Abstract
The SARS-CoV-2 virus, which causes Coronavirus disease 2019 (COVID-19), has resulted in millions of worldwide deaths. When the SARS-CoV-2 virus emerged from Wuhan, China in December 2019, reports of patients with COVID-19 revealed that hospitalized patients had acute changes in mental status, cognition, and encephalopathy. Neurologic complications can be a consequence from overall severity of the systemic infection, direct viral invasion of the SARS-CoV-2 virus in the central nervous system, and possible immune mediated mechanisms. We will examine the landscape regarding this topic in this review in addition to current understandings of COVID-19 and hemostasis, treatment, and prevention, as well as vaccination.
Key Words: COVID-19, Anticoagulation, SARS-CoV-2, Neurologic outcomes
Overview of hemostasis
Human blood vessel structure is divided into three anatomical tunicae: intima, media, adventitia. In most vessels, the intima consists of the endothelium, basal lamina, and a cell free subendothelial space. Endothelial cells play a pivotal role in hemostasis by promoting thrombus formation while also simultaneously producing thrombolytic factors.1 The endothelium provides a structural barrier between the circulation and surrounding tissue and regulates vascular hemodynamics by releasing both vasodilators [nitric oxide (NO) and prostacyclin (PGI2)] and vasoconstrictors [endothelin (ET) and platelet-activating factor (PAF)]. The endothelium also serves to facilitate blood flow by inhibiting platelet adhesion and clotting.2
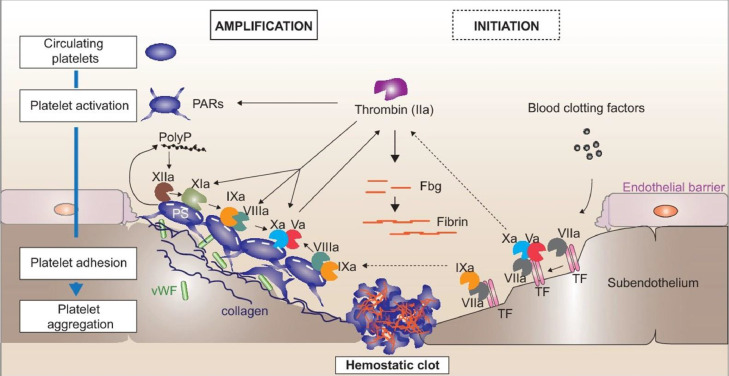
Hemostasis is a normal physiologic process by which the body forms an initial platelet plug at the site of vessel injury through complex interactions between the vessel wall, platelets, and adhesive proteins. It is a rapid, localized, and carefully regulated response to limit blood loss following vascular injury.3 As described in Fig. 1 the initiation and amplification of the coagulation cascade is a complex and detailed process.4 The endothelial cells lining the vascular wall serve as an antithrombotic barrier with negatively charged glycosaminoglycans, coagulation inhibitors, and activators of fibrinolysis. Thrombogenic proteins such as collagen, Von Willebrand factor, and thrombospondin, which are involved in platelet adhesion, are subsequently located within the subendothelial layer. This delicate balance between thrombogenic and anti-thrombogenic factors disrupts as endothelial injury, exposing subendothelial elements such as collagen, recruiting circulating platelets to the site of injury. Simultaneously, tissue factor initiates blood coagulation by generating thrombin and fibrin. Both processes lead to platelet adherence, activation, and secretion. Thrombosis, due to excessive quantities of thrombin formation, can occur when pathologic processes overwhelm the regulatory mechanisms of hemostasis.5 , 6 Table 1 describes selected thrombogenic and anti-thrombogenic components that occur naturally in the body.5 Under normal physiologic conditions, the coagulation process is naturally inhibited, but this delicate equilibrium can become interrupted, leading to thrombus formation and propagation when procoagulant activity is increased or antithrombotic activity is decreased.5
Fig. 1.
Initiation and amplification of the coagulation cascade. Initiation (right): upon endothelial damage, subendothelial tissue factor (TF) is exposed to the bloodstreamand binds factor VII, which is activated to factor VIIa. The TF:VIIa complex enables subsequent activation of factor X. Activated factor Xa interacts with factor Va to form the “prothrombinase” complex on the surface of TF bearing cells and converts prothrombin into thrombin. Amplification (right): small amounts of thrombin activate platelets via protease-activated receptors (PARs) and will activate the factor XIa-IXa feedback loop on the surface (PS = phosphatidylserine) of adherent/aggregated platelets. Factor IXa with factor VIIIa forms the “tenase” complex which will then activate additional factor X. Simultaneously, the trace amounts of thrombin will activate factors Xia, VIII(cofactor to factor IX) and V (cofactor to factor X), which dramatically enhances the catalytic activity of factors IX and X. Finally, thrombin (factor IIa) activation leads to fibrin formation within the hemostatic plug which can further be stabilized by thrombin activated factor XIII (not shown). In parallel, local polyphosphate (polyP) release by activated platelets stimulates the contact pathway of coagulation by activation of factor XII. Fig. reproduced with permission from Gaertner, F. and Massberg, S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol. 2016.
Table 1.
Thrombogenic and anti-thrombogenic components in the body.
| Site | Thrombogenic | Antithrombogenic |
| Vessel wall | Subendothelium | Endothelium |
| Heparin | ||
| Tissue Factor | Thrombomodulin | |
| Collagen | Tissue plasminogen activator | |
| Circulating elements | Platelets | Antithrombin |
| Platelet activating factor | Protein C and S | |
| Clotting factor | Plasminogen | |
| Prothrombin | ||
| Fibrinogen | ||
| Von Willebrand Factor | ||
*Adapted from Palta et al Indian J Anaesth. 2014;58(5):515-523.
Inflammatory response and cytokine storm
The ability for viral infections to trigger massive cytokine responses, leading to dysregulation of the immune system is not a new phenomenon. Experiences with Influenza H1N1 and SARS-CoV-1 revealed that the surge of cytokines that are released in response to these infections cause endothelial damage and alteration of microvascular permeability; this results in systemic coagulopathy and multi-organ failure.7 There have been three highly pathogenic strains of human coronavirus (CoV) that have been identified (SARS-CoV, MERS-CoV, and SARS-CoV-2), each very similar in their genome composition, transmission, and clinical manifestations.8 Cytokine release syndrome (CRS), or cytokine storm, is an acute systemic inflammatory response that ensure due to a variety of factors such as immunomodulatory drugs, haploidentical donor stem cell transplantation, and graft-versus-host disease. Clinically, fever, multiple organ dysfunction and a CRS-like syndrome characterize CRS that may develop due to a severe viral infection, including COVID-19.9
Cytokines are a diverse group of small proteins secreted by cells for the purpose of intercellular signaling and communication, leading to control of cell proliferation and differentiation, regulation of angiogenesis, and immune and inflammatory responses.10 Major cytokines associated with cytokine storm include interferons, interleukins, chemokines, colony-stimulating factors, and tumor necrosis factor (Table 2 ). Inflammation associated with a cytokine storm begins locally with increased blood flow to enable vascular leukocytes and plasma proteins to reach extravascular sites of injury and then spreads throughout the body. These responses can cause tissue damage to the site of local response as tissue edema can subsequently increase extravascular pressures resulting in reduction in tissue perfusion. Anti-inflammatory cytokines aid in tissue and organ function restoration; however, severe inflammation damage can heal with fibrosis resulting in persistent organ damage or dysfunction.10
Table 2.
Major types and actions of cytokines.
| Structural group of cytokines | Role | Comments |
|---|---|---|
| Interferons | Regulation of innate immunity, activation of antiviral properties, anti-proliferative effects | –20 known interferons – Produced by many cells in response to infection – Type I: INFα, INFβ, – Type II: INFγ |
| Interleukins (IL) | Growth and differentiation of leukocytes | –35 known interleukins (IL 1- IL 35) – Produced by leukocytes to act on other leukocytes – Pro-inflammatory: IL-1α, IL-1β, IL-6, IL-8 (IL-6 is a key mediator of cytokine storm) – Anti Inflammatory: IL-4, IL-10, IL-13 |
| Chemokines | Control of chemotaxis, leukocyte recruitment; many are proinflammatory | |
| Tumor necrosis factor (TNF) | Proinflammatory, activates cytotoxic T lymphocytes, Immune cell activation, differentiation, growth, and cell death | –19 known Tumor necrosis factors – Produced by mast cells, macrophages, and T cells – TNFα- major pro-inflammatory cytokines; involved in potent activation of cytotoxic T cells during infection and inflammation; prominent role in cytokine storm |
| Colony-stimulating factors | Stimulation of hematopoietic progenitor cell proliferation and differentiation | - M-CSF: Monocyte Colony-stimulating factor - GM-CSF: Granulocyte Macrophage Colony-stimulating factor (growth/differentiation of dendritic cells) - G-CSF: Granulocyte Colony-stimulating factor (growth/differentiation of neutrophils) |
*Adapted from Tisonick et al, Microbiol Mol Biol Rev. 2012;76(1):16-32.
