Abstract
Background
Extraintestinal pathogenic Escherichia coli (ExPEC) causes various infections, including urinary tract infection (UTI), sepsis, and neonatal meningitis. ExPEC strains have virulence factors (VFs) that facilitate infection by allowing bacterial cells to migrate into and multiply within the host. We compared the microbiological characteristics of ExPEC isolates from blood and urine specimens from UTI patients.
Methods
We conducted a single-center, prospective study in an 855-bed tertiary-care hospital in Korea. We consecutively recruited 80 hospitalized UTI patients with E. coli isolates, which were isolated from blood and/or urine, and urine alone between March 2019 and May 2020. We evaluated the 80 E. coli isolates for the presence of bacterial genes encoding the sequence types (STs), antimicrobial resistance, and VFs using whole-genome sequencing (WGS).
Results
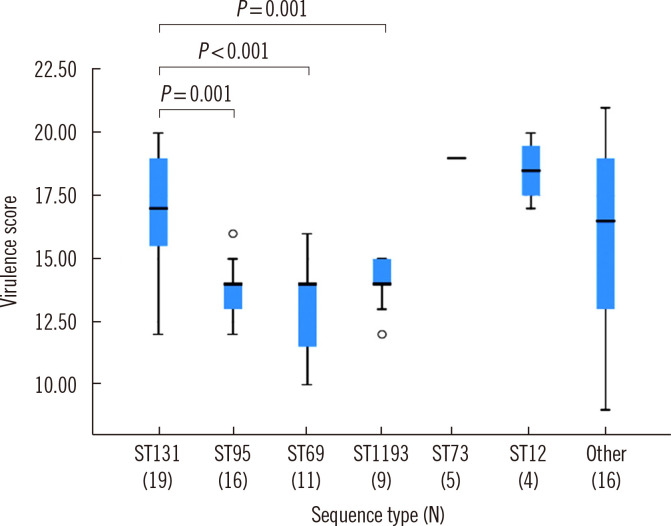
We found no significant differences in STs, antimicrobial resistance patterns, or VFs between isolates from blood and urine specimens. ST131, a pandemic multidrug-resistant clone present in both blood and urine, was the most frequent ST (N=19/80, 24%), and ST131 isolates carried more virulence genes, especially, tsh and espC, than non-ST131 isolates. The virulence scores of the ST131 group and the ST69, ST95, and ST1193 groups differed significantly (P<0.05).
Conclusions
We found no STs and VFs associated with bacteremia in WGS data of E. coli isolates from UTI patients. ST131 was the most frequent ST among UTI causing isolates and carried more VF genes than non-ST131 isolates.
Keywords: Escherichia coli, ST131, Urinary tract infection, Whole-genome sequencing, tsh, Virulence factors
INTRODUCTION
Extraintestinal pathogenic Escherichia coli (ExPEC) causes various infections, including urinary tract infection (UTI), sepsis, and neonatal meningitis [1, 2]. The mechanisms underlying ExPEC transmission and the selection of resistant clones are poorly understood.
Among multidrug-resistant ExPEC strains, the most frequent sequence type (ST) is ST131, which is globally disseminated and resistant to multiple antibiotics [3, 4]. Recently, ST1193, which is resistant to fluoroquinolones, has spread rapidly [4]. ExPEC strains have virulence factors (VFs), including adhesion molecules, iron acquisition systems, invasion proteins, and toxins, which facilitate infection by allowing bacterial cells to migrate into and multiply within the host. Several ExPEC VF genes, including pap, vat, kpsMII, ibeA, and clbB/N, are significantly associated with the dissemination of ExPEC isolates [5, 6]. The yersiniabactin siderophore receptor encoded by fyuA is involved in the efficient uptake of iron from the bloodstream and invasion of the bloodstream from the urinary tract [7]. Pneumonia-specific E. coli isolates carry higher proportions of the VF genes sfa/foc, papGIII, hlyC, cnf1, and iroN than bacteremia isolates [8]. The gene cnf1 encodes cytotoxic necrotizing factor 1, a toxin associated with sepsis severity, whereas the presence of fyuA is associated with mortality. Further, the presence of P fimbriae is associated with improved bacterial survival [9, 10]. The presence of hek/hra may predict clinical outcomes, as one study showed an association between the presence of these genes and mortality in newborns [11].
ExPEC strains involved in UTIs are believed to express a diverse repertoire of VFs to colonize and infect the urinary tract in an ascending manner [1]. However, the role of VFs in the pathogenesis and clinical outcomes of E. coli bacteremia remains to be investigated. We hypothesized that some VFs of ExPEC are involved in bacterial movement into the bloodstream in UTI patients, resulting in urosepsis. Therefore, we compared the repertoire of STs and VF genes between isolates from urine and blood from UTI patients using whole-genome sequencing (WGS) analysis.
