Abstract
Background & Aims
Coronavirus disease 2019 (COVID-19) pandemic lockdown and restrictions had significant disruption to patient care. We aimed to evaluate the impact of COVID-19 restrictions on hospitalizations of patients with alcoholic and nonalcoholic cirrhosis as well as alcoholic hepatitis (AH) in Alberta, Canada.
Methods
We used validated International Classification of Diseases (ICD-9 and ICD-10) coding algorithms to identify liver-related hospitalizations for nonalcoholic cirrhosis, alcoholic cirrhosis, and AH in the province of Alberta between March 2018 and September 2020. We used the provincial inpatient discharge and laboratory databases to identify our cohorts. We used elevated alanine aminotransferase or aspartate aminotransferase, elevated international normalized ratio, or bilirubin to identify AH patients. We compared COVID-19 restrictions (April–September 2020) with prior study periods. Joinpoint regression was used to evaluate the temporal trends among the 3 cohorts.
Results
We identified 2916 hospitalizations for nonalcoholic cirrhosis, 2318 hospitalizations for alcoholic cirrhosis, and 1408 AH hospitalizations during our study time. The in-hospital mortality rate was stable in relation to the pandemic for alcoholic cirrhosis and AH. However, nonalcoholic cirrhosis patients had lower in-hospital mortality rate after March 2020 (8.5% vs 11.5%; P = .033). There was a significant increase in average monthly admissions in the AH cohort (22.1/10,000 admissions during the pandemic vs 11.6/10,000 admissions before March 2020; P < .001).
Conclusions
Before and during COVID-19 monthly admission rates were stable for nonalcoholic and alcoholic cirrhosis; however, there was a significant increase in AH admissions. Because alcohol sales surged during the pandemic, future impact on alcoholic liver disease could be detrimental.
Keywords: COVID-19, Pandemic, Cirrhosis, Alcohol Related Liver Disease, Alcoholic Hepatitis
What You Need to Know.
Background
COVID-19 pandemic lockdown and restrictions had significant disruption to patient care. We do not know the impact of the pandemic-related restrictions on inpatient care among cirrhosis and alcoholic hepatitis patients.
Findings
Hospitalization rates among nonalcohol-related and alcohol-related cirrhosis maintained their increasing trend after March 2020. The alcoholic hepatitis hospitalizations rate had doubled after March 2020, with 9% average monthly increase.
Implications for patient care
The significant increase of alcohol sales and COVID-19 restrictions has led to increasing risk for alcoholic hepatitis. The COVID-19 pandemic might increase the burden of alcoholic liver disease in an alarming trend.
The coronavirus disease 2019 (COVID-19) pandemic has led to a global disruption in all healthcare systems. During the first wave in the spring of 2020, there were significant periods of lockdowns that led to postponing elective care or transitioning to virtual care.1 Such unforeseen and abrupt changes to service delivery methods have led to detrimental effects on patient care.2
Various studies have evaluated the impact that the early pandemic has had on chronic conditions.3, 4, 5, 6, 7 Although the hospitalization rates for patients with heart failure were significantly lower in the first few months after the start of the pandemic, the severity of heart failure among those patients who were admitted was worse.3 , 4 , 8 Similar trends were observed in other critical conditions.5, 6, 7 It was hypothesized that patients were more likely to stay at home rather than seeking medical care because of a “weathering-the-storm” mentality.3 , 4 , 6 Such behavior could lead to an increase in long-term morbidity and mortality.4
There was an observed decline of hospitalization rates among patients with cirrhosis during the month of March and in the first half of April in 2020.9 However, this very early decline in cirrhosis-related hospitalizations does not capture the totality of the detrimental effects of the pandemic on care disruption to patients with cirrhosis.10 There was a striking increase in alcohol sales worldwide that paralleled the start of the pandemic.11 , 12 Such an increase in alcohol sales would ultimately lead to a rising tide of alcohol-related liver disease.11
We aimed to evaluate the impact of COVID-19 restrictions during the first pandemic wave (March–September 2020) on hospitalization trends and health outcomes among patients with alcohol- and nonalcohol-related cirrhosis, as well as alcoholic hepatitis (AH).
Methods
Study Design and Patient Data Source
We conducted a retrospective population-based study to identify adults (≥18 years) with a diagnosis of alcohol-related cirrhosis, nonalcoholic cirrhosis, and AH in Alberta, Canada between March 2018 and September 2020. Alberta Health Services is responsible for providing all healthcare-related services including both inpatient and outpatient care to Albertans. Individual patients are identified by a unique personal health number that is used to track all healthcare utilization across multiple settings and datasets.
