Abstract
Purpose
Lymphadenopathy (LAP) after COVID-19 vaccination in patients with a diagnosis of cancer has been challenging. We analyzed imaging and clinical features from early cases of axillary LAP in six COVID-19 vaccine recipients with a history of breast cancer.
Method
Among the patients with a history of breast cancer and recent COVID-19 vaccine administration, six patients who showed isolated axillary LAP were gathered. Radiologic features were reviewed from breast ultrasound, chest computed tomography, and breast magnetic resonance imaging. Clinical and pathological information were obtained for analysis.
Results
The interval between ultrasound detection of LAP and last COVID-19 vaccine administration ranged from 14 to 28 days (mean 21.67 days). Round shape of the lymph node and irregular cortex were noted in 2 and 0 cases, respectively. Mean maximum cortical thickness, length to width ratio and interval aggravation in maximum cortical thickening were 4.2 mm, 1.34, and 2.81-fold with cut-off value of 3 mm, 1.5, 2.0-fold, respectively.
Conclusion
We observed axillary LAP ipsilateral to a recent vaccine administration persisting longer than what the Centers for Disease Control and Prevention announced. In our patients, COVID-19 vaccine-related LAP tended to show increased cortical thickness without cortical irregularity. Oncologist as well as radiologist should be familiar with the fact that COVID-19 vaccines, regardless of vaccine type or dosage, can frequently cause ipsilateral axillary LAP, showing some suspicious features more often than others, and can persist longer than anticipated so that both over- and underdiagnosis can be avoided. We report our observations in six patients and provide an exhaustive review of the published literature
Keywords: COVID-19, COVID-19 vaccine, COVID-19 vaccine adverse effect, Vaccine-related lymphadenopathy, Oncology lymphadenopathy, Breast cancer lymphadenopathy, mRNA vaccine axillary lymphadenopathy, Pfizer-BioNTech COVID-19 vaccine, Vaxzevria, Oxford/AstraZeneca adenovirus vector COVID-19 vaccine, Moderna COVID-19 vaccine
Abbreviations: BCS, breast conserving surgery; BI-RADS, Breast Imaging-Reporting and Data System; CDC, Centers for Disease Control and Prevention; CSBI, Canadian Society of Breast Imaging; CT, computed tomography; ER, estrogen-receptor; EUSOBI, European Society of Breast Imaging; IMCT, interval change in maximum cortical thickening; IDC, invasive ductal carcinoma; LA, lymphadenopathy; LN, lymph node; L/W, length to width; MCT, maximum cortical thickness; MRI, magnetic resonance imaging; PR, progesterone-receptor; HER2, human epidermal growth factor receptor 2; DCIS, ductal carcinoma in situ; NAC, neoadjuvant chemotherapy; SBI, Society of Breast Imaging; PBV, Pfizer-BioNTech COVID-19 vaccine; TN, triple-negative; UIQ, upper-inner quadrant; UOQ, upper-outer quadrant; US, ultrasound; Vaxzevria, Oxford/AstraZeneca adenovirus vector COVID-19 vaccine
Introduction
Since the beginning of their administration, various reactions and adverse effects of the COVID-19 vaccines have been reported and shared world-wide [1], [2], [3], [4], [5], [6], [7]. While vaccine-related lymphadenopathy (LAP) with other vaccines is rare with a few published articles on H1N1 Influenza, human papillomavirus (HPV), smallpox, Bacille Calmette-Guerin (BCG), and measles vaccines [8], [9], [10], [11], [12], [13], [14], reports of regional LAP in COVID-19 vaccine recipients are gradually increasing along with the rollout of mass COVID-19 vaccination across the world [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. Isolated unilateral axillary LAP can be alarming especially in patients with a history of breast cancer [35,36].
We present 6 challenging cases of COVID-19 vaccine-related axillary LAP in patients with a history of breast cancer. Prior to the routine imaging studies, four patients had received their first dose of the Oxford/AstraZeneca (AZD1222) adenovirus vector COVID-19 vaccine (Vaxzevria), one patient had received her second dose of the Pfizer-BioNTech (BNT 162b2) mRNA COVID-19 vaccine, and one patient had cross-inoculation receiving Vaxzevria first and Pfizer-BioNTech COVID-19 second.
Material and methods
Six patients with history of breast cancer underwent one or more imaging studies including breast ultrasound (US) with linear transducer (iU22, Philips Medical System, Bothell, WA), chest computed tomography (CT) with contrast enhancement (Biograph64, Siemens, Erlangen, Germany) and breast magnetic resonance imaging (MRI) with contrast enhancement (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany) for follow-up or surveillance purposes. Maximum cortical thickness (MCT) of axillary lymph node (LN) was measured from its maximal thickness perpendicular to the inner cortical border on US. Length to width (L/W) ratio was measured on a plane revealing the longest diameter of the targeted LN on CT and MRI. Interval change in maximum cortical thickening (IMCT) was compared with the last follow-up exam on CT and MRI. MCT, L/W ratio and IMCT elicited from the measurements of first and second largest LN if multiple and mean value was calculated in each image modality. Higher number was derived among CT and MRI measurement results for IMCT and lower number for L/W ratio to meet the cut-off value. The cut-of values used for MCT, L/W ratio and IMCT are 3 mm or more (≥3 mm), less than 1.5 (<1.5 mm) and more than doubling (≥2 times), respectively [37,38]. Other imaging features such as shape, marginal irregularity, anatomic location and multiplicity of axillary LAP were included for analysis [39]. Patient information on age, sex, axillary symptom and sign was obtained from the electronic medical record of our facility or asked directly of the patient when performing US by the first author of this article. Prior breast cancer characteristics such as cancer type, subtype, TNM stage, and tumor location were obtained from prior pathologic report or official records from the referring hospital. Date, type, and site of COVID-19 vaccine administration were questioned before and after the imaging study to support interpretation and management.
