PURPOSE
131I-metaiodobenzylguanidine (MIBG) is an active radiotherapeutic for neuroblastoma. The primary aim of this trial was to identify which of three MIBG regimens was likely associated with the highest true response rate.
PATIENTS AND METHODS
Patients 1-30 years were eligible if they had relapsed or refractory neuroblastoma, at least one MIBG-avid site, and adequate autologous stem cells. Patients received MIBG 18 mCi/kg on day 1 and autologous stem cell on day 15. Patients randomly assigned to arm A received only MIBG; patients randomly assigned to arm B received intravenous vincristine on day 0 and irinotecan daily on days 0-4; patients randomly assigned to arm C received vorinostat (180 mg/m2/dose) orally once daily on days 1 to 12. The primary end point was response after one course by New Approaches to Neuroblastoma Therapy criteria. The trial was designed with 105 patients to ensure an 80% chance that the arm with highest response rate was selected.
RESULTS
One hundred fourteen patients were enrolled, with three ineligible and six unevaluable, leaving 105 eligible and evaluable patients (36 in arm A, 35 in arm B, and 34 in arm C; 55 boys; and median age 6.5 years). After one course, the response rates (partial response or better) on arms A, B, and C were 14% (95% CI, 5 to 30), 14% (5 to 31), and 32% (18 to 51). An additional five, five, and four patients met New Approaches to Neuroblastoma Therapy Minor Response criteria on arms A, B, and C, respectively. On arms A, B, and C, rates of any grade 3+ nonhematologic toxicity after first course were 19%, 49%, and 35%.
CONCLUSION
Vorinostat and MIBG is likely the arm with the highest true response rate, with manageable toxicity. Vincristine and irinotecan do not appear to improve the response rate to MIBG and are associated with increased toxicity.
INTRODUCTION
Neuroblastoma is a childhood cancer that typically expresses the norepinephrine transporter (NET).1 131I-metaiodobenzylguanidine (MIBG) is a radiopharmaceutical selectively taken up via NET.2 MIBG has played a role in the treatment of advanced neuroblastoma since the 1980s, with response rates of approximately 30% following 1-2 treatments.3 More recent studies have evaluated the role of MIBG in newly diagnosed high-risk neuroblastoma.4
CONTEXT
Key Objective
Previous trials have shown the feasibility of combining 131I-metaiodobenzylguanidine (MIBG) with putative radiation sensitizer. We sought to select a winning arm associated with highest response rate among three arms: MIBG alone, MIBG plus vincristine and irinotecan, and MIBG plus vorinostat.
Knowledge Generated
Patients randomly assigned to the MIBG plus vorinostat arm had the highest observed response rate (32%) after the first course compared with 14% on either of the other two arms, which met the prespecified threshold for selecting a winning arm. Patients randomly assigned to the MIBG plus vincristine and irinotecan arm had higher rates of toxicity.
Relevance
The combination of MIBG plus vorinostat provides a new option for patients with relapsed or refractory neuroblastoma. Future studies may build upon these findings to investigate the role of epigenetic modifiers as potential radiation sensitizers in this disease and in other cancers.
Several combination strategies have been evaluated to improve upon MIBG's single-agent activity. Preclinical studies showed additive activity when MIBG is combined with topotecan.5,6 As topotecan is used in frontline high-risk therapy in North America,7 this preclinical finding was translated into two clinical trials that showed the feasibility and activity of combining vincristine and irinotecan with MIBG at its usual maximum dose of 18 mCi/kg.8,9 Additional work identified the histone deacetylase (HDAC) inhibitor vorinostat as a promising combination partner on the basis of preclinical evidence of radiosensitizing properties and increased NET expression in neuroblastoma cells treated with vorinostat.10,11 These findings led to a phase I trial demonstrating the tolerability of vorinostat with 18 mCi/kg MIBG.12
In these early phase trials, it has been difficult to determine whether adding these putative radiosensitizers improved the activity of MIBG. We therefore conducted a randomized phase II clinical trial in which all patients received the same dose of MIBG (18 mCi/kg) either as monotherapy or with vincristine and irinotecan or vorinostat. The primary objective was to identify the regimen most likely to have the highest true response rate.
PATIENTS AND METHODS
Eligibility
Patients age 1-30 years with MIBG-avid high-risk neuroblastoma were eligible if they met one of the following responses to frontline therapy: relapsed disease, refractory disease (persistent disease after best overall response of stable disease (SD) after a minimum of four induction cycles), or persistent disease (persistent disease after best overall response of partial response (PR) after a minimum of four induction cycles). Patients had a minimum of 1.5 × 106 CD34+ autologous peripheral blood stem cells (ASCs); a Lansky or Karnofsky score ≥ 50; and adequate hematologic, hepatic, renal, pulmonary, cardiac, and coagulation function. Patients had fully recovered from previous therapy according to protocol requirements (Protocol, online only). Previous MIBG monotherapy ≤ 18 mCi/kg was allowed if > 6 months before and no progression during MIBG therapy. There were no other limits on the number of lines of previous therapy.
Patients with previous total body irradiation, allogeneic transplant, pregnancy, breastfeeding, active or uncontrolled infection, previous noncatheter-associated deep venous thrombosis, or active diarrhea were excluded.
Each local institutional review board approved the Protocol. Patients or legal guardians provided informed consent.
