Abstract
Introduction
Increased antibiotic resistance of non-fermenting Gram-negative bacilli (NFGNB) associated with increased morbidity and mortality makes the infections they produce a major public health problem. This study aims to assess the evolution of antibiotic susceptibility and the level of NFGNB antibiotic resistance.
Methods
We carried out a retrospective study on 994 NFGNB strains which had been isolated in the Clinical Laboratory of the “Sf. Parascheva” Clinical Hospital of Infectious Diseases, Iaşi, during a period of 11 years (2008-2018).
Results
Of the 994 NFGNB analyzed, 322 were Acinetobacter spp. and 672 Pseudomonas aeruginosa. Also, 882 NFGNB were isolated from non-sterile sites, in which there was a higher burden of P. aeruginosa strains (n=617). Acinetobacter spp. presented over 70% resistance to the majority of antibiotics. Three pandrug-resistant P. aeruginosa strains were identified. The rate of colistin resistance was 2.91% for P. aeruginosa and 3.33% for Acinetobacter spp. A comparative analysis of the antibiotic susceptibility of strains isolated from non-sterile sites versus sterile sites revealed statistically significant differences only for Acinetobacter spp. The percentage of resistant strains was significantly higher in tracheobronchial aspirate compared to sputum.
Conclusions
The results show that Acinetobacter spp. is substantially more resistant to antibiotics compared to P. aeruginosa and that the use of medical devices can favor the occurrence of infections with multidrug-resistant strains.
Keywords: Antibiotic resistance, Acinetobacter spp, NFGNB, Pseudomonas aeruginosa
Introduction
In recent years, the rapid increase of antimicrobial resistance has become a global public health challenge, acknowledged by the World Health Organization as one of the three most significant issues for human health.1
Non-fermenting Gram-negative bacilli (NFGNB) are frequently involved in healthcare-associated infections (HCAI), which are on the rise and are known to increase mortality.2 Ventilator-associated pneumonia, sepsis, wound infections, urinary tract infections, and meningitis following neurosurgery are examples of HCAI caused by NFGNB and it is worth mentioning that they are yet more common in immunosuppressed patients.3-5
The frequency of infections caused by Pseudomonas aeruginosa and Acinetobacter baumannii is increasing due to the use of invasive medical devices, intravenous or urinary catheters, as well as to hospitalization in intensive care units.3,5
The annual reports of the European Antimicrobial Resistance Surveillance Network (EARS-Net) show alarming rates of antimicrobial resistance for NFGNB in Romania. For instance, P. aeruginosa has over 50% resistance to most known active antibiotics targeting this microorganism, and its resistance to carbapenems is between 50% and 70%. With these rates, Romania ranks first among European countries, followed closely by Greece (54%) and Slovakia (52%). By contrast, the lowest resistance rates (up to 5%) were reported in the Nordic countries (Norway, Iceland, Denmark). The situation is similar for aminoglycosides, ceftazidime and fluoroquinolones, to which resistance ranges from 50% to 64% in our country.6-8
In the case of Acinetobacter spp. strains, the rate of antibiotic resistance has been included in the EARS-Net annual report only since 2012. In Romania, during 2012-2017, the resistance to carbapenems for Acinetobacter spp. was between 77% and 88%, the highest values being recorded in 2017. With such results, Romania is surpassed only by Greece and Croatia, where resistance has reached approximately 95%. The antibiotic resistance rate for Acinetobacter spp. is much lower in the Nordic countries, where for most antibiotics they are known to have less than 10% resistance.6-8
These increased antibiotic resistance rates and the prevalence of NFGNB infections are what motivated us to research the antibiotic resistance of Acinetobacter spp. and P. aeruginosa using the strains isolated in the Clinical Laboratory of the “Sf. Parascheva” Clinical Hospital of Infectious Diseases in Iaşi, in North-Eastern Romania, during the period 01.01.2008-31.12.2018. The analysis of the data thus obtained can be used in support of making optimal empirical therapy choices. This is all the more important given that infections caused by NFGNB are also associated with higher mortality.
Methods
This is a retrospective study that included all consecutive NFGNB strains isolated in the Clinical Laboratory of the “Sf. Parascheva” Clinical Hospital of Infectious Diseases from Iaşi, in North-Eastern Romania, during the period 01.01.2008-31.12.2018. Isolates taken repeatedly from the same patient were excluded. We analyzed the distribution of strains according to the type of clinical specimen, bacterial species, antibiotic resistance profile, as well as demographic characteristics of the patients with infections caused by NFGNB (gender, age).