The clinical presentation of cytokine storm can vary from mild flu-like symptoms, such as fever, fatigue, headache, rash, arthralgia, and myalgia, to a severe life-threatening systemic response. A severe inflammatory response may cause hypotension, shock, disseminated intravascular coagulation (DIC), coagulopathy, and multi-organ system failure, including neurotoxicity. Laboratory abnormalities may include low blood counts, elevated serum creatinine, transaminitis, and elevated C-reactive protein (CRP), and coagulation abnormalities.11
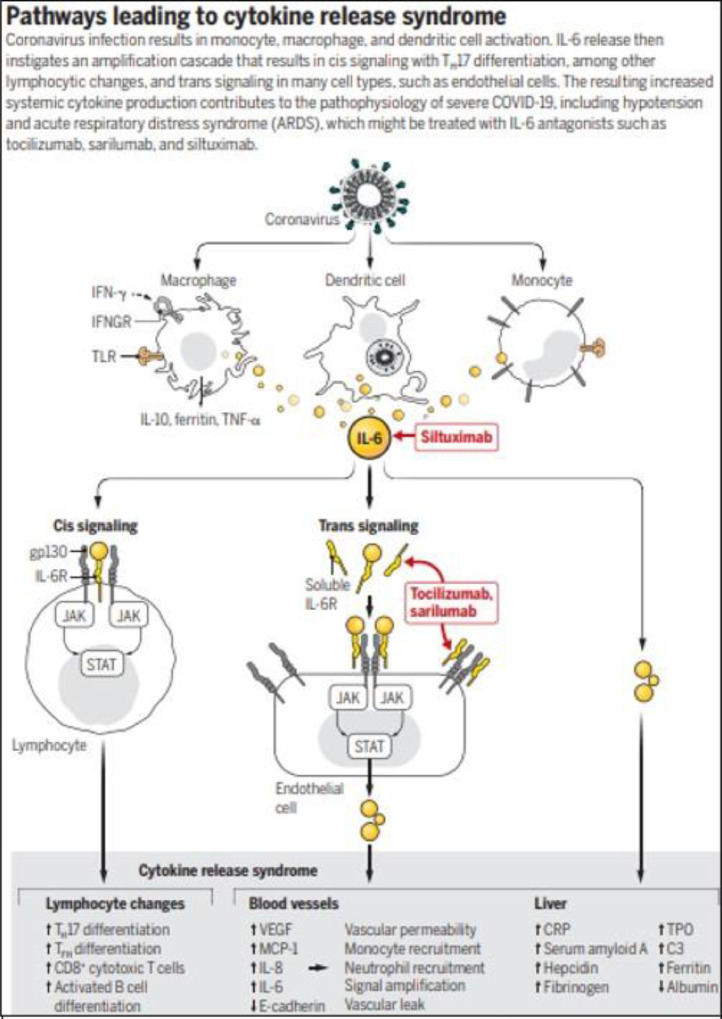
The pathophysiology of CRS and cytokine storm is incompletely understood. Recent reports have described the interplay between the overproduction of early response pro-inflammatory cytokines causing coagulopathy in patients diagnosed with COVID-19.12 One study described initial coagulopathy with prominent elevation of D-dimer and fibrinogen-degradation products in association with COVID-19.13 Furthermore, upon infection by SARS-CoV-2, there is subsequent monocyte, macrophage and dendritic cell activation and release of IL-6 that results in increased systemic cytokine production as described in Fig. 2 . IL-6 also drives up serum CRP levels, leading to a prominent pro-inflammatory process with downstream signaling contributing to CRS.14
Fig. 2.
Pathways leading to cytokine release syndrome. Fig. reproduced with permission from Moore, J. and June, C., 2020. Cytokine Release Syndrome In Severe COVID-19.
Finally, an early retrospective study from Wuhan analyzed patients with confirmed severe COVID-19 pneumonia baseline coagulation parameters during hospitalization.15 They found that non-survivors had significantly higher D-dimer and fibrin degradation product (FDP) levels, prolonged prothrombin time (PT) compared to survivors on admission, and fibrinogen and anti-thrombin (AT) levels were also significantly lower in non-survivors when compared to survivors. Development of DIC was associated with a poor prognosis as 71.4% of non-survivors and 0.6% survivors met the criteria of DIC during their hospital stay.
Overview of thrombosis
In normal physiologic conditions, the endothelium serves to protect against intravascular thrombosis. Sepsis is a well-established cause of DIC and can result in activation of intravascular coagulation. Fibrin deposition leads to platelet and coagulation factor consumption and fibrinolysis resulting in impaired coagulation and bleeding complications. Additionally, monocytes and endothelial cell activation causes cytokine release following vascular injury with expression of tissue factor and secretion of von Willebrand factor and thus initiating the extrinsic pathway of coagulation.16
This circulation of free thrombin, uncontrolled from all physiologic inhibitors, is then able to convert fibrinogen into fibrin, activate platelets, as well as activate factors V, VIII, and XIII.16 In a feedback loop, thrombin complexes with thrombomodulin to form a thrombin:thrombomodulin complex, activating protein C (with cofactor protein S), irreversibly inactivating Factor Va and Factor VIIIa, which serve as highly procoagulant cofactors in the generation of thrombin. The fibrinolytic system is also activated via endothelial release of tissue plasminogen activator (tPA) via catalyzing the conversion of plasminogen to plasmin. Plasmin is then free to degrade factors V, VIII, XIII, developing fibrin clots, and fibrinogen, thus generating fibrin degradation products (FDPs). The simultaneous activation of these multiple pathways allows for both bleeding and thrombosis, particularly in the microcirculation. Inappropriate deposition of fibrin and platelets in the microcirculation may cause organ ischemia.
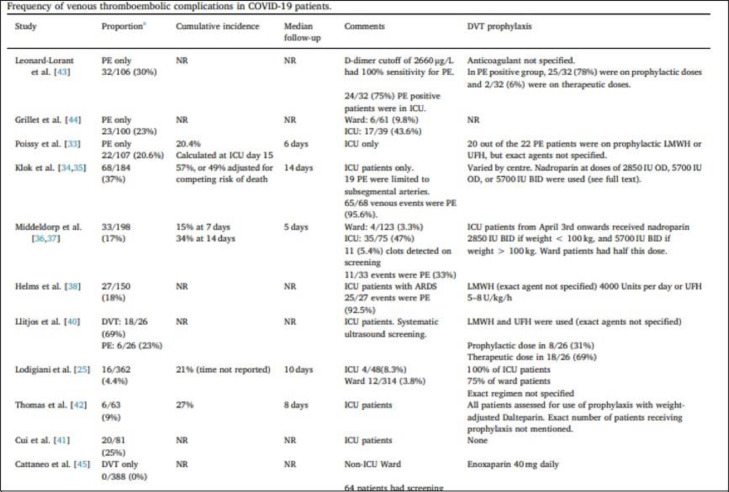
Thrombosis is associated with poorer patient outcomes and can be a frequent complication of infection. Infection-associated thrombosis may produce a stronger inflammation-mediated component caused by the presence of the bacterial or viral pathogen when compared to other etiologies of thrombosis, which then triggers activation of platelets and damage to the endothelium.17 The risk of thrombosis increases between 2-20 times with a systemic or localized infection with infection being an independent risk factor for thromboembolic diseases such as deep vein thrombosis (DVT) and pulmonary embolism (PE) as well as cardiovascular and cerebrovascular events.18 , 19 A review by Al-Ani et al. described the frequency of venous thromboembolic complications in COVID-19 patients as early data demonstrated the possibility of increased incidence of venous thrombosis, particularly in those who were critically ill (Fig. 3 ).20 The frequency of DVT and PE was significantly higher in severely ill patients admitted to the intensive care unit (ICU), compared to patients admitted to the general floor, despite the use of prophylactic anticoagulation (either at standard or higher doses). This review also explored cerebrovascular disease in patients with COVID-19 who presented with acute ischemic stroke and identified a reported overall frequency of stroke in approximately 3% of patients. Oxley et al. reported five cases in New York City hospitals of new-onset large-vessel ischemic stroke in patients younger than 50 years old and all five patients were subsequently diagnosed with SARS-CoV-2 infection.21
Fig. 3.
Frequency of venous thromboembolic complications in COVID 19 patients. Reproduced with permission from Al-Ani F, Chehade S, Lazo-Langner A. Thromb Res. 2020;192:152-160.
SARS-CoV-2 pathogenesis in the nervous system
Neurologic complications resulting from COVID-19 are recognized and the pathogenesis is multifaceted. Animal models of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) have demonstrated how coronaviruses can have direct effects on various cell receptors leading to hyperinflammation or CRS that causes edema and tissue hypoxia to produce a prothrombotic state as discussed previously. Furthermore, data suggests that SARS-CoV-2 has an ability to migrate through axonal and trans-neural transport, as well as migration from lungs to the circulatory system.22 , 23
The spike (S) protein on the viral surface mediates infection by SARS-CoV-2. When the S protein attaches to its receptor, the angiotensin-converting enzyme 2 (ACE2), S protein-ACE2 receptor complex is endocytosed into the cell where fusion of viral membranes and host membranes occur in the endosomal compartment.24 ACE2 receptors commonly found on lung endothelium, reflect common respiratory symptoms reported by those infected by coronavirus. However, ACE2 receptors are also present in the kidney, liver, small intestine, heart, blood vessels, as well as in the brain endothelium as a potential infectious access to the circulatory and nervous system.25 The ubiquity of the ACE2 receptor can help explain the profound systemic inflammation and multi-organ failure seen in severe cases of COVID-19.