Study Design and Treatment
Patients in this prospective, multicenter, randomized phase II trial (NCT02035137) were assigned to arm A, B, or C using a randomized minimization scheme, which was designed to increase the likelihood of 1:1:1 balance overall, and across the margins of four stratification variables: age (< v ≥ 18 years), response to previous frontline therapy (as above), bone marrow involvement at enrollment, and previous receipt of MIBG.13 Stratification did not use MYCN status given the lack of association with response to MIBG.14
All patients received MIBG 18 mCi/kg on day 1 and ASC (minimum dose 1.5 × 106 CD34+ cells/kg) on day 15. Patients on arm A received only MIBG. Patients on arm B additionally received vincristine (2 mg/m2) intravenous on day 0, irinotecan (50 mg/m2) intravenous daily on days 0-4, and cephalosporin diarrhea prophylaxis on days 1 to 6. Patients on arm C additionally received vorinostat (180 mg/m2/dose; maximum 400 mg) orally once daily on days 1 to 12 (14 doses). Patients received standard supportive care including hydration, thyroid blockade, and radiation isolation as previously described.9 Myeloid growth factor was given according to institutional standards. Disease response was evaluated on days 43-50.
Patients without disease progression were initially allowed to receive a second course. Because of financial constraints, the Protocol was amended in June 2017 to allow only one course of therapy.
End Points
The primary end point was objective response after one course by New Approaches to Neuroblastoma Therapy (NANT) Response Criteria v1.2.15 Each of three disease domains (MIBG scan by Curie score, bone marrow by histopathology with immunohistochemistry, soft tissue disease by anatomic imaging, and MIBG uptake) was evaluated, and an overall response of complete response (CR), CR-minimal residual disease (CR-MRD; baseline ≤ 5% marrow involvement that becomes negative), PR, SD, progressive disease, or minor response (patients with CR, CR-MRD, or PR in at least one domain, SD in at least one domain, and no progressive disease in any domain) was assigned. Patients with an overall response of CR, CR-MRD, or PR were considered responders, and all others were considered nonresponders. Central review of computed tomography and/or magnetic resonance imaging scans, MIBG scans, bone marrow slides, and overall response was performed for all eligible and evaluable patients who had an overall response ≥ SD by institutional report. For all patients, central review of imaging and pathology reports was performed.
The secondary end point was toxicity by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4. Neutrophil engraftment was defined as the first date of three consecutive dates with absolute neutrophil counts ≥ 500/μL. Platelet engraftment was defined as the first date of three consecutive dates of platelet count ≥ 20,000/μL following the last platelet transfusion.
Exploratory end points included objective response for each disease domain (soft tissue, bone marrow, and MIBG by relative Curie score [Curie score after first course divided by baseline Curie score]), overall response after two courses, whole-body radiation dose calculated as previously described,16 progression-free survival (PFS), and overall survival (OS). PFS was calculated from date of random assignment to first progression or death from any cause, with patients without progression or death censored at last follow-up. OS was calculated from date of random assignment to death from any cause, with alive patients censored at last follow-up.
Statistical Considerations
The primary analysis followed a modified intent-to-treat (ITT) approach in which all eligible, randomly assigned patients who received any amount of MIBG were considered evaluable and included. Preplanned secondary sensitivity analyses included ITT (all eligible, randomly assigned patients) and per-protocol (received at least 85% of calculated MIBG dose and at least 80% of irinotecan for arm B or vorinostat for arm C) analyses. The study was designed as a pick-the-winner study to select the MIBG regimen most likely associated with the highest true response rate. With this design, formal testing was not planned and P values would not be reported. With 105 eligible and evaluable patients (35 patients/arm), the study was designed to have an 80% chance of selecting the regimen with the highest response rate, assuming that the true response rate of the selected arm was at least 15% higher than the other two arms.17,18
Two interim analyses were performed after 60 and 81 patients completed one course. At each interim analysis, if the posterior probability of one arm having a response rate inferior to the other arms was > .85, then that arm could be dropped. The first interim analysis suggested the potential for subsequently declaring an early winner at the next interim analysis. The Data Safety Monitoring Committee approved modifying the design to add stochastic curtailment principles to permit early selection of a winner at the second interim analysis: if the conditional probability of an arm having a higher response rate than the remaining arms was > .90 across a range of pre-established scenarios, then accrual would be terminated with the conclusion that the true response rate for that arm was likely to be higher than that for the other two arms. None of the stopping rules were met, and the trial fully accrued.
Toxicities were summarized according to randomized arm, for all patients who received any MIBG. PFS and OS were summarized using Kaplan-Meier product-limit calculations.
