Abstract
The COVID-19 pandemic is still raging across the world and vaccination is expected to lead us out of this pandemic. Although the efficacy of the vaccines is beyond doubt, safety still remains a concern.
We report a case of a 65-year-old woman who experienced acute severe autoimmune hepatitis two weeks after receiving the first dose of Moderna-COVID-19 vaccine. Serum immunoglobulin G was elevated and antinuclear antibody was positive (1:100, speckled pattern). Liver histology showed a marked expansion of the portal tracts, severe interface hepatitis and multiple confluent foci of lobular necrosis. She started treatment with prednisolone, with a favorable clinical and analytical evolution.
Some recent reports have been suggested that COVID-19 vaccination can lead to the development of autoimmune diseases. It is speculated that the vaccine can disturb self-tolerance and trigger autoimmune responses through cross-reactivity with host cells. Therefore, healthcare providers must remain vigilant during mass COVID-19 vaccination.
Keywords: COVID-19 vaccine, Moderna mRNA vaccine, Autoimmune hepatitis
There have been some concerns regarding the possibility of COVID-19 vaccine-induced autoimmunity [1]. Molecular mimicry was suggested as a potential mechanism for this association. Indeed, antibodies against the spike protein S1 of SARS-CoV-2 had a high affinity against some human tissue proteins [2]. As vaccine mRNA codes the same viral protein, they can trigger autoimmune diseases in predisposed patients.
We report the case of a 65-year-old woman, with JAK2 V617F-positive polycythemia vera diagnosed in 2006, under pegylated interferon since 2019. Her routine medication also included acetylsalicylic acid 100 mg/day, sertraline 25 mg/day and esomeprazole 20 mg/day for over two years. She had no history of liver disease and was known to have normal routine liver tests (AST 28U/L, ALT 24U/L, GGT 24U/L, ALP 108U/L, total bilirubin 0.72 mg/dL). Moreover, she had no personal or family history of autoimmune disease.
After receiving the first dose of Moderna-COVID-19 vaccine, the patient presented mild abdominal pain. Two weeks later, routine liver function tests showed AST 1056U/L, ALT 1092U/L, GGT 329U/L, ALP 24U/L, total bilirubin 1.14 mg/dL. Complete blood cell count and international normalized ratio were normal. She denied recent changes in drug therapy. The serology for hepatitis A virus, human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus and herpes simplex virus type 1 and 2 were all negative. Polymerase chain reaction for hepatitis B, C and E viruses were also negative. Ceruloplasmin, alpha-1 antitrypsin and iron tests were normal, as well as thyroid function. Antinuclear antibody was positive (1:100, speckled pattern), detected by indirect immunofluorescence assay on HEP-20-10 cells/monkey liver (initial dilution 1/100; final dilution 1/1000). Anti-mitochondrial, anti-smooth muscle, anti-liver-kidney microsomal, anti-soluble liver antigen and antineutrophil cytoplasmic antibodies were all negative. At this point, serum IgA, IgM and IgG levels were normal. Abdominal Doppler ultrasound showed hepatomegaly without cirrhotic morphology, and no biliary dilation or thrombosis.
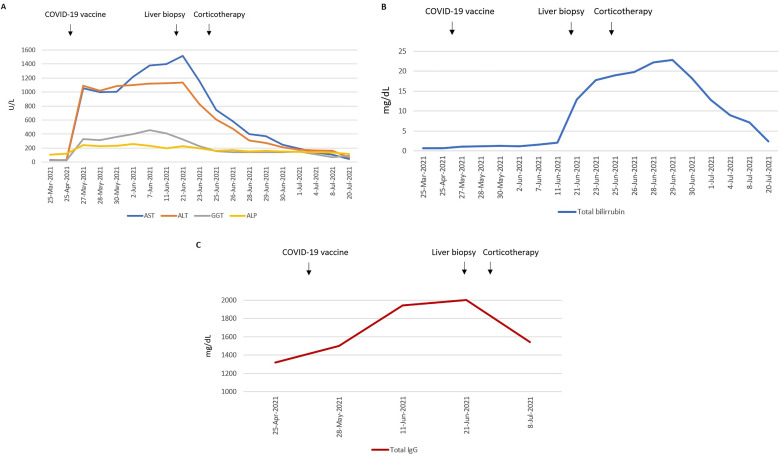
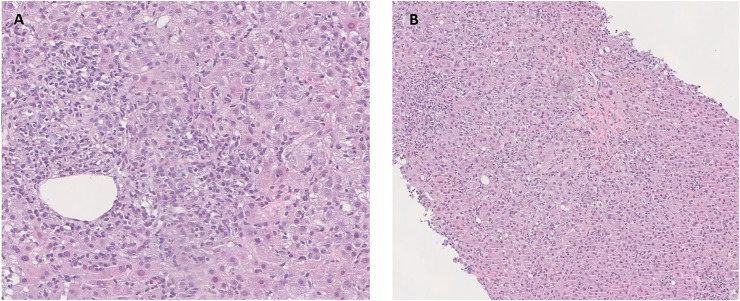
Five weeks after vaccination, the patient presented with jaundice and choluria. Liver profile was worsening and IgG levels were now elevated (Fig. 1 ). The patient was admitted for clinical management. A percutaneous liver biopsy was performed, revealing a marked expansion of the portal tracts due to dense inflammatory infiltrate, with aggregates of plasma cells; severe interface hepatitis and multiple confluent foci of lobular necrosis were also observed (Fig. 2 ).