Fusion of the viral envelope to the host membrane can then generate a massive inflammatory response with release of cytokines including IL-6, IL-12, IL-15, and tumor necrosis factor alpha (TNF-a) as described previously. The inflammatory process can generate lung alveolar damage, which can result in severe hypoxia and further cause cerebral vasodilation, cerebral edema, and ischemia contributing to the wide variety of neurologic symptoms noted in patients presenting with COVID-19. Additionally, as ACE2 receptor complexes are endocytosed, this causes a downregulation of ACE2 receptors, leading to uninhibited ACE1 receptor function resulting in downstream vasoconstriction, salt retention, worsening hypertension that further potentiated cardiovascular risk in patients with pre-existing risk factors. Early in 2020, the use of ACE inhibitors or angiotensin receptor blockers in patients with cardiovascular risk factors did show improvements in all-cause mortality and that patients should continue ACE inhibitor or angiotensin II receptor blocker therapy if diagnosed with COVID-19.26
Mao et al. published a small case series that showed significant neurologic complications in 36.4% of 21 hospitalized patients in Wuhan, China with COVID-19.27 In this study, common neurologic complications included headache, dizziness, impaired consciousness, ataxia, acute cerebrovascular disease, epilepsy, hypogeusia, hyposmia, hypopsia, neuralgia and myalgias. Severe cases of COVID-19 were associated with increased risk of neurologic complications.27 – 28, 29 Finally, due to prevalence of anosmia and hypogeusia, it is postulated that SARS-CoV-2 may enter the nervous system through the olfactory nerves in the cribiform plate, as has been previously demonstrated in animal models for SARS-CoV and MERS-CoV. Previous experiments utilized transgenic mice overexpressing the human ACE2 receptor and upon exposure to intranasal SARS-CoV, they developed severe respiratory failure. Following infection of olfactory bulb, these mice developed rapid infection through transneuronal process causing infection or death of neurons, notably those in the medullary cardiorespiratory centers even in the absence of encephalitis.30 This phenomenon seen in animal models could explain the asymptomatic severe hypoxemic respiratory failure seen in severe COVID-19 cases with severely low oxygen levels in patients presenting with minimal symptoms.31
Pre-existing neurologic conditions and SARS-CoV-2
The prevailing theory for the development of multi-organ failure in COVID-19 begins with profound systemic inflammation. Patients with pre-existing neurologic conditions or receiving chronic immunosuppression due to neurologic conditions may present with more severe COVID-19 symptoms but overall survival is similar to the general population. Gao et al. performed a meta-analysis of studies evaluating immunosuppressed patients (N = 4,007) and showed a 3.25-fold increased risk of severe COVID-19 disease in immunosuppressed patients though a statistical difference was not reached.32 Patients with immunodeficiency (N=3,231) had 1.55-fold increased risk of severe disease though not statistically significant and no correlation was found between immunodeficiency and risk of death in COVID-19 patients. Therefore, patients that are immunosuppressed can be more likely to develop severe COVID-19 disease, but they do not appear to have a worse mortality according to this study. The upcoming sections will review selected COVID-19 cases in patients with pre-existing neurological conditions.
Multiple cclerosis (MS)
There have been several studies addressing COVID-19 in the MS patient population. Sormani et al. presented preliminary results with 232 patients from 38 MS centers in Italy.33 Fifty-seven patients had laboratory confirmed COVID-19, 175 had suspected COVID-19 symptoms without a positive test. Ninety-six percent of patients (N=169) were classified with mild COVID-19 while 5% (N=6) were classified as severe or critical. Safavi et al. reported a cross-sectional survey of 712 patients with relapsing-remitting MS from a tertiary care center in Iran and 34 patients met criteria for a COVID-19 suspect group (ever and cough or fever and shortness of breath, or a presumptive diagnosis based on suggestive chest computed tomography).34 Thirty-two of the 34 COVID-19 suspect patients were receiving a disease modifying treatment: rituximab (N=21, 61.8%), fingolimod (N=5, 14.73%), interferons (B=3, 8.8%), dimethyl fumarate (N=2, 5.9%), or teriflunomide (N=1, 2.9%),. The authors found no statistical difference in rates of SARS-CoV-2 infection amongst the groups but commented that there may be a higher risk of infection seen in patients receiving B-cell depleting antibodies.
Parkinson's disease (PD)
Observational studies from the TriNetX COVID-19 research network of more than fifty million patients, of which about 80,000 positive for SARS-CoV-2, 694 patients also had Parkinson's Disease.35 Case fatality rates were calculated and the PD patients had a21.3% mortality rate (p<0.001. Additionally, higher fatality rates were seen in men vs women, blacks vs whites, and elderly vs younger patients, a similar trend seen in other observational studies.
SARS-CoV-2 and central nervous system (CNS) complications
There are several known CNS complications reported with COVID-19. Alteration of consciousness is often a presenting symptom of COVID-19 that can be a direct result due to cerebral edema or ischemia, toxic encephalopathy, or a consequence of hyper-inflammation. Patients with altered consciousness have a more severe COVID-19 disease course; early studies demonstrated altered consciousness was associated with 14.8% of patients suffered severe disease as compared to 2.4% in non-severe patient cases.27 A study done by Chen et al. noted changes in consciousness occurred as the initial presenting symptom in 22% of fatal COVID-19 cases in Wuhan as compared to 1% of non-fatal cases.36 Later retrospective studies from the US highlight variability of neurologic presentations. Pinna et al. described 50 patients admitted to a neurology service out of 650 hospitalized patients with COVID-19 and estimated a 7.7% prevalence of neurologic manifestations. Notably, this population was more ethnically diverse and characterized a higher cerebrovascular incidence than the previous studies in Wuhan, China.28 Furthermore, another larger retrospective US study by Liotta et al. examined 509 patients, with 134 (26.3%) having severe disease requiring mechanical ventilation.29 Results confirmed similar distribution of symptoms, with neurologic symptoms on presentation found in 215 (42.2%) patients, during hospitalization in 319 (62.7%) patients, and at any time course of disease in 419 (82.3%) patients. Development of neurologic symptoms was associated with increased severity of COVID-19 and younger age. Change in mental status was an independent risk factor for 30 day mortality (OR 0.22; 95% CI 0.11-0.42; P= <0.001).
Hypogeusia and hyposmia
Patients frequently report hypogeusia (alteration of taste) and hyposmia (alteration of smell) when initially diagnosed with COVID-19.37 Mao et al. described a 214-patient cohort from Wuhan, China with COVID-19 pneumonia and hypogeusia was reported in 5.6% of patients and hyposmia noted in 5.1% on presentation.27 A European study by Lechien et al. examined 417 COVID-19 patients and showed that 85.6% described dysfunctions in smell and 88% with dysfunctions in taste on presentation. In 11.8% of cases, hypogeusia and hyposmia where their first presenting symptoms of COVID-19. Early olfactory recovery was seen in 44% and these findings were independent of sinus congestion or obstruction.37
Acute cerebrovascular disease
Initial studies in COVID-19 began to illustrate the potential for increased stroke incidence and several countries issued guidelines underscoring the importance for early recognition and treatment. Ischemic strokes are more common than hemorrhagic strokes in the setting of COVID-19. Several risk factors have been identified from retrospective studies: coagulopathy, thrombocytopenia, and arterial hypertension.38 Known risk factors for ischemic stroke include hypertension, diabetes mellitus, hyperlipidemia, and smoking status.
Several Chinese case series list incidences of acute cerebrovascular disease around 2-11% in COVID-19 patients. Mao et al. reviewed 214 patients, of whom six had acute cerebrovascular disease (2.4%) with correlation toward older age and pre-existing cardiovascular risks such as hypertension.27 Li et al. assessed 221 patients and showed a 5% incidence of ischemic stroke with risk factors of older age (mean age 71 vs 52), female sex, and vascular risk factors including hypertension, diabetes, and prior cerebrovascular disease.31 Stroke patients also had higher CRP (mean 51 mg/L), higher D-dimer (mean 6.9 mg/L) and low white blood cell count than non-stroke patients, which reflects the procoagulant state caused by COVID-19. Five patients died following stroke diagnosis which led to a 38% overall mortality. Aggarawal et al. performed a metanalysis of six Chinese studies and showed the incidence of acute cerebrovascular disease to be 1-6% and there was a non-statistically significant 2.5-fold increase risk of developing severe COVID-19 in those with stroke than those without.39
Oxley et al. described five case reports of severe stroke (mean NIHSS = 17) with large vessel obstruction (LVO) in patients under fifty years old in New York City hospitals. All these patients initially presented with stroke as their presenting symptom for COVID-19.21 Increase in diagnosis of large vessel disease was confirmed in a prospective cohort analysis of 328 patients in a comprehensive stroke center in New Jersey; the authors noted that while incidence of stroke decreased, disease severity at presentation increased.40 This cohort had a median age of 69 with 136 females (42%), 53 patients (16% of cohort) presented during the COVID-19 period. Patients who presented shared similar age, sex, race, vascular risk factors and stroke severity, however new daily stroke diagnoses were lower during the COVID-19 period than pre-COVID-19 (median 1/day vs median 2/day, p=0.04), with more patients exhibiting cortical signs during COVID-19 than prior (68% vs 53%, p=0.04). There was also a 59% decrease in number of mean daily transfers from outside hospitals (p=<0.01). Patients presented during COVID-19 had higher prevalence of presenting with cortical signs, largely driven by increased prevalence of LVO (OR 2.22, 95%CI 1.19-4.15, p=0.01). Notably, the total monthly cases of large vessel occlusion remained stable even as the proportion of patients with LVO grew. It is unlikely that local incidence of stroke decreased during COVID-19 and the authors postulated that despite decrease in incidence of strokes diagnosed, patients with milder symptoms are seeking medical attention in smaller amounts. Similar findings were noted at a stroke center in Florida.41
Frontotemporal hypoperfusion, agitation/delirium
Helms et al. studied 58 ICU patients and noted 84% incidence of concurrent neurologic symptoms at admission. Neurologic symptoms reported included: agitation (69%), corticospinal tract signs (67%), delirium (65%) as assessed by Confusion Assessment Method for ICU (CAM-ICU), and hyperthermia (temp >38.5 C) (16%). Lumbar punctures were performed in seven patients, none of whom had positive cerebral spinal fluid (CSF) for SARS-CoV-2. Brain MRIs showed ischemic strokes in 3 of 13 (23%) patients with two having asymptomatic small ischemic strokes and one with likely pre-existing subacute stroke. Arterial spin labeling perfusion MRI showed bilateral frontotemporal hypoperfusion in eleven patients. Electroencephalography showed diffuse slowing consistent with encephalopathy in one of eight patients. Thirty-three percent of 45 survivors showed symptoms of dysexecutive symptom with alterations of attention, orientation, and poorly organized movements on command, suggesting frontal lobe involvement.42
Acute hemorrhagic necrotizing encephalopathy (AHNE)
One case report described a case of a 58-year-old woman who presented with three days of cough, fever and altered mental status subsequently diagnosed with COVID-19.43 Her CSF showed no bacterial growth, and was negative for herpes simplex 1-2 virus, varicella-zoster virus, and West Nile virus. SARS-CoV-2 could not be tested on CSF. Unenhanced CT of the head showed symmetric low attenuation within bilateral medial thalami with normal CT angiogram and CT venogram. Brain MRI showed hemorrhagic rim-enhancing lesions in bilateral thalami, medial temporal lobes, and subinsular regions. The authors concluded that the patient presented with acute hemorrhagic necrotizing encephalopathy, generally considered a parainfectious disease. AHNE is felt to be caused by a “cytokine storm” with elevation of proinflammatory cytokines leading to brain injury through alterations of the blood brain barrier by trypsin and matrix metalloprotease-9 without vessel wall disruption.
