicity. The primary end point was independent review committee–assessed noninferiority of progression-free survival (PFS).
RESULTS
Overall, 533 patients (acalabrutinib, n = 268; ibrutinib, n = 265) were randomly assigned. At the data cutoff, 124 (46.3%) acalabrutinib patients and 109 (41.1%) ibrutinib patients remained on treatment. After a median follow-up of 40.9 months, acalabrutinib was determined to be noninferior to ibrutinib with a median PFS of 38.4 months in both arms (95% CI acalabrutinib, 33.0 to 38.6 and ibrutinib, 33.0 to 41.6; hazard ratio: 1.00; 95% CI, 0.79 to 1.27). All-grade atrial fibrillation/atrial flutter incidence was significantly lower with acalabrutinib versus ibrutinib (9.4% v 16.0%; P = .02); among other selected secondary end points, grade 3 or higher infections (30.8% v 30.0%) and Richter transformations (3.8% v 4.9%) were comparable between groups and median overall survival was not reached in either arm (hazard ratio, 0.82; 95% CI, 0.59 to 1.15), with 63 (23.5%) deaths with acalabrutinib and 73 (27.5%) with ibrutinib. Treatment discontinuations because of adverse events occurred in 14.7% of acalabrutinib-treated patients and 21.3% of ibrutinib-treated patients.
CONCLUSION
In this first direct comparison of less versus more selective Bruton's tyrosine kinase inhibitors in CLL, acalabrutinib demonstrated noninferior PFS with fewer cardiovascular adverse events.
INTRODUCTION
Bruton's tyrosine kinase (BTK) plays a significant role in survival, proliferation, and adhesion of malignant B lymphocytes in chronic lymphocytic leukemia (CLL).1-3 BTK inhibitors (BTKis) have transformed CLL management.4-7 Ibrutinib, the first inhibitor that irreversibly binds BTK,8 is approved for the treatment of CLL and small lymphocytic lymphoma.9 Long-term pivotal phase III trials of ibrutinib versus chemoimmunotherapy in previously untreated (RESONATE-2) or relapsed (RESONATE) CLL report survival benefits with ibrutinib, but with toxicities leading to ibrutinib discontinuation in 28% and 12% of patients at the median follow-up of 60 and 44 months, respectively.10,11 A systematic review and meta-analysis of randomized ibrutinib trials demonstrated an increased risk of atrial fibrillation and hypertension.12 In the aforementioned analyses of the RESONATE-2 and RESONATE trials, atrial fibrillation rates were 16% and 11%, respectively, and hypertension rates were 26% and 20%, respectively.10,11 Although the cause of cardiac events with ibrutinib is not completely understood, rodent studies have suggested that off-target inhibition of the PI3K-Akt signaling pathway (via tec protein tyrosine kinase) or C-terminal Src kinase may contribute.13,14 In addition, ibrutinib inhibits human epidermal growth factor receptor 2, which is involved in cardiac myocyte homeostasis.15,16 Ibrutinib binds irreversibly to Src kinase and to several non-BTK kinases with analogous cysteine residues at low nanomolar concentrations, which is not seen with the BTKi acalabrutinib,17-19 likely contributing to alternative target adverse events (AEs) with ibrutinib.8,12,18,20
Acalabrutinib is a next-generation, irreversible BTKi approved for the treatment of CLL and small lymphocytic lymphoma with a shorter plasma half-life and greater selectivity for BTK compared with ibrutinib.8,9,17,21 Acalabrutinib demonstrated superior progression-free survival (PFS) versus chemoimmunotherapy in phase III studies in patients with previously untreated (ELEVATE-TN) or relapsed or refractory (ASCEND) CLL, with toxicity-related treatment discontinuations in 9% and 11% of patients at the median follow-up of 28.3 and 16.1 months, respectively.6,7 Treatment discontinuations because of AEs in longer-term analyses of a phase II clinical trial of acalabrutinib monotherapy in previously untreated or relapsed or refractory CLL were reported in 6% and 11% of patients, respectively, at the median follow-up of 53 and 41 months, respectively.22,23 Acalabrutinib has also demonstrated efficacy and tolerability in ibrutinib-intolerant patients with CLL.24
This phase III trial prospectively compared the efficacy and safety of acalabrutinib with ibrutinib in patients with previously treated CLL to test the hypothesis that acalabrutinib was noninferior to ibrutinib in PFS with improved tolerability.
METHODS
Patients
Eligible patients were age 18 years or older, had previously treated CLL, required therapy by International Workshop on CLL criteria,25 had an Eastern Cooperative Oncology Group performance status of 2 or less, and had the presence of del(17)(p13.1) and/or del(11)(q22.3) confirmed by central laboratory testing. Patients with significant cardiovascular disease, concomitant warfarin or equivalent vitamin K antagonist treatment, prior BTK or BCL-2 inhibitor treatment, or requiring treatment with proton-pump inhibitors were excluded. See the Data Supplement (online only) for additional eligibility criteria.
Study Oversight and Conduct
This is a phase III, randomized, multicenter, open-label, noninferiority study (ClinicalTrials.gov identifier: NCT02477696). The Protocol (online only) and informed consent were approved by an Institutional Review Board and Independent Ethics Committee before study initiation. All patients provided a signed informed consent form before enrollment. The study was conducted in accordance with the protocol, applicable local regulations, and the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practices principles.
Random Assignment and Treatment
An interactive web response system randomly assigned eligible patients in a 1:1 ratio to receive oral acalabrutinib 100 mg twice daily or ibrutinib 420 mg once daily (open-label) until disease progression or unacceptable toxicity. Dose modifications were allowed for AE management (Data Supplement). Random assignment was stratified by del(17)(p13.1) status (yes or no), Eastern Cooperative Oncology Group performance status score (2 v 1 or less), and number of prior therapies (1-3 v 4 or more). Crossover between treatment groups was not permitted. An independent review committee (IRC) centrally assessed progression and response data in a blinded manner. An independent data monitoring committee periodically reviewed unblinded safety and efficacy data. The study team was blinded to data at the aggregate level from the start of the study until after the final data transfer from the IRC and finalization of the statistical analysis plan. The study sponsor performed aggregated analyses by treatment group after final results were received from the IRC.
Study End Points and Assessments
The primary end point was IRC-assessed PFS, defined as the time from random assignment until disease progression or death from any cause. Response assessments followed International Workshop on CLL 2008 criteria,25 with treatment-related lymphocytosis in the absence of other signs of disease progression not considered progressive disease (see the Data Supplement). Secondary end points were the incidences of atrial fibrillation (any grade), infections (grade 3 or higher), and Richter transformation and overall survival (OS) (time from random assignment to any-cause death). Additional end points are described in the Data Supplement.
Safety was assessed by AE, laboratory, and clinical assessments across the treatment-emergent period, defined as the time from the first study drug dose until 30 days after the last dose or the date a patient started a new anticancer therapy, whichever was earlier. AE severity was graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.03.
Statistical Analysis
Sample size was calculated assuming a hazard ratio (HR) scale margin of 1.429 (noninferiority margin of 30%) between the acalabrutinib and ibrutinib groups for IRC-assessed median PFS, using a fixed margin method.26 Assuming an exponential distribution for PFS events, 500 patients (randomly assigned 1:1 to each arm) would provide 80% power at a one-sided, 0.025 significance level to test the primary study hypothesis that acalabrutinib is noninferior to ibrutinib in IRC-assessed PFS.