Three databases were used to identify potential cirrhosis and AH cases. The Physician Claims Database records claims submitted by Alberta physicians for services provided to patients who are registrants of the Alberta Health Care Insurance Plan, which is inclusive of 99% of Albertans.13 The inpatient Discharge Abstract Database contains demographics, diagnoses, procedures, intensive care unit (ICU) admissions, and mortality information on all discharges from hospitals in Alberta. Finally, we integrated the Alberta Provincial Laboratory database, which included general lab tests such as clinical chemistry (liver tests), hepatitis serology, and immunology tests.
Study Population
We used the International Classification of Diseases (ICD) Ninth Revision-Clinical Modification (ICD-9-CM) and ICD Tenth Revision (ICD-10) to identify our conditions of interest. Specifically, alcohol-related cirrhosis cases were identified if patient’s primary diagnosis for inpatient admission had the following validated codes (ICD-9-CM 571.2 and ICD-10 K70.3) or a decompensated cirrhosis validated diagnosis code (Supplementary Table 1).14 , 15 If the decompensated cirrhosis code was the primary diagnostic code, an alcohol-related cirrhosis code must be a secondary code for the admission to be categorized as alcohol-related cirrhosis. Similarly, an admission is classified as nonalcohol-related cirrhosis if the main diagnosis code was one of the following validated codes (ICD-9CM 571.5 and ICD-10 K71.7, K74.6) or if a decompensated cirrhosis diagnosis code was the main code with a secondary diagnosis code of nonalcohol-related cirrhosis.14 , 15 AH was identified if the admission main diagnosis code was ICD-9-CM 571.1 or ICD-10 K70.1 and had at least 3 of the following criteria: (1) an elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >50 IU/L, (2) AST:ALT ratio ≥2, (3) an elevated international normalized ratio (INR) ≥1.2, or (4) elevated total bilirubin >30 μmol/L. We used laboratory criteria to improve the validity of AH identification, because using AH diagnosis codes with decompensated cirrhosis codes alone has a poor positive predictive value (∼75%).16 The proposed AH algorithm was validated through a review of 100 medical records from patients who fulfilled the algorithm criteria. The proposed algorithm has a positive predictive value of 87% (95% confidence interval [CI], 81%–93%).
Outcomes and Covariates
The primary outcome was defined as the monthly hospitalization rate for alcohol-related cirrhosis, nonalcohol-related cirrhosis, and AH. Trends for each condition were analyzed by temporal relation to the COVID-19 pandemic (the pre–COVID-19 period was from March 2018 to February 2020, and the COVID-19 period was from March 2020 to September 2020), which was our main exposure variable. We also assessed secondary outcomes, which included predictors of ICU admissions and in-hospital mortality among each condition of interest in relation to our primary exposure. We evaluated being admitted during the COVID-19 era as a predictor of increased admissions to ICU and in-hospital mortality.
Other covariates included age, sex, patient residence (rural vs urban), history of chronic hepatitis B or C infection (defined on the basis of positive serology), the Charlson comorbidity index (excluding chronic liver disease), decompensated cirrhosis including ascites, hepatic encephalopathy, variceal bleeding, hepatorenal syndrome, spontaneous bacterial peritonitis, or hepatocellular carcinoma; and Model for End-Stage Liver Disease (MELD) score on admission.17 Maddrey discriminant function score (DF) was used to evaluate AH severity on admission.18 We assessed major laboratory investigations attributed to AH including ALT, AST, serum bilirubin, gamma-glutamyl transpeptidase, INR, and leukocytosis/white blood cell count that were conducted at the time of hospitalization.