Meningoencephalitis/seizures
Overall presentation of COVID-19 with meningitis/seizures appears to be rare, despite the high neurotropic potential of SARS-CoV-2. Notable in published case reports, CSF RT-PCR was positive in very rare instances despite clinical diagnoses of encephalitis or seizures, suggesting encephalitis may be due to inflammatory injury rather than direct viral infection. Additionally, the use of CSF RT-PCR in diagnosis of COVID-19 has not been studied and may not be sensitive for detecting disease and further studies are warranted in this population.44 – 45, 46
SARS-CoV-2 and peripheral nervous system (PNS) complications
Guillain-barre syndrome (GBS)
There are two published case reports of GBS in the setting of COVID-19, characterized by rapid onset muscle weakness as a result of demyelination of the PNS by the immune system usually as a response to infection, less commonly by surgery or vaccination. Symptoms typically start peripherally in the feet or hands and progress to the rest of the body in the course of hours to weeks. The first case of GBS associated with COVID-19 was reported in a 61-year-old patient who presented with bilateral lower extremity weakness and severe fatigue. On examination, they had symmetric weakness and areflexia of both legs and feet (MRC 4/5) which progressed over three days (MRC 3/5). Lumbar puncture revealed normal values of CSF cell counts. Nerve conduction studies showed delayed distal latencies and absent F waves consistent with a demyelinating neuropathy. They were treated with intravenous immunoglobulin. On hospital day eight, nasal swabs were positive for SARS-COV-2 suggesting a para-infectious profile.47
Another study reported five patients with GBS from three hospitals in Northern Italy. In general, GBS occurred 5-10 days after onset of COVID-19 symptoms, which is the typical post-infectious timeline for GBS.48 Clinical neurophysiology was consistent with axonal-type GBS in three cases, demyelinating-type in two cases. Patients were treated with IVIG, two patients received two cycles. Similar incidence and treatments were seen in MERS outbreak in 2012.49
Miller fisher syndrome, polyneuritis cranialis
Miller Fisher syndrome is a rare, acquired nerve disorder considered a variant of GBS characterized by abnormal gait, paralysis of eye muscles and absence of tendon reflexes. Rare cases have been described in the context of COVID-19. One case reported two patients from Madrid, Spain presenting with PNS involvement.50 They described one 50-year-old patient who initially presented with two days of fever, vertical diplopia, perioral paresthesias, anosmia, ageusia, unsteadiness. On examination, they had a broad ataxic gait and global areflexia, right hypertropia in all fields of gaze, severe limitation of adduction and down-gaze movements of right eye, left eye nystagmus on left gaze consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy. CSF showed no cell counts, protein 80 mg/dL. CSR RT-PCR was negative for SARs-COV-2. Anti-ganglioside antibody GD1b-IgG and nasal SARS-CoV-2 were positive, thus, the patient was diagnosed with Miller Fisher syndrome and treated with IVIG. The second patient was 39 years old with acute onset diplopia. They presented with a history of diarrhea, fever, ageusia, fatigue and was positive for SARS-CoV-2 by nasal swab. Neuro-ophthalmic exam was consistent with bilateral abducens palsy. Neurologic examination revealed global areflexia without motor weakness. All CSF tests were negative, including SARS-CoV-2 PCR. CT brain was normal. The patient made a spontaneous recovery two weeks later of the initial presenting symptoms: diplopia, ageusia and areflexia. They were diagnosed with polyneuritis cranialis or incomplete Miller Fisher syndrome sine ataxia. Like GBS, cranial nerve involvement appears to be para-infectious, likely from immune mediated response to GD1B antibodies, and have a positive response to intravenous immunoglobulin.
Myelitis
There are limited available details of SARS-CoV-2 causing myelitis. One study reported a case of 66-year-old patient with fever and fatigue for two days.51 After a high fever (40 C) they developed acute lower extremity flaccid paralysis along with urinary and bowel incontinence. Physical examination revealed decreased motor strength in bilateral upper extremities (MRC 3/5) with normal bilateral upper extremity reflexes and bilateral flaccid paralysis in lower extremities (MRC 0/5) with bilateral hyporeflexia without pathologic reflexes. Sensation was intact in upper extremities, but globally impaired in lower extremities at the T10 level. CT brain showed basal ganglia and paraventricular lacunar infarcts. The authors postulated that a possible cytokine storm response to SARS-CoV-2 was diagnosed based on high fever, leukocytosis, elevated serum ferritin (>2000 ng/ml) and high levels of CRP, procalcitonin, and IL-2. Muscle strength improved with treatment in upper extremities (4/5) though minimal improvement in lower extremities (MRC 1/5). Infectious causes of myelitis, Mycoplasma pneumonia, chlamydia pneumonia, EBV, influenza A/B, parainfluenza virus, adenovirus, coxsackieviruses, cytomegalovirus, and respiratory syncytial virus were ruled out. Tuberculosis infection was negative. Muscle biopsy was not performed. The authors concluded that this myelitis was due to direct SARS-CoV-2 involvement given presence of ACE2 receptors on surface of membranes of spinal cord neurons.51
Myopathies
The diagnosis for myopathy is also rare in cases of COVID-19, though case series in China do note a significant incidence of myalgia's (36%) and elevated creatinine kinase (CK) levels (33%). However, these patients did not have full work up for myopathy with electromyography or muscle histopathology for full diagnosis of myopathy. There have been rare cases of rhabdomyolysis noted in the literature.
Jin et al. reported a 60-year-old patient with COVID-19 who developed severe symptomatic rhabdomyelitis while in the hospital. To note, admission CK levels were normal, as was renal and hepatic function. Myoglobin was >12000 ug/L (REF: <90 ug/L), CK was 11,842 U/L (REF: 22-198 U/L), LDH was 2,347 U/L (REF: 140-280 U/L), alanine aminotransferase was 111 U/L (REF: 0-35 U/L) and aspartate aminotransferase was 213 U/L (REF: 0-35 U/L). The patient was treated aggressively with intravenous fluids to prevent renal failure.52
Treatments of COVID-19 related coagulopathy and stroke
In a short period since the inception of the pandemic in December 2019, there have also been various treatments of said conditions, with the primary goal of such therapies ranging from primary prevention of infection to treatment, mitigation of progression to critical illness, and treatment of the critically ill, requiring intensive care. SARS-CoV, the virus causing SARS initially discovered in April 2003 affected 8,100 people worldwide resulting in approximately 775 deaths, with about 80% of the cases occurring in China. Below current published regimens to prevent, treat, or mitigate the morbidity and decrease the mortality of COVID-19 will be reviewed.
Pre-exposure prophylaxis (PrEP)
Presently, no viable interventions exist to prevent infection with SARS-CoV-2; however, there are a myriad of clinical trials in progress (Table 3 ). Peg-interferon Lambda-1a is being assessed for the prevention and treatment of COVID-19 infection by Johns Hopkins University Hospital. As well, multiple other universities are assessing the use of topical iodine intranasally as PrEP.
Table 3.
International Society of Thrombosis and Hemostasis (ISTH) Sepsis-Induced Coagulopathy (SIC) Scoring System.
| INR | ≤1.2 | 0 |
| >1.2-1.4 | 1 | |
| >1.4 | 2 | |
| Platelet Count cells x109/L | ≥150 | 0 |
| 100-150 | 1 | |
| <100 | 2 | |
| Total SOFA Score | 0 | 0 |
| 1 | 1 | |
| ≥2 | 2 |
*Adapted from Ding et al. Blood Coagulation & Fibrinolysis: 2018 (29) 6:551-558
Post-exposure prophylaxis (PEP)
Currently there are no viable agents that have been proven or recommended against the progression of infection after suspected or known exposure to someone already infected with COVID-19.
Treatment of hospitalized COVID-19 patients: pharmacology
Hydroxychloroquine and Azithromycin
The anti-malarial drug hydroxychloroquine (HCQ) was hypothesized to help treat COVID-19. Initial data early in the pandemic; of 3,737 patients screened, 3,119 patients received HCQ-azithromycin (AZ), 200mg PO TID and 500mg day 1 followed by 250mg daily for 5 days, respectively.53 The data published that this regimen decreased mortality and risk of transfer to the ICU, (Hazard ratio [HR], 0.18 0.11 to 0.27), a decreased risk of hospitalization ≥ 10 days (95% Confidence interval [CI], 0.38 0.27 to 0.54) and shorted duration of viral shedding (time to negative PCR: HR 1.29, 1.17 to 1.42). This data has been debunked and refuted as several flaws were assessed in this publication. In a statement from International Society of Antimicrobial Chemotherapy (ISAC), the publication “does not meet the Society's expected standard, especially relating to the lack of better explanations of the inclusion criteria and the triage of patients to ensure patient safety.”54 However, prior to this discovery, the FDA initially approved an emergency use authorization, which has since been revoked as several large center studies have found no overall benefit in the use of HCQ or the regimen of HCQ-AZ in the treatment of COVID-19.