MATERIALS AND METHODS
Patients and definitions
We conducted a prospective study in the 855-bed Hanyang University Hospital, Seoul, Korea. We consecutively recruited hospitalized UTI patients with E. coli isolates from blood and/or urine (N=40) and with E. coli isolates from urine alone (N=40), between March 2019 and May 2020. Patients with UTI were defined as those who met all of the following criteria: (i) fever (body temperature ≥37.8°C), (ii) pyuria (≥5–9 white blood cells per high-power field), (iii) clinical symptoms or signs relevant to UTI as judged by an infectious disease specialist, and (iv) absence of other medical conditions that can cause fever or pyuria. We collected clinical data, including demographic data (age and sex), time lag between fever or UTI symptoms (dysuria, frequency, urgency, or nocturia) and bacterial culture sampling, history of antibiotic use prior to bacterial culture sampling, and existence of underlying urinary tract abnormalities. Antibiotic regimens were considered concordant, if they included at least one antibiotic active against the causative organisms based on in vitro susceptibility testing. The clinical factors that may have influenced the culture results of blood and urine specimens, are listed in Table 1. No significant differences were found in these clinical factors. This study was approved by the Hanyang University Hospital Institutional Review Board, with waiver of informed consent (IRB N 201905054).
Table 1.
Clinical factors possibly influencing the culture results of blood and urine specimens
| Clinical factor (%) | Blood (N=40) | Urine (N=40) | P |
|---|---|---|---|
| Age, median (IQR) | 72.5 (59–81) | 70 (54.5–78.5) | 0.473 |
| Male sex, N (%) | 4 (10.0) | 2 (5.0) | 0.675 |
| Time lag between fever or UTI symptom onset and bacterial culture sampling, days, median (IQR) | 2 (1–3.75) | 1 (0–4) | 0.382 |
| History of antibiotic use within three days prior to bacterial culture sampling | 8/39 (20.5) | 7 (17.5) | 0.733 |
| Antimicrobial use concordant with the antimicrobial susceptibility of the causative organisms | 3/39 (7.7) | 1 (2.5) | 0.359 |
| Existence of any underlying urinary tract abnormalities | 18 (45.0) | 17 (42.5) | 0.822 |
| Benign prostatic hypertrophy among male patients | 1/4 (25.0) | 2/2 (100) | 0.400 |
| Neurogenic bladder | 1 (2.5) | 2 (5.0) | 1.000 |
| Urolithiasis | 12 (30.0) | 5 (12.5) | 0.056 |
| Urinary retention | 0 (0) | 1 (2.5) | 1.000 |
| Vesicoureteral reflux | 0 (0) | 0 (0) | - |
| Bladder diverticulum | 0 (0) | 1 (2.5) | 1.000 |
| Prolapse of vagina among female patients | 0 (0) | 0 (0) | - |
| Intubated urinary tract at the time of culture sampling | 5 (12.5) | 9 (22.5) | 0.239 |
| Intermittent catheterization | 0 (0) | 2 (5.0) | 0.494 |
| History of urinary catheterization during one month prior to inclusion | 4 (10.0) | 3 (7.5) | 1.000 |
| Polycystic kidney | 0 (0) | 0 (0) | - |
| Kidney tumor | 0 (0) | 1 (2.5) | 1.000 |
| Urinary tract surgery during three months prior to inclusion | 2 (5.0) | 1 (2.5) | 1.000 |
| Surgically reconstructed bladder | 0 (0) | 0 (0) | - |
Abbreviations: IQR, interquartile range; UTI, urinary tract infection.
Bacterial culture and antimicrobial susceptibility testing
Blood culture bottles were incubated in the BACT/ALERT VIRTUO System (bioMérieux, Marcy l’Étoile, France). Urine specimens were inoculated on blood agar and MacConkey agar plates, and the plates were incubated at 35°C for 24 hours. Bacterial species were identified by matrix-assisted laser desorption/ionization (MALDI) time-of-flight mass spectrometry using the MALDI Biotyper system and related software (version 2.3, Bruker Daltonics, Bremen, Germany). Antimicrobial susceptibility was tested using the MicroScan Walkaway system (Beckman Coulter, Brea, CA, USA), and the results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, with intermediate isolates designated as resistant [12]. All isolates were maintained in 20% skim milk at –80°C.
WGS analysis
Bacterial isolates cultured on MacConkey agar plates were sent to Macrogen (Seoul, Korea) for WGS that was performed using the Illumina system (Illumina, Inc., San Diego, CA, USA). De novo assembly and assembly validation were performed for all 80 isolates. WGS data were analyzed using the Center for Genomic Epidemiology website (http://www.genomicepidemiology.org/), and multilocus ST (MLST), serotype, fimH type, antimicrobial resistance gene repertoire, and VF repertoire were determined. The presence of several virulence traits was determined using the VF analyzer pipeline and VF database (http://www.mgc.ac.cn/VFs/) [13]. Virulence scores were calculated as the sum of VFs present in each isolate [5].
Statistical analysis
The chi-square test (Fisher’s exact test) was used to compare clinical factors, ST, antimicrobial resistance rates between blood and urine groups, and the Kruskal–Wallis test was used to compare virulence scores according to ST. Data was analyzed using SPSS 26 (SAS Institute Inc., Cary, NC, USA), and P<0.05 was considered statistically significant.
