Abstract
Background
Urine tissue inhibitor of metalloproteinases-2/insulin-like growth factor-binding protein 7 (TIMP-2/IGFBP7) (NephroCheck, Ortho Clinical Diagnostics, Raritan, NJ, USA) is a US Food and Drug Administration-approved biomarker for risk assessment of acute kidney injury (AKI) in critically ill adult patients in intensive care units; however, its clinical impact in the emergency department (ED) remains unproven. We evaluated the utility of NephroCheck for predicting AKI development and short-term mortality in the ED.
Methods
This was a prospective, observational, five-center international study. We consecutively enrolled ED patients admitted with ≥30% risk of AKI development (assessed by ED physician ED score) or acute diseases. Serum creatinine was tested on ED arrival (T0), day 1, and day 2 (T48); urine for NephroCheck was collected at T0 and T48. We performed ROC curve and reclassification analyses.
Results
Among the 529 patients enrolled (213 females; median age, 65 years), AKI developed in 59 (11.2%) patients. The T0 NephroCheck value was higher in the AKI group than in the non-AKI group (median 0.77 vs. 0.29 (ng/m)2/1,000, P=0.001), and better predicted AKI development than the ED score (area under the curve [AUC], 0.64 vs. 0.53; P=0.04). In reclassification analyses, adding NephroCheck to the ED score improved the prediction of AKI development (P<0.05). The T0 NephroCheck value predicted 30-day mortality (AUC, 0.68; P<0.001).
Conclusions
NephroCheck can predict both AKI development and short-term mortality in at-risk ED patients. NephroCheck would be a useful biomarker for early ruling-in or ruling-out of AKI in the ED.
Keywords: Acute kidney injury, Mortality, Emergency department, TIMP-2/IGFBP7, NephroCheck
INTRODUCTION
Acute kidney injury (AKI) is an increasingly common yet serious condition that can emerge without warning symptoms. The incidence of AKI is 22% in hospital settings and as high as 57% in intensive care units (ICUs) [1, 2]. AKI is defined as an abrupt decline in kidney function from baseline over hrs to days and is often diagnosed in the context of other acute diseases in the emergency department (ED) [3]. In the clinical evaluation of acutely ill ED patients, early detection of AKI is crucial as it negatively impacts clinical outcomes, and subclinical kidney cell injury may be reversed if recognized early [4, 5]. After ruling in AKI, various therapeutic strategies are recommended by Kidney Disease Improving Global Outcomes (KDIGO), including managing fluids and diuretic dosage, modifying antibiotics or other drugs, and delaying some procedures until the kidneys function normally [3, 6, 7]. Immediately ruling out AKI could help deploy optimal diagnostic and therapeutic procedures in a timely manner or reduce inappropriate hospital admission and related costs.
Current diagnostic criteria of AKI are based on measurements of serum creatinine (sCr) value and urine output [3, 6, 8]. However, sCr values are highly influenced by many confounding factors, such as muscular mass, liver function, diet, sex, and age, and sCr and urine output tests detect kidney dysfunction, not injury [9]. sCr values take 48-72 hours to rise, reflecting extensive kidney cell damage, and by the time this increase is evident, >50% of nephrons are already affected [9, 10]. AKI evolves quickly, and in patients with acute diseases, the ED physician’s immediate clinical judgment often fails to rule in or rule out AKI. Before the diagnosis of AKI is confirmed after 48 hours, patients should be triaged at ED arrival to identify those at high risk of AKI development. Laboratory testing for ongoing tubular damage is imperative in clinical practice to rapidly rule in or rule out AKI in the ED setting.
Urine tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) are promising early markers of kidney damage; both are involved in G1 cell-cycle arrest during the early phases of cell injury. Their combined product, expressed as [TIMP-2] · [IGFBP7], has shown better diagnostic performance in assessing AKI risk than other injury biomarkers, such as plasma and urine neutrophil gelatinase-associated lipocalin (NGAL), urine kidney injury molecule 1 (KIM-1), and urine interleukin 18 (IL-18) [11, 12]. In 2014, soon after two multicenter ICU cohort studies, TIMP-2/IGFBP7 became the first US Food and Drug Administration (FDA)-approved biomarker for risk assessment of AKI in ICU patients >21 years of age with cardiovascular or respiratory compromise within the prior 24 hours [11, 13-15]. NephroCheck (Ortho Clinical Diagnostics, Raritan, NJ, USA) is a commercially available test for TIMP-2/IGFBP7 [16]. The cutoffs for risk assessment of AKI development validated in an ICU cohort are 0.3 (ng/mL)2/1,000 for high sensitivity/high negative predictive value and 2.0 (ng/mL)2/1,000 for high specificity/high positive predictive value [11, 12]. However, its clinical impact in the ED is unproven.
We evaluated the utility of the urine NephroCheck in predicting AKI development and short-term mortality in ED patients with acute diseases.
