Abstract
Background:
Prepectoral implant-based breast reconstruction is an alternative to subpectoral/dual-plane reconstruction.
Methods:
This study examined outcomes of prepectoral reconstruction using a meta-analysis of data pooled with data from our previous review. Thirty studies were included.
Results:
Explantation, seroma, and infection were the most common complications with no animation deformity reported. Significantly lower odds of infection were observed with prepectoral compared with dual-plane reconstruction.
Conclusions:
Current literature suggests that prepectoral reconstruction may be associated with lower rates of postsurgical infections.
INTRODUCTION
Implant-based breast reconstruction (IBBR) is the most common method of breast reconstruction following mastectomy.1 Advancements in implant technology, improved mastectomy techniques, autologous fat grafting, and acellular dermal matrices (ADM) have contributed to favorable outcomes. Such outcomes have made IBBR a standard of practice in breast reconstruction.2,3 In 2020, 103,485 IBBR procedures were performed in the United States, of which 59,247 (57%) used an ADM for reinforcement of weakened thin tissue.4
Two-stage, dual-plane breast reconstruction, in which the breast implant is placed partially behind the pectoralis major muscle and partially behind the lower mastectomy skin flap with lower pole reinforcement using ADM, has been the most common technique of breast reconstruction in the United States. Although placing the prosthetic device under the pectoralis major muscle provides good coverage of the superior pole, it can be associated with postoperative pain, animation deformity, and surgical morbidity following detachment of the inferior origin of the pectoralis major muscle.5
Prepectoral breast reconstruction is an alternate technique that involves placing the implant above the pectoralis major muscle with or without mesh reinforcement. Because prepectoral breast reconstruction does not involve pectoral muscle detachment, it minimizes many of the untoward events that follow a dual-plane breast reconstruction such as animation, discomfort resulting from muscle spasm, and implant lateralization.6,7 Additional benefits include optimal placement of the prosthetic device on the chest wall to mimic that of the natural breast. These benefits, along with evolving surgical techniques, are making prepectoral breast reconstruction a viable alternative approach in IBBR for patients.5,8
We previously conducted a review of the literature and meta-analysis to evaluate early postsurgical outcomes and analyze trends in complication rates associated with prepectoral breast reconstruction.9 The literature review included 14 studies and demonstrated an overall low incidence of complications with the prepectoral technique, consistent with those generally associated with breast reconstruction. The meta-analysis of four head-to-head studies found similar complication rates between the prepectoral and dual-plane techniques, suggesting that prepectoral breast reconstruction is a feasible option for selected patients.9 Since the completion of our study, there has been a growth in the body of literature reporting outcomes with prepectoral breast reconstruction.5,10–25 None of these studies are large, well-controlled trials that provide definitive answers regarding the use of prepectoral or dual-plane for breast reconstruction; however, they add valuable information to a growing body of literature. The objective of this study was to provide updated evidence based on a literature review of outcomes following prepectoral breast reconstruction, and a comparative analysis of complication rates between studies that directly compared prepectoral and dual-plane breast reconstruction.
MATERIALS AND METHODS
Search Strategy, Selection Criteria, and Screening
The PubMed/MEDLINE database was used for a literature search to identify studies published from November 19, 2016 through June 25, 2018, using the following search terms: {[({[(prepectoral) OR subcutaneous] OR prepectoral} OR subglandular) AND breast reconstruction] AND implant}. Manual searches were also conducted to identify conference abstracts/posters. Inclusion criteria were as follows: studies reporting on IBBR that were mesh-assisted (ADM or synthetic), studies conducted in humans, and single arm or comparative studies reporting outcomes of prepectoral technique. Exclusion criteria included reviews, commentary, opinion pieces and narratives, surveys, non–English-language publications, and small sample size (≤10 patients). Studies identified from the current search were combined with the 11 studies6,7,26–34 from our previous search of studies published between January 1, 2010 and November 18, 2016.9 The same search criteria were used in the previous search, with the exception that there was no minimum sample size. Therefore, when applying the current criteria to the studies from the initial search, two of the 14 studies from previous analysis were excluded in this analysis. In addition, an abstract from the previous search was removed from the analysis because the final peer-reviewed article of the same data was included in the current search. Although not primary objectives of this analysis, the studies were reviewed for patient-reported outcomes and health resource utilization outcomes. However, analyses pertaining to these measures were not included given the small number of studies reporting on these outcomes and the variability in patient-reported outcome instruments and follow-up time points, surgical techniques, and hospital settings.