Case 1
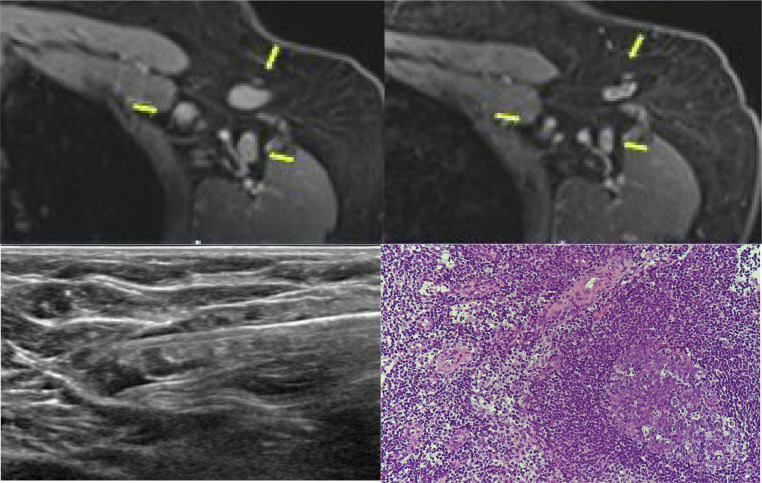
A 61-year-old woman with a history of right breast cancer with ipsilateral axillary LN metastasis underwent breast conserving surgery (BCS) with axillary dissection followed by adjuvant chemotherapy and radiation therapy. The cancer type was estrogen-receptor (ER)-negative, progesterone-receptor (PR)-negative and human epidermal growth factor receptor 2 (HER2)-positive invasive ductal carcinoma (IDC) with medullary features and she received trastuzumab therapy. Unexpectedly in a routine 5-year follow-up multiple, enlarged lymph nodes were observed in left axillary region on imaging studies. She didn't have any other suspicious findings in both breasts and right axilla on US, mammography, CT and MRI. She was asked about recent vaccination history before further intervention, and she reported she had received her first dose of Vaxzevria in the left arm 16 days before the imaging studies. We presumed this new left axillary LAP was most likely due to the vaccine, but the patient desired a definitive answer urgently. US-guided 14-gauge gun biopsy was performed and the pathology reported benign hyperplasia (Fig. 1 ).
Fig. 1.
Images and biopsy result from Case 1.
A 61-year-old woman with a history of right breast cancer with right axillary metastasis underwent her routine 5-year follow-up imaging studies after treatment. Multiple left axillary lymph nodes were observed in axillary level I (upper left), showing significant increase in cortical thickness of lymph nodes compared with her previous breast MRI (upper right, arrows highlight the lymph nodes). She had received her first dose of Vaxzevria in the left arm 16 days before the breast MRI scan and 22 days before the breast ultrasound. Pathologic confirmation and ultrasound guided 14-gauge gun biopsy was performed (lower left), with a diagnosis of benign hyperplasia (lower right).
Case 2
A 75-year-old woman with a history of ER-positive, PR-positive, and HER2-negative IDC with mucin production in her right breast without axillary LN metastases received proper treatment and came back to the hospital for her 2.5-year follow-up. Multiple enlarged, round, and coffee bean-shaped lymph nodes were detected in levels I and II of the left axilla, showing a MCT of 5.38 mm on US and mean L/W ratio less than 1.5 on CT and MRI. IMCT had more than doubled compared with last imaging studies. She reported having had her second dose of the Pfizer-BioNTech COVID-19 vaccine 14 days prior to the hospital visit and the pathologic confirmation was reactive hyperplasia.
Case 3
A 71-year-old woman with a history of ER-positive, PR-positive, and HER2-positive IDC in the right breast with no axillary metastasis had 8-year surveillance exams after proper treatment. She didn't have any abnormalities on physical exam, but the breast US demonstrated one isolated left axillary level I LN with smooth and diffuse enlargement and borderline MCT of 3 mm. On CT, the LN showed a L/W ratio less than 1.5 and IMCT greater than 2 times compared with previous scans. She reported having had her first dose of Vaxzevria in the left arm 8 and 14 days prior to current CT and US evaluation, respectively. An US-guided biopsy was performed with patient consent approximately 27 days after Vaxzevria injection, confirming persistent axillary LAP with little change. Benign hyperplasia was confirmed as the final diagnosis.
Case 4
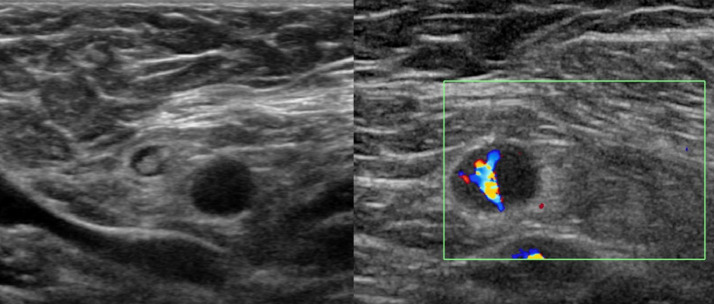
A 73-year-old woman with a history of ductal carcinoma in situ (DCIS) in her left breast without axillary metastasis came to our institution for her 10-year surveillance. She had received her prior treatment at an outside hospital and her medial record was limited. She underwent breast US and mammography without chest CT or breast MRI in this follow-up. These studies noted several enlarged lymph nodes in level I of her right axilla with one LN showing round shape. New unilateral axillary lymphadenopathy showed a mean MCT of 6.4 mm, L/W ratio of 1.3 and IMCT near 3 times greater than her last US exam. She had received her first dose of Vaxzevria and second dose of Pfizer-BioNTech COVID-19 vaccine with an 11-week interval. The current US exam had been performed 28 days after her last dose of Vaxzevria – Pfizer-BioNTech COVID-19 Vaccine cross-inoculation. Given she had no other evidence of tumor recurrence or infection, the patient was advised to undergo 4–12 weeks follow-up with axillary US (Fig. 2 ).
Fig. 2.
Ultrasound images from Case 4.
A 73-year-old woman with history of left breast cancer without axillary metastasis underwent her 10-year surveillance after treatment. There was isolated round shape unilateral lymphadenopathy in her right axilla level I with loss of fatty hilum, MCT of 6.4 mm (cut-off value, 3 mm), L/W ratio of 1.3 (cut-off value, <1.5) and IMCT near 3 times greater than her last exam (cut-off value, ≥2 times) on ultrasound. She had cross-inoculation with a first dose of Vaxzevria and a second dose of Pfizer-BioNTech COVID-19 vaccine 28 days before this ultrasound.
Case 5
A 62-year-old woman with history of triple-negative (TN) IDC in the right breast with ipsilateral axillary metastases underwent BCS with axillary dissection after complete response to neoadjuvant chemotherapy (NAC). On her 2.5-year follow-up, new unilateral left axillary LAP was noted, and others were unremarkable. On US, the largest LN measured about 12.13 × 7.61 mm with MCT up to 3.99 mm. Mean 2.9-fold enlargement in IMCT and 1.3 L/W ratio was calculated on MRI. She had been vaccinated with her first dose of Vaxzevria in the left arm 3 weeks prior to the hospital visit and she agreed on follow-up with axillary US in 4–12 weeks.
Case 6
A 61-year-old woman with history of ER-positive, PR-negative and HER2-negative IDC of the left breast with ipsilateral axillary LN metastasis had BCS with axillary dissection followed by radiation therapy and chemotherapy. New right axillary LAP was detected on 3-year follow-up imaging studies and she reported having received her first dose of Vaxzevria in the right arm 19 days prior to the hospital visit. She agreed on a 3-month follow-up with axillary US.
