Dear Editor,
The COVID-19 pandemic, characterized by severe acute respiratory syndrome (SARS), represents an enormous challenge for healthcare systems throughout the world. The SARS-CoV-2 virus mainly affects the respiratory system, and can give rise to acute respiratory distress syndrome (ARDS). Ninety-five percent of all patients admitted to intensive care have received ventilatory support – fundamentally invasive mechanical ventilation (IMV).1 The effectiveness of noninvasive ventilation (NIV) in influenza virus A (H1N1) pneumonia has been evidenced in small series,2 though not so in larger series3; as a result, its utilization has not been recommended.4 A further aspect to be taken into account is its reported failure in Middle East Respiratory Syndrome (MERS).5 On the other hand, NIV generates aerosols that would increase the viral transmission risk for healthcare professionals.6, 7 In view of the above, the recommendations referred to SARS-CoV-2 pneumonia are to not use NIV (or to do so only in selected cases), in favor of high-flow oxygen therapy (HFOT), and its utilization in ARDS is not advised.6, 7
We present our series of 27 patients admitted to the Department of Intensive Care Medicine (DICM) between March and May 2020. Twenty-one patients received NIV, 5 received IMV, and one patient received HFOT (IMV was not required). The noninvasive support measures comprised BiPAP V60® (Respironics Inc., Pennsylvania, USA), the Puritan Bennett® 980 critical care respirator (Covidien, Mansfield, USA), and the CPAP-Boussignac® device on one occasion (Vygon, Ecouen, France). The respirators were assembled with non-humidified tubing, and the expiratory port was protected with an antibacterial–antiviral filter.6 Upon admission of the patient to the DICM, support was started in continuous positive airway pressure (CPAP) mode (in the case of BiPAP V60® and CPAP-Boussignac®) or as pressure support ventilation (PSV) over positive end-expiratory pressure (PEEP) in the case of the critical care respirator. The CPAP/PEEP levels used were between 12.5 and 20 cmH2O. Noninvasive ventilation was applied on a continuous basis, except during hydration and hygiene. The FiO2 level was adjusted to secure a minimum SatcO2 of 94%. Once clinical-blood gas improvement was observed, allowing the reduction of FiO2/CPAP, weaning was started with HFOT. In the patients subjected to IMV, the ventilatory parameters were adjusted according to the evolution towards ARDS: tidal volume (V t) between 4 and 7 ml/kg, increased PEEP (>10 cmH2O) and FiO2 to achieve SatcO2 94%. Prone decubitus sessions were performed in situations of manifest hypoxemia.7
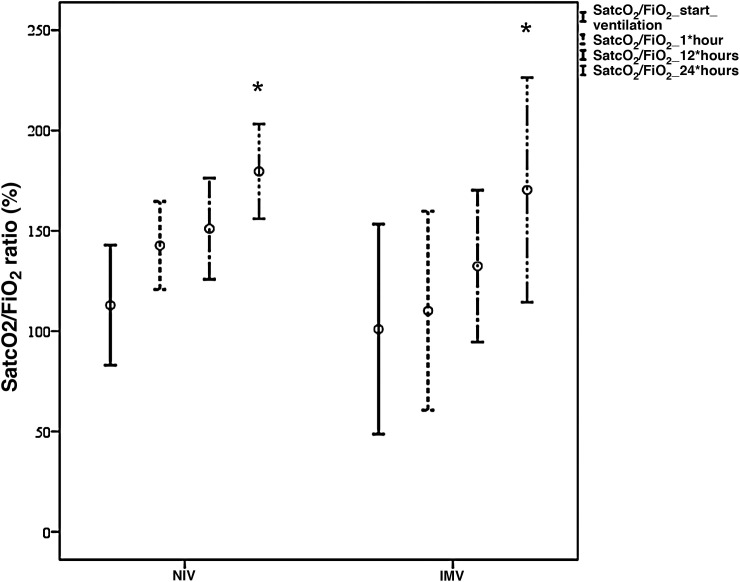
The study sample mainly consisted of males, with a mean age of 66 ± 14 years, arterial hypertension (63%), a mortality risk estimated by the Simplified Acute Physiological Score (SAPS) III of 50 ± 4 points (probability 18%), and an organ failure risk calculated by the Sequential Organ Failure Assessment (SOFA) score of 4 ± 1 points. The patients mostly came from the hospital ward (88.5%), where they mainly received conventional oxygen therapy (58%). Upon admission to the Intensive Care Unit (ICU), the main manifestations were severe hypoxemia accompanied by tachypnea, with no systemic repercussions. Comparison of the demographic parameters, comorbidities and clinical variables between the NIV and IMV groups only evidenced a higher SOFA score in the IMV group (6 ± 2 vs 4 ± 1 in the NIV group, p < 0.05). Both devices [Fig. 1 ] afforded significant improvement of the SatcO2/FiO2 ratio (p < 0.002) after 24 h, with no significant differences between them. As can be seen in Table 1 , the IMV group showed a greater incidence of complications, a longer duration of MV and of hospital stay, and a greater ICU and hospital mortality rate than the NIV group – though statistical significance was not reached. Failure of NIV was recorded in 48% of the patients, with more complications than in those cases where NIV proved successful, but with an incidence similar to that seen in the IMV group. Five healthcare professionals became infected with SARS-CoV-2, though none of them had assisted patients with NIV/CPAP.