The primary analysis was conducted after completion of enrollment and accrual of approximately 250 IRC-assessed PFS events. The acalabrutinib/ibrutinib HR estimate and corresponding 95% CI for IRC-assessed PFS were computed using a Cox proportional-hazards model stratified by del(17)(p13.1) status (yes or no) and number of prior therapies (1-3 v 4 or more). All other stratified analyses used the same strata as the primary analysis.
The primary end point, IRC-assessed PFS, was assessed first for noninferiority. The gate-keeping strategy was implemented to control the family-wise error rate at the 0.05 level given the multiple testing approach for primary and secondary end points. If acalabrutinib was noninferior to ibrutinib on the primary end point (upper bound of HR two-sided 95% CI below 1.429), acalabrutinib superiority on the secondary end points was tested at a two-sided 0.05 significance level in the following prespecified order: (1) incidence of any-grade atrial fibrillation, (2) incidence of grade 3 or higher infections, (3) incidence of Richter transformation, and (4) OS. If noninferiority on the primary end point was not met or when superiority on a secondary end point was not met, the P values for all subsequent end points are presented as descriptive. Additional analyses were not adjusted for multiplicity. Between-group differences were assessed using two-sided Cochran-Mantel-Haenszel tests adjusted for del(17)(p13.1) status (yes or no) and number of prior therapies (1-3 v 4 or more) for all secondary end points except OS, which was assessed using Kaplan-Meier methods and a stratified log-rank test. Additional statistical methodologies are described in the Data Supplement.
Efficacy analyses were performed for the intent to-treat population (all randomly assigned patients). Safety analyses, including the safety secondary end points, were performed for the safety population (all patients who received at least one dose of study drug).
RESULTS
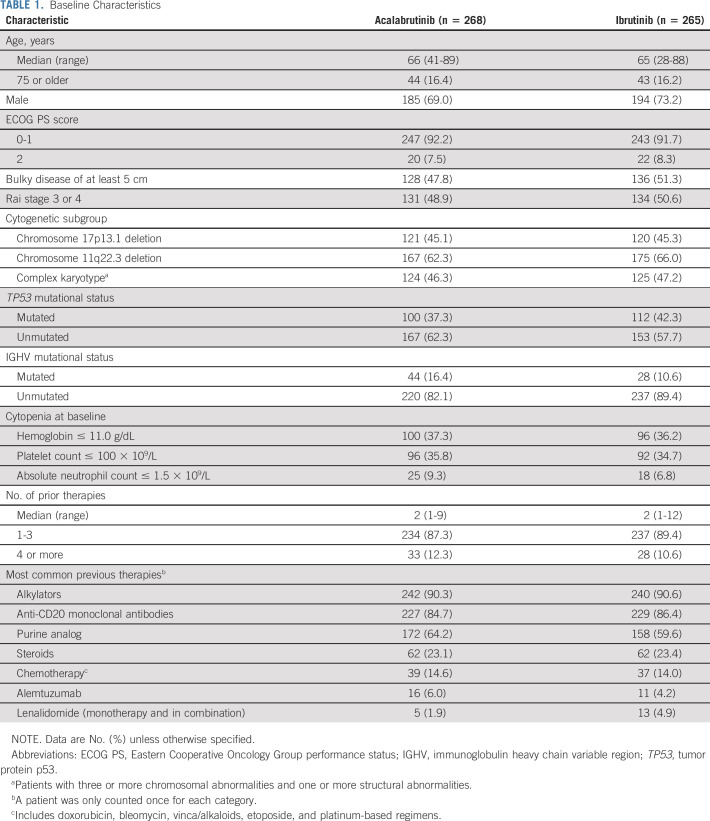
From October 2015 to November 2017, 808 patients were screened for eligibility and 533 patients were randomly assigned at 124 centers in 15 countries to receive acalabrutinib (n = 268) or ibrutinib (n = 265) (Fig 1). Baseline demographics and disease characteristics were balanced between groups (Table 1; Data Supplement). Overall, the median age was 66 years (range, 28-89 years), 241 (45.2%) patients had del(17)(p13.1), and 342 (64.2%) had del(11)(q22.3). The median number of prior therapies was two in both arms (overall range, 1-12; Table 1; Data Supplement).
FIG 1.
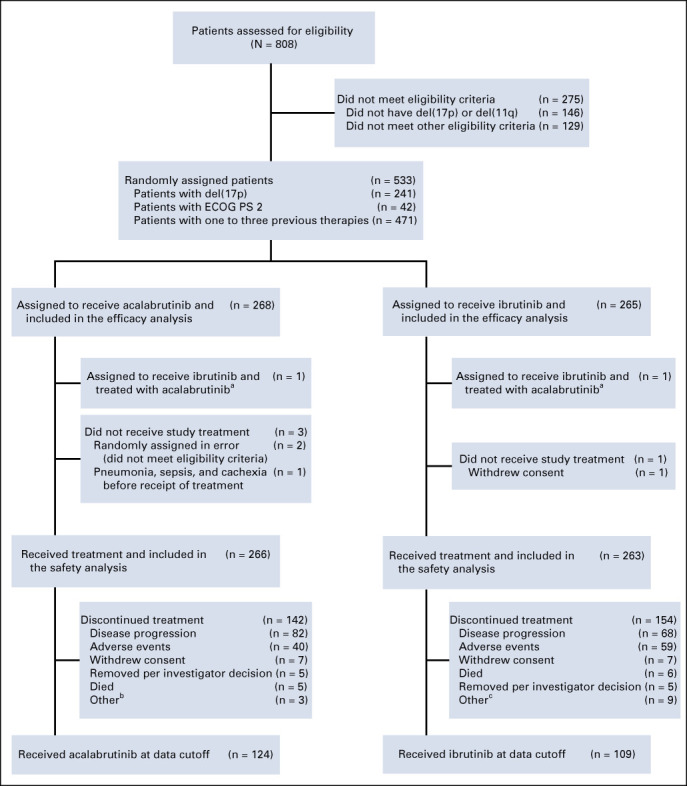
CONSORT diagram. aOne patient who was randomly assigned to the ibrutinib treatment arm received acalabrutinib and ibrutinib during the study and was included in the acalabrutinib safety population. bIncludes patients who discontinued treatment because of relocation (n = 1), medical monitor decision (n = 1), and starting therapy with ibrutinib (n = 1) but agreed to remain on study for follow-up. cIncludes patients who discontinued treatment because of noncompliance (n = 2), withdrawal of consent for treatment or follow-up (n = 1), refusal of medication (n = 1), relocation (n = 2), medical monitor decision (n = 1), early termination because of second primary malignancy (n = 1), and IRC- and medical monitor– or sponsor-confirmed progressive disease (n = 1) but agreed to remain on study for follow-up. ECOG PS, Eastern Cooperative Oncology Group performance status; IRC, Independent Review Committee.
TABLE 1.