Data Extraction
Variables extracted from each study included study characteristics (year, country, single-site versus multi-site, study design, sample size), patient characteristics [age, body mass index (BMI), comorbidities, previous breast surgery, chemotherapy/radiation exposure], surgical characteristics (technique, name and size of ADM/mesh, stage and type of reconstruction), and postsurgical characteristics (rates of infection, seroma, hematoma, dehiscence, implant exposure/removal, flap necrosis, animation deformity, and capsular contracture).
Data Synthesis and Statistical Considerations
For the literature review, patient characteristics and outcomes for prepectoral technique were extracted from publications, and weighted averages were calculated according to sample size of individual studies. Patient characteristics were weighted by number of patients, and clinical outcomes and follow-up time were weighted by number of breasts.
For studies that did not report follow-up time, weighted average of the follow-up times reported in other studies was used. For studies that reported median follow-up time (as well as age, BMI, and other factors) along with ranges, mean and SD were calculated using the formula mean = (a + 2m + b)/4, where a = minimum end of the range, m = median, b = upper end of the range, and SD = range/2. If the median was presented along with the interquartile range, the mean was calculated using the following formula: mean = (q1+m+q3)/3, where q1 and q3 represent the first and third quartile, respectively, and m = median; SD = interquartile range/1.35.35 Bivariate analyses were performed on prepectoral data to identify whether any patient or surgical factors significantly correlated with complications. A heat map was created using RStudio (RStudio, Inc., Boston, Mass.) for models, where a significant correlation was found. A P value of less than 0.05 was considered a significant correlation. For studies that conducted head-to-head comparison of outcomes between prepectoral and dual-plane techniques, comparative analyses were conducted by pooling outcomes data and calculation of weighted averages. Weighted averages were taken according to sample size of individual studies. T-test and chi-square tests were performed to compare pooled data between prepectoral and dual-plane techniques.
A meta-analysis was conducted to compare complication rates between prepectoral and dual-plane breast reconstruction in head-to-head studies. A weighted estimator, inversely proportional to the variance in each study, was used to weight individual effect sizes. Weighted mean odds ratios (OR) were calculated to determine the comparative risk of complications between prepectoral and dual-plane breast reconstruction. Outcomes were adjusted per person-year to control for variable follow-up times across studies and groups. Homogeneity of effect sizes across studies was assessed using a chi-square test on Cochran’s Q statistic (significant at P < 0.05). This test determined whether a fixed effects or random effects approach was taken.36
RESULTS
Literature Review
Study Characteristics
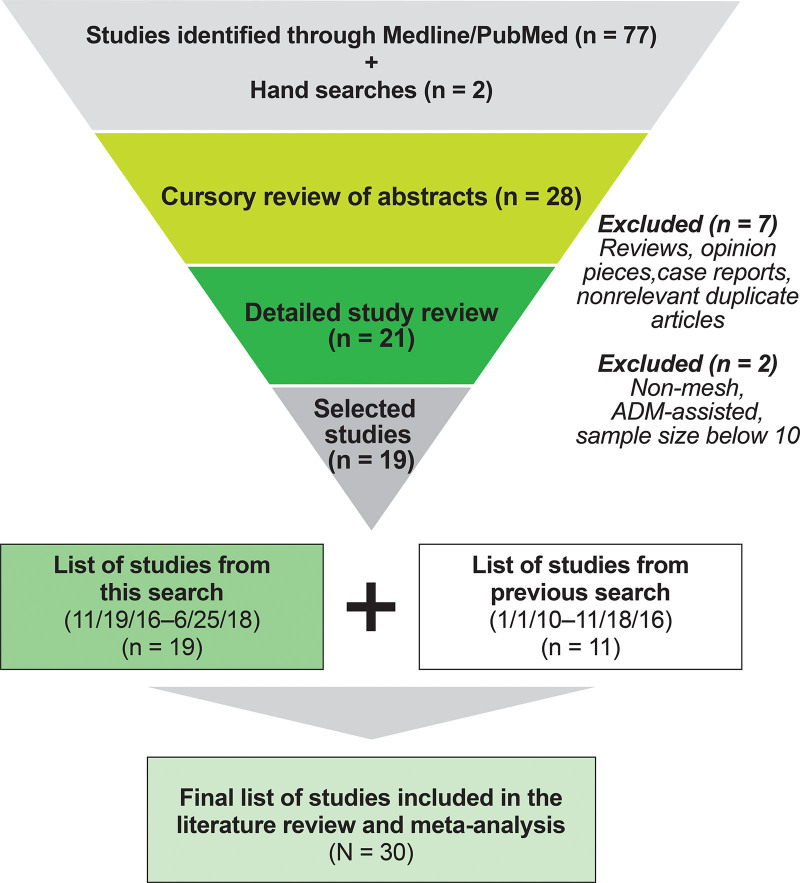
In total, 77 studies were identified from the PubMed/MEDLINE search of studies published from November 19, 2016 through June 25, 2018, and two additional studies were identified through manual searches. After a review of abstract titles, 28 citations were identified and further evaluated. Of these, seven were excluded because they were review articles, duplicates, opinion commentaries, or nonrelevant. Two additional articles were excluded because of a sample size of 10 or fewer or because they did not report use of an ADM or mesh for IBBR. The remaining 19 articles published between November 2016 and June 2018 were combined with 11 studies6,7,26–34 from our previous search that met these study criteria,9 resulting in a total of 30 studies being included in the current literature review (Figure 1).