MATERIALS AND METHODS
Study design and patient selection
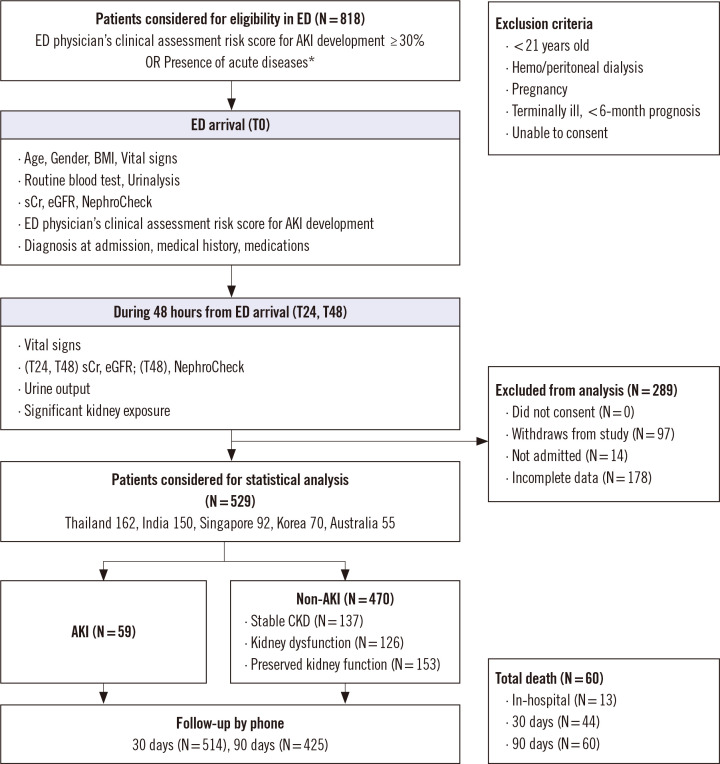
This was a prospective, observational, active comparator, non-randomized, multicenter international study. Five hospitals in five countries (Ramathibodi Hospital, Bangkok, Thailand; Yashoda Hospital, Secunderabad, India; National University Hospital, Singapore; Konkuk University Medical Center, Seoul, Korea; Prince of Wales Hospital, Sydney, Australia) participated in the study. The bed capacity of the hospitals ranged from 500 to 1,500. Between October 2018 and October 2019, we enrolled ED patients based on the following inclusion criteria: (1) a ≥30% risk of AKI development (ED physician’s clinical assessment risk score for AKI development [ED score] noted as a percentage from 0% to 100% [17, 18]); or (2) the presence of acute diseases (confirmed or suspected sepsis, acute decompensated heart failure, significant gastrointestinal loss from vomiting or diarrhea, major trauma, major bleeding, severe burns, diabetic crises, decompensated liver cirrhosis, acute coronary syndrome, emergency need for iodinated contrast studies [e.g., acute abdomen, acute pulmonary embolism, aortic dissection], or shock from any cause) (Fig. 1). Patients were enrolled only when the ED physician of the participating hospital was available in the ED and could make a clinical decision. We excluded patients <21 years, undergoing hemodialysis or peritoneal dialysis, pregnant, terminally ill with less than six-month prognosis, or unable to consent to the study. Of 818 patients who were considered eligible, 529 patients (213 females; median age, 65 years) were finally included in the statistical analysis. The duration of enrollment was set to be 12 months, with an additional three months for follow-up.
Fig. 1.
Flow diagram of the study, patient distribution, and mortality.
Abbreviations: see Table 1.
The study protocol was designed following the criteria of the Declaration of Helsinki and was reviewed by the Independent Ethics Committee or Institutional Review Board of each center (approval nos.: Thailand, ID 02-61-59; India, RP/03/2017; Singapore, NHG DSRB Ref: 2017/01118; Korea, KUH1200094; Australia, HREC 18/040 (LNR/187/POWH/104). Written informed consent was obtained from each patient at ED arrival prior to enrollment. The study was conducted in compliance with Good Clinical Practice guidelines, including the archiving of essential documents.
Data collection and kidney function classification
On ED arrival (T0), demographic information, vital signs, and basic laboratory results were obtained via the routine clinical practice for all patients. The presence of concomitant medical conditions, such as chronic kidney disease (CKD), congestive heart failure, liver disease, and diabetes mellitus, was evaluated from the clinical history. Kidney exposure to antibiotics, contrast media, non-steroidal anti-inflammatory drugs, or other nephrotoxic agents was recorded in the ED and during hospital stay up to 48 hours. sCr values were measured at T0, on day 1 (T24), and day 2 (T48); urine for NephroCheck was collected at T0 and T48 (Fig. 1). sCr values were measured using different enzymatic assays that are traceable with the isotope dilution mass spectrometry reference method. The within-laboratory imprecision of each sCr test was <2% during the study period. Urine output was monitored continuously during the first 48 hours to determine cumulative urine output in 6-hour intervals. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and a reduced eGFR was defined as eGFR (CKD-EPI)<60 mL/min/1.73 m2 [19]. In-hospital mortality was recorded, and survival was checked through phone calls on days 30 and 90. Designated investigator staff entered the data required by the protocol into the e-FORM 5 validated software platform, and queries were verified by the coordinating center.