RESULTS
Patients
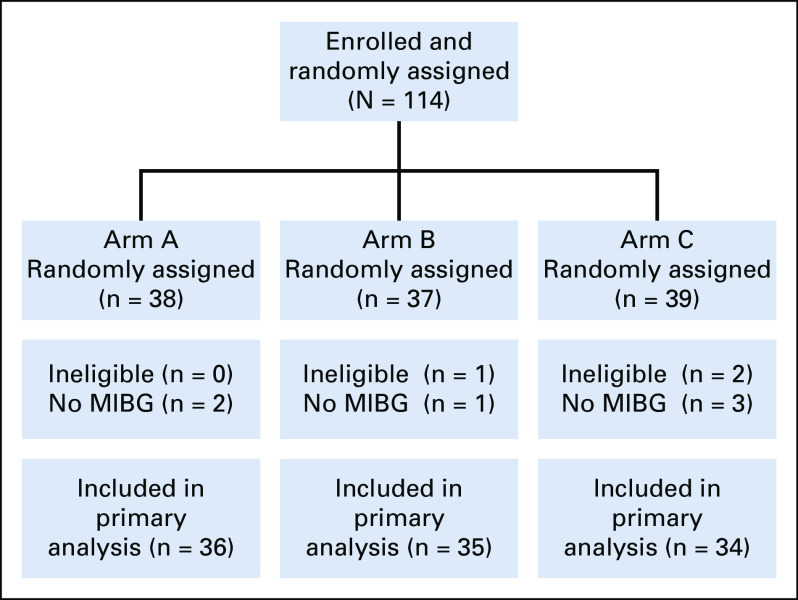
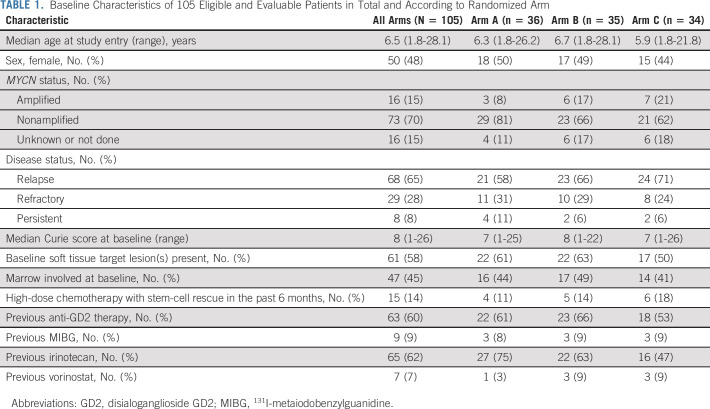
From July 2014 to November 2019, 114 patients were enrolled and randomly assigned (Fig 1). Three patients were ineligible (no MIBG disease, n = 1; inadequate washout of previous medications, n = 2). Six patients never received MIBG and were therefore unevaluable for primary analysis (postrandomization and pre-MIBG progression on arm A, n = 2; MIBG manufacturing issue on arm B, n = 1; withdrawal of consent pre-MIBG on arm C, n = 2; and urethral trauma during bladder catheter placement on arm C, n = 1). The analytic cohort therefore included 105 eligible and evaluable patients. The characteristics of these 105 patients (Table 1) were generally well-balanced between arms, although there were fewer patients with MYCN-amplified tumors on arm A and fewer patients with previous irinotecan treatment on arm C. Median Curie score and percent of patients with marrow involvement were similar between arms, although slightly fewer patients on arm C had soft tissue target lesions.
FIG 1.
CONSORT diagram showing random assignment of 114 patients to yield 105 eligible and evaluable patients. MIBG, 131I-metaiodobenzylguanidine.
TABLE 1.
Baseline Characteristics of 105 Eligible and Evaluable Patients in Total and According to Randomized Arm
Response
Response rates after one course on arms A, B, and C were 14% (95% CI, 5 to 30), 14% (5 to 31), and 32% (18 to 51), respectively (Table 2). An additional five, five, and four patients met minor response criteria on arms A, B, and C, respectively. All patients in the primary modified ITT analysis received MIBG doses within protocol requirements to be included in the per-protocol secondary analysis (median MIBG doses in arms A, B, and C: 18.2, 18.2, and 17.8 mCi/kg, respectively). One patient on arm C received inadequate vorinostat to be included in the per-protocol secondary analysis. Response rates according to ITT and per-protocol preplanned secondary analyses (Appendix Table A1, online only) were consistent with the primary analysis.
TABLE 2.
Overall Response to One Course of Therapy According to Randomized Arm
We evaluated response after one course according to the three disease domains (Appendix Table A2, online only). Response by relative Curie score is shown in Figure 2, with rates of 25%, 31%, and 41% for arms A, B, and C, respectively. The median relative Curie score after the first course was 0.95 (range 0-1.47), 0.94 (0-1.50), and 0.83 (0-2.83) for arms A, B, and C, respectively. Response rates at soft tissue sites were 7%, 15%, and 16% for arms A, B, and C, respectively. Response rates in the bone marrow were 5%, 18%, and 14% for arms A, B, and C, respectively.
FIG 2.
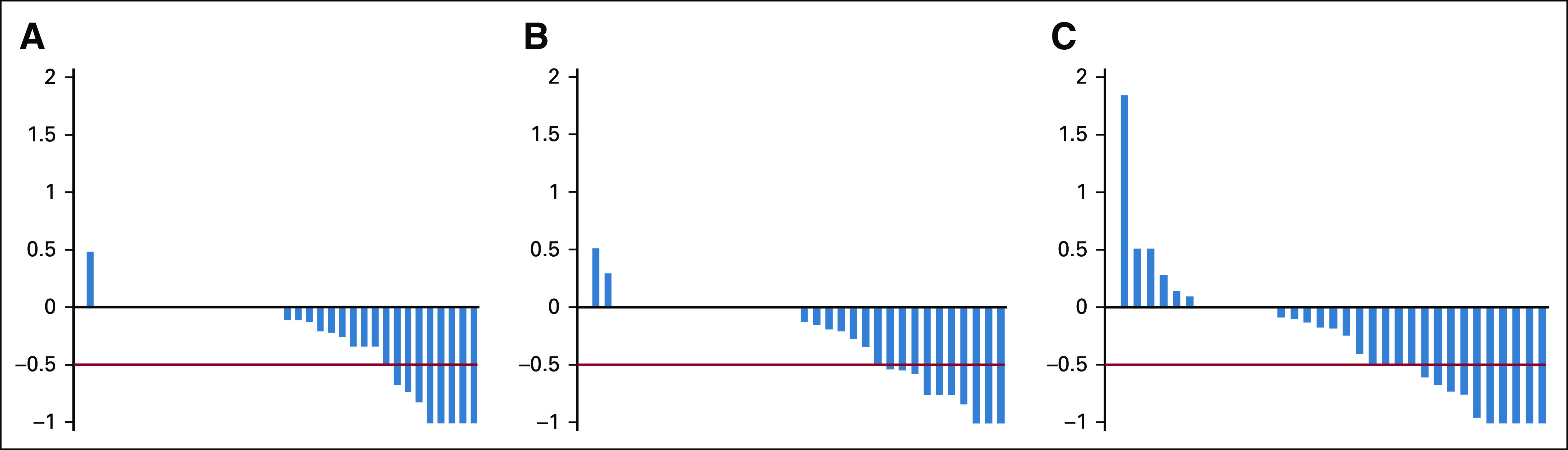
Waterfall plot according to randomized arm showing the sum of relative Curie score (Curie score after first course divided by baseline Curie score) minus 1 such that a value of 0 represents no change from baseline, a value of –1 represents a complete response by Curie score, and positive values represent increases in Curie score. A, B, and C correspond to arms A, B, and C. The heavy red line represents threshold for being considered to have had a partial response or better by Curie score.