Fig. 1.
Flow of the systematic literature search.
In the combined studies encompassing the current dataset, 27 studies were from peer-reviewed journals and three were abstracts (Table 1); Nineteen7,11–17,20,21,24,26-30,32,33,37 of these were retrospective studies, whereas 115,6,10,18,19,22,23,25,31,34,38 were prospective studies. An almost equal number of studies were conducted in the United States [16/30 (53.33%)] and Europe [14/30 (46.67%)]. One-stage reconstruction was reported in 18 (60.00%) studies, which was particularly driven by the studies from Europe, where 11 reported one-stage reconstruction. Among the studies conducted in the United States, seven reported on one-stage reconstruction [7/16 (43.75%)], five on two-stage reconstruction [5/16 (31.25%)], and four on a combination of one-stage and two-stage reconstruction [4/16 (25.00%)]. A majority of the studies reported the use of ADM (90%) versus synthetic/absorbable mesh (13.33%) (Table 1). The mean follow-up time reported was 14.41 months (range 3–25 months) following prepectoral breast reconstruction.
Table 1.
Study Characteristics
| Studies, N = 30 | |
|---|---|
| Types of publication, n (%) | |
| Congress abstract | 3 (10.00) |
| Peer-reviewed journal article | 27 (90.00) |
| Location, n (%) | |
| United States | 16 (53.33) |
| Europe | 14 (46.67) |
| Procedure type, n (%) | |
| One-stage reconstruction | 18 (60.00) |
| Two-stage reconstruction | 6 (20.00) |
| Both | 5 (16.67) |
| Unreported/unknown | 1 (3.33) |
| Mesh type, n (%) | |
| ADM only | 25 (83.33) |
| Synthetic/absorbable only | 3 (10.00) |
| Both | 2 (6.67) |
| Unreported | 0 |
| Mesh name*, n (%) | |
| Alloderm [LifeCell Corporation (an AbbVie affiliate), Bridgewater, N.J.] | 11 (36.67) |
| Braxon (Medical Biomaterial Products GmbH, Neustadt-Glewe, Germany) | 7 (23.33) |
| Strattice [LifeCell Corporation (an AbbVie affiliate), Bridgewater, N.J.] | 4 (13.33) |
| TiLoop Bra [Produkte für die Medizin AG, Cologne, Germany] | 3 (10.00) |
| FlexHD [Musculoskeletal Transplant Foundation, Edison, N.J.] | 2 (6.67) |
| Artia [LifeCell Corporation (an AbbVie affiliate), Bridgewater, N.J.] | 2 (6.67) |
| Vicryl (Ethicon, Somerville, N.J.) | 1 (3.33) |
| Native (Medical Biomaterial Products GmbH, Neustadt-Glewe, Germany) | 1 (3.33) |
| Unreported/unknown | 3 (10.00) |
*Multiple meshes may have been used in a single study.
Patient Characteristics and Complication Rates
A total of 1501 patients (2316 breasts) across 30 studies were identified. The mean (SD) age and BMI of patients were 49.62 (11.58) years and 26.82 (4.35) kg/m2, respectively. At the time of the study, 6.76% of all patients were smokers. A quarter of the patients had undergone pre- or postoperative chemotherapy, whereas 5.84% and 15.96% of the patients received pre- or postoperative radiation, respectively. Overall, 90.17% of patients underwent ADM-assisted breast reconstruction. Prior breast surgery was reported in 52.58% of patients based on 115,14,21,24,25,27–32 studies providing this information; however, the procedure type and time since the earlier procedure were generally not reported (Table 2).21
Table 2.