Kidney function was classified by two independent physicians (one nephrologist and one ED doctor) at the coordinating center who were blinded to the NephroCheck values. The AKI group was determined based on the KDIGO criteria: an increase in sCr value by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 hours or to ≥1.5 times the baseline, or urine volume <0.5 mL/kg/hr for six hours [3]. The non-AKI group was subdivided into three categories: (1) stable CKD: patients with a reduced eGFR who did not meet the criteria for AKI over 48 hours; (2) kidney dysfunction: patients with evidence of increased blood urea nitrogen levels (patients with dehydration and prerenal azotemia); and (3) preserved kidney function: patients with normal eGFR who did not meet the criteria for any of the other categories [18].
TIMP-2/IGFBP7 biomarker analysis
Random urine samples were centrifuged within one hour of collection using polypropylene urine collection cups. Within four hours of collection or after storage at 2-8°C for up to 20 hours, TIMP-2/IGFBP7 was measured in urine supernatants using the VITROS NephroCheck immunoassay on a VITROS 3600 immunodiagnostic system (Ortho Clinical Diagnostics) according to the manufacturer’s instructions. Certified laboratory technicians blinded to clinical data performed the analyses.
Statistical analysis
Data are expressed as mean±standard deviation or median and interquartile range (IQR) for continuous variables, and as percentage for categorical or binary variables. Continuous variables were tested for normality using Kolmogorov-Smirnov nonparametric tests. We compared clinical and laboratory data between AKI and non-AKI groups using Student’s t-test or Mann-Whitney U test for continuous variables and chi-square test or Fisher’s exact test for categorical variables, as appropriate. For statistical comparisons of serial changes within a group, the Friedman test with multiple comparisons was used. Percentage changes from T0 to T48 were calculated as (T48-T0)/T0×100%. For comparison among four subgroups (AKI group and three subgroups in non-AKI group), we used Kruskal-Wallis test and post-hoc pairwise comparisons with Bonferroni correction.
We first conducted an area under the curve (AUC) of ROC curve analysis of T0 NephroCheck values for AKI development within 48 hours. The AUC is a measure of the discriminative ability of a prediction model or continuous test in a certain population, quantifying the separation of the risk distributions of diseased and non-diseased individuals; it is not a measure of clinical utility [20]. Reclassification analyses using net reclassification improvement (NRI) and integrated discrimination improvement (IDI), which provide incremental information on new biomarkers over the AUC, were used to confirm the added value of NephroCheck on top of the conventional ED score [21]. Next, we conducted ROC curve analysis of the T0 NephroCheck values to predict short-term mortality. IBM SPSS Statistics (version 22, Armonk, NY, USA), MedCalc Software (version 20, MedCalc, Ostend, Belgium), and R Statistics (version 3.3.1, The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses. Two-tailed P<0.05 was considered statistically significant.
RESULTS
Comparison of the AKI and non-AKI groups
AKI was confirmed in 59 out of 529 (11.2%) patients. The patient characteristics are presented in Table 1. There were no significant differences in sex, age, body mass index, blood pressure, or ED score between the AKI and non-AKI groups. The medical history was similar between the two groups, and the main diagnosis at admission was confirmed or suspected sepsis (62.7% in AKI vs. 56% in non-AKI, P=0.32). During the first 48 hours, at least one significant kidney exposure was recorded in 431 (87.8%) patients (92.2% in AKI vs. 87.3% in non-AKI, P=0.37). Inotrope or vasopressor use was significantly higher in the AKI group than in the non-AKI group (41.2% vs. 23.9%, P=0.01).
Table 1.
