Abstract
During the COVID-19 pandemic, the use of alcohol-based hand sanitizers (ABHS) increased worldwide among the public as well as the health care workers in pursuit to prevent the spread of SARS-CoV-2, the causative virus of COVID-19. Hand hygiene is one of the primary preventive measures to prevent the spread of harmful germs. Although ABHS are effective hand hygiene products and help reduce the transmission of pathogenic microorganisms, appropriate use of such products is necessary to ensure the maximum killing of pathogens and to prevent hazards associated with ABHS. The effectiveness of ABHS against different microorganisms, including SARS-CoV-2 is also documented, but proper knowledge on hand hygiene techniques, selection of appropriate hand sanitizer product, and safe handling of ABHS are required to avoid their adverse effects such as allergies, skin irritation, lung injury, fire hazards, and toxicities. The effectiveness of ABHS is dependent on several factors including its appropriate usage, manufacturing methods, the choice of active agents, and the appropriateness of the agent on the target pathogen. This article highlights the importance of proper usage, handling, and appropriate ABHS selection for maximum efficacy against intended pathogens and safe use of ABHS. User awareness can help promote the appropriate usage of ABHS and prevent its hazards, which ultimately can help in preventing the transmission of pathogenic microorganisms.
Keywords: Sanitizer, alcohol-based hand sanitizer, COVID-19, hand hygiene
Introduction
The coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has highlighted the importance of preventive infection control measures in decreasing transmission, specifically: maintenance of proper hygiene, use of masks and other facial coverings, social distancing and self-isolation of patients presenting symptoms of acute respiratory infection. Human coronaviruses, including SARS-CoV-2, have been reported to survive up to 28 days at room temperature on surfaces including banknotes, steel, glass and vinyl.1 Similarly, human hands are considered a key vector for transmission of infectious microorganisms enabling cross-transmission of the organisms when appropriate hand hygiene is not maintained.2 Hand hygiene is a basic approach to prevent the transmission of pathogenic microorganisms that include several multidrug-resistant pathogens and coronaviruses.3-5 This is the reason for the emphasis on hand hygiene and surface disinfection in preventing the spread of germs where substances such as sanitizers play a key role.1,6 Therefore, regulatory authorities, including the World Health Organization (WHO), emphasize on hand sanitisation to minimize the spread of microorganisms, as a primary measure of infection control.7 Hand sanitizer can either be an alcohol-based or alcohol-free preparation (liquid, gel or foam) intended for quick application on the hands, by rubbing, to kill microorganisms.8 Such preparations, after application on hands by rubbing, allow rapid drying that eliminates the need for soap, water and drying aids.9 Alcohol-free preparations are based on non-alcoholic active agents such as povidone-iodine or chlorhexidine gluconate whereas the alcohol-based hand sanitizers (ABHS) primarily contain one or more alcohol (such as ethanol and isopropanol) as the active ingredient, together with excipients and humectants.8 ABHS is preferable to the alcohol-free preparations in controlling spread of COVID-19 due to the lower efficacy and narrower spectrum of the alcohol-free preparations.10 Moreover, products containing chlorhexidine gluconate, a non-alcoholic substance as the main ingredient, do not show sufficient activity against SARS-CoV-2.11-12 Similarly, hydrogen peroxide alone at concentrations of 1.5% and 3.0% has been found to show suboptimal viricidal activity against SARS-CoV-2 while povidone-iodine at concentration of 0.5% completely inactivated SARS-CoV-2 in vitro.13
ABHS are among the most effective hand sanitizers due to their rapid antimicrobial efficacy, convenience and good tolerance on the skin.14-15 At the beginning of the pandemic, there was a surge in the use of hand sanitizers which is most likely not because of their effectiveness but rather due to the initial fear and lack of unbiased and scientific information regarding their appropriate use.16 For example, from the last week of February 2020 to the second week of March 2020, the sale of hand sanitizers increased by 561% in Italy.10 Augmentation in hand sanitizer sales was reported by Nielsen, after a market search, that there was a 300% to 470% increase during February to March 2020 compared to the same time in the previous year.10 Interestingly, ‘hand sanitizer’ was also the most searched keyword in Google search engine compared to the ‘pill’, ‘medicine’, and ‘drug’ during the initial peak pandemic time (March 2020).10 Although ABHS is essential in controlling the spread of pathogenic microorganisms; untoward incidences can happen as the ingredients of ABHS are flammable as well as toxic. Moreover, inappropriate use of ABHS can reduce its effectiveness in killing microorganisms. User awareness regarding the appropriate use and the potential hazards of hand sanitizer products is therefore important. There are reports on lack of knowledge, non-adherence to usage directions, and misuse of hand sanitizers.17 Therefore, a good understanding of the ingredients, proper usage, handling, and selection of appropriate ABHS is necessary – all of which is detailed in this article. The information will be useful for scientific community, manufacturers as well as the users of ABHS products.