Patient Characteristics
| No. Studies | Variable | Value* |
|---|---|---|
| 30 | No. patients | 1501 |
| 30 | No. breasts | 2316 |
| 27 | Age (SD) | 49.62 (11.58) |
| 25 | BMI (SD) | 26.82 (4.35) |
| 24 | Smokers† | 6.76% |
| 16 | Prior chemotherapy | 22.83% |
| 23 | Prior radiation | 5.84% |
| 14 | Postoperative chemotherapy | 23.79% |
| 22 | Postoperative radiation‡ | 15.96% |
| 11 | Prior breast surgery | 52.58% |
| 30 | ADM-assisted | 90.17% |
| 28 | Follow-up time, mo (range) | 14.41 (3–25) |
*Weighted by sample size (number of patients) except for follow-up time, which is weighted by the number of breasts.
†% smokers is reported as current smokers if current and former smokers are presented separately or as all smokers if not reported separately.
‡Postoperative radiation is reported as either after expander placement or after implant placement.
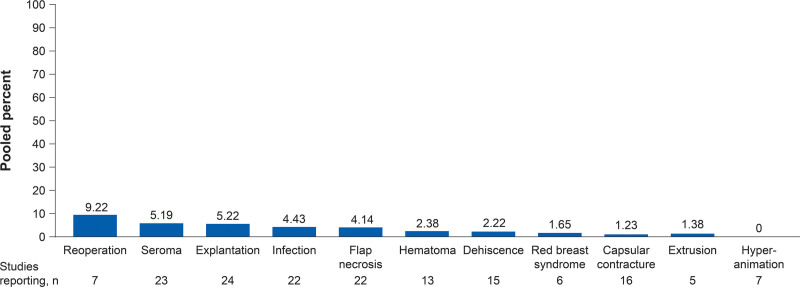
The pooled complication rates showed that the most common complications associated with prepectoral breast reconstruction were explantation (5.22%), seroma (5.19%), infection (4.43%), and flap necrosis (4.14%) (Fig. 2). Other complications included dehiscence (2.22%), red breast syndrome (1.65%), capsular contracture (1.23%), and extrusion (1.38%). Reoperation was performed in 9.22%, as reported in seven5,11,13,16,18,21,31 studies. Of the seven7,17,20,21,27,29,37 studies that evaluated animation deformity, no cases were reported in patients who underwent prepectoral breast reconstruction (Fig. 2).
Fig. 2.
Complication rates associated with prepectoral breast reconstruction. Pooled estimates weighted by sample size (number of breasts). Complication rates are reported post expander placement if the study separates complications after expander and complications after implant. Flap necrosis includes skin necrosis and nipple necrosis.
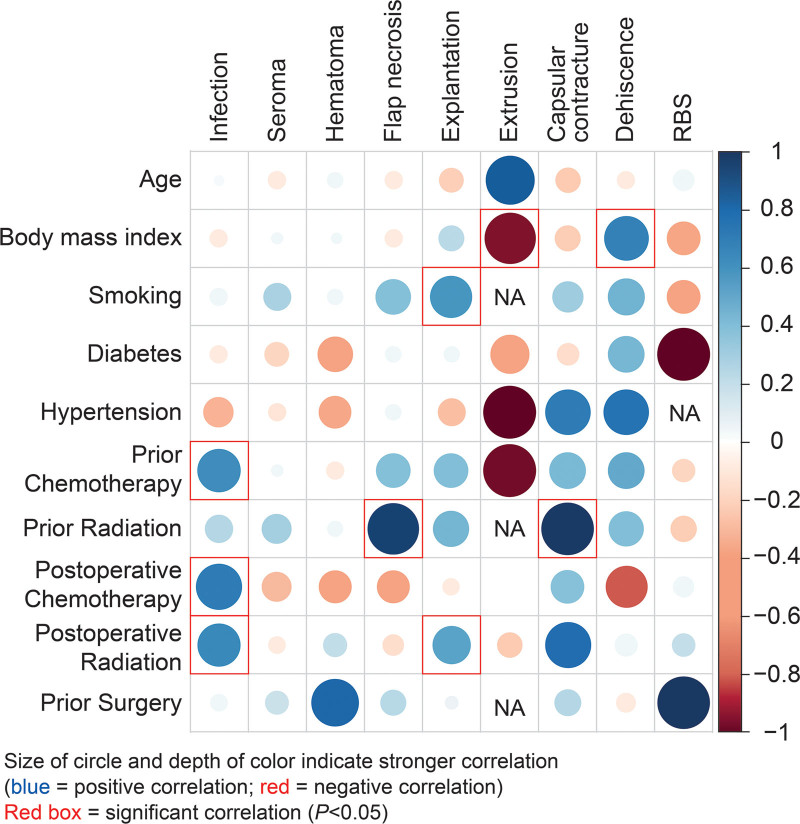
Bivariate analysis of patient characteristics (pre- and postoperative treatment, comorbidities) and complications showed a significant positive correlation between smoking and explantation, preoperative radiation and flap necrosis, postoperative chemotherapy and infection, as also observed in our original study.9 Furthermore, there was also a significant positive correlation between preoperative chemotherapy and infection, preoperative radiation and capsular contracture, postoperative radiation and explantation, and postoperative radiation and infection (Fig. 3).