The identification was based on: microscopic, culture, and biochemical characters.
The evaluation of antibiotic susceptibility was performed according to Clinical and Laboratory Standards Institute (CLSI) for the period 2008-2016 and according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) for the period 2017-2018.
Antibiotic susceptibility was assessed using the disk diffusion method for the following antibiotics: ceftazidime (CAZ), cefepime (FEP), ampicillin-sulbactam (SAM), piperacillin-tazobactam (TZP), ciprofloxacin (CIP), levofloxacin (LEV), gentamicin (G), tobramycin (TOB), amikacin (AK), imipenem (IMI), meropenem (MEM), aztreonam (ATM), trimethoprim-sulfamethoxazole (SXT). For colistin (CT), beginning with 2016 the microdilution method was used, with the determination of the minimum inhibitory concentration (MIC). Antibiotics were selected based on the strain tested. For the antibiogram quality control P. aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used for all the antibiotics except for colistin, in which case we employed E. coli NCTC 13846.
Research ethics
The study follows the international ethical principles and recommendations of the World Medica Association Declaration of Helsinki regarding medical research involving human subjects. The study was also formally approved by the Research Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy Iaşi and the Ethics Committee of the Infectious Diseases Hospital “Sf. Parascheva”, Iaşi, Romania. All data collected from laboratory registers, and information of the patients were anonymized.
Statistical analysis
The data were collected and processed using the software SPSS version 18.0. (SPSS Inc, USA). Both descriptive and analytical methods were part of the statistical analysis. The ANOVA and the t-student tests with a 95% confidence interval were conducted to compare sample averages. The Kruskal Wallis test was used to compare variables categories in the same group. To compare two or more frequency distributions from the same population, the χ2 test was chosen. Skewness and kurtosis tests (−2<p<2) helped determine whether the variables were continuous or not. The threshold for statistical significance was set at p≤0.05.9
Results
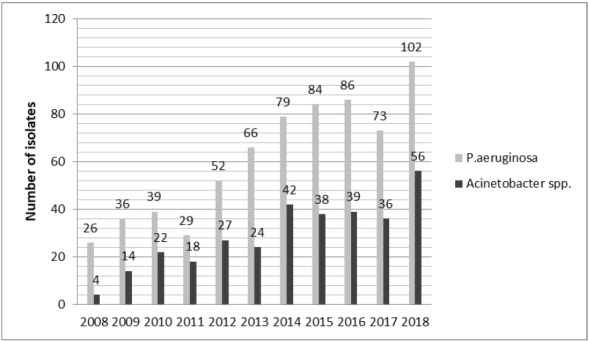
Overall, 994 NFGNB strains isolated from various clinical specimens were included in the study. Of them 322 (32.39%) were identified as Acinetobacter spp. and 672 (67.61%) as P. aeruginosa. The yearly distribution of the number of strains is shown in Figure 1.
Figure 1. The yearly distribution of the number of identified strains.
The strains were isolated from clinical specimens collected from sterile sites (blood, cerebrospinal fluid (CSF), ascites fluid, pleural fluid) and non-sterile sites (urine, pus, tracheobronchial aspirate, sputum) as summarized in Table 1.
Table 1. The distribution of strains according to the collection site.
| Collection site | Species | Total | |||||
|---|---|---|---|---|---|---|---|
| P. aeruginosa | Acinetobacter spp. | ||||||
| No. | % | No. | % | No. | % | ||
| Sterile sites | Blood | 40 | 4.02 | 35 | 3.52 | 112 | 11.27 |
| Cerebrospinal fluids | 9 | 0.91 | 15 | 1.51 | |||
| Other products | 6 | 0.60 | 7 | 0.70 | |||
| Non-sterile sites | Respiratory tract | 86 | 8.65 | 77 | 7.75 | 882 | 88.73 |
| Urine | 296 | 29.78 | 81 | 8.15 | |||
| Pus | 235 | 23.64 | 107 | 10.76 | |||
| Total | 672 | 67.61 | 322 | 32.39 | 994 | 100 | |
Demographically, the age of the patients varied widely from 0.1 to 96 years old in the P. aeruginosa group (coefficient of variation 32.70%) and from 2 to 96 years old in the Acinetobacter spp. group (coefficient of variation 31.12%) respectively. Skewness and kurtosis test results were in the range [-2; 2] and the mean age of the groups (59.27 vs. 59.31 years, p=0.976) close to the median value (63 years) suggests that the age value series was homogeneous, so significance tests could be applied for continuous variables. Concerning the patients’ gender, the samples had come mostly from male patients: 58.8% in the P. aeruginosa group and 56.3% in the Acinetobacter spp. group (p=0.255).