Patient characteristics
| Entire cohort (N=529) | AKI (N=59) | Non-AKI (N=470) | P | ||
|---|---|---|---|---|---|
| Women | 213 (40.3) | 23 (39.0) | 190 (40.4) | 0.94 | |
| Age, yr | 65.0 (53.0-78.0) | 65.0 (59.0-79.5) | 65.0 (53.0-77.0) | 0.06 | |
| BMI, kg/m2 | 23.7 (21.3-26.6) | 23.1 (20.4-25.6) | 23.7 (21.3-26.6) | 0.37 | |
| Systolic BP, mm Hg | 121 (100-145) | 124 (100-148) | 121 (100-145) | 0.52 | |
| Diastolic BP, mm Hg | 70 (60-85) | 70.0 (61-88) | 70 (60-84) | 0.80 | |
| ED score (1-100) | 30.0 (30.0-32.0) | 30.0 (30.0-31.5) | 30.0 (30.0-32.0) | 0.38 | |
| Medical history | |||||
| CKD | 95 (18.0) | 13 (22.0) | 82 (17.4) | 0.26 | |
| CHF | 87 (16.4) | 12 (20.3) | 75 (16.0) | 0.25 | |
| Pulmonary diseases | 93 (17.6) | 10 (16.9) | 83 (17.7) | 1.00 | |
| Neurologic diseases | 91 (17.2) | 10 (16.9) | 81 (17.2) | 1.00 | |
| Liver diseases | 37 (7.0) | 3 (5.1) | 34 (7.2) | 0.78 | |
| Diabetes mellitus | 156 (29.5) | 22 (37.3) | 134 (28.5) | 0.08 | |
| Principal diagnosis on ED admission | |||||
| Sepsis | 300 (56.7) | 37 (62.7) | 263 (56.0) | 0.32 | |
| AHF | 69 (13.0) | 11 (18.6) | 58 (12.3) | 0.22 | |
| ACS | 45 (8.5) | 4 (6.8) | 41 (8.7) | 0.80 | |
| GI diseases | 55 (10.4) | 2 (3.4) | 53 (11.3) | 0.07 | |
| Stroke | 32 (6.0) | 1 (1.7) | 31 (6.6) | 0.09 | |
| Miscellaneous | 28 (5.3) | 4 (6.8) | 24 (5.1) | 0.84 | |
| SKE up to 48 hr* | |||||
| Antibiotics | 338 (68.8) | 40 (78.4) | 298 (67.7) | 0.15 | |
| Contrast media | 123 (25.0) | 6 (11.8) | 117 (26.6) | 0.02 | |
| NSAIDs | 30 (6.1) | 5 (9.8) | 25 (5.7) | 0.22 | |
| Other nephrotoxic drugs | 81 (16.5) | 9 (17.6) | 72 (16.4) | 0.84 | |
| Inotrope or vasopressor use | 126 (25.7) | 21 (41.2) | 105 (23.9) | 0.01 | |
| Any SKE | 431 (87.8) | 47 (92.2) | 384 (87.3) | 0.37 | |
| Laboratory data up to 48 hr | |||||
| sCr, mg/dL | T0 | 1.05 (0.80-1.50) | 1.46 (0.97-2.10) | 1.02 (0.80-1.48) | 0.003 |
| T24 | 1.00 (0.80-1.43) | 1.84 (1.19-2.48) | 1.00 (0.75-1.28) | 0.001 | |
| T48 | 1.00 (0.72-1.36) | 2.00 (1.45-3.01) | 0.91 (0.70-1.20) | <0.001 | |
| % change | -7.1 (-22.0-4.1) | 42.3 (29.4-71.2) | -10.5 (-24.6-0.0) | <0.001 | |
| eGFR, mL/min/1.73 m2 | T0 | 68.0 (40.0-90.2) | 49.0 (28.8-82.3) | 69.8 (42.9-91.0) | 0.002 |
| T24 | 73.9 (43.0-92.0) | 34.0 (21.9-53.5) | 77.6 (50.0-95.0) | <0.001 | |
| T48 | 78.0 (49.0-96.0) | 29.4 (19.6-42.0) | 83.0 (54.6-97.9) | <0.001 | |
| % change | 4.7 (-2.7-24.4) | -31.2 (-44.2--25.9) | 9.4 (0.0-28.6) | <0.001 | |
| NC, (ng/mL)2/1,000 | T0 | 0.31 (0.10-0.98) | 0.77 (0.20-1.91) | 0.29 (0.09-0.88) | 0.001 |
| T48† | 0.19 (0.08-0.50) | 0.36 (0.12-2.10) | 0.18 (0.07-0.47) | 0.001 | |
| % change† | -23.7 (-77.2-82.6) | 0.0 (-79.0-125.0) | -28.9 (-76.6-75.0) | 0.575 | |
Data are presented as number (%) or median (interquartile range).
*Total N=491 (N=51 in the AKI group, N=440 in the non-AKI group); †Total N=447 (N=50 in the AKI group, N=397 in the non-AKI group); % change=(T48-T0)/T0x100.
Abbreviations: AKI, acute kidney injury; BMI, body mass index; BP, blood pressure; ED, emergency department; ED score, ED physician’s clinical assessment risk score for AKI development; CKD, chronic kidney diseases; CHD, congestive heart failures; AHF, acute heart failures; ACS, acute coronary syndromes; GI, gastrointestinal; SKE, serious kidney exposure; NSAIDs, non-steroidal anti-inflammatory drugs; sCr, serum creatinine; eGFR, estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation; NC, NephroCheck.
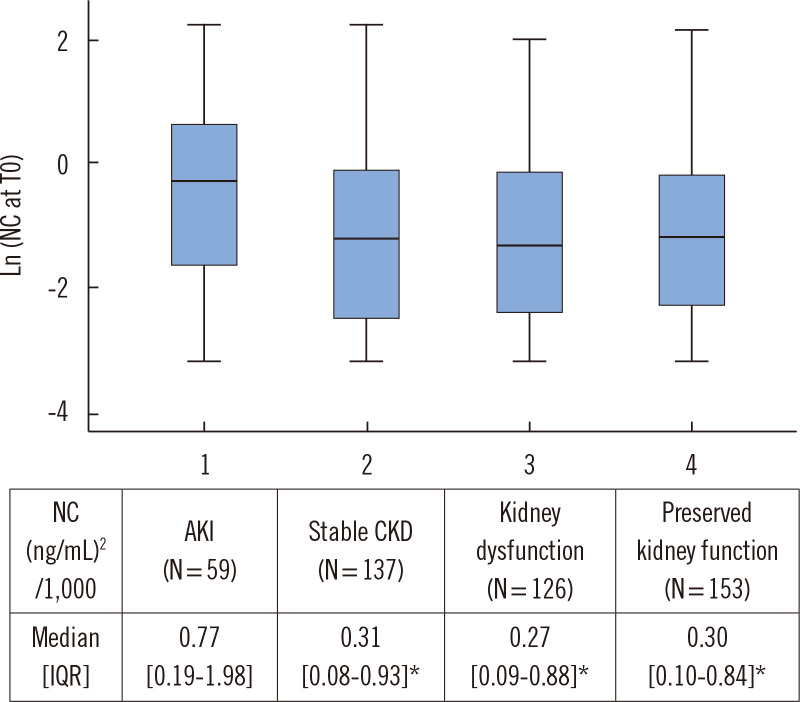
The NephroCheck value was significantly higher in the AKI group than in the non-AKI group at each time point (all P<0.05). In the AKI group, the NephroCheck value was relatively static (P=0.27), whereas in the non-AKI group, it declined significantly from T0 to T48 (P<0.001). Next, each subgroup of the non-AKI group was compared with the AKI group in terms of the T0 NephroCheck value (Fig. 2). All non-AKI subgroups showed significantly lower T0 NephroCheck values than the AKI group (P<0.001).