Statistical Analysis
Patient characteristics for each condition of interest were summarized, stratified by admission time (before and during COVID-19). Chi-square and Kruskal-Wallis tests were used to identify statistical differences between the exposure groups at the time of hospitalization. Monthly admission rate of each condition of interest was determined per 10,000 hospitalizations in the province of Alberta. Temporal trends in alcohol-related cirrhosis, nonalcohol-related cirrhosis, and AH hospitalizations were evaluated by calculating the monthly percent change and implementing a generalized linear model that assumed a Poisson distribution. We evaluated the overall monthly hospitalization rate in Alberta (monthly hospitalizations/Alberta population) during the study time. A Joinpoint regression model was used to assess whether significant inflection points existed in the monthly temporal trends. Joinpoint analysis assumes the dependent variable counts follow a Poisson distribution (and variance). We used the admission month as the predictor variable and pre-specified the need for at least 5 data points between any 2 joinpoints.19 Adjusted multivariable logistic regression models for demographics and the comorbidity index were used to identify the independent predictors of ICU admission and in-hospital mortality in our cohorts. All estimates were presented as unadjusted/adjusted odds ratios (ORs/adjusted ORs) with 95% CIs. All analyses were performed by using SAS 9.4 (SAS Institute Inc, Cary, NC) and Joinpoint Regression Program 4.0.1 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD). We conducted sensitivity analysis exploring the study outcomes in a subgroup of AH defined cohort according to the definition of the National Institute of Alcohol Abuse and Alcoholism (NIAAA).20 The study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (REB18-1249). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Study Populations
Between March 2018 and September 2020, total admissions in Alberta included 2916 for nonalcohol-related cirrhosis, 2318 for alcohol-related cirrhosis, and 1408 for AH. Patient demographic and clinical characteristics in relation to the COVID-19 pandemic for each cohort are summarized in Table 1 . Among the nonalcohol-related cirrhosis cohort, the mean age at admission was 62.3 years (standard deviation [SD] ± 14.9 years), and 55.7% were men. Patient demographics, disease severity measured via MELD score, and comorbidities did not differ in relation to the pandemic. However, the mortality rate was lower among patients admitted during the COVID-19 pandemic, compared with patients admitted before the pandemic (8.5% [n = 53] vs 11.5% [n = 265], respectively; P = .033). For alcohol-related cirrhosis, the mean age at admission was 54.9 years (SD 11.8), and 64.5% were men. The only significant difference in relation to COVID-19 was that patients admitted during the pandemic had lower rates of previous or current hepatitis C virus infection (23.1% [115] vs 28.4% [518]; P = .019). The mortality rate was stable at 13.9% [n = 69] during pandemic vs 12.3% [n = 224] before pandemic; P = .347. Among the AH cohort, patients admitted after the start of COVID-19 were younger (mean age, 43.6 [12.0] vs 46 [12.2]; P < .001), were more likely to be living in rural areas (38.9% [160] vs 22.7% [225]; P < .001), had a lower prevalence of hepatitis C virus (8.6% [36] vs 13.2% [131]; P = .015), and have lower comorbidity rates (no comorbidity: 57.1% [238] vs 53.6% [531]; P = .022) compared with patients admitted before the pandemic. The DF score was similar in relation to the pandemic (median DF, 26.7 vs 24.4; P = .64). The inpatient mortality rate was stable at 6.2% (n = 26) during pandemic vs 7.7% (n = 76) before pandemic; P = .343. There were no significant differences in ICU admissions among the 3 cohorts (Table 1).
Table 1.
Patient Characteristics for the 3 Study Cohorts According to the COVID-19 Pandemic