RESULTS
Molecular epidemiological characteristics
The MLST results revealed a diverse population comprising 18 STs (Table 2). The most frequent STs were ST131 (N=19, 24%), ST95 (N=16, 20%), ST69 (N=11, 14%), and ST1193 (N=9, 11%). We detected five ST73 isolates and two ST127 isolates. There were no significant differences in ST frequencies between the blood and urine specimens. ST12 was more frequently found in urine specimens than in blood specimens (P=0.04), but only four isolates were obtained.
Table 2.
Number of isolates (%) according to MLST in blood and urine specimens
| ST-fimH-serotype | Blood (N=40) | Urine (N=40) | P |
|---|---|---|---|
| ST131 | 8 (20) | 11 (28) | 0.431 |
| ST131-fimH30-O25:H4 | 6 (15) | 8 (0.556) | 0.556 |
| ST131-fimH41-O16:H5 | 2 (5) | 2 (1.000) | 1.000 |
| ST131-fimH99-O25:H4 | 0 (0) | 1 (0.314) | 0.314 |
| ST95 | 9 (23) | 7 (18) | 0.576 |
| ST95-fimH27-O2:H4 | 6 (15) | 3 (0.288) | 0.288 |
| ST95-fimH27-O2:H7 | 1 (3) | 1 (1.000) | 1.000 |
| ST95-fimH41 | 2 (5) | 2 (1.000) | 1.000 |
| ST95-fimH54 | 0 (0) | 1 (0.314) | 0.314 |
| ST69-fimH27 | 8 (20) | 3 (8) | 0.105 |
| ST1193 | 4 (10) | 5 (13) | 0.723 |
| ST1193-fimH64-O75:H5 | 3 (8) | 4 (0.692) | 0.692 |
| ST1193-fimH64-O18:H5 | 1 (3) | 1 (1.000) | 1.000 |
| ST73 | 3 (8) | 2 (5) | 0.644 |
| ST12 | 0 (0) | 4 (10) | 0.040 |
| ST38 | 1 (3) | 2 (5) | 0.556 |
| ST127 | 0 (0) | 2 (5) | 0.152 |
| ST357 | 1 (3) | 1 (3) | 1.000 |
| Others* | 6 (15) | 3 (8) | 0.288 |
*Nine isolates belonging to ST10, ST14, ST70, ST372, ST394, ST421, ST457, ST998, and ST2613.
Abbreviations: ST, sequence type; MLST, multilocus sequence type.
FimH typing revealed that fimH27 isolates were the most frequent (N=22, 28%), followed by fimH30 isolates (N=14, 18%). We also determined fimH-OH serotype clonal groups among the ST isolates. Among the 19 ST131 isolates, the ST131-fimH30-O25:H4 group was predominant (N=14, 74%). Among the 16 ST95 isolates, ST95-fimH27 isolates were the most frequent (N=11, 69%). All nine ST1193 isolates were fimH64, whereas this type was not found among non-ST1193 isolates.
Antimicrobial resistance patterns and antimicrobial resistance genes
The ampicillin and ampicillin/sulbactam resistance rates were 72.5% and 40.0%, respectively, for blood isolates and 75.0% and 45.0%, respectively, for urine isolates. In addition, 20.0% of blood isolates and 27.5% of urine isolates were extended spectrum β-lactamase (ESBL) producers. We found no significant differences in antimicrobial resistance rates between blood and urine isolates (Table 3). However, ST131 and ST1193 isolates showed higher resistance rates than the other isolates. The cefotaxime, ceftazidime, aztreonam, and cefepime resistance rates of ST131 isolates (78.9%) were higher than those of non-ST131 isolates (0%–12.0%). Among the 19 ST131 isolates, 15 carried ESBL-associated genes including CTX-M-15, CTX-M-27, CTX-M-55, and CTX-M-14. CTX-M-14 genes were detected in ST95, ST38, and ST457, and the DHA-1 gene was detected in one ST12 isolate (Table 4). Of the 80 E. coli isolates, 27 (33.8%) were resistant to fluoroquinolone. Among them, the most prevalent clonal group was ST131-fimH30, followed by ST1193-fimH64. All ST1193 isolates were resistant to ciprofloxacin. Quinolone resistance analysis revealed quinolone resistance-determining region (QRDR) point variants in gyrA, parC, and parE. All 27 fluoroquinolone-resistant isolates carried the variations S83L and D87N or D87Y in gyrA;S80I and/or E84V in parC were detected in 24/27 (89%) isolates, and I529L, L416F, and S458A in parE were detected in 23/27 (78%) isolates.
Table 3.