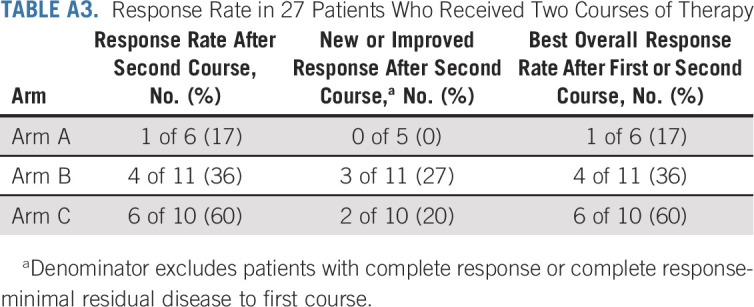
We evaluated best overall response to either a first or second course, accounting for 27 patients who received a second course of therapy (6, 11, and 10 patients in arms A, B, and C, respectively). The best overall response rate was 5 of 36 (14%) on arm A, 6 of 35 (17%) on arm B, and 12 of 34 (35%) on arm C. Among the 27 patients who received a second course, overall response rates after the second course were 1 of 6 (17%), 4 of 11 (36%), and 6 of 10 (60%) for arms A, B, and C, respectively (Appendix Table A3, online only).
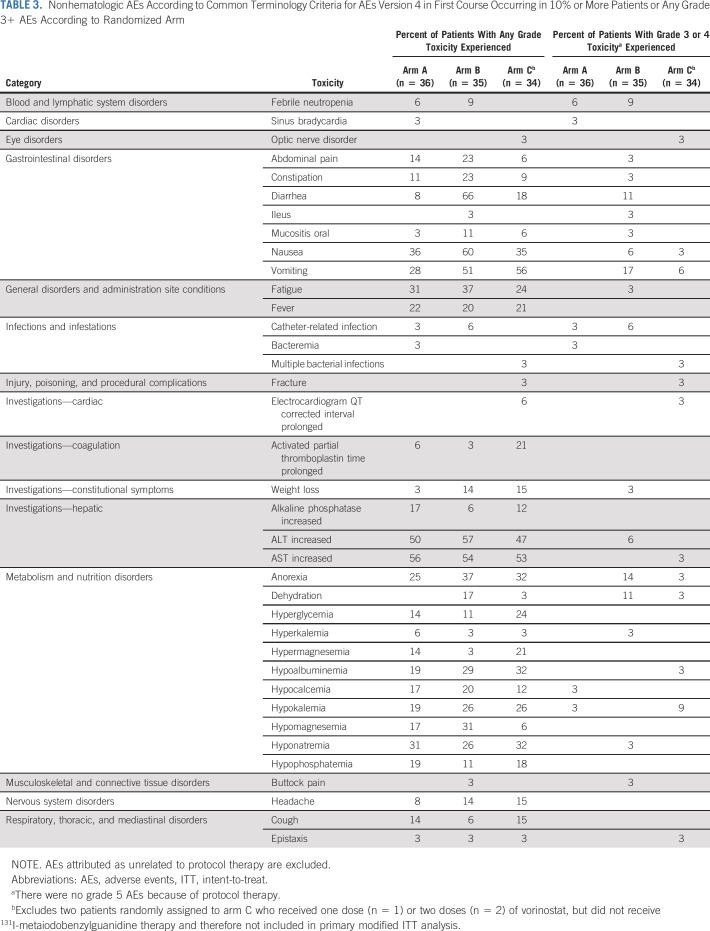
Toxicity
First-course nonhematologic adverse events (AEs) (excluding those attributed as unrelated) are shown by randomized arm in Table 3. On arms A, B, and C, rates of any grade 3+ nonhematologic toxicity were 19%, 49% and 35%. On arm B, 66% of patients developed diarrhea of any grade, with 11% grade 3+. No patients on arm A or C developed grade 3+ diarrhea. Other gastrointestinal toxicities, anorexia, and dehydration were generally more common on arm B. One patient on arm C developed grade 3 QTc prolongation. The only first-course grade 4 nonhematologic AEs were febrile neutropenia (n = 1; arm B), hypocalcemia (n = 1; arm A), hypokalemia (n = 2; arm C), and optic nerve disorder in a patient on arm C with progressive orbital tumor. Three patients (one on arm B and two on arm C) developed myelodysplastic syndrome or acute myeloid leukemia at 12, 12, and 43 months after receiving 1, 2, and 1 courses of protocol therapy and died 7, 5, and 7 months from second malignancy diagnosis, respectively. There were no grade 5 AEs because of protocol therapy.
TABLE 3.