Fig. 3.
Heatmap showing correlation between comorbidities and complications. NA, not applicable; RBS, red breast syndrome.
Head-to-Head Comparison of Prepectoral versus Dual-plane Reconstruction
Patient Characteristics
Nine studies10,13,16,24–26,28,30,31 were identified that directly compared outcomes for patients undergoing prepectoral versus dual-plane reconstruction [n = 362 patients (538 breasts) in the prepectoral group; n = 471 patients (698 breasts) in the dual-plane group]. Patient characteristics such as preoperative radiation and chemotherapy, previous breast surgery, and follow-up time after breast reconstruction were generally similar between groups, although there was a significantly higher proportion of smokers (4.74% prepectoral versus 11.71% dual-plane) and postoperatively radiated patients (13.33% versus 21.04%) in the dual-plane group, and a higher proportion of diabetic patients (7.38% versus 3.07%) in the prepectoral group (Table 3).10,13,16,24–26,28,30,31 Eight of the nine studies reported complication rates. In a comparison of weighted means from these studies, complication rates with prepectoral versus dual-plane IBBR were similar, except that the occurrence of capsular contracture was significantly less in the prepectoral group (Table 4).10,13,24–26,28,30,31
Table 3.
Patient Characteristics between Prepectoral and Dual-plane Groups among Comparative Studies10,13,16,24–26,28,30,31
| Variable | Prepectoral* | Dual-plane* |
|---|---|---|
| No. patients | 362 | 471 |
| No. breasts | 538 | 698 |
| Age (SD) | 50.99 (12.12) | 49.86 (11.13) |
| BMI (SD)† | 26.70 (4.58) | 25.86 (5.02) |
| Unilateral procedure | 57.26% | 58.06% |
| Smokers‡ | 4.74% | 11.71% |
| Diabetes‡ | 7.38% | 3.07% |
| Hypertension | 19.67% | 16.10% |
| Prior chemotherapy | 18.51% | 18.04% |
| Prior radiation | 6.63% | 5.88% |
| Postoperative chemotherapy | 24.12% | 23.64% |
| Postoperative radiation‡ | 13.33% | 21.04% |
| Prior breast surgery | 14.78% | 15.03% |
| Follow-up time, mo (range) | 7.00 (3–14) | 8.06 (3–14) |
*Weighted by sample size (number of patients) except for follow-up time, which is weighted by number of breasts.
†One study did not report BMI; however, the proportion of high BMI (>35) was significantly lower in the prepectoral group.
‡Significant difference determined by chi-square test (P < 0.05).
Walia et al13 did not report % unilateral. All procedures were assumed to be unilateral.
Table 4.
Comparison of Weighted Averages* of Complication Rates between Prepectoral and Dual-plane Techniques.3,10,13,24–26,28,30,31
| Complication | Prepectoral (n = 8 studies, n = 338 patients, n = 499 breasts) | Dual-plane (n = 8 studies, n = 436 patients, n = 641 breasts) |
|---|---|---|
| Infection | 3.75% | 5.62% |
| Seroma | 4.41% | 4.08% |
| Hematoma | 1.81% | 2.14% |
| Flap necrosis | 5.49% | 5.80% |
| Explantation | 4.90% | 5.09% |
| Capsular contracture† | 0.00% | 14.79% |
| Dehiscence | 2.93% | 2.59% |
*Pooled estimates weighted by sample size (number of breasts); significance not determined in pooled results.
†Significant difference determined by chi-square test (P < 0.05).