Baseline Characteristics
Efficacy
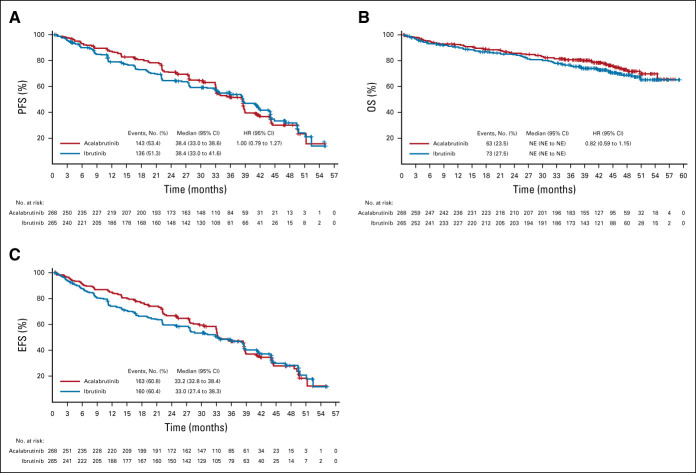
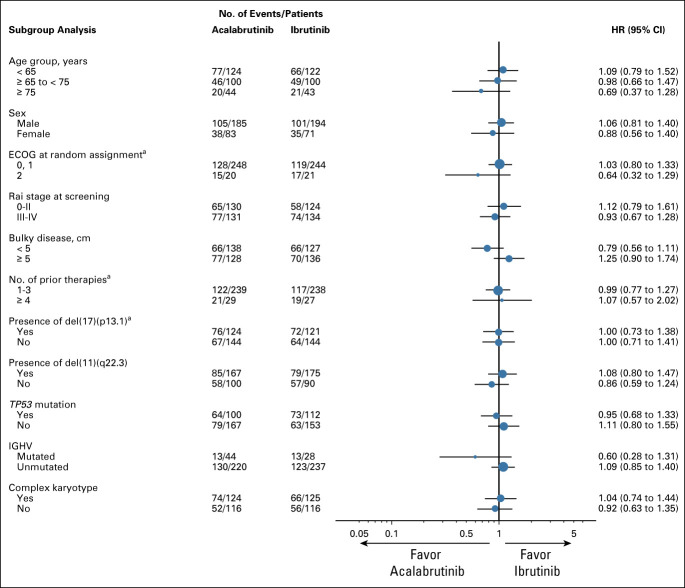
At the data cutoff for the final analysis (September 15, 2020), 124 (46.3%) acalabrutinib patients and 109 (41.1%) ibrutinib patients remained on treatment. After a median follow-up of 40.9 months (range, 0.0-59.1), the prespecified criterion for noninferiority was met; the median IRC-assessed PFS was 38.4 months in both arms (acalabrutinib: 95% CI, 33.0 to 38.6; ibrutinib: 95% CI, 33.0 to 41.6; HR 1.00; 95% CI, 0.79 to 1.27; Fig 2A). IRC-assessed PFS was generally comparable across prespecified subgroups (Fig 3) including patients with del(17)(p13.1) and del(11)(q22.3) (Data Supplement) and regardless of the number of prior therapies. Median OS was not reached in either arm, with 63 (23.5%) deaths with acalabrutinib and 73 (27.5%) with ibrutinib (HR, 0.82; 95% CI, 0.59 to 1.15; Fig 2B; Data Supplement). The IRC-assessed overall response rate was 81.0% (217 of 268; 95% CI, 75.8 to 85.2) for acalabrutinib and 77.0% (204 of 265; 95% CI, 71.5 to 81.6) for ibrutinib (Data Supplement). Investigator-assessed PFS, IRC- and investigator-assessed event-free survival, and investigator-assessed overall response rate were also similar between arms (Fig 2C; Data Supplement). Subsequent anticancer therapy for CLL was initiated by 60 (23.3%) acalabrutinib patients and 56 (22.2%) ibrutinib patients (Data Supplement); median time to next treatment was similar between groups (Data Supplement).
FIG 2.
PFS, OS, and EFS. (A) Kaplan-Meier curve of IRC-assessed PFS (primary end point). (B) Kaplan-Meier curve of OS (secondary end point). (C) Kaplan-Meier curve of IRC EFS. The Kaplan-Meier curves for IRC-assessed PFS cross at 33 months, indicating a violation of the proportional hazards assumption. A sensitivity analysis on the basis of RMST, which is valid under nonproportional hazards, confirmed that acalabrutinib was noninferior to ibrutinib, with a difference in RMST (acalabrutinib-ibrutinib) of 1.1 month (95% CI: −2.17 to 4.36) over 55 months. The lower bound of the 95% CI was compared with an RMST noninferiority margin of −5.83 months, derived from the HR noninferiority margin of 1.429. For the PFS analysis, three ibrutinib-treated patients were censored because of PD or death immediately after missing two or more consecutive visits, and seven acalabrutinib and eight ibrutinib patients were censored at random assignment because of no baseline assessment and/or no adequate postbaseline assessment. EFS, event-free survival; HR, hazard ratio; IRC, Independent Review Committee; NE, not estimable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; RMST, restricted mean survival time.
FIG 3.
Prespecified subgroup analysis of IRC-assessed PFS. aPer interactive voice-web response system record. ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable region; IRC, Independent Review Committee; PFS, progression-free survival.
Safety
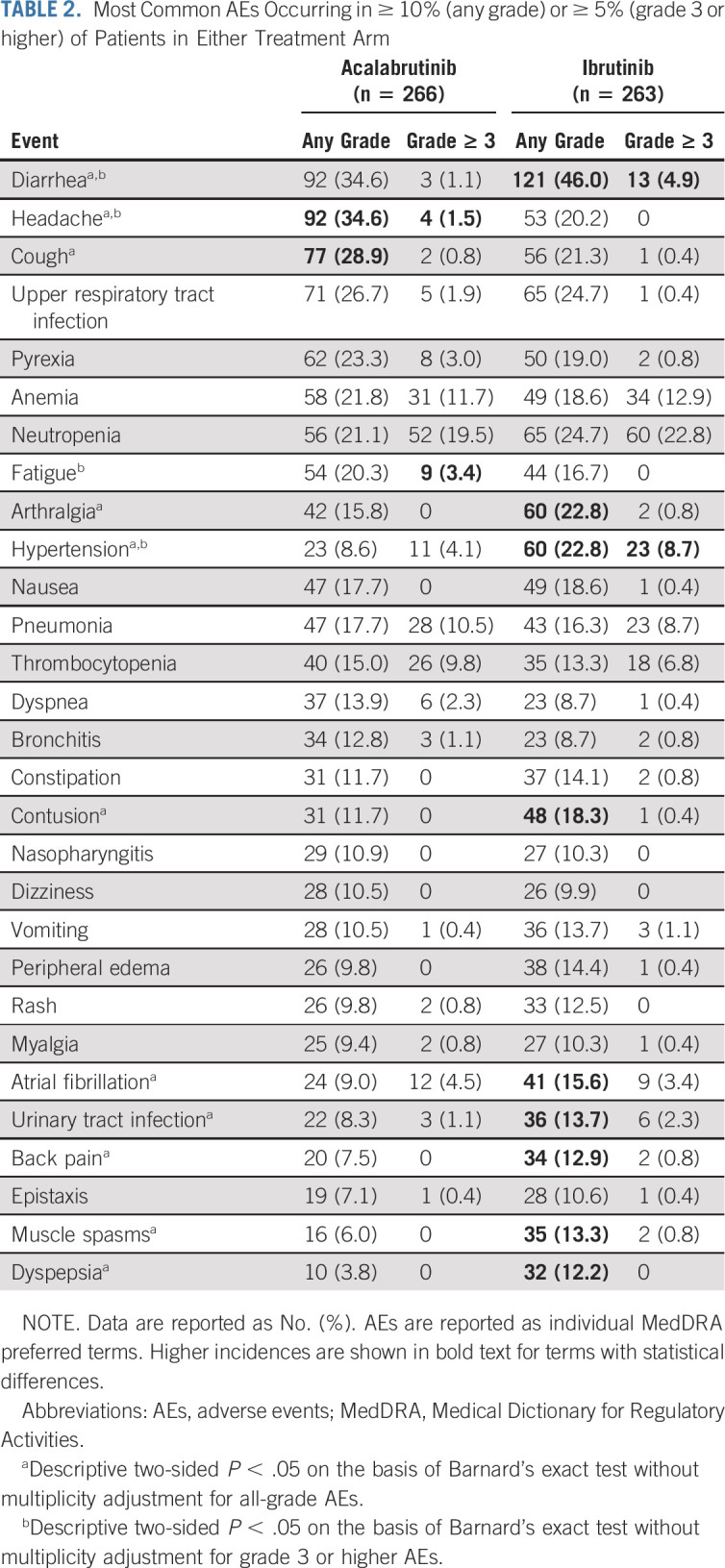
The median treatment exposure duration was 38.3 months (range, 0.3-55.9 months) with acalabrutinib and 35.5 months (range, 0.2-57.7 months) with ibrutinib (Data Supplement). The most common any-grade AEs in at least 10% of patients in either arm included diarrhea, headache, cough, arthralgia, contusion, atrial fibrillation, hypertension, urinary tract infection, back pain, muscle spasms, and dyspepsia (Table 2; Data Supplement). Diarrhea, arthralgia, contusion, atrial fibrillation, hypertension, back pain, muscle spasms, and dyspepsia occurred less