Ibrutinib is the oral, covalently binding inhibitor of Bruton's tyrosine kinase (BTK) that revolutionized therapy for patients with chronic lymphocytic leukemia (CLL). Ibrutinib's significant efficacy and enhanced tolerability compared with standard chemoimmunotherapy regimens were driving factors in the shift to targeted therapies as the preferred CLL treatment option for most patients.1-3 For a period, ibrutinib was considered optimal therapy for many patients with CLL. As ibrutinib is intended to be taken continuously for an indefinite period, tolerability of the drug is quite important. With longer follow-up, notable toxicities were documented for patients with CLL, including atrial fibrillation (11%-16%) and hypertension (20%-26%).1,2 Toxicities were cited as the cause of ibrutinib discontinuation in up to 28% of patients in phase III studies.1 With the goals of maintaining the efficacy seen with ibrutinib but reducing toxicity through more selective BTK inhibition, second-generation BTK inhibitors were developed.
Acalabrutinib is an oral covalently binding BTK inhibitor that is approved by the US Food and Drug Administration (FDA) for marketing in the treatment of CLL. This approval was based on acalabrutinib's ability to prolong progression-free survival (PFS) when compared with standard chemoimmunotherapy regimens in phase III studies.4,5 Acalabrutinib discontinuations because of toxicity were only seen in up to 11% of patients in these studies.4,5 It was hypothesized that acalabrutinib would have similar efficacy and less toxicity to ibrutinib. Therefore, a direct head-to-head comparison was undertaken.
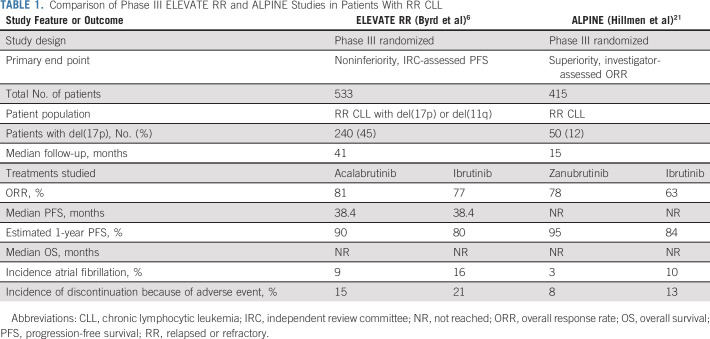
In the article accompanying this editorial, ELEVATE RR study is a phase III, randomized, open-label, noninferiority study with a primary end point of independent review committee-assessed noninferiority of PFS between acalabrutinib and ibrutinib.6 The study enrolled 533 patients with previously treated CLL and prognostic factors of del(17p) (45%) and/or del(11q) (64%), which were both considered high-risk factors at the time of study design [Del(11q) has since been associated with favorable prognosis in the setting of ibrutinib therapy.7] After a median follow-up 41 months, acalabrutinib was found to be noninferior to ibrutinib with median PFS of 38.4 months in both arms (hazard ratio, 1.00). As anticipated, toxicities were experienced less by patients receiving acalabrutinib, including diarrhea, arthralgia, bruising, muscle spasm, and dyspepsia. Especially notable, atrial fibrillation and hypertension occurred less frequently with acalabrutinib.
Incidence of any grade atrial fibrillation was lower with acalabrutinib (9.4% v 16.0%; P = .02). De novo cases of atrial fibrillation were 2.4 times higher in patients receiving ibrutinib. The mechanism underlying ibrutinib-induced atrial fibrillation is not completely understood. Proposed mechanisms are off-target inhibition of cardiac phosphoinositide 3-kinase or receptor tyrosine-protein kinase erbB-2 (human epidermal growth factor receptor 2).8-11 Regardless of mechanism, it is known that atrial fibrillation increases the risk of all-cause and cardiovascular mortality. Therefore, it is important to limit the risk of atrial fibrillation in patients with CLL.12
Medical management of atrial fibrillation not only involves rate control, but also prophylactic anticoagulation. Ibrutinib has been associated with bleeding of any grade in approximately 40% of patients receiving therapy.13 BTK (along with other Tec family kinase members) is involved in platelet aggregation via glycoprotein signaling.14-16 Blockade of BTK decreases platelet adhesion on von Willebrand factor.17 In early ibrutinib clinical trials, some patients receiving warfarin and ibrutinib experienced severe hemorrhage (including subdural bleeding). Therefore, it is strongly recommended not to use warfarin in combination with ibrutinib.18 In the ELEVATE RR study, all-grade bleeding events were experienced less frequently in patients receiving acalabrutinib (38%) compared with ibrutinib (51%). Warfarin use was an exclusion criterion for this study, but the reduced risk of bleeding seen with acalabrutinib makes this a slightly safer choice for patients who need other anticoagulants secondary to atrial fibrillation or other causes.