The profiling of antibiotic resistance in the case of P. aeruginosa strains isolated during 2008-2018 revealed the following rates: 53.38% for CAZ, 59.49% for FEP, 33.32% for TZP, 65.46% for CIP, 66.67% for LEV, 52.27% for G, 59.71% for TOB, 41.10% for AK, 63.14% for IMI, 64.09% for MEM, 44.43% for ATM and 2.91% for CT (17 colistin-resistant strains were isolated).
The results obtained for Acinetobacter spp. indicate the following levels of antibiotic resistance: 58.97% for SAM, 81.32% for TZP, 82.04% for CAZ, 81.3% for FEP, 83.33% for CIP, 70.24% for LEV, 79.91% for G, 43.23% for TOB, 69.35% for AK, 73.76% for IMI, 80.15% for MEM, 72.33% for SXT and for CT 3.33%. Only 4 strains were resistant to colistin.
The analysis of antibiotic resistance profiles resulted in the following assessment. For Acinetobacter spp. 48.8% of strains were qualified as extensively drug-resistant (XDR), meaning that they were resistant to at least one antibiotic in all categories except at least two or fewer antimicrobial categories. Another 37% of strains were multidrug-resistant (MDR), meaning that they were resistant to at least one antibiotic in three or more antimicrobial categories. In the case of P. aeruginosa strains, 36.01% were MDR, 23.07% were XDR, and 3 strains were resistant to all antibiotics (otherwise known as pandrug resistant or PDR). Colistin was the only active antibiotic in XDR strains. The definition of the three categories was made according to the definitions given by Magiorakos et al. in 2012.10
The comparative analysis of antibiotic susceptibility of the strains isolated from non-sterile sites versus those from sterile sites revealed statistically significant differences only in the case of Acinetobacter spp. (Table 2).
Table 2. The antibiotic susceptibility of strains from non-sterile sites compared to sterile sites.
| Antibiotic | Acinetobacter spp. | Pseudomonas aeruginosa | ||||
|---|---|---|---|---|---|---|
| Non-sterile sites | Sterile sites | p | Non-sterile sites | Sterile sites | p | |
| R% | R% | R% | R% | |||
| SAM | 59 | 50 | 0.603 | - | - | |
| CAZ | 84.2 | 78 | 0.444 | 51.85 | 58.18 | 0.908 |
| FEP | 84.7 | 54.5 | 0.001 | 59 | 64.29 | 0.256 |
| TZP | 85 | 76 | 0.153 | 34 | 25 | 0.871 |
| CIP | 85.4 | 74.5 | 0.112 | 66.7 | 51.92 | 0.339 |
| LEV | 71.6 | 60.71 | 0.134 | 67 | 62.96 | 0.885 |
| G | 82.5 | 63.46 | 0.015 | 56 | 59.6 | 0.991 |
| TOB | 46 | 25 | 0.003 | 59.8 | 58 | 0.901 |
| AK | 73.9 | 50 | 0.011 | 41.96 | 43.6 | 0.505 |
| IMI | 77 | 54.4 | 0.035 | 62.77 | 67.3 | 0.888 |
| MEM | 81.7 | 68.63 | 0.049 | 63.75 | 68 | 0.509 |
| ATM | - | - | - | 43.6 | 52.3 | 0.411 |
| SXT | 73.4 | 62.5 | 0.172 | - | - | - |
| CT | 3.84 | 2.13 | 0.678 | 2.82 | 3.8 | 0.995 |
AK - amikacin; ATM - aztreonam; CAZ - ceftazidime; CIP - ciprofloxacin; CT - colistin; FEP - cefepime; G - gentamicin; IMI - imipenem; LEV - levofloxacin; MEM - meropenem; SAM - ampicillin-sulbactam; SXT - trimethoprim-sulfamethoxazole; TOB - tobramycin; TZP - piperacillin-tazobactam.