Antimicrobial resistance rates (%) according to ST and specimen type
| Antimicrobial agent | ST (N of isolates) | Specimen type (N isolates) | |||||
|---|---|---|---|---|---|---|---|
| ST131 (19) | ST95 (16) | ST69 (11) | ST1193 (9) | Others (25) | Blood (40) | Urine (40) | |
| Ampicillin | 89.5 | 31.3 | 81.8 | 88.9 | 80.0 | 72.5 | 75.0 |
| Piperacillin | 84.2 | 31.3 | 81.8 | 88.9 | 72.0 | 72.5 | 67.5 |
| Cefoxitin | 0 | 0 | 0 | 11.1 | 4.0 | 0 | 5.0 |
| Ceftazidime | 78.9 | 6.3 | 0 | 0.0 | 12.0 | 20.0 | 27.5 |
| Cefotaxime | 78.9 | 6.3 | 0 | 11.1 | 12.0 | 20.0 | 30.0 |
| Cefuroxime | 78.9 | 6.3 | 9.1 | 11.1 | 12.0 | 22.5 | 30.0 |
| Aztreonam | 78.9 | 6.3 | 0 | 11.1 | 12.0 | 22.5 | 27.5 |
| Cefepime | 78.9 | 6.3 | 0.0 | 0.0 | 12.0 | 20.0 | 27.5 |
| Ampicillin/sulbactam | 31.6 | 12.5 | 63.6 | 55.6 | 56.0 | 40.0 | 45.0 |
| Amoxicillin/clavulanate | 26.3 | 0.0 | 9.1 | 11.1 | 12.0 | 2.5 | 22.5 |
| Piperacillin/tazobactam | 5.3 | 0 | 9.1 | 0 | - | 2.5 | 2.5 |
| Doripenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ertapenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Meropenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imipenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amikacin | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin | 47.4 | 0 | 27.3 | 22.2 | 16.0 | 25.0 | 20.0 |
| Tobramycin | 52.6 | 0.0 | 27.3 | 22.2 | 8.0 | 22.5 | 20.0 |
| Trimethoprim/sulfamethoxazole | 52.6 | 12.5 | 45.5 | 33.3 | 40.0 | 42.5 | 32.5 |
| Ciprofloxacin | 73.7 | 6.3 | 9.1 | 100.0 | 8.0 | 30.0 | 37.5 |
| Levofloxacin | 73.7 | 0 | 0 | 88.9 | 8.0 | 22.5 | 37.5 |
| Chloramphenicol | 0 | 6.3 | 0 | 0.0 | 8.0 | 2.5 | 5.0 |
| Fosfomycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetracycline | 57.9 | 12.5 | 18.2 | 33.3 | 32.0 | 30.0 | 35.0 |
| Minocycline | 0 | 0 | 0 | 11.1 | 16.0 | 5.0 | 7.5 |
| Tigecycline | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colistin | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ESBL positive | 78.9 | 6.3 | 0 | 0 | 12.0 | 20.0 | 27.5 |
Resistance rates >50% are indicated in bold.
Abbreviations: ST, sequence type; ESBL, extended-spectrum β-lactamase.
Table 4.
Antimicrobial resistance mechanisms of ESBL-producing and fluoroquinolone-resistant isolates according to ST-fimH-serotype
| ST-fimH-serotype | Antimicrobial resistance mechanism | N isolates | |||
|---|---|---|---|---|---|
| ESBL gene | QRDR variation in | ||||
| gyrA | parC | parE | |||
| ST131-fimH30-O25:H4 | CTX-M-15 | S83L, D87N | S80I, E84V | I529L | 8 |
| CTX-M-14, 27 | S83L, D87N | S80I, E84V | I529L | 2 | |
| CTX-M-55 | S83L, D87N | S80I, E84V | I529L | 2 | |
| - | S83L, D87N | S80I, E84V | I529L | 1 | |
| ST131-fimH41-O16:H5 | CTX-M-27 | S83L | - | I529L | 2 |
| ST131-fimH99-O25:H4 | CTX-M-27 | S83L, D87N | S80I, E84V | I529L | 1 |
| ST95-fimH41-O1:H7 | CTX-M-14 | - | - | - | 1 |
| ST95-fimH27-O2:H4 | - | S83L, D87Y | S80R | - | 1 |
| ST38-fimH27-O86:H18 | CTX-M-14 | S83L, D87Y | S80I | S458A | 1 |
| ST38-fimH65-O25:H15 | - | S83L, D87N | S80I, E84V | - | 1 |
| ST457-fimH145-O11:H25 | CTX-M-14 | - | - | - | 1 |
| ST12-fimH27-O4:H5 | DHA-1 | - | - | - | 1 |
| ST1193-fimH64-O75:H5 | - | S83L, D87N | S80I | L416F | 5 |
| - | S83L, D87N | - | - | 2 | |
| ST1193-fimH64-O18:H5 | - | S83L, D87N | S80I | L416F | 1 |
| - | S83L, D87N | - | - | 1 | |
| ST69-fimH27-O17:H18 | - | S83L, D87N | S80I | - | 1 |
Abbreviations: ESBL, extended-spectrum β-lactamase; QRDR, quinolone resistance-determining region; -, not detected; ST: sequence type.