The incidence of hypertension was lower in patients receiving acalabrutinib (9%) versus ibrutinib (23%). The mechanism of hypertension is not well understood at this time. Hypertension is a particularly important toxicity to avoid as ibrutinib-related hypertension has been associated with increased rates of morbidity and mortality.19
Overall, on the ELEVATE RR study, discontinuations of treatment because of adverse events occurred less frequently in acalabrutinib-treated patients (15%) than in ibrutinib-treated patients (21%). In the management of CLL, is acalabrutinib simply the best? Is it better than all the rest? There are other notable contenders for this designation.
Zanubrutinib is another oral covalently binding selective BTK inhibitor that has been approved by the US FDA for marketing in the treatment of mantle cell lymphoma, but not yet for CLL. The ASPEN study was a phase III study comparing treatment with zanubrutinib versus ibrutinib for patients with relapsed or refractory Waldenstrom macroglobulinemia.20 After a median follow-up of 33 months, this study found no statistically significant difference in response rates, duration of response, PFS, or OS between the two agents. Notably, atrial fibrillation, bruising, bleeding, diarrhea, edema, muscle spasm, and pneumonia were all experienced less commonly in patients who received zanubrutinib versus ibrutinib. Only 2% of patients receiving zanubrutinib experienced any grade of atrial fibrillation in contrast to 15% of those receiving ibrutinib (one-sided P = .002). Adverse events leading to treatment discontinuation were numerically less common in patients receiving zanubrutinib (4%) versus ibrutinib (9%). The study concluded that in the treatment of patients with Waldenstrom macroglobulinemia, zanubrutinib led to similar efficacy and less toxicity.20
Early data from a similar study (ALPINE) comparing zanubrutinib to ibrutinib in the treatment of patients with relapsed or refractory CLL were presented at the 2021 European Society of Hematology Meeting.21 The authors concluded that zanubrutinib was actually superior to ibrutinib in terms of both efficacy and safety. Similarly, this study found that the incidence of all-grade atrial fibrillation was lower in patients treated with zanubrutinib (3%) versus ibrutinib (10%; two-sided P = .0014).21 Generally, the rates of atrial fibrillation in both arms were lower than what was seen in the ELEVATE RR study, potentially secondary to shorter follow-up. Incidence of discontinuation secondary to adverse events was also numerically lower in patients receiving zanubrutinib (8%) versus ibrutinib (13%). There are several notable differences in the ALPINE study and the ELEVATE RR study for patients with CLL including study design, patient population, and length of follow-up (Table 1).
1. Study design: The ALPINE study was designed to detect superiority of zanubrutinib with a primary end point of overall response rate (ORR: including only complete remission and partial remission, but not partial remission with lymphocytosis). This study found that the ORR of zanubrutinib (78%) was higher compared with ibrutinib (63%; two-sided P = .0006). In the ELEVATE RR study, the ORR of acalabrutinib and ibrutinib was 81% and 77%, respectively.
2. Study population: The ALPINE study enrolled all-comers with relapsed or refractory CLL, whereas the ELEVATE RR only enrolled patients considered to have high-risk disease [del(17p) or del(11q)]. Twelve percent and 45% of patients enrolled on ALPINE and ELEVATE RR had del(17p), respectively.