frequently with acalabrutinib, whereas headache and cough occurred less frequently with ibrutinib. Kaplan-Meier analysis showed that diarrhea and arthralgia had lower cumulative incidences with acalabrutinib versus ibrutinib over time (Data Supplement). Grade 3 or higher AEs were observed in 68.8% (n = 183) of patients treated with acalabrutinib and 74.9% (n = 197) treated with ibrutinib; the most common grade 3 or higher AEs in at least 5% of patients in either arm were cytopenias, pneumonia, and hypertension (Table 2; Data Supplement). The most common serious AEs in at least 5% of patients in either arm (acalabrutinib v ibrutinib) were pneumonia (n = 27 [10.2%] and n = 26 [9.9%]), anemia (n = 14 [5.3%] and n = 13 [4.9%]), and atrial fibrillation (n = 6 [2.3%] and n = 14 [5.3%]; Data Supplement). AEs led to treatment discontinuation in 14.7% (n = 39) of patients treated with acalabrutinib and 21.3% (n = 56) treated with ibrutinib (Data Supplement); AEs leading to dose interruption or dose reduction occurred at similar frequencies in both arms (Data Supplement).
TABLE 2.
Most Common AEs Occurring in ≥ 10% (any grade) or ≥ 5% (grade 3 or higher) of Patients in Either Treatment Arm
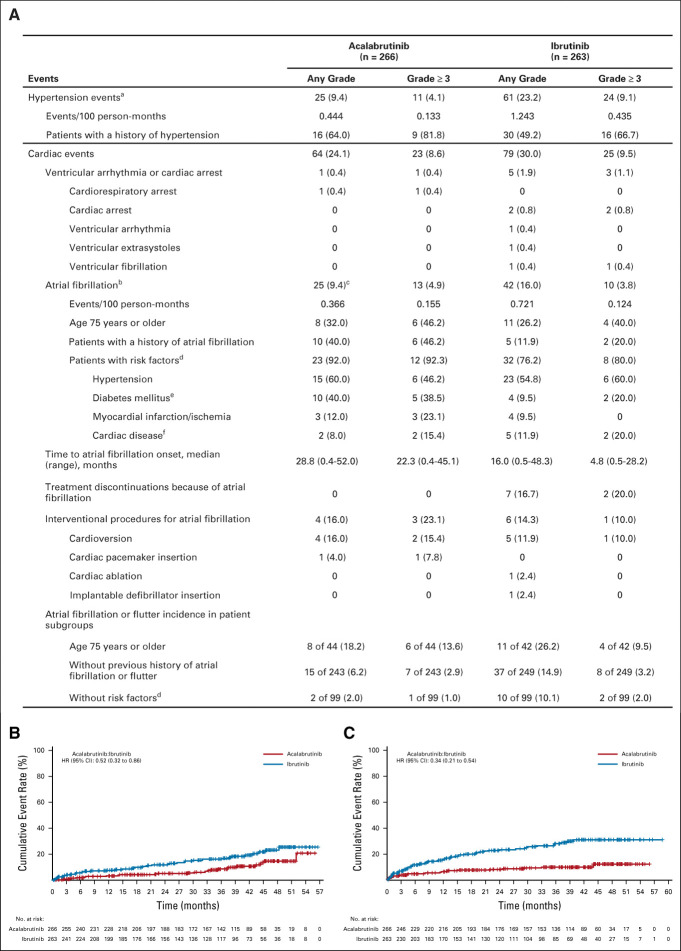
Atrial fibrillation or atrial flutter of any grade was statistically significantly less frequent for acalabrutinib versus ibrutinib (n = 25 [9.4%] v n = 42 [16.0%]; P = .02; Fig 4A); median time to any-grade (28.8 v 16.0 months) and grade 3 or higher (22.3 v 4.8 months) atrial fibrillation or atrial flutter events was longer for acalabrutinib. Among acalabrutinib and ibrutinib patients with atrial fibrillation or flutter, eight (32.0%) and 11 (26.2%) were 75 years or older, respectively, 10 (40.0%) and five (11.9%) had a history of atrial fibrillation, and 15 (60.0%) and 23 (54.8%) had a history of hypertension. Among patients without a prior history of atrial fibrillation or flutter, 15 of 243 (6.2%) and 37 of 249 (14.9%) patients had atrial fibrillation or flutter events with acalabrutinib or ibrutinib, respectively (Fig 4A). No atrial fibrillation events led to treatment discontinuation with acalabrutinib versus seven (2.7%) with ibrutinib. Total cardiac events (acalabrutinib: n = 64 [24.1%] v ibrutinib: n = 79 [30.0%]; Fig 4A; Data Supplement) and hypertension (n = 25 [9.4%] v n = 61 [23.2%], respectively) occurred more frequently with ibrutinib (Fig 4A); grade 3 or higher hypertension incidence was higher with ibrutinib versus acalabrutinib (n = 24 [9.1%] v n = 11 [4.1%], respectively). Exposure-adjusted frequencies of any-grade atrial fibrillation or atrial flutter and hypertension were approximately two-fold and three-fold higher with ibrutinib, respectively. Kaplan-Meier analyses of cumulative incidence revealed HRs of 0.52 (95% CI, 0.32 to 0.86) and 0.34 (95% CI, 0.21 to 0.54) favoring acalabrutinib for atrial fibrillation or atrial flutter (Fig 4B) and hypertension (Fig 4C), respectively. The cumulative incidences of total cardiac events also trended toward acalabrutinib (HR, 0.72; 95% CI, 0.52 to 1.0; Data Supplement). Any-grade cardiac events led to treatment discontinuation in two (0.8%) acalabrutinib-treated patients compared with 11 (4.2%) ibrutinib-treated patients (Data Supplement). No ventricular tachyarrhythmia events occurred with acalabrutinib; one ibrutinib patient experienced grade 4 ventricular fibrillation. One case of sudden cardiac death was reported with ibrutinib in a 55-year-old male with no prior cardiac history after approximately 8 months on treatment.
FIG 4.