Current clinical trials are ongoing assess the efficacy of HCQ in the treatment after exposure and as well the treatment of symptomatic COVID-19. Currently there is no data showing HCQ is beneficial in the treatment of COVID-19. The National Institute of Health (NIH) conducted The Outcomes Related to COVID-19 treated with hydroxychloroquine among In-patients with symptomatic Disease or the ORCHID study.55 The goal of the trial included to recruit 500 patients, and at the time of its cessation, the trial had recruited 470 patients, with the protocol of giving HCQ to patients 400mg twice a day on day one, followed by 200mg daily on days 2-5 with a total of five days treatment. This study was halted early when the drug was determined to not provide any significant benefit in preventing the progression of the disease in hospitalized patients.
Remdesivir
On October 22, 2020, the FDA approved remdesivir for use in adult and pediatric treatment of patient's symptomatic COVID-19 requiring hospitalization. Remdesivir was initially developed for treatment of the Ebola virus. Remdesivir is a nucleoside analogue prodrug, developed in the treatment of COVID-19. In October 2020, the ACTT-1 Trial, a double-blind, randomized, placebo-controlled trial of for the use of intravenous remdesivir in adults hospitalized with COVID-19 with evidence of lower respiratory tract involvement.56 The trial reported on 1062 patients who were randomized, with the treatment arm receiving remdesivir 200mg on day one, followed by 100mg daily for nine additional days vs placebo for ten days, with a primary outcome of the study of time to recovery, defined by discharge from the hospital or continued hospitalization without need for supplemental oxygen or ongoing medical care. Preliminary data showed those who received remdesivir had a median recovery time of 11 days (95% CI, 9 to 12), as compared with 15 days (95% CI, 13 to 19) in those who received placebo (rate ratio for recovery, 1.32; 95% CI, 1.12 to 1.55; P<0.001).
Another study by Spinner et al. published data comparing treatment of COVID-19 with remdesivir for five vs ten days compared with standard care on clinical status on day eleven after initiation of treatment. This was an open-label trial in hospitalized patients across 105 hospitals in the United States, Asia, and European hospitals being treated for moderate COVID-19 induced pneumonia, based in imaging showing pulmonary infiltrates and an oxygen saturation >94%. 596 patients were assessed in this trial, randomized in a 1:1:1 ratio to receive a 10-day course of remdesivir (n=197), a 5-day course of remdesivir (n=199), or standard care (n= 200), at a 100mg per day dose for the treatment regimen. With the conclusion of the study by day 11, patients in the 5-day remdesivir group were noted to have statistically meaningfully greater odds of an improved clinical status distribution compared to the patients who received standard care (odds ratio [OR], 1.65; 95% CI, 1.09-2.48; P = .02).57 To date, there are still a handful of other actively recruiting trials assessing the efficacy of remdesivir in the treatment of COVID-19 (Table 4).
Dexamethasone
In the RECOVERY TRIAL, the low dose dexamethasone arm, patients received 6mg daily for ten days or until discharged, whichever is shorter, orally and/or intravenously, assessing the primary outcome of 28-day mortality.58 2,104 patients were recruited to this arm and received dexamethasone and 4132 patients received usual care, with data showing a lower incidence of mortality (29.3% vs. 41.4%) in patients requiring mechanical ventilation and patients requiring oxygen support without mechanical ventilation (23.3% vs. 26.2%) thus showing promise.
Convalescent plasma
It is hypothesized that plasma obtained from recovered COVID-19 patients could be infused in symptomatic COVID-19 patients requiring hospitalization through the concept of passive antibody-based immunity. This requires the collected plasma to contain a high number of neutralizing antibodies and to be given upon the immediate onset of symptoms for maximum efficacy to prevent progression of current symptoms. The FDA has determined that for a donor to be eligible, specific criteria would have to be met including the following59:
-
iEvidence of COVID-19 documented by a laboratory test either by:
-
•A diagnostic test (e.g., nasopharyngeal swab) at the time of illnessOR
-
•A positive serological test for SARS-CoV-2 antibodies after recovery, if prior diagnostic testing was not performed at the time COVID-19 was suspected.
-
•
-
ii
Complete resolution of symptoms at least 14 days before the donation. A negative result for COVID-19 by a diagnostic test is not necessary to qualify the donor.
-
iii
Male donors, or female donors who have not been pregnant, or female donors who have been tested since their most recent pregnancy and results interpreted as negative for HLA antibodies.
-
iv
SARS-CoV-2 neutralizing antibody titers, if available
-
v
When measurement of neutralizing antibody titers is available, neutralizing antibody titers of at least 1:160. A titer of 1:80 may be considered acceptable if an alternative matched unit is not available.
An open-label trial in China reported on 103 patients to randomly receive standard treatment with or without convalescent plasma.60 Results indicated that although there was decreased time of viral RNA clearance at 72 hours based on nasopharyngeal swab versus standard treatment (87% vs 38%), there was no significant difference in clinical improvement (52% versus 43%, HR for improvement 1.4, 95% CI, 0.79 to 2.49) or mortality (16% versus 24%, OR 0.59, 95% CI, 0.22 to 1.59) by 28 days. According to the American Society of Hematology, the incidence of severe adverse events upon receipt of convalescent plasma is less than 1%, with the known general risks being allergic reactions, transfusion-associated circulatory overload, and transfusion-associated acute lung injury.61 Recommendations to use convalescent plasma are left to the discretion of the treating provider; as this treatment typically has a low incidence of complications, the potential benefits may outweigh the risks of its use. Finally, there are multiple international trials underway assessing the efficacy of convalescent plasma in the treatment of COVID-19 (Table 5).
Anti-inflammatory pathways therapy
Patients with COVID-19, especially those with severe cases with multi-organ failure, have been shown to have markedly elevated inflammatory markers (i.e., Ferritin, D-Dimer) and pro- inflammatory cytokines (i.e., IL-1, IL-2, IL-6), therefore it has been hypothesized that blocking these pro- inflammatory pathways will help mitigate progression of disease. Currently, there are multiple trials underway to evaluate this hypothesis (Table 6).
Several studies have analyzed the benefit of blocking the IL-6 pathway, with two humanized monoclonal antibodies: Tocilizumab and Sarilumab. Tocilizumab is an antagonist to the IL-6 receptor and Sarilumab is a direct inhibitor of IL-6. A retrospective cohort study assessed the benefit of tocilizumab in patients with severe COVID-19 admitted to tertiary care centers in Italy between February and April 2020.62 A total of 544 patients were included in the study; 57 (16%) of 365 patients in the standard care group needed mechanical ventilation, compared with 33 (18%) of 179 patients treated with tocilizumab (p=0.41; 16 (18%) of 88 patients treated intravenously and 17 (19% of 91 patients treated subcutaneously). Seventy-three (20%) patients in the standard care group died, compared with 13 (7%; p<0.0001) patients treated with tocilizumab (6 [7%] treated intravenously and 7 [8%] treated subcutaneously). Tocilizumab treatment was associated with a reduced risk of invasive mechanical ventilation or death (adjusted HR 0.61, 95% CI, 0.40 to 0.92; p=0.020). Twenty-four (13%) of 179 patients treated with tocilizumab were diagnosed with new infections, versus 14 (4%) of 365 patients treated with standard of care alone (p<0.0001). Of note, multiple trials have shown increased risk of secondary infections after the use of IL-6 modulators. Lastly, conflicting data published by Campochiaro et al. evaluated the safety and efficacy of patients given tocilizumab over a 28-day period. In this retrospective study, 65 patients were included, with 32 patients receiving tocilizumab. Mortality was 15% in the tocilizumab group versus 33% in standard treatment group (p = 0.15); bacterial or fungal infections were recorded in 13% of tocilizumab patients and in 12% of standard treatment patients.63
Additionally, further investigations are studying the effects of IL-1 blockade, a pro-inflammatory cytokine responsible for a variety of immunological responses noted to be elevated in COVID-19 patients. One anti-inflammatory compound, anakinra, and IL-1 receptor antagonist, currently approved for the treatment of rheumatoid arthritis. One case-control study compared outcomes in 52 consecutive patients with COVID-19 acute respiratory distress syndrome (ARDS) treated with anakinra and 44 historical controls from March 24 to April 6, 2020. Patients received the standard dose of 100mg subcutaneously daily for 7 days. The primary outcome of ICU admission for mechanical ventilation or death occurred among 13 case patients (25%) and 32 control patients (73%) (HR 0.22; 95% CI, 0.11 to 0.41). CRP levels decreased by day four among those receiving anakinra. Thromboembolic events occurred in ten patients (19%) who received anakinra and in five control patients (11%).64 While this data is promising in treatment of the CRS noted in COVID-19, more data and studies are needed to solidify the place of anakinra in the treatment of COVID-19.
IL-2, a cytokine that is responsible for T-cell differentiation, has also been hypothesized to be a potential target in COVID-19 treatment. Many patients infected with COVID-19 present with lymphopenia, often marked and progressive in the critically ill. In a publication by Shi et al. in June 2020, 54 patients were assessed and divided into three groups: common, severe, and critically ill based on their clinical condition. Each patient's peripheral blood mononuclear cells (PBMC) were analyzed by mass cytometry and cytokine levels were quantified. Results showed the sum of T cells, B cells, and NK cells was distinctly diminished in critical patients versus the normal controls, and the percentage of CD8+ T cells was also profoundly decreased in critically ill patients compared to the common and severe patients with COVID-19 pneumonia.65 Currently, there is an active phase II study assessing low dose IL-2 (LD-IL-2) vs placebo, with the primary objective to investigate the therapeutic benefit of LD-IL-2 as a T-reg inducer for controlling SARS-CoV-2-related ARDS. All patients will receive the experimental treatment daily for ten days after being randomized to two arms, LD-IL-2 versus placebo.66
Anticoagulation in the hospitalized COVID-19 patient
Patients with COVID-19 can suffer from a multitude of hematologic effects. Patients with COVID-19, especially the critically ill with ARDS, are presumably at higher risk when hospitalized for thrombotic complications. VTE is a significant concern for severe COVID-19 patients due to multiple factors: immobility, baseline hypercoagulable state due to sepsis and the cytokine release syndrome, and requirements of central venous catheters.