A total number of 296 strains of P. aeruginosa were isolated from urine, out of which 65 had been collected from the urethral catheter. For Acinetobacter spp., 81 strains were isolated from urine, 19 of them being collected from the urethral catheter. A comparative analysis of the antibiotic resistance of strains isolated from urine collected using a bladder catheter vs. without a bladder catheter indicated statistically significant differences only for SXT (94.74% vs 65.96%, p=0.006), in the case of Acinetobacter spp. strains and for IMI (81.54% vs 67.97%, p=0.05) in the case of P. aeruginosa strains.
Among the strains isolated from patients with lower respiratory tract infections, there were 86 strains of P. aeruginosa (30 from tracheobronchial aspirate, and 56 from sputum), and 77 strains of Acinetobacter spp. (42 from tracheobronchial aspirate, and 33 from sputum). Statistically significant differences were noticed between the levels of antibiotic resistance of strains isolated from sputum vs. tracheobronchial aspirate in both bacterial species, as can be seen in Table 3.
Table 3. The antibiotic resistance of non-fermenting Gram-negative bacilli from sputum versus tracheobronchial aspirate.
| Sputum | Tracheobronchial aspirate | p | |||
|---|---|---|---|---|---|
| %Resistant | %Resistant | ||||
| P. aeruginosa | CAZ | 42.59 | 58.62 | 0.884 | |
| FEP | 39.47 | 73.91 | 0.001 | ||
| TZP | 13.73 | 50.00 | 0.001 | ||
| CIP | 28.85 | 70.00 | 0.003 | ||
| LEV | 18.52 | 71.43 | 0.001 | ||
| G | 29.41 | 58.62 | 0.011 | ||
| TOB | 29.17 | 63.33 | 0.015 | ||
| AK | 29.63 | 53.33 | 0.005 | ||
| IMI | 53.85 | 73.33 | 0.05 | ||
| MEM | 45.45 | 73.33 | 0.041 | ||
| ATM | 40.54 | 44.44 | 0.901 | ||
| Acinetobacter spp. | SAM | 44 | 77.8 | 0.197 | |
| CAZ | 69 | 100 | 0.155 | ||
| FEP | 60 | 94 | 0.001 | ||
| TZP | 57.1 | 100 | 0.001 | ||
| CIP | 67.7 | 97.6 | 0.006 | ||
| LEV | 31 | 85 | 0.001 | ||
| G | 63 | 88 | 0.163 | ||
| TOB | 31 | 41 | 0.001 | ||
| AK | 50 | 70 | 0.025 | ||
| IMI | 72 | 90 | 0.441 | ||
| MEM | 63 | 95 | 0.037 | ||
| SXT | 50 | 81.08 | .001 | ||
AK — amikacin; ATM — aztreonam; CAZ — ceftazidime; CIP — ciprofloxacin; FEP — cefepime; G — gentamicin; IMI — imipenem; LEV — levofloxacin; MEM — meropenem; SAM — ampicillin-sulbactam; SXT — trimethoprim-sulfamethoxazole; TOB — tobramycin; TZP — piperacillin-tazobactam.
The 2008-2018 interval was divided into three periods: 2008-2012 (T1), 2013-2016 (T2) and 2017-2018 (T3), due to the increase of the number of isolates since 2013, and the change of the interpretation standard in 2017, from CLSI to EUCAST, both with a possible impact on the antibiotic resistance rates. We compared each pair combination of the three datasets T1, T2, T3 and we analyzed antibiotic resistance separately for P. aeruginosa and Acinetobacter spp. The results obtained by analyzing the considered time intervals indicated statistically significant differences only for some antibiotics (p<0.05) – Tables 4 and 5.
Table 4. Comparative analysis of antibiotic resistance of P. aeruginosa isolates from different time periods T1 (2008-2012), T2 (2013-2016), T3 (2017-2018).