Figure 1.
Comparative evolution of the SatcO2/FiO2 ratio between NIV (n = 15 patients) and IMV (n = 4 patients) during the first hours of ventilation.
*p < 0.002 SatcO2/FiO2 start ventilation versus 24 h of ventilation.
SatcO2, transcutaneous oxygen saturation; FiO2, fraction of inspired oxygen; NIV, noninvasive ventilation; IMV, invasive mechanical ventilation.
Table 1.
Analysis of global results and comparative analysis of noninvasive ventilation (success and failure) versus invasive mechanical ventilation.
| Global (n = 26) | NIV (n = 21) | NIV success (n = 11) | NIV failure (n = 10) | IMV (n = 5) | |
|---|---|---|---|---|---|
| SOFA 5th daya | 5 ± 3 | 5 ± 3 | 3 ± 1 | 7 ± 3e | 6 ± 2 |
| Pneumothorax, n (%) | 2 (8) | 2 (9.5) | 0 (0) | 2 (20) | 0 (0) |
| Cardiac arrest, n (%) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (20) |
| Tracheostomy, n (%) | 5 (19) | 4 (19) | 0 (0) | 4 (40)f | 1 (20) |
| Blood transfusion, n (%) | 5 (19) | 3 (14) | 0 (0) | 3 (30) | 2 (40) |
| Type of ARDS (%) | |||||
| Mild | 1 (4) | 1 (6) | 1 (9) | 0 (0) | 0 (0) |
| Moderate | 13 (50) | 12 (57) | 7 (63) | 5 (50) | 1 (20) |
| Severe | 12 (46) | 8 (38) | 3 (27) | 5 (50) | 4 (80) |
| Acute kidney injury, n (%) | |||||
| No AKI, n (%) | 20 (77) | 19 (90) | 11 (100) | 8 (80) | 1 (20)b |
| AKI 1, n (%) | 2 (8) | 1 (5) | 0 (0) | 1 (10) | 1 (20)b |
| AKI 2, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)b |
| AKI 3, n (%) | 4 (15) | 1 (5) | 0 (0) | 1 (10) | 3 (60)b |
| Ventilator-associated pneumonia, n (%) | 5 (19) | 4 (19) | 0 (0) | 4 (40)f | 1 (20) |
| Urinary tract infection, n (%) | 4 (15) | 4 (19) | 1 (9) | 3 (30) | 0 (0) |
| Bacteremia, n (%) | 4 (15) | 2 (9.5) | 0 (0) | 2 (20) | 2 (40) |
| Pressure ulcers, n (%) | 9 (33) | 7 (33) | 0 (0) | 7 (70)e | 2 (40) |
| Readmission to ICU,cn (%) | 2 (7) | 2 (9.5) | 2 (18) | 0 (0) | 0 (0) |
| Global duration MV,d days | 6 (4–13) | 5 (3–12) | 3 (1–5) | 10 (6–24)e | 10 (8–28) |
| ICU stay, days | 6 (5–14) | 7 (5–15) | 5 (4–6) | 16 (9–39)e | 14 (9–24) |
| Hospital stay, days | 23 (13–36) | 25 (15–44) | 26 (23–36) | 20 (10–55) | 19 (9–21) |
| ICU mortality, n (%) | 8 (31) | 5 (24) | 0 (0) | 5 (50)e | 3 (60) |
| Cause ICU mortality | |||||
| MOF | 4 | 3 | 0 | 3 | 1 |
| Cardiac arrest | 1 | 0 | 0 | 0 | 1 |
| Refractory hypoxemia | 1 | 1 | 0 | 1 | 0 |
| Shock | 2 | 1 | 0 | 1 | 1 |
| Hospital mortality, n (%) | 9 (35) | 6 (29) | 1 (9) | 5 (50) | 3 (60) |
Mean ± SD, the rest median and interquartile range 25–75.
p ≤ 0.01 NIV vs IMV.
Reason for readmission: peritonitis (n = 1), acute respiratory failure with admission to Department of Anesthesia (n = 1).
Sum of all time on NIV or IMV to complete suspension of ventilatory support.
p ≤ 0.01 NIV success vs NIV failure.
p ≤ 0.05 NIV success vs NIV failure.
NIV: noninvasive ventilation; IMV: invasive mechanical ventilation; SOFA: Sequential Organ Failure Assessment; ARDS: acute respiratory distress syndrome; AKI: acute kidney injury; ICU: Intensive Care Unit; MV: mechanical ventilation; MOF, multiorgan failure.