Composition, dosage forms and mode of action of hand sanitizers
Ethanol, isopropyl alcohol, and n-propanol are the active components in ABHS with or without excipients like hydrogen peroxide, gelling agent, humectant, fragrance, and colorants (Figure 1).8 Sometimes, more than one alcohol can be present in one formulation while the other excipients may vary with different dosage forms like gel, liquid and foam.8 Dosage forms are dependent on the formulation design, e.g., gel forms contain thickening agents, but the liquid/foam dosage forms do not. Hydrogen peroxide, used at a concentration of 3%, inactivates the spores present in the bulk solutions or additives. Humectant, such as glycerol and aloe vera gel, helps to moisten the skin and prevent skin dryness caused by using alcohol alone. A lower concentration of humectant is preferred in sanitizer preparations since a higher concentration is likely to make the formulation sticky.8 Thickening agents, such as xanthan gum and polyethylene glycol, are used to enhance the viscosity, whereas fragrance and colorants are used for aesthetic properties. The WHO recommends two preparations containing ethanol or isopropyl alcohol as the main alcohol constituent, together with hydrogen peroxide and glycerol, for local production of ABHS (Table 1).18
Figure 1.
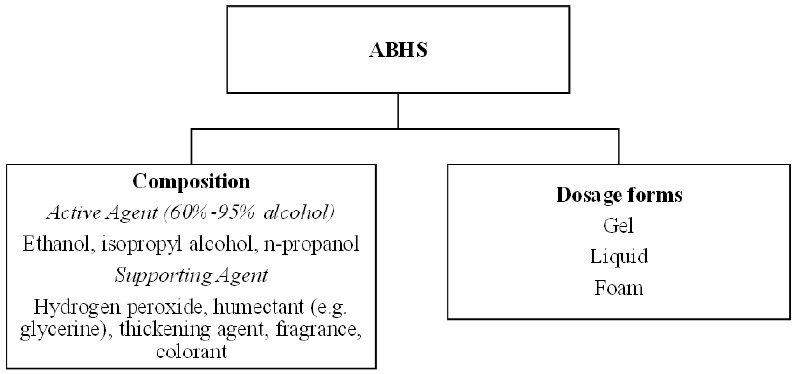
Composition and dosage forms of alcohol-based hand sanitisers (ABHS)
Table 1. WHO recommended formulations for local production of ABHS. Reproduced with permission from www.who.int18.
| Ingredients | Amount | |
|---|---|---|
| Formulation 1 | Formulation 2 | |
| Ethanol 96% | 8333 mL | - |
| Isopropyl alcohol 99.8% | - | 7515 mL |
| Hydrogen peroxide 3% | 417 mL | 417 mL |
| Glycerol 98% | 145 mL | 145 mL |
| Sterile distilled or boiled cold water | Up to 10000 mL | Up to 10000 mL |
For optimum bactericidal and viricidal activity of ABHS, alcohol concentration of 60%-95% by volume is recommended.8 Alcohol has broad-spectrum effectiveness against vegetative forms of bacteria, fungi as well as enveloped viruses. Alcohol dissolves the lipids membrane, denatures the proteins of microorganisms, and inactivates them. Absolute alcohol or alcohol that contains less than 1% of water is less effective against microorganisms because water is crucial in the denaturation process.19 Figure 2 illustrates the denaturation process and the killing of bacteria and viruses by ABHS by disrupting their lipid membrane. Alcohol alone may not be effective against spore-containing bacteria that are mainly present in raw materials. Such bacteria with spores can be inactivated by the addition of hydrogen peroxide (3%) in the ABHS formulation.
Figure 2.