Nonhematologic AEs According to Common Terminology Criteria for AEs Version 4 in First Course Occurring in 10% or More Patients or Any Grade 3+ AEs According to Randomized Arm
All patients except for one arm C patient with early progression received ASC support. Sixty-one patients received myeloid growth factor support (53% in arm A, 69% in arm B, and 58% in arm C). Eighteen (50%), 8 (23%), and 9 (26%) patients never developed grade 4 neutropenia in course one on arms A, B, and C, respectively. Among patients with grade 4 neutropenia, the median time to neutrophil recovery after first ASC was 22, 14, and 20 days on arms A, B, and C, respectively. Six percent, 9%, and 0% of patients developed febrile neutropenia after first course on arms A, B, and C, respectively. Twenty-two (61%), 15 (43%), and 14 (41%) patients never developed first course platelets < 20,000/μL on arms A, B, and C, respectively. Among patients with platelets < 20,000/μL, the median time to platelet recovery after first ASC was 15, 15, and 14 days on arms A, B, and C, respectively.
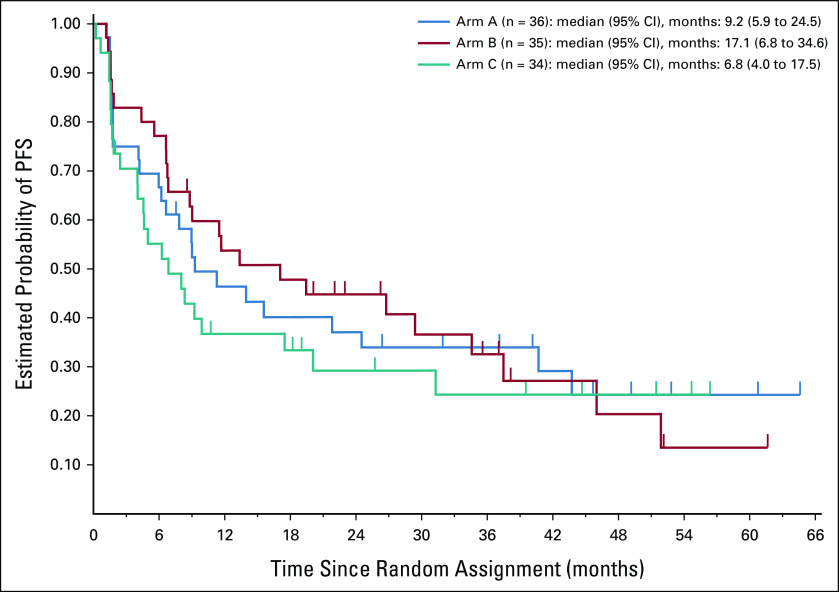
PFS and OS
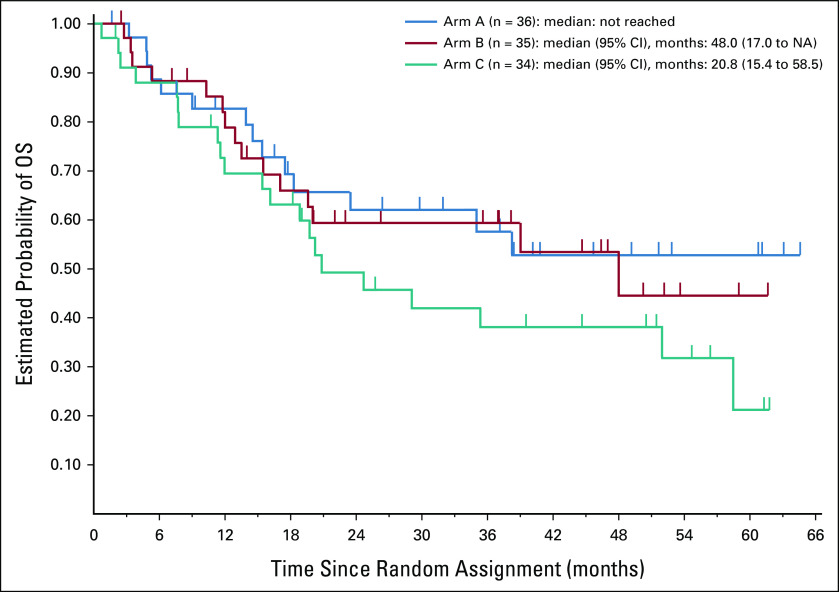
Estimated rates of PFS and OS according to randomized arm are shown in Appendix Figures A1 and A2 (online only), respectively. Patients on arm C had nominally lower PFS and OS compared with patients on arm A or B.
Whole-Body Radiation Dose
First-course dosimetry data were available for 95 patients. The median (range) whole-body radiation dose was 238.0 cGy (104.0-471.2). The median whole-body radiation dose was similar according to randomized arm (258.7, 232.0, and 230.1 for arms A, B, and C, respectively).
DISCUSSION
In this first randomized trial of MIBG in patients with relapsed or refractory neuroblastoma, we demonstrate that MIBG/vorinostat is likely the arm with the highest true response rate after one course of therapy. Additionally, this finding met the prespecified 15% improvement in overall response rate compared with the other arms of the trial. Additional data support the role of vorinostat in this context, including higher response rate by Curie score and higher response rate after up to two courses. MIBG/vorinostat was well-tolerated compared with MIBG monotherapy, although some grade 3+ nonhematologic toxicities were only seen in patients on arm C. By contrast, the addition of vincristine/irinotecan to MIBG increased toxicity without improving response rates.