Fig. 2.
Box-and-whisker plot of NC at ED arrival (T0) based on kidney function classification: 1, AKI; 2, stable CKD; 3, kidney dysfunction; and 4, preserved kidney function. The table shows the median [IQR] of T0 NC values. *P<0.05 vs. AKI by independent-samples Kruskal–Wallis test and post-hoc pairwise comparisons with Bonferroni correction. NC values were log-transformed for the graphical display.
Abbreviations: see Table 1.
Prediction of AKI development
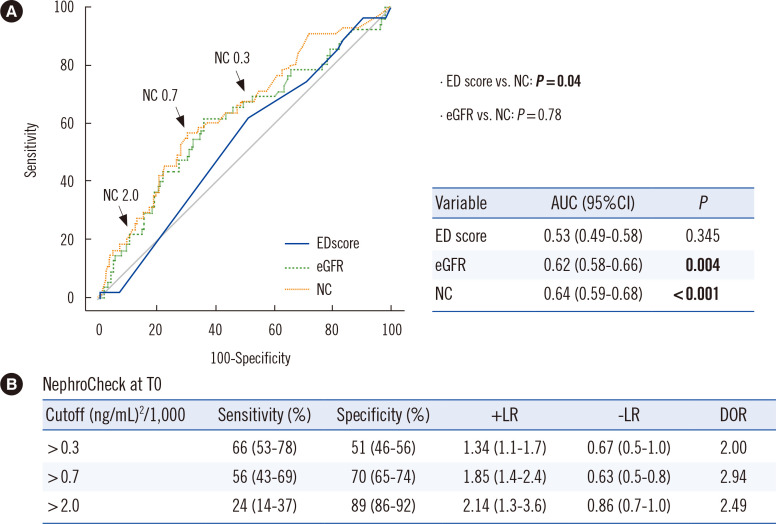
The T0 NephroCheck value predicted AKI development (AUC, 0.64; P<0.001), as did the eGFR value (AUC, 0.62; P=0.004), but not the ED score (AUC, 0.54; P=0.345). The NephroCheck value surpassed the ED score (P=0.04) (Fig. 3A). In the ROC curve of NephroCheck in Fig. 3B, the sensitivities and specificities of the two validated cutoffs for AKI risk assessment (0.3 or 2.0) as well as the Youden index-associated optimal cutoff of 0.7 are indicated. In the reclassification analyses, adding the NephroCheck value to the ED score or eGFR significantly improved the prediction of AKI development (all P<0.05) (Table 2).
Fig. 3.
Prediction of AKI development based on three parameters measured at ED arrival (T0). (A) In ROC curve analyses, NC better predicted AKI development than the ED score (P=0.04). (B) The sensitivity, specificity, and LR of the two validated NC cutoffs (>0.3 or >2.0) and an optimal cutoff (>0.7) are presented as % (95% CI).
Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC curve; CI, confidence interval; LR, likelihood ratio; DOR, diagnostic odds ratio; see Table 1.
Table 2.
Performance of NephroCheck as a biomarker of AKI development added to the conventional variables at T0
| Discrimination | Reclassification | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | P (vs. initial model*) | NRI (%, 95% CI) | P | IDI (%, 95% CI) | P | |
| ED score + NC | 0.71 (0.65-0.78) | 0.023 | 33.8 (4.3-60.2) | 0.012 | 2.4 (0.4-4.5) | 0.02 |
| eGFR + NC | 0.65 (0.57-0.74) | 0.14 | 27.4 (1.8-52.9) | 0.036 | 2.3 (0.2-4.3) | 0.031 |
The initial models are underlined, the updated models additionally included NC, P values are vs. the initial model.
Abbreviations: NRI, net reclassification improvement; IDI, integrated discrimination improvement; see Table 1.
Among the patients with a reduced eGFR, 37 out of 218 (17%) patients developed AKI, as predicted by the T0 NephroCheck value (AUC, 0.61; 95% confidence interval [CI], 0.50-0.71; P=0.043). The optimal cutoff NephroCheck value in patients with a reduced eGFR was 1.04, with a sensitivity of 49% and specificity of 72%. Even among the patients with a normal eGFR (≥60 mL/min/1.73 m2), 22 out of 311 (7.1%) patients developed AKI, as predicted by the T0 NephroCheck value (AUC, 0.65; 95% CI, 0.52-0.77; P=0.027). The optimal cutoff was 0.73 with sensitivity 55% and specificity 77%. In patients with confirmed or suspected sepsis, 37 of 300 (12.3%) patients developed AKI, which was predicted by the T0 NephroCheck value (AUC, 0.62; 95% CI, 0.56-0.67; P=0.018). The optimal NephroCheck value cutoff was 0.68, with a sensitivity of 60% and specificity of 62%.