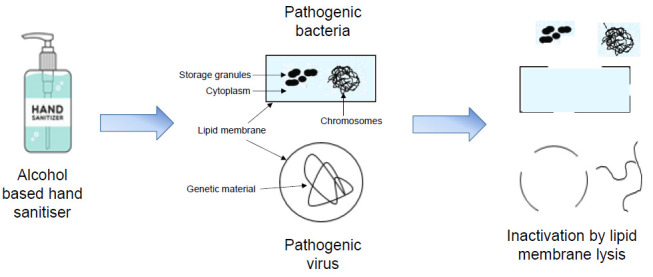
Schematic representation of the bactericidal and viricidal effect of alcohol-based hand sanitisers (ABHS) by inactivation of lipid membrane lysis
ABHS is effective against bacteria and viruses including Escherichia coli, Enterococcus faecalis, Acinetobacter baumannii, Candida albicans, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, SARS-CoV, Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV) and Zika virus.20-22 In a recent study, the WHO recommended ABHS preparations were found to be effective against SARS-CoV-2, the virus causing COVID-19.14 The minimum concentration of ethanol and 2-propanol recommended for activity against SARS-CoV-2 was 80% v/v and 75% v/v, respectively. In contrast, alcohol or ABHS has been found ineffective against some bacteria, viruses as well as parasites such as Clostridioides difficile, cryptosporidium, norovirus, rotavirus, adenovirus, rhinovirus, parvovirus, polyomavirus, human enterovirus 71 and coxsackie virus A7.23 Further studies are required to determine the effectiveness of ABHS against coronaviruses on hands and surfaces, as well as the efficacy of hand hygiene on COVID-19 transmission.24
Harmful impacts of ABHS
Hand sanitizer can play a key role in preventing the transmission of pathogenic microorganisms but may also lead to harmful impacts on the human body if not used appropriately. The ingredients used in hand sanitizer preparations may cause serious health problems if not used carefully.
Minimal systemic toxicity has been reported for dermal contact of ethanol-based hand sanitizer, but tolerability varies from person to person, and this is why it is difficult to predict the actual degree of ethanol toxicity of hand sanitizer.25 Although there is no evidence to suggest that the use of topical ethanol is associated with an increased risk of skin cancer,26 care must be taken to avoid the hazards of ethanol. Studies have also revealed that acute exposure of ethanol-based hand sanitizer is not toxic, but chronic exposure may lead to toxicity.27-28 Acute ethanol toxicity occurs in the case of ingestion of a large volume of alcohol and may affect the respiratory and the nervous system, cause cardiac arrest, induce liver injury, and may also result in death.29 For isopropyl alcohol, the harmful effects are similar to that from ethanol but more intense because of its higher molecular weight.30
Dermal contact with ethanol is responsible for skin irritation and allergic reaction in people who are sensitive to ethanol and those with skin disorders including fissures.31 Higher incidence of hand-skin problems was particularly reported among health care workers during the COVID-19 pandemic, which was associated with the increased frequency of hand sanitizer use.32 Such incidences of hand-skin problems included mainly the dryness of the skin and other conditions such as redness, burning pain and itching. Although there is an increased risk of hand-skin problems among health care workers due to increased use of hand sanitizers, this effect should not discourage alcohol-based hand hygiene in health care workers during the pandemic, which otherwise would increase the risk of transmission of infectious microorganisms. Therefore, in health care settings, it is more important to consider precautions regarding adverse effects of ABHS and implement measures to identify and treat hand-skin problems as early as possible.
Alcohol is highly flammable and can result in fire hazards if used near fire or exposed to high temperatures. Hydrogen peroxide toxicity depends on its concentration used in the ABHS. A low concentration (3%) of hydrogen peroxide may cause mild irritation of the eyes and skin when used externally and when ingested, may result in irritation of the inside of the mouth and the gastrointestinal tract, and may also result in air embolism in rare cases.33-35 Excessive use of ABHS may result in a rise of other viral diseases and antimicrobial resistance due to the selection of resistant strains, particularly for bacteria. In fact, antimicrobial resistance, due to overuse of ABHS, has been reported in different parts of the globe.31
Children are more vulnerable to health hazards from ABHS as they are unaware of its potential hazards. Moreover, children may be attracted to ABHS, which may sometimes be present in attractive coloured bottles and have an appealing fragrance that results in accidental ingestion.31 From 2011 to 2014, 65,293 cases related to exposure (ingestion) of alcohol-based hand sanitizer in children (aged ≤12 years) in the USA were reported, and during the COVID-19 pandemic, the total exposure was 9,496 from January 2020 to May 2020 (Figure 3).31 When normalized, this data suggests that per year incidence of accidental ABHS ingestion increased from approximately 16,323 cases per year in 2011-2014 to approximately 22,790 cases per year in 2020, when the pandemic struck in the first half of the year.