A large body of preclinical data demonstrate a role for HDAC inhibitors as radiation sensitizers. One mechanism that has been posited is reduction in expression of DNA damage repair proteins, leading to increased double-stranded DNA breaks following radiation exposure.19-21 This pattern was seen in neuroblastoma cells in vitro,10 which motivated initial clinical evaluation of vorinostat/MIBG, along with the finding that vorinostat increased NET protein expression and therefore MIBG uptake in neuroblastoma cells.11 Vorinostat has been combined with external beam radiation in other clinical contexts, including in gastrointestinal carcinoma, brain metastasis, pancreatic cancer, glioblastoma, and head and neck carcinoma.22-26 These nonrandomized trials have generally concluded that this approach is tolerable, with mixed conclusions about whether vorinostat improves antitumor activity. To our knowledge, our results provide the first proof-of-concept randomized results showing beneficial radiation sensitizing properties of vorinostat. Vorinostat has shown limited clinical activity in patients with neuroblastoma,27,28 reinforcing its role as a radiation sensitizer rather than as an agent with intrinsic antineuroblastoma activity.
The overall response rate with MIBG monotherapy in this trial (14%) was lower than anticipated. The largest phase II trial in patients with relapsed neuroblastoma reported a 36% response rate,14 and a systematic review reported a pooled response rate of 32%.3 This finding may reflect a combination of factors, including additional treatment options now available to patients before they pursue MIBG therapy,29,30 and differences in response criteria. We note that the MIBG response rate by a Curie score of 25% for MIBG monotherapy in our trial is more consistent with previously reported response rates. Our current response rates were lower with vincristine and irinotecan and higher with vorinostat compared with the results from our previous single-arm trials, emphasizing the role for comparative trials.8,9,12
Although our preclinical data suggest one or more mechanisms by which vorinostat might have improved the activity of MIBG, we are not yet able to confirm the mechanism of the observed clinical benefit. Additional work to understand our finding through companion biology studies, including radiation-related biomarkers,31 will be the focus of future manuscripts. The impact of adding vorinostat was greatest in Curie score response, whereas response in bone marrow and soft tissue was not clearly superior. This translated to a higher overall response rate in the vorinostat arm since all patients had MIBG-positive disease, whereas only subsets had bone marrow or soft tissue disease. Vorinostat did not improve PFS or OS, and patients randomly assigned to arm C had the lowest PFS and OS estimates. This finding is perhaps not surprising given that MIBG is administered for up to two courses, rather than being administered until progression. Slightly higher rates of MYCN amplification in patients on arm C might have contributed to this finding, since MYCN amplification is an independent risk factor for poor PFS postrelapse.15,32 The NANT consortium has previously shown that PFS and OS are similar for patients with responses of ≥ SD by NANT response criteria, such that higher response rates on arm C may not necessarily translate to superior PFS or OS.15 In the context of an ultrarare disease, we designed the trial using a pick-the-winner strategy rather than with a design to obtain definitive statistical evidence of improved outcome. Nevertheless, the improvement in response rate with vorinostat met the prespecified benchmark to declare a winner. As in previous NANT trials,8,12,27 we used NANT response criteria15 as our primary end point as they provided more quantitative domain–specific criteria compared with the International Neuroblastoma Response Criteria version in use at the start of the study.33 A final limitation is that we did not collect data on molecular features in the three patients with myelodysplastic syndrome and/or acute myeloid leukemia.
Potential future steps might include evaluation of next-generation HDAC inhibitors in this context or evaluation of this regimen in patients with newly diagnosed high-risk neuroblastoma who appear to be particularly responsive to MIBG therapy.4 There are ongoing studies of MIBG with anti-GD2 monoclonal antibodies, and previous reports have shown that HDAC inhibition increases GD2 expression in neuroblastoma,34 suggesting a potential role for the use of an HDAC inhibitor in that context. Our results in the vincristine and irinotecan arm may call into question the role of camptothecins as radiosensitizers in this disease,35 although our results cannot address the role of other chemotherapy agents in this context. Beyond neuroblastoma, one might consider further evaluation of HDAC inhibition as a strategy for other radiosensitive tumors, including those treated with other radiopharmaceuticals.
APPENDIX
FIG A1.
PFS from time of random assignment for 105 patients according to randomized arm. PFS, progression-free survival.
FIG A2.
OS from time of random assignment for 105 patients according to randomized arm. NA, not available; OS, overall survival.
TABLE A1.
Response Rates After First Course in Preplanned Secondary ITT and Per-Protocol Analyses
TABLE A2.
Response After One Course According to Site of Disease Involvement and Randomized Arm
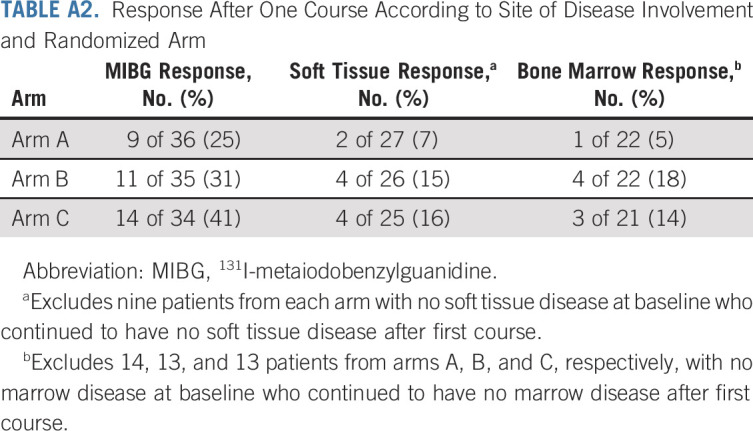
TABLE A3.