(A) Summary of hypertension and selected cardiac events and cumulative incidence of (B) atrial fibrillation and (C) hypertension. NOTE. Data are reported as no. (%) unless otherwise specified; within each event type (hypertension, cardiac events, and atrial fibrillation), percentages are based on the number of patients with the event. aIncludes events with the preferred terms of hypertension, blood pressure increased, and blood pressure systolic increased; two-sided P value on the basis of Barnard’s exact test without multiplicity adjustment, P < .001 (any-grade) and P = .0214 (grade 3 or higher). bIncludes events with the preferred terms of atrial fibrillation and atrial flutter (a patient was only counted once if he or she experienced both types of events); atrial flutter was reported in one patient in the acalabrutinib arm and two patients in the ibrutinib arm (one of the two ibrutinib patients also had an atrial fibrillation event and was counted only once for the combined atrial fibrillation or flutter term). cPart of the multiple testing procedure; difference in any-grade incidence rates was −6.6% (95% CI: −12.2 to −0.9), P = .02. dRisk factors for atrial fibrillation were based on medical review. eIncludes patients with a history of diabetes mellitus or type 2 diabetes mellitus. fIncludes patients with a history of coronary artery bypass, coronary artery disease, cardiomyopathy, cardiac failure chronic, or cardiac failure congestive. HR, hazard ratio.
Rates of grade 3 or higher infections were comparable with acalabrutinib (n = 82 [30.8%]) and ibrutinib (n = 79 [30.0%]) (Data Supplement); the most common grade 3 or higher infections in at least 2% of patients in either arm were pneumonia, sepsis, and urinary tract infection. The cumulative incidences of any-grade and grade 3 or higher infections are shown by arm in the Data Supplement. Fungal opportunistic infection occurred in 10 (3.8%) acalabrutinib patients, including five with pneumocystis jirovecii pneumonia and five with Aspergillus infections, and five (1.9%) ibrutinib patients, including two with Aspergillus infections.
Richter transformation, most commonly manifested as diffuse large B-cell lymphoma, occurred in 10 (3.8%) acalabrutinib and 13 (4.9%) ibrutinib patients; the median time to onset was 7.1 months (range, 2.0-44.7 months) and 11.5 months (range, 2.2-43.6 months), respectively (Data Supplement). Six patients who developed Richter transformation in each arm had del(17)(p13.1).
Bleeding events were less frequent with acalabrutinib (n = 101 [38.0%]) versus ibrutinib (n = 135 [51.3%]; Data Supplement). Rates of major bleeding events were comparable (acalabrutinib: n = 12 [4.5%]; ibrutinib: n = 14 [5.3%]; Data Supplement). Second primary malignancies excluding nonmelanoma skin cancers occurred in 24 (9.0%) and 20 (7.6%) acalabrutinib and ibrutinib patients, respectively (Data Supplement); incidences of all second primary malignancies are shown in the Data Supplement.
Deaths because of AEs within the treatment-emergent period were reported in 17 (6.4%) acalabrutinib and 25 (9.5%) ibrutinib patients (Data Supplement).
DISCUSSION
Herein, we describe the first randomized, phase III trial comparing ibrutinib with acalabrutinib in patients with relapsed CLL. In this study, acalabrutinib, a more selective BTKi, demonstrated similar efficacy versus ibrutinib on the primary end point of IRC-assessed PFS. Concomitantly, lower frequencies of common AEs (such as diarrhea, arthralgia, contusion, back pain, muscle spasms, and dyspepsia) and overall cardiac events, including hypertension and significant decreases in atrial fibrillation, were observed with acalabrutinib. There was increased treatment exposure to acalabrutinib with fewer serious AEs. Other events typically associated with CLL natural history, such as Richter transformation and noncutaneous malignancies, were similar between arms. Collectively, this study met the primary end point of noninferiority and demonstrated that acalabrutinib has similar efficacy to ibrutinib but is generally better tolerated in patients with higher-risk relapsed or refractory CLL.
There has been a paradigm shift in relapsed or refractory CLL management with ibrutinib therapy; however, treatment until disease progression makes tolerability and long-term safety crucial components of therapy, especially in CLL, which tends to affect an older population with comorbidities.27 The cumulative risk of developing atrial fibrillation has emerged as an important issue in patients treated with ibrutinib indefinitely.28,29 A retrospective cohort study of patients with CLL found atrial fibrillation to be the most common toxicity to cause ibrutinib discontinuation.30 Atrial fibrillation is associated with an increased risk of all-cause and cardiovascular mortality, including stroke and other cardiac complications.31 The management of atrial fibrillation is challenging because of increased bleeding risks with prophylactic anticoagulation medication given concomitantly with BTKis and drug-drug interaction potential with anticoagulants.9,21,32 In this study, there was a significantly lower incidence of atrial fibrillation (9.4% v 16.0% with ibrutinib; P = .02) and a 48% lower cumulative atrial fibrillation risk with acalabrutinib. Clinical studies have suggested that different covalent BTKis have comparable activity but variable AE profiles,6,7,10,11,33 which may relate to the degree of BTK selectivity. This was most recently demonstrated by data from a phase III study of zanubrutinib compared with ibrutinib in patients with Waldenstrom's macroglobulinemia, where despite a short study follow-up, the findings also support a reduced incidence of atrial fibrillation with more selective BTK inhibition.33 Hypertension events were also less frequent with acalabrutinib versus ibrutinib (9.4% v 23.2%). Hypertension with ibrutinib has been previously associated with morbidity and mortality.34 Additionally, treatment discontinuation because of cardiac events was more than five-fold higher with ibrutinib compared with acalabrutinib. Moreover, de novo atrial fibrillation or flutter cases were 2.4 times higher with ibrutinib compared with acalabrutinib, and median time to onset for atrial fibrillation or flutter was longer with acalabrutinib versus ibrutinib. Overall, discontinuations because of AEs were numerically lower with acalabrutinib (14.7%) compared with ibrutinib (21.3%). Of note, the incidence of grade ≥ 3 AEs, leading to discontinuation, was similar in both treatment arms. These findings underscore the substantial impact that lower-severity AEs, such as atrial fibrillation, can have on patients receiving chronic therapies. In the present study, survival outcomes were not analyzed by cause of treatment discontinuation to determine if differences in treatment discontinuations because of AEs would affect survival.
Other study limitations include the use of an open-label versus blinded study design that enabled patients and treating physicians to know which treatment each patient received. However, the impact on treatment discontinuation was minimized as both drugs belong to the same class and crossover between groups was not allowed. In addition, the IRC was blinded to treatment assignment, which should facilitate unbiased assessments; assessments of quantifiably observed toxicities such as atrial fibrillation and hypertension should be relatively independent of bias.
This study was performed in patients with relapsed CLL with del(17)(p13.1) or del(11)(q22.3), which are considered high-risk prognostic factors per National Comprehensive Cancer Network guidelines,35 although the value of del(11)(q22.3) as a prognostic factor is questionable with BTKi therapies.36 However, these findings are potentially even more relevant to individuals earlier in the disease course. Patients with previously untreated disease have longer expected survival times37 and therefore potentially longer time on BTKi therapy. The improved tolerability of acalabrutinib suggests that it would be an equally effective yet safer initial treatment than ibrutinib in treatment-naive patients, particularly in those with pre-existing cardiovascular complications.
In summary, in this first directly comparative phase III trial of ibrutinib with acalabrutinib in CLL, acalabrutinib is noninferior in PFS and provides improved safety with fewer atrial fibrillation events and discontinuations because of AEs versus ibrutinib. These clinical trial findings demonstrate that acalabrutinib is better tolerated and has similar efficacy to ibrutinib in previously treated patients with CLL.