Figure 3.
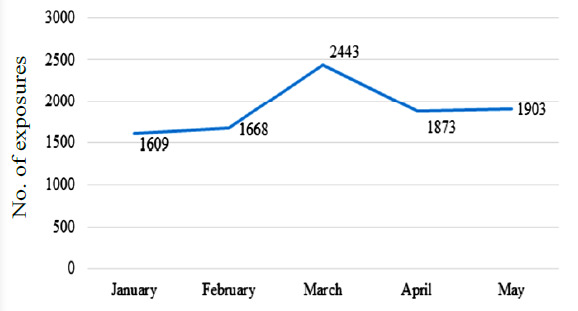
Accidental hand sanitizer exposure by ingestion in children in the USA (January 2020 – May 2020)31
Selection and considerations
Awareness of the safe and appropriate use of ABHS is crucial to achieving its maximum effectiveness. Adulterated and falsified ABHS will be ineffective against microorganisms and the source of potential harm to the user. The product label is the vital source of information that helps the user select and buy the right ABHS product. The following things described in Figure 4 should be checked on the label of ABHS products.
Figure 4.
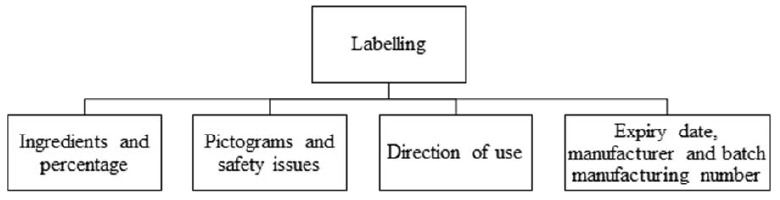
Important information to check on the label of an ABHS product
Ingredients and their composition
Although ABHS contains alcohol, it is not edible and not safe for consumption by humans. Ethanol, isopropyl alcohol, and n-propanol are generally safer compared to methanol, but all alcohols can lead to toxicity in humans if exposed accidentally or in excess amounts.36 Methanol, among other alcohols, is particularly toxic to humans because systemic toxicity can occur as a result of skin exposure leading to erythema, dermatitis, eczema as well as metabolic acidosis upon chronic skin exposure, which can be equivalent to the symptoms from acute ingestion of methanol.37 Methanol is thus highly toxic and can cause severe systemic toxicity and even death when ingested, inhaled, or exposed to via the skin.38-39 However, for optimum bactericidal activity, the desired concentration of alcohol by volume in sanitizer preparations is 60%-95%.8 ABHS can be of substandard quality if the alcohol content in the product is less than 60%.40 The label should specify the name of alcohol (e.g., ethanol, isopropyl alcohol or methanol), and its percentage to inform the user of its active ingredients. This ensures quality and the effectiveness of the hand sanitizer product. A safety survey conducted in Dubai revealed that six of the 102 ABHS products studied contained undeclared/unlisted methanol, and others had less than 60% alcohol content, although the labels had claimed an alcohol content of 70%.40 A literature review emphasized that the presence of undeclared/unlisted methanol in ABHS or the use of substandard ABHS can pose serious health risks in humans.41 Similarly, there are reports of deaths following the ingestion of ABHS containing methanol in countries like the USA, China, Canada, and Hong Kong.41 A survey study conducted on 18 commercial ABHS in Italy during April 2020 has found no specific alcohol name and content in 44% of all surveyed samples.10 Therefore, while purchasing an ABHS product, the primary concern should be on the ingredients and their concentration.
Pictograms and safety issues
ABHS label contains hazard pictograms to indicate the nature of the product and the hazards associated with them as defined and classified by the Globally Harmonized System of Classification and Labelling of Chemicals (GHS).42 GHS has set two sets of pictograms, among which the first set is for the labelling of containers and workplace hazard warnings, whereas the second is for the transport of dangerous goods. Suitable pictograms should appear on ABHS product labels to inform the users of the possible hazards of the product. Pictograms do not contain description, but rely on the symbolic interpretation of the hazard. ABHS labels mainly contain one or both of the flammable and exclamation mark pictograms; however, other pictograms may also be present depending on the characteristics of the ABHS formulation (Figure 5).