Results
All 6 cases presented with asymptomatic unilateral axillary LAP contralateral to their prior breast cancer and ipsilateral to the recent COVID-19 vaccine injection. The age of six patients ranged from 61 to 75 years old (median 68 years old, mean 67.2 years old) and none of the patients complained about symptoms or signs concerning for axillary LAP before the routine examinations.
Five of the six patients had a prior history of IDC with one case of DCIS; ER, PR and HER2 status varied. TNM stages of the breast cancers in this study group were early in five but advanced for case 5 who presented with clinical stage T2N2 disease but experienced a complete response to NAC with ypT0N0 staging at the time of surgery. On pathologic examination all tumors were smaller than 2 cm in the longest dimension and 3 cases had ipsilateral axillary LN metastasis before treatment but none of the cases had contralateral axillary LN metastasis. The locations of the tumor were upper-outer quadrant (UOQ) in 3 cases, upper-inner quadrant (UIQ) in 2 cases and central in one case.
Vaccine types were first dose of Vaxzevria in 4 cases, second dose of Pfizer-BioNTech COVID-19 vaccine in one case and cross-inoculation with Vaxzevria - Pfizer-BioNTech COVID-19 vaccine in one case. The interval between US detection of LAP and last COVID-19 vaccine administration ranged from 14 to 28 days (median 21 days, mean 21.67 days).
In radiologic feature analysis, round shape of the LN was noted in 2 cases but irregular cortical margins were not detected in any of the cases. Mean MCT, L/W ratio and aggravated IMCT ranged from 3.0 mm to 6.4 mm, 1.6 to 2.09, and 1.7 to 3.75 times, respectively (mean values were 4.2 mm, 1.34, and 2.81 times, respectively). All, 5 of 6, and 5 of 6 tumors met the cut-off value of MCT, L/W ratio and IMCT aggravation, respectively (Table 1 ).
Table 1.
Clinical information, pathologic results and radiologic features of 6 patients.
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|---|
| Patient factors | |||||||
| Sex | F | F | F | F | F | F | |
| Age | 61 | 75 | 71 | 73 | 62 | 61 | |
| Axillary LAP-related symptoms and signs | None | None | None | None | None | None | |
| Prior breast cancer characters | |||||||
| Cancer type | IDC | IDC | IDC | DCIS | IDC | IDC | |
| Cancer subtype | ER-/PR-/HER2+ | ER+/PR+/HER2- | ER+/PR+/HER2+ | NA⁎⁎ | TN | ER+/PR-/HER2- | |
| TNM stage | pT1N1 | pT1cN0 | pT1N0 | pTisN0 | ypT0N0† | T1cN1 | |
| Tumor location | UOQ | UIQ | UIQ | UOQ | UOQ | Central | |
| COVID-19 vaccine types and intervals | |||||||
| 1st dose | Vaxzevria | PBV | Vaxzevria | Vaxzevria | Vaxzevria | Vaxzevria | |
| 2nd dose | Not yet | PVB | Not yet | PVB | Not yet | Not yet | |
| Interval between US detection of LAP and last COVID-19 administration (days) | 22 | 19 | 14 | 28 | 21 | 26 | |
| Side of vaccination/axillary LAP/prior breast cancer | L/L/R | L/L/R | R/R/L | L/L/R | L/L/R | R/R/L | |
| Radiologic features of LAP | |||||||
| Round shape | No | Yes | No | Yes | No | No | |
| Irregular margins | No | No | No | No | No | No | |
| MCT ≥ 3mm on US (mean, mm)‡ | Yes (3.35) | Yes (5.38) | Yes (3.0) | Yes (6.4) | Yes (4.0) | Yes (3.08) | |
| L/W < 1.5 on CT and/or MRI (mean)‡§ | Yes (1.43) | Yes (1.3) | Yes (1.09) | Yes (1.3)‖ | Yes (1.32) | No (1.6) | |
| IMCT ≥ 2X on CT and/or MRI (mean, time)‡¶ | Yes (3.75) | Yes (3.4) | Yes (2.19) | Yes (2.9)‖ | Yes (2.91) | No (1.7) | |
| Involved axillary level | Level I | Level I and II | Level I | Level I | Level 1 | Level I | |
| Axillary level | Multiple | Multiple | Single | Multiple | Multiple | Single | |
| US-guided biopsy results of LAP | Benign | Benign | Benign | NA†† | NA †† | NA†† | |
CT = computed tomography; DCIS = ductal carcinoma in situ; ER = estrogen-receptor; F = female; HER2 = human epidermal growth factor receptor 2; IDC = invasive ductal carcinoma; IMCT = interval change in maximum cortical thickening; L = left; LAP = lymphadenopathy; L/W = Length to width; MCT = maximum cortical thickness; MRI = magnetic resonance imaging; NA = not applicable; PBV = Pfizer-BioNTech COVID-19 vaccine; PR = progesterone-receptor; R = right; TN = triple negative; UIQ = upper-inner quadrant; UOQ = upper-outer quadrant; US = ultrasound.
Clinical stage before NAC was cT2N2.
Mean value if multiple.
Lowest number among values on CT or MRI or both.
Highest number among values on CT or MRI or both.
Values from US only as no CT and MRI scans available.
Limited medical record from outside hospital.
Short-term follow-ups were recommended instead of biopsy.
Discussion with review of literature
Publications
As of early October 2021, over 80 articles are accessible on-line or in-print focusing on COVID-19 vaccine-related LAP. Over 60% of them are case reports or case series reporting less than 10 cases each. Less than 20 original studies were published from January to early October 2021 analyzing clinical and radiological features of LAP. Around one dozen articles review the phenomenon and updated clinical guidelines [15,17,19,20,[22], [23], [24], [25], [26], [27], [28], [29],[31], [32], [33], [34],38,[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90]]. While more than half of the literature was published in radiology journals, the first authors varied from oncologists and medical physician to pathologists and radiologists. Some cases of LAP were symptomatic but most cases in large groups were asymptomatic with LAP incidentally recognized during cancer workup or in screening settings. Most studies reported on patients in the USA, probably owing to different socio-economical environments for the vaccination (Table 2 ).
Table 2.