Prediction of short-term morality
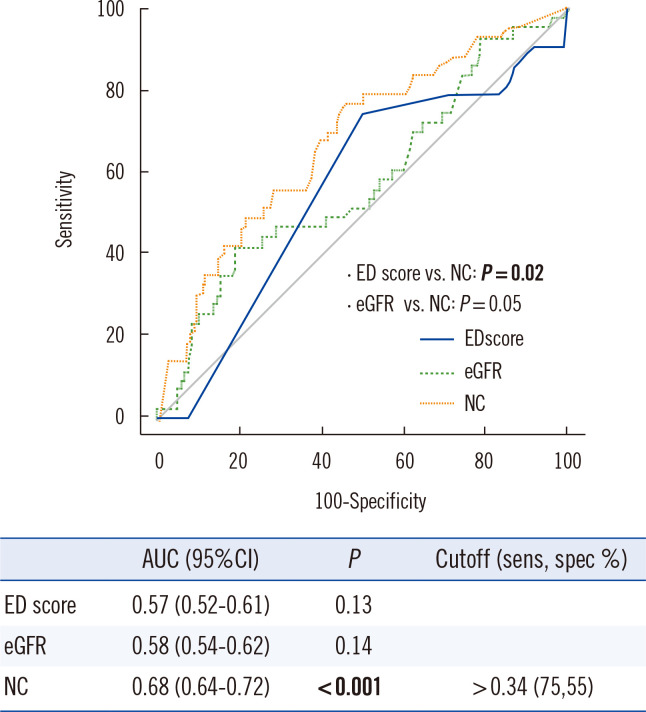
During the 90-day follow-up, 60 out of 425 (14%) patients (54 AKI, 371 non-AKI, and 104 missing patients) died, including 44 patients who died within 30 days and 13 who died in hospital. All-cause mortality was significantly higher in the AKI group than in the non-AKI group (16 patients [30%] vs. 44 patients [12%], P<0.001). The T0 NephroCheck value predicted the 30-day mortality (P<0.001), with an optimal cutoff of 0.34 (sensitivity, 75%; specificity, 55%) (Fig. 4). The T0 NephroCheck value also predicted the 90-day mortality (P=0.045), with an optimal cutoff of 0.99 (sensitivity, 42%; specificity, 77%).
Fig. 4.
Prediction of short-term mortality based on three parameters measured on ED arrival (T0). Thirty-day mortality was observed in 44 (8.3%) of the 529 patients. The AUC (95% CI) and optimal NC cutoff value (sensitivity and specificity) are presented in the table.
DISCUSSION
This is the first study to evaluate the predictive value of NephroCheck for AKI development and short-term mortality in a multicenter ED cohort. The NephroCheck value at T0 predicted the risk of AKI development, with an optimal cutoff of 0.7. Adding the T0 NephroCheck value to the ED score improved the latter’s predictive power for AKI (P=0.023). The T0 NephroCheck value predicted the 30-day mortality significantly better than the ED score (P=0.02), with an optimal cutoff of 0.3.
Since 2014, three key German studies on NephroCheck utility in ED patients have been published [22-24]. Kimmel, et al. [22] analyzed 298 patients admitted to the internal medicine service from the ED; stage 2-3 AKI developed in 15% (36/298) of the patients, and NephroCheck demonstrated additional diagnostic value to the clinical model; however, including both NephroCheck and sCr in the model was not significantly better than including either biomarker alone. In another ED study, stage 2-3 AKI developed in 28% (11/40) of patients with acute decompensated heart failure, and NephroCheck on the first day discriminated AKI at cutoffs of 0.3 (sensitivity, 86%) and 2.0 (specificity, 95%) [23]. More recently, a NephroCheck-guided randomized controlled intervention with nephrologist consultation in the ED in 100 patients with NephroCheck values >0.3 has been reported; this study did not predict significantly different AKI incidence between groups, possibly because of the use of a low rule-in NephroCheck value cutoff of >0.3 (ng/mL)2/1,000 [24].
Our study is unique in that we enrolled all ED patients, not only those with critical heart diseases, but also those with various acute diseases, in five Asia-Pacific countries. The AKI incidence (11.2%) was relatively low, probably due to heterogeneous etiologies and the large spectrum of conditions (ICU, 67%; general ward, 31%); it may reflect a real-world ED setting that requires patient triage at arrival. Compared with a similar study in an European ED population where the overall AKI incidence in ED patients was 7%, the proportion of AKI was higher in Asian ED patients with acute diseases [18]. In line with the German study results, NephroCheck was comparable to the eGFR in predicting AKI development, and their AUCs were marginal [22]. Although the AUC is the most popular metric, sole reliance on the AUC cannot reveal the clinical value or improvement of new biomarkers. The NRI and IDI should also be considered, and improvements in AUC, IDI, and NRI should lead to the same conclusions when assessing the performance of newer biomarkers [21].