| Variables | Nonalcohol-related cirrhosis |
Alcohol-related cirrhosis |
Alcoholic hepatitis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before COVID (n = 2295) | COVID (n = 621) | P value | Before COVID (n = 1821) | COVID (n = 497) | P value | Before COVID (n = 991) | COVID (n = 417) | P value | |
| Male sex, n (%) | 1274 (55.5) | 350 (56.4) | .71 | 1167 (64.1) | 327 (65.8) | .48 | 610 (61.6) | 273 (65.5) | .17 |
| Age at admission, y, mean (±SD) | 62.5 (14.9) | 61.5 (14.9) | .13 | 55 (11.8) | 54.5 (11.8) | .46 | 46 (12.2) | 43.6 (12) | <.01 |
| Rural residence, n (%) | 618 (26.9 | 151 (24.3) | .35 | 470 (25.8) | 141 (28.4) | .21 | 225 (22.7) | 160 (38.4) | <.01 |
| Hepatitis B infection, n (%) | 385 (16.8) | 101 (16.3) | .76 | 175 (9.6) | 48 (9.7) | .97 | 30 (3) | 8 (1.9) | .24 |
| Hepatitis C infection, n (%) | 755 (32.9) | 189 (30.4) | .24 | 518 (28.4) | 115 (23.1) | .02 | 131 (13.2) | 36 (8.6) | .02 |
| MELD score,a median (IQR) | 25 (21–30) n = 1981 | 25 (21–30) n = 478 | .32 | 28 (24-34) n = 1799 | 29 (24–36) n = 441 | .08 | 28 (22–34) n = 937 | 27 (22–33) n = 385 | .39 |
| Having decompensated cirrhosis, n (%) | 1926 (83.9) | 520 (83.7) | .91 | 1111 (61.0) | 279 (56.1) | .06 | 250 (25.2) | 100 (24.0) | .62 |
| Ascites | 761 (33.2) | 200 (32.2) | .65 | 719 (39.5) | 178 (35.8) | .15 | 167 (16.9) | 66 (15.8) | .64 |
| Hepatic decompensation | 725 (31.6) | 215 (34.6) | .15 | 203 (11.2) | 48 (9.7) | .37 | 35 (3.5) | 17 (4.1) | .62 |
| Esophageal/gastric varices | 378 (16.5) | 80 (12.9) | .03 | 292 (16.0) | 74 (14.9) | .58 | 63 (6.4) | 27 (6.5) | .93 |
| Hepatocellular carcinoma | 541 (23.6) | 143 (23.0) | .78 | 121 (6.6) | 29 (5.8) | .61 | 6 (0.6) | 1 (0.2) | .37 |
| Maddrey DF score, median (IQR) | 26.7 (7.5–54.0) | 24.4 (6.9–52.8) | .64 | ||||||
| Charlson comorbidity score, n (%) | |||||||||
| 0 | 1524 (66.4) | 406 (65.4) | .45 | 882 (48.4) | 263 (52.9) | .08 | 531 (53.6) | 238 (57.1) | .02 |
| 1 | 290 (12.6) | 89 (14.3) | 408 (22.4) | 118 (23.7) | 316 (31.9) | 121 (29) | |||
| 2 | 28 (1.2) | 4 (0.6) | 50 (2.7) | 10 (2) | 31 (3.1) | 24 (5.8) | |||
| ≥3 | 453 (19.7) | 122 (19.6) | 481 (26.4) | 106 (21.3) | 113 (11.4) | 34 (8.2) | |||
| Intensive care unit admission, n (%) | 189 (8.2) | 50 (8.1) | .88 | 230 (12.6) | 74 (14.9) | .19 | 111 (11.2) | 47 (11.3) | .97 |
| Inpatient mortality, n (%) | 265 (11.5) | 53 (8.5) | .03 | 224 (12.3) | 69 (13.9) | .35 | 76 (7.7) | 26 (6.2) | .34 |
COVID-19, coronavirus disease 2019; IQR, interquartile range; Maddrey DF, Maddrey discriminant function; MELD, Model for End-Stage Liver Disease; SD, standard deviation.
n = available patients for MELD calculation.
Among the 3 cohorts, whereas cirrhosis decompensation rates were stable in relation to the pandemic, patients with nonalcohol-related cirrhosis had lower rates of admission during the COVID-19 pandemic (12.9% vs 16.5%; P = .032).
Hospitalization Temporal Trends
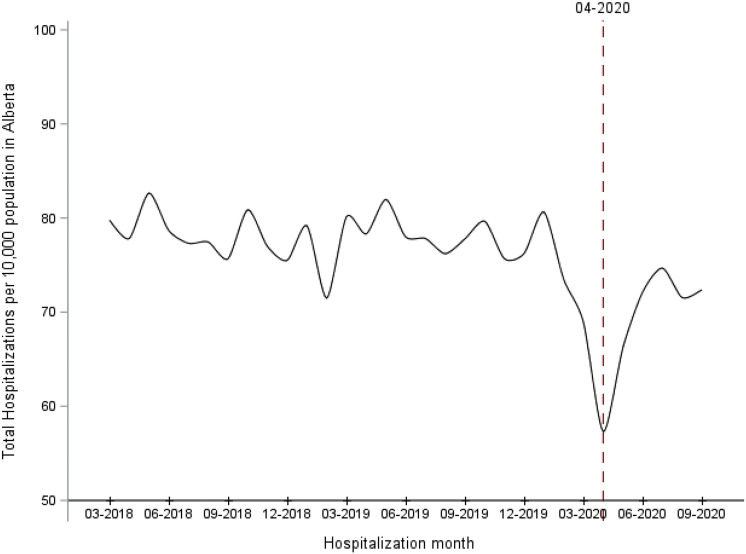
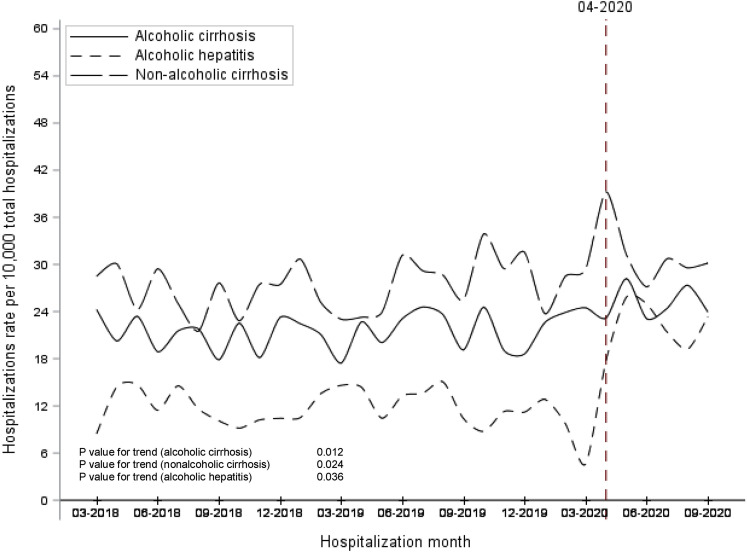
Over the study time, the overall monthly hospitalization rate in Alberta declined after the onset of the pandemic (monthly average, 77.8 hospitalizations/10,000 persons before COVID-19 vs 69.0 hospitalizations/10,000 persons after March 2020; P < .01; Figure 1 ). The average monthly hospitalization rate for each condition of interest increased significantly among the 3 cohorts, P < .05 (Figure 2 ). Furthermore, the pandemic had a significant effect on the hospitalization rates among the 3 cohorts. The average hospitalization rate during the pandemic was significantly higher compared with pre-pandemic among nonalcohol-related cirrhosis (average, 31.4 [SD 4.1]/10,000 admissions vs 27.2 [SD 3.2]/10,000 admissions; P = .013), alcohol-related cirrhosis (average, 25.0 [SD 2.2]/10,000 admissions vs 21.6 [SD 2.3]/10,000 admissions; P = .003), and almost doubled among AH patients (average, 22.1 [SD 3.2]/10,000 admissions vs 11.6 [SD 2.5]/10,000 admissions; P < .001).