Characters of published literatures on COVID-19 vaccine-related lymphadenopathy.*
| Included number of cases in the articles | Number of articles |
|---|---|
| 1 case | 31 |
| 2–5 cases | 15 |
| 6–10 cases | 5 |
| Over 50 cases | 7 |
| 11–50 cases | 9 |
| Field of journal⁎⁎ | Number of articles |
| Biology | 2 |
| Cytopathology | 1 |
| Epidemiology | 1 |
| General Medicine | 6 |
| Hematology | 2 |
| Internal Medicine | 1 |
| Laryngo-Otology | 1 |
| Nuclear Medicine | 17 |
| Oncology | 9 |
| Oral and Maxillofacial Surgery | 1 |
| Pulmonology | 2 |
| Radiology | 28 |
| Study Design⁎⁎ | Number of articles |
| Brief Report | 1 |
| Case Report | 14 |
| Case Series | 5 |
| Clinical Perspective | 2 |
| Clinical Picture | 1 |
| Clinical Practice | 1 |
| Editorial | 2 |
| Hematology Image | 1 |
| Image of the month | 4 |
| Images in Radiology | 3 |
| Interesting Image | 7 |
| Letter to the Editor | 3 |
| Original Research | 17 |
| Review Article | 2 |
| Short Communication | 1 |
| Short Report | 1 |
| Special Report | 1 |
| Teaching case studies | 1 |
| Region⁎⁎ | Number of articles |
| Brazil | 1 |
| Canada | 4 |
| Germany | 1 |
| Israel | 8 |
| Italy | 6 |
| Poland | 1 |
| Portugal | 1 |
| Singapore | 1 |
| South Korea | 1 |
| Spain | 4 |
| Switzerland | 2 |
| UK | 7 |
| USA | 30 |
| Time of creation [2021] | Number of articles |
| January | 2 |
| February | 9 |
| March | 11 |
| April | 5 |
| May | 7 |
| June | 6 |
| July | 12 |
| August | 8 |
| September | 6 |
| October | 1 |
Only articles with clear demonstration of the cases and full-text accessibility were included between January 1 and October 1, 2021.
In alphabetical order.
Incidence
Moderna COVID-19 (mRNA-1273) vaccine-related clinical LAP was reported up to 16% [91]. Barda et al. reported 78.4 events per 100,000 persons (95% CI, 64.1–89.3) of Pfizer-BioNTech COVID-19 vaccine-related clinical LAP in 884,828 cases of nationwide setting in Israel [43]. Reported imaging prevalence of COVID-19 vaccine-related LAP with or without clinical symptom varies from 3% (23 out of 750 patients with or without malignancy on mammography) [72] to 9% (21 out of 232 patients with history of thoracic malignancy on CT) [67]. Reported nuclear imaging prevalence of COVID-19 vaccine-related LAP is higher up to 66% (180 out of 274 with underlying oncologic condition) [68]. As evidence of COVID-19 vaccine-related benign LAP accumulates, many authors are suggesting that the incidence of subclinical LAP will rise on screening or diagnostic imaging studies along with world-wide mass rollout of COVID-19 vaccines [55]. Faermann et al. Reported 394% increase in predominantly subclinical LAP in screening US and or mammography compared with the 2 prior years, with 77.8% attributable to COVID-19 vaccination [54].
Age and sex
Cohen et al. observed higher number of vaccine-related LAP in patients younger than 55 years than in older patients [92]. They also observed the incidence of LAP dropped significantly in patients older than 85 years, however, the severity of LAP increased in these elderly patients. In a 120-case analysis, Ferrari et al. also observed an increased number of LAP in younger patients [57]. Nishino et al. reported higher frequency of LAP amongst females with the difference with statistically significant compared to males [67]. Abou-Foul et al. also observed a higher frequency of cervical LAP in female patients [41].
Pathophysiology
Tintle et al. described mRNA vaccines for COVID-19 as effective in driving a potent adaptive immune response to the spike glycoprotein epitope which mediates viral entry by binding to the angiotensin-converting enzyme 2 (ACE2) receptors in host cells. And administration of COVID‐19 vaccine generates potent activation of the Langerhans cells that then migrate into lymph nodes. Given this, in the early phase of acute viral infections or vaccinations, the stimulation with neoantigens will induce reactive follicular hyperplasia which results in LN enlargement [82]. McIntosh et al. also explained that the vaccine-related LAP could be the result of an active immune responses to the COVID-19 vaccines and this could be even more so with mRNA vaccines owing to their presumed higher immunogenic nature [20]. These phenomenon could sometimes cause borderline findings both in radiology and pathology with prominent multiple nodal enlargement and atypical cytopathologic features [82].
Location
Anatomic location of COVID-19 vaccine-related LAP is mostly in the ipsilateral axilla, supraclavicular, infraclavicular, and cervical areas (Table 3). Plaza et al. demonstrated that the natural physiologic course of lymphatic drainage to the axilla that explains how lymphatics from the upper arm drain to the lateral nodal group of the axilla while lymphatics from the anterior chest and breast drain to anterior nodal group of the axilla, both converging in the central nodal group and finally to the apical nodal group of the axilla [93]. They suggested that from this anatomic difference in the lymphatic drainage pattern, one could carefully tell apart metastatic LAP from vaccine-related LAP injected on the ipsilateral deltoid muscle. Orevi et al. observed 112 cases of bilateral axillary LAP in 268 cases of COVID-19 vaccine-related LAP [68]. Bass et al. reported one case of unilateral iliac and inguinal LAP after Pfizer-BioNTech COVID-19 vaccine injection on the ipsilateral thigh muscle [44]. Pudis et al. reported one case of systemic LAP after Pfizer-BioNTech COVID-19 vaccine injection in right deltoid muscle and Nawwar et al. reported one case of axillary LAP with diffuse splenic and bone marrow uptake on PET/CT after vaccine administration on the left deltoid muscle [65,71].
Table 3.
Summary of 67 published reports on COVID-19 vaccine-related lymphadenopathy.*
| Type of vaccine | Number of cases |
|---|---|
| Pfizer-BioNTech (BNT 162b2) mRNA COVID-19 vaccine | 2859 |
| Moderna (mRNA-1273) COVID-19 vaccine | 103 |
| Oxford/AstraZeneca (AZD1222) adenovirus vector COVID-19 vaccine | 12 |
| mRNA vaccine without specification⁎⁎⁎ | 8 |
| COVID-19 vaccine without specification† | 88 |
| Johnson & Johnson's Janssen COVID-19 vaccine | 1 |
| Gam-COVID-Vac (Sputnik V) vaccine | 1 |
| Location of lymphadenopathy | Number of cases |
| Axillary only | 2319 |
| Supraclavicular and/or infraclavicular only | 44 |
| Cervical area only | 25 |
| Axillary and peri-clavicular‡ area together | 12 |
| Axillary and cervical area together | 1 |
| Axillary, peri-clavicular‡ and cervical area together | 3 |
| Axillary and pectoral area together | 3 |
| Systemic§ | 4 |
| Specific location not provided in the article¶ | 660 |
| Ipsilateral inguinal and iliac lymphadenopathy after injection on thigh muscle‖ | 1 |
| Total number of cases | 3072†† |
Only articles with clear demonstration of the cases and full-text accessibility were included between January 1 and October 1, 2021** Number of cases described as COVID-19-related lymphadenopathy in the article, not total number or enrolled number of the study in each literature.