In July 2020, a retrospective multicenter study reported the risk of ischemic stroke in patients with COVID-19 requiring emergency department visits or hospitalizations from March 4, 2020, to May 2, 2020 versus patients with emergency department visits or hospitalizations due to influenza January 1, 2016, through May 31, 2018, in New York City, New York.67 There were 1,916 COVID-19 patients analyzed in the study and 31 (1.6%; 95% CI, 1.1%-2.3%) were diagnosed with an acute ischemic stroke; the primary reason for admission for 8 (26%) patients in this cohort was for ischemic stroke. However, in the influenza group, only 3 out of 1486 patients presenting with confirmed influenza symptoms were diagnosed with acute ischemic stroke (0.2%; 95% CI, 0.0%-0.6%). Therefore, it can be hypothesized that patients with COVID-19 may be at increased risk for stroke compared to influenza.
To mitigate the risk of stroke and/or VTE in high-risk patients (Table 3 ), multiple studies have been performed to assess the efficacy of anticoagulation, prophylactic or therapeutic, specifically in the hospitalized patient population. In 2017, a novel scoring system to help risk stratify patients with sepsis associated DIC labeled the Sepsis Induced Coagulopathy Score (SIC) (Table 7).68 , 69 Based on this scoring system, patients who were assessed with a score of 0-1 had an approximate 28-day mortality risk of 0%, a score of 2 with a 20% risk, a score of 3 with a 19% risk, a score of 4 with a 30% risk, a score of 5 with a 32% risk, and a score of 6 with a 46% risk. This scoring system to predict mortality risk has been validated by two other studies.70 , 71
Unfractionated heparin (UFH) and its derivatives
In March 2020, Lin et al found that treatment with low molecular weight heparin (LWMH) due to the rise in D-Dimer and inflammatory markers at days 7-14 showed a decrease in the mortality rate.72 The authors proposed that high-risk patients with a D-dimer greater than four times the upper limit of normal to recommended administration of LWMH 100U/kg twice a day for at least 3-5 days. The European Society of Cardiology in April 2020 published new recommended guidelines for hospitalized COVID-19 patients and proposed a standardized anticoagulation algorithm. In this publication, it was emphasized that patients with a high thrombotic risk, defined as those with dyspnea, respiratory rate > 24 breaths per minute, oxygen saturation < 90%, elevated CRP, rising D-dimer levels, and elevated fibrinogen levels, then higher level anticoagulation is warranted. In those with high thrombotic risk requiring ICU care, those patients are recommended to be managed on a heparin drip with the PE protocol with an aPTT goal of 60-85s, and in those patients not requiring ICU level care however still with high thrombotic risk or a D-dimer>3mcg/mL fibrinogen equivalent units, it was recommended LMWH 1mg/kg twice a day if renal function is normal. If those with a positive point of care ultrasound (POCUS), then they should continue treatment with therapeutic anticoagulation, and in those where the POCUS is negative, it is recommended to de-escalate to LWMH if eligible at 40mg twice a day.73
In October 2020, the American Society of Hematology (ASH) and the American College of Chest Physicians (CHEST) published preliminary guidelines for clinicians regarding COVID-19 and coagulopathy. ASH proposed in critically ill patients and acutely ill patients, hospitalized for COVID-19, the committee recommended prophylactic-intensity anticoagulation over intermediate-intensity or therapeutic-intensity anticoagulation in patients who do not have a suspected or confirmed venous thromboembolic event.74 CHEST recommended a in acutely ill patients, anticoagulant thromboprophylaxis is recommended with LWMH or fondaparinux over unfractionated heparin; the favoring of the former agents over UFH is decrease exposure time for staff. In critically ill patients, anticoagulant thromboprophylaxis with LMWH or UFH are recommended over fondaparinux and DOACs, with LMWH preferred still over UFH again to limit exposure. However, UFH is preferred over fondaparinux and DOACs because in the critically ill population, there is a high incidence and higher risk of acute kidney injury, hemodynamic instability, and drug-to-drug interactions. As well, mechanical thromboprophylaxis is still recommended if feasible and pharmacological thromboprophylaxis is contraindicated. CHEST however recommends against routine screening for asymptomatic DVTS with point of care ultrasounds, compared to The European Society of Cardiology.75
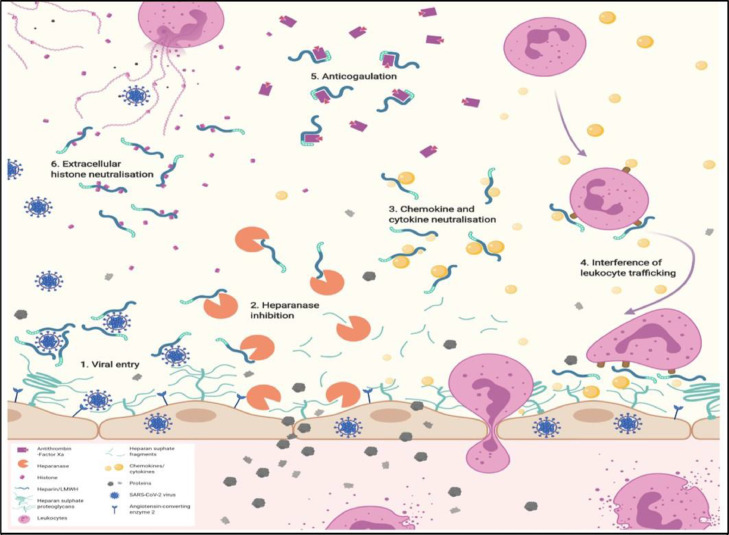
It has been hypothesized that UFH & LMWH play a large role inhibition of multiple factors that lead to ARDS and hypercoagulability. Specifically, in COVID-19, these compounds have been shown to inhibit heparinase (HPSE), an enzyme with the ability to degrade heparin sulfate in the endothelial glycocalyx (Fig. 4 ).76 During normal physiologic maintenance of the endothelium, the endothelial glycocalyx inhibits leakage of proteins by maintenance of a charge balance, as well as filtration via diameter dependent exclusion. One of the most prolific components of the glycocalyx, heparin sulfate, contributes to the greatest extent in the charge balance of the glycocalyx. HPSE is able to degrade this component; therefore, prolonged increased activity leads to degradation of the endothelial barrier, resulting in protein and fluid leak into the interstitial spaces and increased permeability in blood vessels, as seen with ARDS and advanced proteinuria.77 , 78 It has been established that UFH and LMWH have been proven to be effective inhibitors of HPSE.79
Fig. 4.
Summary of the potential beneficial mechanisms of heparin/low molecular weight heparin (LMWH) underlying treatment of COVID-19 patients. 1. Reducing viral entry. Heparan sulfate, and heparin/LMWH have been shown to interact with SARS-CoV-2 spike glycoprotein. 2. Inhibition of heparanase activity. Heparin/LMWH has been shown to inhibit heparanase activity, which is increased in COVID-19 and associated with disease severity. 3. Neutralization of the biological effect of chemokines, and cytokines. Heparin/LMWH interact with chemokines, and cytokines, including those produced in the ‘cytokine storm’ in COVID-19. 4. Interference with leukocyte trafficking. Heparin/LMWH neutralization of chemokine, and cytokines may impact on leukocyte recruitment and trafficking to sites of inflammation, either via neutralization of chemokine, and cytokines or through direct interaction with leukocyte cell surface ligands, i.e. selectins, and integrins, to prevent leukocyte attachment, and extravasation. 5. Anticoagulation. Heparin/LMWH promotes anticoagulation via anti-thrombin III binding. 6. Neutralization of extracellular cytotoxic histones. Heparin/LMWH act as a neutralizing compound for histones via ionic interactions of the negatively charged chemical groups with the positively charged extracellular histones released during COVID-19. (B. Buijsers et al. / EBioMedicine 59 (2020) 102969, Permission obtained from original author for distribution provided original work cited.)
Furthermore, UFH and its derivatives may possess anti-inflammatory characteristics.80 – 81, 82, 83 As stated previously, the endothelial glycocalyx provides a barrier to prevent protein leakage from the vessel membrane, however, it also moderates multiple inflammatory processes. The specific sulphation patterns of glycosaminoglycans (GAGs) in a healthy endothelial glycocalyx weakens binding of chemokines and leukocytes to the endothelial surface. Nevertheless, the configuration of GAGs is altered under the influence of inflammatory environments, which enables the binding of chemokines, selectins, and integrins displayed on the cell surface by leukocytes. Increased HPSE activity is implicated in the generation of a pro-inflammatory glycocalyx. Cells that encounter HPSE also display an amplified reaction to stimuli, such as pro-inflammatory cytokines and chemokines. COVID-19 is associated with the generation and release of inflammatory chemokines and cytokines, which include TNF-a, IFN-g, IL-6, and IL-8. One study by Mummery and Rider demonstrated, using an ELISA approach, recombinant human and murine IL-6 bind to an immobilized heparin-bovine serum albumin complex and at certain concentration's human IL-6 can be displaced by soluble heparin.84 This mechanism supports the use of heparin-based anticoagulation if there are no contraindications in the setting of a COVID-19 induced CRS. Furthermore, it has been demonstrated that UFH and LMWH inhibit IL-8 by competitively binding to heparin sulfate's cell surface receptor leading to the conclusion that UFH and its derivatives are effective in a myriad of ways in the treatment of COVID-19 induced coagulopathy.85
Tissue plasminogen activator (tPA)
There are currently no adequate studies or data to support the use of tPA in the use of COVID-19 associated ARDS or coagulopathy. However, there are several case reports published. A study by Wang et al. published three cases of patient's with COVID-19 associated ARDS were administered tPA. It has been hypothesized that in COVID-19 related ARDS, especially those admitted to the ICU, a microvascular occlusive phenomenon contributes to their distinctive form of respiratory failure.86 Of the three cases, all of them showed initial improvement in their partial pressure of oxygen/FiO2 (P/F) ratio, however two of the cases showed only transient improvement with subsequent increased requirements in oxygen support.