Figure 1.
Monthly hospitalizations rate in Alberta between March 2018 and September 2020.
Figure 2.
Monthly hospitalizations rates for the 3 study cohorts between March 2018 and September 2020.
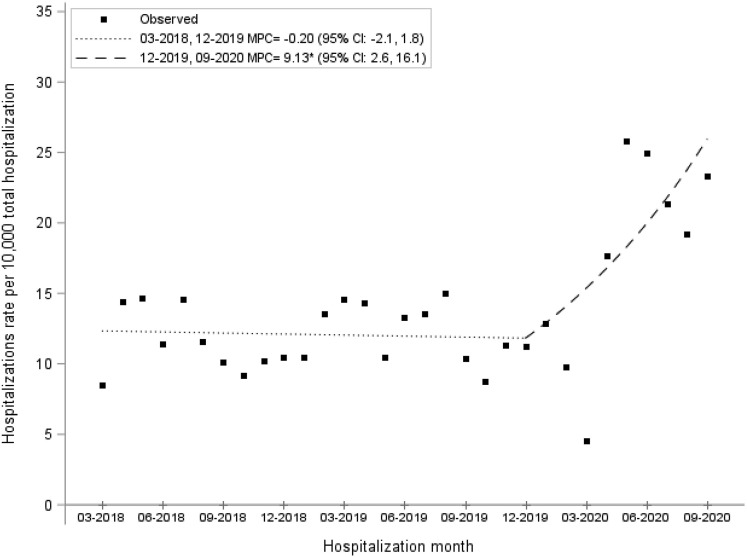
There was a monthly percent increase of 0.62% (95% CI, 0.12%–1.12%; P = .011) among the nonalcohol-related cirrhosis cohort during the study period. Similarly, there was a significant monthly percent increase in alcohol-related cirrhosis admissions during the study time of 0.59% (95% CI, 0.15%–1.03%; P = .017). Among the AH cohort, there was a stability of admissions before March 2020 (monthly percent change, –0.20%; 95% CI, –2.14% to 1.77%; P = .83). However, on Joinpoint regression, there was a significant increase in the monthly percentage increase of AH hospitalizations in March 2020 after the start of the pandemic (monthly percentage increase, 9.13%; 95% CI, 2.59%–16.09%; P < .01), coinciding with the start of the pandemic (Figure 3 ).
Figure 3.
Alcoholic hepatitis hospitalizations increased during the COVID-19 pandemic (infection point: March 2020).
Intensive Care Unit Admissions
The ICU admission rates remained stable among the 3 conditions of interest in relation to the pandemic. Specifically, before and during COVID-19 ICU admissions among hospitalized nonalcohol-related cirrhosis, alcohol-related cirrhosis, and AH were 8.2% vs 8.1% (P = .89), 12.6% vs 14.9% (P = .25), and 11.2% vs 11.3% (P = .97), respectively. Being admitted after the start of the pandemic was not a significant predictor of ICU admissions among nonalcohol-related cirrhosis (OR, 0.93; 95% CI, 0.65–1.33), alcohol-related cirrhosis (OR, 1.18; 95% CI, 0.87–1.59), and AH patients (OR, 0.98; 95% CI, 0.67–1.43).
In-Hospital Mortality
Although the in-hospital mortality rates pre-pandemic and during COVID-19 were similar for alcohol-related cirrhosis (12.3% vs 13.9%; P = .41) and AH (7.7% vs 6.2%; P = .38), the in-hospital mortality for nonalcohol-related cirrhosis rate was lower during pandemic (8.5% vs 11.5%; P = .048). In the univariate analysis, being admitted after the start of the pandemic was not a significant predictor of in-hospital mortality among alcohol-related cirrhosis (OR, 1.15; 95% CI, 0.86–1.54) and AH patients (OR, 0.84; 95% CI, 0.53–1.33). However, being admitted during the pandemic was significantly associated with lower in-hospital mortality among nonalcohol-related cirrhosis (OR, 0.73; 95% CI, 0.53–0.99). In the adjusted logistic regression models for age, sex, residence, history of hepatitis C virus and hepatitis B virus infection, and comorbidities, being admitted during pandemic was still associated with lower in-hospital mortality among nonalcohol-related cirrhosis cohort (adjusted OR, 0.68; 95% CI, 0.49–0.94).