| P. aeruginosa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | TZP | CIP | G | TOB | AK | IMI | MEM | |||||||||
| %R | p | %R | p | %R | p | %R | p | %R | p | %R | p | %R | p | %R | p | |
| T1 vs. T2 | 47.31 | 0.4 | 41.53 | 0.05 | 63.1 | 0.9 | 55.41 | 0.75 | 52 | 0.34 | 44.79 | 0.41 | 54 | 0.04 | 67.95 | 0.76 |
| 57.65 | 25 | 68.9 | 58.82 | 66.4 | 38.64 | 71 | 65.05 | |||||||||
| T2 vs. T3 | 57.65 | 0.43 | 25 | 0.05 | 68.9 | 0.89 | 58.82 | 0.85 | 66.4 | 0.55 | 38.64 | 0.19 | 71 | 0.05 | 65.05 | 0.24 |
| 48 | 41.04 | 61.8 | 52.84 | 52.6 | 45.71 | 59 | 58.8 | |||||||||
| T1 vs. T3 | 47.31 | 0.75 | 41.53 | 0.99 | 63.1 | 0.76 | 55.41 | 0.65 | 52 | 0.75 | 44.79 | 0.88 | 54 | 0.79 | 67.95 | 0.46 |
| 48 | 41.04 | 61.8 | 52.84 | 52.6 | 45.71 | 51 | 58.8 | |||||||||
AK — amikacin; CAZ — ceftazidime; CIP — ciprofloxacin; G — gentamicin; IMI — imipenem; MEM — meropenem; TOB — tobramycin; TZP — piperacillin-tazobactam.
Table 5. Comparative analysis of antibiotic resistance of Acinetobacter spp. isolates from different time periods T1 (2008-2012), T2 (2013-2016), T3 (2017-2018).
| Acinetobacter spp. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | G | TOB | AK | IMI | MEM | SXT | ||||||||
| %R | p | %R | p | %R | p | %R | p | %R | p | %R | p | R% | p | |
| T1 vs T2 | 83 | 0.84 | 78.3 | 0.72 | 18.97 | 0.007 | 75 | 0.16 | 54.5 | 0.001 | 81.5 | 0.24 | 67 | 0.99 |
| 84 | 80.6 | 37.41 | 65.44 | 80.9 | 80 | 67 | ||||||||
| T2 vs T3 | 84 | 0.91 | 80.6 | 0.60 | 37.41 | 0.05 | 65.44 | 0.36 | 80.9 | 0.49 | 80 | 0.73 | 67 | 0.036 |
| 83 | 76.9 | 64.44 | 71.74 | 76.1 | 77 | 81 | ||||||||
| T1 vs T3 | 83 | 0.99 | 78.3 | 0.86 | 16.97 | 0.001 | 75 | 0.74 | 54.5 | 0.049 | 81.5 | 0.48 | 67 | 0.036 |
| 83 | 76.9 | 64.44 | 71.74 | 76.1 | 77 | 81 | ||||||||
AK — amikacin; CIP — ciprofloxacin; G — gentamicin; IMI — imipenem; MEM — meropenem; SXT — trimethoprim-sulfamethoxazole; TOB — tobramycin.
Discussion
The extensive use of antibiotics in the hospital environment has led to the selection of strains with resistance to multiple antibiotics.11 Carbapenems are frequently used for the empirical treatment of severe bacterial infections. In recent decades, the emergence of carbapenem-resistant strains has increased the risk of therapeutic failure with such empirical treatment.12,13 Carbapenems can only be administered intravenously, so these antibiotics are used only in the hospital setting.14 Concurrently, colistin has increasingly been used to treat infections caused by MDR isolates due to a lack of new antibiotics active on Gram-negative bacteria. Currently, colistin resistance is relatively low.15
In this study, the number of strains isolated annually increased from 2008 to 2018 while the distribution of species per year was uneven with P. aeruginosa more frequent that Acinetobacter spp. (Figure 1). This upward trend has also been noticed in other studies; for example: in Italy in the period 2008-2013 the number of A. baumannii strains increased from 20% to 58%. In a Chinese study recorded P. aeruginosa strains doubled from 2013-2017 compared to 2008-2012.16,17 Xu T et al. conducted another study in China over a period of 4 years and identified an increase in the number of A. baumannii strains from 7% in 2008 to 18.8% in 2011.18
Both mentioned microorganisms have developed resistance to multiple antibiotics, leaving colistin as the best therapeutic option in many situations (97.1% of P. aeruginosa strains and 96.66% of Acinetobacter spp. were found susceptible to colistin). However, the use of this antibiotic is associated with important side effects, and it is ineffective in lung infections, due to low penetration in the lung parenchyma, thus being administered as aerosols.19 The use of colistin in monotherapy can lead to the selection of strains which carry resistance genes.20
In our study, 40% of urinary tract infections were associated with the use of a urinary catheter. Urinary infections are the most common infections and occur both in the community and in the hospital environment, 70-80% of their incidence is attributed to the use of the urethral catheter, which means that the catheter can be a favorable factor for the occurrence of infections with MDR strains.21,22 The result of the study by Shrestha LB et al. suggests that bladder catheterization may favor colonization with MDR bacteria (in hospital settings), the selection of MDR strains under antibiotherapy.22 Therefore, the indication for catheterization needs to be reviewed more strictly and performed with utmost care only when such an invasive maneuver is required.