ACKNOWLEDGMENT
The authors would like to thank the investigators and coordinators at each of the clinical sites and the patients who participated in this trial and their families. Medical writing assistance, funded by AstraZeneca, was provided by Allison Green, PhD, and Cindy Gobbel, PhD, of Peloton Advantage, LLC, an OPEN Health Company.
John C. Byrd
Stock and Other Ownership Interests: Vincerx Pharma
Honoraria: Pharmacyclics, AstraZeneca, Novartis, Syndax, Trillium Therapeutics
Consulting or Advisory Role: Acerta Pharma, Janssen, Kura Oncology, Novartis, Syndax, AstraZeneca
Research Funding: Acerta Pharma, Pharmacyclics, Zencor
Patents, Royalties, Other Intellectual Property: OSU Patents
Travel, Accommodations, Expenses: Gilead Sciences, Janssen, Novartis, Pharmacyclics, TG Therapeutics
Peter Hillmen
Honoraria: Janssen, AbbVie, Roche
Research Funding: Janssen, Pharmacyclics, Roche, Gilead Sciences, AbbVie
Travel, Accommodations, Expenses: Janssen, AbbVie
Paolo Ghia
Honoraria: AbbVie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, Juno/Celgene/Bristol Myers Squibb, MSD, Lilly, Roche
Consulting or Advisory Role: AbbVie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, MSD, Lilly, Roche
Research Funding: AbbVie, Janssen Oncology, Gilead Sciences, Sunesis Pharmaceuticals, Novartis, AstraZeneca
Arnon P. Kater
Consulting or Advisory Role: Janssen Oncology, AbbVie/Genentech, AstraZeneca, Bristol Myers Squibb/Celgene, Lava Therapeutics
Research Funding: Janssen Oncology, Roche/Genentech, Bristol Myers Squibb/Celgene, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: Acerta Pharma/AstraZeneca, Roche/Genentech
Asher Chanan-Khan
Stock and Other Ownership Interests: Matthew & Asher Inc, NanoDev Therapeutics, STARTON Therapeutics
Honoraria: BeiGene, Ascentage Pharma
Research Funding: Ascentage Pharma
Patents, Royalties, Other Intellectual Property: Patent on PSMB9 biomarker
Richard R. Furman
Honoraria: Janssen, AstraZeneca
Consulting or Advisory Role: Pharmacyclics, Janssen Biotech, Genentech/Roche, Loxo, TG Therapeutics, Verastem, Acerta Pharma, AstraZeneca, Beigene, Incyte, OncoTracker, AbbVie, MorphoSys, Sanofi
Research Funding: Acerta Pharma, TG Therapeutics
Expert Testimony: AbbVie, Janssen Oncology
Travel, Accommodations, Expenses: TG Therapeutics, Janssen Oncology
Other Relationship: Incyte, Janssen Biotech
Susan O'Brien
Employment: University of California, Irvine
Honoraria: Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, AbbVie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Eisai, TG Therapeutics, Nova Research Company
Consulting or Advisory Role: Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie/Genentech, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis Pharmaceuticals
Research Funding: Acerta Pharma, Regeneron, Gilead Sciences, Pfizer, TG Therapeutics, Pharmacyclics, Kite, a Gilead company, Sunesis Pharmaceuticals
Travel, Accommodations, Expenses: Celgene, Janssen, Gilead Sciences, Regeneron, Janssen Oncology
Mustafa Nuri Yenerel
Consulting or Advisory Role: Pfizer, Alexion Pharmaceuticals
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Pfizer
Arpad Illés
Consulting or Advisory Role: Janssen, Takeda, Novartis, Pfizer, Roche, Celgene
Research Funding: Takeda, Seattle Genetics
Travel, Accommodations, Expenses: Novartis, Janssen, Pfizer, Roche
Neil Kay
Consulting or Advisory Role: MorphoSys, Celgene, Agios, CytomX Therapeutics, AstraZeneca, Pharmacyclics, DAVA Pharmaceuticals, Juno Therapeutics, Rigel, Oncotracker, Bristol Myers Squibb, AbbVie, Targeted Oncology, Acerta Pharma/AstraZeneca, MEI Pharma, Sunesis Pharmaceuticals, TG Therapeutics, Tolero Pharmaceuticals, Janssen Biotech, Genentech/AbbVie
Research Funding: Pharmacyclics/Janssen, Tolero Pharmaceuticals, Acerta Pharma, MEI Pharma, Celgene, Genentech, Sunesis Pharmaceuticals, AbbVie, TG Therapeutics, Bristol Myers Squibb
Jose A. Garcia-Marco
Consulting or Advisory Role: AbbVie, Janssen, AstraZeneca Spain
Speakers' Bureau: AbbVie, Janssen, AstraZeneca Spain
Research Funding: AbbVie, Janssen
Travel, Accommodations, Expenses: AbbVie, Janssen
Anthony Mato
Consulting or Advisory Role: TG Therapeutics, AbbVie/Genentech, Celgene, Pharmacyclics, Adaptive Biotechnologies, Verastem, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Loxo/Lilly, Curio/Vaniam Group, Merck, Bristol Myers Squibb/Pfizer
Research Funding: Regeneron, TG Therapeutics, Sunesis Pharmaceuticals, LOXO, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Genmab, Nurix
Javier Pinilla-Ibarz
Honoraria: Novartis, Pharmacyclics, Janssen, AbbVie, Takeda, AstraZeneca
Consulting or Advisory Role: Novartis, Pharmacyclics, Janssen, AbbVie
Speakers' Bureau: Pharmacyclics/Janssen, AbbVie, Takeda, AstraZeneca
Patents, Royalties, Other Intellectual Property: From a WT vaccine patent by Memorial Sloan Kettering Cancer Center
John F. Seymour
Honoraria: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda
Consulting or Advisory Role: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda, AstraZeneca, BMS, Gilead Sciences, MEI Pharma, MorphoSys
Speakers' Bureau: AbbVie, Roche
Research Funding: AbbVie, Celgene, Janssen, Roche
Expert Testimony: Roche
Travel, Accommodations, Expenses: AbbVie, Roche
Stephan Stilgenbauer
Honoraria: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Speakers' Bureau: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Research Funding: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Tadeusz Robak
Honoraria: AbbVie
Consulting or Advisory Role: AbbVie
Research Funding: AbbVie/Genentech
Wayne Rothbaum
Leadership: Iovance Biotherapeutics
Stock and Other Ownership Interests: Acerta Pharma/AstraZeneca, Telios, Kartos Therapeutics
Patents, Royalties, Other Intellectual Property: Telios Pharma and Kartos Therapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Kartos Therapeutics
Uncompensated Relationships: Kartos Therapeutics, Telios
Raquel Izumi
Employment: Acerta Pharma, Vincerx Pharma
Stock and Other Ownership Interests: Acerta Pharma, Vincerx Pharma
Patents, Royalties, Other Intellectual Property: Patents pending for Acerta Pharma
Expert Testimony: Diablo Valley Oncology
Ahmed Hamdy
Employment: Acerta Pharma/AstraZeneca
Stock and Other Ownership Interests: Acerta Pharma
Patents, Royalties, Other Intellectual Property: Acalabrutinib multiple patents
Priti Patel
Employment: AstraZeneca, Neoleukin Therapeutics
Leadership: Neoleukin Therapeutics
Stock and Other Ownership Interests: AstraZeneca, Neoleukin Therapeutics
Kara Higgins
Employment: AstraZeneca, PROMETRIKA LLC
Sophia Sohoni
Employment: AstraZeneca, Portola Pharmaceuticals
Stock and Other Ownership Interests: Theravance
Wojciech Jurczak
Consulting or Advisory Role: Janssen-Cilag, Roche, AstraZeneca, Debiopharm Group, Epizyme
Research Funding: Acerta Pharma, TG Therapeutics, Incyte, Bayer, Sandoz-Novartis, Roche, Takeda, Epizyme, Janssen-Cilag, BeiGene, Debiopharm Group, MorphoSys, MEI Pharma
No other potential conflicts of interest were reported.