Cases from articles describing the vaccine only as ‘mRNA COVID-19 vaccine’ without specification.
Cases from articles describing the vaccine only as ‘COVID-19 vaccine’ without specification.
Including supraclavicular, infraclavicular and sub-clavicular area as mentioned in the articles.
Cases showing contralateral axillary lymphadenopathy or any other area of the body except ipsilateral axilla, peri-clavicular and cervical area to vaccine injection site [65,71,94].
Cases from one article describing lymphadenopathy only as ‘lymphadenopathy’ without anatomical location [43].
Considered as local reaction given the case had COVID-19 vaccine injection on thigh muscle instead of usual deltoid muscle [44].
All the cases without clear number and corresponding localization of the lymphadenopathy were excluded from this summary table.
Vaccine types
A larger number of vaccine-related LAP have been reported with the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine than with other vaccines such as Vaxzevria or Johnson & Johnson's Janssen COVID-19 Vaccine (Table 3). This could be due to different levels of immunogenicity or due to different supply and permission status among the vaccines. The Centers for Disease Control and Prevention and Nishino et al. reported higher number of LAP with the Moderna COVID-19 vaccine compared with Pfizer-BioNTech COVID-19 vaccine [7,67,91]. On the contrary, Cocco et al. reported COVID-19 vaccine-related LAP in 24 cases with no statistical difference amongst the 3 different kinds of vaccines (Pfizer-BioNTech COVID-19 vaccine, Vaxzevria and Moderna COVID-19 vaccine) [49]. Ferrari et al. also observed no statistically significant difference between the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine regarding vaccine-related LAP on PET/CT scans [57].
Dose
Orevi et al. observed an increase in the number of axillary LAP cases in the patients after the second vaccination (141/203, 69%) than in the patients after the first vaccination (39/71, 55%) without a significant difference in SUVmax on PET/CT studies [68]. Robinson et al. reported no statistically significant difference between the first and second dose of the vaccination regarding the incidence of vaccine-related axillary LAP.
Onset and duration
The Centers for Disease Control and Prevention reported that the onset of COVID-19 vaccine-related LAP is as soon as 1 to ∼3 days after the injection and that the duration could last as long as 16 days, varying between the vaccine types [91]. However, some authors have reported a much longer duration for LAP [22,29]. In our cohort we also observed axillary LAP lasting up to 28 days after vaccine administration. Eshet et al. observed hypermetabolic LAP up to 11 weeks on PET/CT in 19% of 169 cases with vaccine-related LAP [95]. According to Hanneman et al., the hypermetabolic activity of LAP resolved faster than the enlarged lymph node assessed volumetrically [22]. This may suggest perceptible lymph node enlargement could persist beyond 11 weeks on imaging studies. Orevi et al. observed a longer duration of LAP after the second than the first vaccination [68].
Modality
The most sensitive modality for LAP detection is by far PET/CT scans. Many nuclear imaging studies have revealed hypermetabolic activity in LAP even in lymph nodes with normal size and shape, including a case report from Schapiro et al [74]. CT and MRI are considered to be of good value for detection and assessment on anatomic location and number of involved lymph nodes. US is the best imaging modality for detailed evaluation of superficially located LN characters and for on-site fine needle aspiration or gun biopsy guidance. Mammography reveals less of axillary LAP than other imaging modalities depending on the location of the lymph node and technical position of the patient. Robinson et al. observed only 3% of axillary LAP in 750 women [72].
Imaging findings
Among more than 30 articles regarding PET/CT findings of the COVID-19-related LAP, Orevi et al. analyzed a large group of 268 cases out of 1281. LN size ranged between 0.2 and 5.3 cm (mean: 0.8 ± 0.6 cm) and SUVmax ranged from 0.6 to 17.8 (mean: 3.3±2.7) on PET/CT. They observed simultaneously increased activity in the arm and in the ipsilateral axillary LN, defined as a "double sign" in 20% of vaccinated patients with 100% of specificity and PPV [68]. Nishino et al. analyzed CT images and reported vaccine-related LAP in 21 out of 232 cases (9.0%). The median short-axis diameter of the largest node was 7 mm (range: 5–14 mm). The median number of increased nodes was 4 (range: 1–10). The median time to the post-vaccine scan revealing lymphadenopathy was 1.7 weeks (range: -2.9 to 6.6) from the second dose [67].
Cocco et al. analyzed US features of 24 cases with vaccine-related LAP. The median size was 0.9 cm ± 0.19 on US and the number of nodes was less than 3 in 83.3%. The prevalent morphology was ovular in 18 patients (75%) and round in six patients (25%). The prevalent cortex–hilum pattern was asymmetric cortex with hilum evidence (9, 37.5%), followed by the absence of hilum (8, 33.3%) and symmetric cortex with hilum evidence (7, 29.2%). Elastosonography patterns were like the surrounding tissue in 15 cases (71.4%) and prevalently hard in 9 cases (28.6%) [49].
While not many articles describe findings of mammography compared with other modalities, Locklin et al. reported 3 rare findings of mammography and US other than LN enlargement associated with COVID-19 vaccine. They described 2 cases showing breast edema with skin thickening which corresponded with increased non-mass like density and trabeculation on mammography. One case showed increased fat infiltration in axillary fossa on US [96]. These results from literatures demonstrate the heterogeneity of imaging features of vaccine-related LAP that can mimic malignant lymph nodes.
Clinical significance and published recommendations
The Society of Breast Imaging (SBI) stated proper assessment and management guideline in the field of breast radiology and this was endorsed by the Canadian Society of Breast Imaging (CSBI) [97,98]. In the screening setting, isolated axillary LAP with recent history of COVID-19 vaccine administration should be categorized as Breast Imaging-Reporting and Data System (BI-RADS) category 3, warranting a short-term FU in 4–12 weeks to assure resolution of the findings. After excluding other significant cause for the axillary LAP, acknowledgement of COVID-19 vaccine-related LAP can prevent unnecessary medical expenditures and relieve patient anxiety. Recent articles even suggested BIRADS category 2 for vaccine-related LAP. Without history of vaccine administration, BI-RADS category 0 is appropriate and further evaluation for differential diagnosis is mandatory.