Figure 5.

Example of typical hazard pictograms present on ABHS labels
Studies have shown that the presence of pictograms on a product label improves, to a certain degree, the comprehension of the information.36 However, all consumers may not have much knowledge about pictograms, and they may not be able to correctly identify the pictograms.43 Besides the use of pictograms, other safety information is provided in the form of text to indicate the possible hazards of the product, such as ‘flammable’, ‘skin irritant’, ‘eye irritant’ and ‘keep away from children’. Nevertheless, the users of ABHS are encouraged to look for safety information on the product label or seek advice from reliable sources, including chemists, pharmacists and other health care professionals.
Expiry date, manufacturer, and batch manufacturing number
The expiry date of ABHS is vital as the alcohol content may decrease by evaporation over time and upon storage.44 As per US Food and Drug Administration (FDA), over-the-counter topical antiseptic products, including hand sanitizer products, are required to have an expiration date or shelf life listed on the product label unless stability data of more than 3 years is available.45 The name of the manufacturer is crucial to ensure that the manufacturer is authorised. One can check the manufacturer’s authenticity through government-listed websites. The batch manufacturing number is a unique code of each prepared formulation batch. The appearance of a batch manufacturing number on the label is an indication that the manufacturer followed good manufacturing practice and has retained the record of preparation. It is also possible to check and trace the authenticity of the product through the manufacturer using the specific batch manufacturing number.
Directions for use
The effectiveness of ABHS also depends on the application technique. There are no hard and fast rules, but it is always recommended to rub both hands until they are dry. Several studies have recommended that, to achieve effective killing of microorganisms, a single application of hand sanitizer must be in volumes ranging from 1.1 to 3 mL, whereas the FDA recommends 2.4 mL as an appropriate volume of hand sanitizer required in a single application.46 For hand sanitizers to be effective and to ensure the maximum killing of germs, an optimum application time is necessary for the duration of which the user must rub their hands with the sanitizer to dry. The government, public health organizations recommend rubbing hands with sanitizer for at least 20 seconds for good efficacy against germs.47-48 There have been reports that suggest inadequate knowledge of proper hand hygiene can result in the inaccurate application of hand sanitizer.49 This is because viable microorganisms can be present on hands even after the application of hand sanitizer since all areas of the hand and palm were not covered during sanitizer application.49 The WHO recommended hand hygiene guide using ABHS is shown in Figure 6.
Figure 6.
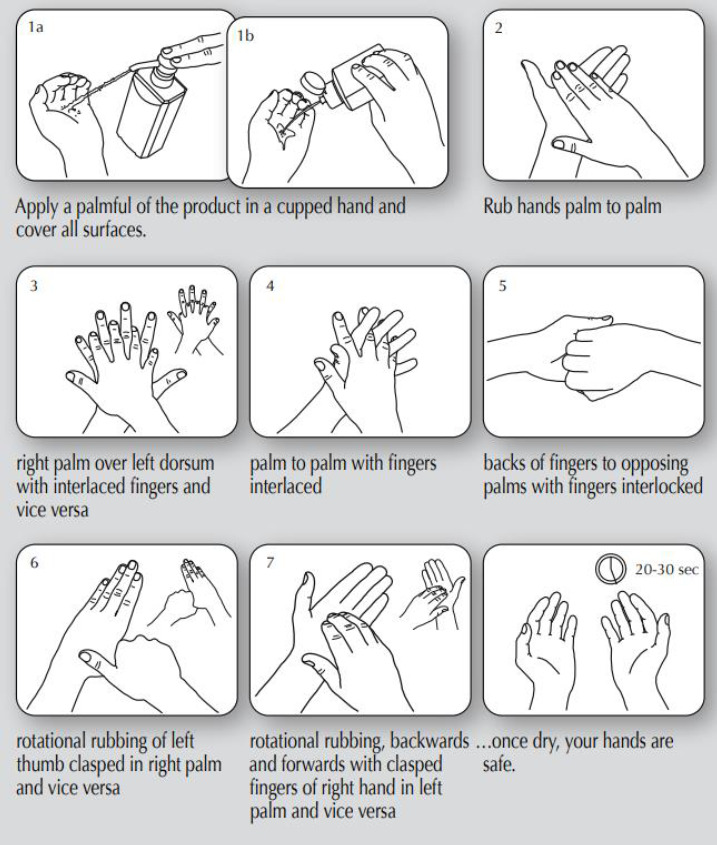
Hand hygiene technique with alcohol-based hand sanitisers (ABHS). Reproduced with permission from www.who.int (WHO guidelines on hand hygiene in health care (advanced draft): a summary.50
Recommendations
Awareness regarding the composition, hazards and appropriate use of hand sanitizer products can prevent untoward accidents that can happen during preparation, handling, and use of ABHS.17 Simple yet important considerations for the safe production and use of ABHS are summarized as follows.