Sensitivity Analysis
We identified that 770 AH admissions (539 pre-COVID-19 and 231 during COVID-19) fulfilled the laboratory criteria of NIAAA definition. These cohort characteristics in relation to the pandemic are presented in Supplementary Table 2. In this subgroup, AH is more severe (median DF, 44.9; interquartile range, 25.4–66.4) and had higher rates of decompensated cirrhosis (37.9%) compared with our AH cohort. There were no significant changes of ICU admissions or inpatient mortality in relation to the pandemic. Similar to the AH main cohort, admission rates were significantly higher during the pandemic (average, 13.3 [SD 2.9]/10,000 admissions vs 6.9 [SD 2.4]/10,000 admissions; P < .001), with a significant inflection point in March 2020.
Discussion
In this large population-based study, we evaluated the impact of the COVID-19 pandemic restrictions on hospitalization trends and health outcomes among patients with nonalcohol-related cirrhosis, alcohol-related cirrhosis, and AH. We demonstrated that the beginning of the pandemic was associated with a statistically significant inflection point, with drastically increasing AH hospitalizations in the subsequent months. There were also steadily increasing trends of hospitalizations among patients with alcohol-related and nonalcohol-related cirrhosis, although the pandemic did not alter the trajectory of these trends in our Canadian cohorts.
The hospitalizations rates, morbidity, and mortality of cirrhosis patients have been growing significantly over the last 2 decades.21 , 22 In fact, there were alarming projections regarding the increasing burden of both nonalcohol-related and alcohol-related cirrhosis before the start of the pandemic.23 , 24 In our cohort, the significant trends of cirrhosis patient hospitalizations observed during the pandemic are consistent with the trends before March 2020 and to previous literature. There are several possible explanations as to why we did not observe a decline in hospitalizations among cirrhosis patients, as was observed among other chronic diseases (eg, heart failure). These possible reasons include (1) during the first wave in Alberta, there was not a significant burden of COVID-19 cases on the healthcare system25 and (2) the hospitalization rates and ICU admissions in Alberta were not overwhelmed by the COVID-19 cases during the first wave.26 While implementing restrictions for providing elective and outpatient services, we did not observe increasing admissions to manage patients with decompensated cirrhosis during the first wave of the pandemic. In fact, among nonalcohol-related cirrhosis, there were fewer admissions to manage variceal bleeding.
The concerning increase of AH patients has been expected because of the effect of the pandemic on mental health and acute increases in habitual alcohol consumption.27 Multiple studies have warned of the effects of the pandemic and that the increase in alcohol sales could be critical for the health of the population.11 , 12 , 28 In our study, we report that the impact of increased alcohol consumption was substantial and was observed early in the course of the pandemic. Specifically, we observed a monthly hospitalization increase of ∼10% per month for AH. With applying rigorous definition of AH based on NIAAA criteria, we identified AH with severe disease likely as NIAAA criteria require total bilirubin ≥3.0 mg/dL and AST to ALT >1.5. Furthermore, the AH defined cohort based on NIAAA criteria was smaller because we only used laboratory investigations on admission to define our cohort. Some AH patients may have worse clinical course and laboratory investigations during the admission.
There were no significant differences between the characteristics of hospitalized cirrhosis patients admitted during the pandemic as compared with before the pandemic. However, patients with AH admitted during the pandemic were younger, had lower rates of comorbidities and a lower prevalence of hepatitis C, and were more likely to live in a rural area. This shift to more frequent cases in patients living in rural areas might reflect an increased impact of social isolation of the pandemic restrictions as compared with urban areas. These differences in demographics and clinical features of hospitalized AH patients suggest that alcohol use disorder and alcohol-related liver disease may expand to broader populations and differentially impact vulnerable populations. Because the association between alcohol-related liver disease and alcohol consumption is dose dependent, it is more urgent than ever before that we identify patients who are at risk for alcohol-related liver diseases and implement risk-reduction interventions.23 , 29
In our study, we evaluated decompensated cirrhosis, ICU admissions, and in-hospital mortality as indirect indicators for the severity of hospitalized patients. Among the 3 cohorts, the rates of decompensated cirrhosis and ICU admissions were similar in relation to the pandemic. Furthermore, we did not observe an increase in in-hospital mortality among patients with AH or alcohol-related cirrhosis. However, patients with nonalcohol-related cirrhosis had lower in-hospital mortality during the pandemic compared with patients who were admitted in the 2 years prior. This observed decline in mortality may be due to patients being admitted to manage early decompensated features or lower rate of variceal bleeding admissions during the pandemic.