The challenge lies when a patient with a history of a malignancy with a tendency to involve axillary LNs, such as breast cancer, melanoma and lymphoma, presents with isolated axillary LAP after a COVID-19 vaccine injection. There are over 160,000 women currently living with metastatic breast cancer in the United States and an even larger number world-wide [99]. Cohen et al. reported that the greatest difficulty in the differential diagnosis between vaccine associated hypermetabolic LAP and malignant LAP were in patients with either breast cancer or lymphoma [92]. Avner et al., Czepczyński et al., and Prieto et al. reported challenging cases of axillary LAP mimicking metastatic melanoma.
Well-known radiologic features of pathologic LAP include cortical thickening more than 3 mm, marked hypoechoic cortices, round shape, loss of fatty hilum, cortical irregularity, L/W ratio less than 1.5 and interval cortical thickening aggravation greater than 2 times in comparison with prior imaging study [39,41]. Furthermore, there can be overlapping “worrisome” features in vaccine-related self-limited LAP such as a round shape, irregular cortex, and stiffness with an aggravated IMCT and L/W ratio < 1.5 as seen in our study [49]. Round shape with loss of hilum and irregular margin of LN were less frequently encountered in our study group.
Given that even a little change in the shape or size of axillary LNs in follow-up or surveillance imaging studies can be of concern to both clinicians and radiologists, common as well as uncommon findings and overlapping malignant features as well as typically benign features of vaccine-related LAP should be shared and recognized.
Practical strategy for injection, medical recording, and more
The European Society of Breast Imaging (EUSOBI) recently announced ten proposals for vaccine-related axillary LAP, reinforcing prior SBI statements and assembling the published literature [89]. These recommendations include vaccine injection in the cancer-free arm, vaccination data collection prior to breast imaging referrals and routine imaging at least 12 weeks after the last vaccine dose. They emphasized managing lymphadenopathy, considering the time frame from vaccination and overall nodal metastatic risk in patients with breast cancer, and a multidisciplinary team approach for complex and unclear cases.
Our six patients received vaccine injections in the cancer-free arm, but still three patients proceeded to invasive pathologic confirmation. In correlation with the result of our study we agree on the need for collecting medical records on the COVID-19 vaccination status of the patient in effort to reduce false positive biopsies [16,29]. In our institution, we started have begun to obtain information that includes the date, dose, type and location of the COVID-19 vaccine injection from all visiting or referred patients before performing breast imaging studies.
Action after vaccine-related LAP
Owing to accumulation of experience on COVID-19 vaccine-related axillary LAP, an increasing number of authors advocate a conservative short-term follow-up over prompt invasive intervention [15,16,19,21,[25], [26], [27], [28], [29],[31], [32], [33], [34]]. We also have been recommending short-term follow-up to the patients showing incidental mild to borderline LAP after COVID-19 vaccinations including cases 4, 5, and 6 of this communication. All such decisions are made after thorough exclusion of any possible cause with clinical significance.
Appropriate timeline for imaging study in the COVID-19 era
If it does not unduly delay care SBI recommends scheduling screening exams prior to the first dose or 4–6 weeks after the second dose of a COVID-19 vaccination [98]. In recent studies, the proposed recommendations on follow-up interval varies from 4 to 12 weeks [31,64]. In oncologic cases, adjusting follow-up or surveillance schedules according to vaccination can be difficult especially for the patient who received Vaxzevria. The World Health Organization (WHO) announced that longer dose intervals in the 8 -12 weeks range are associated with greater vaccine efficacy for Vaxzevria, whereas 21- to 28-day and 28- to 42-day intervals are appropriate for the Pfizer-BioNTech COVID-19 and Moderna COVID-19 vaccines, respectively [42], [43], [44]. Overall in cancer patients, clinicians need to balance among long COVID-19 vaccine dose intervals for efficacy, axillary LAP persisting longer than expected and adequate timing for follow-up exams.
To our knowledge, our study is one of the earliest papers on Vaxzevria-related axillary LAP with pathologic correlation and with multimodality imaging features and is the very first documentation about Vaxzevria and Pfizer-BioNTech COVID-19 vaccine cross-inoculation [19].
Limitations
This is retrospective review of a limited number of patients with a history of early-stage breast cancer and with COVID-19 vaccine-related LAP in single institution. Further experience in a larger group will allow for safer and more discrete guidelines in interpreting and managing isolated LAP in patients with cancer.
Conclusion
Oncologist as well as radiologist should be familiar with the fact that regardless of type or vaccine dose, COVID-19 vaccines can frequently cause ipsilateral axillary LAP, showing some suspicious features more often than others, and lasting longer than the anticipated period of time so that both over- and underdiagnosis can be avoided. And this awareness should receive special consideration in patients with a diagnosis of breast cancer.
Conflicts of interest
The authors declare no conflict of interest.
Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Prior presentation of abstracts at meetings: Western Pacific Regional Conference 2021 of the MWIA, August 20–21, 2021.
References
- 1.Moderna COVID-19 vaccine. 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine (Accessed 8/4/2021).
- 2.Pfizer-BioNTech COVID-19 vaccine. 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.)
- 3.Janssen COVID-19 vaccine. 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.)
- 4.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. 2021. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (Accessed August 4, 2021).
- 8.Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005;294:2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 9.Shirone N, Shinkai T, Yamane T, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 10.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28:1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 11.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 12.Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36:848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman RF, Herweg JC. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA. 1966;198:320–321. [PubMed] [Google Scholar]
- 14.Marais BJ, Wright CA, Schaaf HS, et al. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatr Infect Dis J. 2006;25:142–146. doi: 10.1097/01.inf.0000199259.04970.d1. [DOI] [PubMed] [Google Scholar]
- 15.Washington T, Bryan R, Clemow C. Adenopathy following COVID-19 vaccination. Radiology. 2021;299:E280–E2e1. doi: 10.1148/radiol.2021210236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA vaccine with ipsilateral axillary lymph node reactivity on imaging. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn RW, Mootz AR, Brewington CC, Abbara S. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3 doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46:353–354. doi: 10.1097/RLU.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 19.Nawwar AA, Searle J, Hagan I, Lyburn ID. COVID-19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48:2655–2656. doi: 10.1007/s00259-021-05274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25728. [DOI] [PubMed] [Google Scholar]
- 21.Avner M, Orevi M, Caplan N, Popovtzer A, Lotem M, Cohen JE. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021;48:2659–2660. doi: 10.1007/s00259-021-05278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanneman K, Iwanochko RM, Thavendiranathan P. Evolution of lymphadenopathy at PET/MRI after COVID-19 vaccination. Radiology. 2021;299:E282. doi: 10.1148/radiol.2021210386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson BJ, Van Abel K, Ma D, Johnson DR. FDG avid axillary lymph nodes after COVID-19 vaccination. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghimi S, Wilson D, Martineau P. FDG PET findings post-COVID vaccinations: signs of the times? Clin Nucl Med. 2021;46:437–438. doi: 10.1097/RLU.0000000000003636. [DOI] [PubMed] [Google Scholar]
- 25.Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021;13:e13524. doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–e300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 29.Becker AS, Perez-Johnston R, Chikarmane SA, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300:E323–E3e7. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell OR, Dave R, Bekker J, Brennan PA. Supraclavicular lymphadenopathy following COVID-19 vaccination: an increasing presentation to the two-week wait neck lump clinic? Br J Oral Maxillofac Surg. 2021;59:384–385. doi: 10.1016/j.bjoms.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cellina M, Irmici G, Carrafiello G. Unilateral axillary lymphadenopathy after coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021;216:W27. doi: 10.2214/AJR.21.25683. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez JL, Eberhardt SC, Revels JW. Unilateral axillary lymphadenopathy following COVID-19 vaccination: a case report and imaging findings. Radiol Case Rep. 2021;16:1660–1664. doi: 10.1016/j.radcr.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman CD, D'Alessandro HA, Mendoza DP, Succi MD, Kambadakone A, Lamb LR. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021;18:843–852. doi: 10.1016/j.jacr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dialani V, James DF, Slanetz PJ. A practical approach to imaging the axilla. Insights Imaging. 2015;6:217–229. doi: 10.1007/s13244-014-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters G, Jones CM. Unilateral axillary adenopathy with unremarkable breast imaging—differential diagnoses. 2012;16:3.