In the case of strains found in patients with lower respiratory tract infections, the antibiotic resistance of isolates from tracheobronchial aspirate was higher, compared to that of strains isolated from sputum, with statistically significant differences for most antibiotics in both species of microorganisms. This may be because the tracheobronchial aspirate is collected from mechanically ventilated patients, hospitalized in intensive care units. Mechanical ventilation and hospitalization in intensive care units are known risk factors for contracting infections with microorganisms that have become resistant to multiple antibiotics.
In a multicentric study conducted in Italy, Spain and Greece, P. aeruginosa was isolated from 57 patients with ventilator-associated pneumonia and, upon testing, 75-78% of strains were found to be resistant to carbapenems.23 These results are similar to ours.
Increased antibiotic resistance of the strains isolated from tracheobronchial aspirate and from urine collected from the urethral catheter suggests that the use of such medical devices (endotracheal intubation tubes and urinary catheters) promotes infections with MDR strains.
For the period 2008-2016, the interpretation of antibiograms was done according to CLSI, and since 2017 the EUCAST guide has been used. Although the standard of interpretation has changed, the comparative analysis of the T2 and T3 periods shows an increase in the resistance of Acinetobacter spp. to TOB and SXT and of P. aeruginosa. This could be explained by the change of breakpoints in EUCAST which are higher compared to CLSI.
Although the values of the breakpoints increased due to the changes of the interpretation standard, we noticed that, the resistance to carbapenems of both studied species decreased in the period T3 compared to T2, the differences between these two periods being statistically significant only for IMI (59% vs. 71%, p=0.05) in P. aeruginosa strains.
Between T2 and T1, both microorganisms increased their resistance to carbapenem possibly because in Romania carbapenem consumption was 2.33 times higher in 2015 than in 2011.24
A study conducted in Italy during 2007-2010 found A. baumannii strains that were 84% resistant to ciprofloxacin and 76% to carbapenems. In the same study the sensitivity of P. aeruginosa to different antibiotics was measured at 31% to CAZ, 14% to AK, 42% to CIP, 36% to IMI, and 25% to TZP.25
Given the dynamics of antibiotic consumption and resistance patterns, such research needs to be conducted regularly and repeatedly, for adjustment of empirical treatment.
Conclusions
The number of NFGNB strains isolated from distinct clinical specimens increased constantly during the studied period, which justifies the need for coordinated strategies and programs to prevent the spread of multidrug-resistant microorganisms.
The distribution of NFGNB strains during the 11 years was uneven, with a predominance of strains from non-sterile sites over those from sterile sites; this can be explained by the microbiological characteristics of the two types of sites.
Even if Acinetobacter spp. was found more frequently resistant to carbapenems when compared to P. aeruginosa, recommending more precautions in the prescription and use of carbapenems as empirical therapy could help lower the number of resistant strains, regardless of the species involved.
Furthermore, the high antibiotic resistance of Acinetobacter spp. isolated from tracheobronchial aspirate limits the choice of a therapeutic regimen and reinforces the need for dedicated programs to prevent the spread of resistant strains, especially in hospital settings.
This study contributes to the comprehensive understanding of antibiotic resistance for Acinetobacter spp. and P. aeruginosa, in North-Eastern Romania by analyzing 11 years’ worth of evidence from a major infectious diseases hospital. The results are relevant in the worrying context of rising antibiotic resistance rates across the country, the continent, and the world. The results can be used by clinicians in making appropriate empirical antibiotic therapy choices and by policymakers in providing adequate mitigation and prevention of increased antibiotic resistance.
Acknowledgments:
The authors would like to thank their colleagues from the “Sf. Parascheva” Hospital of Infectious Diseases, Iaşi, Romania whose continuous clinical practice has made this research possible.