Through reclassification and subgroup analyses, the addition of NephroCheck to the conventional parameters at T0 proved to improve the prediction of AKI development (Table 2). In a previous study using multivariate analysis, the initial sCr value was independently associated with AKI development later in the course of sepsis; the risk for AKI could already be estimated on the first day of sepsis, and the risk for AKI development increased 7.5 times when the sCr value was >1 mg/dL [25]. As AKI is currently defined based on a change in sCr, the initial sCr value alone is not sufficient for the diagnosis of AKI. Accordingly, NephroCheck may be useful to triage at-risk patients. Despite concerns about low sensitivity in sepsis patients, who might benefit from timely intervention, this study revealed that the T0 NephroCheck value predicted AKI development in patients with confirmed or suspected sepsis (P=0.02) [26]. Furthermore, our study demonstrated a significant prognostic value of NephroCheck in 30-day or 90-day mortality in a large ED cohort, which was not revealed in previous ED studies [23, 24].
Early rule-in AKI biomarkers should detect subclinical AKI (tubular damage biomarker positivity without dysfunction) [27]. In this study, NephroCheck showed potential as an early rule-in biomarker in two aspects. First, at T0 in the AKI group, an increased NephroCheck value signaled AKI. Second, in patients with normal eGFR (≥60 mL/min/1.73 m2), the T0 NephroCheck value predicted subclinical AKI (P=0.027), with an optimal cutoff of 0.7 (specificity, 77%). Thus, NephroCheck may be an early rule-in AKI biomarker detecting subclinical AKI at ED arrival, with a cutoff of 0.7. NephroCheck could predict the presence of subclinical AKI, satisfying the criteria currently recommended by the Acute Disease Quality Initiative (ADQI). Although the use of the term “subclinical AKI” is still debated, the ADQI has recommended that patients who are biomarker-positive/sCr-negative are classified as AKI 1S [28, 29].
Early rule-out AKI biomarkers should distinguish pre-existing CKD from the ongoing tubular damage superimposed on CKD by AKI. In this study, NephroCheck showed potential as an early rule-out biomarker, especially in patients with pre-existing CKD. The T0 NephroCheck value was significantly lower in the stable CKD group than in the AKI group. Thus, ED physicians may rule out the risk of AKI development (Fig. 2). Similarly, in patients with a reduced eGFR, the T0 NephroCheck value predicted AKI development (P=0.043). The validated cutoff of ≤0.3 was useful, with a negative predictive value of 92% (95% CI, 89%-95%).
In a previous study in critically ill ICU patients, a NephroCheck value >0.3 identified patients at risk of AKI development with a sensitivity and specificity of 92% and 46%, respectively; with increased cutoff of 2.0, the sensitivity was 46%, and the specificity was 95% [13]. In our study, the validated NephroCheck cutoffs of 0.3 and 2.0 identified patients at risk of AKI development, but with relatively low sensitivity and specificity, presumably because of heterogeneous clinical conditions (Fig. 3). In this multicenter ED cohort, the NephroCheck value increased on ED arrival and improved the prediction of AKI development when combined with the ED score. This finding supports biomarker-guided AKI risk assessment in the ED on top of the clinical context. It would be reasonable to rule in patients with a NephroCheck value >0.7. Similarly, AKI can be ruled out in patients with a NephroCheck value ≤0.3, even in the presence of reduced baseline kidney function. Although the validity of NephroCheck for predicting AKI or mortality has been proven in numerous studies, some studies monitoring AKI after surgical interventions using the NephroCheck cutoff of 0.3 showed negative outcomes [4, 30]. Accordingly, results derived from studies with different clinical settings, study purposes, and cutoffs cannot be extrapolated to the ED setting, where NephroCheck would not be utilized for AKI monitoring, but for AKI ruling-in and ruling-out.
This study had several limitations. First, it was conducted in five Asia-Pacific countries; further studies are awaited to generalize the results to other countries worldwide. Second, the ED score is subjective, and we did not evaluate combinations with other biomarkers, such as NGAL, KIM-1, and IL-18 [11, 12]. Although NephroCheck is the best-performing among these biomarkers, comparing the utility of NephroCheck with that of other biomarkers in a future ED cohort will be necessary [12]. Third, there were certain levels of data attrition at T48 and loss to follow-up at 90 days. However, the study purpose was to explore the T0 NephroCheck value for predicting AKI development and 30-day mortality, and for this purpose, the data attrition was inconsequential. Further studies on repeated measurements of biomarkers or new-onset CKD as defined by the persistence of kidney disease for >90 days are warranted [31]. Fourth, the subset of patients who were already recovering from AKI at ED arrival was not dealt with; a larger study population may be required to address whether the T0 NephroCheck value can detect this subset. Fifth, we used the KDIGO criteria to define AKI in the CKD subgroup. The current KDIGO definition may increase diagnostic false-positives in patients with stable CKD because of inherent laboratory and biological variabilities of sCr [32]. A new recommendation is to use a 20% change to define AKI when the sCr value is >1 mg/dL, and it has been independently correlated with mortality [33, 34]. In all AKI patients in the CKD group (13/95 patients, 22%), the sCr change (median, 42.3%) fulfilled this new recommendation. In conclusion, this was the first multicenter ED cohort study on NephroCheck, where NephroCheck could predict both AKI development and short-term (30- and 90-day) mortality. The application of NephroCheck in the ED may be useful for early ruling-in and ruling-out of AKI, with respective cutoffs of 0.7 and 0.3.