Prevalence of VFs
Reference strains were uropathogenic E. coli (UPEC) strains (E. coli CFT073, E. coli O25b:H4-ST131, and E. coli VR50), adherent and invasive E. coli strains (E. coli UM146 and E. coli O83:H1 strain NRG 857C), neonatal meningitis E. coli (NMEC) strains (E. coli O7:K1 strain CE10 and E. coli O45:K1:H7), and an enterohemorrhagic E. coli strain (E. coli O157:H7 strain EDL933). In total, 56 VFs were examined and categorized as adhesion molecules, autotransporter systems, invasion proteins, or toxins (Table 5). All isolates carried E. coli common pilus, hemorrhagic E. coli pilus, and EaeH. The frequencies of individual VFs according to ST ranged from 91%–100% for type I fimbriae and 64%–100% for P fimbriae. F1C or S fimbriae were not detected in prevalent isolates, such as ST131, ST95, ST69, and ST1193. Among VFs associated with autotransporter systems, the temperature-sensitive hemagglutinin gene (tsh) was detected in all ST131 and ST1193 isolates, 6% of ST95 isolates, and 0% of ST69 isolates. Among invasion VFs, ibeA, associated with neonatal meningitis, was detected in only four (5%) isolates, including ST357, ST372, and ST998 isolates. Iron uptake systems, such as the sitABCD pump and chuA system, were detected in nearly all isolates (98%) (Table 5). Yersiniabactin, aerobactin, and salmochelin siderophores, which are indirect iron uptake systems, were detected in 99%, 63%, and 29% of the isolates, respectively.
Table 5.
Proportions (%) of VFs according to ST and specimen type
| VF | VF gene | ST (N isolates) | Specimen type (N isolates) | Total (80) | |||||
|---|---|---|---|---|---|---|---|---|---|
| ST131 (19) | ST95 (16) | ST69 (11) | ST1193 (9) | Others (25) | Blood (40) | Urine (40) | |||
| Adherence | |||||||||
| E. coli common pilus | ecp | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Hemorrhagic E. coli pilus | hcp | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| EaeH | eaeH | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Type I fimbriae | fim | 100 | 100 | 91 | 100 | 100 | 100 | 100 | 100 |
| P fimbriae | pap | 95 | 94 | 64 | 100 | 80 | 85 | 88 | 86 |
| F1C fimbriae | foc | 0 | 0 | 0 | 0 | 52 | 13 | 20 | 16 |
| Afimbrial adhesin AFA-I | afa, draP | 16 | 6 | 9 | 0 | 32 | 18 | 15 | 16 |
| S fimbriae | sfa | 0 | 0 | 0 | 0 | 40 | 10 | 15 | 13 |
| AAF/II fimbriae | aaf | 21 | 0 | 9 | 0 | 8 | 15 | 3 | 9 |
| E. coli laminin-binding fimbriae | elf | 0 | 0 | 0 | 0 | 24 | 8 | 8 | 8 |
| CFA/I fimbriae | cfa | 0 | 0 | 0 | 0 | 16 | 5 | 5 | 5 |
| AAF/III fimbriae | agg3 | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 1 |
| K88 fimbriae | fae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Curli fibers | cgs, csg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EtpA | etpA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dispersin | aap | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intimin | eae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Porcine attaching-effacing associated protein | paa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ToxB | toxB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Autotransporters | |||||||||
| UpaG adhesin, trimeric AT | upaG | 95 | 100 | 100 | 100 | 88 | 95 | 95 | 95 |
| EhaB, AIDA-I type | ehaB | 89 | 88 | 100 | 100 | 88 | 93 | 90 | 91 |
| Antigen 43, AIDA-I type | agn43 | 89 | 69 | 45 | 100 | 60 | 70 | 73 | 71 |
| Temperature-sensitive hemagglutinin | tsh | 100 | 6 | 0 | 100 | 32 | 40 | 53 | 46 |
| Sat | sat | 79 | 0 | 55 | 89 | 32 | 55 | 38 | 46 |
| Vacuolating autotransporter gene | vat | 0 | 81 | 9 | 44 | 68 | 45 | 43 | 44 |
| Cah, AIDA-I type | cah | 63 | 38 | 18 | 0 | 44 | 35 | 43 | 39 |
| EspC, SPATE | espC | 100 | 6 | 0 | 0 | 16 | 25 | 35 | 30 |
| Enteroaggregative immunoglobulin repeat protein | air/eaeX | 0 | 0 | 100 | 0 | 24 | 28 | 15 | 21 |
| Contact-dependent growth inhibition system | cdi | 11 | 0 | 0 | 0 | 48 | 10 | 25 | 18 |
| UpaH, AIDA-I type | upaH | 0 | 0 | 9 | 33 | 12 | 10 | 8 | 9 |
| Pic | pic | 0 | 0 | 0 | 0 | 24 | 13 | 3 | 8 |
| AIDA-I | aida | 11 | 0 | 0 | 0 | 0 | 5 | 0 | 3 |
| EhaA, AIDA-I type | ehaA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EspI, SPATE | espl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pet, SPATE | pet | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AIDA-I type | tibA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AatA, AIDA-I type | aatA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EspP | espl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Invasion | |||||||||
| Invasion of brain endothelial cells | ibeABC | 0 | 0 | 0 | 0 | 16 | 5 | 5 | 5 |
| Tia/Hek | tia | 53 | 88 | 18 | 0 | 68 | 53 | 55 | 54 |