Direct oral anticoagulants (DOACs)
There are currently no sufficient data to support the use of DOACs in the treatment of COVID-19 coagulopathy. This class of drugs has been avoided thus far for the most part because of the concern for drug-to-drug interactions, including azoles, CYP3A4 Inhibitors, and antiplatelet therapies. However, this does not include the use of DOACs in the treatment of thromboses discovered during the treatment of those hospitalized with COVID-19 or in the outpatient setting. As with any patient on anticoagulation, bleeding risk and risk of ICH should be carefully assessed prior to implementation by the treating team.
Vaccination
The greatest hope in eradicating COVID-19, as with many viruses since the 20th century, is to create a vaccine that is efficacious is producing long term immunity. With previous viral outbreaks, including SARS and MERS, prior research helped pave the way to the production of multiple vaccines. Tozinameran, created by BioNTech and Pfizer, is the first vaccine approved for mass production and distribution in the United States and Canada and a myriad of other countries. The second approved vaccine for distribution in the US was mRNA-1273, created by the biotechnology company Moderna. The mechanism for both vaccines is the same, nucleoside-modified messenger RNA (modRNA) compound, which induces immunity to SARS-CoV-2 by encoding a prefusion stabilized spike (S) protein naturally present on the surface of SARS-CoV-2 particles. Three other vaccines are also approved for distribution in other countries, including China and Russia. In China, there are two approved vaccines for emergency use or full authorization, BBIBP-CorV and CoronaVac, produced by Sinopharm and Sinovac, respectively. The mechanism is the same for both vaccines, via chemically inactivated whole virus.
When comparing the vaccines by Pfizer and Moderna to the two produced by Sinopharm and Sinovac, there are pro and cons for both vaccines. Those produced by Pfizer and Moderna have data thus far that have shown a very high efficacy of greater than 90% in preventing infection. Both are also given in two doses, with the Pfizer a second dose is given 21 days the initial dose, whereas the Moderna vaccine requires a second dose at 28 days after the initial dose. However, the disadvantage of the Pfizer vaccine is the requirement of ultracold storage at 2 to 8°C (36 to 46°F) and transport, between −80 and −60°C (−112 and −76°F). This storage temperature is not feasible for developing countries, so while the Pfizer has the highest efficacy at greater than 95 percent, it would likely not be able to be distributed in unindustrialized developing countries. On August 23, 2021, the Food and Drug Administration (FDA) officially approved the Pfizer-BionTech vaccine for use in individuals sixteen years and older. Of note, no major side effects have been reported in the Pfizer or Moderna vaccines, while mild symptoms include temporary injection site swelling and pain, fevers, rigors, chills, fatigue, and myalgias were noted in both in both doses, with the symptoms at greater frequency after the second dose.
Following the development of subsequent variants, including the highly contagious Delta variant, there was concern whether the Pfizer and Moderna vaccines would have the same efficacy. After multiple studies, it was learned that most people with these two noted vaccines had waxing passive immunity with time. With the respect to the Pfizer vaccine, it was noted to have 79% and 83% efficacy against preventing asymptomatic and symptomatic disease, respectively, from the delta variant, compared to 92% efficacy in preventing asymptomatic and symptomatic disease due to the alpha variant, along with 95% efficacy in preventing hospitalization from the alpha variant versus 96% against the delta variant. As of August 2021, a third booster dose of the Pfizer vaccine has been offered in multiple countries. Israel required a third dose within six months of the second dose to maintain the “green pass” vaccine passport. In the United States, booster doses have been offered, especially to those immunocompromised, however there is still conflicting opinion on this as there has not been any evidence to show benefit in otherwise healthy individuals, citing while antibody-mediated immunity may diminish over time, cell-mediated immunity theoretically should remain.
In reference to the Moderna vaccine, efficacy has been noted to be as high as 90% and 94% in preventing symptomatic disease and hospitalization, respectively. However, as with the Pfizer vaccine, waxing antibody-mediated immunity was noted, and efficacy decreased to 66% against the delta variant. A booster has also been offered in multiple countries to those immunocompromised, however like the Pfizer vaccine, no sufficient data has been produced to support widespread booster administration.
BBIBP-CorV and CoronaVac have the advantage of practicality, as these two vaccines can be disseminated at normal refrigeration temperatures. The technology is also tried and true, used in many previous vaccines that have eradicated debilitating disease (i.e., polio, measles, mumps, rubella); inactivated virus technology has been well studied. The efficacy of the BBIBP-CorV vaccines is not as significant as those by Pfizer and Moderna, with preliminary data showing 86% efficacy against COVID-19 infection, however also a 99% sero-conversion rate of neutralizing antibodies, as well 100% success in preventing moderate and severe cases of the disease. CoronaVac has had more promising data compared to that of BBIBP-CorV. In June 2020, Zhang et al. published data showing seroconversion of neutralizing antibodies was seen for 109 (92%) of 118 participants in the 3 μg group, 117 (98%) of 119 in the 6 μg group, and two (3%) of 60 in the placebo group at day 14 after the days 0 and 14 schedule, whereas at day 28 after the days 0 and 28 schedule, seroconversion was seen in 114 (97%) of 117 in the 3 μg group, 118 (100%) of 118 in the 6 μg group, and none (0%) of 59 in the placebo group.87
In Russia, a vaccine produced by Gamaleya Institute has also been developed for distribution, Gam-COVID-Vac, which is a viral two-vector vaccine constructed from two human adenoviruses that encode for the spike protein of SARS-CoV-2. This vaccine uses the recombinant adenovirus type-26 (rAd26, component I) and adenovirus type-5 (rAd5, Component II); the rAd26 based vaccine is administered on the Day 1 and the rAd5 vaccine is administered on the Day 21 as a booster injection.88 The data for this vaccine's efficacy has been called into question, as the phase 1 trial only included 76 participants, however in an article published in Lancet February 2021, it was reported to have 92% efficacy against symptomatic disease.89 Currently the phase III data has not been completely published, therefore it has not been approved by the World Health Organization (WHO). In December 2020, the phase III included over 22,000 participants.90 There are many vaccines in clinical trials and awaiting approval (Table 8).
There have been two other viral vector vaccines derived from human adenovirus. The Ad26.COV2.S, produced by Janssen pharmaceuticals, and ChAdOx1 nCoV-19/AZD1222, produced by Oxford-AstraZeneca, contain replication-incompetent adenoviral vectors, human Ad26.COV2.S and chimpanzee ChAdOx1, respectively, that encode the spike glycoprotein on SARS-CoV-2. The Janssen vaccine has EUA approval in the United States and has a conditional approval with the European Union. With respect to efficacy, the Janssen vaccine, which is given in a single dose, is noted to have 66% and 85% efficacy against symptomatic disease and severe disease requiring hospitalization. The Oxford-AstraZeneca, also given in two doses, has been shown to have an efficacy of 81% against symptomatic disease with the alpha variant, however decreased to 61% with the delta variant.