3. Length of follow-up: At the time of presentation, the ALPINE study had a much shorter median follow-up of 15 months compared with 41 months in the ELEVATE RR study. The length of follow-up may alter interpretation of the ALPINE study authors' statement that zanubrutinib prolongs PFS in this population compared with ibrutinib. The study reported that estimated 1-year PFS was 95% in zanubrutinib-treated patients versus 84% in ibrutinib-treated patients (hazard ratio, 0.4 [95% CI, 0.23 to 0.69]; P = .0007). After 40 months of follow-up, the ELEVATE RR study reported equivalent PFS between acalabrutinib and ibrutinib. However, if you look closely at Figure 2A, there is a separation of the PFS curves with an advantage to acalabrutinib over ibrutinib starting at about 9 months and concluding at about 33 months, where the PFS curves overlap. Longer follow-up of the ALPINE study is needed to determine if the same overlapping zanubrutinib and ibrutinib curves will be seen over time.
TABLE 1.
Comparison of Phase III ELEVATE RR and ALPINE Studies in Patients With RR CLL
The results of the ALPINE study and the ELEVATE RR study indicate that both of the more selective BTK inhibitors, zanubrutinib and acalabrutinib, demonstrate a favorable toxicity profile and at least equivalence in terms of efficacy when compared with ibrutinib in patients with relapsed and refractory CLL. Although patients with previously treated CLL and in the ELEVATE RR study had del(17p) or del(11q) were included, these results can likely be extrapolated to other subgroups of patients with CLL. These results beg the question, in which patients would you still select ibrutinib, acalabrutinib, or zanubrutinib? At the time of manuscript preparation, zanubrutinib is not FDA-approved for marketing in CLL, but this may change over the coming year. Patients who would clearly benefit from ibrutinib over acalabrutinib or zanubrutinib are those who can be more compliant with a once-daily dosing (ibrutinib) than twice-daily dosing (acalabrutinib and zanubrutinib) and those who have severe gastroesophageal reflux requiring proton-pump inhibitor therapy (interferes with absorption of acalabrutinib). At this time, there are more data specifically for patients with TP53 mutations and younger patients using ibrutinib, which are potential subgroups that could benefit from ibrutinib over acalabrutinib, but this may become more clear with longer follow-up of current studies.3,22 The general length of follow-up data for patients with CLL receiving ibrutinib is also much longer at this time (over 8 years) compared with patients with CLL receiving acalabrutinib or zanubrutinib (3-4 years).5,23,24 Of course, any benefit of receiving ibrutinib should be balanced with the potential risk of adverse events experienced over the long period of ibrutinib exposure possible in patients.
To avoid adverse effects that are commonly associated with BTK inhibitors, there are additional available therapies that are also alternatives to BTK inhibitors, including venetoclax, phosphoinositide 3-kinase inhibitors, and noncovalent BTK inhibitors (such as pirtobrutinib and ARQ531).25-28 Additional therapeutic strategies include combination with BTK inhibitors with venetoclax with a goal of time-limited therapy, which could reduce adverse effects seen over time.29 Detailed discussion of these alternative therapies and strategies are outside the scope of this article.
In summary, the ELEVATE RR study is a bold comparison of ibrutinib and acalabrutinib in patients with relapsed or refractory CLL. The study found that acalabrutinib was not inferior to ibrutinib in terms of PFS and has a more favorable safety profile. The list of patients in whom ibrutinib is preferable over acalabrutinib is becoming smaller. The ongoing ALPINE study is a similar comparison of zanubrutinib and ibrutinib in patients with relapsed or refractory CLL. The list of efficacious and tolerable medications for the treatment of CLL continues to grow. Which agent is simply the best therapy for patients with CLL? Time and further studies hope to better answer this question.
Deborah M. Stephens
Honoraria: Genentech
Consulting or Advisory Role: Pharmacyclics/Janssen, Karyopharm Therapeutics, BeiGene, Innate Pharma, Epizyme, TG Therapeutics, Adaptive Biotechnologies, AstraZeneca
Research Funding: Acerta Pharma, Gilead Sciences, Karyopharm Therapeutics, Verastem, Juno Therapeutics, ArQule, MingSight
No other potential conflicts of interest were reported.