Meta-analysis of Complication Rates
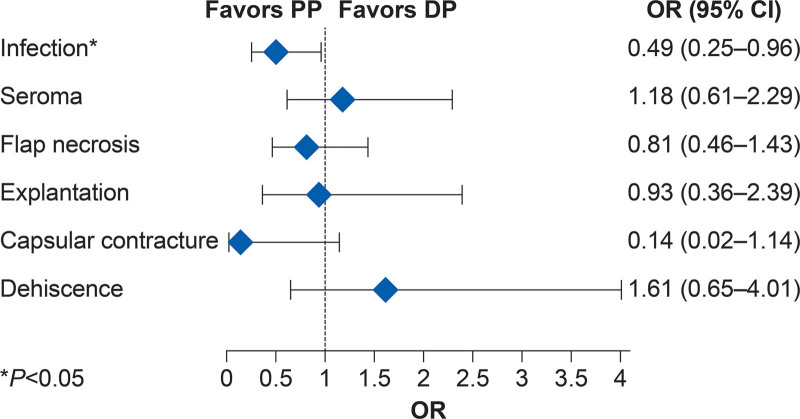
The meta-analysis results revealed no significant differences in the incidence of seroma, hematoma, flap necrosis, explantation, and dehiscence between the prepectoral and dual-plane groups. The prepectoral group had statistically lower odds of infection compared with the dual-plane group (OR 0.49; 95% CI 0.25–0.96; Figure 4). Capsular contracture trended lower for the prepectoral group compared with the dual-plane group, but results did not reach statistical significance (OR 0.14; 95% CI 0.02–1.14).
Fig. 4.
Meta-analysis of complication rates between prepectoral and dual-plane techniques. OR less than one indicates a lower risk of complication in the PP group compared with the DP group. For calculation of OR in the meta-analysis, if there was no incidence of a complication, the number of exposures was counted as 0.5. DP, dual-plane; PP, prepectoral.
DISCUSSION
In our recent updated search of the IBBR literature, 77 new studies published over a period of 20 months since our initial search were screened, of which 19 were selected for the literature review. In contrast, our initial research9 identified 95 articles published over 6 years, of which 11 were included in this analysis. While our initial meta-analysis included only four26,28,30,31 head-to-head comparative studies between prepectoral and dual-plane breast reconstruction, the current search identified five10,13,16,24,25 more, increasing the power of the meta-analysis by pooling of data from nine studies.
The trend we observed in our first literature analysis for a substantial number of studies using one-stage versus two-stage breast reconstruction with prepectoral technique continued in the updated analysis. This largely reflects the proportion of studies from Europe, where one-stage is the standard approach. Although there has been an increase in the incidence of one-stage IBBR in the United States (2016: 10.8% versus 2019: 17.8%), the majority of breast reconstruction procedures are two-stage (2016: 89.2% versus 2019: 82.2%) based on recent statistics reported by the American Society of Plastic Surgeons.4,39 In the current analysis, 11 of 16 studies from the United States reported performing one-stage or a combination of one- and two-stage IBBR.
This literature review update, which includes almost four times the number of patients from our original analysis, continues to show that prepectoral breast reconstruction is associated with an overall low incidence of complications, largely consistent with the incidence of complications with IBBR using the ADM-assisted dual-plane technique. Subcutaneous implant placement without disruption of the pectoralis major muscle has been associated with absence of animation deformity,27 which is further supported by our findings across seven studies7,15,17,20,21,27,29 reporting no cases of animation deformity on follow-up after prepectoral breast reconstruction. Associations between patient characteristics/comorbidities and surgical outcomes identified by the bivariate analysis are consistent with the findings in our original study. BMI, use of tobacco, pre- and postoperative radiation, and chemotherapy were found to be positively associated with complications.
Comparison of weighted means of complications between the two techniques demonstrated a significantly lower rate of capsular contracture following prepectoral breast reconstruction compared with dual-plane. The current meta-analysis included only two studies30,31 [median follow-up (range): 9.56 months (140–589)30; median follow-up: 25 months (16–40)31] that showed a trend for a lower rate of capsular contracture with prepectoral IBBR; however, it did not reach statistical significance. Although all other complications were found to be comparable between the techniques, the meta-analysis showed that the odds of infection were significantly less with prepectoral versus dual-plane IBBR. These differences may be attributed to a statistically significant, higher percentage of smokers and postoperatively radiated patients in the dual-plane versus prepectoral groups. Therefore, these results should be interpreted with caution because it is not possible to control for this variability without patient-level data. Additional studies with longer follow-up periods are needed to confirm the trend of lower capsular contracture rates associated with prepectoral breast reconstruction. It is postulated that prepectoral placement will decrease the amount of implant movement that is typically seen when implants are placed under the pectoralis major muscle.