See accompanying editorial on page 3419
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology (ASCO) Virtual Annual Meeting, June 4-8, 2021; European Hematology Association (EHA) 2021 Virtual Congress, June 9-17, 2021; and the International Conference on Machine Learning (ICML) Virtual Meeting, July 18-24, 2021.
SUPPORT
Supported by the National Cancer Institute R35 CA197734, Four Winds Foundation, Sullivan CLL Foundation, and the D. Warren Brown Foundation.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: John C. Byrd, Peter Hillmen, Paolo Ghia, Arnon P. Kater, Susan O'Brien, Tadeusz Robak, Wayne Rothbaum, Raquel Izumi, Ahmed Hamdy, Sophia Sohoni, Wojciech Jurczak
Administrative support: Ahmed Hamdy
Provision of study materials or patients: John C. Byrd, Peter Hillmen, Paolo Ghia, Arnon P. Kater, Asher Chanan-Khan, Arpad Illés, Jose A. Garcia-Marco, John F. Seymour, Stephane Lepretre, Stephan Stilgenbauer, Tadeusz Robak
Collection and assembly of data: John C. Byrd, Peter Hillmen, Paolo Ghia, Arnon P. Kater, Asher Chanan-Khan, Susan O'Brien, Mustafa Nuri Yenerel, Arpad Illés, Jose A. Garcia-Marco, Anthony Mato, Javier Pinilla-Ibarz, John F. Seymour, Stephane Lepretre, Stephan Stilgenbauer, Tadeusz Robak, Raquel Izumi, Priti Patel, Sophia Sohoni, Wojciech Jurczak
Data analysis and interpretation: John C. Byrd, Peter Hillmen, Paolo Ghia, Arnon P. Kater, Asher Chanan-Khan, Richard R. Furman, Susan O'Brien, Neil Kay, Jose A. Garcia-Marco, Anthony Mato, Javier Pinilla-Ibarz, John F. Seymour, Stephan Stilgenbauer, Tadeusz Robak, Priti Patel, Kara Higgins, Sophia Sohoni, Wojciech Jurczak
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
John C. Byrd
Stock and Other Ownership Interests: Vincerx Pharma
Honoraria: Pharmacyclics, AstraZeneca, Novartis, Syndax, Trillium Therapeutics
Consulting or Advisory Role: Acerta Pharma, Janssen, Kura Oncology, Novartis, Syndax, AstraZeneca
Research Funding: Acerta Pharma, Pharmacyclics, Zencor
Patents, Royalties, Other Intellectual Property: OSU Patents
Travel, Accommodations, Expenses: Gilead Sciences, Janssen, Novartis, Pharmacyclics, TG Therapeutics
Peter Hillmen
Honoraria: Janssen, AbbVie, Roche
Research Funding: Janssen, Pharmacyclics, Roche, Gilead Sciences, AbbVie
Travel, Accommodations, Expenses: Janssen, AbbVie
Paolo Ghia
Honoraria: AbbVie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, Juno/Celgene/Bristol Myers Squibb, MSD, Lilly, Roche
Consulting or Advisory Role: AbbVie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, MSD, Lilly, Roche
Research Funding: AbbVie, Janssen Oncology, Gilead Sciences, Sunesis Pharmaceuticals, Novartis, AstraZeneca
Arnon P. Kater
Consulting or Advisory Role: Janssen Oncology, AbbVie/Genentech, AstraZeneca, Bristol Myers Squibb/Celgene, Lava Therapeutics
Research Funding: Janssen Oncology, Roche/Genentech, Bristol Myers Squibb/Celgene, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: Acerta Pharma/AstraZeneca, Roche/Genentech
Asher Chanan-Khan
Stock and Other Ownership Interests: Matthew & Asher Inc, NanoDev Therapeutics, STARTON Therapeutics
Honoraria: BeiGene, Ascentage Pharma
Research Funding: Ascentage Pharma
Patents, Royalties, Other Intellectual Property: Patent on PSMB9 biomarker
Richard R. Furman
Honoraria: Janssen, AstraZeneca
Consulting or Advisory Role: Pharmacyclics, Janssen Biotech, Genentech/Roche, Loxo, TG Therapeutics, Verastem, Acerta Pharma, AstraZeneca, Beigene, Incyte, OncoTracker, AbbVie, MorphoSys, Sanofi
Research Funding: Acerta Pharma, TG Therapeutics
Expert Testimony: AbbVie, Janssen Oncology
Travel, Accommodations, Expenses: TG Therapeutics, Janssen Oncology
Other Relationship: Incyte, Janssen Biotech
Susan O'Brien
Employment: University of California, Irvine
Honoraria: Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, AbbVie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Eisai, TG Therapeutics, Nova Research Company
Consulting or Advisory Role: Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie/Genentech, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis Pharmaceuticals
Research Funding: Acerta Pharma, Regeneron, Gilead Sciences, Pfizer, TG Therapeutics, Pharmacyclics, Kite, a Gilead company, Sunesis Pharmaceuticals
Travel, Accommodations, Expenses: Celgene, Janssen, Gilead Sciences, Regeneron, Janssen Oncology
Mustafa Nuri Yenerel
Consulting or Advisory Role: Pfizer, Alexion Pharmaceuticals
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Pfizer
Arpad Illés
Consulting or Advisory Role: Janssen, Takeda, Novartis, Pfizer, Roche, Celgene
Research Funding: Takeda, Seattle Genetics
Travel, Accommodations, Expenses: Novartis, Janssen, Pfizer, Roche
Neil Kay
Consulting or Advisory Role: MorphoSys, Celgene, Agios, CytomX Therapeutics, AstraZeneca, Pharmacyclics, DAVA Pharmaceuticals, Juno Therapeutics, Rigel, Oncotracker, Bristol Myers Squibb, AbbVie, Targeted Oncology, Acerta Pharma/AstraZeneca, MEI Pharma, Sunesis Pharmaceuticals, TG Therapeutics, Tolero Pharmaceuticals, Janssen Biotech, Genentech/AbbVie
Research Funding: Pharmacyclics/Janssen, Tolero Pharmaceuticals, Acerta Pharma, MEI Pharma, Celgene, Genentech, Sunesis Pharmaceuticals, AbbVie, TG Therapeutics, Bristol Myers Squibb
Jose A. Garcia-Marco
Consulting or Advisory Role: AbbVie, Janssen, AstraZeneca Spain
Speakers' Bureau: AbbVie, Janssen, AstraZeneca Spain
Research Funding: AbbVie, Janssen
Travel, Accommodations, Expenses: AbbVie, Janssen
Anthony Mato
Consulting or Advisory Role: TG Therapeutics, AbbVie/Genentech, Celgene, Pharmacyclics, Adaptive Biotechnologies, Verastem, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Loxo/Lilly, Curio/Vaniam Group, Merck, Bristol Myers Squibb/Pfizer
Research Funding: Regeneron, TG Therapeutics, Sunesis Pharmaceuticals, LOXO, AbbVie/Genentech, Pharmacyclics, Adaptive Biotechnologies, Johnson & Johnson, Acerta Pharma/AstraZeneca, DTRM, Genmab, Nurix
Javier Pinilla-Ibarz
Honoraria: Novartis, Pharmacyclics, Janssen, AbbVie, Takeda, AstraZeneca
Consulting or Advisory Role: Novartis, Pharmacyclics, Janssen, AbbVie
Speakers' Bureau: Pharmacyclics/Janssen, AbbVie, Takeda, AstraZeneca
Patents, Royalties, Other Intellectual Property: From a WT vaccine patent by Memorial Sloan Kettering Cancer Center
John F. Seymour
Honoraria: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda
Consulting or Advisory Role: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda, AstraZeneca, BMS, Gilead Sciences, MEI Pharma, MorphoSys
Speakers' Bureau: AbbVie, Roche
Research Funding: AbbVie, Celgene, Janssen, Roche
Expert Testimony: Roche
Travel, Accommodations, Expenses: AbbVie, Roche
Stephan Stilgenbauer
Honoraria: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Speakers' Bureau: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Research Funding: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Celgene, Gilead Sciences, GlaxoSmithKline, Roche, Janssen
Tadeusz Robak
Honoraria: AbbVie
Consulting or Advisory Role: AbbVie
Research Funding: AbbVie/Genentech
Wayne Rothbaum
Leadership: Iovance Biotherapeutics
Stock and Other Ownership Interests: Acerta Pharma/AstraZeneca, Telios, Kartos Therapeutics
Patents, Royalties, Other Intellectual Property: Telios Pharma and Kartos Therapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Kartos Therapeutics
Uncompensated Relationships: Kartos Therapeutics, Telios
Raquel Izumi