Regulatory control on ‘over the counter’ sanitizer products such as ABHS should be a priority to ensure good manufacturing practices are followed and to ensure protection of consumer rights and safety of users.
Awareness among users is necessary. Purchasing and use of substandard or counterfeit products should be discouraged, while knowledge sharing on appropriate hand hygiene techniques using ABHS should be promoted. Online news media, social media, television, and radio are excellent platforms for the rapid dissemination of information during a pandemic.
Use of soap and water, rather than ABHS, for hand hygiene and sanitisation is recommended in the case of greasy and visibly dirty hands.
Before disposing empty ABHS containers into the general waste bins, these should be rinsed with cold water.
Use of alcoholic beverages as an alternative to ABHS is discouraged because beverages do not contain sufficient concentration of alcohol to achieve effective killing of microorganisms. Instead, such practice can result in hazards and adverse effects such as skin diseases.
Use of ABHS can lead to skin irritation and allergic reactions. However, it can be emphasized that this effect should not discourage alcohol-based hand hygiene in healthcare workers during the pandemic.32
Conclusions
Proper hand hygiene is the best preventive strategy to lower the spread of pathogenic microorganisms. ABHS products are widely used as hand hygiene aids and have been proven effective against coronaviruses. Despite ABHS being an important commodity in preventing the spread of pathogenic microorganisms during pandemics, it is important that it is handled or used appropriately to ensure its safety to humans and effectiveness against microorganisms. User awareness is necessary regarding the appropriate use and handling, selection of the right ABHS product for specific microorganisms, and the possible hazards associated with ABHS products. It is important to use the right technique of ABHS application on hands for effective killing of microorganisms.
Footnotes
Authors’ contributions: TS and PK collected the data, performed the background literature review for the manuscript, drafted and revised the manuscript. TS and PK contributed equally to this work. SD supervised the project and revised the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest: none to declare.
Funding: None to declare.
References
- 1.Marzoli F, Bortolami A, Pezzuto A, et al. A systematic review of human coronaviruses survival on environmental surfaces. Sci Total Environ. 2021 Jul;15(778):146191. doi: 10.1016/j.scitotenv.2021.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzyood M, Jackson D, Aveyard H, Brooke J. COVID-19 reinforces the importance of handwashing. J Clin Nurs. 2020;29:2760–1. doi: 10.1111/jocn.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manigandan S, Wu MT, Ponnusamy VK, Raghavendra VB, Pugazhendhi A, Brindhadevi K. A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19. Process Biochem. 2020;98:233–40. doi: 10.1016/j.procbio.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarka P, Gutkowska K, Nitsch-Osuch A. Assessment of tolerability and acceptability of an alcohol-based hand rub according to a WHO protocol and using apparatus tests. Antimicrob Resist Infect Control. 2019;8:191. doi: 10.1186/s13756-019-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci. 2014;6:2–9. doi: 10.4103/0975-7406.124301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020 Mar;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Güner R, Hasanoğlu I, Aktaş F. COVID-19: Prevention and control measures in community. Turk J Med Sci. 2020;50:571–7. doi: 10.3906/sag-2004-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing JLJ, Pei Yi T, Bose RJC, McCarthy JR, Tharmalingam N, Madheswaran T. Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int J Environ Res Public Health. 2020;17:3326. doi: 10.3390/ijerph17093326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abuga K, Nyamweya N. Alcohol-based hand sanitizers in COVID-19 prevention: a multidimensional perspective. Pharmacy (Basel) 2021;9:64. doi: 10.3390/pharmacy9010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berardi A, Perinelli DR, Merchant HA, et al. Hand sanitizers amid COVID-19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int J Pharm. 2020;584:119431. doi: 10.1016/j.ijpharm.2020.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies K, Buczkowski H, Welch SR, et al. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J Gen Virol. 2021;102:001578. doi: 10.1099/jgv.0.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assis MS, Araújo RAAM, Lopes AMM. Safety alert for hospital environments and health professional: chlorhexidine is ineffective for coronavirus. Rev Assoc Med Bras (1992) 2020;66(2):124–9. doi: 10.1590/1806-9282.66.s2.124. [DOI] [PubMed] [Google Scholar]