| Iron uptake | |||||||||
| Iron/manganese transport | sitA | 100 | 100 | 100 | 100 | 92 | 98 | 98 | 98 |
| Heme uptake | chuA | 100 | 100 | 91 | 100 | 96 | 98 | 98 | 98 |
| Yersiniabactin siderophore | fyuA | 100 | 100 | 91 | 100 | 100 | 98 | 100 | 99 |
| Aerobactin siderophore | iucC | 74 | 44 | 73 | 89 | 52 | 73 | 53 | 63 |
| Iron-regulated element | ireA | 5 | 88 | 18 | 0 | 44 | 40 | 30 | 35 |
| Salmochelin siderophore | iroN | 5 | 13 | 27 | 0 | 68 | 28 | 30 | 29 |
| Toxins | |||||||||
| Hemolysin/cytolysin A | hlyE/clyA | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Colicin-like Usp | usp | 100 | 100 | 0 | 100 | 72 | 73 | 83 | 78 |
| Enterotoxin SenB/TieB | senB | 79 | 38 | 45 | 56 | 52 | 53 | 58 | 55 |
| Alpha-hemolysin | hlyABD | 53 | 0 | 27 | 0 | 60 | 28 | 43 | 35 |
| Cytotoxic necrotizing factor 1 | cnf1 | 53 | 0 | 0 | 0 | 48 | 20 | 35 | 28 |
| Cytolethal distending toxin | cdtB | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 1 |
| Shiga-like toxin | stx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Heat-labile enterotoxin | elt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Heat-stable enterotoxin 1 | astA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enterotoxin 1 | set1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: ST, sequence type; VF, virulence factor; CFA, colonization factor antigen; AAF, aggregative adhesion fimbria; SPATE, serine protease autotransporters of Enterobacteriaceae; sat, secreted autotransporter toxin; AIDA, adhesin involved in diffuse adherence.
In ST131 and ST1193 isolates, yersiniabactin and aerobactin siderophores were more frequent than salmochelin siderophores. In contrast, salmochelin siderophores were more frequent (68%) in other isolates than ST131 (5%) and ST1193 (0%) isolates. Several toxin genes were detected in UTI-associated isolates. The hlyE gene was detected in all isolates. The usp and senB genes were predominant in 78% and 55%, respectively, of the isolates. The alpha-hemolysin genes, hlyA, hlyB, and hlyD, were detected in 35% of the isolates. These toxin genes were more frequent in the ST131 group (53%) than in the non-ST131 group (30%), but the difference was not significant (P=0.07). The cnf1 gene was more frequent in the ST131 group (53%) than in the non-ST131 groups (20%) (P<0.05).
The median virulence score for all 80 isolates was 15 (range, 0–21). Virulence scores differed significantly among ST groups (Fig. 1). ST12 and ST73 isolates had high virulence scores, ranging from 17–20.
Fig. 1.
Box plot with median and interquartile range of virulence scores according to ST. Virulence scores were calculated based on the number of VFs present and compared using the Kruskal–Wallis test.
Abbreviations: ST, sequence type; VF, virulence factor.
DISCUSSION
We hypothesized that we could define specific VFs of E. coli linked to bacterial movement from the urinary tract to the circulation. However, virulence properties as well as molecular epidemiological traits and antimicrobial resistance patterns showed no significant differences between blood and urine isolates from our cohort of UTI patients, suggesting that specific VFs of E. coli do not contribute to bacterial dissemination from the urinary tract to the circulation. However, specific isolates, such as ST131, carried more VF genes and exhibited higher antimicrobial resistance than the other isolates evaluated.
We classified isolates according to ST-fimH-OH serotype clonal groups using WGS. The ST131-fimH30-O25:H4 type was the most predominant and was highly resistant to several antibiotics, including β-lactams and fluoroquinolones. Among the 14 ST131-fimH30-O25:H4 isolates, 12 produced CTX-M type EBSLs and carried variations in gyrA, parC, and parE. The next three most prevalent ST-fimH isolates were ST95-fimH27, ST69-fimH27, and ST1193-fimH64. ST131 is the predominant E. coli lineage among multidrug-resistant ExPEC isolates worldwide [14]. Most ST131 isolates are fluoroquinolone-resistant, many are co-resistant to aminoglycosides and cotrimoxazole, and a minority produce ESBLs, such as CTX-M enzymes [5, 14]. In our study, 78.9% of the ST131 isolates produced CTX-M ESBLs and were resistant to fluoroquinolones, and 52.6% of these were co-resistant to aminoglycosides and cotrimoxazole. After the rapid expansion of ST131 E. coli strains, fluoroquinolone-resistant ST1193 isolates emerged [4]. ST1193 strains, derived from ST14, are commonly resistant to fluoroquinolones. fimH64 is a highly specific marker for ST1193, a clonal group that may be entirely fluoroquinolone-resistant [4]. Consistent herewith, all ST1193-fimH64 isolates in our study were resistant to fluoroquinolones.