- 37.Clark DA. Preoperative ultrasound of axillary lymph nodes in patients with breast cancer. ANZ J Surg. 2008;78:307–308. doi: 10.1111/j.1445-2197.2008.04443.x. [DOI] [PubMed] [Google Scholar]
- 38.Lernevall A. Imaging of axillary lymph nodes. Acta Oncol. 2000;39:277–281. doi: 10.1080/028418600750013014. [DOI] [PubMed] [Google Scholar]
- 39.Saffar B, Bennett M, Metcalf C, Burrows S. Retrospective preoperative assessment of the axillary lymph nodes in patients with breast cancer and literature review. Clin Radiol. 2015;70:954–959. doi: 10.1016/j.crad.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Aalberg JJ, Collins TP, Dobrow EM. Axillary lymphadenopathy in a renal cell carcinoma patient after COVID-19 vaccination. Radiol Case Rep. 2021;16:2164–2167. doi: 10.1016/j.radcr.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abou-Foul AK, Ross E, Abou-Foul M, George AP. Cervical lymphadenopathy following coronavirus disease 2019 vaccine: clinical characteristics and implications for head and neck cancer services. J Laryngol Otol. 2021:1–6. doi: 10.1017/S0022215121002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albano D, Volpi G, Dondi F, Giubbini R, Bertagna F. COVID-19 vaccination manifesting as unilateral lymphadenopathies detected by 18F-Choline PET/CT. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bass D, Puri S. FDG-avid ipsilateral iliac and inguinal lymphadenopathy after COVID-19 vaccination with thigh injection. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.26491. [DOI] [PubMed] [Google Scholar]
- 45.Brophy J, Henkle G, Rohren EM. DOTATATE uptake in an axillary lymph node after COVID-19 vaccination. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown A, Shah S, Dluzewski S, et al. Unilateral axillary adenopathy following COVID-19 vaccination: a multimodality pictorial illustration and review of current guidelines. Clin Radiol. 2021;76:553–558. doi: 10.1016/j.crad.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canan A, Kukkar V. Unilateral lymphadenopathy after COVID-19 vaccination. Lung India. 2021;38:294. doi: 10.4103/lungindia.lungindia_91_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardoso F, Reis A, Osorio C, Scigliano H, Nora M. A case of cervical lymphadenopathy after vaccination against COVID-19. Cureus. 2021;13:e15050. doi: 10.7759/cureus.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cocco G, Delli Pizzi A, Fabiani S, et al. Lymphadenopathy after the anti-COVID-19 Vaccine: multiparametric ultrasound findings. Biology (Basel) 2021;10 doi: 10.3390/biology10070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen D, Hazut Krauthammer S, Cohen YC, et al. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021;48:3540–3549. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czepczyński R, Szczurek J, Mackiewicz J, Ruchała M. Interference of COVID-19 vaccination with PET/CT leads to unnecessary additional imaging in a patient with metastatic cutaneous melanoma-case report. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.690443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Auria D, Fulgione L, Romeo V, Stanzione A, Maurea S, Brunetti A. Ultrasound and shear-wave elastography patterns of COVID-19 mRNA vaccine-related axillary, supra and subclavicular lymphadenopathy. Clin Transl Imaging. 2021:1–7. doi: 10.1007/s40336-021-00441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eifer M, Eshet Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology. 2021;299:E248. doi: 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faermann R, Nissan N, Halshtok-Neiman O, et al. COVID-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: analysis of 163 cases. Acad Radiol. 2021;28:1191–1197. doi: 10.1016/j.acra.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felices-Farias JM, Martínez-Martínez JF, Guzmán-Aroca F. Unusual lymphadenopathies secondary to the BNT162b2 mRNA Covid-19 vaccine. Med Clin (Barc) 2021 doi: 10.1016/j.medcle.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Prada M, Rivero-Calle I, Calvache-Gonzalez A, Martinon-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.10.2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrari C, Nappi AG, Santo G, et al. The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management. Cancers (Basel) 2021;13 doi: 10.3390/cancers13174340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garreffa E, York J, Turnbull A, Kendrick D. Regional lymphadenopathy following COVID-19 vaccination: considerations for primary care management. Br J Gen Pract. 2021;71:284–285. doi: 10.3399/bjgp21X716117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Granata V, Fusco R, Setola SV, et al. Lymphadenopathy after BNT162b2 Covid-19 vaccine: preliminary ultrasound findings. Biology (Basel) 2021;10 doi: 10.3390/biology10030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021;151:w20557. doi: 10.4414/smw.2021.20557. [DOI] [PubMed] [Google Scholar]
- 61.Kim B, Park Y, Kim EK, Lee SH. Supraclavicular lymphadenopathy after COVID-19 vaccination in Korea: serial follow-up using ultrasonography. Clin Imaging. 2021;79:201–203. doi: 10.1016/j.clinimag.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y. DOTATATE -avid bilateral axilla and subpectoral lymphadenopathy induced from COVID-19 mRNA vaccination visualized on PET/CT. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell OR, Couzins M, Dave R, Bekker J, Brennan PA. COVID-19 vaccination and low cervical lymphadenopathy in the two week neck lump clinic—a follow up audit. Br J Oral Maxillofac Surg. 2021;59:720–721. doi: 10.1016/j.bjoms.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mortazavi S. COVID-19 vaccination-associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021;217:857–858. doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 65.Nawwar AA, Searle J, Lyburn ID. Features of systemic immune response from COVID-19 vaccination on 18F-FDG PET/CT. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003859. [DOI] [PubMed] [Google Scholar]