Employment: Acerta Pharma, Vincerx Pharma
Stock and Other Ownership Interests: Acerta Pharma, Vincerx Pharma
Patents, Royalties, Other Intellectual Property: Patents pending for Acerta Pharma
Expert Testimony: Diablo Valley Oncology
Ahmed Hamdy
Employment: Acerta Pharma/AstraZeneca
Stock and Other Ownership Interests: Acerta Pharma
Patents, Royalties, Other Intellectual Property: Acalabrutinib multiple patents
Priti Patel
Employment: AstraZeneca, Neoleukin Therapeutics
Leadership: Neoleukin Therapeutics
Stock and Other Ownership Interests: AstraZeneca, Neoleukin Therapeutics
Kara Higgins
Employment: AstraZeneca, PROMETRIKA LLC
Sophia Sohoni
Employment: AstraZeneca, Portola Pharmaceuticals
Stock and Other Ownership Interests: Theravance
Wojciech Jurczak
Consulting or Advisory Role: Janssen-Cilag, Roche, AstraZeneca, Debiopharm Group, Epizyme
Research Funding: Acerta Pharma, TG Therapeutics, Incyte, Bayer, Sandoz-Novartis, Roche, Takeda, Epizyme, Janssen-Cilag, BeiGene, Debiopharm Group, MorphoSys, MEI Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wen T, Wang J, Shi Y, et al. : Inhibitors targeting Bruton's tyrosine kinase in cancers: Drug development advances. Leukemia 35:312-332, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kil LP, de Bruijn MJ, van Hulst JA, et al. : Bruton's tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. Am J Blood Res 3:71-83, 2013 [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij MF, Kuil A, Geest CR, et al. : The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 119:2590-2594, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Tedeschi A, Barr PM, et al. : Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373:2425-2437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Brown JR, O'Brien S, et al. : Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371:213-223, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghia P, Pluta A, Wach M, et al. : ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 38:2849-2861, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Sharman JP, Egyed M, Jurczak W, et al. : Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 395:1278-1291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barf T, Covey T, Izumi R, et al. : Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 363:240-252, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Imbruvica [package insert]. Sunnyvale, CA; Horsham, PA, Pharmacyclics; Janssen Biotech, 2020 [Google Scholar]
- 10.Byrd JC, Hillmen P, O'Brien S, et al. : Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 133:2031-2042, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Barr PM, Robak T, et al. : Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 34:787-798, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldeira D, Alves D, Costa J, et al. : Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One 14:e0211228, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMullen JR, Boey EJ, Ooi JY, et al. : Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 124:3829-3830, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Xiao L, Salem JE, Clauss S, et al. : Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation 142:2443-2455, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salem JE, Manouchehri A, Bretagne M, et al. : Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol 74:1667-1678, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Kinoshita T, Sukbuntherng J, et al. : Ibrutinib inhibits ERBB receptor tyrosine kinases and HER2-amplified breast cancer cell growth. Mol Cancer Ther 15:2835-2844, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Harrington B, O'Brien S, et al. : Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 374:323-332, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond DA, Woyach JA: Targeting BTK in CLL: Beyond ibrutinib. Curr Hematol Malig Rep 14:197-205, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Patel V, Balakrishnan K, Bibikova E, et al. : Comparison of acalabrutinib, a selective Bruton tyrosine kinase inhibitor, with ibrutinib in chronic lymphocytic leukemia cells. Clin Cancer Res 23:3734-3743, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caron F, Leong DP, Hillis C, et al. : Current understanding of bleeding with ibrutinib use: A systematic review and meta-analysis. Blood Adv 1:772-778, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calquence [package insert]. Wilmington, DE, AstraZeneca Pharmaceuticals, 2019 [Google Scholar]
- 22.Byrd JC, Woyach JA, Furman RR, et al. : Acalabrutinib in treatment-naïve chronic lymphocytic leukemia. Blood 137:3327-3328, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd JC, Wierda WG, Schuh A, et al. : Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood 135:1204-1213, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awan FT, Schuh A, Brown JR, et al. : Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv 3:1553-1562, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallek M, Cheson BD, Catovsky D, et al. : Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446-5456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Non-inferiority Clinical Trials to Establish Effectiveness. Guidance for Industry. Silver Spring, MD, US Department of Health and Human Services; Food and Drug Administration, 2016 [Google Scholar]
- 27.Eichhorst B, Goede V, Hallek M: Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 50:171-178, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Brown JR, Moslehi J, O'Brien S, et al. : Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica 102:1796-1805, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiczer TE, Levine LB, Brumbaugh J, et al. : Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv 1:1739-1748, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mato AR, Nabhan C, Barr PM, et al. : Outcomes of CLL patients treated with sequential kinase inhibitor therapy: A real world experience. Blood 128:2199-2205, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Wolf PA, D'Agostino RB, et al. : Impact of atrial fibrillation on the risk of death: The Framingham heart study. Circulation 98:946-952, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Chai KL, Rowan G, Seymour JF, et al. : Practical recommendations for the choice of anticoagulants in the management of patients with atrial fibrillation on ibrutinib. Leuk Lymphoma 58:2811-2814, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Tam CS, Opat S, D'Sa S, et al. : A randomized phase 3 trial of zanubrutinib versus ibrutinib in symptomatic Waldenström macroglobulinemia:the ASPEN study. Blood 136:2038-2050, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickerson T, Wiczer T, Waller A, et al. : Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 134:1919-1928, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Version 3.2021. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2021 [Google Scholar]
- 36.Kipps TJ, Fraser G, Coutre SE, et al. : Long-term studies assessing outcomes of ibrutinib therapy in patients with del(11q) chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk 19:715-722.e6, 2019 [DOI] [PubMed] [Google Scholar]
- 37.O'Brien S, Furman RR, Coutre S, et al. : Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: A 5-year experience. Blood 131:1910-1919, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]