Adhesins, including fimbriae and afimbrial adhesins, play a significant role in host cell colonization [7, 15]. We detected type I fimbriae, E. coli common pilus, hemorrhagic E. coli pilus, and EaeH in all isolates. P fimbriae were detected in 80% of the isolates. ExPEC can cause sepsis and infections under very low iron availability, as this pathotype has developed many strategies for obtaining iron [16]. The sitABCD system is a membrane pump system, and the ChuA transporter enables Fe uptake directly from extracellular heme. In addition, ExPEC have siderophores, such as salmochelin, yersiniabactin, and aerobactin, which are small molecules with high affinity for Fe ions that indirectly uptake Fe [17]. Yersiniabactin contributes to the pathogenicity of UPEC during urinary tract colonization [18, 19]. In our study, 98% isolates had yersiniabactin, the sitABCD system, and the chuA transporter. Aerobactin receptors are substantially more efficient at capturing Fe than enterobactin receptors [7]. In this study, 74% and 89% of the ST131 and ST1193 isolates, respectively, had the gene encoding aerobactin. An ExPEC salmochelin marker gene, iroN, was detected in 5% of the ST131 isolates, but in none of the ST1193 isolates.
Some of the most frequently detected toxin genes in ExPEC are hlyA, hlyD, hlyF, cdtB, tsh, sat, pic, vat, and astA [7]. hlyF, cdtB, and tsh have been detected in NMEC strains [20]. While all isolates carried hlyE in this study, alpha-hemolysins encoded by hlyABCD were detected in only 53% of ST131 and 27% of ST69 isolates. The cnf1 gene was detected in 53% of the ST131 isolates. The presence of these genes in ST131 isolates suggests that they exhibit high virulence and toxicity compared with non-ST131 isolates. The IbeA protein recognizes surface receptors on brain capillary endothelial cells, allowing the pathogen to invade the nervous system and cause neonatal meningitis [21–23]. Therefore, ibeA is a representative VF gene in NMEC strains. In our study, three of each of ST357, ST372, and ST998 isolates (5%) carried ibeA. Major UTI pathogens, such as ST131 and ST1193, may not carry ibeA and therefore, do not cause meningitis in neonates.
ST131 isolates carried more VF genes, including tsh and espC, than non-ST131 isolates. The VF genes in ST131 isolates may play a role in pathogenesis. In a previous study, tsh was present in >50% of avian pathogenic E. coli isolates, 4.5% of UPEC isolates, and 11.5% of NMEC isolates [15]. In this study, all ST131 and ST1193 isolates among UTI-associated E. coli isolates carried tsh. Although the function of the temperature-sensitive hemagglutinin encoded by tsh in human infections is unclear, in Bacteroides fragilis, this protein contributes to abscess formation in intra-abdominal infections [24]. ST127 isolates can cause bacteremia in adults and are highly virulent in experimental models of invasive E. coli infection. Therefore, ST127 isolates should be monitored because of their greater pathogenic potential than more widely recognized clones, including ST73, ST95, and ST131 [25]. Two ST127 isolates were recovered from urine in this study and had high virulence scores of 20 and 17.
ST131 isolates reportedly had a higher virulence potential than other E. coli types in a murine sepsis model [5]. Similarly, in this study, ST131 isolates had higher virulence scores than ST1193, ST95, and ST69 isolates (P<0.05). Shah, et al. [26] suggested a significant association between VFs and antimicrobial resistance in UPEC. Consistent herewith, we found that ST131 isolates of UTI patients had several VFs and a high resistance rate to several antibiotics.
Our study had some limitations, mainly related to the relatively small specimen size. Because of the limited number of E. coli bacteremia isolates reported, larger studies are needed to accurately determine the VF composition of these isolates in relation to their clonal characteristics. Further studies are needed to clarify the association between virulence and antibiotic resistance in urosepsis-related E. coli strains. Second, WGS analysis and web-based assessment are subject to experimental error. Third, the association between VFs and disease severity could not be clarified in this study.
In conclusion, E. coli bacteremia isolates from UTI patients were analyzed for a broad range of VFs, molecular backgrounds, and antibiotic resistance genes using WGS. We found no STs and VFs associated with bacteremia in WGS data of E. coli isolated from UTI patients. ST131, a pandemic multidrug-resistant clone present in both blood and urine specimens, was the most frequent ST and carried more VF genes, especially tsh and espC, than non-ST131 isolates.
ACKNOWLEDGEMENTS
None.
Footnotes
AUTHOR CONTRIBUTIONS
Lee Y and Kim B designed the study; Kim J collected and identified clinical isolates and performed molecular studies; Lee Y, Kim B, and Kim J analyzed the data; Lee Y and Kim B wrote, edited, and reviewed the manuscript. All authors revised and accepted the final version of the manuscript.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this article were reported.
RESEARCH FUNDING
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1F1A1061400).