Our study has several strengths, including a longitudinal population-based evaluation of hospitalizations before and during the pandemic. This allowed for the examination of temporal trends of admissions among each cohort of interest. We studied the hospitalization rates in a large province-wide healthcare system that is inclusive to almost all Albertans, ensuring reliable assessment of the impacts that the pandemic had on a single payer healthcare system. We used validated definitions to define alcohol-related and nonalcohol-related cirrhosis. For AH, using only ICD codes for case classification is associated with modest accuracy.16 Therefore, we integrated laboratory databases into our AH case definition, and this validated algorithm achieved a good accuracy with ≥85% positive predictive value. Using rigorous NIAAA criteria to define AH in our sensitivity analysis confirmed the significant increase in hospitalizations during the pandemic. One major strength of our study is that we evaluated the severity of disease through hepatic decompensation, MELD, and Maddery DF scores in our cohorts. Furthermore, extending our analysis to September 2020, we had sufficient time to observe the variations in hospitalizations among patients with end-stage liver disease or AH during the first wave of the pandemic.
Our study also has some limitations. First, we evaluated the hospitalization trends in Alberta, which had a low healthcare burden during the first COVID-19 wave, so these could differ significantly compared with other jurisdictions that had overwhelming COVID-19 hospitalizations and ICU admissions. Second, we only focused on the first wave of the pandemic. It is clear that the pandemic waves varied significantly across different healthcare systems, and the full impact of the pandemic on patient care is unknown.11 , 30 Studies examining the burden of the pandemic waves on the healthcare system with a wider scope are needed. These future studies should focus on the impact of the pandemic on outpatient and inpatient care among patients with chronic liver disease.
In conclusion, using 3 large population-based cohorts, we have shown the effects of the COVID-19 pandemic on hospitalizations of patients with AH, nonalcohol-related and alcohol-related cirrhosis. Our findings suggest that the burden of cirrhosis, particularly among patients with alcohol-related liver disease, might increase in the coming months. Importantly, there is an urgent need for interventions to decrease alcohol use at the population level and secondary prevention measures to reduce advanced liver fibrosis or cirrhosis among patients with high-risk alcohol consumption.
CRediT Authorship Contributions
Abdel-Aziz Shaheen, MBBCh, MPH, FRCPC (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Validation: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Kristine Kong (Formal analysis: Equal; Methodology: Equal; Validation: Equal)
Christopher Ma (Formal analysis: Equal; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Chelsea Doktorchik (Writing – original draft: Equal; Writing – review & editing: Equal)
Carla S. Coffin (Supervision: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Mark G. Swain (Methodology: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Kelly W. Burak (Methodology: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Stephen E. Congly (Methodology: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Samuel S. Lee (Methodology: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Matthew Sadler (Writing – original draft: Supporting; Writing – review & editing: Supporting)
Meredith Borman (Writing – original draft: Supporting; Writing – review & editing: Supporting)
Juan G. Abraldes (Conceptualization: Equal; Formal analysis: Equal; Methodology: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by a Canadian Institutes of Health Research (CIHR) Project Grant.
Note: To access the supplementary material accompanying this article, please click here.
Supplementary Material
Supplementary Table 1.
Validated International Classification of Diseases Diagnoses Codes for Decompensated Cirrhosis1, 2, 3
| Condition | ICD-9-CM codesa | ICD-10 codesa |
|---|---|---|
| Ascites | 789.5 | R18 |
| Spontaneous bacterial peritonitis | 567.2 | K65.2 |
| Hepatic encephalopathy | 572.2 | G93.4, K72.1, K72.9 |
| Esophageal or gastric varices | 456.0, 456.20 | I85.0, I85.9, I86.4, I98.20, I98.21 |
| Hepatorenal syndrome | 572.4 | K76.7 |
| Hepatocellular carcinoma | 155.0 | C22.0, C22.9 |
International Classification of Diseases (ICD) Ninth Revision-Clinical Modification [ICD-9-CM] and ICD Tenth Revision [ICD-10].
Supplementary Table 2.