See accompanying article on page 3441
SUPPORT
D.S. is supported by NIH/NCI Grant K23 CA212271.
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Second-Generation Bruton's Tyrosine Kinase Inhibitors: Simply the Best Treatments for Chronic Lymphocytic Leukemia?
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Deborah M. Stephens
Honoraria: Genentech
Consulting or Advisory Role: Pharmacyclics/Janssen, Karyopharm Therapeutics, BeiGene, Innate Pharma, Epizyme, TG Therapeutics, Adaptive Biotechnologies, AstraZeneca
Research Funding: Acerta Pharma, Gilead Sciences, Karyopharm Therapeutics, Verastem, Juno Therapeutics, ArQule, MingSight
No other potential conflicts of interest were reported.
REFERENCES
- 1.Byrd JC, Hillmen P, O'Brien S, et al. : Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 133:2031-2042, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Barr PM, Robak T, et al. : Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 34:787-798, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Wang XV, Kay NE, et al. : Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381:432-443, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharman JP, Egyed M, Jurczak W, et al. : Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 395:1278-1291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghia P, Pluta A, Wach M, et al. : ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 38:2849-2861, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Hillmen P, Ghia P, et al. : Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J Clin Oncol 39:3441-3452, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kipps TJ, Fraser G, Coutre SE, et al. : Long-Term studies assessing outcomes of ibrutinib therapy in patients with del(11q) chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk 19:715-722.e6, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Pretorius L, Du XJ, Woodcock EA, et al. : Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol 175:998-1009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMullen JR, Boey EJ, Ooi JY, et al. : Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 124:3829-3830, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Kinoshita T, Sukbuntherng J, et al. : Ibrutinib inhibits ERBB receptor tyrosine kinases and HER2-amplified breast cancer cell growth. Mol Cancer Ther 15:2835-2844, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Salem JE, Manouchehri A, Bretagne M, et al. : Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol 74:1667-1678, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Wolf PA, D'Agostino RB, et al. : Impact of atrial fibrillation on the risk of death: The framingham Heart study. Circulation 98:946-952, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Brown JR, Moslehi J, Ewer MS, et al. : Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: An integrated analysis. Br J Haematol 184:558-569, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quek LS, Bolen J, Watson SP: A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol 8:1137-1140, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Futatani T, Watanabe C, Baba Y, et al. : Bruton's tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br J Haematol 114:141-149, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Fitzgerald ME, Berndt MC, et al. : Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood 108:2596-2603, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levade M, David E, Garcia C, et al. : Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 124:3991-3995, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Wang ML, Rule S, Martin P, et al. : Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369:507-516, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickerson T, Wiczer T, Waller A, et al. : Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 134:1919-1928, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CS, Opat S, D'Sa S, et al. : A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 136:2038-2050, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillmen P, Eichhorst B, Brown JR, et al. : First interim analysis of ALPINE study: Results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma, European Hematology Association Annual Meeting (Virtual), June 11, 2021 (abstr LB1900)
- 22.Ahn IE, Tian X, Wiestner A: Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med 383:498-500, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd JC, Furman RR, Coutre SE, et al. : Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: Final analysis of the pivotal phase ib/II PCYC-1102 study. Clin Cancer Res 26:3918-3927, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam CS, Trotman J, Opat S, et al. : Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 134:851-859, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kater AP, Wu JQ, Kipps T, et al. : Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-Year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol 38:4042-4054, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gribben JG, Jurczak W, Jacobs RW, et al. : Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Results from the phase 3 unity-CLL study. Blood 136:37-39, 2020 [Google Scholar]
- 27.Mato AR, Shah NN, Jurczak W, et al. : Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 397:892-901, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Woyach J, Stephens DM, Flinn IW, et al. : Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood 134, 2019. (abstr 4298) [Google Scholar]
- 29.Wierda WG, Tam CS, Allan JN, et al. : Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL): 1-Year disease-free survival (DFS) results from the MRD cohort of the phase 2 CAPTIVATE study. Blood 136:16-17, 2020 [Google Scholar]