Vaccine-induced immune thrombotic thrombocytopenia
One major side effect has been noted with both Janssen and Oxford-AstraZeneca vaccines.91, 92 Termed vaccine-induced immune thrombotic thrombocytopenia (VITT), the pathophysiology parallels that of heparin-induced thrombocytopenia (HIT), however this mechanism is independent of heparin and heparin-related products. VITT is caused by autoantibodies that target platelet factor 4 (PF4) attached to platelets, which bind to the FcγIIa receptors on IgG and subsequently activate the platelets and leading to thrombocytopenia and thromboses. It is believed that leakage of DNA from the adenovirus infected cells binds to platelet factor 4 (PF4) and triggers the production of autoantibodies. Characteristics of the VITT antibodies include IgG subclass, independent of heparin exposure. As well, these antibodies recognize PF4 and are detectable in PF4/polyanion and PF4 enzyme-linked immunosorbent assay (ELISA) and in functional assays, which leads to platelet activation. The epidemiology and possible risk factors are still being assessed; however, early data is showing most patients assessed have been on average greater than 30 years old. In a case series by Greinacher et al.93 Of 11 patients diagnosed with VITT, the median age was noted to be 36 years old (range 22-49 years). Range of presentation after vaccination was 5-16 days after vaccination with the AstraZeneca vaccine in Germany and Austria. In a series by Schultz et al.94, five patients who presented with venous thrombosis and thrombocytopenia 7 to 10 days after the AstraZeneca vaccine. The range of age of the patients were health care workers who were 32 to 54 years of age. Presenting symptoms included visual changes, abdominal pain, nausea and vomiting, chest pain, back pain, shortness of breath and increased oxygen requirement, leg pain and/or extremity swelling, unexplained petechiae, bleeding, or bruising, severe/acute headache, changes in speech, thrombosis, and unilateral weakness. The time of presentation has been identified in most cases as 5-30 days after vaccination. The most common sites of thrombosis included cerebral venous thrombosis (CVST), splanchnic vein thrombosis (including mesenteric vein, portal vein, splenic vein, hepatic vein), adrenal vein thrombosis, pulmonary embolism, deep venous thrombosis, and acute arterial thrombosis. Per the American Society of Hematology, the diagnostic criteria include:95
-
1
COVID vaccine (Janssen or AstraZeneca only to date) 4 to 30 days previously
-
2
Venous or arterial thrombosis (often cerebral or abdominal)
-
3
Thrombocytopenia
-
4
Positive PF4 HIT antibody testing via Enzyme-linked immunosorbent assay (ELISA)
A positive PF4 Elisa is the most reliable confirmatory test thus far. It is to be noted, per most articles published with cases of VITT, the rapid HIT immunoassays are most often negative, therefore should not be used in the diagnosis of patients suspected for VITT and no recent heparin exposure. Currently there are no consensus guidelines in treatment of this syndrome, as it is novel, and the incidence is still being determined.96, 97 Treatment recommendations suggest the use of IVIG 1 g/kg daily for two days, and non-heparin anticoagulation. Because of the unknown effect of heparin on exacerbation of this syndrome and the similarity to HIT, currently the consensus is avoidance of the use of heparin. Corticosteroids have been administered along with IVIG in some cases, however currently there is no consensus on the need for this concurrent therapy, standardized dosage, and whether it adds to recover. Lastly, plasma exchange (PLEX) is controversial as different expert groups have conflicting stances on its efficacy and utility. The American Society of Hematology advises against PLEX. “The large extravascular volume of distribution of IgG antibodies, causative in both HIT and TTS, prevents rapid or complete removal via PE, and the concurrent bleeding complications in TTS may make catheter placement and prolonged apheresis challenging.” The International Society of Thrombosis and Hemostasis (ISTH) however recommended PLEX in cases of severe or refractory thrombocytopenia or recurrent thrombosis.94, 98
Conclusion
In this rapidly evolving setting, increasing research and clinical discovery is being published regarding SARS-CoV-2 and COVID-19. Potential complications of COVID-19 are increasingly being understood including potential ways to prevent them. The neurological complications of COVID-19 can be minor such as losing sense of taste or smell or fatal (acute ischemic stroke) and early recognition of signs and symptoms in patients are essential. Many patients who become infected with COVID-19 may have neurologic sequelae. Acute cerebral vascular disease, specifically acute ischemic stroke, reflects this hypercoagulable state which has been recognized with patients at the largest risk having severe illness and other co-morbidities that increase the risk of stroke. True virus encephalitis is rare but can occur and results in altered mental status and further testing is warranted. Furthermore, more post-infectious causes are being recognized like potential Guillain-Barré syndrome or acute disseminated myelitis. Further study for potential neurologic complications in patients in the post infectious period are warranted in this population. Vaccines against COVID-19 have also had complications regarding thrombosis and thrombocytopenia in multiple reports and we are unsure of any neurological sequelae at this time.
Footnotes
Grant support: none
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2021.106163.
Appendix. Supplementary materials
References
- 1.Tennant M, McGeachie JK. Blood vessel structure and function: a brief update on recent advances. Aust N Z J Surg. 1990;60(10):747–753. doi: 10.1111/j.1445-2197.1990.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 3.Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50(4):326–336. doi: 10.3109/10409238.2015.1050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol. 2016;28(6):561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–523. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 7.Cantan B, Luyt CE. Martin-Loeches I. Influenza infections and emergent viral infections in intensive care unit. Semin Respir Crit Care Med. 2019;40(4):488–497. doi: 10.1055/s-0039-1693497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YY, Li BR, Ning BT. The comparative immunological characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 coronavirus infections. Front Immunol. 2020;11:2033. doi: 10.3389/fimmu.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 15.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchens CS. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC) Hematology Am Soc Hematol Educ Program. 2009:240–246. doi: 10.1182/asheducation-2009.1.240. [DOI] [PubMed] [Google Scholar]
- 17.Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, et al. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol. 2019;10:2569. doi: 10.3389/fimmu.2019.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeeth L, Cook C, Thomas S, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 19.Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129(13):1387–1396. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 20.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020;27(9):1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinna P, Grewal P, Hall JP, et al. Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415 doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e93–e95. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021 doi: 10.1002/ana.26028. [published online ahead of printJan 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Schultz JL, Aldridge GM, Simmering JE, Narayanan NS. Coronavirus disease 2019 case fatality and Parkinson's disease. Mov Disord. 2020;35(11):1914–1915. doi: 10.1002/mds.28325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 40.Siegler JE, Heslin ME, Thau L, et al. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strasser S, Miskolczi L, Cunha J, Justynski L. COVID-19 impact on stroke presentations. J Stroke Cerebrovasc Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karimi N, Sharifi Razavi A, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3) [Google Scholar]
- 45.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behavior and Immunity. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Shen D, Zhou H, et al. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? The Lancet Neurology. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 51.Zhao K, Huang J, Dai D, et al. Acute myelitis after SARS-CoV2 infection: a Case report. medRxiv Prep. 2020 doi: 10.1101/2020.03.16.20035105. [DOI] [Google Scholar]
- 52.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Media MTC. International Society of Antimicrobial Chemotherapy. April 3, 2020. Statement on IJAA paper. www.isac.worldPublishedhttps://www.isac.world/news-and-publications/official-isac-statement. [Google Scholar]
- 55.Kiley J. NIH halts clinical trial of hydroxychloroquine. National Institutes of Health (NIH). Published June 20, 2020. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine
- 56.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collaborative Group RECOVERY, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - preliminary report. N Engl J Med. 2020 NEJMoa2021436. [Google Scholar]
- 59.Center for Biologics Evaluation and Research . U.S. Food and Drug Administration; 2020. Investigational COVID-19 Convalescent Plasma - Emergency INDs. PublishedAccessed October 25, 2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma#Collection%20of%20COVID-19. [Google Scholar]
- 60.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.COVID-19 and Convalescent Plasma. Accessed October 25, 2020. https://www.hematology.org/covid-19/covid-19-and-convalescent-plasma
- 62.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi H, Wang W, Yin J, et al. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. 2020;11(6):429. doi: 10.1038/s41419-020-2636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Low Dose of IL-2 In Acute Respiratory Distress Syndrome Related to COVID-19 - Accessed October 25, 2020. https://clinicaltrials.gov/ct2/show/NCT04357444
- 67.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1–7. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and international society on thrombosis and haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29(6):551–558. doi: 10.1097/MBC.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamakawa K, Yoshimura J, Ito T, et al. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb Haemost. 2019;119(2):203–212. doi: 10.1055/s-0038-1676610. [DOI] [PubMed] [Google Scholar]
- 71.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):260–261. doi: 10.1093/ehjcvp/pvaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: chest guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buijsers B, Yanginlar C, Maciej-Hulme ML, et al. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226(4):562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 77.Garsen M, Rops AL, Rabelink TJ, Berden JH, van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transplant. 2014;29(1):49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 78.Bar-Ner M, Eldor A, Wasserman L, et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70(2):551–557. [PubMed] [Google Scholar]
- 79.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Mu S, Li X, Liang Y, Wang L, Ma X. Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. J Surg Res. 2019;238:175–185. doi: 10.1016/j.jss.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 82.Vasavada VA, Praveen MR, Shah SK, Trivedi RH, Vasavada AR. Anti-inflammatory effect of low-molecular-weight heparin in pediatric cataract surgery: a randomized clinical trial. Am J Ophthalmol. 2012;154(2):252–258. doi: 10.1016/j.ajo.2012.02.021. e4. [DOI] [PubMed] [Google Scholar]
- 83.Mummery RS, Rider CC. Characterization of the heparin-binding properties of IL-6. J Immunol. 2000;165(10):5671–5679. doi: 10.4049/jimmunol.165.10.5671. [DOI] [PubMed] [Google Scholar]
- 84.Frevert CW, Kinsella MG, Vathanaprida C, et al. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28(4):464–472. doi: 10.1165/rcmb.2002-0084OC. [DOI] [PubMed] [Google Scholar]
- 85.Ramdin L, Perks B, Sheron N, Shute JK. Regulation of interleukin-8 binding and function by heparin and alpha2-macroglobulin. Clin Exp Allergy. 1998;28(5):616–624. doi: 10.1046/j.1365-2222.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.https://clinicaltrials.gov/ct2/show/NCT04436471
- 89.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/s0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.The Sputnik V vaccine's efficacy is confirmed at 91.4% based on data analysis of the final control point of clinical trials. Sputnikvaccine.com. Published. 2020 https://sputnikvaccine.com/newsroom/pressreleases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-po Accessed December 22, 2020. [Google Scholar]
- 91.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. New England J Med. 2021;384(22):2092–2101. doi: 10.1056/nejmoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. New England Journal of Medicine. 2021;384(22):2124–2130. doi: 10.1056/nejmoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimabukuro T. Thrombosis with Thrombocytopenia Syndrome (TTS) Following Janssen COVID-19 Vaccine Advisory Committee on Immunization Practices (ACIP) CDC COVID-19 Vaccine Task Force Vaccine Safety Team. 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-04-23/03-COVID-Shimabukuro-508.pdf
- 94.Pavord D, Lester W, Makris M, Scully M, Hunt B. Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT). https://b-s-h.org.uk/media/19718/guidance-v20-20210528-002.pdf [DOI] [PMC free article] [PubMed]
- 95.Ana Catarina PINHO. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low platelets - European Medicines Agency. European Medicines Agency. April 6, 2021 https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood Published Accessed June 18, 2021. [Google Scholar]
- 96.Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. Centers for Disease Control and Prevention. Published 2021. Accessed June 18, 2021. https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html
- 97.Thrombosis with Thrombocytopenia Syndrome - Hematology.org. Hematology.org. Published 2021. Accessed June 18, 2021. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia
- 98.Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Following Adenovirus Vector COVID-19 Vaccination - Ontario COVID-19 Science Advisory Table. Ontario COVID-19 Science Advisory Table. Published May 10, 2021. Accessed June 18, 2021. https://covid19-sciencetable.ca/sciencebrief/vaccine-induced-immune-thrombotic-thrombocytopenia-vitt-following-adenovirus-vector-covid-19-vaccination/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.