Footnotes
Authors’ contributions statement: ERB: study concept and design; collected and centralization of data, analyzed data; original draft preparation; writing – original draft; approval of the final version to be published. EVN: approval of study design; methodology, supervision, review and editing, approval of the final version to be published. CL: Review of the article, approval of the final version to be published. AB: Review of the article, approval of the final version to be published. EM: Review of the article, approval of the final version to be published. LSI: Approval of study design; supervision; review, approval of the final version to be published. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Bitew A. High Prevalence of multi-drug resistance and extended spectrum beta lactamase production in non-fermenting Gram-negative bacilli in Ethiopia. Infect Dis (Auckl) 2019;12:1178633719884951. doi: 10.1177/1178633719884951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tucaliuc D, Alexa O, Tuchiluş CG, et al. Antibiotic resistance spectrum of non fermenting Gram negative bacilli isolated in the Orthopedic Traumatology Clinic of “Sf. Spiridon” Clinical Emergency Hospital Iaşi. Rev Med Chir Soc Med Nat Iaşi. 2015;119:536–43. [PubMed] [Google Scholar]
- 3.Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 4.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–73. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 5.Gokale SK, Metgud SC. Characterization and antibiotic sensitivity pattern of nonfermenting gram-negative bacilli from varius clinical samples in a tertiary care hospital, Belgaum. J Pharm Biomed Sci. 2012;17:1–5. [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2013. [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2015. [Google Scholar]
- 8.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2017. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2018. [Google Scholar]
- 9.Boiculese V, Dimitriu G, Moscalu M. Elemente de biostatistica. Analiza statistică a datelor biologice. Editura Pim. 2007 [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol Rev. 2018;42:fux053. doi: 10.1093/femsre/fux053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baughman RP. The use of carbapenems in the treatment of serious infections. J Intensive Care Med. 2009;24:230–41. doi: 10.1177/0885066609335660. [DOI] [PubMed] [Google Scholar]
- 13.Perez F, ElChakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17:761–81. doi: 10.1517/14656566.2016.1145658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun SD, Dorneanu OS, Vremeră T, Reißig A, Monecke S, Ehricht R. Carbapenemase-producing Enterobacteriaceae: a 2-year surveillance in a hospital in Iaşi, Romania. Future Microbiol. 2016;11:391–401. doi: 10.2217/fmb.15.148. [DOI] [PubMed] [Google Scholar]
- 15.Malekzadegan Y, Abdi A, Heidari H, Moradi M, Rastegar E, Sedigh Ebrahim-Saraie H. In vitro activities of colistin, imipenem and ceftazidime against drug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii isolates in the south of Iran. BMC Res Notes. 2019;12:301. doi: 10.1186/s13104-019-4344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agodi A, Barchitta M, Quattrocchi A, et al. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008-2013. Antimicrob Resist Infect Control. 2015;4:43. doi: 10.1186/s13756-015-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian L, Zhang Z, Sun Z. Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: a 20-year surveillance study (1998-2017) Antimicrob Resist Infect Control. 2019;8:86. doi: 10.1186/s13756-019-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu T, Xia W, Rong G, Pan S, Huang P, Gu B. A 4-year surveillance of antimicrobial resistance patterns of Acinetobacter baumanni in a university-affiliated hospital in China. J Thorac Dis. 2013;5:506–12. doi: 10.3978/j.issn.2072-1439.2013.08.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min KL, Son ES, Kim JS, Kim SH, Jung SM, Chang MJ. Risk factors of colistin safety according to administration routes: Intravenous and aerosolized colistin. PLoS One. 2018;13:e0207588. doi: 10.1371/journal.pone.0207588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin Microbiol Infect. 2008;14:816–27. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 21.Gomila A, Shaw E, Carratalà J, et al. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections. Antimicrob Resist Infect Control. 2018;7:111. doi: 10.1186/s13756-018-0401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha LB, Baral R, Khanal B. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infect Drug Resist. 2019;12:957–63. doi: 10.2147/IDR.S200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez A, Gato E, Pérez-Llarena J, et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2019;74:1244–52. doi: 10.1093/jac/dkz030. [DOI] [PubMed] [Google Scholar]
- 24.Popescu GA, Şerban R, Niculcea A. Consumul de antibiotice, Rezistenţa microbiană şi Infecţii Nosocomiale în România - 2015 (CARMIN ROMÂNIA 2015) 2017 [Google Scholar]
- 25.De Francesco MA, Ravizzola G, Peroni L, Bonfanti C, Manca N. Prevalence of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in an Italian hospital. J Infect Public Health. 2013;6:179–85. doi: 10.1016/j.jiph.2012.11.006. [DOI] [PubMed] [Google Scholar]