Limitations identified in our initial analysis,9 including the small number of randomized comparative studies and the potential for selection bias in retrospective studies; variable, short follow-up times leading to underestimation of complication rates; variation in the use of one-stage versus two-stage breast reconstruction; and type of ADM/mesh used, remain applicable to this analysis. In addition, the inability to control for patient comorbidities, such as smoking and postoperative radiation, that influence outcomes including infection, seroma, and capsular contracture should be recognized in the overall assessment of the data. Furthermore, many postoperative complications after IBBR occur because the mastectomy skin flap has significant ischemia and/or thermal injury. This variable is extremely difficult to standardize in any meta-analysis, yet may contribute more to postoperative complications than the placement position of the implant. Finally, our study did not take into consideration the additional cost of mesh for prepectoral versus dual-plane IBBR. There have been several cost-effectiveness analyses in breast reconstruction comparing different surgical approaches.9,40–42 Consequently, and especially with possible clinical benefits such as the lack of animation deformities, future studies comparing the cost-effectiveness of prepectoral implant placement to dual-plane IBBR are necessary.
CONCLUSIONS
Lower odds of infection after prepectoral reconstruction in comparison with dual-plane reconstruction suggest that the prepectoral technique may have a positive effect on postsurgical complications, bearing in mind that patient characteristics and comorbidities can affect outcomes following reconstruction. There remains a need for large, prospective, comparative studies between breast reconstruction techniques with long-term follow-up.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided to the authors by Yashodhara Dasgupta, PhD, and Susan Sutch, PharmD, CMPP, of Evidence Scientific Solutions, Inc, Philadelphia, Pa., and Linda Romagnano, PhD, of Peloton Advantage (an OPEN Health company at Parsippany, N.J.). Sara Higa, PharmD, MS, of AbbVie, Irvine, Calif. created the heat map. All authors meet the ICMJE authorship criteria. Neither honoraria nor other form of payments were made for authorship.
Footnotes
Published online 26 October 2021.
Disclosure: Dr. Chatterjee is a consultant for Allergan, KCI, and Biom’Up. Dr. Nahabedian is a consultant for Allergan and Stryker. Dr. Gabriel is a consultant for Allergan and KCI. Dr. Sporck was a research fellow at Allergan during the time of this analysis. Drs. Parekh, Macarios, and Hammer are employees of AbbVie and may have AbbVie stock. Dr. Sigalove is a speaker and consultant for Galatea Surgical, Sientra, and KCI, and an editor of Prepectoral Techniques in Reconstructive Breast Surgery (Wolters Kluwer, Philadelphia, Pa.). This study was sponsored by Allergan plc, Irvine, Calif. (prior to its acquisition by AbbVie).
REFERENCES
- 1.Rebowe RE, Allred LJ, Nahabedian MY. The evolution from subcutaneous to prepectoral prosthetic breast reconstruction. Plast Reconstr Surg Glob Open. 2018;6:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salibian AA, Frey JD, Choi M, et al. Subcutaneous implant-based breast reconstruction with acellular dermal matrix/mesh: a systematic review. Plast Reconstr Surg Glob Open. 2016;4:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan NM, Chatterjee A, Rosenkranz KM, et al. The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2014;67:468–476. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Plastic Surgeons. 2019 Plastic Surgery Statistics Report. Available at https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. Accessed September 7, 2021.