Fig. 1.
– Evolution of liver function tests (A), total bilirubin (B) and total IgG levels (C) over time. AST - aspartate aminotransferase, ALT - alanine aminotransferase, GGT - gamma-glutamyl transferase, ALP - alkaline phosphatase, IgG - Immunoglobulin G.
Fig. 2.
Liver biopsy findings – (A) Marked portal tract inflammation with intense lymphoplasmacytic infiltrate and interface hepatitis (HE, 30x). The inflammation consists primarily of lymphocytes and aggregates of plasma cells, with few eosinophils. (B) Intense lobular activity associated with centrilobular necrosis (HE 20x).
The score of simplified diagnostic criteria of the International Autoimmune Hepatitis Group was 8 [definite diagnosis of autoimmune hepatitis (AIH)]. Treatment with prednisolone 60 mg/day was started with a quick improvement of liver function tests and normalization of IgG levels. One month after initial diagnosis, the patient remains well on a tapering course of corticosteroids.
Recently, some cases of AIH that developed after COVID-19 vaccination have been reported [3,4]. There are some similarities between the previously described cases and the present case, such as a short interval between vaccination and symptoms onset [3]. Although our patient received the Moderna mRNA vaccine, there are already other reports of AIH induced by the Pfizer-BioNTech and Oxford-AstraZeneca vaccines, supporting the idea that the COVID-19 vaccine triggers autoimmune phenomena regardless of its mechanism of action [4].
In contrast to other cases, our patient did not have confounding factors such as pregnancy or autoimmune conditions [3,4]. However, she was under treatment with pegylated interferon for polycythemia vera. In fact, this drug has been linked to induction of autoimmune conditions, including AIH [5]. Nevertheless, this side effect usually arises within 1–2 months of starting therapy and our patient had already started pegylated interferon for over two years. In addition, the timing of the liver injury, the extensive exclusion of other causes of hepatic disease and the normalization of liver function tests and IgG levels after treatment, make it very likely that AIH was triggered by COVID-19 vaccination.
Only long-term follow-up will confirm whether COVID-19 vaccination increases the risk of AIH. Nevertheless, it should not distract healthcare providers from the overwhelming benefits of mass COVID-19 vaccination.
Credit author statement
Susana Lopes: Conceptualization. Rodrigo Liberal: Conceptualization. Isabel Garrido: Writing – original draft, Visualization, Project administration. Joanne Lopes: Writing – original draft. Susana Lopes, Writing – review & editing. Manuel Sobrinho Simões, Writing – review & editing. Rodrigo Liberal: Writing – review & editing. Fátima Carneiro: Supervision. Guilherme Macedo: Supervision. Isabel Garrido did literature review and drafted the manuscript. Isabel Garrido, Susana Lopes, Manuel Sobrinho Simões, Rodrigo Liberal, Fátima Carneiro, Joanne Lopes and Guilherme Macedo have critically revised and finalized the manuscript. All authors have approved the final version of the manuscript.
Author’s contributions
Isabel Garrido did literature review and drafted the manuscript. Isabel Garrido, Susana Lopes, Manuel Sobrinho Simões, Rodrigo Liberal, Fátima Carneiro, Joanne Lopes and Guilherme Macedo have critically revised and finalized the manuscript. All authors have approved the final version of the manuscript.
Financial support
None.
Declaration of competing interest
The authors have no disclosures to report.
References
- 1.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021 doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casualty. J. Hepatol. 2021;S0168–8278(21):412–418. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LiverTox Clinical and research information on drug-induced liver injury. https://www.ncbi.nlm.nih.gov/books/NBK547867/ [Internet]. Available from: Accessed. [PubMed]