The experience gained in previous epidemics showed us that the patients presented hypoxemic acute respiratory failure without evident signs of labored breathing – this inclining us to use those devices (CPAP/NIV) affording high PEEP levels.2 We used NIV as first therapeutic choice in 80.8% of the patients – this percentage being far higher than in most published series, with figures between 0 and 61.9% (mean 11.3%).1 Invasive mechanical ventilation implied longer stay and was not without risks, since these patients had a higher incidence of nosocomial infections, advanced renal failure and pressure ulcers. The high mortality rate associated to IMV, together with the improved oxygenation evidenced with NIV, the reduction of intubation by 52%, and the fewer complications in the NIV group (though with more complications in the case of failed NIV), cause us to believe that there is an opportunity for the use of NIV in those patients in which direct IMV is initially contemplated.
The recommendation regarding the use of HFOT versus NIV6, 7 was fundamented upon a multicenter clinical trial comparing conventional oxygen therapy, NIV and HFOT. In the subgroup of patients with PaO2/FiO2 ≤ 200, the intubation and mortality rates were lower with HFOT (53%, 58% and 35%, respectively; p = 0.009, and 22%, 28% and 12%, respectively; p = 0.03) than with NIV.8 We observed that 13% (14/106) of the patients in the HFOT group were subjected to NIV, and that the NIV group received relatively low levels of PSV and PEEP (8 ± 3 cmH2O and 5 ± 1 cmH2O, respectively), and with short periods of use (median 8 h/day). Of note is the fact that the support levels recommended for reducing respiratory effort are higher (>15 cmH2O), and that it is advised not to suspend NIV in prolonged periods in order to avoid setback to the original stage.9 Likewise, we wish to point out that early weaning from NIV with conversion to HFOT may prove harmful, since two patients required intubation after using HFOT, probably due to lung derecruitment.10
The results obtained in our study question the recent recommendations regarding restriction of the use of NIV.6, 7 In conclusion, we have seen that in a considerable percentage of cases, the use of NIV in patients with SARS-CoV-2 pneumonia avoids intubation, as well as its complications. The technique should be applied by trained staff, with the adoption of correct safety and barrier measures, and with careful patient selection.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Footnotes
Please cite this article as: Belenguer Muncharaz A, Hernández-Garcés H, López-Chicote C, Ribes-García S, Ochagavía-Barbarín J, Zaragoza-Crespo R. Eficacia de la ventilación no invasiva en pacientes ingresados por neumonía por SARS-CoV-2 en una unidad de cuidados intensivos. Med Intensiva. 2021;45:e56–e58.
References
- 1.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emergency literature. Crit Care. 2020;24:285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belenguer-Muncharaz A., Reig-Valero R., Altaba-Tena S., Casero-Roig P., Ferrándiz-Sellés A. Utilización de la ventilación mecánica no invasiva en neumonía grave por virus H1N1. Med Intensiva. 2011;35:470–477. doi: 10.1016/j.medin.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez A., Ferri C., Martín-Loeches I., Díaz E., Masclans J.R., Gordo F., et al. Risk factors for noninvasive ventilation failure in critically ill subjects with confirmed influenza infection. Respir Care. 2017;62:1307–1315. doi: 10.4187/respcare.05481. [DOI] [PubMed] [Google Scholar]
- 4.Rochwerg B., Brochard L., Elliott M.W., Hess D., Hill N.S., Nava S., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 5.Alraddadi B.M., Qushmaq I., Al-Hameed F.M., Mandourah Y., Almekhlaf Gh, Jose J., et al. Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses. 2019;13:382–390. doi: 10.1111/irv.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinesi C., Peñuelas O., Luján M., Egea C., Masa J.F., García J., et al. Recomendaciones de consenso respecto al soporte respiratorio no invasivo en el paciente adulto con insuficiencia respiratoria aguda secundaria a infección por SARS-CoV-2. Med Intensiva. 2020 doi: 10.1016/j.medin.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballesteros Sanz M.A., Hernández-Tejedor A., Estella A., Jiménez Rivera J.J., González de Molina Ortiz F.J., Sandiumenge Camps A., et al. Recomendaciones de «hacer» y «no hacer» en el tratamiento de los pacientes críticos ante la pandemia por coronavirus causante de COVID-19 de los Grupos de Trabajo de la Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias (SEMICYUC) Med Intensiva. 2020;44:371–388. doi: 10.1016/j.medin.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frat J.P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., et al. FLORALI Study Group and the REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 9.Brochard L., Lefebvre J.C., Cordioli R.L., Akoumianaki E., Richard J.C. Noninvasive ventilation for patients with hypoxemic respiratory failure. Semin Respir Crit Care Med. 2014;35:492–500. doi: 10.1055/s-0034-1383863. [DOI] [PubMed] [Google Scholar]
- 10.Belenguer-Muncharaz A., Hernández-Garcés H. Fracaso de la ventilación no invasiva tras empleo de oxigenoterapia de alto flujo en pacientes con neumonía por SARS-CoV-2. Med Intensiva. 2020 doi: 10.1016/j.medin.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]