REFERENCES
- 1.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–60. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front Microbiol. 2012;3:9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaik S, Ranjan A, Tiwari SK, Hussain A, Nandanwar N, Kumar N, et al. Comparative genomic analysis of globally dominant ST131 clone with other epidemiologically successful extraintestinal pathogenic Escherichia coli (ExPEC) lineages. mBio. 2017;8:e01596–17. doi: 10.1128/mBio.01596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tchesnokova VL, Rechkina E, Larson L, Ferrier K, Weaver JL, Schroeder DW, et al. Rapid and extensive expansion in the United States of a new multidrug-resistant Escherichia coli clonal group, sequence type 1193. Clin Infect Dis. 2019;68:334–7. doi: 10.1093/cid/ciy525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JR, Porter SB, Zhanel G, Kuskowski MA, Denamur E. Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infect Immun. 2012;80:1554–62. doi: 10.1128/IAI.06388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E, Lee Y. Prevalence of Escherichia coli carrying pks islands in bacteremia patients. Ann Lab Med. 2018;38:271–3. doi: 10.3343/alm.2018.38.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, et al. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Combe B, Clermont O, Messika J, Eveillard M, Kouatchet A, Lasocki S, et al. Pneumonia-specific Escherichia coli with distinct phylogenetic and virulence profiles, France, 2012-2014. Emerg Infect Dis. 2019;25:710–8. doi: 10.3201/eid2504.180944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora-Rillo M, Fernández-Romero N, Navarro-San Francisco C, Díez-Sebastián J, Romero-Gómez MP, Fernández FA, et al. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence. 2015;6:93–100. doi: 10.4161/21505594.2014.991234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauréguy F, Carbonnelle E, Bonacorsi S, Clec'h C, Casassus P, Bingen E, et al. Host and bacterial determinants of initial severity and outcome of Escherichia coli sepsis. Clin Microbiol Infect. 2007;13:854–62. doi: 10.1111/j.1469-0691.2007.01775.x. [DOI] [PubMed] [Google Scholar]
- 11.Cole BK, Ilikj M, McCloskey CB, Chavez-Bueno S. Antibiotic resistance and molecular characterization of bacteremia Escherichia coli isolates from newborns in the United States. PLoS One. 2019;14:e0219352. doi: 10.1371/journal.pone.0219352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI, author. CLSI supplement M100S. 30th ed. Wayne, PA; Clinical and Laboratory Standards Institute: 2020. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 13.Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47:D687–92. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y, Johnson JR, Yamamoto M, Nagao M, Tanaka M, Takakura S, et al. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J Antimicrob Chemother. 2015;70:1639–49. doi: 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 15.Dhanji H, Doumith M, Rooney PJ, O'Leary MC, Loughrey AC, Hope R, et al. Molecular epidemiology of fluoroquinolone-resistant ST131 Escherichia coli producing CTX-M extended-spectrum beta-lactamases in nursing homes in Belfast, UK. J Antimicrob Chemother. 2011;66:297–303. doi: 10.1093/jac/dkq463. [DOI] [PubMed] [Google Scholar]
- 16.Antão EM, Wieler LH, Ewers C. Adhesive threads of extraintestinal pathogenic Escherichia coli. Gut Pathog. 2009;1:22. doi: 10.1186/1757-4749-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Wang X, Xu H, Xu Y, Ling J, Zhang D, et al. Roles of iron acquisition systems in virulence of extraintestinal pathogenic Escherichia coli: salmochelin and aerobactin contribute more to virulence than heme in a chicken infection model. BMC Microbiol. 2012;12:143. doi: 10.1186/1471-2180-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caza M, Lépine F, Dozois CM. Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli. Mol Microbiol. 2011;80:266–82. doi: 10.1111/j.1365-2958.2011.07570.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JR, Magistro G, Clabots C, Porter S, Manges A, Thuras P, et al. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 ('clonal group A') cystitis isolate in murine models of urinary tract infection and sepsis. Microb Pathog. 2018;120:128–31. doi: 10.1016/j.micpath.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Mellata M, Johnson JR, Curtiss R., 3rd Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections. Zoonoses Public Health. 2018;65:103–13. doi: 10.1111/zph.12376. [DOI] [PubMed] [Google Scholar]
- 22.Bonacorsi S, Bingen E. Molecular epidemiology of Escherichia coli causing neonatal meningitis. Int J Med Microbiol. 2005;295:373–81. doi: 10.1016/j.ijmm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhao WD, Liu DX, Wei JY, Miao ZW, Zhang K, Su ZK, et al. Caspr1 is a host receptor for meningitis-causing Escherichia coli. Nat Commun. 2018;9:2296. doi: 10.1038/s41467-018-04637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto BR, van Dooren SJ, Dozois CM, Luirink J, Oudega B. Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and heme-dependent growth of Bacteroides fragilis. Infect Immun. 2002;70:5–10. doi: 10.1128/IAI.70.1.5-10.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alghoribi MF, Gibreel TM, Dodgson AR, Beatson SA, Upton M. Galleria mellonella infection model demonstrates high lethality of ST69 and ST127 uropathogenic E. coli. PLoS One. 2014;9:e101547. doi: 10.1371/journal.pone.0101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019;19:204. doi: 10.1186/s12866-019-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]