Patient Characteristics for Alcoholic Hepatitis (NIAAA Definition) According to the COVID-19 Pandemic
| Variables | Alcoholic hepatitis |
||
|---|---|---|---|
| Before COVID (n = 539) | COVID (n = 231) | P value | |
| Male sex, n (%) | 312 (57.9) | 143 (61.9) | .30 |
| Age at admission, y, median (IQR) | 47 (39–55) | 43 (36–53) | .04 |
| Rural residence, n (%) | 110 (20.4) | 77 (33.3) | <.01 |
| Hepatitis B infection | 15 (2.8) | 5 (12.2) | .62 |
| Hepatitis C infection | 81 (15.0) | 24 (10.4) | .09 |
| Having decompensated cirrhosis | 207 (38.4) | 85 (36.8) | .67 |
| Ascites | 142 (26.4) | 60 (26.0) | .92 |
| Hepatic decompensation | 32 (5.9) | 15 (6.5) | .77 |
| Esophageal/gastric varices | 53 (9.8) | 20 (8.7) | .61 |
| Hepatocellular carcinoma | 5 (0.9) | 1 (0.4) | .47 |
| Maddrey DF score, median (IQR) | 44.2 (25.4–64.8) | 47.5 (25.4–70.9) | .50 |
| Charlson comorbidity score | |||
| 0 | 272 (50.5) | 123 (53.3) | .42 |
| 1 | 179 (33.2) | 74 (32.0) | |
| 2 | 17 (3.2) | 11 (4.8) | |
| ≥3 | 71 (13.2) | 23 (10.0) | |
| Intensive care unit admission | 71 (13.2) | 29 (12.6) | .82 |
| Inpatient mortality | 63 (11.7) | 26 (11.3) | .86 |
COVID-19, coronavirus disease 2019; IQR, interquartile range; Maddery DF, Maddery discriminant function.
References
- 1.Wu K., Smith C.R., Lembcke B.T., et al. Elective surgery during the Covid-19 pandemic. N Engl J Med. 2020;383:1787–1790. doi: 10.1056/NEJMclde2028735. [DOI] [PubMed] [Google Scholar]
- 2.Chudasama Y.V., Gillies C.L., Zaccardi F., et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14:965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson C., Gerds T., Fosbol E., et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007274. [DOI] [PubMed] [Google Scholar]
- 4.Hall M.E., Vaduganathan M., Khan M.S., et al. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail. 2020;26:462–463. doi: 10.1016/j.cardfail.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghaus T.M., Karschnia P., Haberl S., et al. Disproportionate decline in admissions for exacerbated COPD during the COVID-19 pandemic. Respir Med. 2020:106120. doi: 10.1016/j.rmed.2020.106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Filippo O., D’Ascenzo F., Angelini F., et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansagra A.P., Goyal M.S., Hamilton S., et al. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383:400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromage D.I., Cannata A., Rind I.A., et al. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22:978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmud N., Hubbard R.A., Kaplan D.E., et al. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology. 2020;159:1134–1136 e3. doi: 10.1053/j.gastro.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper E.B., Asrani S.K. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard M.S., Tucker J.S., Green H.D., Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shield K. Canadian Institutes of Health Research; 2021. Alcohol consumption and the COVID-19 pandemic: synthesizing knowledge for policy action. [Google Scholar]
- 13.Data disclosure handbook. Alberta Health and Wellness; Edmonton, Canada: 2003. pp. 1–15. [Google Scholar]
- 14.Lapointe-Shaw L., Georgie F., Carlone D., et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip G., Djerboua M., Carlone D., et al. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang J.X., Ross E., Borman M.A., et al. Validation of coding algorithms for the identification of patients hospitalized for alcoholic hepatitis using administrative data. BMC Gastroenterol. 2015;15:116. doi: 10.1186/s12876-015-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M., Szatrowski T.P., Peterson J., et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.Maddrey W.C., Boitnott J.K., Bedine M.S., et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 19.Kim H.J., Fay M.P., Feuer E.J., et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D., Cholankeril G., Li A.A., et al. Trends in hospitalizations for chronic liver disease-related liver failure in the United States, 2005-2014. Liver Int. 2019;39:1661–1671. doi: 10.1111/liv.14135. [DOI] [PubMed] [Google Scholar]
- 22.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien J., Ayer T., Bethea E.D., et al. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health. 2020;5:e316–e323. doi: 10.1016/S2468-2667(20)30062-1. [DOI] [PubMed] [Google Scholar]
- 24.Estes C., Razavi H., Loomba R., et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim E. Regional variability in COVID-19 case fatality rate in Canada, February-December 2020. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18041839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberta Health Services . 2021. COVID-19 Alberta statistics. [Google Scholar]
- 27.Pan K.Y., Kok A.A.L., Eikelenboom M., et al. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: a longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry. 2021;8:121–129. doi: 10.1016/S2215-0366(20)30491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Da B.L., Im G.Y., Schiano T.D. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72:1102–1108. doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 29.Aberg F., Puukka P., Salomaa V., et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology. 2020;71:835–848. doi: 10.1002/hep.30864. [DOI] [PubMed] [Google Scholar]
- 30.Moreno C., Wykes T., Galderisi S., et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7:813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Nehra M.S., Ma Y., Clark C., et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip G., Djerboua M., Carlone D., et al. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapointe-Shaw L., Georgie F., Carlone D., et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201120. [DOI] [PMC free article] [PubMed] [Google Scholar]