- 13.Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont. 2020;29:599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kratzel A, Todt DV, kovski P, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–5. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenters N, Eikelenboom-Boskamp A, Hines J, McGeer A, Huijskens EGW, Voss A. Product dose considerations for real-world hand sanitizer efficacy. Am J Infect Control. 2020;48:503–6. doi: 10.1016/j.ajic.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Miron VD. Drugs in COVID-19 – life-saving or potentially hazardous approaches. J Contemp Clin Pract. 2020;6:47. doi: 10.18683/jccp.2020.1055. [DOI] [Google Scholar]
- 17.Gharpure R, Hunter CM, Schnall AH, et al. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention - United States, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:705–9. doi: 10.15585/mmwr.mm6923e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Guide to local production: WHO-recommended handrub formulations. 2010. Accessed on: 11 June 2021. Available at: https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf.
- 19.Morton HE. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann N Y Acad Sci. 1950;53:191–6. doi: 10.1111/j.1749-6632.1950.tb31944.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramasethu J. Prevention and treatment of neonatal nosocomial infections. Matern Health Neonatol Perinatol. 2017;3:5. doi: 10.1186/s40748-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Muzio M, Cammilletti V, Petrelli E, Di Simone E. Hand hygiene in preventing nosocomial infections:a nursing research. Annali Di Igiene: Medicina Preventiva E Di Comunita. 2015;27:485–91. doi: 10.7416/ai.2015.2035. [DOI] [PubMed] [Google Scholar]
- 22.Malherbe H, Nugier A, Clé ment J, Lamboy B. [Evidence-based and promising interventions to prevent infectious diseases among youth as a result of poor hand hygiene in schools: a literature review] Sante Publique. 2013;25(1):57–63. doi: 10.3917/spub.130.0057. [DOI] [PubMed] [Google Scholar]
- 23.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J Hosp Infect. 2018;98:331–8. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leslie RA, Zhou SS, Macinga DR. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am J Infect Control. 2021;49:401–2. doi: 10.1016/j.ajic.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis-Caleo T, Burstein S. A case of hand sanitizer intoxication. Proceedings of UCLA Healthcare. 2017 [Google Scholar]
- 26.Lachenmeier DW. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol. 2008;3:26. doi: 10.1186/1745-6673-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirschner MH, Lang RA, Breuer B, et al. Transdermal resorption of an ethanol- and 2-propanol-containing skin disinfectant. Langenbecks Arch Surg. 2009;394:151–7. doi: 10.1007/s00423-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 28.Kramer A, Below H, Bieber N, et al. Quantity of ethanol absorption after excessive hand disinfection using three commercially available hand rubs is minimal and below toxic levels for humans. BMC Infect Dis. 2007;7:117. doi: 10.1186/1471-2334-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaHood AJ, Kok SJ. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Accessed on: 11 June 2021]. Ethanol Toxicity. Available at: https://www.ncbi.nlm.nih.gov/books/NBK557381/ [Google Scholar]