- 66.Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021;48:2657–2658. doi: 10.1007/s00259-021-05279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishino M, Hatabu H, Ricciuti B, Vaz V, Michael K, Awad MM. Axillary lymphadenopathy after coronavirus disease 2019 vaccinations in patients with thoracic malignancy: incidence, predisposing factors, and imaging characteristics. J Thorac Oncol. 2021 doi: 10.1016/j.jtho.2021.08.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orevi M, Chicheportiche A, Ben-Haim S. Lessons learned from post-COVID-19 vaccination PET/CT studies. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Placke JM, Reis H, Hadaschik E, et al. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow-up: a monocentre study. Eur J Cancer. 2021;154:167–174. doi: 10.1016/j.ejca.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prieto PA, Mannava K, Sahasrabudhe DM. COVID-19 mRNA vaccine-related adenopathy mimicking metastatic melanoma. Lancet Oncol. 2021;22:e281. doi: 10.1016/S1470-2045(21)00197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pudis M, Vercher Conejero JL, Martín Marcuartu JJ, Cortés Romera M. 68Ga-DOTATOC-avid lymphadenopathies induced from COVID-19 mRNA vaccination. Jpn J Clin Oncol. 2021 doi: 10.1093/jjco/hyab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson KA, Maimone S, Gococo-Benore DA, Li Z, Advani PP, Chumsri S. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA Oncol. 2021;7:1395–1397. doi: 10.1001/jamaoncol.2021.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roca B, Rambla M, Roca MM. Supraclavicular lymphadenopathy secondary to COVID-19 BNT162b2 vaccine. J Postgrad Med. 2021;67:180–181. doi: 10.4103/jpgm.JPGM_254_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schapiro R, Moncayo VM, Meisel JL. Case report of lymph node activation mimicking cancer progression: a false positive F(18) FDG PET CT after COVID-19 vaccination. Curr Probl Cancer Case Rep. 2021;4 doi: 10.1016/j.cpccr.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schroeder DG, Jang S, Johnson DR, et al. Frequency and characteristics of nodal and deltoid FDG and (11)C-Choline uptake on PET performed after COVID-19 vaccination. AJR Am J Roentgenol. 2021:1–11. doi: 10.2214/AJR.21.25928. [DOI] [PubMed] [Google Scholar]
- 76.Singh B, Kaur P, Kumar V, Maroules M. COVID-19 vaccine induced axillary and pectoral lymphadenopathy on PET scan. Radiol Case Rep. 2021;16:1819–1821. doi: 10.1016/j.radcr.2021.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skawran S, Gennari AG, Dittli M, et al. [(18)F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2021:1–9. doi: 10.1007/s00330-021-08122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith MV, Yang M. Reactive axillary lymphadenopathy to COVID-19 vaccination on (18)F-FDG PET/CT. J Nucl Med Technol. 2021;49:286–287. doi: 10.2967/jnmt.121.262008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suleman A, Bilbily A, Cheung M, Chodirker L. Hypermetabolic lymphadenopathy on positron emission tomography scan following COVID-19 vaccination: a mimicker of disease progression in Hodgkin lymphoma. EJHaem. 2021 doi: 10.1002/jha2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Surasi DSS, Lin L, Ravizzini G, Wong F. Supraclavicular and axillary lymphadenopathy induced by COVID-19 vaccination on 18F-fluorthanatrace, 68Ga-DOTATATE, and 18F-Fluciclovine PET/CT. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 81.Tan NJH, Tay KXJ, Wong SBJ, Nga ME. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn Cytopathol. 2021 doi: 10.1002/dc.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tintle S, Chen M. Lymphadenopathy with florid lymphoid and Langerhans cell hyperplasia and hemophagocytosis mimicking lymphoma after COVID-19 mRNA vaccination. EJHaem. 2021 doi: 10.1002/jha2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viana JA, Fonseca E, Sawamura MVY. Post COVID-19 vaccine adenopathy: first Brazilian report. J Bras Pneumol. 2021;47 doi: 10.36416/1806-3756/e20210206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown AH, Shah S, Groves AM, Wan S, Malhotra A. The challenge of staging breast cancer with PET/CT in the era of COVID vaccination. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delgado Bolton RC, Calapaquí Terán AK, Erba PA, Giammarile F. Medical imaging in times of pandemic: focus on the cornerstones of successful imaging. Eur J Nucl Med Mol Imaging. 2021;48:1724–1725. doi: 10.1007/s00259-021-05331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garver K. Managing the risk of delayed breast cancer screening versus COVID-19 vaccination associated axillary lymphadenopathy. Acad Radiol. 2021;28:1198–1199. doi: 10.1016/j.acra.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ko G, Hota S, Cil TD. COVID-19 vaccination and breast cancer surgery timing. Breast Cancer Res Treat. 2021;188:825–826. doi: 10.1007/s10549-021-06293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehman CD, Lamb LR, D'Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol. 2021;217:584–586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 89.Schiaffino S, Pinker K, Magni V, et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) Insights Imaging. 2021;12:119. doi: 10.1186/s13244-021-01062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tu W, Gierada DS, Joe BN. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imaging Cancer. 2021;3 doi: 10.1148/rycan.2021210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prevention Ca. The Moderna COVID-19 vaccine's local reactions, systemic reactions, adverse events, and serious adverse events. 2021: Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
- 92.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plaza MJ, Wright J, Fernandez S. COVID-19 vaccine-related unilateral axillary lymphadenopathy: pattern on screening breast MRI allowing for a benign assessment. Clin Imaging. 2021;80:139–141. doi: 10.1016/j.clinimag.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McIntosh LJ, Rosen MP, Mittal K, et al. Coordination and optimization of FDG PET/CT and COVID-19 vaccination; lessons learned in the early stages of mass vaccination. Cancer Treat Rev. 2021;98 doi: 10.1016/j.ctrv.2021.102220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300:E345–E3e7. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Locklin JN, Woodard GA. Mammographic and sonographic findings in the breast and axillary tail following a COVID-19 vaccine. Clin Imaging. 2021;80:202–204. doi: 10.1016/j.clinimag.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seely JM, Barry MH. The Canadian Society of Breast Imaging Recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination - update. Can Assoc Radiol J. 2021 doi: 10.1177/0846537121998949. [DOI] [PubMed] [Google Scholar]
- 98.Committee SoBIPCaD. SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. 2021.
- 99.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:809–815. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]