Response Rate in 27 Patients Who Received Two Courses of Therapy
Steven G. DuBois
Consulting or Advisory Role: Bayer
Research Funding: Merck, Roche/Genentech, Lilly, Curis, Loxo, BMS, Eisai, Pfizer, Turning Point Therapeutics, Bayer, Salarius Pharmaceuticals
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Ymabs Therapeutics Inc
Susan Groshen
Employment: Pfizer
Scarlett Czarnecki
Speakers' Bureau: United Therapeutics
Yael P. Mosse
Consulting or Advisory Role: ASCO, Pfizer, Lilly, Auron Therapeutics, Jumo
Research Funding: Pfizer
John M. Maris
Stock and Other Ownership Interests: Tantigen BIO Inc
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs, Neuroblastoma antigens
Suzanne Shusterman
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline, Intuitive Surgical, Kindred Biosciences, Merck, Nuvasive, OPKO Health, Regeneron, Surgalign, Amgen, BioTelemetry, RTI Surgical Holdings, EFOFEM biosciences, InVitae
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Stryker, Amgen, Pfizer, AbbVie, Amgen, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summary
Brian Weiss
Research Funding: Novartis, Genentech, Exelixis
Travel, Accommodations, Expenses: Exelixis, Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/620363
Gregory A. Yanik
Research Funding: Jazz Pharmaceuticals
Meredith S. Irwin
Honoraria: Bayer
Daphne A. Haas-Kogan
Consulting or Advisory Role: Leidos Biomedical Research, SRA International, Gerson Lehrman Group, Guidepoint Global
Araz Marachelian
Research Funding: Pfizer, United Therapeutics
Katherine K. Matthay
Consulting or Advisory Role: Resonance Health
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 American Society of Clinical Oncology (ASCO) Virtual Annual Meeting, May 29-31, 2020 and the Advances in Neuroblastoma Research (ANR) 2021 Virtual Meeting, January 25-27, 2021.
SUPPORT
Supported by National Cancer Institute Grant Nos. P01 CA081403, P01 CA217959, 1R01 CA172067, and R35 CA220500; Alex's Lemonade Stand Foundation; Friends for Life; Children's Neuroblastoma Cancer Foundation; Dougherty Foundation; Evan Dunbar Foundation; Pediatric Cancer Research Foundation; Merck & Co Inc; V Foundation for Cancer Research; St Baldrick's Foundation; and an anonymous private donor. The funders played no role in study design, study conduct, manuscript preparation, or decision to submit for publication.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Steven G. DuBois, M. Meaghan Granger, Susan Groshen, Lingyun Ji, Judith G. Villablanca, Yael P. Mosse, John M. Maris, Gregory A. Yanik, Daphne A. Haas-Kogan, Julie R. Park, Araz Marachelian, Katherine K. Matthay
Financial support: Steven G. DuBois, M. Meaghan Granger
Administrative support: M. Meaghan Granger, Scarlett Czarnecki, Araz Marachelian
Provision of study materials or patients: Steven G. DuBois, M. Meaghan Granger, Navin Pinto, Yael P. Mosse, Suzanne Shusterman, Susan L. Cohn, Kelly C. Goldsmith, Meredith S. Irwin, Daphne A. Haas-Kogan, Araz Marachelian, Katherine K. Matthay
Collection and assembly of data: Steven G. DuBois, M. Meaghan Granger, Susan Groshen, Scarlett Czarnecki, Rachel Berkovich, Hiroyuki Shimada, Judith G. Villablanca, Kieuhoa T. Vo, Navin Pinto, Suzanne Shusterman, Susan L. Cohn, Kelly C. Goldsmith, Brian Weiss, Clare J. Twist, Meredith S. Irwin, Daphne A. Haas-Kogan, Julie R. Park
Data analysis and interpretation: Steven G. DuBois, M. Meaghan Granger, Denice Tsao-Wei, Anasheh Shamirian, Fariba Goodarzian, Judith G. Villablanca, Navin Pinto, Yael P. Mosse, Gregory A. Yanik, Daphne A. Haas-Kogan, Julie R. Park, Katherine K. Matthay
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steven G. DuBois
Consulting or Advisory Role: Bayer
Research Funding: Merck, Roche/Genentech, Lilly, Curis, Loxo, BMS, Eisai, Pfizer, Turning Point Therapeutics, Bayer, Salarius Pharmaceuticals
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Ymabs Therapeutics Inc
Susan Groshen
Employment: Pfizer
Scarlett Czarnecki
Speakers' Bureau: United Therapeutics
Yael P. Mosse
Consulting or Advisory Role: ASCO, Pfizer, Lilly, Auron Therapeutics, Jumo
Research Funding: Pfizer
John M. Maris
Stock and Other Ownership Interests: Tantigen BIO Inc
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals, Jubilant DraxImage
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs, Neuroblastoma antigens
Suzanne Shusterman
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline, Intuitive Surgical, Kindred Biosciences, Merck, Nuvasive, OPKO Health, Regeneron, Surgalign, Amgen, BioTelemetry, RTI Surgical Holdings, EFOFEM biosciences, InVitae
Susan L. Cohn
Stock and Other Ownership Interests: Merck, Stryker, Amgen, Pfizer, AbbVie, Amgen, Lilly, Sanofi, Accelerated Medical Diagnostics, Novo Nordisk, Gilead Sciences, United Health Group, Teva
Honoraria: Y-mAbs Therapeutics
Research Funding: United Therapeutics, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/physician/46569/summary
Brian Weiss
Research Funding: Novartis, Genentech, Exelixis
Travel, Accommodations, Expenses: Exelixis, Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/620363
Gregory A. Yanik
Research Funding: Jazz Pharmaceuticals
Meredith S. Irwin
Honoraria: Bayer
Daphne A. Haas-Kogan
Consulting or Advisory Role: Leidos Biomedical Research, SRA International, Gerson Lehrman Group, Guidepoint Global
Araz Marachelian
Research Funding: Pfizer, United Therapeutics
Katherine K. Matthay
Consulting or Advisory Role: Resonance Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dubois SG, Geier E, Batra V, et al. : Evaluation of norepinephrine transporter expression and metaiodobenzylguanidine avidity in neuroblastoma: A report from the Children's Oncology Group. Int J Mol Imaging 2012:250834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glowniak JV, Kilty JE, Amara SG, et al. : Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med 34:1140-1146, 1993 [PubMed] [Google Scholar]
- 3.Wilson JS, Gains JE, Moroz V, et al. : A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 50:801-815, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Kraal KC, Bleeker GM, van Eck-Smit BL, et al. : Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer 76:188-196, 2017 [DOI] [PubMed] [Google Scholar]