- 30.Wilson ME, Guru PK, Park JG. Recurrent lactic acidosis secondary to hand sanitizer ingestion. Indian J Nephrol. 2015;25:57–9. doi: 10.4103/0971-4065.135351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood A, Eqan M, Pervez S, et al. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci Total Environ. 2020;742:140561. doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altunisik Toplu S, Altunisik N, Turkmen D, Ersoy Y. Relationship between hand hygiene and cutaneous findings during COVID-19 pandemic. J Cosmet Dermatol. 2020;19:2468–73. doi: 10.1111/jocd.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon JM, Chun BJ, Min YI. Hemorrhagic gastritis and gas emboli after ingesting 3% hydrogen peroxide. J Emerg Med. 2006;30:403–6. doi: 10.1016/j.jemermed.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Sung J, Cossarini F, Palaiodimos L, Benson B, Meholli M. Extra oxygen leads to bubble trouble: portal vein gas embolism from 3% hydrogen peroxide ingestion. Cureus. 2018;10:e2136. doi: 10.7759/cureus.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watt BE, Proudfoot AT, Vale JA. Hydrogen peroxide poisoning. Toxicol Rev. 2004;23:51–7. doi: 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial. 2014;27:395–401. doi: 10.1111/sdi.12237. [DOI] [PubMed] [Google Scholar]
- 37.Moon CS. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann Occup Environ Med. 2017;29:44. doi: 10.1186/s40557-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi JH, Lee SK, Gil YE, et al. Neurological complications resulting from non-oral occupational methanol poisoning. J Korean Med Sci. 2017;32:371–6. doi: 10.3346/jkms.2017.32.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paasma R, Hovda KE, Tikkerberi A, Jacobsen D. Methanol mass poisoning in Estonia: outbreak in 154 patients. Clin Toxicol (Phila) 2007;45:152–7. doi: 10.1080/15563650600956329. [DOI] [PubMed] [Google Scholar]
- 40.Jairoun AA, Al-Hemyari SS, Shahwan M. The pandemic of COVID-19 and its implications for the purity and authenticity of alcohol-based hand sanitizers: The health risks associated with falsified sanitizers and recommendations for regulatory and public health bodies. Res Social Adm Pharm. 2021;17:2050–1. doi: 10.1016/j.sapharm.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan APL, Chan TYK. Methanol as an unlisted ingredient in supposedly alcohol-based hand rub can pose serious health risk. Int J Environ Res Public Health. 2018;15:1440. doi: 10.3390/ijerph15071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boelhouwer E, Davis J, Franco-Watkins A, Dorris N, Lungu C. Comprehension of hazard communication: effects of pictograms on safety data sheets and labels. J Safety Res. 2013;46:145–55. doi: 10.1016/j.jsr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Montagne M. Pharmaceutical pictograms: a model for development and testing for comprehension and utility. Res Social Adm Pharm. 2013;9:609–20. doi: 10.1016/j.sapharm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson A. Does hand sanitizer expire? 2020. [Accessed on: 11 June 2021]. Available from: https://www.singlecare.com/blog/news/does-hand-sanitizer-expire/#:~:text=The%20short%20answer%20is%3A%20Yes%2C%20hand%20sanitizer%20does%20expire.&text=As%20an%20over%2Dthe%2Dcounter,shelf%20life%20of%20three%20years.
- 45.US Food and Drug Administration. 2020. Q&A for Consumers. Hand Sanitizers and COVID-19. [Accessed on: 11 June 2021]. Available at: https://www.fda.gov/drugs/information-drug-class/qa-consumers-hand-sanitizers-and-covid-19.
- 46.Gold NA, Mirza TM, Avva U. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Accessed on: 11 June 2021]. Alcohol sanitizer. Available at: https://www.ncbi.nlm.nih.gov/books/NBK513254/ [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. 2020. Hand sanitizer use out and about. [Accessed on: 11 June 2021]. Available at: https://www.cdc.gov/handwashing/hand-sanitizer-use.html.
- 48.New Zealand Government. 2020. Protect yourself and others from COVID-19. [Accessed on: 11 June 2021]. Available at: https://covid19.govt.nz/health-and-wellbeing/protect-yourself-and-others/wash-your-hands/#how-to-use-hand-sanitiser.
- 49.Christie S, Sidhu B. The efficacy of alcohol-based hand sanitizers used in a series, modifying the ASTM E2755 method with a shorter hand sanitizer application time. BCIT Environ Public Health J. 2014 doi: 10.47339/ephj.2014.147. [DOI] [Google Scholar]
- 50.World Health Organization. WHO guidelines on hand hygiene in health care (advanced draft): a summary. World Health Organization: 2005. [Accessed on: 11 June 2021]. Available at: http://apps.who.int/iris/bitstream/handle/10665/69143/WHO_EIP_SPO_QPS_05.2.pdf.