- 5.Highton L, Johnson R, Kirwan C, et al. Prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705. [DOI] [PubMed] [Google Scholar]
- 7.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. [DOI] [PubMed] [Google Scholar]
- 8.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee A, Nahabedian MY, Gabriel A, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: a literature review and meta-analysis. J Surg Oncol. 2018;117:1119–1130. [DOI] [PubMed] [Google Scholar]
- 10.Cattelani L, Polotto S, Arcuri MF, et al. One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: functional and cost evaluation. Clin Breast Cancer. 2018;18:e703–e711. [DOI] [PubMed] [Google Scholar]
- 11.Elswick SM, Harless CA, Bishop SN, et al. Prepectoral implant-based breast reconstruction with postmastectomy radiation therapy. Plast Reconstr Surg. 2018;142:1–12. [DOI] [PubMed] [Google Scholar]
- 12.Woo A, Harless C, Jacobson SR. Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J. 2017;23:545–553. [DOI] [PubMed] [Google Scholar]
- 13.Walia GS, Aston J, Bello R, et al. Prepectoral versus subpectoral tissue expander placement: a clinical and quality of life outcomes study. Plast Reconstr Surg Glob Open. 2018;6:e1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidya R, Cawthorn SJ. Muscle-sparing ADM-assisted breast reconstruction technique using complete breast implant coverage: a dual-institute UK-based experience. Breast Care (Basel). 2017;12:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction and postmastectomy radiotherapy: short-term outcomes. Plast Reconstr Surg Glob Open. 2017;5:e1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson EC, Kang V, Cesarz A, et al. Evolution of the surgical technique for direct to implant breast reconstruction: transitioning from dual plane to pre-pectoral implant placement [abstract]. Plast Reconstr Surg Glob Open. 2017;5:109. [Google Scholar]
- 17.Pittman TA, Abbate OA, Economides JM. The P1 method: prepectoral breast reconstruction to minimize the palpable implant edge and upper pole rippling. Ann Plast Surg. 2018;80:487–492. [DOI] [PubMed] [Google Scholar]
- 18.Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing “wrap” technique: a single-center experience. J Plast Reconstr Aesthet Surg. 2017;70:1527–1536. [DOI] [PubMed] [Google Scholar]
- 19.Maruccia M, Di Taranto G, Onesti MG. One-stage muscle-sparing breast reconstruction in elderly patients: a new tool for retaining excellent quality of life. Breast J. 2018;24:180–183. [DOI] [PubMed] [Google Scholar]
- 20.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):31S–38S. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J. 2018;38:519–526. [DOI] [PubMed] [Google Scholar]
- 22.Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon. Gland Surg. 2017;6:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casella D, Di Taranto G, Marcasciano M, et al. Nipple-sparing bilateral prophylactic mastectomy and immediate reconstruction with TiLoop Bra mesh in BRCA1/2 mutation carriers: a prospective study of long-term and patient reported outcomes using the BREAST-Q. Breast. 2018;39:8–13. [DOI] [PubMed] [Google Scholar]
- 24.Bettinger LN, Waters LM, Reese SW, et al. Comparative study of prepectoral and subpectoral expander-based breast reconstruction and clavien IIIb score outcomes. Plast Reconstr Surg Glob Open. 2017;5:e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker BG, Irri R, MacCallum V, et al. A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast Reconstr Surg. 2018;141:1077–1084. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86. [DOI] [PubMed] [Google Scholar]
- 27.Kobraei EM, Cauley R, Gadd M, et al. Avoiding breast animation deformity with pectoralis-sparing subcutaneous direct-to-implant breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabriel A, Sigalove S, Sigalove N, et al. Does surgical technique impact post-operative outcomes of breast reconstruction? Presented at: the Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research; May 21–25, 2016; Washington, DC. [Google Scholar]
- 29.Downs RK, Hedges K. An alternative technique for immediate direct-to-implant breast reconstruction—a case series. Plast Reconstr Surg Glob Open. 2016;4:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra K, Houssock C, Cai SS, et al. Submuscular versus prepectoral immediate implant based breast reconstruction. Abstract presented at: the Annual Meeting of the Northeastern Society of Plastic Surgeons; October 14–16, 2016; Baltimore, MD. [Google Scholar]
- 31.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. 2015;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg. 2017;87:493–498. [DOI] [PubMed] [Google Scholar]
- 33.Becker H, Lind JG, II, Hopkins EG. Immediate implant-based prepectoral breast reconstruction using a vertical incision. Plast Reconstr Surg Glob Open. 2015;3:e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casella D, Calabrese C, Bianchi S, et al. Subcutaneous tissue expander placement with synthetic titanium-coated mesh in breast reconstruction: long-term results. Plast Reconstr Surg Glob Open. 2015;3:e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macarios D, Griffin L, Chatterjee A, et al. A meta-analysis assessing postsurgical outcomes between aseptic and sterile AlloDerm regenerative tissue matrix. Plast Reconstr Surg Glob Open. 2015;3:e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139:287–294. [DOI] [PubMed] [Google Scholar]
- 38.Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017;23:670–676. [DOI] [PubMed] [Google Scholar]
- 39.American Society of Plastic Surgeons. Plastic Surgery Statistics Report 2016. Available at https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed June 15, 2017.
- 40.Asban A, Homsy C, Chen L, et al. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with single stage implant reconstruction in the treatment of breast cancer. Breast. 2018;41:159–164. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee A, Offodile II AC, Asban A, et al. A cost-utility analysis comparing oncoplastic breast surgery to standard lumpectomy in large breasted women. Adv Breast Cancer Res. 2018;7:187–200. [Google Scholar]
- 42.Chatterjee A, Asban A, Jonczyk M, et al. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with free flap reconstruction in the treatment of breast cancer. Am J Surg. 2019;218:597–604. [DOI] [PubMed] [Google Scholar]