- 5.McCluskey AG, Boyd M, Ross SC, et al. : [131I]meta-iodobenzylguanidine and topotecan combination treatment of tumors expressing the noradrenaline transporter. Clin Cancer Res 11:7929-7937, 2005 [DOI] [PubMed] [Google Scholar]
- 6.McCluskey AG, Boyd M, Pimlott SL, et al. : Experimental treatment of neuroblastoma using [131I]meta-iodobenzylguanidine and topotecan in combination. Br J Radiol 81:S28-S35, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Park JR, Kreissman SG, London WB, et al. : Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: A randomized clinical trial. JAMA 322:746-755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuBois SG, Chesler L, Groshen S, et al. : Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: A new approaches to neuroblastoma therapy trial. Clin Cancer Res 18:2679-2686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuBois SG, Allen S, Bent M, et al. : Phase I/II study of (131)I-MIBG with vincristine and 5 days of irinotecan for advanced neuroblastoma. Br J Cancer 112:644-649, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller S, Yang X, Sottero TL, et al. : Cooperation of the HDAC inhibitor vorinostat and radiation in metastatic neuroblastoma: Efficacy and underlying mechanisms. Cancer Lett 306:223-229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.More SS, Itsara M, Yang X, et al. : Vorinostat increases expression of functional norepinephrine transporter in neuroblastoma in vitro and in vivo model systems. Clin Cancer Res 17:2339-2349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuBois SG, Groshen S, Park JR, et al. : Phase I study of vorinostat as a radiation sensitizer with 131I-metaiodobenzylguanidine (131I-MIBG) for patients with relapsed or refractory neuroblastoma. Clin Cancer Res 21:2715-2721, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115, 1975 [PubMed] [Google Scholar]
- 14.Matthay KK, Yanik G, Messina J, et al. : Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 25:1054-1060, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Villablanca JG, Ji L, Shapira-Lewinson A, et al. : Predictors of response, progression-free survival, and overall survival using NANT Response Criteria (v1.0) in relapsed and refractory high-risk neuroblastoma. Pediatr Blood Cancer 65:e26940, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthay KK, Panina C, Huberty J, et al. : Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med 42:1713-1721, 2001 [PubMed] [Google Scholar]
- 17.Sobel M, Huyett MJ: Selecting the best one of several binomial populations. Bell Syst Tech J 36:537-576, 1957 [Google Scholar]
- 18.Bechhofer RE, Santner TJ, Goldsman DM: Design and Analysis of Experiments for Statistical Selection, Screening, and Multiple Comparisons. Wiley Series in Probability and Statistics Applied Probability and Statistics. Hoboken, NJ, Wiley, 1995, pp 325, xii [Google Scholar]
- 19.Munshi A, Tanaka T, Hobbs ML, et al. : Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther 5:1967-1974, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. : Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 62:223-229, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Shabason JE, Tofilon PJ, Camphausen K: Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med 15:2735-2744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ree AH, Dueland S, Folkvord S, et al. : Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: The Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol 11:459-464, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Shi W, Lawrence YR, Choy H, et al. : Vorinostat as a radiosensitizer for brain metastasis: A phase I clinical trial. J Neurooncol 118:313-319, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Chan E, Arlinghaus LR, Cardin DB, et al. : Phase I trial of vorinostat added to chemoradiation with capecitabine in pancreatic cancer. Radiother Oncol 119:312-318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galanis E, Anderson SK, Miller CR, et al. : Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro Oncol 20:546-556, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teknos TN, Grecula J, Agrawal A, et al. : A phase 1 trial of vorinostat in combination with concurrent chemoradiation therapy in the treatment of advanced staged head and neck squamous cell carcinoma. Invest New Drugs 37:702-710, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Pinto N, DuBois SG, Marachelian A, et al. : Phase I study of vorinostat in combination with isotretinoin in patients with refractory/recurrent neuroblastoma: A new approaches to neuroblastoma therapy (NANT) trial. Pediatr Blood Cancer 65:e27023, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouladi M, Park JR, Stewart CF, et al. : Pediatric phase I trial and pharmacokinetic study of vorinostat: A Children's Oncology Group phase I consortium report. J Clin Oncol 28:3623-3629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mody R, Yu AL, Naranjo A, et al. : Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: A report from the Children's Oncology Group. J Clin Oncol 38:2160-2169, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mody R, Naranjo A, Van Ryn C, et al. : Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol 18:946-957, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell K, Karski EE, Olow A, et al. : Peripheral blood biomarkers associated with toxicity and treatment characteristics after (131)I- metaiodobenzylguanidine therapy in patients with neuroblastoma. Int J Radiat Oncol Biol Phys 99:468-475, 2017 [DOI] [PubMed] [Google Scholar]
- 32.London WB, Castel V, Monclair T, et al. : Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J Clin Oncol 29:3286-3292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodeur GM, Pritchard J, Berthold F, et al. : Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Kroesen M, Bull C, Gielen PR, et al. : Anti-GD2 mAb and vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology 5:e1164919, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaze MN, Chang YC, Flux GD, et al. : Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm 20:195-199, 2005 [DOI] [PubMed] [Google Scholar]