ACKNOWLEDGEMENTS
We acknowledge the technical and administrative assistance of Ress K and Sackett H, New South Wales Health Pathology, Pearse J and Gray Z, Department of Nephrology, and Murphy D and Davis M, Department of Emergency Medicine, Prince of Wales Hospital, Sydney, Australia; Jee Y and Khaing NW, Emergency Medicine Department, National University Hospital, National University Health System, Singapore; Choi SK, Department of Laboratory Medicine, Konkuk University Medical Center, Seoul, Korea; and all the other laboratory and clinical personnel from Thailand and India.
Footnotes
AUTHOR CONTRIBUTIONS
Yang HS analyzed the data and wrote the draft; Hur M and Di Somma S conceived and designed the study, analyzed the data, and finalized the draft; Lee KR, Kim H, Kim HY, Kim JW, Chua MT, Kuan WS, Chua HR, Kitiyakara C, Phattharapornjaroen P, Werayachankul T, Chittamma A, Anandh U, Herath S, Endre Z, Horvath AR, and Antonini P collected the samples, analyzed the data, and participated in the drafting. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
None declared
RESEARCH FUNDING
This study (registered at ClinicalTrials.gov; NCT03754023) was supported by Ortho Clinical Diagnostics (Raritan, NJ, USA).
REFERENCES
- 1.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–93. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Group KDIGOAKIW. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. doi: 10.1053/j.ajkd.2013.02.349,. [DOI] [PubMed] [Google Scholar]
- 4.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–61. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervellin G, di Somma S. Neutrophil gelatinase-associated lipocalin (NGAL): the clinician's perspective. Clin Chem Lab Med. 2012;50:1489–93. doi: 10.1515/cclm-2012-0433. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative Workgroup, author. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 10.Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am J Physiol Renal Physiol. 2011;301:F697–707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan W, Ankawi G, Zhang J, Digvijay K, Giavarina D, Yin Y, et al. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med. 2019;57:567–76. doi: 10.1515/cclm-2018-0776. [DOI] [PubMed] [Google Scholar]
- 13.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–9. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration letter to Astute Medical, author. [Updated on Sep 2014]; http://www.accessdata.fda.gov/cdrh_docs/pdf13/den130031.pdf .
- 15.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, et al. Clinical use of the urine biomarker [TIMP-2]×[IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilaria G, Kianoush K, Ruxandra B, Francesca M, Mariarosa C, Davide G, et al. Clinical adoption of Nephrocheck in the early detection of acute kidney injury. Ann Clin Biochem. 2021;58:6–15. doi: 10.1177/0004563220970032. [DOI] [PubMed] [Google Scholar]
- 17.McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–22. doi: 10.1161/01.CIR.0000025242.79963.4C. [DOI] [PubMed] [Google Scholar]
- 18.Di Somma S, Magrini L, De Berardinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013;17:R29. doi: 10.1186/cc12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssens ACJW, Martens FK. Reflection on modern methods: revisiting the area under the ROC curve. Int J Epidemiol. 2020:1397–403. doi: 10.1093/ije/dyz274. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino Sr RB, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. discussion 207–12. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel M, Shi J, Latus J, Wasser C, Kitterer D, Braun N, et al. Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol. 2016;11:938–46. doi: 10.2215/CJN.10551015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40:485–91. doi: 10.1002/clc.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schanz M, Wasser C, Allgaeuer S, Schricker S, Dippon J, Alscher MD, et al. Urinary [TIMP-2]∙[IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant. 2019;34:1902–9. doi: 10.1093/ndt/gfy186. [DOI] [PubMed] [Google Scholar]
- 25.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–30. doi: 10.1097/01.ASN.0000059863.48590.E9. [DOI] [PubMed] [Google Scholar]
- 26.Johnson ACM, Zager RA. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol. 2018;29:2157–67. doi: 10.1681/ASN.2018030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16:313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 29.Albert C, Haase M, Albert A, Zapf A, Braun-Dullaeus RC, Haase-Fielitz A. Biomarker-guided risk assessment for acute kidney injury: time for clinical implementation? Ann Lab Med. 2021;41:1–15. doi: 10.3343/alm.2021.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267:1013–20. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 31.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–57. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Fernandez H, Shashaty MG, Negoianu D, Testani JM, Berns JS, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10:1723–31. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Khoury JM, Hoenig MP, Jones GRD, Lamb EJ, Parikh CR, Tolan NV, et al. AACC Guidance Document on laboratory investigation of acute kidney injury. J Appl Lab Med. 2021 May 11;:jfab020. doi: 10.1093/jalm/jfab020. doi: 10.1093/jalm/jfab020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Yeh HC, Lo YC, Ting IW, Chu PL, Chang SN, Chiang HY, et al. 24-hour serum creatinine variation associates with short- and long-term all-cause mortality: a real-world insight into early detection of acute kidney injury. Sci Rep. 2020;10:6552. doi: 10.1038/s41598-020-63315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]